Abstract
Purpose:
To study the efficacy of the Aurolab aqueous drainage implant (AADI) compared to Ahmed glaucoma valve (AGV) in patients with refractory glaucoma.
Methods:
This was a prospective, randomized controlled trial. Thirty-eight adult patients (>18 years) scheduled for a glaucoma drainage device (GDD) were randomized into two groups to receive either AGV or AADI. Primary outcome measures: intraocular pressure (IOP) control and requirement of antiglaucoma medications; secondary outcome measures: final best correct visual acuity (log MAR), visual field (Visual field index [VFI], mean deviation [MD] and pattern standard deviation [PSD]), postoperative complications and additional interventions. Complete success was defined as IOP ≥5–≤18 mmHg without antiglaucoma medications/laser/additional glaucoma surgery or any vision threatening complications.
Results:
There were 19 age and sex-matched patients in each group. Both groups had comparable IOP before surgery (P = 0.61). The AGV group had significantly lower IOP compared to AADI group (7.05 ± 4.22 mmHg vs 17.90 ± 10.32 mmHg, P = <0.001) at 1 week. The mean postoperative IOP at 6 months was not significantly different in the two groups (13.3 ± 4.2 and 11.4 ± 6.8 mmHg respectively; P = 0.48). At 6 months, complete success rate according to antiglaucoma medication criteria was 78.94% in AADI and 47.36% in AGV groups. AGV group required 1.83 times more number of topical medications than AADI group. There was no significant difference in early (P = 0.75) and late (P = 0.71) postoperative complications in the AADI and AGV group. The complete success rate was higher in AADI group (68.42%) than AGV group (26.31%) (P = 0.034).
Conclusion:
In this study, AADI appears to have comparable efficacy versus AGV implant with higher complete success rate at 6 months follow-up.
Keywords: Ahmed glaucoma valve, Aurolab aqueous drainage implant, refractory glaucoma
Glaucoma drainage devices (GDD) were initially reserved for patients with failed trabeculectomy, but are now becoming the primary choice for surgery in refractory glaucoma.[1,2] Typically, GDDs create alternate pathways by channeling aqueous from the anterior chamber to an equatorial plate through a long tube and promote bleb formation posteriorly. Ahmed glaucoma valve (AGV; New World Medical Inc., Rancho Cucamonga, USA) is the most commonly used valve worldwide. It is made up of silicone, has a smaller surface area of 184 mm2, and maintains a tapered profile for easy insertion. It has a unique, nonobstructive valve system to prevent excessive drainage and chamber collapse, but provides immediate reduction of intraocular pressure.
Aurolab aqueous drainage implant (AADI) has been introduced recently for clinical use in India by Aurolab, a manufacturing division of Aravind Eye Institute, Madurai, Tamil Nadu. AADI is a nonvalved GDD made up of silicone and modeled on the principle of Baerveldt implant with characteristic feature of large surface area of 350 mm2.[3,4] The 32-mm long end plate extends beyond 2 clock hours of circumference on the equatorial sclera. Though the implant is available for use in India, there are only a couple of published data about the safety or efficacy of this implant;[5,6] however, none of the studies were compared same with AGV implant. The purpose of this study was to assess the safety and efficacy of AADI and compare it with AGV.
Methods
This was a prospective randomized interventional study, recruiting refractory glaucoma patients who presented to the tertiary care institute from August 2013 to March 2014. The study followed the declaration of Helsinki guidelines and was approved by the Institutional Ethics Committee. Informed consent was obtained from all patients. The patients were assigned to either of the two groups using random number tables, groups A (AADI) and B (AGV), recruiting 20 patients in each group. The total number of patients were arbitrarily selected as 40 because the magnitude of difference (effect size) between AADI and AGV implant was not known.
The patients above 18 years of age, with IOP >18 mmHg, poorly controlled with maximum antiglaucoma medications, failed SLT or trabeculectomy with significant optic nerve damage and visual field loss due to glaucoma, prior history of filtering surgery with significant conjunctival scarring were included. Patients with no light perception, previous cyclodestructive procedure/previous glaucoma drainage devices, corneal abnormalities that precluded accurate IOP readings, uncontrolled systemic diseases, and any other active ocular disease, (active uveitis, ocular infection) were excluded.
All patients underwent detailed baseline evaluation at presentation which included Snellen visual acuity, slit-lamp biomicroscopy examination of the anterior and posterior segment, intraocular pressure measurement (Goldmann applanation tonometry, average of 2 measurements with 1 mmHg or less difference), gonioscopy, and standard automated perimetry (Humphrey's Field Analyzer HFA 750 II, Carl Zeiss-Humphrey Systems, Dublin, CA) (if Snellen's visual acuity of ≥6/60). 24-2 testing protocol in majority/10-2, if needed.
Surgical details and technique
All procedures were performed by a single surgeon (SSP). The basic surgical steps were similar for both the procedures. The subtenon space was exposed in superotemporal quadrant. The implant was inserted in the subtenon space, and the end plate was secured to sclera using 5-0 Dacron suture (Polyester, Green Braided, Alcon Laboratories, Inc. Fort worth, Texas, USA) with the anterior edge of the device 8–10 mm posterior to the limbus. Anterior chamber entry was made with a 23-G needle to create a track of around 1.5 mm in length parallel to the iris plane. Tube was trimmed to the desired length and inserted into the anterior chamber through a 1.5 mm long track created with a 23-G needle. The tube was positioned in the anterior chamber away from the corneal endothelium without touching the iris. Anterior part of the tube was covered with a donor scleral patch graft 4 mm × 5 mm in size, which was sutured with 10-0 non absorbable suture. Conjunctiva was sutured to the limbus with 8- 0 vicryl sutures. (Braided coated polyglactin 910 violet, Ethicon, Johnson & Johnson Ltd. India).
AADI
AADI was used in all cases randomized to the group A. Both superior and lateral rectii muscles were isolated and the lateral expansions of the implant plate were positioned beneath them. A 1-0 nylon (monofilament polyamide black, Ethilon, Ethicon, Johnson and Johnson Ltd. India) nonabsorbable suture was placed alongside parallel to the tube as stent suture. Tube and stent suture was ligated together (externally) near the tube plate junction with 6-0 vicryl (Braided coated polyglactin 910 violet, Ethicon, Johnson and Johnson Ltd. India), and complete occlusion was ensured by injecting balanced salt solution through the tube. The other free end of stent suture was positioned in lower fornix so that it can be pulled out later on.
AGV
The basic surgical steps were the same as described above. AGV Model FP7 was used in all cases randomized to the treatment group B after priming the valve with balanced salt solution. All patients received topical Moxifloxacin 0.5% eyedrops (Vigamox, Alcon Laboratories, Inc. Fortworth, Texas, USA) three times a day for 4–6 weeks.
All patients also received topical betamethasone 0.1% eyedrops (Betnesol N, GlaxoSmithKline, India) 4 times a day which was tapered over 4–6 weeks. All antiglaucoma medications were continued in the postoperative period in the AADI group for 4–6 weeks, and then they were gradually tapered. In the AGV group, all antiglaucoma medications were stopped on the first postoperative day and reintroduced, as and when required.
All patients had scheduled postoperative follow-up examinations at day 1, 1 week, 1 month, 3 months, and 6 months after surgery. Visual fields were done at 3 month and 6 months follow-up. Complete success at final follow-up was defined as achievement of final IOP of ≥5 and ≤18 mmHg without any medication, with no vision threatening complications or additional glaucoma surgery/laser procedure and visual loss less than 2 Snellen acuity lines; qualified success if similar IOP control required 1 or 2 topical antiglaucoma medications with no vision threatening complications or additional glaucoma surgery/laser procedure and visual loss more than 2 Snellen lines but no progression to no light perception, and treatment failure if IOP was <5 mmHg or >18 mmHg and required 3 topical or systemic antiglaucoma medications, presence of vision threatening complications, or additional glaucoma procedures or if vision loss progressed to no light perception. We defined complications recorded during follow-up as early (within 3 months) and late (after 3 months). The postoperative data was collected by the same observer in all patients who was not blinded to the choice of implant used in particular patient.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences program (IBM SPSS version 20.0 for windows, Chicago, IL, USA). The normality of quantitative data was checked using Kolmogorov–Smirnov test. Quantitative parameters at each point of time for two groups were compared by Student's t-tests and their descriptive data were presented by Mean ± SD. Qualitative data was described as frequencies and proportions and was analysed for its association with the groups using Chi-square test. A P value of less than 0.05 was considered significant.
Results
Of 40 patients enrolled in the study, 38 (19 in each group) who had minimum 6 months follow-up were enrolled for analysis. The demographic data and baseline characteristics of both the groups are summarized in Tables 1 and 2. The baseline and follow-up IOPs for the 2 groups are illustrated in Fig. 1. The baseline IOP in AADI group was 25.89 ± 8.42 (95% CI: 22.10, 29.69) mmHg, whereas the baseline IOP in AGV group was 24.26 ± 10.78 (95% CI: 19.42, 29.18) mmHg. There was significant reduction in IOP at 6 months in both the groups with 54% and 48% reduction from the baseline, respectively. The AGV group had significantly lower IOP compared to the AADI group (7.05 ± 4.22 mmHg vs 17.90 ± 10.32 mmHg, P = <0.001) at 1 week. The mean IOP in the AADI group was approximately 1 mmHg lower than that of the AGV group at 6 month follow-up; however, this difference was not statistically significant (P = 0.48).
Table 1.
Preoperative demographics of refractory glaucoma patients
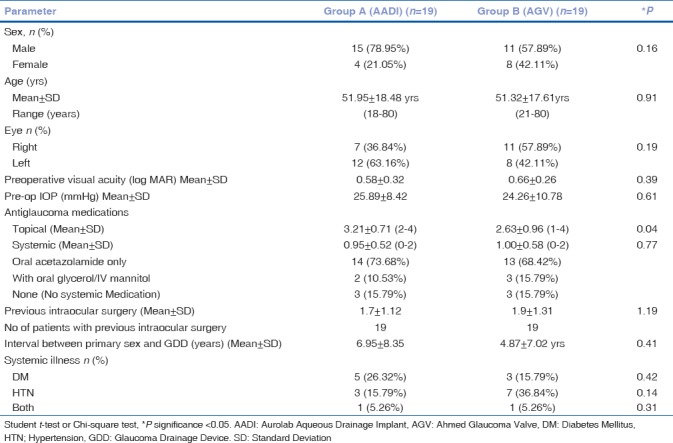
Table 2.
Preoperative diagnosis and previous surgeries in two groups
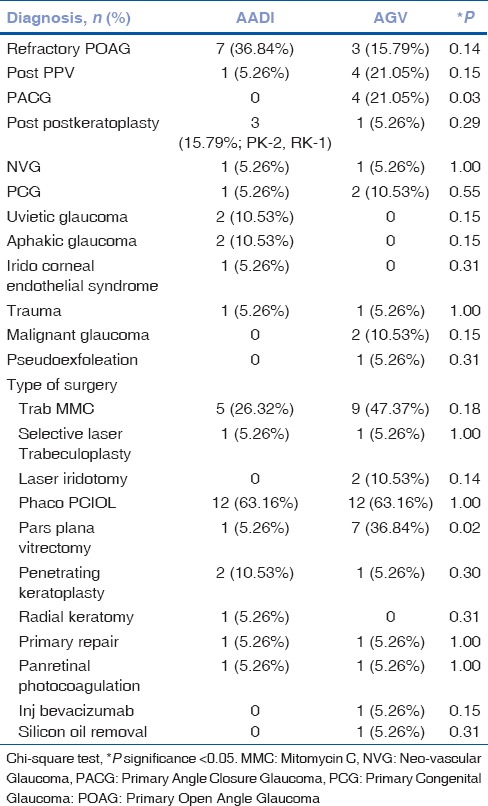
Figure 1.
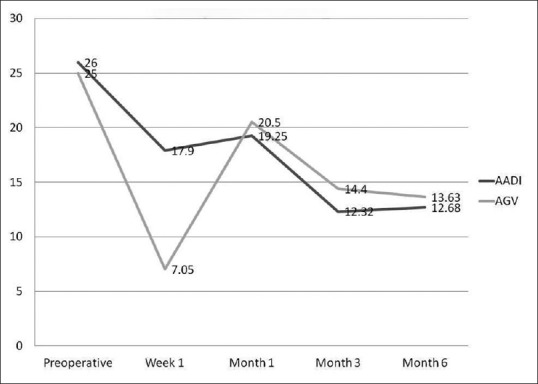
IOP trends following AADI and AGV implantation
The patients who were implanted with AADI had higher number of mean preoperative topical medication vs AGV group (P = 0.02). There was a significant reduction in the need for medical therapy in both the groups at 6 months with 72% and 38% reduction from the baseline in AADI and AGV groups, respectively. At 6 months, complete success rate according to antiglaucoma medication criteria was 78.94% and 47.36%, respectively, in AADI and AGV group. AGV group required 1.83 times more number of topical medication than AADI group (1.63 ± 0.90 vs 0.89 ± 0.94; P = 0.02), but there was no significant difference in the requirement of systemic medication (0.21 ± 0.419 vs 0.16 ± 0.375; P = 0.69) between the two groups at 6 months.
In AADI group, stent suture was used in 19 patients for a mean duration of 5.44 ± 2.77 weeks (range, 2–14 weeks). In one patient due to high IOP of 28 mmHg at 2 weeks, early stent removal was performed (by pulling out the forniceal end of stent suture with a tooth forceps) whereas in another patient stent removal was delayed till 14 weeks due to persistent low IOP of 10 mmHg. Mean intraocular pressure decreased from 21.61 ± 10.28 mmHg before the removal of stent to 8.89 ± 5.05 mmHg after the removal of stent.
The mean baseline visual acuity (Snellen best corrected visual acuity was converted into logarithm of minimum angle of resolution (log MAR) for analysis by using vision acuity conversion table; http://publicfiles.jaeb.org/drcrnet/Misc/VAScoreConversionChart.pdf) in AADI group was 0.59 ± 0.32 (95% CI: 0.45, 0.73) while in AGV group was 0.64 ± 0.28 (95% CI: 0.51, 0.77). Improvement by two or more Snellen lines was seen in 18 patients (94.73%) in AADI group and 17 patients (89.47%) in AGV group. There was no significant difference in improvement in mean BCVA (log MAR) at 6 months follow-up between the two groups (P = 0.863). None of the patients lost light perception. The mean pre and postoperative global indices in AADI group was as follows, VFI = 45.80 ± 31.69 and 42.88 ± 30.82, MD = −20.10 ± 7.49 and −18.16 ± 7.46 and PSD = 5.55 ± 1.75 and 6.59 ± 2.01. While mean pre and postoperative global indices in AGV group was VFI = 44.75 ± 33.97 and 47.00 ± 34.37, MD = −13.63 ± 14.60 and −18.09 ± 7.94, and PSD = 6.12 ± 2.49 and 5.84 ± 2.72. No significant differences in visual field global indices were observed both intra/intergroups.
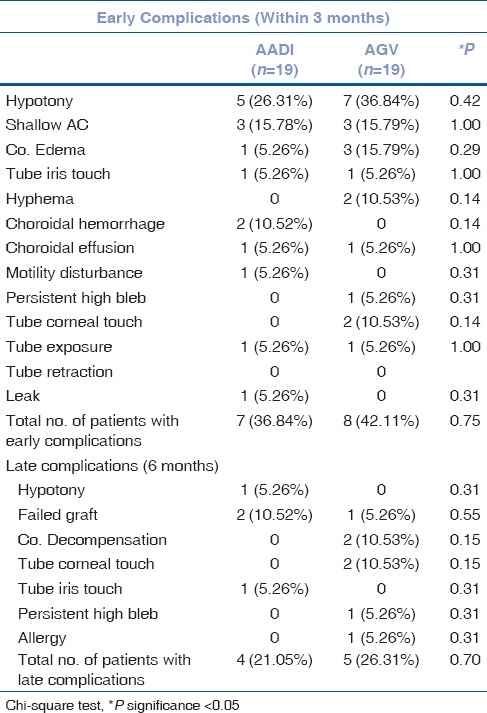
No significant intraoperative complications were noted. Early complications were seen in 8 patients in AGV group (42.11%) vs 7 patients in the AADI group (36.84%) (P = 0.752). Most common early postoperative complication were hypotony (26.31%) followed by shallow anterior chamber (15.78%) in the AADI group. In AADI group, 2 (10.52%, noticed at day 1 and 1 week post operatively) patients had choroidal hemorrhage and 1 (5.26%, noticed at day 1) had serous choroidal detachment. One of the patient with choroidal hemorrhage and other with serous choroidal detachment had hypotony on day one, which might lead to choroidal hemorrhage or detachment. There was no significant difference for late postoperative complications which occurred in 5 patients in the AGV group (26.31%) and 4 patients in the AADI group (21.05%) (P = 0.705). Table 3 lists early (during 3 months) and late (at 6 months follow-up) postoperative complications.
Table 3.
Comparison of early and late post-operative complications in AADI and AGV groups
Re-surgeries for complications were performed in 7 (36.8%) patients in the AADI group and in 5 (26.3%) patients in the AGV group (P = 0.490). The reasons and timing for reoperations in the AADI group included suprachoroidal hemorrhage drainage in 2 (10.52%) patients at 1 month, tube ligation for hypotony in 1 (5.26%) at 3 months, patch graft for tube exposure in 1 (5.26%) at 3 months, tube reposition secondary to tube-lens touch in 1 (5.26%), and optical keratoplasty for graft failure (visual rehabilitation) in 2 (10.52%) patients at 6 months. In the AGV group, complications included conjunctival resuturing for tube exposure in 1 (5.26%) at 1 month, tube reposition for tube-corneal touch in 1 (5.26%) at 3 months, optical keratoplasty for corneal decomposition, or pseudophakic bullous keratopathy done in 3 (15.78%) at 6 months. None of the patients required implant removal secondary to any cause.
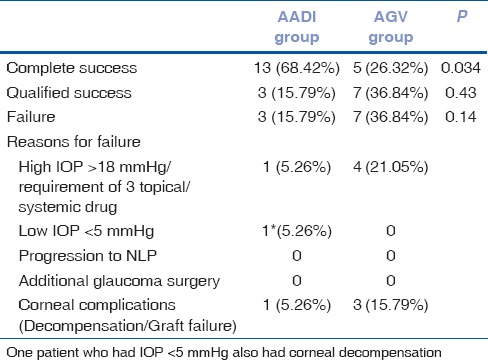
There was significant difference in outcome in terms of complete success between the two groups at 6 month of follow-up (P = 0.034). However, there was no significant difference in the overall success (complete and qualified) between the two groups (0.14) [Table 4].
Table 4.
Reasons for failure in 6 months of follow-up
Discussion
Our study compared the efficacy and safety of two aqueous drainage devices used for refractory glaucoma, the AGV, and the new implant AADI. To our knowledge, this is the first randomized comparative study on safety and efficacy of AADI and AGV implants.
The design of the AADI is quite different from AGV and shares some features with Baerveldt implant. AADI is a valve-less drainage device without any flow restrictor,[3,4] requiring intraoperative tube ligation to prevent postoperative hypotony. This results in persistence of high IOP in postoperative period until the ligation-suture dissolves or is removed. Once patent, the nonvalved implants achieve good IOP reduction owing to its large filtration surface area,[7] but hypotony and its resultant complications are much more common if flow is not restricted by using a suture ligature.[8,9,10] This suture usually dissolves in 5–6 weeks postoperatively.
On the other hand, AGV has a restrictive valve designed to prevent hypotony. This is particularly important in the immediate postoperative period before a capsule forms around the end plate. Encapsulation leading to hypertensive phase has been reported with AGV, requiring higher post-operative medications.[11,12,13] This may be a result of the smaller surface area of the Ahmed implant compared with the Baerveldt implant, more thickness of implant, additional resistance created by the valve mechanism, and early exposure of bleb to inflammatory mediators in the aqueous. End plates of all the implants develop a surrounding capsule,[14] but the degree to which it encapsulates is the main determinant of implant function. In both valved and non-valved drainage devices, capsule is the main point of resistance to aqueous flow. Surface area of the external plate is one of the important variables that may influence IOP control. The larger surface area of the implant provides a greater potential drainage bleb area for aqueous absorption and lower IOP.[8,15] However, relation between IOP lowering and implant size is not linear. Other influencing factors include thickness, material, and flexibility of the drainage device.[16]
In our study, IOP was higher in the AADI group, in the first 4-6 weeks after surgery, requiring more number of medications. The mean IOP in AADI group, though not statistically significant, was lower than AGV group at 6 month follow-up visit by 1 mmHg. This observation is similar to Ahmed Baerveldt Comparison study and Ahmed Versus Baerveldt study, in which more IOP reduction from baseline was found in Baerveldt Glaucoma Implant group, even though the two populations and patient profiles were different.[8,17,18]
There was significant difference in the outcome in terms of complete success in AADI versus AGV groups at 6 months. Though the overall success rate was higher i.e. 73.6% in AADI group and 63.1% in AGV group (P = 0.48), but AADI group has more complete success rate 63.15% in comparison to AGV group, i.e. 26.3% (P < 0.034). Complete success rate of AADI in our study was comparable (66.6%) with the recently published study by Ray et al.[6]
The rates of failure were high in AGV group (36.84%) vs AADI (15.79%). It was similar to those reported in previous studies on AGV and BGI, in which Ahmed implantation had a failure rate of 16% to 38% and Baerveldt implantation had a failure rate of 8% to 36%.[19,20,21] This difference was likely a result of the more stringent IOP target of 18 mmHg that we used compared to an IOP of 21 mmHg in other studies. We chose 18 mmHg as upper limit because all of our patients had advanced glaucoma with lower target IOP requirements.
There was significantly greater topical as well as systemic antiglaucoma medications requirement in AADI group compared to AGV in first month, but after that the AGV group required more topical and systemic medications during the 6 month follow-up in our study. Though the patients included in AADI group were on significantly higher topical antiglaucoma medication preoperatively, but at 6 months, it was the AGV group that required 1.83 times the topical medication compared to AADI group. This could be related to the hypertensive phase in AGV. There was no significant difference in postoperative complications between the two groups. Most postoperative complications reported with these implants were transient and resolved completely with conservative management.[17,18,19,20,22]
Serious complications such as suprachoroidal hemorrhage was seen in 2 patients with AADI implantation (10.52%), whereas serous choroidal effusion extending beyond equator was seen in 1 (5.26%) patient each undergoing AADI and AGV implantation. Other tube related complications include tube iris touch, tube corneal touch, tube retraction, and tube exposure. Nguyen et al. reported suprachoroidal hemorrhage in 4% of the patients and choroidal effusions requiring surgical drainage in 2% patients undergoing Baervedlt implantation.[23] In a study by Colemann et al., suprachoroidal hemorrhage was seen in 2% and serous choroidal detachment in 22% patients undergoing AGV implantation.[24] Re-surgeries for complications were performed in 7 (36.8%) patients in the AADI group and in 5 (26.3%) patients in the AGV group. In a study by Ray et al.,[6] the authors reported 25.9% of repeat procedures for early and late complications in patients undergoing AADI implantation. Higher percentage of complications and re-surgeries in the present study could be attributed to small sample of study population. Apart from that we suggest that prevention of hypotony, proper positioning of tube in the anterior chamber, proper placement of patch graft, and meticulous conjunctival closure can prevent these potentially serious complications.
Apart from the randomized, prospective nature of the study, all surgeries were performed by a single surgeon, so that the difference in surgical experience which was one of the potential limitation of ABC study was eliminated.[9,19,22]
Limitations in the current study are small sample size as well as short-term follow-up of the patients. Longer follow-up are required to evaluate the safety of any implants or procedure or long-term efficacy. Another potential limitation was hypertensive phase not defined though a couple of patients had IOP spikes. The efficacy of glaucoma procedures in reducing IOP must be evaluated in light of adverse events associated with their use.
Conclusion
In our study, we found AADI group had similar IOP control compared to AGV with lesser need for antiglaucoma medications at 6 months follow-up leading to higher complete success rate compared to AGV group at 6 months follow-up. Longer follow-up will provide additional information on the relative efficacy of these two implants on long-term IOP control and evaluate the risk–benefit ratio of both the devices in the surgical management of refractory glaucoma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ramulu PY, Corcoran KJ, Corcoran SL, Robbin Al. Utilization of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995 to 2004. Ophthalmology. 2007;114:2265–70. doi: 10.1016/j.ophtha.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Desai MA, Gedde SJ, Feuer WJ, Shi W, Chen PP, Parrish RK., 2nd Practice preferences for glaucoma surgery: A survey of the American Glaucoma Society in 2008. Ophthalmic Surg Lasers Imaging. 2011;42:202–8. doi: 10.3928/15428877-20110224-04. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd MA, Baerveldt G, Fellenbaum PS, Sidoti PA, Minckler DS, Martone JF, et al. Intermediate-term results of a randomized clinical trial of the 350- versus the 500-mm2 Baerveldt implant. Ophthalmology. 1994;101:1456–63. doi: 10.1016/s0161-6420(94)31152-3. [DOI] [PubMed] [Google Scholar]
- 4.Krishna R, Godfrey DG, Budenz DL, Escalona-Camaaño E, Gedde SJ, Greenfield DS, et al. Intermediate-term outcomes of 350-mm(2) Baerveldt glaucoma implants. Ophthalmology. 2001;108:621–6. doi: 10.1016/s0161-6420(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 5.Kaushik S, Kataria P, Raj S, Pandav SS, Ram J. Safety and efficacy of low cost glaucoma drainage devices for refractory childhood glaucoma. Br J Ophthalmol. 2017;101:1623–27. doi: 10.1136/bjophthalmol-2017-310276. [DOI] [PubMed] [Google Scholar]
- 6.Ray VP, Rao DP. Surgical Outcomes of a New Low-Cost Nonvalved Glaucoma Drainage Device in Refractory Glaucoma: Results at 1 Year. J Glaucoma. 2018;27:433–9. doi: 10.1097/IJG.0000000000000930. [DOI] [PubMed] [Google Scholar]
- 7.Roy S, Ravinet E, Mermoud A. Baerveldt implant in refractory glaucoma: Long-term results and factors influencing outcome. Int Ophthalmol. 2001;24:93–100. doi: 10.1023/a:1016335313035. [DOI] [PubMed] [Google Scholar]
- 8.Budenz DL, Barton K, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, et al. Ahmed Baerveldt Comparison Study Group. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology. 2011;118:443–52. doi: 10.1016/j.ophtha.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christakis PG, Tsai JC, Zurakowski D, Kalenak JW, Cantor LB, Ahmed II. The Ahmed versus Baerveldt study: Design, baseline patient characteristics, and intraoperative complications. Ophthalmology. 2011;118:2172–9. doi: 10.1016/j.ophtha.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Seah SK, Gazzard G, Aung T. Intermediate-term outcome of Baerveldt glaucoma implants in Asian eyes. Ophthalmology. 2003;110:888–94. doi: 10.1016/S0161-6420(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 11.Huang MC, Netland PA, Coleman AL, Siegner SW, Moster MR, Hill RA. Intermediate- term clinical experience with the Ahmed Glaucoma Valve implant. Am J Ophthalmol. 1999;127:27–33. doi: 10.1016/s0002-9394(98)00394-8. [DOI] [PubMed] [Google Scholar]
- 12.Nouri-Mahdavi K, Caprioli J. Evaluation of the hypertensive phase after insertion of the Ahmed Glaucoma Valve. Am J Ophthalmol. 2003;136:1001–8. doi: 10.1016/s0002-9394(03)00630-5. [DOI] [PubMed] [Google Scholar]
- 13.Ayyala RS, Zurakowski D, Smith JA, Monshizadeh R, Netland PA, Richards DW, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology. 1998;105:1968–76. doi: 10.1016/S0161-6420(98)91049-1. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd MA, Baerveldt G, Nguyen QH, Minckler DS. Long-term histologic studies of the Baerveldt implant in a rabbit model. J Glaucoma. 1996;5:334–9. [PubMed] [Google Scholar]
- 15.Britt MT, LaBree LD, Lloyd MA, Minckler DS, Heuer DK, Baerveldt G, et al. Randomized clinical trial of the 350-mm2 versus the 500-mm2 Baerveldt implant: longer term results: Is bigger better? Ophthalmology. 1999;106:2312–8. doi: 10.1016/S0161-6420(99)90532-8. [DOI] [PubMed] [Google Scholar]
- 16.Ayyala RS, Michelini-Norris B, Flores A, Haller E, Margo CE. Comparison of different biomaterials for glaucoma drainage devices: Part 2. Arch Ophthalmol. 2000;118:1081–4. doi: 10.1001/archopht.118.8.1081. [DOI] [PubMed] [Google Scholar]
- 17.Barton K, Feuer WJ, Budenz DL, Schiffman J, Costa VP, Godfrey DG, et al. Three-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2014;121:1547–57. doi: 10.1016/j.ophtha.2014.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budenz DL, Barton K, Gedde SJ, Feuer WJ, Schiffman J, Costa VP, et al. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122:308–16. doi: 10.1016/j.ophtha.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christakis PG, Kalenak JW, Tsai JC, Zurakowski D, Kammer JA, Harasymowycz PJ, et al. The Ahmed versus Baerveldt Study: Five-Year treatment outcomes. Ophthalmology. 2016;123:2093–102. doi: 10.1016/j.ophtha.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 20.Rockwood EJ. The Ahmed Baerveldt Comparison (ABC) Study: Long-term results, successes, failures, and complications. Am J Ophthalmol. 2016;163:xii–xiv. doi: 10.1016/j.ajo.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz KS, Lee RK, Gedde SJ. Glaucoma drainage implants: A critical comparison of types. Curr Opin Ophthalmol. 2006;17:181–9. doi: 10.1097/01.icu.0000193080.55240.7e. [DOI] [PubMed] [Google Scholar]
- 22.Budenz DL, Feuer WJ, Barton K, Schiffman J, Costa VP, Godfrey DG, et al. Postoperative Complications in the Ahmed Baerveldt Comparison study during five years of follow-up. Am J Ophthalmol. 2016;163:75–82.e3. doi: 10.1016/j.ajo.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen QH, Budenz DL, Parrish RK., 2nd Complications of Baerveldt glaucoma drainage implants. Arch Ophthalmol. 1998;116:571–5. doi: 10.1001/archopht.116.5.571. [DOI] [PubMed] [Google Scholar]
- 24.Coleman AL, Hill R, Wilson MR, Choplin N, Kotas-Neumann R, Tam M, et al. Initial clinical experience with the Ahmed Glaucoma Valve implant. Am J Ophthalmol. 1995;120:23–31. doi: 10.1016/s0002-9394(14)73755-9. [DOI] [PubMed] [Google Scholar]


