Abstract
Background:
Multiple sclerosis (MS) is a chronic central nervous system inflammatory demyelinating disease characterized by multiple lesions disseminated in time and space. The lesions often have characteristic imaging findings on magnetic resonance (MR) imaging and cerebrospinal fluid findings that lead to their diagnosis. At times, these lesions may resemble tumors due to their large size (>2 cm), significant vasogenic edema, and ring-enhancing MR imaging findings. Such lesions are described as tumefactive demyelinating lesions or tumefactive MS, and they are generally seen in aggressive forms of MS associated with rapid progression.
Case Description:
We report an uncommon but clinically significant case of transtentorial brain herniation secondary to malignant cerebral edema from tumefactive MS in a 50-year-old woman. After the initial diagnosis of MS, the patient continued to have progression of her white matter lesions suggesting evolution of her MS despite treatment with intravenous (IV) steroids, IV immunoglobulin, and plasmapheresis. She was admitted to the hospital with a new, large, ring-enhancing lesion that displayed significant mass effect from vasogenic edema and progressed, necessitating a decompressive hemicraniectomy.
Conclusion:
Tumefactive MS presents a unique pathology that can often mimic primary brain tumors. Although these lesions affect white matter and infrequently cause a significant amount of mass effect, they can act like a tumor, causing edema that generates sufficient intracranial pressure to cause transtentorial herniation.
Keywords: Decompressive hemicraniectomy, transtentorial herniation, tumefactive demyelinating lesion, tumefactive multiple sclerosis
INTRODUCTION
Primary demyelinating disease of the central nervous system presents in many different forms such as multiple sclerosis (MS), myelinoclastic diffuse sclerosis, and acute demyelinating encephalomyelitis. MS is a chronic inflammatory demyelinating disease of the central nervous system that is characterized by multiple lesions disseminated in time and space. Typical MS-associated lesions have a predilection for the periventricular areas, cerebellum, brainstem, spinal cord, and optic nerves and are visualized on T1 contrast-enhanced sequences as partially ring-enhancing on the basis of their acuteness.[2,9,11]
The term “tumefactive demyelinating lesion” in conjunction with MS was first introduced by Kepes in 1993.[10] These lesions occur more frequently in women, especially during the second or third decade of life. They are often misdiagnosed on neuroimaging due to their ill-defined margins, variable degree of perilesional edema, mass effect, central zones of necrosis, and cystic degeneration. The characteristic histopathological features of these lesions are the presence of foamy macrophages, reactive gliosis, and perivascular lymphocytic infiltrate.
Malignant edema from tumefactive MS lesions has been described in previous case reports. Most tumors and demyelinating lesions show considerable fluid-attenuated inversion recovery signal changes on magnetic resonance (MR) imaging that denote clinically significant vasogenic edema.[9] Most case reports describe lesions leading to subfalcine herniation.[14,18,22] Only two cases have been described in which MS presented with transtentorial brain herniation. The first case was described in 2001 of a cystic ring-enhancing frontal lesion that showed moderate mass effect on the lateral ventricles.[2] The second case involved a 22-year-old woman who presented with a 3-day course of headache and quickly worsened neurologically within the first 24 h of admission.[19] The patient had tumefactive demyelination with transtenorial herniation. Such a rapid decline in mentation and progression to a stuporous state is a rare circumstance that has not been reported in the literature.
We describe an uncommon case of confirmed tumefactive MS that mimicked a brain tumor in which the development of malignant edema led to transtentorial herniation.
CASE DESCRIPTION
This is a 50-year-old woman with an established diagnosis of MS manifested by transverse myelitis. She had been treated with intravenous (IV) steroids and glatiramer acetate with excellent results in the past for episodes of left-sided weakness after MR imaging of the brain done 1 year before this presentation revealed a deep white matter lesion with an incomplete ring-enhancing pattern without midline shift or edema [Figure 1].
Figure 1.
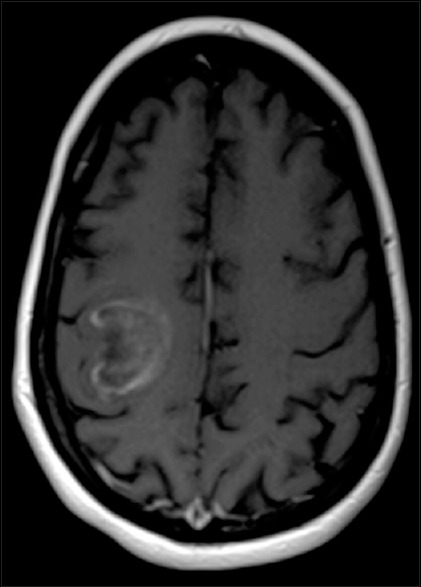
Axial T1-weighted contrast-enhanced magnetic resonance image demonstrating a right temporoparietal, partial ring-enhancing lesion in the white matter
On the current admission, she presented with nausea, vomiting, and lethargy. A computed tomographic (CT) scan of the head demonstrated subfalcine herniation [Figure 2] secondary to extensive vasogenic edema, which explained her obtundation. She was admitted to the intensive care unit and placed on 1000 mg of IV methylprednisone on a daily basis. Considerable improvement in her mentation was noted over the next day.
Figure 2.
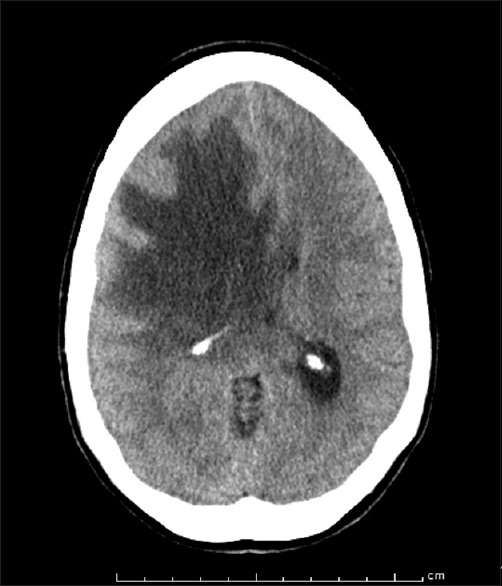
Axial computed tomographic image obtained at the time of admission, before high-dose steroid therapy was started, showing subfalcine herniation
On the third day of her stay in the intensive care unit, she developed a nonreactive dilated right pupil and was found to be somnolent. A repeat CT scan revealed worsening edema, causing transtentorial herniation [Figure 3]. She was taken immediately to the operating room for a right decompressive hemicraniectomy.
Figure 3.
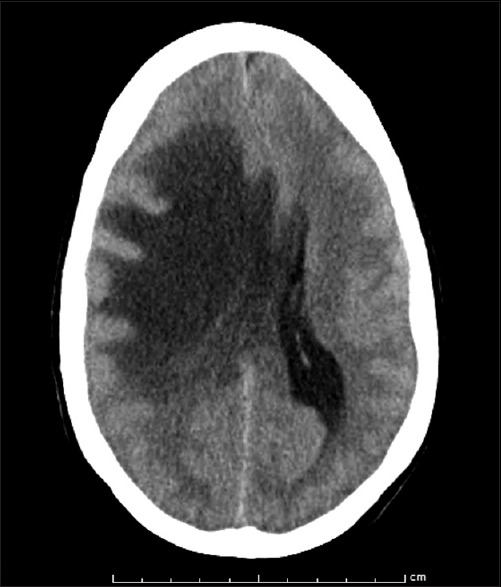
Axial computed tomographic image showing aggressive development of edema causing transtentorial herniation
Intraoperatively, after removal of the bone flap, the right cerebral hemisphere herniated through the bony defect. Underlying the dura, there was a significant amount of abnormal brain tissue that was grayish in color. A biopsy of the tissue was performed, and the skin edges were closed. The patient was continued on the high-dose steroid therapy and remained under close observation in the intensive care unit. Over the next 3 days, she became more alert and her right pupil returned to normal size and reactivity. She regained her previous baseline neurologic status with a left hemiparesis and was able to communicate easily.
The results of the pathologic examination were consistent with demyelinating disease. Due to the enhancing nature of the lesion as well as the extensive edema, there was a concern for an underlying pathology that was not obvious. It was decided to continue treatment with immunosuppressive therapies and repeat neuroimaging in a few weeks after interval resolution of the edema. After 5 weeks, the patient's skin flap had become sunken; however, given her ongoing immunotherapy, a cranioplasty was not performed. She was transferred to a rehabilitation facility.
DISCUSSION
Tumefactive MS lesions present as large intracranial lesions that may resemble brain tumors. The incidence is thought to be 0.3 cases per 100,000 patients per year.[16] There is no clear gender predilection for tumefactive lesions. Patients in their 20s and 30s are most likely to be affected, although cases involving pediatric and older patients have also been described.[15] These lesions are typically larger than 2 cm in diameter and usually cause considerable edema and mass effect.[1,6,18,20] They are commonly seen as ring-enhancing lesion[3,6] which makes their diagnosis challenging because tumors and infections have a similar appearance on MR imaging. An open ring-enhancing lesion is almost pathognomonic for MS and affected patients can frequently present with localized neurological deficits, such as hemiparesis, aphasia, ataxia, headaches, and seizures. The ring enhancement is thought to be an advancing area of active inflammation away from a central and more chronic nonenhancing core.[8] Histologically active lesions consist of areas of demyelination with hypercellularity and reactive astrocytes that may contain multiple nuclei (Creutzfeldt cells) closely intermingled with myelin-containing foamy macrophages.[15]
Cerebrospinal fluid testing may be normal or demonstrate elevation of the immunoglobulin G index and oligoclonal bands. These findings have been demonstrated in 11–33% of the cases of tumefactive MS.[16] Biopsy often helps differentiate these demyelinating lesions from other pathologies. It is also worth noting that biopsy results can be misleading if not interpreted by an experienced neuropathologist. Initial biopsy results may be interpreted incorrectly in as many as 31% of the cases; most frequently, the lesion is misdiagnosed as a low-grade astrocytoma.[16]
Treatment of tumefactive lesions is generally challenging and recovery is often incomplete. Treatment choices include IV methylprednisolone, β-interferons, plasma exchange, rituximab, and natalizumab (monoclonal antibody). IV steroid therapy is generally initiated for any acute, symptomatic tumefactive lesion. Plasmapheresis should be tried in the event of a relapse or no response to steroid therapy.[17,21] Available data suggest that disease-modifying therapies like interferon-β and glatiramer acetate may be tried.[5,12] There are no data to suggest superiority of one over the other. There are certain case reports where patients with relapsing-remitting MS undergoing treatment with fingolimod (sphingosine-1-phosphate receptor modulator) have developed tumefactive lesions on discontinuation of therapy.[5] This observation needs further study. Rituximab and cyclophosphamide have shown benefit in tumefactive lesions.[4,7] Series consisting of cases of biopsy-confirmed tumefactive MS suggest that lesions without significant mass effect may help differentiate MS lesions from other space-occupying lesions.[13,14,18,22]
To our knowledge, our case represents the second reported case[19] of cerebral herniation from tumefactive MS. This case illustrates the importance of recognizing the catastrophic complications that may arise as a result of mass effect from vasogenic edema if this condition is not diagnosed and treated early and aggressively.
CONCLUSION
Tumefactive MS presents a unique pathology that can often mimic primary brain tumors. Although these lesions affect white matter and infrequently cause a significant amount of mass effect, there are now two cases that show these lesions can act like tumors causing edema that generates sufficient intracranial pressure to cause transtentorial herniation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank Paul H. Dressel BFA for the preparation of the illustrations and Debra J. Zimmer for editorial assistance.
Footnotes
Contributor Information
Kunal Vakharia, Email: kvakharia@ubns.com.
Haris Kamal, Email: hariskamalmd@gmail.com.
Gursant S. Atwal, Email: gursant@gmail.com.
James L. Budny, Email: jbudny@ubns.com.
REFERENCES
- 1.Almalki DM, Mudhafar OY, Alsaman AS, Mahmoud AA. Diagnostic uncertainty of tumefactive cystic demyelinating lesions. Neurosciences (Riyadh) 2013;18:176–7. [PubMed] [Google Scholar]
- 2.Censori B, Agostinis C, Partziguian T, Gazzaniga G, Biroli F, Mamoli A, et al. Large demyelinating brain lesion mimicking a herniating tumor. Neurol Sci. 2001;22:325–9. doi: 10.1007/s10072-001-8176-5. [DOI] [PubMed] [Google Scholar]
- 3.Dagher AP, Smirniotopoulos J. Tumefactive demyelinating lesions. Neuroradiology. 1996;38:560–5. doi: 10.1007/BF00626098. [DOI] [PubMed] [Google Scholar]
- 4.Dastgir J, DiMario FJ., Jr Acute tumefactive demyelinating lesions in a pediatric patient with known diagnosis of multiple sclerosis: Review of the literature and treatment proposal. J Child Neurol. 2009;24:431–7. doi: 10.1177/0883073808324769. [DOI] [PubMed] [Google Scholar]
- 5.Faissner S, Hoepner R, Lukas C, Chan A, Gold R, Ellrichmann G, et al. Tumefactive multiple sclerosis lesions in two patients after cessation of fingolimod treatment. Ther Adv Neurol Disord. 2015;8:233–8. doi: 10.1177/1756285615594575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Given CA, 2nd, Stevens BS, Lee C. The MRI appearance of tumefactive demyelinating lesions. AJR Am J Roentgenol. 2004;182:195–9. doi: 10.2214/ajr.182.1.1820195. [DOI] [PubMed] [Google Scholar]
- 7.Haupts MR, Schimrigk SK, Brune N, Chan A, Ahle G, Hellwig K, et al. Fulminant tumefactive multiple sclerosis: Therapeutic implications of histopathology. J Neurol. 2008;255:1272–3. doi: 10.1007/s00415-008-0883-x. [DOI] [PubMed] [Google Scholar]
- 8.He J, Grossman RI, Ge Y, Mannon LJ. Enhancing patterns in multiple sclerosis: Evolution and persistence. AJNR Am J Neuroradiol. 2001;22:664–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Kaeser MA, Scali F, Lanzisera FP, Bub GA, Kettner NW. Tumefactive multiple sclerosis: An uncommon diagnostic challenge. J Chiropr Med. 2011;10:29–35. doi: 10.1016/j.jcm.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kepes JJ. Large focal tumor-like demyelinating lesions of the brain: Intermediate entity between multiple sclerosis and acute disseminated encephalomyelitis? A study of 31 patients. Ann Neurol. 1993;33:18–27. doi: 10.1002/ana.410330105. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Shimizu Y, Shibata N, Uchiyama S. Gadolinium enhancement patterns of tumefactive demyelinating lesions: Correlations with brain biopsy findings and pathophysiology. J Neurol. 2014;261:1902–10. doi: 10.1007/s00415-014-7437-1. [DOI] [PubMed] [Google Scholar]
- 12.La Mantia L, Di Pietrantonj C, Rovaris M, Rigon G, Frau S, Berardo F, et al. Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev. 2016;11:CD009333. doi: 10.1002/14651858.CD009333.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Puma D, Llufriu S, Sepúlveda M, Blanco Y, Ribalta T, Graus F, et al. Long-term follow-up of immunotherapy-unresponsive recurrent tumefactive demyelination. J Neurol Sci. 2015;352:127–8. doi: 10.1016/j.jns.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Liao PY, Chen CY. Regarding tumefactive demyelinating lesion, its image diagnosis, and discussion. Am J Emerg Med. 2015;33:290–1. doi: 10.1016/j.ajem.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, Weigand S, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131:1759–75. doi: 10.1093/brain/awn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masdeu JC, Moreira J, Trasi S, Visintainer P, Cavaliere R, Grundman M, et al. The open ring. A new imaging sign in demyelinating disease. J Neuroimaging. 1996;6:104–7. doi: 10.1111/jon199662104. [DOI] [PubMed] [Google Scholar]
- 17.Meca-Lallana JE, Hernández-Clares R, León-Hernández A, Genovés Aleixandre A, Cacho Pérez M, Martín-Fernández JJ, et al. Plasma exchange for steroid-refractory relapses in multiple sclerosis: An observational, MRI pilot study. Clin Ther. 2013;35:474–85. doi: 10.1016/j.clinthera.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Ninomiya S, Hara M, Morita A, Teramoto H, Momose M, Takahashi T, et al. Tumefactive demyelinating lesion differentiated from a brain tumor using a combination of magnetic resonance imaging and (11) C-methionine positron emission tomography. Intern Med. 2015;54:1411–4. doi: 10.2169/internalmedicine.54.3712. [DOI] [PubMed] [Google Scholar]
- 19.Ragel BT, Fassett DR, Baringer JR, Browd SR, Dailey AT. Decompressive hemicraniectomy for tumefactive demyelination with transtentorial herniation: Observation. Surg Neurol. 2006;65:582–3. doi: 10.1016/j.surneu.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Sinha MK, Garg RK, Bhatt ML, Chandra A. Tumefactive demyelinating lesion: Experience with two unusual patients. J Postgrad Med. 2010;56:146–9. doi: 10.4103/0022-3859.65292. [DOI] [PubMed] [Google Scholar]
- 21.Weiner HL, Dau PC, Khatri BO, Petajan JH, Birnbaum G, McQuillen MP, et al. Double-blind study of true vs. sham plasma exchange in patients treated with immunosuppression for acute attacks of multiple sclerosis. Neurology. 1989;39:1143–9. doi: 10.1212/wnl.39.9.1143. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita S, Kimura E, Hirano T, Uchino M. Tumefactive multiple sclerosis. Intern Med. 2009;48:1113–4. doi: 10.2169/internalmedicine.48.2046. [DOI] [PubMed] [Google Scholar]


