To the Editor: Previous studies showed that the rate of retears after repair of rotator cuff injury was 38–94%, owing to the low healing capability of tendon–bone and the long duration.[1] Adipose-derived mesenchymal stem cells (ADSCs) have been adopted to repair the tendon and ligament tears, which acquired effective repair improvement.[2] Although ADSCs could effectively promote the healing of degenerative rotator cuff injury, the poor self-renewal ability and uncertain safety still disturb patients with rotator cuff tears. Therefore, a new or substitute treatment method remains to be investigated. Several studies demonstrated that the adipose stromal vascular fraction (SVF) treatment, which contained adipose stem cells, showed better therapy potential than adipose stem cell treatment in promoting healing of connective tissue injury in animal models.[3]
In the present study, 36 adult, male, New Zealand white rabbits weighing 2.0–2.5 kg were randomly balloted into the control group (n = 18) and the SVF fibrin glue (SVF-FG) group (n = 18). All samples were anesthetized by intraperitoneal injection of 1 ml/kg 3% pentobarbital sodium. Then, their inguinal yellow-white adipose tissue was obtained, washed with the same amount of phosphate-buffered saline, added with type I collagenase, and followed by digestion and centrifugation to obtain SVF suspension, which was mixed with FG to form a gel-like slow-release compound (SVF-FG) to release it slowly at the tendon–bone junction. A 4-cm longitudinal incision was made above the shoulder to expose the rotator cuff. The supraspinatus tendon was picked out using a cured clamp, and the insertion of the supraspinatus tendon was severed from the greater tubercle with a blade or scissors. Then, in the tendon–bone interface, the severed supraspinatus tendon was sutured to the tubercle through the bones using the mattress suture method, followed by tightening and knotting. In the experimental group, rabbit SVF-FG was injected and made to uniformly fill into the tendon–bone interface, with a total volume of l ml per side, which could be automatically solidified after about 10 s [Figure 1a]. In the control group, only FG was injected into the tendon–bone interface. After the operation, all animals were fixed with plaster and removed after 3 weeks. Subsequently, at postoperative week 8, the animals were executed, and then the supraspinatus tendon–bones were sampled from the bilateral shoulders. Since the surgical model was developed in bilateral shoulders of rabbits, 36 samples were obtained from each group. Then, in each group, six samples were randomly selected for slice staining and immunohistochemistry, while the rest of thirty samples were used for the biomechanical test.
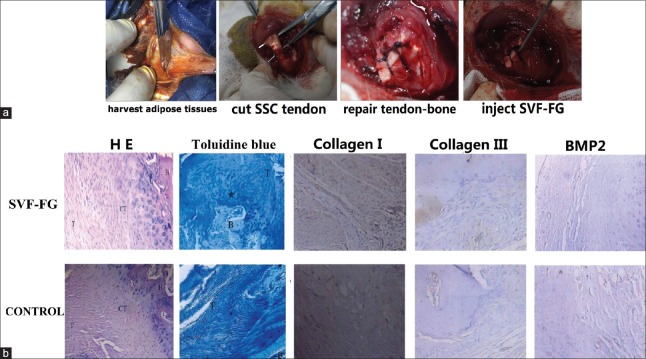
Figure 1.
Animal experiment (a); H&E staining (original magnification ×100), toluidine blue staining (×20), immunohistochemical staining for Collagen I, Collagen II, and BMP2 (×100) (b). T: Tendon; B: Bone; CT: Chondral–tendon interface; *Fibrocartilage.
For the histological evaluation, all sample slices were carried out by hematoxylin and eosin staining and toluidine blue staining for histological observation using an Olympus biological microscope (OLYMPUS BH-2, Tokyo, Japan). For expressions of I and III collagen tests, after observing the sections under the polarized light microscope and locating the positive areas, images were extracted with the color camera and put into image analysis software named Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA). The positive staining was brown. The areas of collagen I and collagen III were measured, and the area ratio of collagen I to collagen III was calculated according to the distribution of collagen fibers. For expressions of the bone morphogenetic protein-2 (BMP-2) test, positioning the positive area, images were extracted by the color camera and put into the image analyzer measuring system. After accurately segmenting the positive area, the unit gray value of the area was measured. Each slice was taken in the up, down, left, right, and central visual fields for quantitative detection. In each group, the tendon–bone healing was comprehensively quantified using the JUNJIDE tendon–bone maturity score system.[4] A MTS858 multifunctional biomaterial tester (Instron 8847, Norwood, Massachusetts, UK) was used to measure the maximum load, stiffness, and maximum strength of the tendon–bone specimens. Tendon–bone interface maturity scores were conducted by the t-test. Biomechanical parameters were first performed normality and variance homogeneity test, followed by analyzing the differences between different groups using analysis of variance. P < 0.05 was considered statistically significant.
Tendon–bone interface with SVF-FG treatment formed a four-layer structure similar to the direct dead point, which successively includes the tendon, fibrocartilage, calcified cartilage, and bone tissue. However, osteoblastic reaction and a large number of fibroblasts were visible in the tendon–bone interface in the control group. The results of toluidine blue staining revealed that cartilage cells in the tendon–bone interface gradually penetrated into the bone cell areas, which formed a “bone-fibrocartilage-tendon-like” transition area in the SVF-FG treatment group, whereas the tendon–bone interface was mainly composed of collagen fiber connection, with a small amount of blue cells similar to chondrocytes in the control group [Figure 1b]. In addition, the results of JUNJIDE tendon–bone maturity showed that the tendon–bone healing maturity scores of the SVF-FG group (19.1 ± 0.3) were significantly enhanced, compared with those of the control group (178.6 ± 0.8, P < 0.01).
We detected type I and type III collagen and BMP2 expression after rotator cuff tears in rabbits. The results showed that the expression level of collagen I in the SVF-FG treatment group was obviously higher than the expression in the control group. Consistently, the expression level of collagen III in the SVF-FG treatment group was slightly higher than the expression in the control group. The type I collagen/type III collagen area ratio of the experimental group (1.69 ± 0.43) was significantly increased, compared with that of the control group (1.31 ± 0.34, P = 0.031). In addition, the expression of BMP-2 was increased in the SVF-FG treatment group, and the BMP-2 quantified gray value of the experimental group (64.5 ± 3.8) was markedly enhanced, compared with that of the control group (46.8 ± 3.4, P = 0.006).
We detected the maximum load, maximum strength, and stiffness in rabbits. The results demonstrated that the maximum load of the SVF-FG group was significantly increased, compared with that of the control group at postoperative week 8. Meanwhile, the maximum strength of the SVF-FG group was much higher than that of the control group at postoperative week 8. In addition, we also studied the stiffness of each group after treatment in the model of rotator cuff tears. The results showed that stiffness in the SVF-FG treatment group was remarkably promoted, compared with control groups in the rotator cuff tear model [Table 1].
Table 1.
Biomechanical results of the SVF-FG and control groups (n = 18 in each group)
| Parameters | SVF-FG | Control | t | P |
|---|---|---|---|---|
| Maximum load (N) | 166.89 ± 11.62 | 99.40 ± 5.70 | 6.943 | 0.000 |
| Stiffness (N/mm) | 34.85 ± 3.00 | 24.57 ± 5.72 | 3.308 | 0.022 |
| Maximum strength (Pa) | 8.22 ± 1.90 | 5.82 ± 0.68 | 3.758 | 0.011 |
Data were presented as mean ± SD. SVF-FG: Stromal vascular fraction fibrin glue; SD: Standard deviation.
Adipose SVF is a group of stromal cells isolated and enriched from adipose tissue. It removes mature adipocytes, which contains a variety of cellular components, including adipose stem cells, endothelial cells, pericytes, fibroblasts, and a small number of undetected mature adipocytes. Adipose SVF has shown greater potential in the treatment of connective tissue injury diseases and plastic filling.[5]
The area ratiosof type I collagen and type III collagen of the experimental group were higher than those of the control group at postoperative week 8, revealing that type I collagen fibers gradually dominated while the type III collagen fibers relatively decreased after the SVF-FG treatment. The area ratio of type I/type III collagen significantly increased in the experimental group, which suggested that the experimental group showed faster tendon–bone healing progress and took the lead in transforming into collagen fibers, compared with the control group. In the experimental group, microscopic observation revealed that the tendon–bone was more maturely healed, compared with the control group, which indicated that except for the nondistance suture of the tendon and bones, the tendon-bone healing also required adequate cell secretion to accelerate the generation of collagen fibers, maintain tendon strength, and reduce the probability of postoperative re-rupture. Nevertheless, whether SVF promotes the repair of the dead point of the rotator cuff through neovascularization is still unknown, and further study should be conducted.
At postoperative week 8, the BMP-2 quantified gray values of experiments were higher than those of the control group, which was consistent with the increased proportion of type I collagen fibers. The results suggested that SVF could promote BMP-2 expression in the tendon–bone interface in rabbits that undergo operation for the rupture of the supraspinatus tendon, hereby to promote the repair of injured tendon–bone. According to the comparative results of important parameters representing biomechanical features of the tendon–bone, the postoperative biomechanics recovery of the experimental group was stronger and the recovery rate was faster than those of the control group. These results suggested that SVF could promote repair of tendon–bone after operation for rupture of the rotator cuff in rabbits.
In conclusion, the SVF injected into the shoulder cuff insertion tendon–bone interface can accelerate the formation of “tidal line” at 8 weeks after operation, which is similar to the structure of the direct insertion point (bone–cartilage transitional zone), accelerate the transformation of collagen fiber types, and promote the BMP-2 expression to a certain extent. Therefore, it is hypothesized that autologous SVF could accelerate the regeneration of “bone–cartilage” in the interface during the healing of rotator cuff insertion.
Financial support and sponsorship
This work was supported by a grant of the Key Specialty Construction Project of Pudong Health and Family Planning Commission of Shanghai (No. PWZzk2017-25).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: An anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23:662–9. doi: 10.1016/j.arthro.2007.02.018. doi: 10.1016/j.arthro.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Kryger GS, Chong AK, Costa M, Pham H, Bates SJ, Chang J, et al. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg Am. 2007;32:597–605. doi: 10.1016/j.jhsa.2007.02.018. doi: 10.1016/j.jhsa.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Tan Q, Lui PP, Rui YF, Wong YM. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A. 2012;18:840–51. doi: 10.1089/ten.tea.2011.0362. doi: 10.1089/ten.TEA.2011.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ide J, Kikukawa K, Hirose J, Iyama K, Sakamoto H, Fujimoto T, et al. The effect of a local application of fibroblast growth factor-2 on tendon-to-bone remodeling in rats with acute injury and repair of the supraspinatus tendon. J Shoulder Elbow Surg. 2009;18:391–8. doi: 10.1016/j.jse.2009.01.013. doi: 10.1016/j.jse.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–10. doi: 10.1002/stem.1076. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]