Abstract
Objective:
To propose a new definition of the pericollapse stage of osteonecrosis of the femoral head (ONFH) and review its significance in disease diagnosis and treatment selection.
Data Sources:
A search for eligible studies was conducted in three electronic databases including PubMed, Cochrane Library, and Embase up to August 10, 2018, using the following keywords: “osteonecrosis”, “prognosis”, and “treatment”.
Study Selection:
Investigations appraising the clinical signs, symptoms, and imaging manifestations in different stages of ONFH were included. Articles evaluating the prognosis of various joint-preserving procedures were also reviewed.
Results:
The pericollapse stage refers to a continuous period in the development of ONFH from the occurrence of subchondral fracture to early collapse (<2 mm), possessing specific imaging features that mainly consist of bone marrow edema and joint effusion on magnetic resonance imaging (MRI), crescent signs on X-ray films, and clinical manifestations such as the sudden worsening of hip pain. Accumulating evidence has indicated that these findings may be secondary to the changes after subchondral fractures. Of note, computed tomography provides more information for identifying possible subchondral fractures than does MRI and serves as the most sensitive tool for grading the pericollapse lesion stage. The pericollapse stage may indicate a high possibility of progressive disease but also demonstrates satisfactory long- and medium-term outcomes for joint-preserving techniques. In fact, if the articular surface subsides more than 2 mm, total hip arthroplasty is preferable.
Conclusions:
The pericollapse stage with distinct clinical and imaging characteristics provides a last good opportunity for the use of joint-preserving techniques. It is necessary to separate the pericollapse stage as an independent state in evaluating the natural progression of ONFH and selecting an appropriate treatment regimen.
Keywords: Joint-Preserving Procedure, Osteonecrosis of the Femoral Head, Pericollapse, Prognosis
摘要
目的:
本文提出了股骨头坏死围塌陷期的最新定义并回顾了其在疾病诊断和治疗方案选择中的意义。
数据来源:
通过使用”骨坏死”、”预后”和”治疗”三个关键词,我们对包括PubMed,Cochrane图书馆和Embase(截止到2018年8月10日)在内的三个数据库进行了系统的检索,寻找合适的文献。
研究选择:
纳入评价股骨头坏死不同时期的临床症状、体征和影像学表现的研究。评估不同保髋治疗预后的文章也纳入了本系统回顾。
结果:
围塌陷期指的是从软骨下骨骨折到轻微塌陷(小于2mm)出现这之间的一段连续时期。这一时期的特征性表现包括核磁共振(MRI)上的骨髓水肿和关节积液,X线上的新月征和临床表现上突发的疼痛加重。越来越多的证据表明这些表现继发于软骨下骨骨折后的改变。值得注意的是,CT能够在鉴定软骨下骨骨折方面提供比MRI更多的信息,因此被认为是对围塌陷期分期最敏感的工具。围塌陷的出现表明股骨头坏死进一步进展的可能性大,同时在该期实施保髋手术有着较好的中远期效果。实际上,一旦塌陷高度超过2mm,全髋关节置换可能是更好的选择。
结论:
具有明确的临床和影像特征的围塌陷期为股骨头坏死的保髋治疗提供了最后的良机。把围塌陷期作为股骨头坏死自然进展中的一个独立时期进行评估并指导治疗方案的选择是必须的。
INTRODUCTION
Osteonecrosis is a refractory and debilitating disease and is associated with high mortality. Once the femoral head collapses and acetabular degeneration or secondary arthritis is present, a total hip arthroplasty (THA) is often the last and most reliable option.[1,2,3] Although studies of THA have normally demonstrated excellent results in greater than a 10-year follow-up period compared with that in patients with traumatic osteonecrosis of the femoral head (ONFH), those with nontraumatic ONFH tend to be younger and physically more active, and as a result, replaced hip joint may need to be revised.[1,3,4,5,6,7,8]
To salvage the patients’ own natural hip joints and delay or avoid joint arthroplasty is one of research focuses in the treatment of ONFH. A successful joint-preserving procedure depends on accurate and early diagnosis (especially those at Association Research Circulation Osseous [ARCO] Stage I/II) and skillful techniques.[1,2,3] However, ARCO Stages I and II are also known as subclinical states because lesions at these stages are often insidious, and the symptoms and signs are minimal and nonspecific, which leads to the fact that those patients rarely make a first visit to an orthopedist. Meanwhile, surgical intervention of asymptomatic ONFH is controversial due to the observation of spontaneous repair of necrotic lesions, great variability of progression (with only 7% of small lesions and 80% of large lesions collapsing by the 8th year after diagnosis) at the early stage, and limited evidence for treatment.[3,9,10] On the other hand, the “wait-and-watch” policy may miss the critical window of opportunity to prevent head depression. For children receiving chemotherapeutic treatment for malignancies, up to 72% of patients have already been at Stage IV by the time they are diagnosed.[11] Defining specific biomarkers in the development of ONFH is of great importance to guarantee both diagnostic accuracy and joint-preserving success rate.
The three most commonly used grading systems for the evaluation of ONFH include the Ficat system, ARCO system, and Steinberg system.[12] The Ficat classification system mainly considers collapse observed on standard radiographs, making it impossible to quantify the lesion size and assess disease progression.[13] The classification system proposed by the ARCO consists of four stages with comprehensive reference to X-ray, magnetic resonance imaging (MRI), computed tomography (CT), bone scintigraphy, and histologic findings.[14] In the ARCO system, Stage III indicates evidence of subchondral fracture, and the modified Nijmegen ARCO system further subdivides this stage into an early and late phase according to the existence of femoral head collapse.[15] Mont et al.[16] defined the stages of ONFH as precollapse, early collapse (head depression ≤2 mm), late collapse (head depression >2 mm), and acetabular changes.[1] However, to evaluate the status of ONFH, these classification systems are apparently not sufficient. The pericollapse stage is a conception that is first recommended in an attempt to assess a continuous period before and after head collapse as a whole.[17] At first, this term refers to ARCO Stage II ONFH with necrotic lesions located at the anterolateral part of the femoral head as well as ARCO Stage III ONFH with head depression <4 mm observed on an anteroposterior film, a faint sclerotic band, and a pain history of <6 months. It reflects a gray area lasting from the extensive trabecular fracture to early collapse of ONFH.[17] However, this definition has several flaws in its application of clinical diagnosis and treatment guidance. First, ARCO Stage II ONFH is normally asymptomatic and has a variable development process according to this classification system.[3,9,10] Second, by searching related electronic databases, we found that currently, there were no clinical reports regarding the treatment of pericollapse ONFH according to this definition. Therefore, authors of review articles tend to use Ficat Stages II–III or ARCO Stages II–III ONFH to approximately evaluate the pericollapse period, which may impose a big selection bias.[17] Last but not least, the complexity of this definition prevents it from broadly popularizing.
To better guide the diagnosis of nontraumatic ONFH, the clinical and imaging manifestations of the pericollapse stage ought to be characteristic and specific; meanwhile, the pericollapse stage should be able to discriminate between reversible and progressive ONFH lesions, providing the opportunity for joint-preserving procedures. By reviewing former studies and summarizing the clinical experiences of the Centre for Osteonecrosis and Joint-Preserving and Reconstruction of China-Japan Friendship Hospital (CJFH), we proposed a new definition of the pericollapse stage of ONFH, which begins with the occurrence of subchondral fracture and ends after the head collapse exceeds 2 mm. Thus, the pericollapse stage is equivalent to approximately Stage III of the Steinberg classification system or Stage IIIa of the ARCO classification system.
CHARACTERISTICS OF THE PERICOLLAPSE STAGE OF OSTEONECROSIS OF THE FEMORAL HEAD
A subchondral fracture exhibits a high T2-weighted signal on MRI but is often obscured by bone marrow edema (BME) and joint effusion. BME is an ill-defined, diffuse abnormality in the femoral head and neck presenting as decreased signal intensity on T1-weighted images. Accordingly, increased signal intensity is observed on T2-weighted images, manifesting elevated free-water content or bleeding in the bone marrow. In contrast to the recognition that BME is the early change in ONFH, BME accompanies disease progression and the onset of collapse, even before subchondral fracture becomes apparent on imaging studies.[18,19,20,21,22,23,24,25,26,27] More cases of subchondral fractures could be detected on additional CT and axial reconstruction is clearer than coronal reconstruction is.[20,21] Meier et al. analyzed 37 symptomatic hips in 27 consecutive adult patients with both ONFH and associated BME. Although only 19 cases showed fracture lines on MRI, subchondral fractures were observed in all patients on CT, which recategorized the remaining 18 cases to ARCO Stage III/IV diseases.[20] Studies in pediatric and adolescent patients also demonstrated that the presence of BME on MRI in symptomatic ONFH patients correlated with the occurrence of early subchondral fractures.[18] Delineation of the necrotic area using the linear low-density band on T1-weighted images and the double-line sign on T2-weighted images on MRI with no collapse identified on anteroposterior and frog-leg lateral radiographs are widely used criteria that constitute the diagnosis of ARCO Stage I/II ONFH, which may result in the underdiagnosis of subchondral fractures.[3,28,29] A crescent sign or interruption of the outline of the femoral head appearing on the X-ray film also denotes intra-articular fracture and subchondral collapse, indicating Stage III of the Steinberg classification system.[30] Nevertheless, crescent signs may not be present or only appear in specific planes; under normal conditions, by the time, X-ray radiographs reveal macroscopic epiphyseal fractures, joint motion impairment has become apparent, and core decompression (CD) is less successful.[15] Clinical manifestations after occurrence of subchondral fractures include sudden pain in the groin or hips, painful limping, and aggravated pain with strong internal rotation; most patients are first referred to orthopedists due to these symptoms and signs.[21,26]
It should be noticed that although aggravated pain, the presence of BME on short-tau inversion recovery (STIR), and accompanied joint effusion on MRI may indicate a high possibility of the appearance of a subchondral fracture, the gold standard technique for diagnosing the pericollapse stage remains CT. These imaging and clinical findings testify extensive trabecular fractures, accompanied structural instability, and further collapse [Figure 1].[10,31]
Figure 1.
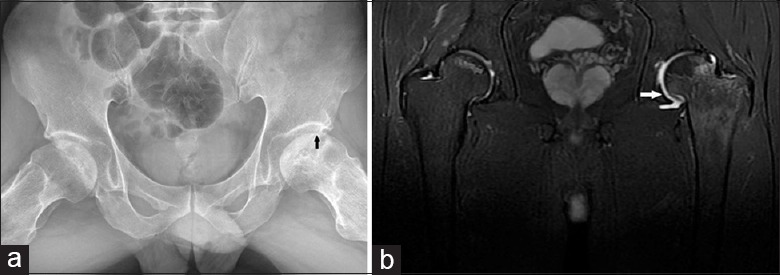
Frog-leg lateral X-ray film shows a subchondral fracture (arrowheads) (a). Coronal T2-weighted fat-saturated fast spin-echo image (TR/TE: 1600/68) shows extensive bone marrow edema (arrowheads) in the adjacent right head and neck and joint effusion (b).
PERICOLLAPSE STAGE MAY FORECAST STRUCTURAL INSTABILITY OF THE FEMORAL HEAD
Close relationships exist among subchondral fractures, ONFH-associated BME of the proximal femur, worsening of clinical symptoms and signs, hip joint effusion, and the ultimate collapse of the femoral head [Figure 2].[18,19,20,21,22,23,24,25,26,27,32,33,34,35] In fact, the latter four phenomena may occur secondary to subchondral fractures, which forecast the progression of structural instability of the femoral head.
Figure 2.
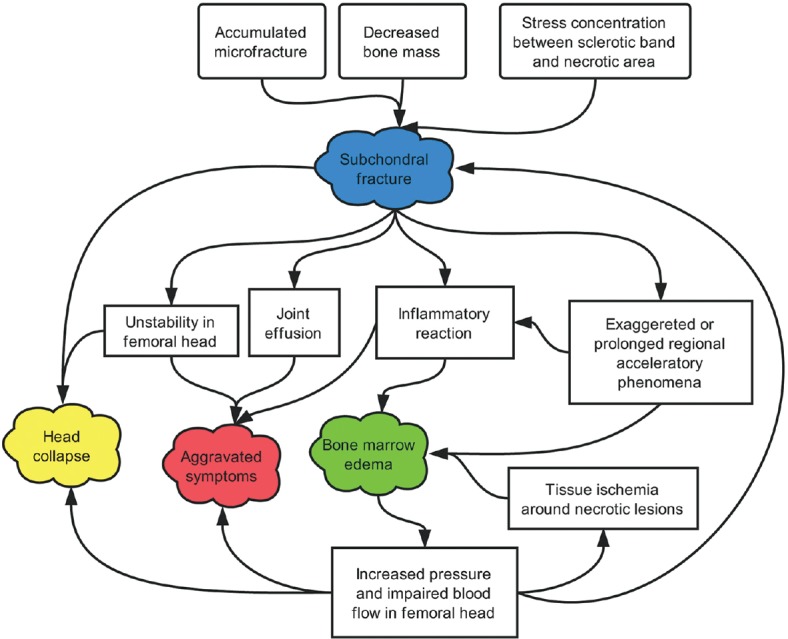
Pathogenetic pathways of a subchondral fracture, head collapse, aggravated symptoms, and bone marrow edema in the development of ONFH are shown. A subchondral fracture develops due to the accumulated microfractures, decreased bone mass, and stress concentration between the sclerotic band and necrotic area, which act as the central step in the formation of bone marrow edema, aggravated clinical symptoms/signs, and eventual head collapse. ONFH: Osteonecrosis of the femoral head.
With the development of ONFH, mechanical strength decreases in the necrotic and cystic zone due to accumulated fatigue fractures of necrotic trabeculae after sustained loads and increased trabecular fragility; meanwhile, shear stress concentrates at the junctional zone between the normal bone and sclerotic zone and the necrotic area, which causes a loss of structural stability of the femoral head.[18,19,20,21,22,23,24,25,26,27,32] In these processes, the subchondral fracture impairs the immobilization function of subchondral bone to articular cartilage and initiates head collapse.[15,24,26,32,36] Focal resorption of the broken subchondral plate and secondary compaction of necrotic cancellous bone result in the formation of a cavum below the subchondral plate, which appears as a crescent sign or radiolucent line on X-ray radiographs and joint effusion and closely correlates with the occurrence of BME and hip pain.[18,19,20,21,22,23,24,25,26,27]
Primary BME of the hip in most cases runs a self-limiting and transient course; however, ONFH accompanied by BME tends to have a larger necrotic volume compared with ONFH without BME, and it was suggested that BME was the most significant risk factor for aggravated pain of osteonecrotic hips.[21,26] In Theruvath et al.'s[18] report about pediatric and adolescent leukemia patients with glucocorticoid-induced ONFH, during follow-up, 70% of patients with BME eventually progressed to head collapse, even though edema might completely resolve later during follow-up; surprisingly, however, of 24 patients without BME, only one collapse was observed. Multiple theories such as disruption of sensory nerves within the bone marrow, venous hypertension, regional acceleratory phenomena (acceleratory rapidity of the ordinary biological regional processes after fracture, arthrodesis, or osteotomy), and trauma have been proposed, but no one mechanism has been authenticated for the development of BME.[33,34] Given the former findings, it is also rational to assume that BME in ONFH might be an inflammatory change to mechanical stress of subchondral fractures or to tissue ischemia around the necrotic area.[18,26,34]
Causes and severity of pain in patients with ONFH are varied and multifactorial in different stages. Most ARCO Stage I/II cases are asymptomatic, but with the aggravation of bone mass loss, bone marrow necrosis, and bone infarction, intermedullary pressure rises and dull pain may occur at the hips, groin, thighs, or knees in a few cases.[15,37] While at a later stage, due to the degenerative osteoarthritic changes, severe pain persistently exists during either physical activity or resting. In the observation of the natural progression of asymptomatic ONFH, obvious pain and other suddenly aggravated symptoms are normally related to the occurrence of femoral head collapse.[10,37,38,39] Min et al.[38] reported that after the pain developed, all 62 hips had progressed to Steinberg III or later stages, and of them, 44 cases continued to deteriorate sufficiently to accept hip arthroplasty in 1 year. Possible conjectures for the aggravation of clinical symptoms in the pericollapse stage include deteriorative structural instability of the femoral head, enhanced secretion of inflammatory mediators, elevated intramedullary pressure, and increase in hyperstatic pressure caused by joint effusion, etc.[18,19,20,22,33,35] There are, of course, exceptions. In a systematic review including 16 studies involving 664 asymptomatic hips, 26 cases (4%) were identified as Ficat Stage III disease, but the patients did not have pain. Therefore, multiple examinations should be considered to obtain a definitive diagnosis of the pericollapse stage.
Considering these specific manifestations and high possibility for further collapse, the pericollapse stage should begin with the occurrence of the subchondral fracture instead of with the classification of ARCO Stage II. However, from the onset of femoral head necrosis, how long does it take to progress to the pericollapse stage? How long will the pericollapse stage last?
SIZE AND LOCATION OF OSTEONECROSIS AND PROGRESSION OF THE PERICOLLAPSE STAGE
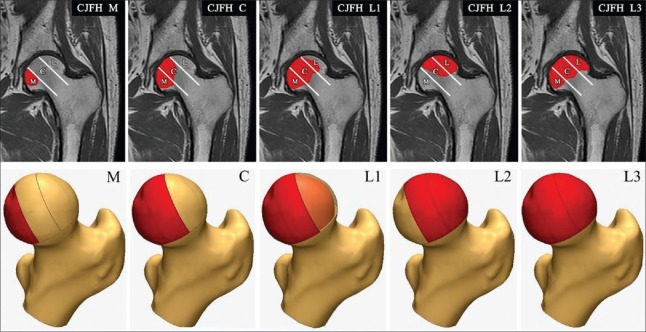
It has been widely acknowledged that a large-sized necrotic lesion is a crucial risk factor for the prevalence of collapse.[37,40] Meanwhile, when the lesions extend laterally to the acetabular edge, which is considered as the weight-bearing portion, head depression often becomes unavoidable.[37,40,41,42] Several classification systems such as the Steinberg classification, modified Kerboul classification, and Japanese Investigation Committee (JIC) classification attempt to quantify and categorize ONFH lesions and therefore predict collapse and prognosis.[14] The lesion volume is the primary reference index of the Steinberg classification system while Kerboul et al.'s[14,43] modified method calculates an angle by considering the necrotic portion in both the mid-coronal image and mid-sagittal image of the MRI. The JIC classification considers the location of the necrotic lesion first, and the lesion size is also involved because it is reported that large lesions are prone to locate laterally.[44,45] The CJFH classification system is based on the three-pillar structure theory, in which the medial pillar, central pillar, and lateral pillar represent 30%, 40%, and 30% of the femoral head, respectively. According to the involved site of the necrotic lesion, ONFH can be classified as a medial (M) type (only medial pillar involved), central (C) type (medial and central pillars involved), and lateral (L) type (lateral pillar involved). The L type is further divided into an L1 type (partial lateral pillar preserved [sublateral]), L2 type (the lateral pillar involved only [extralateral]), and L3 type (all three pillars involved) [Figure 3]. Preservation of the lateral pillar is the keystone for forestalling the collapse of the femoral head.[46]
Figure 3.
The three-pillar theory of the femoral head and China-Japan Friendship Hospital classification system are shown. According to the involved site of the necrotic lesion, ONFH can be classified as a medial (M) type (only medial pillar involved), central (C) type (medial and central pillars involved), and lateral (L) type (lateral pillar involved). This L type is further divided into an L1 type (partial lateral pillar preserved [sublateral]), L2 type (the lateral pillar involved only [extralateral]), and L3 type (all three pillars involved). ONFH: Osteonecrosis of the femoral head.
The duration of the pericollapse stage and the average intervals from the onset of ONFH to the pericollapse stage varies across the published literature. In the majority of patients who reach the pericollapse stage, head collapse appears within a short period of time. Min reported that for 31 asymptomatic ONFH hips that finally progressed to symptomatic ones, 26 (83.8%) of them collapsed with a mean time of 8 months (range, 1–36 months).[37] In Iida et al.'s study,[24] approximately a 4-month interval (range, 1–7 months) existed from the onset of pain to head collapse. However, Theruvath et al.[18] described 14 patients with subchondral fracture, and 12 patients experienced collapse with a mean follow-up of 2.6 years. The duration of the pericollapse stage should be further confirmed by long-term follow-up. The time needed from the onset of ONFH to the pericollapse stage is mainly determined by the classification, which is consistent with the occurrence of head collapse.[1,2,3,12] Concerning the data about the natural progression of severe acute respiratory syndrome patients with ONFH, it was observed that no CJFH type M lesions, 6 of 45 (13.3%) CJFH type C lesions, and 20 of 89 (22.5%) CJFH type L1 lesions progressed to the pericollapse stage within 7 years, while all CJFH type L2 diseases and 86.1% of CJFH type L3 diseases progressed to the pericollapse stage within 3 years [Table 1].[46] Nam et al.[38] reported that lesion size is a predictor of developing pain in asymptomatic ONFH: 1 of 21 (4.8%) hips with a small lesion, 11 of 24 (45.8%) hips with a medium-sized lesion, and 50 of 60 (83.3%) hips with a large lesion became symptomatic by the last follow-up. In Mont et al.'s[47] systematic review, the weighted mean time to the appearance of pain in originally asymptomatic ONFH patients was 30 months (range, 1–134 months).
Table 1.
Natural progression of SARS patients from ONFH onset to the pericollapse stage
| Stage | Type M | Type C | Type L1 | Type L2 | Type L3 |
|---|---|---|---|---|---|
| Pericollapse/total patients | 0/13 | 6/45 | 20/89 | 9/9 | 31/36 |
| Percentage | 0.0 | 13.3 | 22.5 | 100.0 | 86.1 |
| Time needed, n | |||||
| <1 year | / | / | / | 2 | 5 |
| <2 years | / | / | 1 | 2 | 13 |
| <3 years | / | / | 4 | 5 | 13 |
| <4 years | / | 2 | 4 | / | / |
| <5 years | / | 2 | 11 | / | / |
| <6 years | / | 1 | / | / | / |
| <7 years | / | 1 | / | / | / |
| Mean | / | 5.2 | 4.3 | 2.3 | 2.3 |
SARS: Severe acute respiratory syndrome; ONFH: Osteonecrosis of the femoral head.
THE PERICOLLAPSE STAGE AND JOINT-PRESERVING PROCEDURES OF OSTEONECROSIS OF THE FEMORAL HEAD
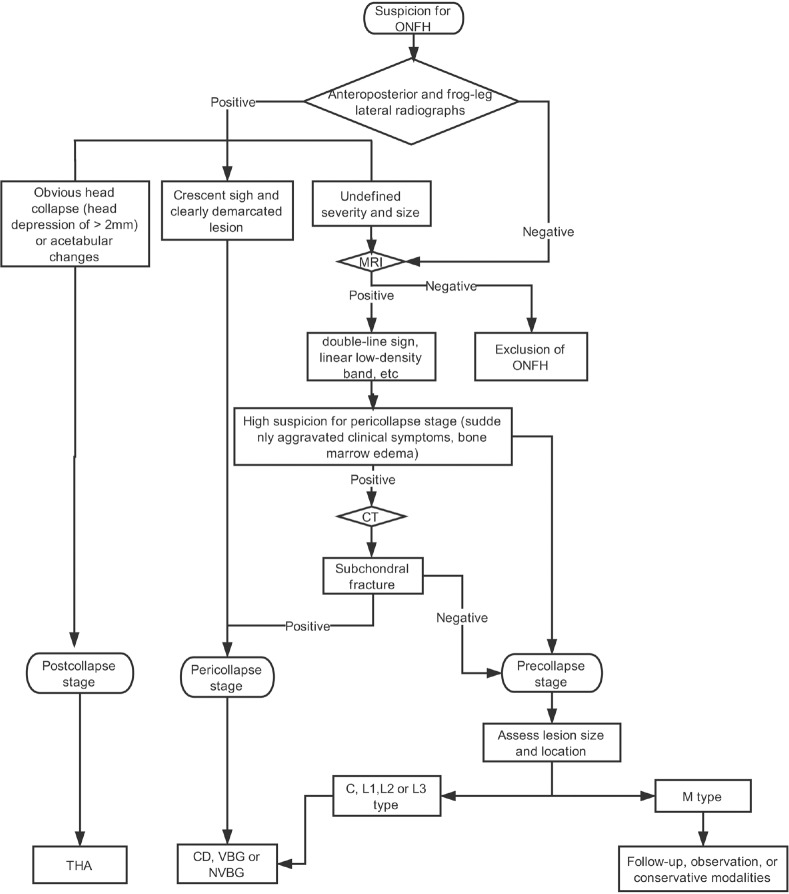
If left untreated, almost all symptomatic ONFH will progress to collapse and eventually disabling arthritis that requires hip arthroplasty; however, to date, for the intervention concerning asymptomatic ONFH, there is still no consensus.[10,39,47,48,49] The Chinese guideline for the diagnosis and treatment of ONFH recommends that the same principles should be applied to both symptomatic and silent hips; namely, only those patients with Stage I/II M type ONFH could wait for spontaneous repair with mere follow-up.[2] However, Mont et al.[3] advocated no more than observation for asymptomatic diseases. Given that the principle for the treatment of early-stage ONFH is to preserve one's natural joints, the therapeutic regimen for early-stage ONFH should be decided by classification, stages, and risk factors rather than symptoms [Figure 4]. Any lesions susceptible to collapse need to be managed as soon as possible to regain structural stability of the femoral head and therefore halt the ongoing progression.[2,46] Currently, joint-preserving techniques for pericollapse and postcollapse ONFH mainly include CD (normally combined with bone grafting), femoral osteotomy, nonvascularized bone grafting (NVBG), and vascularized bone grafting (VBG), with many studies showing successful outcomes at medium-term and long-term follow-up periods.[3,49]
Figure 4.
A flow diagram for the diagnosis and treatment of ONFH based on the pericollapse theory is shown. The therapeutic regimen for ONFH should be decided by classification, stages, and risk factors rather than symptoms. For postcollapse lesions, THA is preferable. Any lesions susceptible to collapse (pericollapse stage; CJFH L1, L2, L3, and C type precollapse stage lesions) need to be managed as soon as possible to regain the internal stability of the femoral head and therefore halt the ongoing progression. The CJFH M-type precollapse stage lesions can be followed up with mere observation. ONFH: Osteonecrosis of the femoral head; THA: Total hip arthroplasty; CJFH: China-Japan Friendship Hospital.
Core decompression
CD as well as the modified multiple percutaneous drilling procedure are the most commonly performed joint-preserving procedures, with the tenet of reducing intraosseous pressure and enhancing blood flow in the necrotic area.[3] Such techniques are mainly indicated in the treatment of small-to-medium-sized (<15% of the femoral head or a Kerboul angle <200%) ARCO Stage I/II lesions while the survivorship of lesions in the postcollapse stage (Ficat III) is only 23–35% in published studies.[50,51] For lesions in the pericollapse and postcollapse stages, CD combined with bone grafting is normally applied.[41,51]
Femoral osteotomy
The rationale of femoral osteotomy is to transpose the healthy portion of the femoral head to the weight-bearing area and therefore restore joint congruity, which is mainly suitable for ONFH patients of a young age (<40 years), low body mass index (<25 kg/m2), early-stage lesions, and a large postoperative intact ratio.[15] Even in the promising long-term results of angular intertrochanteric and transtrochanteric rotational osteotomies reported in multiple studies in which ARCO Stage IIIa lesions or late-stage osteonecrosis (ON) is present, the progressive collapse of a relocated necrotic lesion was significantly associated with a larger level of preoperative collapse (2.1 ± 1.0 mm in the progressive collapse group vs. 4.4 ± 1.4 mm in the nonprogressive collapse group).[52,53,54] Meanwhile, osteotomy is less frequently used in China and Western countries due to the complexity of the operation and the high rate of complications (nonunion, delayed union, fixation failure, and increased difficulty in future THA, etc.).[55,56]
Nonvascularized bone grafting
Replacing the necrotic bone of the femoral head with cancellous or cortical bone grafting via a window (light bulb technique) at the femoral neck base or decompression tracts (Phemister technique) aims to achieve necrotic area decompression and provide strong structural support for lesion healing and subchondral bone rebuilding. Compared with CD alone, NVBG is more suitable for ONFH at the pericollapse stage.[49,57,58,59] In Steinberg et al.'s[60] study, for lesions in Steinberg Stage III, CD combined with autologous bone grafting could decrease the need for THA from 82% to 23%, while the joint-preserving rate in Steinberg IV is only approximately 50%. Zuo et al.[61] reported that the clinical failure rates of the light bulb technique in patients at ARCO Stage II and IIIa were 25.9% and 16.2%, respectively, and as high as 61.5% at ARCO Stage III (b + c).
Vascularized fibular grafting
VBG mainly consists of the following three types: muscle pedicle grafting, vascularized fibular grafting, and vascularized iliac grafting.[62,63,64,65] In addition to providing structural support, VBG also attempts to reconstruct the blood supply to the necrotic lesions. In 2006, a study[66] of vascularized iliac bone grafting reported a 66.7% (8/12) success (no progression) rate with a follow-up duration of more than 3 years for the Steinberg Stage III hips but only 37.5% for Steinberg Stage IV hips. A midterm study using free vascularized fibular grafting observed that symptoms in 91.7% (11/12) of Steinberg Stage II cases and 85.7% (24/28) of Steinberg Stage III cases improved after the operation; however, only 45% (9/20) of Steinberg Stage IV cases improved, and because the duration of follow-up (24–40 months) was not long enough, only one case that progressed to THA was observed.[67] Zeng et al.[68] used a vascularized greater trochanter bone graft combined with a free iliac flap and impaction bone grafting to treat ONFH, with a 100% good-to-excellent rate for ARCO IIIa Stage lesions. These results are in accordance with a series of other reports.[64,66,69,70,71,72,73]
From the abovementioned studies, we noticed a satisfactory prognosis of ONFH in the pericollapse stage after joint-preserving techniques and sharply increased failure rates once evident femoral head collapse occurred [Table 2]. Therefore, the pericollapse stage should end with a head collapse more than 2 mm instead of 4 mm. The pericollapse stage may be the last good opportunity for the use of joint-preserving techniques. Of note, treatment regimens for ONFH should be individualized with consideration of the age and personal needs of patients in addition to staging and classification. For the CJFH L2 and L3 types, the pericollapse stage lesions in patients aged >50 years with severe pain and joint disturbance as well as a high functional requirement, THA may be a good choice.
Table 2.
Bone grafting outcome
| Author | Surgical procedures | Hips, n | Mean age (years) | Mean follow-ups (range) (years) | Definition of failure | |
|---|---|---|---|---|---|---|
| NVBG | ||||||
| Level I | ||||||
| Yang et al.[74] | Phemister technique | 56 | 38.6 | NR (3.0–6.5) | Conversion into THA or onset/progression of collapse or progressive osteoarthritis | |
| Level III | ||||||
| Steinberg et al.[60] | Phemister technique | 312 | NR | NR (2–14) | Conversion to THA | |
| Level IV | ||||||
| Zhang et al.[56] | Phemister technique | 85 | 31.4 | NR (2–NR) | Disease progression | |
| Wei and Ge[75] | Phemister technique | 223 | 33.5 | 2.0 (0.6–3.5) | A Harris hip score of <80 | |
| Wang et al.[76] | Light bulb technique | 138 | 32.4 | 2.1 (0.6–3.5) | A Harris hip score of <80 | |
| Zuo et al.[61] | Light bulb technique | 119 | NR | 2.8 (0.3–5.4) | Conversion into THA or onset/progression of collapse or progressive osteoarthritis or a Harris hip score of <70 | |
| VBG | ||||||
| Level III | ||||||
| Feng et al.[67] | Vascularized fibular grafting | 60 | 37 | 2.2 (2.0–3.2) | A Harris hip score of <80 | |
| Yen et al.[66] | Vascularized fibular grafting | 39 | 40 | NR (3–NR) | Disease progression | |
| Vascularized iliac grafting | 22 | 38 | NR (4–NR) | Disease progression | ||
| Level IV | ||||||
| Ding et al.[64] | Vascularized fibular grafting | 78 | 27.8 | 5.6 (2–NR) | Disease progression | |
| Aoyama et al.[65] | Vascularized iliac grafting | 9 | 31.7 | 2 | Disease progression | |
| Zeng et al.[68] | Vascularized iliac grafting and greater trochanteric flap | 64 | 31 | 3 (1–5) | A Harris hip score of <80 | |
| Yin et al.[70] | Vascularized fibular grafting | 14 | 34 | 3.3 (1.5–4.2) | Conversion into THA or a Harris hip score of <70 | |
| Chen et al.[73] | Vascularized iliac grafting | 33 | 37 | 5.8 (0.7–13.8) | Conversion into THA | |
| Dailiana et al.[72] | Vascularized fibular grafting | 86 | 35 | 4.0 (0.6–13.7) | Conversion into THA | |
| Author | Precollapse stage disease | Pericollapse stage disease | Postcollapse stage disease | |||
| Number | Survivorship | Number | Survivorship | Number | Survivorship | |
| NVBG | ||||||
| Level I | ||||||
| Yang et al.[74] | 48 | 91.70% | 8 | 50.00% | NR | NR |
| Level III | ||||||
| Steinberg et al.[60] | 198 | 68.20% | 13 | 76.90% | 95 | 49.50% |
| Level IV | ||||||
| Zhang et al.[56] | 71 | 90.10% | 9 | 88.90% | 5 | 25.00% |
| Wei and Ge[75] | 134 | 77.60% | 89 | 85.50% | NR | NR |
| Wang et al.[76] | 67 | 74.60% | 71 | 62.00% | NR | NR |
| Zuo et al.[61] | 27 | 74.10% | 105 | 83.80% | 26 | 38.50% |
| VBG | ||||||
| Level III | ||||||
| Feng et al.[67] | 12 | 91.70% | 28 | 85.70% | 20 | 45.00% |
| Yen et al.[66] | 11 | 72.70% | 12 | 66.67% | 16 | 37.50% |
| 4 | 100.00% | 11 | 81.82% | 7 | 71.43% | |
| Level IV | ||||||
| Ding et al.[64] | 19 | 78.95% | 36 | 80.56% | 23 | 65.22% |
| Aoyama et al.[65] | NR | NR | 5 | 100.00% | 4 | 50% |
| Zeng et al.[68] | 16 | 100.00% | 22 | 90.90% | 26 | 76.92% |
| Yin et al.[70] | 3 | 100.00% | 9 | 89.00% | 2 | 50.00% |
| Chen et al.[73] | NR | NR | 26 | 30.77% | 7 | 0.00% |
| Dailiana et al.[72] | 8 | 50.00% | 15 | 73.33% | 63 | 57.14% |
THA: Total hip arthroplasty; NR: Not reported; VBG: Vascularized bone grafting; NVBG: Non-VBG.
Limitations of this research concerning pericollapse ONFH merit consideration. First, there are some common flaws existing in now available joint-preserving studies, for example, no powered randomized controlled trials, the inclusion of ONFH with different risk factors (i.e., glucocorticoid, alcohol, trauma, caisson, and sickle cell disease), and a lack of validated patient-oriented evaluation parameters and variability with respect to indications, staging system, surgical procedures, determination of success (progression or conversion to THA), and follow-up duration.[3,8] Second, there is a paucity of large-scale (even >10 subjects) studies with long-term follow-up (>10 years) investigating the pericollapse stage. Most studies involving joint-preserving techniques were mainly targeted at ARCO or Steinberg Stage I/II ON, and a large number of investigators have adapted the Ficat system, which was incapable of discriminating the pericollapse stage as a distinctive stage.[1,3] Last but not least, for many studies using Steinberg or ARCO classification systems to assess ONFH, the CT results were missing.[18,21,22,24,25,37,39] In these studies, many alleged Steinberg or ARCO I/II ONFH cases with obvious hip pain may have been actually at the pericollapse stage. A high index of suspicion is required when younger patients present with worsening hip pain and BME, but no apparent collapse observed on X-ray.
CONCLUSION
The pericollapse stage begins with the occurrence of the subchondral fracture and ends with head depression of more than 2 mm. It is necessary to consider the pericollapse stage as an independent stage for the evaluation of the natural progression of ONFH and selection of the treatment regimen. The clinical characteristics of the pericollapse stage are serious pain in the groin that occurs suddenly, painful limping, and aggravated pain with strong internal rotation, while the imaging manifestations include a band-like low signal with T1-weighted images, BME with STIR and joint effusion on MRI, fracture of the subchondral bone plate on CT, or crescent signs with no visible collapse on plain radiographs. Among the various tools, CT is the gold standard for identifying pericollapse stage.
Furthermore, in the development of ONFH, all collapsed femoral heads will experience the pericollapse stage, which presents a high possibility of progressive disease and a relatively satisfactory prognosis if joint-preserving techniques are applied promptly. In fact, if the articular surface subsides more than 2 mm, THA is preferable. The pericollapse stage with distinct clinical and imaging characteristics provides a last good opportunity for the implementation of joint-preserving techniques. High-quality prospective investigations are still needed to further confirm the efficacy of these joint-preserving procedures in the treatment of pericollapse ONFH.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Chughtai M, Piuzzi NS, Khlopas A, Jones LC, Goodman SB, Mont MA, et al. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J. 2017;99-B:1267–79. doi: 10.1302/0301-620X.99B10.BJJ-2017-0233.R2. doi: 10.1302/0301-620X.99B10.BJJ-2017-0233.R2. [DOI] [PubMed] [Google Scholar]
- 2.Joint Surgery Group of the Orthopaedic Branch of the Chinese Medical Association. Guideline for diagnostic and treatment of osteonecrosis of the femoral head. Orthop Surg. 2015;7:200–7. doi: 10.1111/os.12193. doi: 10.1111/os.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mont MA, Cherian JJ, Sierra RJ, Jones LC, Lieberman JR. Nontraumatic osteonecrosis of the femoral head: Where do we stand today? A Ten-year update. J Bone Joint Surg Am. 2015;97:1604–27. doi: 10.2106/JBJS.O.00071. doi: 10.2106/JBJS.O.00071. [DOI] [PubMed] [Google Scholar]
- 4.Lee SH, Lee GW, Seol YJ, Park KS, Yoon TR. Comparison of outcomes of total hip arthroplasty between patients with ankylosing spondylitis and avascular necrosis of the femoral head. Clin Orthop Surg. 2017;9:263–9. doi: 10.4055/cios.2017.9.3.263. doi: 10.4055/cios.2017.9.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedard NA, Callaghan JJ, Liu SS, Greiner JJ, Klaassen AL, Johnston RC, et al. Cementless THA for the treatment of osteonecrosis at 10-year follow-up: Have we improved compared to cemented THA? J Arthroplasty. 2013;28:1192–9. doi: 10.1016/j.arth.2012.09.008. doi: 10.1016/j.arth.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Byun JW, Yoon TR, Park KS, Seon JK. Third-generation ceramic-on-ceramic total hip arthroplasty in patients younger than 30 years with osteonecrosis of femoral head. J Arthroplasty. 2012;27:1337–43. doi: 10.1016/j.arth.2011.07.004. doi: 10.1016/j.arth.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Kim YH, Kim JS, Park JW, Joo JH. Contemporary total hip arthroplasty with and without cement in patients with osteonecrosis of the femoral head: A concise follow-up, at an average of seventeen years, of a previous report. J Bone Joint Surg Am. 2011;93:1806–10. doi: 10.2106/JBJS.J.01312. doi: 10.2106/JBJS.J.01312. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman JR, Engstrom SM, Meneghini RM, SooHoo NF. Which factors influence preservation of the osteonecrotic femoral head? Clin Orthop Relat Res. 2012;470:525–34. doi: 10.1007/s11999-011-2050-4. doi: 10.1007/s11999-011-2050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura J, Harada Y, Oinuma K, Iida S, Kishida S, Takahashi K, et al. Spontaneous repair of asymptomatic osteonecrosis associated with corticosteroid therapy in systemic lupus erythematosus: 10-year minimum follow-up with MRI. Lupus. 2010;19:1307–14. doi: 10.1177/0961203310372951. doi: 10.1177/0961203310372951. [DOI] [PubMed] [Google Scholar]
- 10.Nishii T, Sugano N, Ohzono K, Sakai T, Haraguchi K, Yoshikawa H, et al. Progression and cessation of collapse in osteonecrosis of the femoral head. Clin Orthop Relat Res. 2002;400:149–57. doi: 10.1097/00003086-200207000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Mayer SW, Mayer BK, Mack Aldridge J, Urbaniak JR, Fitch RD, Lark RK, et al. Osteonecrosis of the femoral head in childhood malignancy. J Child Orthop. 2013;7:111–6. doi: 10.1007/s11832-012-0471-6. doi: 10.1007/s11832-012-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop. 2015;6:590–601. doi: 10.5312/wjo.v6.i8.590. doi: 10.5312/wjo.v6.i8.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jawad MU, Haleem AA, Scully SP. In brief: Ficat classification: Avascular necrosis of the femoral head. Clin Orthop Relat Res. 2012;470:2636–9. doi: 10.1007/s11999-012-2416-2. doi: 10.1007/s11999-012-2416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takashima K, Sakai T, Hamada H, Takao M, Sugano N. Which classification system is most useful for classifying osteonecrosis of the femoral head? Clin Orthop Relat Res. 2018;476:1240–9. doi: 10.1007/s11999.0000000000000245. doi: 10.1007/s11999.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo KH, Mont MA, Jones LC. Osteonecrosis. Heidelberg: Springer Berlin Heidelberg; 2014. [Google Scholar]
- 16.Schmitt-Sody M, Kirchhoff C, Mayer W, Goebel M, Jansson V. Avascular necrosis of the femoral head: Inter – And intraobserver variations of ficat and ARCO classifications. Int Orthop. 2008;32:283–7. doi: 10.1007/s00264-007-0320-2. doi: 10.1007/s00264-007-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Zhou C, Chen L, Sun Y, He W. A summary of hip-preservation surgery based on peri-collapse stage of osteonecrosis of femoral head (in Chinese) Chin J Repar Reconst Surg. 2017;31:1010–5. doi: 10.7507/1002-1892.201611084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theruvath AJ, Sukerkar PA, Bao S, Rosenberg J, Luna-Fineman S, Kharbanda S, et al. Bone marrow oedema predicts bone collapse in paediatric and adolescent leukaemia patients with corticosteroid-induced osteonecrosis. Eur Radiol. 2018;28:410–7. doi: 10.1007/s00330-017-4961-2. doi: 10.1007/s00330-017-4961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jianchuan W, Lei Y, Benjie W, Dewei Z. Study on correlation between bone marrow edema, stage of necrosis and area ratio of necrosis with the hip pain grading in nontraumatic osteonecrosis of the femoral head. Open Med (Wars) 2015;10:440–4. doi: 10.1515/med-2015-0076. doi: 10.1515/med-2015-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier R, Kraus TM, Schaeffeler C, Torka S, Schlitter AM, Specht K, et al. Bone marrow oedema on MR imaging indicates ARCO stage 3 disease in patients with AVN of the femoral head. Eur Radiol. 2014;24:2271–8. doi: 10.1007/s00330-014-3216-8. doi: 10.1007/s00330-014-3216-8. [DOI] [PubMed] [Google Scholar]
- 21.Ito H, Matsuno T, Minami A. Relationship between bone marrow edema and development of symptoms in patients with osteonecrosis of the femoral head. AJR Am J Roentgenol. 2006;186:1761–70. doi: 10.2214/AJR.05.0086. doi: 10.2214/AJR.05.0086. [DOI] [PubMed] [Google Scholar]
- 22.Huang GS, Chan WP, Chang YC, Chang CY, Chen CY, Yu JS, et al. MR imaging of bone marrow edema and joint effusion in patients with osteonecrosis of the femoral head: Relationship to pain. AJR Am J Roentgenol. 2003;181:545–9. doi: 10.2214/ajr.181.2.1810545. doi: 10.2214/ajr.181.2.1810545. [DOI] [PubMed] [Google Scholar]
- 23.Fujioka M, Kubo T, Nakamura F, Shibatani M, Ueshima K, Hamaguchi H, et al. Initial changes of non-traumatic osteonecrosis of femoral head in fat suppression images: Bone marrow edema was not found before the appearance of band patterns. Magn Reson Imaging. 2001;19:985–91. doi: 10.1016/s0730-725x(01)00424-6. [DOI] [PubMed] [Google Scholar]
- 24.Iida S, Harada Y, Shimizu K, Sakamoto M, Ikenoue S, Akita T, et al. Correlation between bone marrow edema and collapse of the femoral head in steroid-induced osteonecrosis. AJR Am J Roentgenol. 2000;174:735–43. doi: 10.2214/ajr.174.3.1740735. doi: 10.2214/ajr.174.3.1740735. [DOI] [PubMed] [Google Scholar]
- 25.Kim YM, Oh HC, Kim HJ. The pattern of bone marrow oedema on MRI in osteonecrosis of the femoral head. J Bone Joint Surg Br. 2000;82:837–41. doi: 10.1302/0301-620x.82b6.10740. [DOI] [PubMed] [Google Scholar]
- 26.Koo KH, Ahn IO, Kim R, Song HR, Jeong ST, Na JB, et al. Bone marrow edema and associated pain in early stage osteonecrosis of the femoral head: Prospective study with serial MR images. Radiology. 1999;213:715–22. doi: 10.1148/radiology.213.3.r99dc06715. doi: 10.1148/radiology.213.3.r99dc06715. [DOI] [PubMed] [Google Scholar]
- 27.Turner DA, Templeton AC, Selzer PM, Rosenberg AG, Petasnick JP. Femoral capital osteonecrosis: MR finding of diffuse marrow abnormalities without focal lesions. Radiology. 1989;171:135–40. doi: 10.1148/radiology.171.1.2928517. doi: 10.1148/radiology.171.1.2928517. [DOI] [PubMed] [Google Scholar]
- 28.Lee GC, Khoury V, Steinberg D, Kim W, Dalinka M, Steinberg M, et al. How do radiologists evaluate osteonecrosis? Skeletal Radiol. 2014;43:607–14. doi: 10.1007/s00256-013-1803-4. doi: 10.1007/s00256-013-1803-4. [DOI] [PubMed] [Google Scholar]
- 29.van der Jagt D, Mokete L, Pietrzak J, Zalavras CG, Lieberman JR. Osteonecrosis of the femoral head: Evaluation and treatment. J Am Acad Orthop Surg. 2015;23:69–70. doi: 10.5435/JAAOS-D-14-00431. doi: 10.5435/JAAOS-D-14-00431. [DOI] [PubMed] [Google Scholar]
- 30.Choi HR, Steinberg ME, Y Cheng E. Osteonecrosis of the femoral head: Diagnosis and classification systems. Curr Rev Musculoskelet Med. 2015;8:210–20. doi: 10.1007/s12178-015-9278-7. doi: 10.1007/s12178-015-9278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–74. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Hamada H, Takao M, Sakai T, Sugano N. Subchondral fracture begins from the bone resorption area in osteonecrosis of the femoral head: A micro-computerised tomography study. Int Orthop. 2018;42:1479–84. doi: 10.1007/s00264-018-3879-x. doi: 10.1007/s00264-018-3879-x. [DOI] [PubMed] [Google Scholar]
- 33.Trevisan C, Ortolani S, Monteleone M, Marinoni EC. Regional migratory osteoporosis: A pathogenetic hypothesis based on three cases and a review of the literature. Clin Rheumatol. 2002;21:418–25. doi: 10.1007/s100670200112. doi: 10.1007/s100670200112. [DOI] [PubMed] [Google Scholar]
- 34.Patel S. Primary bone marrow oedema syndromes. Rheumatology (Oxford) 2014;53:785–92. doi: 10.1093/rheumatology/ket324. doi: 10.1093/rheumatology/ket324. [DOI] [PubMed] [Google Scholar]
- 35.Elder GJ. From marrow oedema to osteonecrosis: Common paths in the development of post-transplant bone pain. Nephrology (Carlton) 2006;11:560–7. doi: 10.1111/j.1440-1797.2006.00708.x. doi: 10.1111/j.1440-1797.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 36.Board TN. Subchondral insufficiency fracture of the femoral head and acetabulum: Indications for total hip arthroplasty. J Bone Joint Surg Am. 2003;85-A:572. doi: 10.2106/00004623-200303000-00036. [DOI] [PubMed] [Google Scholar]
- 37.Min BW, Song KS, Cho CH, Lee SM, Lee KJ. Untreated asymptomatic hips in patients with osteonecrosis of the femoral head. Clin Orthop Relat Res. 2008;466:1087–92. doi: 10.1007/s11999-008-0191-x. doi: 10.1007/s11999-008-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nam KW, Kim YL, Yoo JJ, Koo KH, Yoon KS, Kim HJ, et al. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90:477–84. doi: 10.2106/JBJS.F.01582. doi: 10.2106/JBJS.F.01582. [DOI] [PubMed] [Google Scholar]
- 39.Hernigou P, Habibi A, Bachir D, Galacteros F. The natural history of asymptomatic osteonecrosis of the femoral head in adults with sickle cell disease. J Bone Joint Surg Am. 2006;88:2565–72. doi: 10.2106/JBJS.E.01455. doi: 10.2106/JBJS.E.01455. [DOI] [PubMed] [Google Scholar]
- 40.Kang JS, Moon KH, Kwon DG, Shin BK, Woo MS. The natural history of asymptomatic osteonecrosis of the femoral head. Int Orthop. 2013;37:379–84. doi: 10.1007/s00264-013-1775-y. doi: 10.1007/s00264-013-1775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larson E, Jones LC, Goodman SB, Koo KH, Cui Q. Early-stage osteonecrosis of the femoral head: Where are we and where are we going in year 2018? Int Orthop. 2018;42:1723–8. doi: 10.1007/s00264-018-3917-8. doi: 10.1007/s00264-018-3917-8. [DOI] [PubMed] [Google Scholar]
- 42.Kubo Y, Motomura G, Ikemura S, Sonoda K, Hatanaka H, Utsunomiya T, et al. The effect of the anterior boundary of necrotic lesion on the occurrence of collapse in osteonecrosis of the femoral head. Int Orthop. 2018;42:1449–55. doi: 10.1007/s00264-018-3836-8. doi: 10.1007/s00264-018-3836-8. [DOI] [PubMed] [Google Scholar]
- 43.Koo KH, Kim R. Quantifying the extent of osteonecrosis of the femoral head. A new method using MRI. J Bone Joint Surg Br. 1995;77:875–80. [PubMed] [Google Scholar]
- 44.Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K, et al. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7:601–5. doi: 10.1007/s007760200108. doi: 10.1007/s007760200108. [DOI] [PubMed] [Google Scholar]
- 45.Mont MA, Marulanda GA, Jones LC, Saleh KJ, Gordon N, Hungerford DS, et al. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):16–26. doi: 10.2106/JBJS.F.00457. doi: 10.2106/JBJS.F.00457. [DOI] [PubMed] [Google Scholar]
- 46.Sun W, Li ZR, Wang BL, Liu BL, Zhang QD, Guo WS, et al. Relationship between preservation of the lateral pillar and collapse of the femoral head in patients with osteonecrosis. Orthopedics. 2014;37:e24–8. doi: 10.3928/01477447-20131219-12. [DOI] [PubMed] [Google Scholar]
- 47.Mont MA, Zywiel MG, Marker DR, McGrath MS, Delanois RE. The natural history of untreated asymptomatic osteonecrosis of the femoral head: A systematic literature review. J Bone Joint Surg Am. 2010;92:2165–70. doi: 10.2106/JBJS.I.00575. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 48.Hernigou P, Poignard A, Nogier A, Manicom O. Fate of very small asymptomatic stage-I osteonecrotic lesions of the hip. J Bone Joint Surg Am. 2004;86-A:2589–93. doi: 10.2106/00004623-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Liu LH, Zhang QY, Sun W, Li ZR, Gao FQ. Corticosteroid-induced osteonecrosis of the femoral head: Detection, diagnosis, and treatment in earlier stages. Chin Med J. 2017;130:2601–7. doi: 10.4103/0366-6999.217094. doi: 10.4103/0366-6999.217094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maus U, Roth A, Tingart M, Rader C, Jäger M, Nöth U, et al. S3 guideline. Part 3: Non-traumatic avascular necrosis in adults-surgical treatment of atraumatic avascular femoral head necrosis in adults. Z Orthop Unfall. 2015;153:498–507. doi: 10.1055/s-0035-1545902. doi: 10.1055/s-0035-1545902. [DOI] [PubMed] [Google Scholar]
- 51.Pierce TP, Jauregui JJ, Elmallah RK, Lavernia CJ, Mont MA, Nace J, et al. Acurrent review of core decompression in the treatment of osteonecrosis of the femoral head. Curr Rev Musculoskelet Med. 2015;8:228–32. doi: 10.1007/s12178-015-9280-0. doi: 10.1007/s12178-015-9280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubo Y, Motomura G, Ikemura S, Sonoda K, Yamamoto T, Nakashima Y, et al. Factors influencing progressive collapse of the transposed necrotic lesion after transtrochanteric anterior rotational osteotomy for osteonecrosis of the femoral head. Orthop Traumatol Surg Res. 2017;103:217–22. doi: 10.1016/j.otsr.2016.10.019. doi: 10.1016/j.otsr.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Sugioka Y, Yamamoto T. Transtrochanteric posterior rotational osteotomy for osteonecrosis. Clin Orthop Relat Res. 2008;466:1104–9. doi: 10.1007/s11999-008-0192-9. doi: 10.1007/s11999-008-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon TR, Abbas AA, Hur CI, Cho SG, Lee JH. Modified transtrochanteric rotational osteotomy for femoral head osteonecrosis. Clin Orthop Relat Res. 2008;466:1110–6. doi: 10.1007/s11999-008-0188-5. doi: 10.1007/s11999-008-0188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Issa K, Johnson AJ, Naziri Q, Khanuja HS, Delanois RE, Mont MA, et al. Hip osteonecrosis: Does prior hip surgery alter outcomes compared to an initial primary total hip arthroplasty? J Arthroplasty. 2014;29:162–6. doi: 10.1016/j.arth.2013.04.028. doi: 10.1016/j.arth.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 56.Hungerford DS. Treatment of osteonecrosis of the femoral head: Everything's new. J Arthroplasty. 2007;22:91–4. doi: 10.1016/j.arth.2007.02.009. doi: 10.1016/j.arth.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Zhang HJ, Liu YW, Du ZQ, Guo H, Fan KJ, Liang GH, et al. Therapeutic effect of minimally invasive decompression combined with impaction bone grafting on osteonecrosis of the femoral head. Eur J Orthop Surg Traumatol. 2013;23:913–9. doi: 10.1007/s00590-012-1141-6. doi: 10.1007/s00590-012-1141-6. [DOI] [PubMed] [Google Scholar]
- 58.Sun W, Li Z, Gao F, Shi Z, Zhang Q, Guo W, et al. Recombinant human bone morphogenetic protein-2 in debridement and impacted bone graft for the treatment of femoral head osteonecrosis. PLoS One. 2014;9:e100424. doi: 10.1371/journal.pone.0100424. doi: 10.1371/journal.pone.0100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang YH, Hu CC, Chen DW, Ueng SW, Shih CH, Lee MS, et al. Local cancellous bone grafting for osteonecrosis of the femoral head. Surg Innov. 2009;16:63–7. doi: 10.1177/1553350608330398. doi: 10.1177/1553350608330398. [DOI] [PubMed] [Google Scholar]
- 60.Steinberg ME, Larcom PG, Strafford B, Hosick WB, Corces A, Bands RE, et al. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;386:71–8. doi: 10.1097/00003086-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 61.Zuo W, Sun W, Zhao D, Gao F, Su Y, Li Z, et al. Correction: Investigating clinical failure of bone grafting through a window at the femoral head neck junction surgery for the treatment of osteonecrosis of the femoral head. PLoS One. 2016;11:e0160163. doi: 10.1371/journal.pone.0160163. doi: 10.1371/journal.pone.0156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plakseychuk A. CORR insights®: Free vascularized fibular grafting improves vascularity compared with core decompression in femoral head osteonecrosis: A randomized clinical trial. Clin Orthop Relat Res. 2017;475:2241–4. doi: 10.1007/s11999-017-5425-3. doi: 10.1007/s11999-017-5425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ligh CA, Nelson JA, Fischer JP, Kovach SJ, Levin LS. The effectiveness of free vascularized fibular flaps in osteonecrosis of the femoral head and neck: A systematic review. J Reconstr Microsurg. 2017;33:163–72. doi: 10.1055/s-0036-1594294. doi: 10.1055/s-0036-1594294. [DOI] [PubMed] [Google Scholar]
- 64.Ding H, Chen SB, Gao YS, Lin S, Zhang CQ. Free vascularized fibular grafting for patients receiving postoperative corticosteroids. Orthopedics. 2014;37:e357–61. doi: 10.3928/01477447-20140401-56. doi: 10.3928/01477447-20140401-56. [DOI] [PubMed] [Google Scholar]
- 65.Aoyama T, Goto K, Kakinoki R, Ikeguchi R, Ueda M, Kasai Y, et al. An exploratory clinical trial for idiopathic osteonecrosis of femoral head by cultured autologous multipotent mesenchymal stromal cells augmented with vascularized bone grafts. Tissue Eng Part B Rev. 2014;20:233–42. doi: 10.1089/ten.teb.2014.0090. doi: 10.1089/ten.TEB.2014.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yen CY, Tu YK, Ma CH, Yu SW, Kao FC, Lee MS, et al. Osteonecrosis of the femoral head: Comparison of clinical results for vascularized iliac and fibula bone grafting. J Reconstr Microsurg. 2006;22:21–4. doi: 10.1055/s-2006-931902. doi: 10.1055/s-2006-931902. [DOI] [PubMed] [Google Scholar]
- 67.Feng Y, Wang S, Jin D, Sheng J, Chen S, Cheng X, et al. Free vascularised fibular grafting with osteoSet®2 demineralised bone matrix versus autograft for large osteonecrotic lesions of the femoral head. Int Orthop. 2011;35:475–81. doi: 10.1007/s00264-009-0915-x. doi: 10.1007/s00264-009-0915-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeng YR, He S, Feng WJ, Li FL, Li J, Jian LY, et al. Vascularised greater trochanter bone graft, combined free iliac flap and impaction bone grafting for osteonecrosis of the femoral head. Int Orthop. 2013;37:391–8. doi: 10.1007/s00264-012-1773-5. doi: 10.1007/s00264-012-1773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao D, Wang B, Guo L, Yang L, Tian F. Will a vascularized greater trochanter graft preserve the necrotic femoral head? Clin Orthop Relat Res. 2010;468:1316–24. doi: 10.1007/s11999-009-1159-1. doi: 10.1007/s11999-009-1159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin S, Zhang C, Jin D, Chen S, Sun Y, Sheng J, et al. Treatment of osteonecrosis of the femoral head in lymphoma patients by free vascularised fibular grafting. Int Orthop. 2011;35:1125–30. doi: 10.1007/s00264-010-1031-7. doi: 10.1007/s00264-010-1031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang YS, Yin L, Lu ZD, Wu XJ, Liu HJ. Analysis of long-term outcomes of double-strut bone graft for osteonecrosis of the femoral head. Orthop Surg. 2009;1:22–7. doi: 10.1111/j.1757-7861.2008.00005.x. doi: 10.1111/j.1757-7861.2008.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dailiana ZH, Toth AP, Gunneson E, Berend KR, Urbaniak JR. Free vascularized fibular grafting following failed core decompression for femoral head osteonecrosis. J Arthroplasty. 2007;22:679–88. doi: 10.1016/j.arth.2006.12.042. doi: 10.1016/j.arth.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 73.Chen CC, Lin CL, Chen WC, Shih HN, Ueng SW, Lee MS, et al. Vascularized iliac bone-grafting for osteonecrosis with segmental collapse of the femoral head. J Bone Joint Surg Am. 2009;91:2390–4. doi: 10.2106/JBJS.H.01814. doi: 10.2106/JBJS.H.01814. [DOI] [PubMed] [Google Scholar]
- 74.Yang S, Wu X, Xu W, Ye S, Liu X, Liu X, et al. Structural augmentation with biomaterial-loaded allograft threaded cage for the treatment of femoral head osteonecrosis. J Arthroplasty. 2010;25:1223–30. doi: 10.1016/j.arth.2009.08.019. doi: 10.1016/j.arth.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 75.Wei BF, Ge XH. Treatment of osteonecrosis of the femoral head with core decompression and bone grafting. Hip Int. 2011;21:206–10. doi: 10.5301/HIP.2011.6525. doi: 10.5301/HIP.2011.6525. [DOI] [PubMed] [Google Scholar]
- 76.Wang BL, Sun W, Shi ZC, Zhang NF, Yue DB, Guo WS, et al. Treatment of nontraumatic osteonecrosis of the femoral head using bone impaction grafting through a femoral neck window. Int Orthop. 2010;34:635–9. doi: 10.1007/s00264-009-0822-1. doi: 10.1007/s00264-009-0822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]