Abstract
Background
Previous studies have shown that T cell immunoglobulin domain and mucin-3 (Tim-3) and interleukin-17 (IL-17) are implicated in the development of several autoimmune diseases. However, it is unclear whether these proteins contribute to the pathogenesis of systemic lupus erythematosus (SLE). The purpose of this study was to evaluate SLE patient serum Tim-3 and IL-17 levels, and to assess correlations between these proteins and major clinical parameters of SLE.
Material/Methods
Overall, 55 SLE patients and 55 healthy controls were recruited in a case-control study. Serum Tim-3 and IL-17 levels were quantified using an enzyme-linked immunosorbent assay (ELISA) kit.
Results
Serum Tim-3 and IL-17 levels in SLE patients were significantly elevated relative to healthy controls (all P<0.05). Serum Tim-3 levels were significantly lower in SLE patients with nephritis than in those SLE without nephritis (P<0.05), while no statistically significant correlation between serum IL-17 and nephritis was detected (P>0.05). Serum Tim-3 with IL-17 levels were positively correlated in SLE patients (rs=0.817, P<0.01); however, no statistically significant correlation was found between serum Tim-3 or IL-17 levels and systemic lupus erythematosus disease activity index (SLEDAI) scores in those with SLE (all P>0.05). In addition, serum Tim-3 was associated with central lesions in SLE patients, while there were no significant correlations between serum Tim-3 or IL-17 levels and other SLE clinical parameters.
Conclusions
Increased serum Tim-3 and IL-17 levels and their clinical associations in SLE patients suggest their possible role in this disease.
MeSH Keywords: Immunoglobulin Allotypes; Interleukin-17; Lupus Vasculitis, Central Nervous System
Background
Systemic lupus erythematosus (SLE) is a typical autoimmune disease, characterized by a large number of autoantibodies and the deposition of immune complexes, with diverse clinical manifestations causing persistent illness [1]. SLE prevalence is estimated to be up to 241/100 000 people, mainly affecting women of childbearing age [2]. Regulatory imbalances in helper T cells and their cytokines play important roles in the pathogenesis of SLE [3].
T cell immunoglobulin domain and mucin-3 (Tim-3) is a member of the Tim gene family discovered in 2003 [4]. Tim-3 is the first known transmembrane glycoprotein that specifically identifies Th1 cells in mice and humans, and is a Th1 cell-specific type 1 membrane protein. Previous studies have reported that Tim-3 is mainly expressed on differentiated Th1 cells and Th17 cells but not on Th2 cells. Th1 cells and Th2 cells could previously only be recognized by their secretion of specific cytokines, but Tim-3 can also be used as a unique surface molecule to distinguish them, making it a valuable reference for T cell status [5]. In vivo administration of Tim-3 antibody not only aggravates the severity of autoimmune disease mediated by Th1 cells, but also increases the number and activation level of macrophages [6]. Moreover, Tim-3 is associated with nephritis [7] and disease activity [8] in SLE patients, but no significant relationship between Tim-3 mRNA and SLE was found in a separate study [9]. These inconsistent results suggest that the role of Tim-3 in SLE is complex and deserves further exploration.
Interleukin-17A (IL-17), a major effect factor of Th17 cells, is a precursor to proinflammatory cytokines identified in recent years that are secreted by CD4 + T cells. Elevated IL-17 levels are associated with a higher experimental autoimmune meningitis (EAE) risk [10], increased multiple sclerosis (MS) severity [11], serious rheumatoid arthritis (RA) inflammation [12], and higher SLE disease activity [13], while decreased IL-17 levels can lead to lupus or RA-like symptoms [14]. However, the reliability of these findings remains controversial [15]. Therefore, the exact role of IL-17 in SLE warrants additional investigation.
Herein, to further examine the roles of Tim-3 and IL-17 in the pathogenesis of SLE, we evaluated serum Tim-3 and IL-17 levels in patients with SLE. Moreover, we analyzed their correlations with major clinical parameters.
Material and Methods
Subjects
A total of 110 participants, including 55 SLE patients and 55 healthy controls, were recruited from the Affiliated Yijishan Hospital of Wannan Medical College. SLE was defined based on the classification criteria revised by the American College of Rheumatology (ACR) in 1997 [16]. Disease activity was assessed based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) [17]. Patients in the more active SLE group were those with SLEDAI-2K score >4, while those with less active SLE were those with a SLEDAI-2K score ≤4 [18,19]. Nephritis diagnosis was made according to the ACR criteria, which were as follows: 1) persistent proteinuria ≥0.5 g/day 2) the presence of active cellular casts, or 3) biopsy evidence of lupus nephritis. Exclusion criteria for all patients were as follows: a) patients with other connective tissue diseases, b) patients with malignancies, c) patients with other autoimmune diseases, and d) patients with viral infectious diseases. Patients were also excluded if they did not meet the inclusion criteria. Patients were randomly selected from among eligible cases in the ward of the hospital’s Rheumatology Department based on patient bed numbers and a random number table. Sample sizes were calculated based on the previous study of Montaigne et al. [20]. Our hypothesis was that there would be a 50% (SD 51%) relative difference in serum Tim-3 levels between SLE patients and controls. We therefore recruited 55 patients and 55 controls to achieve a power of 80% for a significance level of 5% with a 2-tailed test in this study. The healthy control group was age- and sex-matched with the SLE group. Disease duration was calculated since the time of diagnosis. General demographic information and clinical parameters were obtained through epidemiological interviews and hospital records.
This study was approved by the Ethics Committee of Wannan Medical College, and informed consent was obtained from all participants.
Serum isolation and enzyme-linked immunosorbent assay (ELISA)
Serum was separated from 5 ml of whole blood collected from all participants, and was stored at −80°C. Serum levels of Tim-3 and IL-17 were assayed via quantitative sandwich ELISAs, following the manufacturer’s instructions for the Tim-3 and IL-17 kits (R&D Systems, Inc.). All ELISA results are expressed as cytokine concentrations (pg/ml). Serum from SLE patients and controls were analyzed together in the same laboratory, and the laboratory personnel were blind to patient disease status.
Statistical analysis
Numerical data conforming to normal distributions are presented as means± standard deviation (SD), while those not normally distributed are presented as medians (interquartile range, IQR). Mann-Whitney rank sum tests or t tests were performed to estimate differences between groups for continuous variables. Differences in categorical variables among groups were assessed using the chi-squared test or Fisher’s exact test. Correlation analyses were performed using Spearman’s rank correlation coefficient. Statistical analyses were conducted using SPSS software version 18.0 (SPSS, Inc, Chicago, IL). A 2-tailed P<0.05 was viewed as significantly different.
Results
Study subject demographics
The general characteristics of study subjects are presented in Table 1. The age (P=0.903) and sex (P=1.000) between the SLE group and the control group were not statistically different. The average disease duration and SLEDAI-2K scores for the SLE group were 4.42 years and 16.00 years, respectively. Participants with lupus nephritis accounted for 54.55% of SLE patients.
Table 1.
The general characteristics of study subjects.
| SLE group | Control group | |
|---|---|---|
| Number | 55 | 55 |
| Age (years) | 37.7±13.6 | 37.4±12.2 |
| Female, n (%) | 52 (94.55) | 52 (94.55) |
| Duration (years) | 4.42 (0.07, 9.00) | NA |
| SLEDAI | 16.00 (10.00, 20.00) | NA |
| Lupus Nephritis, n (%) | 30 (54.55) | NA |
| Arthritis, n (%) | 26 (47.27) | NA |
| Rash, n (%) | 30 (54.55) | NA |
| Alopecia, n (%) | 24 (43.64) | NA |
| Central lesions, n (%) | 12 (21.82) | NA |
| Visually impairment, n (%) | 5 (9.10) | NA |
| Oral ulcers, n (%) | 11 (20.00) | NA |
| Fever, n (%) | 37 (67.27) | NA |
| Headache, n (%) | 6 (10.91) | NA |
| Thrombocytopenia, n (%) | 12 (21.82) | NA |
| Leukopenia, n (%) | 8 (14.55) | NA |
| Cast, n (%) | 9 (16.36) | NA |
| Hematuria, n (%) | 30 (54.55) | NA |
| Proteinuria, n (%) | 28 (50.91) | NA |
| Anti-dsDNA, n (%) | 35 (63.64) | NA |
| Anti-Sm, n (%) | 24 (43.64) | NA |
| Anti-SSA, n (%) | 39 (70.91) | NA |
| Anti-SSB, n (%) | 9 (16.36) | NA |
| Anti-RNP, n (%) | 24 (43.64) | NA |
| Anti-Ribosomal P, n (%) | 18 (32.73) | NA |
| C3 | 0.65 (0.47, 0.84) | NA |
| C4 | 0.10 (0.04, 0.21) | NA |
| ESR | 41.00 (22.00, 68.50) | NA |
| CRP | 5.86 (1.98, 17.22) | NA |
| IgA | 2.39 (1.63, 3.27) | NA |
| IgG | 12.77 (8.86, 20.91) | NA |
| IgM | 1.01 (0.70, 1.38) | NA |
| Corticosteroids≤ 30mg/day, n (%) | 28 (50.91) | NA |
| Corticosteroids > 30mg/day, n (%) | 27 (49.09) | NA |
| Antimalarials, n (%) | 49 (89.09) | NA |
| Azathioprine | 8 (14.55) | NA |
| Methotrexate | 9 (16.36) | NA |
| Cyclophosphamide | 11 (20) | NA |
SLE – systemic lupus erythematosus; SLEDAI-2K – systemic lupus erythematosus disease activity index 2000; NA – not applicable. Numerical data conforming to the normal distribution are presented as means± standard deviation (SD); otherwise data are presented as medians (interquartile range, IQR).
Comparison of serum Tim-3 and IL-17 levels between SLE patients and healthy controls, and within SLE patient subgroups
The average levels of serum Tim-3 for SLE patients and controls were 252.71 (214.61, 332.33) and 229.42 (159.40, 297.03) pg/ml, respectively. The average levels of serum IL-17 for these 2 groups were 4.67 (3.74, 5.33) and 4.13 (3.00, 4.91) pg/ml, respectively. The levels of Tim-3 (P=0.029) and IL-17 (P=0.022) in SLE patients were significantly elevated relative to healthy controls (Figure 1).
Figure 1.
Comparison of serum Tim-3 and IL-17 levels between SLE patients and healthy controls. (A) The serum level of Tim-3 in the SLE and healthy control groups, and (B) the serum level of IL-17 in the SLE and healthy control groups. The serum concentrations (median) of Tim-3 and IL-17 in the SLE group were significantly higher than in the healthy control group (both P<0.05).
The serum level of Tim-3 was significantly lower in SLE patients with nephritis as compared to SLE patients without nephritis (P=0.020). However, there was no significant difference in IL-17 levels between SLE patients with or without nephritis (P=0.101). Moreover, no significant differences in Tim-3 and IL-17 levels were identified between patients with less active SLE as compared to those with more active SLE (P=0.344 and P=0.671, respectively) (Table 2).
Table 2.
Comparison of Tim-3 and IL-17 levels between different SLE patient subgroups.
| Group | Number | Tim-3 (pg/ml) | IL-17 (pg/ml) |
|---|---|---|---|
| SLE without nephritis | 25 | 292.54 (277.85–370.70) | 4.89 (4.18–5.43) |
| SLE with nephritis | 30 | 236.65 (206.57–288.02)* | 4.37 (3.65–5.23) |
| Less active SLE | 6 | 198.40 (169.11–264.00) | 3.70 (3.02–4.80) |
| More active SLE | 49 | 270.62 (222.25–332.35) | 4.71 (3.93–5.35) |
SLE – systemic lupus erythematosus; Tim-3 – T cell immunoglobulin domain and mucin-3; IL-17 – interleukin-17. Numerical data conforming to the normal distribution are presented as means ± standard deviation (SD); otherwise data are presented as medians (interquartile range, IQR).
P<0.05 vs. SLE without nephritis.
Correlation of serum Tim-3 level with IL-17 level in SLE patients
We analyzed the correlation between serum Tim-3 levels and IL-17 levels in SLE patients and found that these 2 factors were significantly positively correlated (rs=0.817, P<0.01) (Figure 2), whereas they were not correlated in controls (rs=0.006, P=0.963).
Figure 2.
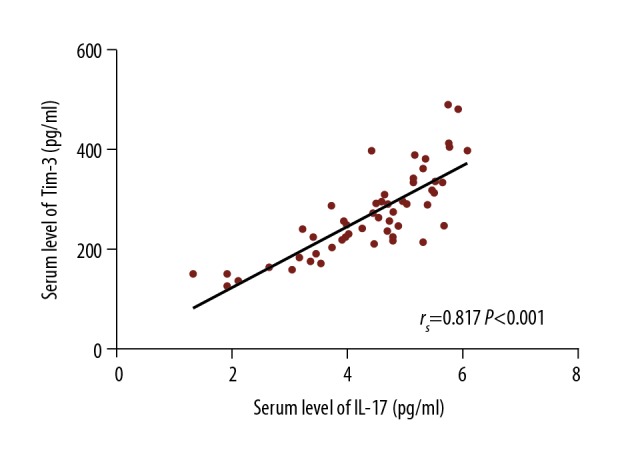
Correlation of serum Tim-3 level with IL-17 level in SLE patients. The individual dots represent paired values of serum Tim-3 and IL-17 levels in SLE patients. The line shown is a linear univariate correlation. A significant positive correlation was observed between the serum levels of Tim-3 and IL-17 (rs=0.817, P<0.001).
Correlations of serum Tim-3 and IL-17 levels with major clinical parameters in SLE patients
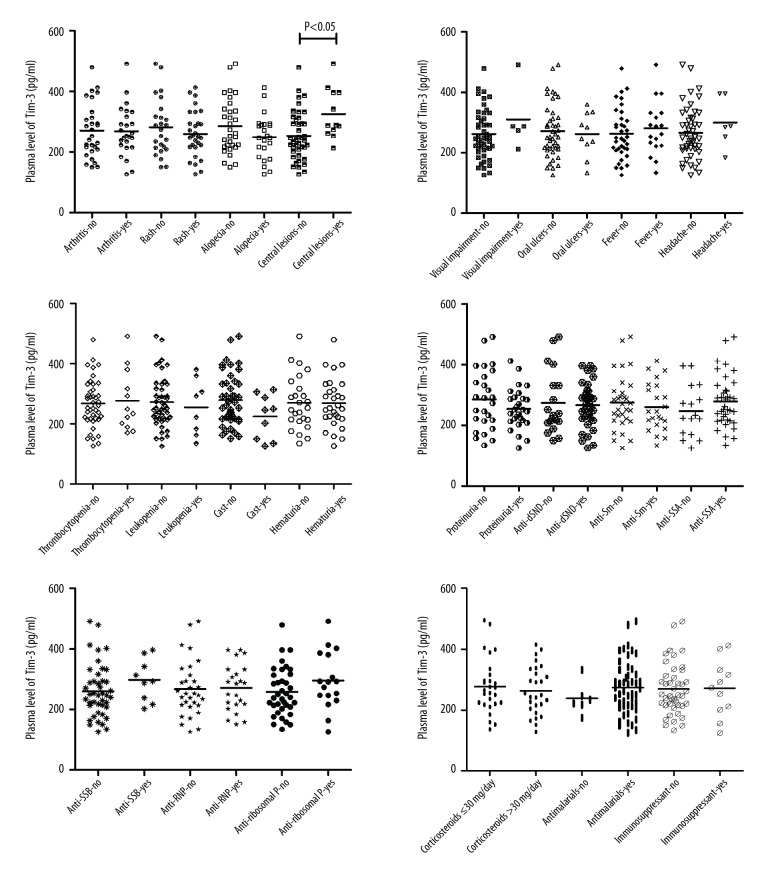
Correlations between serum Tim-3 and IL-17 levels and major clinical parameters in SLE patients were analyzed, revealing that the Tim-3 level in SLE patients with central lesions differed significantly from those in SLE patients without central lesions (P=0.020), but no significant differences in serum Tim-3 or IL-17 levels between other clinical parameters were observed (all P>0.05) (Table 3, Figures 3, 4).
Table 3.
Correlations of serum Tim-3 and IL-17 levels with quantitative clinical parameters in SLE patients.
| Parameters | Tim-3 | IL-17 | ||
|---|---|---|---|---|
| rs | P | rs | P | |
| C3 | 0.237 | 0.081 | 0.147 | 0.284 |
| C4 | −0.027 | 0.849 | −0.092 | 0.510 |
| ESR | 0.105 | 0.453 | 0.177 | 0.204 |
| CRP | 0.129 | 0.359 | 0.139 | 0.321 |
| SLEDAI-2K | 0.028 | 0.840 | −0.005 | 0.973 |
| Disease duration | −0.213 | 0.119 | −0.044 | 0.751 |
| IgA | 0.262 | 0.103 | 0.276 | 0.085 |
| IgG | 0.211 | 0.191 | 0.118 | 0.468 |
| IgM | 0.047 | 0.773 | −0.007 | 0.964 |
SLE – systemic lupus erythematosus; Tim-3 – T cell immunoglobulin domain and mucin-3; IL-17 – interleukin-17; C3 – Complement 3; C4 – Complement 4; ESR – erythrocyte sedimentation rate; CRP – C-reactive protein; SLEDAI-2K – systemic lupus erythematosus disease activity index 2000; IgA – immunoglobulin A; IgG – immunoglobulin G; IgM – immunoglobulin M.
Figure 3.
Comparison of serum Tim-3 levels with different categorical clinical parameters in SLE patients. SLE – systemic lupus erythematosus; Tim-3 – T cell immunoglobulin domain and mucin-3; IL-17 – interleukin-17; dsDNA – double stranded DNA; Sm – Smith; SSA – Sjögren’s syndrome-related antigen A; SSB – Sjögren’s syndrome-related antigen B; RNP – Ribonucleoprotein. Significant differences in serum Tim-3 levels were observed between SLE patients with and without central lesions (P=0.020). No significant differences in other clinical parameters were observed (all P>0.05).
Figure 4.
Comparison of serum IL-17 levels with different categorical clinical parameters in SLE patients. SLE – systemic lupus erythematosus; Tim-3 – T cell immunoglobulin domain and mucin-3; IL-17 – interleukin-17; dsDNA – double stranded DNA; Sm – Smith; SSA – Sjögren’s syndrome-related antigen A; SSB – Sjögren’s syndrome-related antigen B; RNP – Ribonucleoprotein. No significant differences in serum IL-17 level were observed between patients with different clinical parameters (all P>0.05).
Discussion
Monney et al. showed that Tim-3 acts as an immunosuppressive factor in Th1 cells [21]. IL-17 has been reported to be involved in the development of inflammation and plays an important role in autoimmune diseases [22,23]. In the present study, we identified increased serum Tim-3 and IL-17 levels in SLE patients. We further found that serum Tim-3 levels were significantly lower in SLE patients with nephritis than in those without nephritis. Moreover, a positive correlation between serum Tim-3 and IL-17 levels was observed in SLE patients. Serum Tim-3 levels in SLE patients with central lesions were significantly different from those in SLE patients without central lesions, but no significant differences in serum Tim-3 and IL-17 levels were identified for other clinical parameters in this case-control study.
Dysfunctional regulation of Th1 and Th2 cells and an imbalance in the Th1/Th2 cell ratio may be a primary mechanism of SLE development or progression [24], and SLE may also be mediated by differentiated Th1 and Th17 cells expressing Tim-3 [3]. Studies have found that Tim-3 expression in peripheral blood mononuclear cells (PBMCs) and CD3+ CD4+/CD3+ CD4− T cells in SLE patients was significantly higher relative to controls [8,25], similar to our results. This may be due to the upregulation of Tim-3 induced by the immune environment [26]. Other research groups have found that Tim-3 mRNA levels in PBMCs from SLE patients were similar to those in healthy controls, which may be a consequence of the different levels of Tim-3 expression in different PBMC subtypes, including activated Th17 cells, macrophages/monocytes, dendritic cells, and natural killer cells [27]. Furthermore, SLE patient renal pathological grades were positively correlated with Tim-3 expression upon renal biopsy, further providing a pathological basis for the involvement of Tim-3 in the pathogenesis of SLE [28]. Both SLEDAI score and serum levels of complement proteins and C-reactive protein (CRP) can reflect SLE disease activity, and Tim-3 expression has been associated with SLEDAI score and serum levels of C3, C4m, and CRP, suggesting that Tim-3 may reflect SLE disease activity [8,29]. We have also found that central lesions are associated with Tim-3 levels, which may be related to the upregulation of Tim-3 on CD11b+ monocytes and intrinsic microglia infiltrating the central nervous system [30]. Moreover, serum Tim-3 levels were significantly lower in SLE patients with nephritis than in those without nephritis in the present study. This may be because the reduction in soluble Tim-3 is a compensatory protective response in the body to prevent excessive kidney damage. The high levels of soluble Tim-3 in the context of chronic inflammation represent a persistent response in the body due to a persistent failure to return to the non-inflamed steady state. In MRL/lpr lupus mice, a Tim-3 ligand (Galectin-9) has been linked to the severity of various rheumatic symptoms such as nephritis and arthritis due to Galectin-9-induced programmed cell death of Th1 and Th17 effector cells [31,32]. The 1516G>T single-nucleotide polymorphism (SNP) in the Tim-3 promoter region has also been associated with SLE susceptibility [33]. These studies demonstrate that Tim-3 is closely associated with the pathological mechanisms underlying SLE, acting by negatively regulating the Th1/Th17 immune response [34].
There is accumulating evidence that IL-17 is involved in autoimmune diseases such as experimental autoimmune meningococcal (EAE) [35], rheumatoid arthritis (RA) [12,36], autoimmune hepatitis [37], and autoimmune enteropathy [38]. Abdel Galil et al. have identified a positive correlation between IL-17 and 24-h proteinuria and high anti-ds-DNA titers in SLE patients [39]. We observed no significant association between IL-17 and auto-antibody levels or other major clinical manifestations, which may be a consequence of the small sample size and differences in efficacy of immunosuppressive drugs in the present study. Toll-like receptor 2 (TLR2) is an innate immune receptor that recognizes bacterial lipoproteins/lipopeptides, and increased expression of TLR2 promotes IL-17 expression via histone modifications, which result in IL-17 being implicated in early-onset SLE and pediatric SLE [13,40–42]. IL-17 is a major effect factor produced by Th17 cells, and in the present study we observed a significant positive correlation between Tim-3 and IL-17 levels in patient serum, which is consistent with previous work showing that Tim-3 is expressed in differentiated Th17 cells [43]. Tim-3 and IL-17 respond similarly to corticosteroids [44]. Therefore, soluble Tim-3 may be a surrogate marker for IL-17 in SLE patients. Th17 cells are proinflammatory effector CD4+ T cells, and the expression of Tim-3 on such cells has been confirmed [45]. Application of Galectin-9 can reduce levels of stimulated Th17 cells and Tim-3+ CD4+ T cells, and can inhibit IL-17 production, thereby reducing bacterial clearance [46]. Administration of antibodies against Tim-3 can significantly exacerbate the clinical and pathologic severity of EAE and can increase the number and activation level of macrophages [21,47]. The Tim-3/Galectin-9 pathway has been well established, and such signaling can lead to the apoptosis of effector CD4+ and CD8+ T cells [48]. This suggests that the Tim-3/Galectin-9 pathway is an immunosuppressive pathway in response to Th1 and Th17-mediated inflammation. In summary, CD4+ T cells differentiate into Th17 cells when activated in SLE patients, and IL-17 expression is consequently increased. Simultaneously, the expression of Tim-3 is increased and plays an immunosuppressive role via Tim-3/Galectin-9 signaling. These changes mediate the chronic inflammatory response observed in many patients, and targeted therapy influencing the Tim-3/Galectin-9 pathway is thus attractive for use in SLE patients.
The present study has several limitations. First, several SLE patients had established disease rather than being new-onset cases, and these results may therefore be affected by corticosteroids, immunosuppressive therapy, and other confounding factors. Second, the lack of a significant correlation between serum Tim-3/IL-17 levels and SLE disease activity index could be due in part to the relatively small sample size and limited statistical power of this study. Finally, distorted results in epidemiological association studies can result from potential biases in case-control studies. Consequently, further studies are needed to clarify the exact roles of Tim-3 and IL-17 in the pathogenesis of SLE.
Conclusions
Increased serum Tim-3 and IL-17 levels in SLE patients and their correlations with disease parameters suggest that they have key roles in modulating the course of this disease.
Acknowledgements
We thank all the SLE patients and our colleagues who helped make this study possible.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Natural Science Foundation of Anhui Province, China (grant no. 1608085MH219, 1808085QH251) and the Student’s Platform for Innovation and Entrepreneurship Training Program of Anhui Province, China (grant no. 201710368069)
References
- 1.Rees F, Doherty M, Grainge M, et al. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999–2012. Ann Rheum Dis. 2016;75:136–41. doi: 10.1136/annrheumdis-2014-206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rees F, Doherty M, Grainge MJ, et al. LThe worldwide incidence and prevalence of systemic lupus erythematosus: A systematic review of epidemiological studies. Rheumatology. 2017;56:1945–61. doi: 10.1093/rheumatology/kex260. [DOI] [PubMed] [Google Scholar]
- 3.Mak A, Kow NY. The pathology of T cells in systemic lupus erythematosus. J Immunol Res. 2014;2014 doi: 10.1155/2014/419029. 419029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuchroo VK, Umetsu DT, Dekruyff RH, Freeman GJ. The TIM gene family: Emerging roles in immunity and disease. Nat Rev Immunol. 2003;3:454–62. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 5.Li YH, Zhou WH, Tao Y, et al. The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the maternal-fetal interface in early pregnancy. Cell Mol Immunol. 2016;13:73–81. doi: 10.1038/cmi.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Sun D, Chen G, et al. Tim-3 inhibits macrophage control of Listeria monocytogenes by inhibiting Nrf2. Sci Rep. 2017;7:42095. doi: 10.1038/srep42095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai XZ, Huang WY, Qiao Y, et al. Downregulation of TIM-3 mRNA expression in peripheral blood mononuclearcells from patients with systemic lupus erythematosus. Braz J Med Biol Res. 2015;48:77–82. doi: 10.1590/1414-431X20143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song LJ, Wang X, Wang XP, et al. Increased Tim-3 expression on peripheral T lymphocyte subsets and association with higher disease activity in systemic lupus erythematosus. Diagn Pathol. 2015;10:71. doi: 10.1186/s13000-015-0306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Meng J, Wang X, et al. Expression of human TIM-1 and TIM-3 on lymphocytes from systemic lupus erythematosus patients. Scand J Immunol. 2008;67:63–70. doi: 10.1111/j.1365-3083.2007.02038.x. [DOI] [PubMed] [Google Scholar]
- 10.Weaver CT, Murphy KM. The central role of the Th17 lineage in regulating the inflammatory/autoimmune axis. Semin Immunol. 2007;19:351–52. doi: 10.1016/j.smim.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Zhao M, Sun D, Guan Y, et al. Disulfiram and diphenhydramine hydrochloride upregulate miR-30a to suppress IL-17-associated autoimmune inflammation. J Neurosci. 2016;36:9253–66. doi: 10.1523/JNEUROSCI.4587-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kugyelka R, Kohl Z, Olasz K, et al. Enigma of IL-17 and Th17 cells in rheumatoid arthritis and in autoimmune animal models of arthritis. Mediators Inflamm. 2016;2016 doi: 10.1155/2016/6145810. 6145810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rana A, Minz RW, Aggarwal R, et al. Gene expression of cytokines (TNF-α, IFN-γ), serum profiles of IL-17 and IL-23 in paediatric systemic lupus erythematosus. Lupus. 2012;21:1105–12. doi: 10.1177/0961203312451200. [DOI] [PubMed] [Google Scholar]
- 14.Biswas PS, Kang K, Gupta S, et al. A murine autoimmune model of rheumatoid arthritis and systemic lupus erythematosus associated with deregulated production of IL-17 and IL-21. Methods Mol Biol. 2012;900:233–51. doi: 10.1007/978-1-60761-720-4_11. [DOI] [PubMed] [Google Scholar]
- 15.Kurasawa K, Hirose K, Sano H, et al. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum. 2000;43:2455–63. doi: 10.1002/1529-0131(200011)43:11<2455::AID-ANR12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 17.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- 18.Franklyn K, Lau CS, Navarra SV, et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS) Ann Rheum Dis. 2015;75:1615–21. doi: 10.1136/annrheumdis-2015-207726. [DOI] [PubMed] [Google Scholar]
- 19.Ugartegil MF, Wojdyla D, Ponsestel GJ, et al. Remission and Low Disease Activity Status (LDAS) protect lupus patients from damage occurrence: Data from a multiethnic, multinational Latin American Lupus Cohort (GLADEL) Ann Rheum Dis. 2017;76:2017–74. doi: 10.1136/annrheumdis-2017-211814. [DOI] [PubMed] [Google Scholar]
- 20.Montaigne D, Marechal X, Modine T, et al. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: A single-centre propensity-matched cohort study and a randomised study. Lancet. 2017;391(10115):59–69. doi: 10.1016/S0140-6736(17)32132-3. [DOI] [PubMed] [Google Scholar]
- 21.Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–41. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 22.Zhao D, Guo M, Liu B, et al. Frontline science: Tim-3-mediated dysfunctional engulfment of apoptotic cells in SLE. J Leukoc Biol. 2017;102:1313–22. doi: 10.1189/jlb.3HI0117-005RR. [DOI] [PubMed] [Google Scholar]
- 23.Shao X, Chen S, Yang D, et al. FGF2 cooperates with IL-17 to promote autoimmune inflammation. Sci Rep. 2017;7:7024. doi: 10.1038/s41598-017-07597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu M, Lu B, Liu Y, et al. Interference with Tim-3 protein expression attenuates the invasion of clear cell renal cell carcinoma and aggravates anoikis. Mol Med Rep. 2017;15:1103–8. doi: 10.3892/mmr.2017.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng H, Guo X, Tian Q, et al. Distinct role of Tim-3 in systemic lupus erythematosus and clear cell renal cell carcinoma. Int J Clin Exp Med. 2015;8:7029–38. [PMC free article] [PubMed] [Google Scholar]
- 26.Chae SC, Park YR, Lee YC, et al. The association of TIM-3 gene polymorphism with atopic disease in Korean population. Hum Immunol. 2004;65:1427–31. doi: 10.1016/j.humimm.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Meng J, Wang X, et al. Expression of human TIM-1 and TIM-3 on lymphocytes from systemic lupus erythematosus patients. Scand J Immunol. 2008;67:63–70. doi: 10.1111/j.1365-3083.2007.02038.x. [DOI] [PubMed] [Google Scholar]
- 28.Guo L, Yang X, Xia Q, et al. Expression of human T cell immunoglobulin domain and mucin-3 (TIM-3) on kidney tissue from systemic lupus erythematosus (SLE) patients. Clin Exp Med. 2014;14:383–88. doi: 10.1007/s10238-013-0264-3. [DOI] [PubMed] [Google Scholar]
- 29.Hou N, Zhao D, Liu Y, et al. Increased expression of T cell immunoglobulin- and mucin domain-containing molecule-3 on natural killer cells in atherogenesis. Atherosclerosis. 2012;222(1):67–73. doi: 10.1016/j.atherosclerosis.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Anderson AC, Anderson DE, Bregoli L, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318(5853):1141–43. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 31.Jiao Q, Qian Q, Zhao Z, et al. Expression of human T cell immunoglobulin domain and mucin-3 (TIM-3) and TIM-3 ligands in peripheral blood from patients with systemic lupus erythematosus. Arch Dermatol Res. 2016;308:553–61. doi: 10.1007/s00403-016-1665-4. [DOI] [PubMed] [Google Scholar]
- 32.Wiersma VR, Bruyn MD, Helfrich W, Bremer E. Therapeutic potential of Galectin-9 in human disease. Med Res Rev. 2013;33(Suppl 1):E102. doi: 10.1002/med.20249. [DOI] [PubMed] [Google Scholar]
- 33.Chuan-Mei P, Gao H, Xiao-Ye FU. Association analysis of Tim-1 polymorphism with systemic lupus erythematosus in a Han population of Yunnan Province. Chin J Lab Diagn. 2014;18:1961–65. [Google Scholar]
- 34.Luo L, Li D, Wang K, et al. Tim3/galectin-9 alleviates the inflammation of TAO patients via suppressing Akt/NF-κB signaling pathway. Biochem Biophys Res Commun. 2017;491(4):966–72. doi: 10.1016/j.bbrc.2017.07.144. [DOI] [PubMed] [Google Scholar]
- 35.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Röhn A, Jennings GT, Hernandez M, et al. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur J Immunol. 2006;36:2857–67. doi: 10.1002/eji.200636658. [DOI] [PubMed] [Google Scholar]
- 37.Grant CR, Holder BS, Liberal R, et al. Immunosuppressive drugs affect interferon (IFN)-γ and programmed cell death 1 (PD-1) kinetics in patients with newly diagnosed autoimmune hepatitis. Clin Exp Immunol. 2017;189:71–82. doi: 10.1111/cei.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paroni M, Magarotto A, Tartari S, et al. Uncontrolled IL-17 production by intraepithelial lymphocytes in a case of non-IPEX autoimmune enteropathy. Clin Transl Gastroenterol. 2016;7:e182. doi: 10.1038/ctg.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdel Galil SM, Ezzeldin N, El-Boshy ME. The role of serum IL-17 and IL-6 as biomarkers of disease activity and predictors of remission in patients with lupus nephritis. Cytokine. 2015;76(2):280–87. doi: 10.1016/j.cyto.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Zhang Y, Zhang L, et al. Epistatic interaction between genetic variants in susceptibility gene ETS1 correlates with IL-17 levels in SLE patients. Ann Hum Genet. 2013;77:344–50. doi: 10.1111/ahg.12018. [DOI] [PubMed] [Google Scholar]
- 41.Nilsen NJ, Vladimer GI, Stenvik J, et al. A role for the adaptor proteins TRAM and TRIF in toll-like receptor 2 signaling. J Biol Chem. 2015;290:3209–22. doi: 10.1074/jbc.M114.593426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Liao J, Zhao M, et al. Increased expression of TLR2 in CD4+ T cells from SLE patients enhances immune reactivity and promotes IL-17 expression through histone modifications. Eur J Immunol. 2015;45:2683–93. doi: 10.1002/eji.201445219. [DOI] [PubMed] [Google Scholar]
- 43.Hu WK, Lu XX, Yang S, et al. Expression of the Th1-specific cell-surface protein Tim-3 increases in a murine model of atopic asthma. J Asthma. 2009;46:872–77. doi: 10.3109/02770900903199953. [DOI] [PubMed] [Google Scholar]
- 44.Seki M, Oomizu S, Sakata KM, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Kanai Y, Satoh T, Igawa K, Yokozeki H. Impaired expression of Tim-3 on Th17 and Th1 cells in psoriasis. Acta Derm Venereol. 2012;92:367–71. doi: 10.2340/00015555-1285. [DOI] [PubMed] [Google Scholar]
- 46.Wang F, Xu J, Liao Y, et al. Tim-3 ligand galectin-9 reduces IL-17 level and accelerates Klebsiella pneumoniae infection. Cell Immunol. 2011;269:22–28. doi: 10.1016/j.cellimm.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Sabatos CA, Chakravarti S, Cha E. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–10. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 48.Chen TC, Chen CH, Wang CP, et al. The immunologic advantage of recurrent nasopharyngeal carcinoma from the viewpoint of Galectin-9/Tim-3-related changes in the tumour microenvironment. Sci Rep. 2017;7:10349. doi: 10.1038/s41598-017-10386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]