Abstract
Background
Valproic acid (VPA) is an antiepileptic agent with histone deacetylase inhibitor activity shown to enhance overall survival and progression free survival in patients with newly diagnosed glioblastoma (GBM). This reports on the late toxicity of the VPA/radiotherapy (RT)/temozolomide (TMZ) combination in the long-term survivors of a phase 2 study evaluating this regimen.
Methods
37 patients with newly diagnosed GBM were initially enrolled on this trial and received combination therapy. VPA/RT/TMZ related late toxicities were evaluated in the 6 patients that lived greater than 3 years using the Cancer Therapy and Evaluation Program Common Toxicity Criteria (CTC) Version 4.0 for toxicity and adverse event reporting as well as the RTOG/EORTC Radiation Morbidity Scoring Scheme.
Results
The median duration of follow-up for these 6 patients was 69.5m. In this cohort, the median OS was 73.8m (60.8–103.8m) and median PFS was 53.1m (37.3 – 103.8m). The most common late toxicity of VPA in conjunction with RT/TMZ were the CTC classifications of neurological, pain, and blood/ bone marrow toxicity and most were grade 1/2. There were only two grade 3/4 toxicities.
Conclusions
The addition of VPA to concurrent RT/TMZ in patients with newly diagnosed GBM was well tolerated with little late toxicity. Additionally, VPA may result in improved outcomes as compared to historical data and merits further study.
Keywords: Glioblastoma, Late effects, radiation sensitizer, toxicity, Valproic Acid
Glioblastoma (GBM) is the most common primary brain tumor in the United States with 12000 new cases annually. Standard therapy including surgical resection, the combination of radiation therapy (RT) and temozolomide (TMZ) followed by additional monthly TMZ, results in a median survival of 14.6 months.1 Due to this underwhelming result, additional research is needed to improve on this standard protocol.
Most GBMs fail in or adjacent to the initial RT treatment volume (85–90%) suggesting that enhancing the effectiveness of RT could improve response. Recently, we reported on the efficacy of adding the histone deacetylase inhibitor (HDACi) Valproic Acid (VPA) as a radiation sensitizer to the standard RT/TMZ regimen.2 VPA enhances glioma cell radiosensitivity in pre-clinical models and readily crosses the blood brain barrier.3 The median overall survival (OS) reported in our study was 29.6m (21- 63.8m), and the median progression free survival (PFS) was 10.5m (6.8 – 51.2m). The combination was well tolerated by the patients with minimal acute toxicity.
For a radiosensitizer to be of clinical benefit, it should selectively enhance the radiosensitivity of tumor cells over normal cells.3 However, preclinical models of normal tissue toxicity are relatively insensitive especially those evaluating late CNS effects. Thus, to determine if VPA is safe to use as a clinical radiation sensitizer, the late normal tissue toxicity of the triple combination was studied in those patients that lived beyond 3 years (2.5 times median survival for standard therapy) without evidence of tumor recurrence. Reported herein is that late toxicity profile.
Methods and Materials
Patient Eligibility
As reported previously, this open-label, Phase II study (NCI-06-C-0112) was conducted at the National Cancer Institute and Virginia Commonwealth University in patients with histologically confirmed GBM, aged 18 years or older and a life expectancy greater than 8 weeks, with surgery no more than 6 weeks prior to enrollment.2 The protocol was reviewed and approved by the NCI Institutional Review Board, and written informed consent was signed by all patients.
Treatment
Patients were administered VPA 25 mg/kg orally divided into two daily doses concurrent with 6 weeks of RT and TMZ. The first dose of VPA was given 1 week before the first day of RT at 10 to 15 mg/kg/day and subsequently increased up to 25 mg/kg/day. RT was delivered using 3D conformal or IMRT technique 5 days a week in 2 Gy fractions to 60 Gy total. TMZ was given daily at a dose of 75 mg/m2 concurrently with RT. Adjuvant TMZ was given for 5 days at 150 mg/m2 for one cycle and then 200 mg/m2 every 4 weeks if tolerated and no evidence of tumor progression.
Patient Evaluation
Patients were evaluated at baseline, weekly during VPA/RT/TMZ and subsequently at 1 month and then 3 month intervals for the first 2 years and then every six months until failure. Evaluations included history and physical exam, hematological and clinical chemistry studies and MR imaging. Late toxicities were evaluated using the Cancer Therapy and Evaluation Program Common Toxicity Criteria (CTC) version 4.0 for toxicity and adverse event reporting as well as the RTOG/EORTC Radiation Morbidity Scoring Scheme. Treatment response was analyzed per Response Evaluation Criteria in Solid Tumors (RECIST)4 and retrospectively by RANO criteria.5 Time to progression was determined from initiation of treatment on protocol to symptomatic or radiographic progression. Overall survival (OS) was determined from the initiation of treatment on protocol to date of death. Patient’s MR images were fused to their original treatment plan using Eclipse® and brain volumes were calculated within the 100% and 50% dose values.
Statistical Analysis
Kaplan-Meier method was used to estimate OS and PFS.
Results
Patient Characteristics
Six patients had no evidence of progression for a minimum of 3 years and form the cohort to evaluate late toxicities potentially caused by VPA/RT/TMZ. Patient demographics and baseline characteristics are listed in Table 1. Half the patients were male, most were RPA class 3, with a gross total resection and a KPS of 100%. Adjuvant therapy ranged from 7–60 cycles of TMZ. Adequate material to perform molecular analyses was available for 4 of 6 tumors: three were MGMT (+), one was IDH mutated and none carried the EGFR Viii mutation.
Table 1.
Valproic Acid Study Characteristics for 6 Long Term Survivors
| Characteristic | N = 6 | % |
|---|---|---|
| Age (y) Median | 44 (range: 31.1-59.2) | |
| Sex | ||
| Female | 3 | 50 |
| Male | 3 | 50 |
| RPA | ||
| 3 | 5 | 83 |
| 4 | 1 | 17 |
| 5 | 0 | 0 |
| Resection | ||
| GTR | 4 | 67 |
| STR | 2 | 33 |
| Biopsy | 0 | 0 |
| KPS | ||
| Median | 100 | |
| Range | 90-100 | |
| Tumor location | ||
| Frontal lobe | 1 | 17 |
| Parietal lobe | 2 | 33 |
| Temporoparietal | 2 | 33 |
| Temporal lobe | 1 | 17 |
| Completed 6 adjuvant TMZ cycles | 6 | 100 |
| MGMT (+) | 3:4 | 75 |
| IDH (mutated) | 1:4 | 25 |
| EGFR Viii (mutated) | 0:4 | 0 |
| Pseudoprogression | 3 | 50 |
| Failures | ||
| Within 90% isodose line | 3 | 75 |
| Outside 90% isodose line | 1 | 25 |
| No failure | 2 | |
Abbreviations: RPA=recursive partitioning analysis; GTR=gross-total resection; STR=sub-total resection; KPS= Karnofsky performance score; TMZ=temozolomide; MGMT= methyl guanine methyl transferase; IDH= Isocitrate dehydrogenase; EGFR= epidermal growth factor receptor.
Time to Progression and Survival
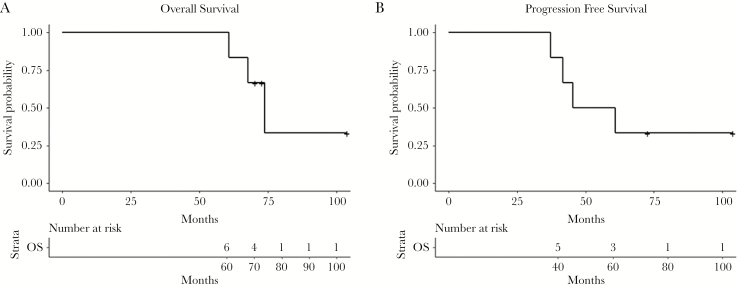
At the time of this analysis, 3 of the 6 patients had died. Median duration of follow-up for the six-patient group was 69.5 months. For this sub-group, using Kaplan-Meier estimates, the median overall survival (OS) was 73.8 months (CI: 60.8–103.8m) (Fig. 1A). Three of six patients had pseudoprogression (Table 1), which is consistent with our overall clinical data at the NCI (43%, personnel communication). The median progression free survival (PFS) was 53.1 months (CI: 37.3–103.8m) as shown in Fig. 1B. At the time of this manuscript 4 of 6 patients had failed, 3 within the 90% isodose line and one outside (Table 1). These data suggest that the rates of pseudoprogression are not higher with the addition of VPA to RT/TMZ.
Fig. 1.
Kaplan-Meier analysis of A) overall survival and B) progression-free survival.
Toxicity
All patients completed the prescribed VPA/RT/TMZ as per protocol. All CTC toxicities beyond 90 days were collected into Table 2A. The most common late toxicities were grade 1/2 neurologic or pain with only 4 of 33 either possibly or probably attributable to the therapy. There were two grade 3/4 toxicities, neither attributable to the VPA/RT/TMZ combination. Each patient was also evaluated on the RTOG/EORTC late morbidity scale (Brain/CNS) and none of the 6 patients at any timepoint had a score greater than 1 (0-no change from baseline, 1-mild headache or slight lethargy) (Table 2B). Evaluation of the MR scans of the six patients at their respective three-year timepoint show changes in the resection cavity consistent with previous surgery and RT but no discernable changes in brain volume in either the high dose regions or within the 50% dose line. The lack of CNS toxicity or volumetric changes on the MR imaging are consistent with minimal to no effect on the normal tissues from the combination of VPA/RT/TMZ therapy. These data suggest that the addition of VPA to the standard RT/TMZ is both safe and effective and warrants a larger Phase III clinical trial.
Table 2A.
Late Toxicity by Cancer Therapy and Evaluation Program Common Toxicity Criteria
| Toxicity | Grade | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Allergy/Immunology | 1/0 | 0/0 | 0/0 | 0/0 |
| Auditory/hearing | 0/0 | 0/0 | 0/0 | 0/0 |
| Blood/bone marrow | 5/5 | 2/2 | 1/0 | 0/0 |
| Cardiovascular | 0/0 | 0/0 | 0/0 | 0/0 |
| Coagulation | 0/0 | 0/0 | 0/0 | 0/0 |
| Constitutional symptoms | 4/2 | 0/0 | 0/0 | 0/0 |
| Dermatology/skin | 1/1 | 0/0 | 0/0 | 0/0 |
| Endocrine | 0/0 | 0/0 | 0/0 | 0/0 |
| Gastrointestinal | 5/3 | 0/0 | 0/0 | 0/0 |
| Hepatobiliary/pancreas | 0/0 | 0/0 | 0/0 | 0/0 |
| Lymphatics | 0/0 | 0/0 | 0/0 | 0/0 |
| Metabolic/laboratory | 5/5 | 2/1 | 0/0 | 0/0 |
| Musculoskeletal | 0/0 | 0/0 | 0/0 | 0/0 |
| Neurology | 13/4 | 8/0 | 0/0 | 0/0 |
| Ocular/visual | 2/1 | 0/0 | 0/0 | 0/0 |
| Pain | 10/0 | 2/0 | 1/0 | 0/0 |
| Renal/Genitourinary | 1/0 | 0/0 | 0/0 | 0/0 |
| Infection | 0/0 | 3/0 | 0/0 | 0/0 |
**The first number in each column represents all AEs that patients developed on study; the second was probably or possibly attributed to concurrent TMZ/VPA and RT.
Table 2B.
Late toxicity by RTOG/EORTC Radiation Morbidity Scoring Scheme
| Grade | 3m-1yr | 1-2yr | 2-3yr |
|---|---|---|---|
| 0–1 | 6 | 6 | 6 |
| 2 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 |
Late toxicity was assessed at 3, 6, 9, 12, 15 18, 21, 24, 30 and 36m.
Discussion
Despite research into the surgical, radiation and drug treatment options for patients with GBM, prognosis remains poor with 20% of patients surviving 5 years.1 As RT is a cornerstone of care, much work has been done developing novel agents that modify the tumor cells response to RT. Identifying the late normal tissue effects of radiation/drug combinations has been difficult in patients with GBM as the time to tumor recurrence is short. However, as drug/radiation combinations extend survival it becomes possible to evaluate the late effects. Thus, we undertook the analysis of the late toxicity of patients that lived greater than 3 years after treatment with VPA/RT/TMZ.
To date there have been two large drug/RT Phase III studies with data mature enough to assess late normal tissue toxicity (Table 3). The first is the EORTC-NCIC trial combining concurrent TMX/RT, which reported late grade 3/4 toxicity in 3/287 patients.1 However, there was no detail on the number of patients that were evaluated at the later time points (3-year PFS 6%) but this sets the baseline for the late toxicity of RT/TMZ. The second trial was RTOG0825 which compared the standard therapy versus the addition of bevacizumab.6 637 patients were randomized but the number of 3-year progression free survivors and their late toxicities were not reported.
Table 3.
Literature Review of Phase II Studies of a Radiation sensitizer plus RT/TMZ
| First Author | # of Patients | % Complete Study Drug | % Delayed RT | Late Grade 3–5 Toxicity | 6m PFS | median OS | % 3-year PFS | Reference |
|---|---|---|---|---|---|---|---|---|
| Stupp, R. | 287 | 85% | 32% | 1% | 54% | 14.6m | 6% | 1 |
| Gilbert, M. | 637 | - | - | - | 82% | 15.7m | - | 6 |
| Brown, P. | 97 | 82% | - | - | - | 15.3m | - | 7 |
| Peereboom, D. | 28 | 74% | - | - | 30% | 8.6m | - | 8 |
| Prados, M. | 66 | - | - | - | 73% | 19.3m | 12% | 9 |
| Chakravarti, A. | 147 | 74% | - | - | 40% | 11.5m | - | 10 |
| Butowski, N. | 66 | - | - | - | 65% | 16.5m | - | 11 |
| Rosenfeld, M. | 97 | - | - | - | - | 17.2m | - | 12 |
| This Trial | 37 | 67% | 0% | 0% | 70% | 29.6m | 16% | Current Study |
For direct comparison to our study, there have been six Phase II studies that combined a radiation modifier and standard RT/TMZ including four studies of EGFR inhibitors, one with enzastaurin, and one with poly-ICLC8–12 (Table 3). None of these six studies reported on the late toxicities observed, with only one reporting on the number of patients surviving to three years (Prados 12%). Thus, our study with a 29.6m OS and 16% 3-year PFS and no late toxicities attributable to the VPA/RT/TMZ combination compares favorably with the historical data.
To classify a drug as a clinically effective radiation sensitizer the enhanced cell killing should be selective for tumor cells over the surrounding normal cells. To date there is no reported data on late toxicity in patients with GBM that survive greater than 3 years. In our study of VPA and RT/TMZ we report little late toxicity in our long-term survivors, however, our study numbers were small (n = 6) with tumors in a variety of locations. This study was important to report as it is the only trial that used VPA at doses high enough to inhibit HDACi activity (25mg/kg BID) compared to other trials that use lower doses of VPA for anti-convulsive therapy (5-10mg/kg BID). Thus, there could have been differences in the late toxicity when using VPA as a radiation sensitizer versus an anti-seizure medication. However, the current measures of late toxicity are insensitive and need to be modified as our patients live longer after initial therapy. In future clinical trials, the addition of neurocognitive testing and patient reported outcomes will be necessary for the assessment of late toxicity. A shortcoming of our study was that we did not collect neurocognition data on our patients throughout the trial. We only had physician scored toxicities that have been documented as less sensitive than those reported by the patients themselves. Additionally, as patients live longer, new methods of quality of life and toxicity assessment will become an important area of research, as long-term survivors are often heavily treated and may present with multifactorial toxicity profiles. However, as presented here the addition of VPA to RT/TMZ appears to improve OS and PFS without accumulation of late toxicities.
Funding
This work was supported in part by the Intramural program of the National Cancer Institute grant number ZIA SC 010373.
Conflicts of interest: No conflicts of interest to disclose.
References
- 1. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Krauze AV, Myrehaug SD, Chang MG, et al. A phase 2 study of concurrent radiation therapy, temozolomide, and the histone deacetylase inhibitor valproic acid for patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2015;92(5):986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tofilon PJ, Camphausen K. Molecular targets for tumor radiosensitization. Chem Rev. 2009;109(7):2974–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 5. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 6. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown PD, Krishnan S, Sarkaria JN, et al. ; North Central Cancer Treatment Group Study N0177 Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26(34):5603–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peereboom DM, Shepard DR, Ahluwalia MS, et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol. 2010;98(1):93–99. [DOI] [PubMed] [Google Scholar]
- 9. Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27(4):579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chakravarti A, Wang M, Robins HI, et al. RTOG 0211: a phase 1/2 study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int J Radiat Oncol Biol Phys. 2013;85(5):1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Butowski N, Chang SM, Lamborn KR, et al. Phase II and pharmacogenomics study of enzastaurin plus temozolomide during and following radiation therapy in patients with newly diagnosed glioblastoma multiforme and gliosarcoma. Neuro Oncol. 2011;13(12):1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenfeld MR, Chamberlain MC, Grossman SA, et al. A multi-institution phase II study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma. Neuro Oncol. 2010;12(10):1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]