Abstract
Forkhead box (FOX) proteins are multifaceted transcription factors that are significantly implicated in cancer, with various critical roles in biological processes. Herein, we provide an overview of several key members of the FOXA, FOXC, FOXM1, FOXO and FOXP subfamilies. Important pathophysiological processes of FOX transcription factors at multiple levels in a context-dependent manner are discussed. We also specifically summarize some major aspects of FOX transcription factors in association with cancer research such as drug resistance, tumor growth, genomic alterations or drivers of initiation. Finally, we suggest that targeting FOX proteins may be a potential therapeutic strategy to combat cancer.
Keywords: FOX proteins, FOXA, FOXC, FOXP, FOXO-FOXM1, hallmarks of cancer, drug resistance, genomic alterations, miRNAs
1. Introduction
The forkhead box (FOX) family comprises diverse tissue- and cell type-specific transcription factors with a conserved winged-helix DNA-binding domain (DBD) or forkhead domain [1]. All members of the FOX family share this DBD but possess distinct transactivation and repression domains [2]. Members of the FOX transcription factor family are generally regarded as important regulators in physiological development during embryogenesis as well as cellular homeostasis, and evolution is the driving force for the diversity of this family. FOX family members participate in the development of the nervous system, kidney, lung, hair, and immune system, among other roles [3]. Many congenital disorders associated with mutations of FOX transcription factors have been reported [4]. In addition, FOX proteins, particularly FOXA1 and FOXA2, are able to recognize some specific patterns in DNA sequences and ultimately bind to chromatin to decompress it and facilitate the activities of other regulators. FOX transcription factors can act as co-activators and transcriptional repressors, although the precise mechanisms remain largely undisclosed. Their roles in regulating the epigenetic processes of cells via DNA methylation, histone acetylation, and non-coding RNA expression have also been documented [1]. Collectively, this family is deeply involved in various complex cellular processes with a high degree of plasticity.
Since the first FOX gene was discovered, 50 FOX-encoding genes in humans have been categorized into 19 subfamilies based on protein sequence homology (FOXA to FOXS) [5]. FOX transcription factors display unusual specificity in biological regulation and present various opposing roles under different oncogenic conditions. Several members of this family, such as FOXA1 and FOXP1, may be either oncogenic or tumor-suppressive depending on how they interact with the distinct transcriptional networks of tissue-specific cancers [6,7]. Generally, FOX proteins influence the cell cycle, proliferation and differentiation, DNA damage repair, metabolism, angiogenesis, cell fate, and senescence [8]. The dysregulation of FOX proteins is associated with cancer initiation, invasion, progression, and drug resistance. They are also capable of regulating other cancer-related pathways that assist cell survival under harsh conditions. For instance, during cellular stress, the FOXO subfamily induces antioxidant enzymes to protect the cell against oxidative stress [9]. Moreover, the regulation of FOXs, e.g., FOXO, may not be limited to the gene expression level but may also include various post-translational modifications, such as acetylation and ubiquitination [10,11]. Although every subfamily of FOX transcription factors exhibits biologically significant roles, FOXA, FOXM1, FOXO, FOXC and FOXP have received the most attention from the scientific community, especially in cancer research [12]. For example, FOXM1 is currently regarded as an essential regulator of various cancers. FOXM1 is involved in at least 12 different cancer types, and its overexpression is important for the initiation, progression, and drug resistance of tumors [13]. The FOXO-FOXM1 axis is considered important in the development of prognostic markers and therapeutics [14]. More recently, the dominant roles of FOXC1, especially in basal-like breast cancer, have been revealed and discussed thoroughly [15]. In addition, FOXC1 is overexpressed in non-small cell lung cancer (NSCLC) cells and is negatively correlated with the survival of the patients. This may be because FOXC1 induces cancer stem cells (CSC)-like properties of the cancer cells via β-catenin [16]. Somatic mutations of FOX transcription factors, such as amplification, point mutation, translocation, deletion and gene fusion, are commonly identified in human cancers [17]. However, the mutation landscape of FOX-binding sites within the regulatory sites of FOX-target genes remains to be elucidated in meticulous detail.
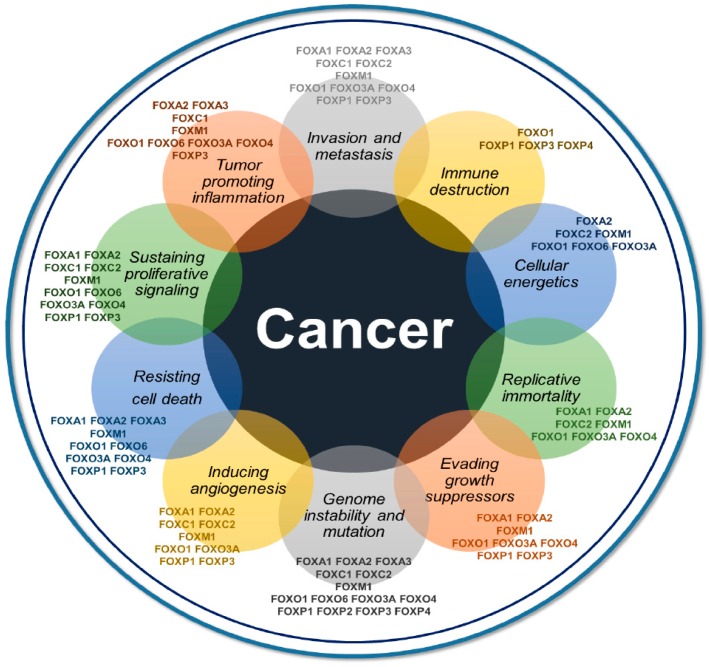
The FOX family contributes to the initiation, maintenance, progression, and metastasis of cancer at different levels of regulation, with highly convoluted and widespread networks. FOX proteins are also associated with major aspects of the hallmarks of cancer, as described for FOXM1 and indicated by our literature text-mining analysis using Cancer Hallmarks Analytics Tools (Figure 1) [18,19]. This article aims to review and emphasize the functions of major FOX transcription factors in various aspects of cancer biology in a context-dependent manner. In addition, we selectively focus on major aspects of FOXs in cancer biology, such as drug resistance, genomic alterations and therapeutics, including applications of microRNA (miRNA) and specific inhibitors for targeting FOX proteins.
Figure 1.
Direct and indirect associations of 14 individual FOX transcription factors and the hallmarks of cancer acquired from Cancer Hallmarks Analytics Tool. FOXO1 appears to be associated with every hallmark while FOXM1, FOXO3a, FOXA2, and FOXP3 are connected to at least eight hallmarks of cancer. FOXP2 is only related to genome instability when FOXP4 is involved in the genome instability and immune destruction process.
2. An Overview of Recent Insights on FOXs in Cancer
2.1. Forkhead Box A (FOXA) in Cancer
FOXA1, FOXA2, and FOXA3 are pioneer factors and play important roles in the development of endoderm and endoderm-derived organs [20,21]. As pioneer factors, they assist other transcription factors in accessing chromatin to elicit their tissue-specific functions [20]. Indeed, FOXA1 and FOXA2 play important roles in tumorigenesis based on their multifaceted activities, mainly in terms of genome instability and mutation, activation of invasion and metastasis, and sustained proliferative signaling. FOXA1 and FOXA2 are also associated with a variety of cancers, and their behaviors are tumor type-specific, with a dependence on the particular transcriptome interactions [22]. The up-regulation of FOXA1 is highly correlated with the malignancy of lung cancer, prostate cancer, and esophageal cancer [23,24]. FOXA1 and FOXA2 also participate in a phenomenon in liver cancer called sexual dimorphism [25]. The two FOXA factors regulate the estrogen-dependent resistance and androgen-mediated facilitation of this disease [26]. FOXA1 is positively associated with estrogen receptor-positive breast cancer as well as androgen receptor-dependent prostate cancer [17]. Interestingly, due to its distinct roles in estrogen and androgen pathways, FOXA1 upregulation is associated with either a good prognosis or poor prognosis in breast cancer patients and prostate cancer patients, respectively [27]. In bladder cancer, however, the reduced expression of FOXA1 is associated with the histological subtypes of muscle-invasive bladder cancer, which later develops into the metastatic stages of the disease [28]. Recently, FOXA transcription factors were found to be involved in the enhancer elements at epithelial signature genes and are repressed by SNAIL1 in colorectal cancer. This repression activity of SNAIL1 facilitates the epithelial-mesenchymal transition (EMT) of the cancer cells, which suggests that FOXA factors are important in maintaining physiological expression of the network of epithelial genes [21]. The mediator forms a complex with cohesin, and together they act as the central cofactors that control the cellular development and differentiation of normal cells [29]. Dysregulation of cohesin has been associated with cancer [30]. Moreover, a recent study has demonstrated that FOXA1 and/or FOXA2, together with other master transcription factors, are essential for the maintenance of cancer cell states through the recruitment of the mediator–cohesin complex [31]. There is also evidence that FOXA2 is linked to lipid and carbohydrate metabolism. In type 2 diabetes, preventing FOXA2 phosphorylation may help control the disorder [32]. These observations support the hypothesis that FOXA factors have essential roles in the disruption of cancer metabolism, and the modulation of FOXAs may provide new opportunities for cancer treatment.
2.2. FOXC in Cancer
The FOXC subfamily is well-known for its functions during cardiovascular development [33]. Mice without FOXC1 or FOXC2 exhibit various abnormal cardiovascular phenotypes that are prenatally lethal, and embryos without FOXC1/FOXC2 die within several days postcoitum [34]. In cancer, FOXC1 and FOXC2 are involved mainly in inducing angiogenesis, invasion and metastasis, invading growth suppressors, genome instability and mutation, and sustaining proliferative signaling. FOXC1 is involved in many cancers such as breast cancer, liver cancer, Hodgkin’s and non-Hodgkin’s lymphoma, pancreatic cancer, and endometrial cancer [15,35,36]. Of note, loss of expression of FOXC1 suppresses cancer cell growth and reverts fibroblast-like cells to epithelial-like cells in a mammary carcinoma model. Furthermore, FOXC1 is positively associated with cancer metastasis and poorer prognosis of basal-like breast cancer patients [37]. In hepatocellular carcinoma, FOXC1 triggers the EMT process, which increases the migration and invasion capacities of the cancer cells. Patients with higher expression levels of FOXC1 tend to have a worse prognosis [38]. FOXC2, similar to FOXC1, also has a vital role in the carcinogenesis of various cancers. FOXC2 is overexpressed in breast cancer, stomach cancer, lung cancer, prostate cancer, cervical cancer, and ovarian cancer [39]. EMT, along with angiogenesis and lymphangiogenesis, is the key phenotypic feature resulting from the interactions of FOXC2 with the cadherin family, kinases, and other regulators. For instance, FOXC2-induced EMT is triggered via activation of the Akt pathway and is related to the expression of Snail and p-(glycogen synthase kinase 3β) GSK-3β [39]. The overexpression of FOXC2 also can induce MET expression and stimulate the hepatocyte growth factor (HGF)-MET signaling pathway, hence inducing the metastasis and invasion of colorectal cancer cells [40]. Both FOXC1 and FOXC2 have essential roles in the EMT process, angiogenesis, and target cancer stem cells [41,42]. Cancer cells with EMT exhibit overlapping features with cancer stem cells and likely develop drug resistance [43]. Taken together, a large body of evidence now suggests that the modes of action of FOXC1 and FOXC2 share some phenotypic features through various signaling pathways that promote tumor progression and metastasis. This suggests an opportunity to explore their roles as cooperative prognostic biomarkers and in cancer management. Finally, it is also important to mention that FOXC2 can modulate the metabolism of cancer cells. The first observation was in nasopharyngeal carcinoma, in which FOXC2 increases glycolysis in cancer cells via the FOXC2-YAP (Yes-Associated Protein) axis to up-regulate hexokinase 2 and ultimately facilitates tumor survival and progression [44]. FOXC2 may also be involved in the lipid alterations of cancer via kinases in lipid metabolism, which represents an interesting research direction [39]. However, the role of FOXC1 in cancer metabolism remains unknown.
2.3. FOXM1 in Cancer
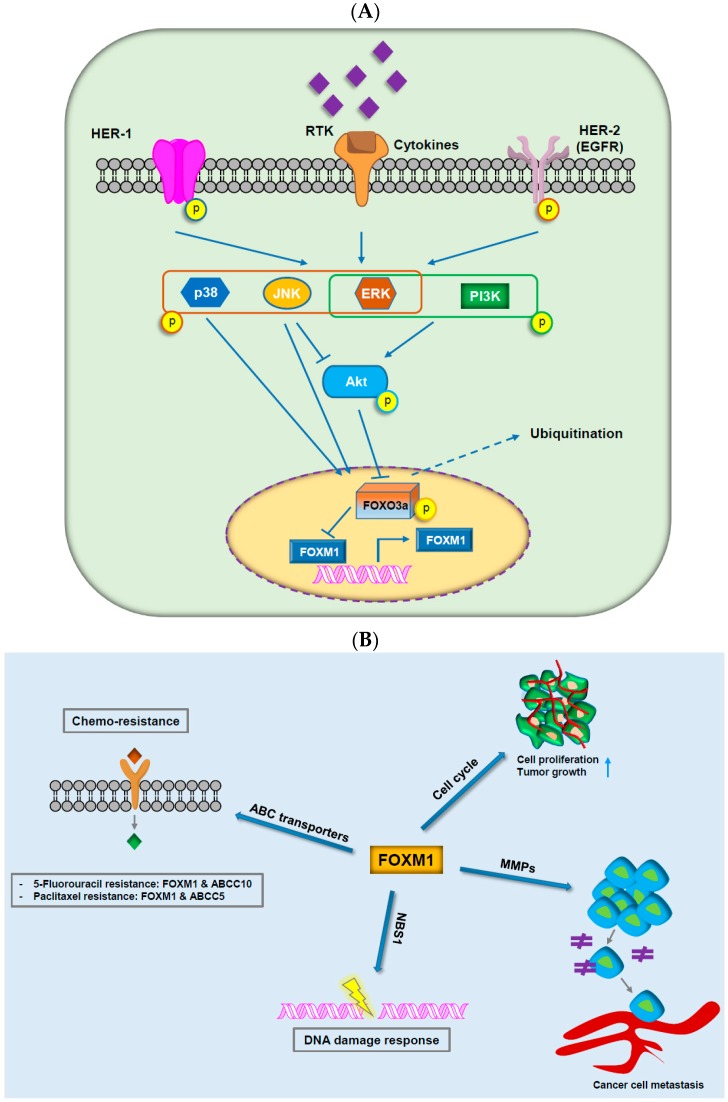
FOXM1 is specifically expressed in proliferating cells and is a master regulator of cancer tumorigenesis and metastasis (Figure 2) [45]. Indeed, overexpression of FOXM1 is common in cancer, and higher expression of FOXM1 is associated with worse survival of patients [46]. The mode of action of FOXM1 is to facilitate evasion of growth suppressors by cancer cells by activating regulators of cell-cycle progression, anti-oxidant genes, and progression through the EMT phenotype, invasion, and pre-metastatic niche formation [47,48,49,50]. FOXM1 and FOXOs are direct and indirect targets of many conventional and novel therapeutics due to their important impact on PI3K-Akt signaling (Figure 2A) [51]. Dysregulation of this axis, such as through inhibition of FOXO3a combined with overexpression of FOXM1, results in drug resistance to some standard therapies [1,13]. Interestingly, the inhibition of FOXM1 alone is supposed to be adequate in targeting multifaceted mechanisms of tumorigenesis [52]. Interest in FOXM1 dysregulation and its impact on cancer management has been maintained in recent years. The connection of FOXM1 with other oncogenic proteins will be discussed in more detail in the later part of this review.
Figure 2.
The critical roles of FOXM1 in cancer progression. (A) Integration of extracellular and intracellular signaling pathways with the axis of forkhead box protein M1 and forkhead box class O3a. (B) FOXM1 contributes to chemo-resistance through ABC transporters, tumor growth and cell proliferation through cell-cycle, cancer cell metastasis through matrix metalloproteinases, and DNA damage response through NBS1.
2.4. FOXO in Cancer
The FOXO subfamily (FOXO1, FOXO2/FOXO6, FOXO3a, and FOXO4) receives arguably the most attention from scientists among FOX proteins. Each FOXO protein possesses various biological functions. For instance, FOXO1 is important in vascular development, whereas FOXO3a plays an essential role in ovarian follicle development [53]. FOXO1, FOXO3a, and FOXO4 are universally expressed among tissues, but FOXO6 is physiologically expressed in brain tissue [54]. Combined, the activities of FOXO proteins regulate almost every phase of the cell cycle [55]. In contrast to the FOXC and FOXM subfamilies, which are genuine oncogenes, FOXO proteins negatively regulate various biological processes at multiple levels, and dysregulation of FOXOs may lead to cancer [55]. Indeed, FOXOs are part of a multitude of oncogenic pathways and cancer hallmarks, including resisting cell death, sustaining proliferative signaling, tumor-promoting inflammation, immune destruction, cellular energetics, replicative immortality, evading growth suppressors, genome instability and mutation, and inducing angiogenesis [14,56,57,58,59,60,61]. Alternatively, even though FOXOs are widely regarded as tumor suppressors, a significant growing number of evidences have been suggested that FOXO transcription factors are oncogenic regulators [62]. For instance, through regulating various processes that are essential for tumorigenesis, FOXOs demonstrate its oncogenic roles in breast cancer [63]. Hence, it is of importance to re-evaluate the context-dependent roles of FOXO transcription factors in cancer. FOXOs are largely regulated via various post-translational modifications. For instance, members of FOXOs are generally modulated by the PI3K/Akt/Insulin (phosphorylated by phosphatidylinositol 3-kinase/RAC-α serine/threonine-protein kinase) signaling pathway [64]. In osteosarcoma, FOXO1 can suppress osteosarcoma oncogenesis through suppression of Wnt/β-catenin pathway [65]. The roles of FOXOs in cancer have been examined in breast cancer, prostate cancer, leukemia, glioblastoma, and rhabdomyosarcoma. Indirect upregulation of FOXOs through inhibition of Akt, ERK, and IKKβ is expected to be particularly effective in the treatment of cancer [66]. The network of FOXO transcriptional target genes has been reviewed extensively [67]. Furthermore, FOXO3a seems to be the representative protein of this subfamily, and its functions in cancer have been extensively studied [68]. A comprehensive review with regard to the roles of FOXO3a in carcinogenesis, e.g., the inactivation and the initiation and progression of cancer, has been recently documented [69]. Interestingly, essential roles of the FOXO subfamily in metabolic reprogramming have recently been uncovered. FOXOs are involved in various metabolic processes, including glucose metabolism, amino acid metabolism, and lipid metabolism. Thus, FOXOs may initiate a broad therapeutic window for the use of metabolic disruptors [70]. However, some atypical exceptions in which FOXO proteins exhibit oncogenic properties have been recorded. For example, high expression of the PAX3-FOXO1 fusion protein may promote tumorigenesis of human myoblasts [53,71].
2.5. FOXP in Cancer
The FOXP proteins (FOXP1, FOXP2, FOXP3, FOXP4) are a functionally diverse subfamily known for their cooperative roles in embryonic development, including brain development [72]. Dysregulation of FOXP1 and FOXP2 has been prominently studied in language and speech disorders [73]. In addition, FOXP3 and regulatory T cell (Treg) dysregulation result in autoimmune diseases [74]. Interestingly, the three FOXP proteins are associated with cancer initiation and progression [17,75,76]. FOXP4 has been also found to be functional in cancer [77]. FOXP-dependent cancer initiation and progression are generally associated with immune destruction, evading growth suppressors, genome instability and mutation, inducing angiogenesis, resisting cell death, sustaining proliferative signaling, and tumor-promoting inflammation. One of the unique properties of the FOXP subfamily is their capability of homo- and heterotypic dimerization with paralogs, known as FOXP1/2/4 interactions [72]. This dimerization may strongly influence their behavior and eventually lead to pathophysiological processes or oncogenic phenomena [76]. FOXP2 mainly acts as a repressor and has a dual role in oncogenesis and cancer progression. For instance, FOXP2 can interact with C-terminal binding protein 1 (CTBP1), a transcriptional corepressor that modulates and targets tumor suppressors expression, such as BAX, PTEN and p16 [64]. FOXP2 may also participate in modulating the expression of various genes involved in tumor signaling pathways, including IGF-1 (insulin-like growth factor 1), NF-ĸB (nuclear factor kappa-light-chain-enhancer of activated B cells), and Wnt [72,78,79]. It is diminished in some cancers such as breast cancer, liver cancer, and gastric cancer but overexpressed in others [76,80,81,82]. ABCA6 and ABCG2, which are direct targets of FOXP2 in its regulatory network, exhibit aberrant expression in various cancers. This suggests that FOXP2 is potentially associated with drug resistance [76]. FOXP1 is generally considered a transcriptional repressor and is a tumor suppressor in epithelial malignancies such as lung cancer and breast cancer. However, FOXP1 is overexpressed in B-cell lymphomas, and patients with higher FOXP1 expression tend to have a worse prognosis. FOXP1 is involved in the development of lymphocytes, particularly B cell proliferation [83]. FOXP3 is a major component of Tregs [84]. Mutations and dysregulation of FOXP3 are linked to immune response abnormalities and carcinogenesis [75]. The functions of FOXP3 may be one of the central mechanisms that help tumor cells escape from immune cells [85]. FOXP3 also acts through vascular endothelial growth factor (VEGF) to inhibit angiogenesis, as observed in breast cancer [86]. The relationship between the expression of FOXP3 and the prognosis of cancer patients is not straightforward. For instance, overexpression of FOXP3 is associated with worse prognosis in NSCLC, colorectal cancer, and cervical cancer but good prognosis in breast cancer, prostate cancer, and gastric cancer [85]. The roles of FOXP4 in cancer have not been well-studied. However, dysregulation of FOXP4 has been associated with breast cancer, kidney cancer, prostate cancer, and NSCLC [77,87,88,89].
2.6. Other Important FOX Transcription Factors in Cancer
FOXD3 can be considered a tumor suppressor since it inhibits tumor growth and angiogenesis of NSCLC and neuroblastoma, whereas its deficiency leads to the induction of EMT and increased invasiveness of breast cancer [90,91,92]. Interestingly, a large body of research indicates a considerable impact of FOXE1 in thyroid cancer [93,94]. In a meta-analysis, Zhu et al. suggested that common genetic variants of FOXE1 are associated with an increased risk of thyroid cancer [95]. FOXF1 is the target of the p53 family, and their interactions play an important role in the migration and invasion of cancer cells [96]. FOXF1 has a positive correlation with lymph node metastasis of NSCLC and promotes the progression of prostate cancer [97,98]. FOXF1 may also trigger the ataxia-telangiectasia mutated (ATM)/ATM- and Rad3-Related (ATR)-medicated DNA damage response and stimulate the p53-p21WAF1 checkpoint pathway in colon cancer cells [99]. However, FOXF1 may be also considered a tumor suppressor since the loss of FOXF1 is associated with poor prognosis in liver cancer patients [100]. FOXL1 is a novel tumor suppressor whose expression and co-expression with other regulators inhibit the aggressiveness of pancreatic cancer, kidney cancer, gallbladder cancer, and osteosarcoma [101,102,103,104] and could be initially implicated in the modulation of the Wnt/APC (Adenomatous Polyposis Coli)/β-catenin pathway [64]. By contrast, FOXQ1 promotes the progression and metastasis of esophageal cancer, breast cancer, pancreatic cancer, and colorectal cancer [105,106,107,108]. A recent review has demonstrated that FOXQ1 works through EMT, cell-cycle progression, cellular proliferation, and other mechanisms to promote cancer initiation and progression [109]. Similarly, the up-regulation of FOXJ1 is linked to higher histological grade and poor prognosis of liver cancer via cell proliferation and cell-cycle progression of the tumor cells [110]. FOXJ1 also induces proliferation and colony formation of bladder cancer cells, due in part to aberrant metabolism of the cancer cells [111]. However, FOXJ1 appears to play dual roles since lower expression of FOXJ1 is associated with worse prognosis of patients with gastric carcinoma [112]. FOXL2 acts as a tumor suppressor in cervical cancer since its overexpression reduces the proliferation of cervical cancer cells [113]. However, FOXL2 may be either an oncogene or tumor-suppressor gene depending on the genetic context in ovarian granulosa cell tumors [114]. Significantly, FOXL2 is a target for the development of new diagnostic approaches for adult-type granulosa cell tumors [115].
3. Major Areas of Focus on FOXs in Cancer
3.1. FOX Proteins in Cancer Drug Resistance
Clinically, the development of resistance to both conventional and newly emerging molecular targeted therapies is a major challenge confronting current cancer treatment [116,117,118,119,120,121,122]. Intriguingly, FOX proteins have also been associated with the mechanisms of resistance to molecular targeted therapies and classical cytotoxic chemotherapies. The associations between FOX proteins and the development of drug resistance generally involve alterations in drug targets, cancer stem cell population, drug metabolism, cell survival and death signals, as summarized in Table 1. For example, changes in the expression levels of FOXM1 or FOXOs are highly associated with chemoresistance and poor prognosis in cancer patients.
Table 1.
Contributions of forkhead box (FOX) proteins to drug resistance of cancer cells.
| FOX Members | Model/Cell Type | Corresponding Drug | Function | Ref |
|---|---|---|---|---|
| FOXM1 | Non-small cell lung cancer (NSCLC) patients | Tyrosine kinase inhibitor (TKI) | Contributes to TKI-resistant NSCLC cells Associated with unfavorable prognosis in NSCLC patients |
[123] |
| Ovarian cancer patients | Platinum | Overexpressed in ovarian cancer cell lines and cancer cells in patients’ ascites Targeting FOXM1 improves the cytotoxicity of paclitaxel and cisplatinum in platinum-resistant ovarian cancer |
[124] | |
| Lung adenocarcinoma | Gefitinib | FOXM1 stimulates acquired resistance to gefitinib in lung adenocarcinoma cells through a MET/Akt-dependent positive feedback loop | [125] | |
| Leukemia patient samples | Chemoresistance | Nuclear FOXM1 contributes to chemoresistance in acute myeloid leukemia (AML) FOXM1 inactivation causes a favorable prognosis and provides fertile ground for strategies to suppress this oncogenic transcription factor in AML |
[126] | |
| Colorectal cancer | 5-Fluorouracil | FOXM1 can evoke 5-fluorouracil resistance depending on ATP binding cassette subfamily C member 10 (ABCC10) | [127] | |
| Glioma cells | Temozolomide | FOXM1-mediated repair gene replication factor 5 promotes temozolomide resistance in glioma cells independent of methylguanine-DNA-methyltransferase activation | [128] | |
| Nasopharyngeal carcinoma cells | Paclitaxel | FOXM1 can contribute to drug efflux and paclitaxel resistance by regulating the gene transcription of ABCC5, one of the ABC transporters | [129] | |
| Ovarian cancer patients | Chemo-resistance | The expression of FOXM1 is highly associated with chemotherapy resistance and adverse prognosis in non-serous epithelial ovarian cancer patients | [130] | |
| Bladder cancer | Chemo-resistance | FOXM1 is proposed to directly active ABC G member 2 to enhance drug resistance and drug efflux activation | [131] | |
| Breast cancer patients | Epirubicin | FOXM1 can target nijmegen breakage syndrome gene to modulate DNA damage-stimulated senescence and epirubicin resistance | [132] | |
| Gastric cancer | Docetaxel | FOXM1 might be a new therapeutic target in docetaxel-resistant gastric cancer and can be used as a marker for predicting patient prognosis and monitoring the response to docetaxel | [133] | |
| Cervical cancer | Chemoresistance | The prolyl isomerase Pin1 can modulate chemoresistance by up-regulating FOXM1 and involvement in the Wnt/β-catenin pathway | [134] | |
| Breast cancer patients | Chemoresistance | Targeting X-linked inhibitor of apoptosis gene (XIAP) and Survivin by FOXM1 may contribute to chemoresistance in breast cancer survivors | [135] | |
| Leukemia | Chemoresistance | FOXM1 is overexpressed in B-acute lymphoblastic leukemia (B-ALL) Inhibition of FOXM1 may sensitize B-ALL cells to chemotherapeutic drugs |
[136] | |
| Breast cancer | Epirubicin | The suppression of ubiquitination and degradation of FOXM1 by ubiquitin thioesterase OTUB1 has been suggested to play a key role in genotoxic agent resistance | [137] | |
| Breast cancer | Paclitaxel | Paclitaxel resistance can be modulated by deregulating FOXM1 expression to regulate kinesin family member 20A in mitotic catastrophe | [138] | |
| Ovarian cancer | Chemoresistance | Overexpression of FOXM1 can enhance the expression and activity of β-catenin in chemoresistant cells, whereas downregulation of FOXM1 may suppress these events | [139] | |
| Gastric cancer | Oxaliplatin | FOXM1-stimulated resistance to oxaliplatin is partially mediated through its target gene Mcl-1 | [140] | |
| Ovarian cancer | Paclitaxel | Upregulation of FOXM1 contributes to paclitaxel resistance by suppressing mitotic catastrophe | [141] | |
| Ovarian cancer | Cisplatin | FOXM1 can contribute to cisplatin sensitivity by modulating exonuclease 1 | [142] | |
| FOXC1 | Breast cancer patients | Endocrine | FOXC1 expression is related to decreased or undetectable estrogen receptor (ER) expression in recurrent tumors FOXC1 is involved in ERα silencing through counteracting GATA binding protein 3 binding and has been implicated in endocrine resistance |
[143] |
| FOXQ1 | Breast cancer | Chemoresistance | Platelet-derived growth factor receptors have been suggested as critical mediators of breast cancer chemoresistance driven by FOXQ1 and have potential implications for investigating novel therapeutic combinations to treat breast cancer | [106] |
| NSCLC | Chemoresistance | Overexpression of FOXQ1 elicits opposing effects on these phenotypes in vivo by regulating epithelial-mesenchymal transition (EMT) and modulating chemosensitivity in NSCLC | [144] | |
| FOXC2 | Ovarian cancer | Cisplatin | FOXC2 stimulates EMT and metastasis in cisplatin-resistant human ovarian cancer cells | [145] |
| FOXC2 promotes the resistance of human ovarian cancer cells to cisplatin by activating the Amkt and MAPK-signaling pathways | [146] | |||
| Nasopharyngeal carcinomas | Chemoresistance | FOXC2 may stimulate chemoresistance through activation of EMT | [147] | |
| FOXD1 | Breast cancer | Chemoresistance | FOXD1 can stimulate breast cancer growth and chemoresistance by modulating p27 | [148] |
| FOXO3a | Lung cancer | Gefitinib | NF-ĸB-driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance | [149] |
| Colorectal cancer | Cetuximab | FOXO3a contributes to cetuximab resistance in RAS wild-type metastasis through c-Myc | [150] | |
| Multi drug resistance cells | Docetaxel and Paclitaxel | Paclitaxel-resistant cancer cell-derived secretomes escape from apoptosis through FOXO3a-driven glycolytic modulation in association with ABCB1 | [151] | |
| HeLa cells | Cisplatin | Butein may sensitize HeLa cells to cisplatin through the ERK/p38 MAPK and Akt pathways by targeting FOXO3a | [152] | |
| Ovarian cancer | Cisplatin | -8-Bromo-7-methoxychrysin-induced apoptosis in cisplatin-sensitive and -resistant cells can occur through modulation of Akt/FOXO3a | [153] | |
| FOXO1 | Hepatocellular carcinoma | Doxorubicin | Expression of Bim is mediated by FOXO1 and indirectly downregulated by thyroid hormone/hormone receptor, causing chemotherapy resistance and doxorubicin-stimulated metastasis of hepatoma cells | [154] |
| Esophageal squamous cell carcinoma | Chemoresistance | Cancer-associated fibroblasts mediate chemoresistance through a FOXO1/TGFβ signaling loop | [155] | |
| Gastric cancer | Lapatinib | Inactivation of FOXO1 is suggested as a determinant of acquired lapatinib-resistance in HER2-positive breast cancer through upregulation of MET | [156] | |
| Gastric cancer | Cisplatin | FOXO1 may contribute to cisplatin resistance by stimulating the phosphoinositide 3-kinase/Akt pathway | [157] | |
| Leukemia | TKI | Overexpressed FOXO1 can contribute to BCR-ABL1 kinase-independent resistance in chronic myeloid leukemia patients | [158] | |
| NSCLC | TKI | FOXO1 acetylation suppresses cell growth and stimulates apoptosis of NSCLC Posttranslational modifications of FOXO1 modulate EGFR-TKI resistance in NSCLC cells |
[159] | |
| FOXJ2 | Prostate cancer | Castration | The phosphorylation of FOXJ2 is associated with increased expression of NEK6 that can mediate castration resistance in prostate cancer | [160] |
| FOXL2 | Gastric cancer | Chemoresistance | The HMGA2-FOXL2 axis can modulate EMT and metastasis of chemoresistant gastric cancer | [161] |
| FOXP3∆3 | Bladder cancer | Cisplatin | Biased expression of the FOXP3∆3 isoform in aggressive bladder cancer contributes to differentiation and cisplatin chemotherapy resistance | [162] |
| FOXP3 | Lung adenocarcinoma | Cisplatin | Downregulation of FOXP3 can enhance chemosensitivity to cisplatin and suppress cell proliferation in human lung adenocarcinoma | [163] |
| FOXP1 | Gastric cancer | Chemoresistance | FOXP1 may interact with nuclear aurora kinase A, which regulates survivin stability by modulating F-box and leucine rich repeat protein 7 in gastric cancer drug resistance and affects prognosis | [164] |
| Ovarian cancer | Chemoresistance | The expression of nuclear FOXP1 is an independent risk factor related to chemotherapy resistance and the prognosis of patients with ovarian cancer | [165] | |
| FOXA1 | Breast cancer | Tamoxifen | Down-regulation of FOXA1 causes cancer stem cell-like properties in tamoxifen-resistant breast cancer cells through stimulation of interleukin-6 | [166] |
| Prostate cancer | Castrate | FOXA1 modulates androgen receptor variant activity in models of castrate-resistant prostate cancer | [167] | |
| FOXF2 | Breast cancer patients | Multidrug resistance | FOXF2 may contribute to multidrug resistance of basal-like breast cancer by suppressing FOXC2-mediated EMT | [168] |
Alternatively, aberrant activation of DNA damage repair may be associated not only with cancer initiation but also with cancer progression and genotoxic drug resistance. Convincing evidence suggests an impact of the FOXOs–FOXM1 forkhead transcription factor axis on the DNA damage response, indicating the therapeutic potential of targeting FOXM1 and FOXOs to overcome genotoxic drug resistance (Table 1 and Figure 2B [127,129]). The expression of FOXM1 may confer genotoxic agent resistance, and its overexpression in DNA-damaging cancer drug-resistant cells has been commonly observed (Table 1) [169]. However, this observation also supports potential exploration for cancer therapy based on FOXM1 overexpression in cancer and in genotoxic resistance. Consistently, various studies have shown that the inhibition of the overexpression of FOXM1 can suppress tumor development and active cell death via various pathways (Table 1). Therefore, approaches based on small peptides have also been developed to directly target FOXM1 [169].
Alternatively, a well-established principle of cancer therapy for overcoming drug resistance and treating cancer is appropriate drug combinations. Recent studies have shown that FOXO3a can be activated by agents targeting its upstream regulatory PI3K-Akt pathway, such as OSU-03012, an Akt inhibitor that has been shown to enhance the dephosphorylation of FOXO3a and nuclear relocation in breast cancer cells [170]. MK-2206, another Akt inhibitor, can also lead to FOXO3a activation and dephosphorylation and potentially synergize with conventional genotoxic drugs such as doxorubicin in liver cancer treatment [169].
In summary, FOX proteins are crucial modulators of chemoresistance in cellular progression, at least under some circumstances, but may also improve resistance to chemotherapeutics. Hence, it is critical to fully understand the FOX protein-mediated transcriptional programs in specific cancer disease states or the upstream regulators, downstream targets and cellular functions of FOX proteins to investigate the most suitable targets for modulating FOX proteins [171].
3.2. FOX Proteins and Genomic Alterations
Human cancers occur in a multi-step manner as a result of the accumulation of genetic alterations and epigenetic changes [17]. Numerous studies have indicated the roles of somatic mutations of FOX family genes in various types of human cancers in relation to transcriptional modulation as well as DNA repair or histone modification [17,172,173]. Additionally, the advancement and spread of exome or whole-genome analyses have provided novel data on somatic mutations as well as point mutations, gene amplifications and translocations of FOX family members.
3.3. FOXM1
The FOXM1 gene on human chromosome 12p13.33 is suggested to be amplified in 5.6% of breast cancer [174] and 58% of malignant peripheral nerve sheath tumors [175] and is frequently upregulated in human cancer [176]. Although FOXM1 may stimulate cell-cycle regulation in the DNA replication S phase (G1/S), it also plays a significant role in the G2/M transition by transactivation of modulators of mitosis and cytokinesis such as polo-like kinase or Aurora B (Figure 2B) [177]. FOXM1 and the promoter regions of cell cycle-contributed genes acquire higher levels of H3K4me3, indicating that epigenetic modulations of these critical regulatory genes can define quiescence of liver cells [178]. The oncogenic transcription factor FOXM1 is activated in various human malignancies and is required for execution of the mitotic program and chromosomal instability (CIN) [177,179]. For example, YAP stimulates and interacts with FOXM1, a master modulator of cell-cycle control, and this YAP/FOXM1 complex drives CIN gene expression and stimulates aneuploidy [180,181]. Additionally, FOXM1, in combination with precancerous cell growth deregulation, allows human keratinocytes to proliferate despite accumulating DNA damage and subsequently stimulates genomic instability (Figure 2B) [132,182]. This may also explain why mutated p53 and deregulated FOXM1 are both frequently selected in cancer.
3.4. FOXO Subfamily Genes
FOXO subfamily members include the FOXO1, FOXO2 (FOXO6), FOXO3 and FOXO4 genes [183], and in the nucleus, FOXOs can bind to their consensus DNA-binding motif to activate the transcription of their target genes, such as BIM (BCL2-like 11), FASLG (Fas ligand) or CDKN1A and CDKN1B [17]. The FOXO1 gene on human chromosome 13q14.11 is fused to either the PAX7 or PAX3 gene as a result of chromosomal translocation in alveolar rhabdomyosarcoma, whereas the FOXO3 gene at 6q21 and FOXO4 gene at Xq13.1 are fused to the MLL gene as a result of chromosomal translocation in secondary leukemia and acute lymphoblastic leukemia (ALL), respectively [17,184]. Translocation of FOXO1 to the nuclear periphery may promote histone modifications that contribute to the transcriptional repression of phosphoenolpyruvate carboxykinase 1 in hepatocytes [185], whereas the formation of the (CREB)-binding protein-FOXO1 complex leads to histone acetylation in cancer and aging [186]. Recently, Jeffery et al. indicated that the depletion of FBXO31 leads to increased expression of FOXM1 transcriptional targets and mimics FOXM1 overexpression. By contrast, co-depletion of FBXO31 and FOXM1 can restore the genomic instability phenotype but not the delay in mitosis, indicating that FBXO31 probably has additional mitotic substrates [187]. FBXO31 has also been implicated in DNA damage repair through its degradation of MDM2, an E3 ligase and negative modulator of p53, and MKK6, an activator of the p38 MAPK [188,189]. Alternatively, DNA damage accrued as a result of elevated reactive oxygen species in FOXO3−/− mutant hematopoietic stem and progenitor cells is at least partially reversible [190]. Recent studies have indicated numerous modulators of FOXO, and a clear and evolutionarily conserved role has emerged for phosphoinositide-3 kinase/protein kinase B (also known as c-Akt) signaling and c-jun N-terminal kinase signaling [191,192]. The tumor suppressor functions of FOXO transcription factors are lost in cancer cells as a result of chromosomal translocations or deletions of FOXO genes or Akt-mediated cytoplasmic sequestration of FOXO proteins [17,192]. Overall, FOXOs appear to contribute to longevity by modulating processes involved in both DNA repair and apoptosis according to cancer progression [191].
3.5. Other FOX Genes
The FOXF1 locus at human chromosome 16q24.1 is deleted in prostate cancer, whereas the FOXA1 gene at human chromosome 14q21.1 is amplified in various different cancers such as anaplastic thyroid cancer, estrogen receptor-positive breast cancer, esophageal cancer, lung cancer or metastatic prostate cancer [17]. Genome doubling and ongoing dynamic CIN are related to intratumor heterogeneity and lead the parallel evolution of driver somatic copy-number alterations, including amplifications of FOXA1, CDK4 and BCL11A [193]. Alternatively, FOXE1 can bind to the thyroperoxidase promoter during thyroid cell differentiation and modify the compacted chromatin structure [194]. In neuroblastoma, intrachromosomal deletions may create FOXR1 fusion genes that contribute to Myc-driven proliferation in mouse neuroblasts and suppress forkhead-box family target genes [195]. Alternatively, the FOXP1 gene is also amplified in diffuse large B-cell lymphoma and MALT lymphoma either with or without translocation [17,196].
4. Negative Modulation of FOX Proteins by miRNAs
miRNAs are a new class of small, non-protein-encoding RNAs with a length of 18–25 nucleotides. Studies in the past decade have revealed that miRNAs are involved in various biological processes such as cell differentiation, stress resistance or tumorigenesis [117,119]. Many studies have indicated the regulation of FOX proteins by miRNAs in cancer patients under various pathological conditions. Recently, several studies have indicated the specific modulation of FOX genes by miRNAs in various different cancers such as colorectal cancer [197], esophageal cancer [198], triple-negative breast cancer [199] and hepatocellular carcinoma [200]. For example, miR-342 may suppress the expression levels of FOXM1 and FOXQ1 through direct binding within the putative 3’-UTR binding sites of these genes, thereby inhibiting the proliferation, migration, and invasion of colorectal cancer cells in a xenograft animal model [197]. FOXM1 is also one of the direct targets of miR-204, and the functional effect of miR-204 on esophageal cancer cells lines is also dependent on FOXM1 [198]. In hepatocellular carcinoma (HCC) cells, suppression of FOXO1 by miR-1269 was related to dysregulation of cyclin D1 and Ki67 expression, suggesting a critical role in the growth of HCC cells [200]. By contrast, the restoration of miR-422a expression significantly suppressed tumor growth and liver metastasis in xenograft tumor models by modulating its direct targets, such as FOXG1, FOXQ1 and FOXE1 [201]. Kumar et al. recently also analyzed the crosstalk between miR-122 and FOX family genes in HepG2 cells and suggested that miR-122 may induce apoptosis by regulating FOX family target genes at various levels to exert its antitumor effects in HCC [202]. Importantly, the combination of miR-6883-5p and miR-149* suppresses CDK4/6-FOXM1 signaling in colorectal cancer cell lines [203]. Together, these studies indicate the existence of an additional level of complexity in the regulation of the FOX protein pathway. Investigating the comprehensive network of miRNAs and FOX proteins in further research will provide better strategies for improving cancer treatment.
5. Targeting FOX Proteins as Potential Therapeutics in Cancers
FOX proteins are involved in various cellular processes, such as the DNA damage response, differentiation, proliferation and drug resistance, and consequently, targeting FOX proteins can significantly contribute to tumorigenesis and tumor progression. Generally, FOX proteins are transcription factors, which are traditionally considered undruggable molecules, and thus these proteins are not easily targeted in traditional drug development approaches [204]. However, on the therapeutic point, several recent studies elicited the selective pharmacological targeting of FOX proteins, indicating the promising strategies in clinical setting and disease treatment [205,206]. Although potential therapeutics targeting FOX proteins have yet to be fully explored, efforts to develop inhibitors of FOX proteins are underway. There are several approaches for modulating FOX protein activity in human cancer cells, as outlined in the following.
5.1. FOX Proteins-Targeting RNAi
RNA interference (RNAi), a process of sequence-specific posttranscriptional gene silencing initiated by double-stranded RNA, has been widely employed in the past decade as an experimental tool to investigate the roles of genes. Recently, the first therapy based on RNAi received approval from the US Food and Drug Administration. Several reports employing experimental human tumor models have displayed the feasibility of RNAi in suppressing the expression of cancer-associated genes, including FOX proteins, due to their advantages of exquisite precision and high efficacy in downregulating gene expression [204]. For example, depletion of FOXM1 expression by small interfering RNA transfection of lung adenocarcinoma cells can significantly decrease DNA replication and mitosis and reduce anchorage-independent growth of cell colonies on soft agar [207]. Silencing of FOXM1 by RNAi also abolished estrogen-stimulated breast cancer cell proliferation and overcame acquired tamoxifen resistance [208]. Alternatively, FOXM1 downregulation by stable or transient knockdown using RNAi or by treatment with proteasome inhibitors that target FOXM1 significantly sensitized human cancer cells of different origin to DNA damage-stimulated apoptosis [209]. Taken together, these findings indicate that targeting FOX proteins, especially FOXM1 with RNAi, a technique capable of specificity, may be a potential strategy for cancer therapy.
5.2. Proteasome Inhibitors
Although there are several drugs that can target the transcriptional activity or gene expression of FOX proteins, proteasome inhibitors appear to work well, but much more basic research is needed to unlock the complex interplay of interactions with FOX family members [5]. Recently, proteasome inhibitors have been widely employed in many clinical trials for cancer treatment as these proteasome inhibitors can selectively suppress cancer cell growth without affecting normal cells. However, their precise mechanism in anticancer activity has not been fully investigated [204,210]. These inhibitors can inhibit cancer cell progression through modulating FOX proteins. For example, several well-known proteasome inhibitors, such as MG132, MG115 and bortezomib, can suppress the transcriptional activity and expression of FOXM1. However, the overexpression of FOXM1 may also specifically protect against bortezomib-stimulated apoptosis but not doxorubicin-stimulated apoptosis [211]. Consequently, FOXM1 has been suggested as a general target for proteasome inhibitors [211,212]. Alternatively, the suppression of the proteasome causes regression of leukemia and abrogates BCR-ABL-stimulated evasion of apoptosis in part through modulation of forkhead tumor suppressors [213]. Other potential therapeutics include bioactive natural products (genistein), peptide inhibitors or thiazole antibiotics [204].
6. Conclusions and Future Perspectives
The use of public databases can facilitate prospective studies of the biological functions of FOX proteins at different omics levels in cancer as well as other disorders [214]. The regulatory networks of the FOX family are tremendously complex since they are involved cooperatively in extensive physiological and pathophysiological processes at multiple levels in a context-dependent manner. Studies designed to elucidate the correlations of FOX proteins and cancer progression are essential. Nevertheless, causative study designs are further required to extend our understanding of the fundamental roles of FOX proteins in cancer. Suitable research platforms that combine genomics and transcriptomics with proteomics and metabolomics are expected to provide fundamental information regarding the genetic structure, functional regions, expression patterns, and functional networks of FOXs in cancer. In parallel, re-analyzing available data with better statistical approaches, such as meta-analysis and cross-platform normalization, will be beneficial for providing more robust results and new opportunities for future investigations [215]. This information is crucial because it will provide clues into the mode of action of these transcription factors in the progression of cancers. Moreover, the diagnostic and prognostic impact of FOX proteins should be considered since their activities are closely related to the initiation, progression and metastasis of cancer. The utility of a single biomarker is limited in terms of the actual potential of biomarker candidates in clinical settings. Multiplex biomarker panels combined with state-of-the-art statistical learning are expected to help improve the clinical usability of FOXs in early detection, diagnosis, prognosis, and treatment of cancer patients [216].
The FOXO subfamily is highly correlated with the cell cycle and is exceptionally regulated by epigenetic effectors. Thus, these proteins are attractive targets for epigenetics-associated therapeutic development. Moreover, translational and clinical studies of FOXM1, particularly the FOXO-FOXM1 axis, should be further extended because of their impacts on a multitude of cellular processes, including tumorigenesis, progression, and drug resistance [169]. In addition, better drug-delivery strategies for not only small-molecule drugs but also RNAi may help improve the effectiveness of cancer treatment [217]. Combinational therapy targeting other therapeutic targets and the FOX family holds profound potential for providing synergistic effects and reduced treatment side effects, eventually improving clinical efficacy [218]. Furthermore, there is an urgent need to explore the roles of FOX proteins in the aberrant metabolism of cancer. Targeting the altered metabolism of cancer using metabolic disruptors is currently a dominant topic in cancer research [219]. Finally, similarities in the gene or protein structures of FOX family members, such as atypical FOXPs, may cause unpredictable complications due to off-target effects of treatment and thus should be taken into account when advancing new drug-development strategies.
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| CIN | chromosomal instability |
| DBD | DNA-binding domain |
| EMT | epithelial-mesenchymal transition |
| FASLG | Fas ligand |
| GSK | glycogen synthase kinase 3 |
| NSCLC | non-small cell lung cancer |
| RNAi | RNA interference |
| YAP | Yes-Associated Protein |
Author Contributions
D.-H.B.: designed, conducted the literature review and co-wrote the manuscript. N.P.L.: conducted the literature review and co-wrote the manuscript. T.-T.-T.L. & N.H.A.: discussed, editted and contributed to the manuscript. S.W.K. & S.K.L.: provided overall supervision and co-editted the manuscript.
Funding
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (NRF-2016M3A9B6903499) (S.K.L.) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1D1A1A02046560) (S.W.K.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Lam E.W.F., Brosens J.J., Gomes A.R., Koo C.-Y. Forkhead box proteins: Tuning forks for transcriptional harmony. Nat. Rev. Cancer. 2013;13:482. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- 2.Coffer P.J., Burgering B.M.T. Forkhead-box transcription factors and their role in the immune system. Nat. Rev. Immunol. 2004;4:889. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 3.Jonsson H., Peng S.L. Forkhead transcription factors in immunology. Cell Mol. Life Sci. 2005;62:397–409. doi: 10.1007/s00018-004-4365-8. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann O.J., Sowden J.C., Carlsson P., Jordan T., Bhattacharya S.S. FOX’s in development and disease. Trends Genet. 2003;19:339–344. doi: 10.1016/S0168-9525(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 5.Jackson B.C., Carpenter C., Nebert D.W., Vasiliou V. Update of human and mouse forkhead box (FOX) gene families. Hum. Genom. 2010;4:345–352. doi: 10.1186/1479-7364-4-5-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi E.J., Seo E.J., Kim D.K., Lee S.I., Kwon Y.W., Jang I.H., Kim K.H., Suh D.S., Kim J.H. FOXP1 functions as an oncogene in promoting cancer stem cell-like characteristics in ovarian cancer cells. Oncotarget. 2016;7:3506–3519. doi: 10.18632/oncotarget.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu M., Bao W., Wang J., Yang T., He X., Liao Y., Wan X. FOXA1 promotes tumor cell proliferation through AR involving the Notch pathway in endometrial cancer. BMC Cancer. 2014;14:78. doi: 10.1186/1471-2407-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam E.W.-F., Gomes A.R. Forkhead box transcription factors in cancer initiation, progression and chemotherapeutic drug response. Front. Oncol. 2014;4:305. doi: 10.3389/fonc.2014.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell E.G., Cotter T.G. Chapter Six—New insight into the role of reactive oxygen species (ROS) in cellular signal-transduction processes. Int. Rev. Cell Mol. Biol. 2015;319:221–254. doi: 10.1016/bs.ircmb.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Obsil T., Obsilova V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene. 2008;27:2263. doi: 10.1038/onc.2008.20. [DOI] [PubMed] [Google Scholar]
- 11.Essaghir A., Dif N., Marbehant C.Y., Coffer P.J., Demoulin J.B. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J. Biol. Chem. 2009;284:10334–10342. doi: 10.1074/jbc.M808848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myatt S.S., Lam E.W.F. The emerging roles of forkhead box (FOX) proteins in cancer. Nat. Rev. Cancer. 2007;7:847. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 13.Koo C.-Y., Muir K.W., Lam E.W.F. FOXM1: From cancer initiation to progression and treatment. Biochim. Biophys. Acta Gene Regul. Mech. 2012;1819:28–37. doi: 10.1016/j.bbagrm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Wilson M.S., Brosens J.J., Schwenen H.D., Lam E.W. FOXO and FOXM1 in cancer: The FOXO-FOXM1 axis shapes the outcome of cancer chemotherapy. Curr. Drug Targets. 2011;12:1256–1266. doi: 10.2174/138945011796150244. [DOI] [PubMed] [Google Scholar]
- 15.Elian F.A., Yan E., Walter M.A. FOXC1, the new player in the cancer sandbox. Oncotarget. 2018;9:8165–8178. doi: 10.18632/oncotarget.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao S., Wang Z., Gao X., He W., Cai Y., Chen H., Xu R. FOXC1 induces cancer stem cell-like properties through upregulation of beta-catenin in NSCLC. J. Exp. Clin. Cancer Res. 2018;37:220. doi: 10.1186/s13046-018-0894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katoh M., Igarashi M., Fukuda H., Nakagama H., Katoh M. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328:198–206. doi: 10.1016/j.canlet.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Baker S., Ali I., Silins I., Pyysalo S., Guo Y., Hogberg J., Stenius U., Korhonen A. Cancer Hallmarks Analytics Tool (CHAT): A text mining approach to organize and evaluate scientific literature on cancer. Bioinformatics. 2017;33:3973–3981. doi: 10.1093/bioinformatics/btx454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halasi M., Gartel A.L. FOX(M1) News—It Is Cancer. Mol. Cancer Ther. 2013;12:245–254. doi: 10.1158/1535-7163.MCT-12-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman J.R., Kaestner K.H. The FOXA family of transcription factors in development and metabolism. Cell Mol. Life Sci. 2006;63:2317–2328. doi: 10.1007/s00018-006-6095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jägle S., Busch H., Freihen V., Beyes S., Schrempp M., Boerries M., Hecht A. SNAIL1-mediated downregulation of FOXA proteins facilitates the inactivation of transcriptional enhancer elements at key epithelial genes in colorectal cancer cells. PLoS Genet. 2017;13:e1007109. doi: 10.1371/journal.pgen.1007109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang G., Zhao Y., Liu Y., Kao L.-P., Wang X., Skerry B., Li Z. FOXA1 defines cancer cell specificity. Sci. Adv. 2016;2:e1501473. doi: 10.1126/sciadv.1501473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin L., Miller C.T., Contreras J.I., Prescott M.S., Dagenais S.L., Wu R., Yee J., Orringer M.B., Misek D.E., Hanash S.M., et al. The hepatocyte nuclear factor 3 alpha gene, HNF3alpha (FOXA1), on chromosome band 14q13 is amplified and overexpressed in esophageal and lung adenocarcinomas. Cancer Res. 2002;62:5273. [PubMed] [Google Scholar]
- 24.Mirosevich J., Gao N., Gupta A., Shappell S.B., Jove R., Matusik R.J. Expression and role of FOXA proteins in prostate cancer. Prostate. 2005;66:1013–1028. doi: 10.1002/pros.20299. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y., Li Z. Interplay of estrogen receptors and FOXA factors in the liver cancer. Mol. Cell Endocrinol. 2015;418:334–339. doi: 10.1016/j.mce.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z., Tuteja G., Schug J., Kaestner Klaus H. FOXA1 and FOXA2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148:72–83. doi: 10.1016/j.cell.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahu B., Laakso M., Ovaska K., Mirtti T., Lundin J., Rannikko A., Sankila A., Turunen J.P., Lundin M., Konsti J., et al. Dual role of FOXA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 2011;30:3962. doi: 10.1038/emboj.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeGraff D.J., Clark P.E., Cates J.M., Yamashita H., Robinson V.L., Yu X., Smolkin M.E., Chang S.S., Cookson M.S., Herrick M.K., et al. Loss of the urothelial differentiation marker FOXA1 is associated with high grade, late stage bladder cancer and increased tumor proliferation. PLoS ONE. 2012;7:e36669. doi: 10.1371/journal.pone.0036669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ball A.R., Chen Y.Y., Yokomori K. Mechanisms of cohesin-mediated gene regulation and lessons learned from cohesinopathies. Biochim. Biophys. Acta Gene Regul. Mech. 2014;1839:191–202. doi: 10.1016/j.bbagrm.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhodes J.M., McEwan M., Horsfield J.A. Gene regulation by cohesin in cancer: Is the ring an unexpected party to proliferation? Mol. Cancer Res. 2011;9:1587. doi: 10.1158/1541-7786.MCR-11-0382. [DOI] [PubMed] [Google Scholar]
- 31.Fournier M., Bourriquen G., Lamaze F.C., Côté M.C., Fournier É., Joly-Beauparlant C., Caron V., Gobeil S., Droit A., Bilodeau S. FOXA and master transcription factors recruit Mediator and Cohesin to the core transcriptional regulatory circuitry of cancer cells. Sci. Rep. 2016;6:34962. doi: 10.1038/srep34962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfrum C., Asilmaz E., Luca E., Friedman J.M., Stoffel M. FOXA2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- 33.Kume T. The cooperative roles of FOXC1 and FOXC2 in cardiovascular development. Adv. Exp. Med. Biol. 2009;665:63–77. doi: 10.1007/978-1-4419-1599-3_5. [DOI] [PubMed] [Google Scholar]
- 34.Papanicolaou K.N., Izumiya Y., Walsh K. Forkhead transcription factors and cardiovascular biology. Circ. Res. 2008;102:16–31. doi: 10.1161/CIRCRESAHA.107.164186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han B., Bhowmick N., Qu Y., Chung S., Giuliano A.E., Cui X. FOXC1: An emerging marker and therapeutic target for cancer. Oncogene. 2017;36:3957. doi: 10.1038/onc.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramani R., Camacho F.A., Levin C.I., Flores K., Clift A., Galvez A., Terres M., Rivera S., Kolli S.N., Dodderer J., et al. FOXC1 plays a crucial role in the growth of pancreatic cancer. Oncogenesis. 2018;7:52. doi: 10.1038/s41389-018-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray P.S., Wang J., Qu Y., Sim M.-S., Shamonki J., Bagaria S.P., Ye X., Liu B., Elashoff D., Hoon D.S., et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 2010;70:3870. doi: 10.1158/0008-5472.CAN-09-4120. [DOI] [PubMed] [Google Scholar]
- 38.Xia L., Huang W., Tian D., Zhu H., Qi X., Chen Z., Zhang Y., Hu H., Fan D., Nie Y., et al. Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology. 2012;57:610–624. doi: 10.1002/hep.26029. [DOI] [PubMed] [Google Scholar]
- 39.Wang T., Zheng L., Wang Q., Hu Y.W. Emerging roles and mechanisms of FOXC2 in cancer. Clin. Chim. Acta. 2018;479:84–93. doi: 10.1016/j.cca.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Cui Y.M., Jiao H.L., Ye Y.P., Chen C.M., Wang J.X., Tang N., Li T.T., Lin J., Qi L., Wu P., et al. FOXC2 promotes colorectal cancer metastasis by directly targeting MET. Oncogene. 2015;34:4379–4390. doi: 10.1038/onc.2014.368. [DOI] [PubMed] [Google Scholar]
- 41.Han B., Qu Y., Jin Y., Yu Y., Deng N., Wawrowsky K., Zhang X., Li N., Bose S., Wang Q., et al. FOXC1 activates smoothened-independent Hedgehog signaling in basal-like breast cancer. Cell Rep. 2015;13:1046–1058. doi: 10.1016/j.celrep.2015.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollier B.G., Tinnirello A.A., Werden S.J., Evans K.W., Taube J.H., Sarkar T.R., Sphyris N., Shariati M., Kumar S.V., Battula V.L., et al. FOXC2 expression links epithelial–mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013;73:1981. doi: 10.1158/0008-5472.CAN-12-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou P., Li B., Liu F., Zhang M., Wang Q., Liu Y., Yao Y., Li D. The epithelial to mesenchymal transition (EMT) and cancer stem cells: Implication for treatment resistance in pancreatic cancer. Mol. Cancer. 2017;16:52. doi: 10.1186/s12943-017-0624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song L., Tang H., Liao W., Luo X., Li Y., Chen T., Zhang X. FOXC2 positively regulates YAP signaling and promotes the glycolysis of nasopharyngeal carcinoma. Exp. Cell Res. 2017;357:17–24. doi: 10.1016/j.yexcr.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 45.Raychaudhuri P., Park H.J. FOXM1: A master regulator of tumor metastasis. Cancer Res. 2011;71:4329. doi: 10.1158/0008-5472.CAN-11-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L., Wu D., Yu Q., Li L., Wu P. Prognostic value of FOXM1 in solid tumors: A systematic review and meta-analysis. Oncotarget. 2017;8:32298–32308. doi: 10.18632/oncotarget.15764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park H.J., Carr J.R., Wang Z., Nogueira V., Hay N., Tyner A.L., Lau L.F., Costa R.H., Raychaudhuri P. FOXM1, a critical regulator of oxidative stress during oncogenesis. EMBO J. 2009;28:2908. doi: 10.1038/emboj.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park H.J., Gusarova G., Wang Z., Carr J.R., Li J., Kim K.H., Qiu J., Park Y.D., Williamson P.R., Hay N., et al. Deregulation of FOXM1B leads to tumour metastasis. EMBO Mol. Med. 2011;3:21. doi: 10.1002/emmm.201000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang I.C., Chen Y.-J., Hughes D., Petrovic V., Major M.L., Park H.J., Tan Y., Ackerson T., Costa R.H. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol. Cell Biol. 2005;25:10875. doi: 10.1128/MCB.25.24.10875-10894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bao B., Wang Z., Ali S., Kong D., Banerjee S., Ahmad A., Li Y., Azmi A.S., Miele L., Sarkar F.H. Over-expression of FOXM1 leads to epithelial–mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J. Cell Biochem. 2011;112:2296–2306. doi: 10.1002/jcb.23150. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Gomes A.R., Zhao F., Lam E.W.F. Role and regulation of the forkhead transcription factors FOXO3a and FOXM1 in carcinogenesis and drug resistance. Chin. J. Cancer. 2013;32:365–370. doi: 10.5732/cjc.012.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radhakrishnan S.K., Gartel A.L. FOXM1: The Achilles’ heel of cancer? Nat. Rev. Cancer. 2008;8:242. doi: 10.1038/nrc2223-c1. [DOI] [PubMed] [Google Scholar]
- 53.Coomans de Brachène A., Demoulin J.B. FOXO transcription factors in cancer development and therapy. Cell Mol. Life Sci. 2016;73:1159–1172. doi: 10.1007/s00018-015-2112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salih D.A., Rashid A.J., Colas D., de la Torre-Ubieta L., Zhu R.P., Morgan A.A., Santo E.E., Ucar D., Devarajan K., Cole C.J., et al. FOXO6 regulates memory consolidation and synaptic function. Genes Dev. 2012;26:2780–2801. doi: 10.1101/gad.208926.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang H., Tindall D.J. Dynamic FOXO transcription factors. J. Cell Sci. 2007;120:2479. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y., Gan B., Liu D., Paik J.H. FOXO family members in cancer. Cancer Biol. Ther. 2011;12:253–259. doi: 10.4161/cbt.12.4.15954. [DOI] [PubMed] [Google Scholar]
- 57.Greer E.L., Brunet A. FOXO transcription factors in ageing and cancer. Acta Physiol. 2007;192:19–28. doi: 10.1111/j.1748-1716.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 58.Maiese K., Chong Z.Z., Shang Y.C., Hou J. A “FOXO” in sight: Targeting FOXO proteins from conception to cancer. Med. Res. Rev. 2008;29:395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kloet D.E.A., Burgering B.M.T. The PKB/FOXO switch in aging and cancer. Biochim. Biophys. Acta Mol. Cell Res. 2011;1813:1926–1937. doi: 10.1016/j.bbamcr.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Ma Y., Wang H. PI3K/Akt/FOXO: A novel participant in signal transduction in bone cells under mechanical stimulation. Cell Biol. Int. 2013;36:923–926. doi: 10.1042/CBI20120078. [DOI] [PubMed] [Google Scholar]
- 61.Maiese K., Chong Z.Z., Shang Y.C., Hou J. Clever cancer strategies with FOXO transcription factors. Cell Cycle. 2008;7:3829–3839. doi: 10.4161/cc.7.24.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hornsveld M., Dansen T.B., Derksen P.W., Burgering B.M.T. Re-evaluating the role of FOXOs in cancer. Semin. Cancer Biol. 2018;50:90–100. doi: 10.1016/j.semcancer.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 63.Hornsveld M., Smits L.M.M., Meerlo M., van Amersfoort M., Groot Koerkamp M.J.A., van Leenen D., Kloet D.E.A., Holstege F.C.P., Derksen P.W.B., Burgering B.M.T., et al. FOXO transcription factors both suppress and support breast cancer progression. Cancer Res. 2018;78:2356–2369. doi: 10.1158/0008-5472.CAN-17-2511. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W., Duan N., Song T., Li Z., Zhang C., Chen X. The emerging roles of forkhead box (FOX) proteins in osteosarcoma. J. Cancer. 2017;8:1619–1628. doi: 10.7150/jca.18778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guan H., Tan P., Xie L., Mi B., Fang Z., Li J., Yue J., Liao H., Li F. FOXO1 inhibits osteosarcoma oncogenesis via Wnt/beta-catenin pathway suppression. Oncogenesis. 2015;4:e166. doi: 10.1038/oncsis.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J.-Y., Hung M.-C. A new fork for clinical application: Targeting forkhead transcription factors in cancer. Clin. Cancer Res. 2009;15:752. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van der Vos K.E., Coffer P.J. The extending network of FOXO transcriptional target genes. Antioxid. Redox Signal. 2011;14:579–592. doi: 10.1089/ars.2010.3419. [DOI] [PubMed] [Google Scholar]
- 68.Nho R.S., Hergert P. FOXO3A and disease progression. World J. Biol. Chem. 2014;5:346–354. doi: 10.4331/wjbc.v5.i3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y., Ao X., Ding W., Ponnusamy M., Wu W., Hao X., Yu W., Wang Y., Li P., Wang J. Critical role of FOXO3A in carcinogenesis. Mol. Cancer. 2018;17:104. doi: 10.1186/s12943-018-0856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yadav R.K., Chauhan A.S., Zhuang L., Gan B. FOXO transcription factors in cancer metabolism. Semin. Cancer Biol. 2018;50:65–76. doi: 10.1016/j.semcancer.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia S.J., Holder D.D., Pawel B.R., Zhang C., Barr F.G. High expression of the PAX3-FKHR oncoprotein is required to promote tumorigenesis of human myoblasts. Am. J. Pathol. 2009;175:2600–2608. doi: 10.2353/ajpath.2009.090192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sin C., Li H., Crawford D.A. Transcriptional regulation by FOXP1, FOXP2, and FOXP4 dimerization. J. Mol. Neurosci. 2015;55:437–448. doi: 10.1007/s12031-014-0359-7. [DOI] [PubMed] [Google Scholar]
- 73.Meerschaut I., Rochefort D., Revençu N., Pètre J., Corsello C., Rouleau G.A., Hamdan F.F., Michaud J.L., Morton J., Radley J., et al. FOXP1-related intellectual disability syndrome: A recognisable entity. J. Med. Genet. 2017;54:613. doi: 10.1136/jmedgenet-2017-104579. [DOI] [PubMed] [Google Scholar]
- 74.Tao J.-H., Cheng M., Tang J.-P., Liu Q., Pan F., Li X.-P. FOXP3, regulatory T cell, and autoimmune diseases. Inflammation. 2017;40:328–339. doi: 10.1007/s10753-016-0470-8. [DOI] [PubMed] [Google Scholar]
- 75.Martin F., Ladoire S., Mignot G., Apetoh L., Ghiringhelli F. Human FOXP3 and cancer. Oncogene. 2010;29:4121. doi: 10.1038/onc.2010.174. [DOI] [PubMed] [Google Scholar]
- 76.Herrero M.J., Gitton Y. The untold stories of the speech gene, the FOXP2 cancer gene. Genes Cancer. 2018;9:11–38. doi: 10.18632/genesandcancer.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teufel A., Wong E.A., Mukhopadhyay M., Malik N., Westphal H. FOXP4, a novel forkhead transcription factor. Biochim. Biophys. Acta. 2003;1627:147–152. doi: 10.1016/S0167-4781(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 78.Vernes S.C., Spiteri E., Nicod J., Groszer M., Taylor J.M., Davies K.E., Geschwind D.H., Fisher S.E. High-throughput analysis of promoter occupancy reveals direct neural targets of FOXP2, a gene mutated in speech and language disorders. Am. J. Hum. Genet. 2007;81:1232–1250. doi: 10.1086/522238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeLaughter D.M., Christodoulou D.C., Robinson J.Y., Seidman C.E., Baldwin H.S., Seidman J.G., Barnett J.V. Spatial transcriptional profile of the chick and mouse endocardial cushions identify novel regulators of endocardial EMT in vitro. J. Mol. Cell Cardiol. 2013;59:196–204. doi: 10.1016/j.yjmcc.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song X.L., Tang Y., Lei X.H., Zhao S.C., Wu Z.Q. miR-618 inhibits prostate cancer migration and invasion by targeting FOXP2. J. Cancer. 2017;8:2501–2510. doi: 10.7150/jca.17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong K.K., Gascoyne D.M., Soilleux E.J., Lyne L., Spearman H., Roncador G., Pedersen L.M., Moller M.B., Green T.M., Banham A.H. FOXP2-positive diffuse large B-cell lymphomas exhibit a poor response to R-CHOP therapy and distinct biological signatures. Oncotarget. 2016;7:52940–52956. doi: 10.18632/oncotarget.9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Campbell A.J., Lyne L., Brown P.J., Launchbury R.J., Bignone P., Chi J., Roncador G., Lawrie C.H., Gatter K.C., Kusec R., et al. Aberrant expression of the neuronal transcription factor FOXP2 in neoplastic plasma cells. Br. J. Haematol. 2010;149:221–230. doi: 10.1111/j.1365-2141.2009.08070.x. [DOI] [PubMed] [Google Scholar]
- 83.Koon H.B., Ippolito G.C., Banham A.H., Tucker P.W. FOXP1: A potential therapeutic target in cancer. Expert. Opin. Ther. Targets. 2007;11:955–965. doi: 10.1517/14728222.11.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sawant D.V., Vignali D.A.A. Once a Treg, always a Treg? Immunol. Rev. 2014;259:173–191. doi: 10.1111/imr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szylberg L., Karbownik D., Marszalek A. The role of FOXP3 in human cancers. Anticancer Res. 2016;36:3789–3794. [PubMed] [Google Scholar]
- 86.Li X., Gao Y., Li J., Zhang K., Han J., Li W., Hao Q., Zhang W., Wang S., Zeng C., et al. FOXP3 inhibits angiogenesis by downregulating VEGF in breast cancer. Cell Death Dis. 2018;9:744. doi: 10.1038/s41419-018-0790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang T., Li H., Thakur A., Chen T., Xue J., Li D., Chen M. FOXP4 modulates tumor growth and independently associates with miR-138 in non-small cell lung cancer cells. Tumour Biol. 2015;36:8185–8191. doi: 10.1007/s13277-015-3498-8. [DOI] [PubMed] [Google Scholar]
- 88.Liu M., Shi X., Wang J., Xu Y., Wei D., Zhang Y., Yang K., Wang X., Liang S., Chen X., et al. Association of FOXP4 gene with prostate cancer and the cumulative effects of rs4714476 and 8q24 in Chinese men. Clin. Lab. 2015;61:1491–1499. doi: 10.7754/Clin.Lab.2015.150313. [DOI] [PubMed] [Google Scholar]
- 89.Howarth K.D., Blood K.A., Ng B.L., Beavis J.C., Chua Y., Cooke S.L., Raby S., Ichimura K., Collins V.P., Carter N.P., et al. Array painting reveals a high frequency of balanced translocations in breast cancer cell lines that break in cancer-relevant genes. Oncogene. 2007;27:3345. doi: 10.1038/sj.onc.1210993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan J.-H., Zhao C.L., Ding L.-B., Zhou X. FOXD3 suppresses tumor growth and angiogenesis in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2015;466:111–116. doi: 10.1016/j.bbrc.2015.08.116. [DOI] [PubMed] [Google Scholar]
- 91.Chu T.-L., Zhao H.-M., Li Y., Chen A.-X., Sun X., Ge J. FoxD3 deficiency promotes breast cancer progression by induction of epithelial–mesenchymal transition. Biochem. Biophys. Res. Commun. 2014;446:580–584. doi: 10.1016/j.bbrc.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 92.Li D., Mei H., Qi M., Yang D., Zhao X., Xiang X., Pu J., Huang K., Zheng L., Tong Q. FOXD3 is a novel tumor suppressor that affects growth, invasion, metastasis and angiogenesis of neuroblastoma. Oncotarget. 2013;4:2021–2044. doi: 10.18632/oncotarget.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mond M., Bullock M., Yao Y., Clifton-Bligh R.J., Gilfillan C., Fuller P.J. Somatic mutations of FOXE1 in papillary thyroid cancer. Thyroid. 2015;25:904–910. doi: 10.1089/thy.2015.0030. [DOI] [PubMed] [Google Scholar]
- 94.Penna-Martinez M., Epp F., Kahles H., Ramos-Lopez E., Hinsch N., Hansmann M.L., Selkinski I., Grunwald F., Holzer K., Bechstein W.O., et al. FOXE1 association with differentiated thyroid cancer and its progression. Thyroid. 2014;24:845–851. doi: 10.1089/thy.2013.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu H., Xi Q., Liu L., Wang J., Gu M. Quantitative assessment of common genetic variants on FOXE1 and differentiated thyroid cancer risk. PLoS ONE. 2014;9:e87332. doi: 10.1371/journal.pone.0087332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tamura M., Sasaki Y., Koyama R., Takeda K., Idogawa M., Tokino T. Forkhead transcription factor FOXF1 is a novel target gene of the p53 family and regulates cancer cell migration and invasiveness. Oncogene. 2013;33:4837. doi: 10.1038/onc.2013.427. [DOI] [PubMed] [Google Scholar]
- 97.Gialmanidis I.P., Bravou V., Petrou I., Kourea H., Mathioudakis A., Lilis I., Papadaki H. Expression of Bmi1, FOXF1, Nanog, and γ-Catenin in relation to Hedgehog signaling pathway in human non-small-cell lung cancer. Lung. 2013;191:511–521. doi: 10.1007/s00408-013-9490-4. [DOI] [PubMed] [Google Scholar]
- 98.Fulford L., Milewski D., Ustiyan V., Ravishankar N., Cai Y., Le T., Masineni S., Kasper S., Aronow B., Kalinichenko V.V., et al. The transcription factor FOXF1 promotes prostate cancer by stimulating the mitogen-activated protein kinase ERK5. Sci. Signal. 2016;9:ra48. doi: 10.1126/scisignal.aad5582. [DOI] [PubMed] [Google Scholar]
- 99.Lo P.-K., Lee J.S., Sukumar S. The p53-p21(WAF1) checkpoint pathway plays a protective role in preventing DNA rereplication induced by abrogation of FOXF1 function. Cell Signal. 2012;24:316–324. doi: 10.1016/j.cellsig.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao Z.G., Wang D.Q., Hu D.F., Li Y.S., Liu S.H. Decreased FOXF1 promotes hepatocellular carcinoma tumorigenesis, invasion, and stemness and is associated with poor clinical outcome. OncoTargets Ther. 2016;9:1743–1752. doi: 10.2147/OTT.S95002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang G., He P., Gaedcke J., Ghadimi B.M., Ried T., Yfantis H.G., Lee D.H., Hanna N., Alexander H.R., Hussain S.P. FOXL1, a novel candidate tumor suppressor, inhibits tumor aggressiveness and predicts outcome in human pancreatic cancer. Cancer Res. 2013;73:5416–5425. doi: 10.1158/0008-5472.CAN-13-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang F.Q., Yang F.P., Li W., Liu M., Wang G.C., Che J.P., Huang J.H., Zheng J.H. FOXL1 inhibits tumor invasion and predicts outcome in human renal cancer. Int. J. Clin. Exp. Pathol. 2014;7:110–122. [PMC free article] [PubMed] [Google Scholar]
- 103.Qin Y., Gong W., Zhang M., Wang J., Tang Z., Quan Z. Forkhead box L1 is frequently downregulated in gallbladder cancer and inhibits cell growth through apoptosis induction by mitochondrial dysfunction. PLoS ONE. 2014;9:e102084. doi: 10.1371/journal.pone.0102084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen X., Deng M., Ma L., Zhou J., Xiao Y., Zhou X., Zhang C., Wu M. Inhibitory effects of forkhead box L1 gene on osteosarcoma growth through the induction of cell cycle arrest and apoptosis. Oncol. Rep. 2015;34:265–271. doi: 10.3892/or.2015.3969. [DOI] [PubMed] [Google Scholar]
- 105.Pei Y., Wang P., Liu H., He F., Ming L. FOXQ1 promotes esophageal cancer proliferation and metastasis by negatively modulating CDH1. Biomed. Pharmacother. 2015;74:89–94. doi: 10.1016/j.biopha.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 106.Meng F., Speyer C.L., Zhang B., Zhao Y., Chen W., Gorski D.H., Miller F.R., Wu G. PDGFRalpha and beta play critical roles in mediating FOXQ1-driven breast cancer stemness and chemoresistance. Cancer Res. 2015;75:584–593. doi: 10.1158/0008-5472.CAN-13-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng X., Luo Z., Kang Q., Deng D., Wang Q., Peng H., Wang S., Wei Z. FOXQ1 mediates the crosstalk between TGF-beta and Wnt signaling pathways in the progression of colorectal cancer. Cancer Biol. Ther. 2015;16:1099–1109. doi: 10.1080/15384047.2015.1047568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhan H.X., Xu J.W., Wang L., Wu D., Zhang G.Y., Hu S.Y. FOXQ1 is a novel molecular target for pancreatic cancer and is associated with poor prognosis. Curr. Mol. Med. 2015;15:469–477. doi: 10.2174/1566524015666150630125247. [DOI] [PubMed] [Google Scholar]
- 109.Tang H., Zhang J., Guo Q. Research progress on the regulation of tumor initiation and development by the forkhead box Q1 gene. J. Cancer Res. Ther. 2018;14:6–11. doi: 10.4103/jcrt.JCRT_701_17. [DOI] [PubMed] [Google Scholar]
- 110.Chen H.W., Huang X.D., Li H.C., He S., Ni R.Z., Chen C.H., Peng C., Wu G., Wang G.H., Wang Y.Y., et al. Expression of FOXJ1 in hepatocellular carcinoma: Correlation with patients’ prognosis and tumor cell proliferation. Mol. Carcinog. 2012;52:647–659. doi: 10.1002/mc.21904. [DOI] [PubMed] [Google Scholar]
- 111.Xian S., Shang D., Kong G., Tian Y. FOXJ1 promotes bladder cancer cell growth and regulates Warburg effect. Biochem. Biophys. Res. Commun. 2018;495:988–994. doi: 10.1016/j.bbrc.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 112.Wang J., Cai X., Xia L., Zhou J., Xin J., Liu M., Shang X., Liu J., Li X., Chen Z., et al. Decreased expression of FOXJ1 is a potential prognostic predictor for progression and poor survival of gastric cancer. Ann. Surg. Oncol. 2015;22:685–692. doi: 10.1245/s10434-014-3742-2. [DOI] [PubMed] [Google Scholar]
- 113.Liu X.L., Meng Y.H., Wang J.L., Yang B.B., Zhang F., Tang S.J. FOXL2 suppresses proliferation, invasion and promotes apoptosis of cervical cancer cells. Int. J. Clin. Exp. Pathol. 2014;7:1534–1543. [PMC free article] [PubMed] [Google Scholar]
- 114.Rosario R., Cohen P.A., Shelling A.N. The role of FOXL2 in the pathogenesis of adult ovarian granulosa cell tumours. Gynecolog. Oncol. 2014;133:382–387. doi: 10.1016/j.ygyno.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 115.Kommoss S., Anglesio M.S., Mackenzie R., Yang W., Senz J., Ho J., Bell L., Lee S., Lorette J., Huntsman D.G., et al. FOXL2 molecular testing in ovarian neoplasms: Diagnostic approach and procedural guidelines. Mod. Pathol. 2013;26:860. doi: 10.1038/modpathol.2012.226. [DOI] [PubMed] [Google Scholar]
- 116.Yao S., Fan L.Y., Lam E.W. The FOXO3-FOXM1 axis: A key cancer drug target and a modulator of cancer drug resistance. Semin. Cancer Biol. 2018;50:77–89. doi: 10.1016/j.semcancer.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bach D.-H., Hong J.Y., Park H.J., Lee S.K. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int. J. Cancer. 2017;141:220–230. doi: 10.1002/ijc.30669. [DOI] [PubMed] [Google Scholar]
- 118.Bach D.-H., Kim D., Bae S.Y., Kim W.K., Hong J.-Y., Lee H.-J., Rajasekaran N., Kwon S., Fan Y., Luu T.-T.-T., et al. Targeting nicotinamide n-methyltransferase and miR-449a in EGFR-TKI-resistant non-small-cell lung cancer cells. Mol. Ther. Nucleic Acids. 2018;11:455–467. doi: 10.1016/j.omtn.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bach D.-H., Lee S.K. Long noncoding RNAs in cancer cells. Cancer Lett. 2018;419:152–166. doi: 10.1016/j.canlet.2018.01.053. [DOI] [PubMed] [Google Scholar]
- 120.Bach D.-H., Park H.J., Lee S.K. The dual role of bone morphogenetic proteins in cancer. Oncolytics. 2018;8:1–13. doi: 10.1016/j.omto.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duc-Hiep B., Lee S.K. The potential impacts of tylophora alkaloids and their derivatives in modulating inflammation, viral infections, and cancer. Curr. Med. Chem. 2018;25:1–16. doi: 10.2174/0929867325666180726123339. [DOI] [PubMed] [Google Scholar]
- 122.Bach D.H., Luu T.T.T., Kim D., An Y.J., Park H.J., Park S., Lee S.K. BMP4 upregulation is associated with acquired drug resistance and fatty acid metabolism in EGFR-mutant non-small cell lung cancer cells. Mol. Ther. Nucleic Acids. 2018;12:817–828. doi: 10.1016/j.omtn.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li M., Yang J., Zhou W., Ren Y., Wang X., Chen H., Zhang J., Chen J., Sun Y., Cui L., et al. Activation of an AKT/FOXM1/STMN1 pathway drives resistance to tyrosine kinase inhibitors in lung cancer. Br. J. Cancer. 2017;117:974–983. doi: 10.1038/bjc.2017.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Westhoff G.L., Chen Y., Teng N.N.H. Targeting FOXM1 improves cytotoxicity of paclitaxel and cisplatinum in platinum-resistant ovarian cancer. Int. J. Gynecol. Cancer. 2017;27:1602–1609. doi: 10.1097/IGC.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 125.Wang Y., Zhang W., Wen L., Yang H., Wen M., Yun Y., Zhao L., Zhu X., Tian L., Luo E., et al. FOXM1 confers resistance to gefitinib in lung adenocarcinoma via a MET/AKT-dependent positive feedback loop. Oncotarget. 2016;7:59245–59259. doi: 10.18632/oncotarget.11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khan I., Halasi M., Zia M.F., Gann P., Gaitonde S., Mahmud N., Gartel A.L. Nuclear FOXM1 drives chemoresistance in AML. Leukemia. 2017;31:251–255. doi: 10.1038/leu.2016.270. [DOI] [PubMed] [Google Scholar]
- 127.Xie T., Geng J., Wang Y., Wang L., Huang M., Chen J., Zhang K., Xue L., Liu X., Mao X., et al. FOXM1 evokes 5-fluorouracil resistance in colorectal cancer depending on ABCC10. Oncotarget. 2017;8:8574–8589. doi: 10.18632/oncotarget.14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peng W.X., Han X., Zhang C.L., Ge L., Du F.Y., Jin J., Gong A.H. FOXM1-mediated RFC5 expression promotes temozolomide resistance. Cell Biol. Toxicol. 2017;33:527–537. doi: 10.1007/s10565-017-9381-1. [DOI] [PubMed] [Google Scholar]
- 129.Hou Y., Zhu Q., Li Z., Peng Y., Yu X., Yuan B., Liu Y., Liu Y., Yin L., Peng Y., et al. The FOXM1-ABCC5 axis contributes to paclitaxel resistance in nasopharyngeal carcinoma cells. Cell Death Dis. 2017;8:e2659. doi: 10.1038/cddis.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tassi R.A., Todeschini P., Siegel E.R., Calza S., Cappella P., Ardighieri L., Cadei M., Bugatti M., Romani C., Bandiera E., et al. FOXM1 expression is significantly associated with chemotherapy resistance and adverse prognosis in non-serous epithelial ovarian cancer patients. J. Exp. Clin. Cancer Res. 2017;36:63. doi: 10.1186/s13046-017-0536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roh Y.G., Mun M.H., Jeong M.S., Kim W.T., Lee S.R., Chung J.W., Kim S.I., Kim T.N., Nam J.K., Leem S.H. Drug resistance of bladder cancer cells through activation of ABCG2 by FOXM1. BMB Rep. 2018;51:98–103. doi: 10.5483/BMBRep.2018.51.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khongkow P., Karunarathna U., Khongkow M., Gong C., Gomes A.R., Yague E., Monteiro L.J., Kongsema M., Zona S., Man E.P., et al. FOXM1 targets NBS1 to regulate DNA damage-induced senescence and epirubicin resistance. Oncogene. 2014;33:4144–4155. doi: 10.1038/onc.2013.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li X., Qiu W., Liu B., Yao R., Liu S., Yao Y., Liang J. Forkhead box transcription factor 1 expression in gastric cancer: FOXM1 is a poor prognostic factor and mediates resistance to docetaxel. J. Transl. Med. 2013;11:204. doi: 10.1186/1479-5876-11-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang T., Liu Z., Shi F., Wang J. Pin1 modulates chemo-resistance by up-regulating FOXM1 and the involvements of Wnt/beta-catenin signaling pathway in cervical cancer. Mol. Cell Biochem. 2016;413:179–187. doi: 10.1007/s11010-015-2651-4. [DOI] [PubMed] [Google Scholar]
- 135.Nestal de Moraes G., Delbue D., Silva K.L., Robaina M.C., Khongkow P., Gomes A.R., Zona S., Crocamo S., Mencalha A.L., Magalhaes L.M., et al. FOXM1 targets XIAP and Survivin to modulate breast cancer survival and chemoresistance. Cell Signal. 2015;27:2496–2505. doi: 10.1016/j.cellsig.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 136.Consolaro F., Basso G., Ghaem-Magami S., Lam E.W., Viola G. FOXM1 is overexpressed in B-acute lymphoblastic leukemia (B-ALL) and its inhibition sensitizes B-ALL cells to chemotherapeutic drugs. Int. J. Oncol. 2015;47:1230–1240. doi: 10.3892/ijo.2015.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Karunarathna U., Kongsema M., Zona S., Gong C., Cabrera E., Gomes A.R., Man E.P., Khongkow P., Tsang J.W., Khoo U.S., et al. OTUB1 inhibits the ubiquitination and degradation of FOXM1 in breast cancer and epirubicin resistance. Oncogene. 2016;35:1433–1444. doi: 10.1038/onc.2015.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khongkow P., Gomes A.R., Gong C., Man E.P., Tsang J.W., Zhao F., Monteiro L.J., Coombes R.C., Medema R.H., Khoo U.S., et al. Paclitaxel targets FOXM1 to regulate KIF20A in mitotic catastrophe and breast cancer paclitaxel resistance. Oncogene. 2016;35:990–1002. doi: 10.1038/onc.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chiu W.T., Huang Y.F., Tsai H.Y., Chen C.C., Chang C.H., Huang S.C., Hsu K.F., Chou C.Y. FOXM1 confers to epithelial-mesenchymal transition, stemness and chemoresistance in epithelial ovarian carcinoma cells. Oncotarget. 2015;6:2349–2365. doi: 10.18632/oncotarget.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hu C.J., Wang B., Tang B., Chen B.J., Xiao Y.F., Qin Y., Yong X., Luo G., Zhang J.W., Zhang D., et al. The FOXM1-induced resistance to oxaliplatin is partially mediated by its novel target gene Mcl-1 in gastric cancer cells. Biochim. Biophys. Acta. 2015;1849:290–299. doi: 10.1016/j.bbagrm.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 141.Zhao F., Siu M.K., Jiang L., Tam K.F., Ngan H.Y., Le X.F., Wong O.G., Wong E.S., Gomes A.R., Bella L., et al. Overexpression of forkhead box protein M1 (FOXM1) in ovarian cancer correlates with poor patient survival and contributes to paclitaxel resistance. PLoS ONE. 2014;9:e113478. doi: 10.1371/journal.pone.0113478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou J., Wang Y., Wang Y., Yin X., He Y., Chen L., Wang W., Liu T., Di W. FOXM1 modulates cisplatin sensitivity by regulating EXO1 in ovarian cancer. PLoS ONE. 2014;9:e96989. doi: 10.1371/journal.pone.0096989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yu-Rice Y., Jin Y., Han B., Qu Y., Johnson J., Watanabe T., Cheng L., Deng N., Tanaka H., Gao B., et al. FOXC1 is involved in ERalpha silencing by counteracting GATA3 binding and is implicated in endocrine resistance. Oncogene. 2016;35:5400–5411. doi: 10.1038/onc.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feng J., Xu L., Ni S., Gu J., Zhu H., Wang H., Zhang S., Zhang W., Huang J. Involvement of FOXQ1 in NSCLC through regulating EMT and increasing chemosensitivity. Oncotarget. 2014;5:9689–9702. doi: 10.18632/oncotarget.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li C., Ding H., Tian J., Wu L., Wang Y., Xing Y., Chen M. Forkhead box protein C2 promotes epithelial-mesenchymal transition, migration and invasion in cisplatin-resistant human ovarian cancer cell line (SKOV3/CDDP) Cell Physiol. Biochem. 2016;39:1098–1110. doi: 10.1159/000447818. [DOI] [PubMed] [Google Scholar]
- 146.Li C., Ding H., Tian J., Wu L., Wang Y., Xing Y., Chen M. Forkhead Box Protein C2 (FOXC2) promotes the resistance of human ovarian cancer cells to cisplatin in vitro and in vivo. Cell Physiol. Biochem. 2016;39:242–252. doi: 10.1159/000445620. [DOI] [PubMed] [Google Scholar]
- 147.Zhou Z., Zhang L., Xie B., Wang X., Yang X., Ding N., Zhang J., Liu Q., Tan G., Feng D., et al. FOXC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transition. Cancer Lett. 2015;363:137–145. doi: 10.1016/j.canlet.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 148.Zhao Y.F., Zhao J.Y., Yue H., Hu K.S., Shen H., Guo Z.G., Su X.J. FOXD1 promotes breast cancer proliferation and chemotherapeutic drug resistance by targeting p27. Biochem. Biophys. Res. Commun. 2015;456:232–237. doi: 10.1016/j.bbrc.2014.11.064. [DOI] [PubMed] [Google Scholar]
- 149.Chiu C.F., Chang Y.W., Kuo K.T., Shen Y.S., Liu C.Y., Yu Y.H., Cheng C.C., Lee K.Y., Chen F.C., Hsu M.K., et al. NF-kappaB-driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance. Proc. Natl. Acad. Sci. USA. 2016;113:E2526–E2535. doi: 10.1073/pnas.1522612113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu Y., Guo M., Wei Y., Yu S., Li H., Wang Y., Xu X., Cui Y., Tian J., Liang L., et al. FOXO3A confers cetuximab resistance in RAS wild-type metastatic colorectal cancer through c-Myc. Oncotarget. 2016;7:80888–80900. doi: 10.18632/oncotarget.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aldonza M.B., Hong J.Y., Lee S.K. Paclitaxel-resistant cancer cell-derived secretomes elicit ABCB1-associated docetaxel cross-resistance and escape from apoptosis through FOXO3a-driven glycolytic regulation. Exp. Mol. Med. 2017;49:e286. doi: 10.1038/emm.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang L., Yang X., Li X., Li C., Zhao L., Zhou Y., Hou H. Butein sensitizes HeLa cells to cisplatin through the AKT and ERK/p38 MAPK pathways by targeting FOXO3A. Int. J. Mol. Med. 2015;36:957–966. doi: 10.3892/ijmm.2015.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ding Q., Chen Y., Zhang Q., Guo Y., Huang Z., Dai L., Cao S. 8bromo7methoxychrysin induces apoptosis by regulating Akt/FOXO3A pathway in cisplatinsensitive and resistant ovarian cancer cells. Mol. Med. Rep. 2015;12:5100–5108. doi: 10.3892/mmr.2015.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chi H.C., Chen S.L., Cheng Y.H., Lin T.K., Tsai C.Y., Tsai M.M., Lin Y.H., Huang Y.H., Lin K.H. Chemotherapy resistance and metastasis-promoting effects of thyroid hormone in hepatocarcinoma cells are mediated by suppression of FOXO1 and Bim pathway. Cell Death Dis. 2016;7:e2324. doi: 10.1038/cddis.2016.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang H., Xie C., Yue J., Jiang Z., Zhou R., Xie R., Wang Y., Wu S. Cancer-associated fibroblasts mediated chemoresistance by a FOXO1/TGFbeta1 signaling loop in esophageal squamous cell carcinoma. Mol. Carcinog. 2017;56:1150–1163. doi: 10.1002/mc.22581. [DOI] [PubMed] [Google Scholar]
- 156.Park J., Choi Y., Ko Y.S., Kim Y., Pyo J.S., Jang B.G., Kim M.A., Lee J.S., Chang M.S., Park J.W., et al. FOXO1 suppression is a determinant of acquired lapatinib-resistance in HER2-positive gastric cancer cells through met upregulation. Cancer Res. Treat. 2018;50:239–254. doi: 10.4143/crt.2016.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Park J., Ko Y.S., Yoon J., Kim M.A., Park J.W., Kim W.H., Choi Y., Kim J.H., Cheon Y., Lee B.L. The forkhead transcription factor FOXO1 mediates cisplatin resistance in gastric cancer cells by activating phosphoinositide 3-kinase/Akt pathway. Gastric Cancer. 2014;17:423–430. doi: 10.1007/s10120-013-0314-2. [DOI] [PubMed] [Google Scholar]
- 158.Wagle M., Eiring A.M., Wongchenko M., Lu S., Guan Y., Wang Y., Lackner M., Amler L., Hampton G., Deininger M.W., et al. A role for FOXO1 in BCR-ABL1-independent tyrosine kinase inhibitor resistance in chronic myeloid leukemia. Leukemia. 2016;30:1493–1501. doi: 10.1038/leu.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu Z.H., Shun W.W., Hang J.B., Gao B.L., Hu J.A. Posttranslational modifications of FOXO1 regulate epidermal growth factor receptor tyrosine kinase inhibitor resistance for non-small cell lung cancer cells. Tumour Biol. 2015;36:5485–5495. doi: 10.1007/s13277-015-3215-7. [DOI] [PubMed] [Google Scholar]
- 160.Choudhury A.D., Schinzel A.C., Cotter M.B., Lis R.T., Labella K., Lock Y.J., Izzo F., Guney I., Bowden M., Li Y.Y., et al. Castration resistance in prostate cancer is mediated by the kinase NEK6. Cancer Res. 2017;77:753–765. doi: 10.1158/0008-5472.CAN-16-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dong J., Wang R., Ren G., Li X., Wang J., Sun Y., Liang J., Nie Y., Wu K., Feng B., et al. HMGA2-FOXL2 axis regulates metastases and epithelial-to-mesenchymal transition of chemoresistant gastric cancer. Clin. Cancer Res. 2017;23:3461–3473. doi: 10.1158/1078-0432.CCR-16-2180. [DOI] [PubMed] [Google Scholar]
- 162.Zhang H., Prado K., Zhang K.X., Peek E.M., Lee J., Wang X., Huang J., Li G., Pellegrini M., Chin A.I. Biased expression of the FOXP3Delta3 isoform in aggressive bladder cancer mediates differentiation and cisplatin chemotherapy resistance. Clin. Cancer Res. 2016;22:5349–5361. doi: 10.1158/1078-0432.CCR-15-2581. [DOI] [PubMed] [Google Scholar]
- 163.Li C., Sun L., Jiang R., Wang P., Xue H., Zhan Y., Gai X. Downregulation of FOXP3 inhibits cell proliferation and enhances chemosensitivity to cisplatin in human lung adenocarcinoma. Pathol. Res. Pract. 2017;213:1251–1256. doi: 10.1016/j.prp.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 164.Kamran M., Long Z.J., Xu D., Lv S.S., Liu B., Wang C.L., Xu J., Lam E.W., Liu Q. Aurora kinase A regulates Survivin stability through targeting FBXL7 in gastric cancer drug resistance and prognosis. Oncogenesis. 2017;6:e298. doi: 10.1038/oncsis.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hu Z., Zhu L., Gao J., Cai M., Tan M., Liu J., Lin B. Expression of FOXP1 in epithelial ovarian cancer (EOC) and its correlation with chemotherapy resistance and prognosis. Tumour Biol. 2015;36:7269–7275. doi: 10.1007/s13277-015-3383-5. [DOI] [PubMed] [Google Scholar]
- 166.Yamaguchi N., Nakayama Y., Yamaguchi N. Down-regulation of Forkhead box protein A1 (FOXA1) leads to cancer stem cell-like properties in tamoxifen-resistant breast cancer cells through induction of interleukin-6. J. Biol. Chem. 2017;292:8136–8148. doi: 10.1074/jbc.M116.763276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jones D., Wade M., Nakjang S., Chaytor L., Grey J., Robson C.N., Gaughan L. FOXA1 regulates androgen receptor variant activity in models of castrate-resistant prostate cancer. Oncotarget. 2015;6:29782–29794. doi: 10.18632/oncotarget.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cai J., Tian A.X., Wang Q.S., Kong P.Z., Du X., Li X.Q., Feng Y.M. FOXF2 suppresses the FOXC2-mediated epithelial-mesenchymal transition and multidrug resistance of basal-like breast cancer. Cancer Lett. 2015;367:129–137. doi: 10.1016/j.canlet.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 169.Nestal de Moraes G., Bella L., Zona S., Burton M.J., Lam E.W. Insights into a critical role of the FOXO3a-FOXM1 axis in DNA damage response and genotoxic drug resistance. Curr. Drug Targets. 2016;17:164–177. doi: 10.2174/1389450115666141122211549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Weng S.C., Kashida Y., Kulp S.K., Wang D., Brueggemeier R.W., Shapiro C.L., Chen C.S. Sensitizing estrogen receptor-negative breast cancer cells to tamoxifen with OSU-03012, a novel celecoxib-derived phosphoinositide-dependent protein kinase-1/Akt signaling inhibitor. Mol. Cancer Ther. 2008;7:800–808. doi: 10.1158/1535-7163.MCT-07-0434. [DOI] [PubMed] [Google Scholar]
- 171.Bullock M. FOXO factors and breast cancer: Outfoxing endocrine resistance. Endocr. Relat. Cancer. 2016;23:R113–R130. doi: 10.1530/ERC-15-0461. [DOI] [PubMed] [Google Scholar]
- 172.Hannenhalli S., Kaestner K.H. The evolution of FOX genes and their role in development and disease. Nat. Rev. Genet. 2009;10:233–240. doi: 10.1038/nrg2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Benayoun B.A., Caburet S., Veitia R.A. Forkhead transcription factors: Key players in health and disease. Trends Genet. 2011;27:224–232. doi: 10.1016/j.tig.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 174.Curtis C., Shah S.P., Chin S.-F., Turashvili G., Rueda O.M., Dunning M.J., Speed D., Lynch A.G., Samarajiwa S., Yuan Y., et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yu J., Deshmukh H., Payton J.E., Dunham C., Scheithauer B.W., Tihan T., Prayson R.A., Guha A., Bridge J.A., Ferner R.E., et al. Array-based comparative genomic hybridization identifies CDK4 and FOXM1 alterations as independent predictors of survival in malignant peripheral nerve sheath tumor. Clin. Cancer Res. 2011;17:1924–1934. doi: 10.1158/1078-0432.CCR-10-1551. [DOI] [PubMed] [Google Scholar]
- 176.Laoukili J., Stahl M., Medema R.H. FOXM1: At the crossroads of ageing and cancer. Biochim. Biophys. Acta. 2007;1775:92–102. doi: 10.1016/j.bbcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 177.Laoukili J., Kooistra M.R., Bras A., Kauw J., Kerkhoven R.M., Morrison A., Clevers H., Medema R.H. FOXM1 is required for execution of the mitotic programme and chromosome stability. Nat. Cell Biol. 2005;7:126–136. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 178.Sato Y., Katoh Y., Matsumoto M., Sato M., Ebina M., Itoh-Nakadai A., Funayama R., Nakayama K., Unno M., Igarashi K. Regulatory signatures of liver regeneration distilled by integrative analysis of mRNA, histone methylation, and proteomics. J. Biol. Chem. 2017;292:8019–8037. doi: 10.1074/jbc.M116.774547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang X., Arceci A., Bird K., Mills C.A., Choudhury R., Kernan J.L., Zhou C., Bae-Jump V., Bowers A., Emanuele M.J. VprBP/DCAF1 regulates the degradation and nonproteolytic activation of the cell cycle transcription factor FOXM1. Mol. Cell Biol. 2017 doi: 10.1128/MCB.00609-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Thompson S.L., Bakhoum S.F., Compton D.A. Mechanisms of chromosomal instability. Curr. Biol. 2010;20:R285–R295. doi: 10.1016/j.cub.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Weiler S.M.E., Pinna F., Wolf T., Lutz T., Geldiyev A., Sticht C., Knaub M., Thomann S., Bissinger M., Wan S., et al. Induction of chromosome instability by activation of yes-associated protein and forkhead box M1 in liver cancer. Gastroenterology. 2017;152:2037–2051. doi: 10.1053/j.gastro.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 182.Molinuevo R., Freije A., de Pedro I., Stoll S.W., Elder J.T., Gandarillas A. FOXM1 allows human keratinocytes to bypass the oncogene-induced differentiation checkpoint in response to gain of MYC or loss of p53. Oncogene. 2016;36:956. doi: 10.1038/onc.2016.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Katoh M., Katoh M. Human FOX gene family (Review) Int. J. Oncol. 2004;25:1495–1500. doi: 10.3892/ijo.25.5.1495. [DOI] [PubMed] [Google Scholar]
- 184.Dansen T.B., Burgering B.M. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 2008;18:421–429. doi: 10.1016/j.tcb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 185.Arai T., Kano F., Murata M. Translocation of forkhead box O1 to the nuclear periphery induces histone modifications that regulate transcriptional repression of PCK1 in HepG2 cells. Genes Cells. 2015;20:340–357. doi: 10.1111/gtc.12226. [DOI] [PubMed] [Google Scholar]
- 186.Daitoku H., Sakamaki J.-i., Fukamizu A. Regulation of FOXO transcription factors by acetylation and protein–protein interactions. Biochim. Biophys. Acta Mol. Cell Res. 2011;1813:1954–1960. doi: 10.1016/j.bbamcr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 187.Jeffery J.M., Kalimutho M., Johansson P., Cardenas D.G., Kumar R., Khanna K.K. FBXO31 protects against genomic instability by capping FOXM1 levels at the G2/M transition. Oncogene. 2017;36:1012–1022. doi: 10.1038/onc.2016.268. [DOI] [PubMed] [Google Scholar]
- 188.Liu J., Han L., Li B., Yang J., Huen M.S., Pan X., Tsao S.W., Cheung A.L. F-box only protein 31 (FBXO31) negatively regulates p38 mitogen-activated protein kinase (MAPK) signaling by mediating lysine 48-linked ubiquitination and degradation of mitogen-activated protein kinase kinase 6 (MKK6) J. Biol. Chem. 2014;289:21508–21518. doi: 10.1074/jbc.M114.560342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Malonia S.K., Dutta P., Santra M.K., Green M.R. F-box protein FBXO31 directs degradation of MDM2 to facilitate p53-mediated growth arrest following genotoxic stress. Proc. Natl. Acad. Sci. USA. 2015;112:8632–8637. doi: 10.1073/pnas.1510929112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bigarella C.L., Li J., Rimmele P., Liang R., Sobol R.W., Ghaffari S. FOXO3 Transcription Factor Is Essential for Protecting Hematopoietic Stem and Progenitor Cells from Oxidative DNA Damage. J. Biol. Chem. 2017;292:3005–3015. doi: 10.1074/jbc.M116.769455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Charitou P., Burgering B.M. Forkhead box(O) in control of reactive oxygen species and genomic stability to ensure healthy lifespan. Antioxid. Redox Signal. 2013;19:1400–1419. doi: 10.1089/ars.2012.4921. [DOI] [PubMed] [Google Scholar]
- 192.Ma J., Matkar S., He X., Hua X. FOXO family in regulating cancer and metabolism. Semin. Cancer Biol. 2018;50:32–41. doi: 10.1016/j.semcancer.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 193.Jamal-Hanjani M., Wilson G.A., McGranahan N., Birkbak N.J., Watkins T.B.K., Veeriah S., Shafi S., Johnson D.H., Mitter R., Rosenthal R., et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 2017;376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 194.Cuesta I., Zaret K.S., Santisteban P. The forkhead factor FOXE1 binds to the thyroperoxidase promoter during thyroid cell differentiation and modifies compacted chromatin structure. Mol. Cell Biol. 2007;27:7302–7314. doi: 10.1128/MCB.00758-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Santo E.E., Ebus M.E., Koster J., Schulte J.H., Lakeman A., van Sluis P., Vermeulen J., Gisselsson D., Ora I., Lindner S., et al. Oncogenic activation of FOXR1 by 11q23 intrachromosomal deletion-fusions in neuroblastoma. Oncogene. 2012;31:1571–1581. doi: 10.1038/onc.2011.344. [DOI] [PubMed] [Google Scholar]
- 196.Goatly A., Bacon C.M., Nakamura S., Ye H., Kim I., Brown P.J., Ruskone-Fourmestraux A., Cervera P., Streubel B., Banham A.H., et al. FOXP1 abnormalities in lymphoma: Translocation breakpoint mapping reveals insights into deregulated transcriptional control. Mod. Pathol. 2008;21:902–911. doi: 10.1038/modpathol.2008.74. [DOI] [PubMed] [Google Scholar]
- 197.Weng W., Okugawa Y., Toden S., Toiyama Y., Kusunoki M., Goel A. FOXM1 and FOXQ1 are promising prognostic biomarkers and novel targets of tumor-suppressive miR-342 in human colorectal cancer. Clin. Cancer Res. 2016;22:4947–4957. doi: 10.1158/1078-0432.CCR-16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sun Y., Yu X., Bai Q. miR-204 inhibits invasion and epithelial-mesenchymal transition by targeting FOXM1 in esophageal cancer. Int. J. Clin. Exp. Pathol. 2015;8:12775–12783. [PMC free article] [PubMed] [Google Scholar]
- 199.Song G.Q., Zhao Y. MicroRNA-211, a direct negative regulator of CDC25B expression, inhibits triple-negative breast cancer cells’ growth and migration. Tumour Biol. 2015;36:5001–5009. doi: 10.1007/s13277-015-3151-6. [DOI] [PubMed] [Google Scholar]
- 200.Yang X.W., Shen G.Z., Cao L.Q., Jiang X.F., Peng H.P., Shen G., Chen D., Xue P. MicroRNA-1269 promotes proliferation in human hepatocellular carcinoma via downregulation of FOXO1. BMC cancer. 2014;14:909. doi: 10.1186/1471-2407-14-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang J., Yang Y., Yang T., Yuan S., Wang R., Pan Z., Yang Y., Huang G., Gu F., Jiang B., et al. Double-negative feedback loop between microRNA-422a and forkhead box (FOX)G1/Q1/E1 regulates hepatocellular carcinoma tumor growth and metastasis. Hepatology. 2015;61:561–573. doi: 10.1002/hep.27491. [DOI] [PubMed] [Google Scholar]
- 202.Kumar S., Batra A., Kanthaje S., Ghosh S., Chakraborti A. Crosstalk between microRNA-122 and FOX family genes in HepG2 cells. Exp. Biol. Med. 2017;242:436–440. doi: 10.1177/1535370216681548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lulla A.R., Slifker M.J., Zhou Y., Lev A., Einarson M.B., Dicker D.T., El-Deiry W.S. miR-6883 family miRNAs target CDK4/6 to induce G1 phase cell-cycle arrest in colon cancer cells. Cancer Res. 2017;77:6902–6913. doi: 10.1158/0008-5472.CAN-17-1767. [DOI] [PubMed] [Google Scholar]
- 204.Huang C., Du J., Xie K. FOXM1 and its oncogenic signaling in pancreatic cancer pathogenesis. Biochim. Biophys. Acta. 2014;1845:104–116. doi: 10.1016/j.bbcan.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Langlet F., Haeusler R.A., Lindén D., Ericson E., Norris T., Johansson A., Cook J.R., Aizawa K., Wang L., Buettner C., Accili D. Selective inhibition of FOXO1 activator/repressor balance modulates hepatic glucose handling. Cell. 2017;171:824–835. doi: 10.1016/j.cell.2017.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cautain B., Castillo F., Musso L., Ferreira B.I., de Pedro N., Rodriguez Quesada L., Machado S., Vicente F., Dallavalle S., Link W. Discovery of a novel, isothiazolonaphthoquinone-based small molecule activator of FOXO nuclear-cytoplasmic shuttling. PLoS ONE. 2016;11:e0167491. doi: 10.1371/journal.pone.0167491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kim I.M., Ackerson T., Ramakrishna S., Tretiakova M., Wang I.C., Kalin T.V., Major M.L., Gusarova G.A., Yoder H.M., Costa R.H., et al. The forkhead box M1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006;66:2153–2161. doi: 10.1158/0008-5472.CAN-05-3003. [DOI] [PubMed] [Google Scholar]
- 208.Millour J., Constantinidou D., Stavropoulou A.V., Wilson M.S., Myatt S.S., Kwok J.M., Sivanandan K., Coombes R.C., Medema R.H., Hartman J., et al. FOXM1 is a transcriptional target of ERalpha and has a critical role in breast cancer endocrine sensitivity and resistance. Oncogene. 2010;29:2983–2995. doi: 10.1038/onc.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Halasi M., Gartel A.L. Suppression of FOXM1 sensitizes human cancer cells to cell death induced by DNA-damage. PLoS ONE. 2012;7:e31761. doi: 10.1371/journal.pone.0031761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nakanishi C., Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 211.Bhat U.G., Halasi M., Gartel A.L. FOXM1 is a general target for proteasome inhibitors. PLoS ONE. 2009;4:e6593. doi: 10.1371/journal.pone.0006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gartel A.L. A new target for proteasome inhibitors: FOXM1. Expert. Opin. Investig Drugs. 2010;19:235–242. doi: 10.1517/13543780903563364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jagani Z., Song K., Kutok J.L., Dewar M.R., Melet A., Santos T., Grassian A., Ghaffari S., Wu C., Yeckes-Rodin H., et al. Proteasome inhibition causes regression of leukemia and abrogates BCR-ABL-induced evasion of apoptosis in part through regulation of forkhead tumor suppressors. Cancer Res. 2009;69:6546–6555. doi: 10.1158/0008-5472.CAN-09-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Martens L., Vizcaíno J.A. A golden age for working with public proteomics data. Trends Biochem. Sci. 2017;42:333–341. doi: 10.1016/j.tibs.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Long N.P., Jung K.H., Yoon S.J., Anh N.H., Nghi T.D., Kang Y.P., Yan H.H., Min J.E., Hong S.S., Kwon S.W. Systematic assessment of cervical cancer initiation and progression uncovers genetic panels for deep learning-based early diagnosis and proposes novel diagnostic and prognostic biomarkers. Oncotarget. 2017;8:109436–109456. doi: 10.18632/oncotarget.22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Long N.P., Yoon S.J., Anh N.H., Nghi T.D., Lim D.K., Hong Y.J., Hong S.S., Kwon S.W. A systematic review on metabolomics-based diagnostic biomarker discovery and validation in pancreatic cancer. Metabolomics. 2018;14:109. doi: 10.1007/s11306-018-1404-2. [DOI] [PubMed] [Google Scholar]
- 217.Lu Z.R., Qiao P. Drug delivery in cancer therapy, quo vadis? Mol. Pharm. 2018 doi: 10.1021/acs.molpharmaceut.8b00860. [DOI] [PubMed] [Google Scholar]
- 218.Rationalizing combination therapies. Nat. Med. 2017;23:1113. doi: 10.1038/nm.4426. [DOI] [PubMed] [Google Scholar]
- 219.Bost F., Decoux-Poullot A.G., Tanti J.F., Clavel S. Energy disruptors: Rising stars in anticancer therapy? Oncogenesis. 2016;5:e188. doi: 10.1038/oncsis.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]