Abstract
Background
Stomach preparation by ischaemic conditioning prior to oesophageal resection represents a potential method of reducing the risk of anastomotic complications. This study compares the results of the anastomotic complications of cervical anastomosis after oesophagectomy with a short interval after ischaemic conditioning (group S) and a long interval (group L).
Methods
Subjects undergoing oesophagectomy for carcinoma after ischaemic conditioning were divided into two groups. Group S had a median interval between ischaemic conditioning and resection of 20 days, while for group L the median interval was 49 days. Anastomotic leak and anastomotic stenosis in relation to the interval between ischaemic conditioning and actual resection were followed.
Results
After ischaemic conditioning, 33 subjects in total underwent surgery for carcinoma; 19 subjects in group S and 14 subjects in group L. Anastomotic leak incidence was comparable in both groups. Anastomotic stenosis occurred in 21% of cases in group S and 7% of cases in group L (not statistically significant).
Conclusions
A long interval between ischaemic conditioning and oesophagectomy does not adversely affect the postoperative complications. A lower incidence of anastomosis stenoses was found in subjects with a longer interval, however, given the size of our sample, the statistical significance was not demonstrated. Both groups seem comparable in surgical procedure course and postoperative complications.
Keywords: Anastomotic leak, Anastomotic stenosis, Oesophageal carcinoma, Minimally invasive oesophagectomy, Stomach ischaemic conditioning
Introduction
The incidence of oesophageal adenocarcinoma has grown significantly. Surgical resection represents one of basic therapeutic modalities, with the exception of early-stage carcinoma.1 Oesophagogastroduodenoscopy and endosonography play an important role in local staging. Chest and abdominal cavity computed tomography (CT) is important for evaluating lymphadenopathy and ruling out metastases. With a positive local finding, resection is the only possible curative procedure. It is an extensive procedure with a high incidence of postoperative complications. A significant portion of the deaths after oesophageal resection is connected to anastomotic leak – 37% according to Briel et al.2 Half of the subjects with a healed leak also suffer from anastomotic stenosis in the future, adversely affecting their quality of life.2 Anastomotic healing after oesophagus resection is adversely affected by concurrent stomach mobilisation with removal of a part of the vascular supply, stomach tubulisation and its mechanical stretching during transposition via mediastinum. The anastomotic site in the stomach fundus area is thereby supplied from the right gastroepiploic artery only by intramural branches and capillaries.3
The possibility of eliminating the current negative effect of these factors and ensuring a better blood supply to the proximal part of the stomach in place of the future anastomosis by ischaemic conditioning of the stomach has been discussed. In 1996, Akiyama et al.4 published work on the preparation of the stomach by radiological embolisation. The surgical approach by artery ligation was described one year later in experimental work by Urschel et al.5 In clinical practice, the positive effect of ischaemic conditioning on the expected reduction in oesophagogastroanastomotic leak incidence has not been clearly demonstrated.6 A shorter interval between ischaemic conditioning and oesophageal resection, 5–12 days, has been preferred.4,6,7 An experimental, animal model study showed that a seven-day interval after ischaemic conditioning does not yet cause the stomach fundus neovascularisation, which occurs after several weeks.8 Indeed, surgical procedure in subjects with a good health status cannot be unreasonably delayed. At the time of diagnosis, some subjects are not fit for early resection for nutritional reasons, or they are indicated for neoadjuvant oncological treatment. These selected subjects may benefit from a prolonged interval between ischaemic conditioning and resection procedure.
One objective of this study was to compare two subjects groups having a different time interval between ischaemic conditioning and minimally invasive resection for oesophageal carcinoma with cervical anastomosis. All subjects underwent staging laparoscopy to rule out tumour generalisation and to interrupt the left gastric artery. A secondary objective was to evaluate whether incorporating neoadjuvant chemotherapy between ischaemic conditioning and resection adversely affects resection procedure outcomes. We found no literature resources dedicated specifically to clinical outcomes of a long interval between ischaemic conditioning of the stomach and oesophageal resection.
Methods
All subjects with an oesophageal tumour were indicated for standard staging examinations: endoscopy with histological sampling, endosonography, CT and in indicated cases positron-emission tomography (PET) or PET-CT. The staging laparoscopy also searched for metastases in the abdominal cavity. At the same time, the left gastric artery was interrupted (Fig 1) and nutrition jejunostomy was established in subjects with eating disorders. Some subjects were only referred to our site after neoadjuvant treatment was finished. In these cases, staging laparoscopy and ischaemic conditioning were performed as a part of restaging. Resection followed at an interval shorter than 25 days after ischaemic conditioning, the same as in subjects without neoadjuvant treatment.
Figure 1.
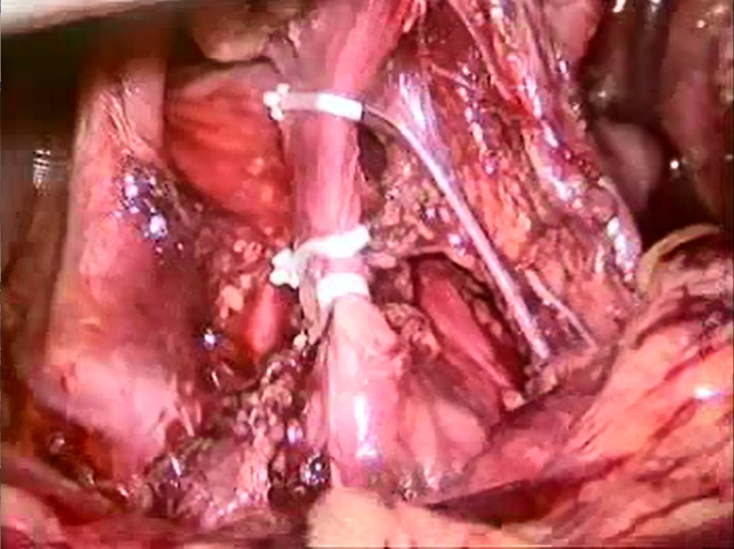
Ligation of the left gastric artery and the position of the clips
In the group in which neoadjuvant treatment was administered after ischaemic conditioning, status was evaluated after restaging examinations. Subjects with generalisation during neoadjuvant treatment and subjects with locally advanced tumour without response to neoadjuvant treatment were not indicated for resection. A longer interval after ischaemic conditioning was also necessary in subjects with malnutrition at the time of diagnosis. In all such cases, resection was performed more than 35 days after ischaemic conditioning.
Minimally invasive oesophagectomy (MIO) was carried out first in the prone position without selective ventilation by right side thoracoscopy, releasing the thoracic oesophagus with lymphadenectomy. A change in position was follow by laparoscopic division of the short gastric arteries, releasing the stomach fundus, gastro-oesophageal junction and the abdominal oesophagus. From the short transverse laparotomy, releasing the remaining part of the stomach was completed with investigation of the arcades around greater curvature, Kocher manoeuvre, lymphadenectomy around the coeliac trunk, pyloroplasty and stomach tubulisation. After preparation of the cervical part of oesophagus and finishing the resection phase, the tubulised stomach was transpositioned by mediastinum and cervical side-to-side anastomosis was performed using a 60-mm linear stapler.
Outcomes of MIO, postoperative complications, incidence of anastomotic leak and stenoses with respect to the length of the interval between the ischaemic conditioning and MIO performance were followed-up. The shorter interval group underwent surgery in less than 25 days (median 20 days); the group with a longer interval had the surgery performed after more than 35 days (median 49 days). The results were statistically evaluated using Statistica 10.0 software. Statistical tests were applied to discover whether the number of anastomotic leaks, surgical revisions and anastomotic stenoses depends on the interval after ischaemic conditioning. This correlation was tested using Fisher’s exact test based on contingency tables. Results with a level of significance P < 0.05 were considered statistically significant. The same test was also used to determine the correlation of the number of anastomotic leaks, surgical revisions and postoperative stenoses with the administration of chemotherapy. The non-parametric Mann-Whitney test was used to compare differences in age, body mass index (BMI) and nutritional parameters in between subjects with shorter and longer intervals after ischaemic conditioning.
Results
From 2012 to June 2014, staging laparoscopy and ischaemic conditioning of the stomach procedures were performed in 45 patients in total. In three patients (6.6%), a previously undiagnosed tumour generalisation was revealed. In these cases, the resection procedure was contraindicated and systematic chemotherapy was initiated.
Of 42 patients without tumour generalisation during staging laparoscopy, a locally non-resectable state was found in 4, lymphadenopathy progression was diagnosed in another 3 and the resection procedure was contraindicated. Two patients were not fit for the resection procedure because of their generally poor health status.
In 2012–14, 33 patients were indicated for MIO after ischaemic conditioning. There was at least one year of postoperative follow up. The short interval (group S) after ischaemic conditioning was used in 19 cases and the long interval (group L) in 14 cases. The data set characteristics are displayed in Table 1.
Table 1.
The data set characteristics.
| Parameter | Value |
| Age (years) | 61.24 ± 8.74 |
| Sex: | |
| Male (n) | 30 |
| Female (n) | 3 |
| Body mass index (kg/m2) | 26.54 ± 5.23 |
| Operation time (minutes) | 315.03 ± 46.39 |
| Anastomotic leaks n (%) | 10 (30.3) |
| Reoperations n (%) | 4 (12.12) |
| Anastomotic stenosis n (%) | 5 (15.15) |
| Patients with neoadjuvant therapy n (%) | 17 (51.5) |
| Patients without neoadjuvant therapy n (%) | 16 (48.5) |
| 30-day postoperative mortality n (%) | 1 (3) |
An anastomotic leak after surgery occurred in 10 patients. With the exception of two cases, its course was uncomplicated, with no signs of sepsis, and output disappeared within one week of local wound treatment. The remaining two cases required surgical revision for leakage of the pathological secretion into the right hemithorax. One patient died and the other needed long-term artificial ventilation with a necessary tracheostomy for bilateral bronchopneumonia. Reoperation was necessary in four cases: two anastomotic leaks, one right side rethoracoscopy with successful ligation of thoracic duct for chylous secretion from a chest drain after resection and one laparotomy resuture. Anastomotic stenoses were resolved by repeated endoscopic dilatation in all five cases. No difference in incidence of postoperative complications in subjects with neoadjuvant treatment and without neoadjuvant treatment was found, regardless of the interval after ischaemic conditioning (Table 2).
Table 2.
Differences in leak incidence, number of reoperations and incidence of stenoses between subjects with neoadjuvant treatment and without neoadjuvant treatment.
| Parameter | Neoadjuvant treatment | P-value | |
| Yes | No | ||
| Patients n (%) | 17 (51.50) | 16 (48.50) | |
| Anastomotic leak (n) | 4 | 6 | 0.383 |
| Reoperations (n) | 1 | 3 | 0.258 |
| Anastomotic stenosis (n) | 2 | 3 | 0.576 |
Absolute values (%); P, significance level (Fisher’s exact test).
A comparison of subject groups divided by the length of the interval between ischaemic conditioning and MIO is displayed in Table 3. The median interval after ischaemic conditioning was 20 days in the case of short intervals and 49 days for long intervals. The histopathologic results are summarised in Table 4.
Table 3.
Characteristics of group S (short interval after ischaemic conditioning) and group L (long interval).
| Parameter | Group S | Group L |
| Interval between MIO and IC (median days) | 20 | 49 |
| Age (years) | 61.37 ± 8.17 | 61.07 ± 9.76 |
| Body mass index (kg/m2) | 27.38 ± 5.87 | 25.47 ± 4.23 |
| Anastomotic leak n (%) | 6 (31.58) | 4 (28.57) |
| Reoperations n (%) | 2 (10.53) | 2 (14.29) |
| Anastomotic stenosis n (%) | 4 (21.05) | 1 (7.14) |
| Neoadjuvant therapy n (%) | 7 (36.84) | 10 (71.43) |
IC, ischaemic conditioning; MIO, minimally invasive oesophagectomy.
Table 4.
Pathological staging of carcinomas in group S (short interval after ischaemic conditioning) and group L (long interval).
| Parameter | Group S | Group L | All patients |
| Patients (n) | 19 | 14 | 33 |
| T stage: | |||
| 1 n (%) | 10 (52.6) | 2 (14.3) | 12 (36.4) |
| 2 n (%) | 4 (21.1) | 5 (35.7) | 9 (27.2) |
| 3 n (%) | 5 (26.3) | 7 (50.0) | 12 (36.4) |
| N stage: | |||
| 0 n (%) | 11 (57.9) | 7 (50.0) | 18 (54.5) |
| 1 n (%) | 6 (31.6) | 4 (28.6) | 10 (30.3) |
| 2 n (%) | 2 (10.5) | 3 (21.4) | 5 (15.2) |
| Grade: | |||
| Well differentiated n (%) | 5 (26.3) | 6 (42.9) | 11 (33.3) |
| Moderately differentiated n (%) | 10 (52.6) | 7 (50.0) | 17 (51.5) |
| Poorly/undifferentiated n (%) | 4 (21.1) | 1 (7.1) | 5 (15.2) |
No difference between both groups in age, BMI and nutritional parameters at the time of surgery were found. Table 5 shows the incidence of leak and anastomotic stenoses. Leak incidence and the necessity of reoperations are comparable in both groups. The number of stenoses is higher in group S (21%) compared with just 7% in group L, but the statistical significance could not be confirmed due to the limited size of the data set.
Table 5.
Differences in incidence of leak, number of revisions and incidence of stenosis in group S (short interval after ischaemic conditioning) and group L (long interval).
| Parameter | Group S | Group L | P-value |
| Patients (n) | 19 | 14 | |
| Anastomotic leak n (%) | 6 (31.5) | 4 (28.5) | 0.850 |
| Reoperations n (%) | 2 (10.5) | 2 (14.3) | 0.740 |
| Anastomotic stenosis n (%) | 4 (21) | 1 (7.1) | 0.271 |
| Death n (%) | 1 (5.2) | 0 | not evaluated |
Absolute values (%); P, significance level (Fisher’s exact test).
For group L, preoperative chemotherapy was administered in six patients and a surgical procedure was performed in eight patients without chemotherapy who needed nutrition preparation. Statistical tests were performed to find out whether chemotherapy administered after ischaemic conditioning influences the resection procedure outcome: no statistically significant difference in complication incidence was found (Table 6). A summary of one- and three-year survival rates after resection in both groups can be seen in Table 7.
Table 6.
Differences in leak incidence, number of reoperations and incidence of stenoses between subjects with chemotherapy after ischaemic conditioning and without chemotherapy after ischaemic conditioning in Group L.
| Parameter | Chemotherapy after ischaemic conditioning | P-value | |
| With | Without | ||
| Patients n (%) | 6 (42.86) | 8 (57.14) | |
| Anastomotic leak (n) | 1 | 3 | 0.393 |
| Reoperations (n) | 1 | 1 | 0.825 |
| Anastomotic stenosis (n) | 1 | 0 | 0.231 |
Absolute values (%); P, significance level (Fisher’s exact test).
Table 7.
One- and three-year survival rates in group S (short interval after ischaemic conditioning) and group L (long interval).
| Survival | Group S (n = 19) | Group L (n = 14) | All patients (n = 33) |
| 1 year (%) | 79 | 82 | 81 |
| 3 years (%) | 47 | 57 | 51 |
Discussion
In recent years, mortality after oesophagal resection has decreased significantly. According to some authors, mortality is below 2% and morbidity is also decreasing.9 It is not easily demonstrated whether this improvement is because of better preoperative preparation, improved anaesthesia or minimally invasive approaches.2 Comparison of the benefits of minimally invasive techniques is limited by their variability.
A benefit from staging laparoscopy is also reported for other malignancies of the digestive tract, such as pancreatic cancer. Within our data set, we found previously undetected generalised tumours in 3 of 45 patients. Laparoscopy also makes it possible to concurrently perform ischaemic conditioning. Radiological methods of ischaemic conditioning were described by Akiyama et al.4 with the interval to resection 12 days. The procedure is performed without anaesthesia, but there is a risk of embolisation material dislocation, which appears more common than the risk of complications after laparoscopic ligation of the left gastric artery.4 Possible revascularisation after radiological embolisation has also been discussed, as against surgical interruption of arteries, which makes revascularisation impossible.10
Several types of ischaemic conditioning surgical procedure have been published: interruption of the arcade around the stomach lesser curvature by vascular stapler or concurrent division of the short gastric arteries with possible complete stomach mobilisation.7 In our data set, we preferred the easiest procedure that would lead to the smallest postoperative changes complicating the resection phase.11 We have not experienced any problems caused by staging laparoscopy and performance of ischaemic conditioning. We prefer to not dissect the left gastric artery origin directly, but we rather insert the clips closer to stomach (Fig 1) so as to not complicate lymphadenectomy around branches of the coeliac trunk. This also eliminates concerns about the long interval between performance of the ischaemic conditioning and resection phases, which some authors attempt to shorten due to concerns about the formation of undesirable adhesions.7
Theoretical works show a better blood supply to the end of the stomach tubulisation in the area of the future anastomosis after performing ischaemic conditioning using various methods. Lower oxygen saturation of the stomach mucosa during complete stomach mobilisation returned to normal within four to five days.12 Laser Doppler flowmetry showed no difference in the perfusion of the upper part of the stomach during resection carried out two weeks from ischaemic conditioning compared with a group with no conditioning in a randomised study with 22 patients in each group.13
The reported interval between ischaemic conditioning and resection is usually less than two weeks.4,6 A very short interval was recommended by Hölscher. Four to six days after a complete laparoscopic release of the stomach, oesophagal resection and anastomosis are performed only by right-sided thoracotomy. Transposition of the stomach into the chest is done without the possibility of directly checking the abdomen status, and therefore must be carried out before significant adhesions have formed.7 This study refers to the documented normalisation of mucous oxygen saturation four to five days after stomach release.12
After complete mobilisation, blood to the proximal part of stomach is not supplied from the main arcade around the greater curvature, but by intramural branches. The focus has therefore been on observing morphological changes to these vessels after ischaemic conditioning. Both the new formation of vessels and their subsequent dilatation were demonstrated. According to Lamas, neovascularisation formation in the area of the stomach fundus peaks 15 days after ischaemic conditioning.14 Mittermaier et al.15 published a study observing stomach vascularisation by fluorescence microscopy. After surgical ligation of the left gastric artery, so-called functional capillary density in the area of the stomach fundus was increasing within eight weeks of the performed procedure. During this period a gradual dilatation of the vascular arcade in the wall of the greater curvature was noted.15 Vasodilatation and angiogenesis in the proximal stomach were also demonstrated by other experimental studies.8 Reavis et al.16 carried out oesophagogastric anastomosis on animal models at various time intervals after ischaemic conditioning with subsequent histological examination. Other than neoangiogenesis and vasodilatation, the smallest collagen deposits were also found during the longest time interval from ischaemic conditioning, thus also the smallest theoretical predisposition for stenosis formation.16 In our data set, we treated anastomosis stenosis in 21% of the patients from group S and only 7% of those from group L but, because of the size of the dataset, the statistical significance of the difference was not confirmed.
Theoretical works thus tend to prefer a longer interval from ischaemic conditioning, which is interesting in the context of a clinical work from Oezcelik et al.17 In 37 patients with 554 oesophagectomies, the vascularisation quality of the tubulised stomach was peroperatively assessed as not suitable for the performance of anastomosis, so anastomosis in the second phase with the creation of temporary cervical oesophagostomy and fixation of the tubulised stomach in the subcutaneous tissue of the cervix was preferred. All pateints survived the procedure and, in 35, oesophagogastric anastomosis was performed after 89–110 days. No case of a leak was reported and stenosis developed in only three patients (9%). This represents excellent results in pateints with primary macroscopic insufficient vascularisation of the stomach conduit.17 Other studies have explored the possibilities of pharmaceutical support of neovascularisation and vasodilatation of stomach conduit during resection of oesophagus; so far with no clear impact on clinical practice.18,19
Neither preoperative chemotherapy nor chemoradiotherapy has any effect on the incidence of postoperative complications.20 In one group of patients in our cohort, chemotherapy was administered between conditioning and stomach resection, with the same results as in patients without chemotherapy. We are aware that this is not a prospective randomised study. It would be difficult to justify a deliberately prolonged interval between ischaemic conditioning and resection of a malignant tumour in patients in overall good health. No statistically significant differences in the incidence of postoperative complications were determined between the groups. Theoretical works on the formation of neovascularisation support the importance of a long interval after conditioning. The same results for both shorter and longer intervals in our data set show that it is possible to perform standard staging laparoscopy with ischaemic conditioning in all patients scheduled for oesophagectomy, regardless of whether the first-indicated treatment modality is neoadjuvant therapy or resection. One of the staging laparoscopy benefits is the chance to rule out generalisation of tumours undetectable with other methods and the performance of nutrition jejunostomy.
Conclusion
Performing ischaemic conditioning of the stomach by laparoscopic ligation of the left gastric artery together with the formation of nutrition jejunostomy is a simple procedure. In 3 of 45 patients, malignancy generalisation was discovered before initiation of oncological treatment. Thus, the general management strategy for these patients, who up to that point were considered candidates for a radical resection procedure, was changed. A long interval between ischaemic conditioning and resection procedure does not have an adverse effect on the surgical procedure course or postoperative complications in subjects who have undergone neoadjuvant chemotherapy. We found a lower incidence of anastomosis stenoses in subjects with a long interval between ischaemic conditioning and MIO, but its statistical significance was not demonstrated in the current size of dataset. We therefore perform staging laparoscopy with interruption of the left gastric artery as standard care in all patients scheduled for resection procedure for oesophageal carcinoma. Other studies on a larger set of subjects could refine recommendations regarding the clinical significance of ischaemic conditioning of the stomach, because the interval between its performance and resection can be a major factor influencing neovascularisation, and therefore can have an effect on lowering the rate of complications resulting from anastomosis healing defects. According to our results, both groups (short and long interval) seem comparable and neither worsened the surgical procedure course or postoperative complications more than the other.
References
- 1.Takeo Y, Yoshida T, Shigemitu T et al. Endoscopic mucosal resection for early esophageal cancer and esophageal dysplasia. Hepatogastroenterology 2001; (38): 453–457. [PubMed] [Google Scholar]
- 2.Briel JW, Tamhankar AP, Hagen JA et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg 2004; (4): 536–541. [DOI] [PubMed] [Google Scholar]
- 3.Liebermann-Meffert DM, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg 1992; (6): 1,110–1,115. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama S, Ito S, Segikuchi H et al. Preoperative embolisation of gastric arteries for esophageal cancer. Surgery 1996; (3): 542–546. [DOI] [PubMed] [Google Scholar]
- 5.Urschel JD, Takita H, Antkowiak JG. The effect of ischemic conditioning on gastric wound healing in the rat: implications for esophageal replacement with stomach. J Cardiovasc Surg 1997; (5): 535–538. [PubMed] [Google Scholar]
- 6.Nguyen NT, Nguyen XM, Reavis KM et al. Minimally invasive esophagectomy with and without gastric ischemic conditioning. Surg Endosc 2012; (6): 1,637–1,641. [DOI] [PubMed] [Google Scholar]
- 7.Hölscher AH, Schneider PM, Gutschow Ch, Schröder W. Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann Surg 2007; (2): 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry KA, Banarjee A, Liu J et al. Gastric ischemic conditioning increases neovascularization and reduces inflammation and fibrosis during gastroesophageal anastomotic healing. Surg Endosc 2013; (3): 753–760. [DOI] [PubMed] [Google Scholar]
- 9.Luketich JD, Pennathur A, Awais O et al. Outcomes after minimally invasive esophagectomy: review of over 1.000 patients. Ann Surg 2012; (1): 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diana M, Hübner M, Vuilleumier H et al. Redistribution of gastric blood flow by embolisation of gastric arteries before esophagectomy. Ann Thorac Surg 2011; (5): 1,546–1,551. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen NT, Longoria M, Sabio A et al. Preoperative laparoscopic ligation of the left gastric vessels in preparation for esophagectomy. Ann Thorac Surg 2006; (6): 2,318–2,320. [DOI] [PubMed] [Google Scholar]
- 12.Bludau M, Hölscher AH, Vallböhmer D et al. Ischemic conditioning of the gastric conduit prior to esophagectomy improves mucosal oxygen saturation. Ann Thorac Surg 2010; (4): 1,121–1,126. [DOI] [PubMed] [Google Scholar]
- 13.Veeramootoo D, Shore AC, Wajed SA. Randomized Controlled trial of laparoscopic gastric ischemic conditioning prior to minimally invasive esophagectomy, the LOGIC trial. Surg Endosc 2012; (7): 1,822–1,829. [DOI] [PubMed] [Google Scholar]
- 14.Lamas S, Azuara D, de Oca J et al. Time course of necrosis/apoptosis and neovascularization during experimental gastric conditioning. Dis Esophagus 2008; (4): 370–376. [DOI] [PubMed] [Google Scholar]
- 15.Mittermair Ch, Klaus A, Scheidl S. Functional capillary density in ischemic conditioning: implications for esophageal resection with the gastric conduit. Am J Surg 2008; (1): 88–92. [DOI] [PubMed] [Google Scholar]
- 16.Reavis KM, Chang EY, Hunter JG, Jobe BA. Utilization of the delay phenomenon improves blood flow and reduces collagen deposition in esophagogastric anastomoses. Ann Surg 2005; (5): 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oezcelik A, Banki F, DeMeester SR et al. Delayed esophagogastrostomy: a safe strategy for management of patients with ischemic gastric conduit at the time of esophagectomy. J Am Coll Surg 2009; (6): 1,030–1,034. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki Y, Edagawa M, Maeda M et al. Beneficial effect of prostaglandin E1 od blood flow to the gastric tube after esophagectomy. Ann Thorac Surg 1999; (4): 908–910. [DOI] [PubMed] [Google Scholar]
- 19.Enestvedt CK, Hosack L, Hoppo T et al. Recombinant vascular endothelial growth factor (165) gene therapy improves anastomotic healing in an animal model of ischemic esophagogastrostomy. Dis Esophagus 2012; (5): 456–464. [DOI] [PubMed] [Google Scholar]
- 20.van Hagen P, Hulshof MC, van Lanschot JJ et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; (22): 2,074–2,084. [DOI] [PubMed] [Google Scholar]


