Abstract
Introduction
Several stoma related complications can occur following ileostomy or colostomy formation. The reported incidence of these conditions varies widely in the literature. A systematic review of randomised controlled trials reporting the incidence of stoma related complications in adults was performed to provide the most comprehensive summary of existing data.
Methods
PubMed, CINAHL® (Cumulative Index to Nursing and Allied Health Literature) and the Cochrane Library were searched for trials assessing the incidence of complications in adults undergoing conventional stoma formation. Data were extracted by two independent reviewers and entered into SPSS® for statistical analysis. The Cochrane Collaboration tool for assessing risk of bias was used to critically appraise each study. Cochran’s Q statistic and the I2 statistic were used to measure the level of heterogeneity between studies.
Results
Overall, 18 trials were included, involving 1,009 patients. The incidence of stoma related complications ranged from 2.9% to 81.1%. Peristomal skin complications and parastomal hernia were the most common complications. End colostomy had the highest incidence of morbidity, followed by loop colostomy and loop ileostomy. There were no trials involving patients with end ileostomy. There was a high level of detection bias and heterogeneity between studies.
Conclusions
This systematic review has summarised the best available evidence concerning the incidence of stoma related morbidity. The high level of heterogeneity between studies has limited the accuracy with which the true incidence of each stoma related complication can be reported. Large, multicentre trials investigating homogenous participant populations are therefore required.
Keywords: Stoma, Ileostomy, Colostomy, Complication, Systematic review
The most common indications for stoma formation in the UK are colorectal cancer, diverticular disease and inflammatory bowel disease. There are a variety of stoma related complications that can occur in patients with an ileostomy or colostomy, associated with both operative and patient related factors.1 Early complications, such as high output stoma, peristomal irritation, stoma infection, ischaemia and retraction, typically occur within one month of surgery. Parastomal hernia, stoma prolapse and stenosis are examples of late complications, which tend to occur after the first postoperative month. These conditions may impact on quality of life, require further surgery or contribute to mortality.2,3 The burden of stoma related morbidity for patients is therefore significant. There are also financial implications for patients and healthcare services.4,5
Recent studies have focused on parastomal hernia prophylaxis with mesh insertion at the time of stoma formation.6–9 To date, there has been no comprehensive and summative review of the incidence of all stoma related complications in patients undergoing conventional ileostomy and colostomy formation. The reported incidence of stoma related complications is variable, with two large cohort studies demonstrating an incidence of 34–56%.10,11 The aim of this study was to perform a systematic review of randomised controlled trials (RCTs) in order to most accurately identify the rate of ileostomy and colostomy related complications.
Methods
The systematic review was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.12,13 PubMed, CINAHL® (Cumulative Index to Nursing and Allied Health Literature) and the Cochrane Library were searched to identify eligible articles. Boolean operators were used to combine the following Medical Subject Headings: “stoma”, “ostomy”, “ileostomy”, “colostomy”, “complication”, “incidence”, “management”, “hernia”, “prolapse”, “high output”, “necrosis” and “stenosis”.
Owing to advancements in surgical practice in recent decades, the database search was limited to identify only studies published after 1 January 1990. Only English language articles were sought as limited resources precluded accurate translation. The final search was carried out on 5 November 2015. A secondary hand search was performed on the bibliographies of all identified RCTs and systematic reviews that reported the incidence of stoma related complications as a primary outcome.
Eligibility criteria
Only RCTs involving patients over 16 years of age were included. There must have been at least 30 participants in each trial, with at least one study group undergoing conventional ileostomy or colostomy formation. Conventional stoma formation was defined as an operation to create a new stoma with no prophylactic intervention (eg mesh reinforcement). Patients must have been followed up to assess for stoma related complications, with the incidence of individual complications reported. Studies were excluded if they reported complications following stoma reversal without reporting those that occurred before closure.
Screening and eligibility assessment
Duplicate records were identified and removed using EndNote® (Clarivate Analytics, Philadelphia, PA, US) and by a secondary manual review. The remaining abstracts were screened to exclude ineligible studies. Where there was doubt about whether to exclude a study, the full text of the article was retrieved for assessment. The full-text articles of all remaining abstracts were retrieved and underwent an eligibility assessment by two reviewers (TM and ABH) independently. Disagreements were generally resolved through discussion. In cases where consensus was not reached, a third reviewer (MJL) made the final decision.
Data extraction
A standardised data extraction form was created using Excel® (Microsoft, Redmond, WA, US). This was pilot tested on five included studies and any relevant variables not considered previously were added. The form included fields for the number of intention-to-treat patients in each randomisation group, patient demographics, indication for surgery, type of stoma formed, incidence of stoma related complications and incidence of mortality.
The reported incidence of stoma stenosis, retraction, prolapse, ischaemia, fistula, dehiscence, infection, varices, parastomal hernia, high output stoma, peristomal irritation and bowel obstruction caused by stoma was recorded individually. Data concerning the incidence of other stoma specific complications were classified as ‘other complications’.
If relevant data were unclearly presented or absent, the trial authors were contacted via email. Data extraction was carried out independently by TM and MJL, and disagreements were resolved through discussion. ABH was consulted if consensus could not be achieved.
Assessment of risk of bias
The Cochrane Collaboration tool for assessing risk of bias in RCTs was used to critically appraise each study.14 The assessment accounted for the risk of selection bias, performance bias, detection bias, attrition bias, reporting bias and other sources of bias. The methods used to generate the random sequence, conceal allocations, blind patients and personnel, blind outcome assessors, ensure that outcome data were complete, avoid selective reporting and minimise other sources of bias were evaluated for each trial. A judgement of low risk, high risk or unclear risk of bias was made for each factor. RevMan version 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark) was used to produce a risk of bias graph and summary table.
Outcome measures
The primary outcome measures were incidence of all stoma related complications and incidence of each specific stoma related complication by stoma type (loop ileostomy, end ileostomy, loop colostomy and end colostomy). Where possible, the incidence was calculated from the intention-to-treat population. Secondary outcome measures were the incidence of 30-day, 1-year and 3-year mortality.
Data analysis
Statistical analyses were performed using SPSS® version 22.0 (IBM, New York, US). The median and range were used for descriptive analysis of incidence data. Heterogeneity between studies was assessed qualitatively by comparison of study populations, interventions, outcomes and design. Quantitative analysis of heterogeneity was performed using Cochran’s Q and I2 tests. In the former, p≤0.10 was considered the limit of statistical significance.14,15 The latter provided a percentage of the variability in effect sizes caused by heterogeneity between studies.16 Cochran’s Q was found using SPSS®. The Q statistic was incorporated into a hand calculation to find I2.
Results
A total of 4,356 records were identified by the initial search and 5 trials were identified by the secondary hand search. Following the removal of duplicates and screening, 22 studies remained, of which 18 fulfilled the eligibility criteria and were included for analysis.17–34 The four trials excluded during the eligibility assessment35–38 were deemed ineligible because they involved the same sample of patients as other included RCTs.21,22,24 The selection process is summarised in Figure 1. Emails to clarify unclear or missing data were sent to 15 of the authors, of whom 3 responded.
Figure 1.
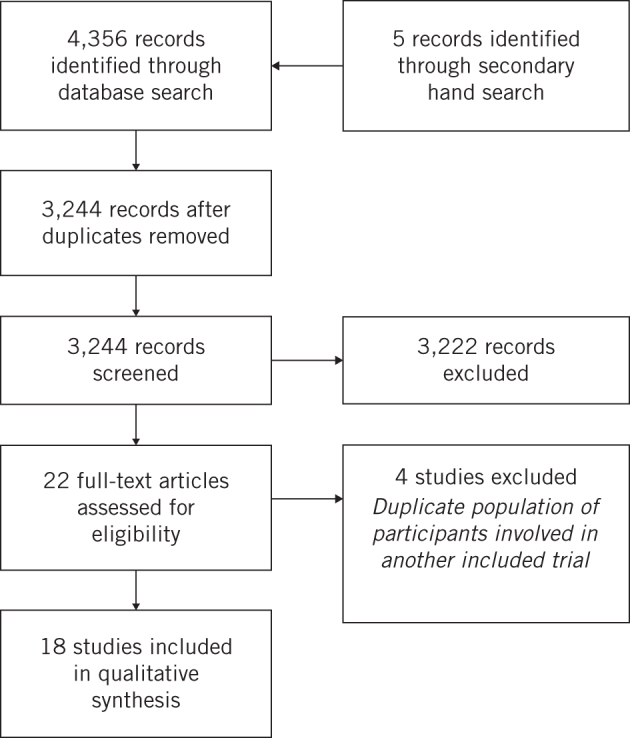
Flowchart of studies included in review
Study characteristics
A total population of 1,009 patients underwent conventional stoma formation in the trials. An overview of study characteristics is shown in Table 1. The number of intention-to-treat patients randomised to have a conventional stoma was not clearly reported in three of the trials.20,21,27 The age of patients was similar across most studies but was reduced in those evaluating patients with more uncommon conditions, such as typhoid, tuberculosis and trauma.17,27 Data concerning patient co-morbidity were limited and several authors made no report of co-morbidities at all.17,19–23,25,27,28,32,33 Some studies rejected patients with a history of stoma surgery, mesh insertion, abdominal wall hernia and other co-morbidities.18,20,26,30,31 Others reported that these conditions were present in their sample.26,29
Table 1.
Summary of studies included in review
| Study | Setting | Number of patients with stoma | Mean age in years (range) | Male-to-female ratio | Indication for stoma | Mean time to stoma closure / end of follow-up (range) |
| Chude, 200817 | Surgical unit, Greece | LI 136 | 55.5 (22–89) | 76:60 | CRC | – |
| Dong, 201218 | Surgical unit, China | EC 62 | 57.9 (SD: 10.3) | 33:29 | CRC | F 6–60 months |
| Edwards, 200119 | Surgical unit, UK | LI 34 LC 36 |
LI 63* (40–85) LC 68* (32–90) |
LI 27:7 LC 2:14 |
CRC | C LI 62* (17–120) days C LC 73* (28–141) days |
| Fleshman, 201420 | University hospitals, US | EI 23** EC 35** |
59.1 (SD: 14.4) | 29:29 | CRC, IBD, other | F 2 years |
| Gonzalez, 200021 | Surgical unit, US | LI 9** LC 78** |
26.4 | All patients 168:8 | Other | – |
| Gooszen, 199822 | 5 Surgical units, Netherlands | LI 37 LC 39 |
LI 63.2 (26–86) LC 64.7 (29–83) |
LI 14:23 LC 13:26 |
CRC, DD, other | – |
| Hardt, 201623 | University hospital, Germany | LI 30 | 50 (19–79) | 19:11 | CRC, IBD, other | F 112 (15–642) days |
| Jänes, 200924 | Surgical unit, Sweden | EC 27 | 71 | 16:11 | CRC, DD, IBD, other | F 5 years |
| Law, 200225 | University hospital, Hong Kong | LI 42 LC 38 |
LI 65.2 LC 67.8 (all patients 38–87) |
LI 26:16 LC 23:15 |
CRC | C LI 183* days C LC 180* days |
| López-Cano, 201226 | University hospital, Spain | EC 17 | 65.9 (SD: 13.9) | 7:10 | CRC | F 317* days |
| Patil, 201227 | University hospital, India | LI 30 | All patients 32.6 (16–63) | 22:8 | Other | F 6 months |
| Roed-Petersen, 199228 | University hospital, Denmark | EC 51 | 71* (38–87) | 30:21 | - | – |
| Salum, 200629 | 15 Surgical units, US | LI 64 | 41.5 (20–84) | 38:26 | - | – |
| Serra-Aracil, 200930 | Surgical unit, Spain | EC 27 | 67.2 (SD: 9.7) | – | CRC | F 29* (13–49) months |
| Speirs, 200631 | Surgical unit, UK | LI 29** | 63 (IQR: 52–70) | – | CRC, other | F 3 months |
| Tang, 200332 | University hospital, Singapore | Phase I: LI 54 Phase II: LI 36 |
Phase I: 62.5* (24–81) Phase II: 68* (37–85) |
Phase I: 33:21 Phase II: 16:20 |
CRC, DD, other | F Phase I: 22* (0.2–45.5) months F Phase II: 6.9* (0.2–37) months |
| Thoker, 201433 | University hospital, India | LI 34 | – | – | CRC | F 6 months |
| Vierimaa, 201534 | 5 Surgical units, Finland | EC 41 | 65.1 (SD: 11.7) | 19:16 | CRC | F 12 months |
C = time to stoma closure; CRC = colorectal cancer; DD = diverticular disease; EC = end colostomy; EI = end ileostomy; F = time to end of follow-up; IBD = inflammatory bowel disease; IQR = interquartile range; LC = loop colostomy; LI = loop ileostomy; SD = standard deviation
*Median
**Not intention-to-treat population
Colorectal cancer was the most common indication for stoma, featuring in 14 of the studies.17–20,22–26,28,30,32–34 Eight of these solely recruited patients with colorectal cancer.17–20,25,26,30,33,34 Diverticular disease featured in three studies,22,24,32 as did inflammatory bowel disease.20,23,24 Other presenting conditions included faecal incontinence, constipation, irritable bowel syndrome, typhoid, tuberculosis, trauma, colovesical fistula and familial adenomatous polyposis syndrome. The urgency of surgery varied between studies, as did the operative approach. Eight studies reported that stoma site marking was performed before stoma formation19,20,23,25,26,30,31,34 whereas the other authors did not mention it.
Four of the trials were multicentre studies.20,22,29,34 Tang et al reported the results of two separate RCTs (phase I and phase II) involving two separate populations of participants.32 The studies varied in their inclusion criteria owing to specific indications for stoma formation. Five studies involving participants with rectal cancer stated that the lesion must be in the lower part of the rectum.17,25,26,30,32 Other authors set much broader criteria.20,22–24,28,31 Exclusion criteria varied considerably, often based on co-morbidities. Uniquely, Edwards et al did not report any inclusion or exclusion criteria.19
The definitions of stoma related complications were poorly reported. Criteria for parastomal hernia were defined in six of the trials although they varied from clinical examination24,30,34 and computed tomography evidence20,26,30,34 to intraoperative findings.20,23 One study reported criteria for stoma retraction31 but there were otherwise no definitions of what constituted a stoma related complication.
Risk of bias
There were concerns related to study bias in multiple domains. Most of the studies were unclear about their randomisation criteria. In one RCT, there was a high risk of selection bias as allocation to a study group was determined by the surgeon intraoperatively.17
There was a high risk of detection bias in studies, which either stated that there was no blinding of outcome assessors or did not report whether outcome assessors were blinded. In addition, the criteria used to diagnose stoma related complications were rarely provided by authors. Diagnostic criteria were best reported for parastomal hernia although these varied from radiological classifications to clinical and intraoperative findings.20,23,24,26,30,34
The risk of reporting bias was mostly unclear. No study protocols were available and in most manuscripts, it was not clearly reported which stoma related complications were being assessed for as an outcome. The risk was high in two studies because outcome data were not reported in their entirety, as specified in the methods.24,33
Miscellaneous sources of bias were also detected. Three trials involved several study centres with teams of varying experience.20,29,34 Some study samples involved patients with different indications for stoma formation.22,23,32 In one trial, control participants were not well matched to the study group with respect to co-morbidities.29 The authors also changed the eligibility criteria, leading to the exclusion of rectal cancer patients partway through the study period, and failed to account for immunosuppressant therapy in patients with inflammatory bowel disease. There were no baseline characteristics of patients reported in another study.33 Finally, one author had a professional role in a corporate organisation with an interest in the outcomes of the research.24
Incidence of stoma related complications
There was considerable variation in reporting of stoma related complications (Fig 2). It was possible to extract incidence data by stoma type from all except two trials.20,21 Excluding these studies, 526 patients underwent loop ileostomy formation, 113 had a loop colostomy and 225 had an end colostomy. There were no data reported for participants with an end ileostomy and no cases of stoma varices. The following incidence data are presented as the median and range across included studies.
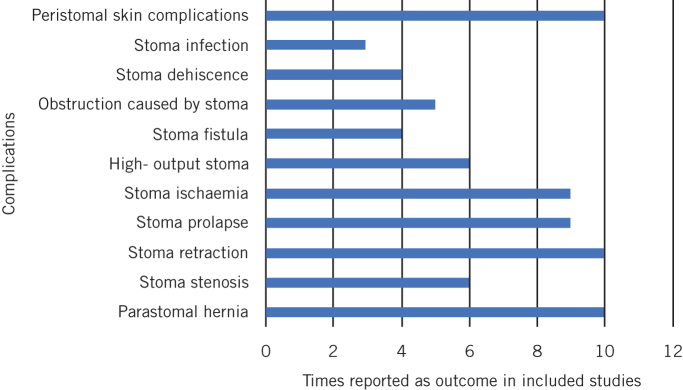
Figure 2.
Number of times each complication was reported as an outcome in included studies
Peristomal skin complications had the highest incidence across all stoma types at 14.0% (2.4–46.2%). This was followed by parastomal hernia, which occurred in 5.5% of patients (0–88.2%). The incidence of all stoma related complications across all stoma types was 26.5% (2.0–100%). End colostomy had the highest incidence of complications, with 62.6% (2.0–100%) of patients affected. This was followed by loop colostomy at 26.3% (13.9–100%) and loop ileostomy at 14.3% (2.9–62.2%). The incidence of specific stoma related complications in patients with a loop ileostomy, loop colostomy and end colostomy are shown in Table 2. Peristomal skin complications were most common in patients with a loop ileostomy (median 14.0%) and loop colostomy (median 32.3%). In end colostomy cases, patients were mostly affected by parastomal hernia (median 59.3%). No deaths were attributed to the formation of a stoma.
Table 2.
Incidence of stoma related complications displayed as median (range)
| Complication | Loop ileostomy | Loop colostomy | End colostomy |
| All complications | 14.3% (2.9–62.2%) | 26.3% (13.9–100%) | 62.6% (2.0–100%) |
| Peristomal skin complications | 14.0% (5.6–37.8%) | 32.3% (18.4–46.2%) | 3.6% (2.4–4.8%) |
| Stoma infection | – | – | 3.1% (2.4–3.7%) |
| Stoma dehiscence | 5.9% (5.9–5.9%) | – | 2.4% (0–5.9%) |
| Obstruction caused by stoma | 3.8% (2.9–4.7%) | – | – |
| Stoma fistula | 0% (0–2.7%) | 4.0% (2.8–5.1%) | 0% (0.0–0.0%) |
| High output stoma | 2.4% (0–18.5%) | 0% (0.0–0.0%) | – |
| Stoma ischaemia | 0% (0–3.3%) | 2.6% (2.6–2.6%) | 5.9% (2.0–7.3%) |
| Stoma prolapse | 0% (0–5.4%) | 7.9% (5.6–41.0%) | 4.1% (3.2–4.9%) |
| Stoma retraction | 3.1% (0–10.8%) | 1.3% (0–2.6%) | 4.8% (0–4.9%) |
| Stoma stenosis | 0.7% (0–3.3%) | 2.6% (2.6–2.6%) | 2.5% (0–4.9%) |
| Parastomal hernia | 2.4% (0–13.3%) | 0% (0–5.6%) | 59.3% (41.5–88.2%) |
Heterogeneity
Qualitative comparison of the trials demonstrated a high level of heterogeneity due to considerable variation in study characteristics. Eligibility criteria varied and there were several indications for surgery. Three different types of stoma were studied in the sample, some created electively and others as an emergency. Stoma site marking was not performed universally and diagnostic criteria were inconsistent. There were both single and multicentre trials included, based at institutions in middle and high income countries alike.
Some studies comprised two eligible intervention arms, both of which were included in the statistical tests for heterogeneity where required.19,22,25,32 These tests demonstrated a considerable level of heterogeneity (I2>75%, p<0.001) between studies reporting incidence data for parastomal hernia, stoma prolapse, high output stoma and peristomal irritation across all stoma types. The tests comparing groups of studies by stoma type mostly suggested substantial heterogeneity (I2>50%).
It was decided that meta-analysis would be inappropriate given the differences in study characteristics, variation in reported outcomes and results of the statistical tests.14,39
Discussion
This systematic review has summarised data from 18 RCTs reporting the incidence of stoma related complications. Recent studies have focused on the use of mesh to prevent parastomal hernia.9,40–42 Other systematic reviews have studied defunctioning loop stomas and outcomes following Hartmann’s procedure in complicated diverticulitis.43,44 There have also been several large cohort studies reporting the incidence of stoma related morbidity.2,10,11,45–52 This review is unique in providing a comprehensive summary of randomised evidence regarding the incidence of stoma related morbidity. No RCTs were identified reporting the incidence of stoma related complications following end ileostomy formation. Observational data have previously demonstrated an incidence of 15.7–56.7% morbidity following end ileostomy.48,53,54
The highest incidence of stoma related complications was seen following end colostomy formation (62.6%). In their systematic review, which was limited to observational data concerning diverticular disease, Salem and Flum reported complications in 10.3% of patients with an end colostomy.43 By contrast, most of the patients having an end colostomy formed in the trials included in our study underwent abdominoperineal excision of the rectum for rectal cancer. When this group of patients was considered in isolation, with benign conditions excluded, the lower limit for the range of incidence of morbidity increased. This perhaps demonstrates a trend for higher complication rates in malignant end colostomy cases.
Peristomal skin complications had the highest incidence across all stoma types, consistent with data reported in observational studies.11,45,48,49 Although this may appear obvious to clinicians, peristomal morbidity is likely to be discussed later in the consent process than, for example, parastomal hernia. Skin complications are common and represent a potentially recurring cost, with impact on stoma management for both the patient and healthcare professionals. Surgical teams should prepare patients adequately for this prior to their operation.
Parastomal hernia is recognised as one of the most common stoma related complications. The particularly high rate demonstrated in this review may be due to the high risk of detection bias across studies. Various diagnostic criteria were used by trial authors to define parastomal herniation, which may have reduced the overall sensitivity of the outcome assessment. In comparable cohort studies, there was no mention of computed tomography diagnosis,24,49,51 suggesting these authors relied on clinical examination alone. This may explain the lower rates of herniation among their patients.
A high level of heterogeneity between studies was evident. Several differences in study methodology contributed to variation between the trials. Reports of patient co-morbidities were highly variable and some studies excluded patients who had a history of stoma surgery or abdominal wall herniation while others included these patients. This is important as the rate of stoma related morbidity has been shown to be higher following secondary operations.55
This review was limited by factors related to the original studies, most notably owing to assessment and detection biases. The majority did not appear to involve a blinded outcome assessor. Definitions of complications were inconsistent and often poorly defined (if at all). The selection of complications reported varied across studies, providing an incomplete picture of outcomes. This is likely to be a significant contributor to the variation seen in results. There is an argument that this review should have included cohort studies to better estimate incidence rates. While these might provide long-term follow-up data, it was felt that a body of predominantly retrospective studies would provide highly selective reporting and lead to significant bias in estimation of incidence.
The results reported are all clinician centric or clinician reported. As highlighted, stoma related morbidity significantly impacts on the quality of life of patients. For this reason, a patient reported outcome measure (PROM) for stoma formation would be ideal. There is no globally applicable stoma PROM. Although a stoma impact score has been developed for colostomy,56 this requires validation in a population outside of Denmark. To our knowledge, there are no other widely used PROMs in this setting. The ongoing UK CIPHER (Cohort study to Investigate the prevention of Parastomal HERnia) trial will provide an accurate estimate of parastomal hernia formation and includes development of a PROM for parastomal hernia. This is likely to be useful to researchers once available. While neither of these is likely to be applicable worldwide, researchers should consider the use of PROMs where an appropriate tool exists.
Given the issues discovered in this review, we should work towards standardised definitions of major stoma related complications, including parastomal hernia, peristomal skin complications and stoma prolapse. It is also important to consider that the outcomes reported in this review are those likely to be of interest to clinicians. For example, parastomal hernia is clearly a topic of interest but does this matter to patients as much as peristomal skin complications? Further work is clearly required to understand which complications matter to patients, surgeons and stoma nurses, and then to develop robust definitions for these using a core outcome or core information set methodology.57 This would improve the quality of future studies and allow more accurate determination of the benefit of interventions on all stoma complication rates.
Conclusions
This systematic review has summarised the best available evidence concerning the incidence of stoma related morbidity in patients with ileostomy and colostomy. Peristomal skin complications, parastomal hernia and stoma retraction were most frequently reported. There was a high level of heterogeneity between studies and there were significant methodological limitations (specifically concerning outcome reporting) that should be addressed in future work.
References
- 1.Bafford AC, Irani JL. Management and complications of stomas. Surg Clin North Am 2013; : 145–166. [DOI] [PubMed] [Google Scholar]
- 2.Harris DA, Egbeare D, Jones S et al. . Complications and mortality following stoma formation. Ann R Coll Surg Engl 2005; : 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiergens TS, Hoffmann V, Schobel TN et al. . Long-term quality of life of patients with permanent end ileostomy: results of a nationwide cross-sectional survey. Dis Colon Rectum 2017; : 51–60. [DOI] [PubMed] [Google Scholar]
- 4.Kwiatt M, Kawata M. Avoidance and management of stomal complications. Clin Colon Rectal Surg 2013; : 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meisner S, Lehur PA, Moran B et al. . Peristomal skin complications are common, expensive, and difficult to manage: a population based cost modeling study. PLoS One 2012; : e37813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sajid MS, Kalra L, Hutson K, Sains P. Parastomal hernia as a consequence of colorectal cancer resections can prophylactically be controlled by mesh insertion at the time of primary surgery: a literature based systematic review of published trials. Minerva Chir 2012; : 289–296. [PubMed] [Google Scholar]
- 7.Shabbir J, Britton DC. Stoma complications: a literature overview. Colorectal Dis 2010; : 958–964. [DOI] [PubMed] [Google Scholar]
- 8.Tam KW, Wei PL, Kuo LJ, Wu CH. Systematic review of the use of a mesh to prevent parastomal hernia. World J Surg 2010; : 2,723–2,729. [DOI] [PubMed] [Google Scholar]
- 9.Wijeyekoon SP, Gurusamy K, El-Gendy K, Chan CL. Prevention of parastomal herniation with biologic/composite prosthetic mesh: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Surg 2010; : 637–645. [DOI] [PubMed] [Google Scholar]
- 10.Nastro P, Knowles CH, McGrath A et al. . Complications of intestinal stomas. Br J Surg 2010; : 1,885–1,889. [DOI] [PubMed] [Google Scholar]
- 11.Park JJ, Del Pino A, Orsay CP et al. . Stoma complications: the Cook County Hospital experience. Dis Colon Rectum 1999; : 1,575–1,580. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; : b2535. [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; : b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 London: Cochrane Collaboration; 2011. [Google Scholar]
- 15.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med 1998; : 841–856. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; : 1,539–1,558. [DOI] [PubMed] [Google Scholar]
- 17.Chude GG, Rayate NV, Patris V et al. . Defunctioning loop ileostomy with low anterior resection for distal rectal cancer: should we make an ileostomy as a routine procedure? A prospective randomized study. Hepatogastroenterology 2008; : 1,562–1,567. [PubMed] [Google Scholar]
- 18.Dong LR, Zhu YM, Xu Q et al. . Clinical evaluation of extraperitoneal colostomy without damaging the muscle layer of the abdominal wall. J Int Med Res 2012; : 1,410–1,416. [DOI] [PubMed] [Google Scholar]
- 19.Edwards DP, Leppington-Clarke A, Sexton R et al. . Stoma-related complications are more frequent after transverse colostomy than loop ileostomy: a prospective randomized clinical trial. Br J Surg 2001; : 360–363. [DOI] [PubMed] [Google Scholar]
- 20.Fleshman JW, Beck DE, Hyman N et al. . A prospective, multicenter, randomized, controlled study of non-cross-linked porcine acellular dermal matrix fascial sublay for parastomal reinforcement in patients undergoing surgery for permanent abdominal wall ostomies. Dis Colon Rectum 2014; : 623–631. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez RP, Falimirski ME, Holevar MR. Further evaluation of colostomy in penetrating colon injury. Am Surg 2000; : 342–346. [PubMed] [Google Scholar]
- 22.Gooszen AW, Geelkerken RH, Hermans J et al. . Temporary decompression after colorectal surgery: randomized comparison of loop ileostomy and loop colostomy. Br J Surg 1998; : 76–79. [DOI] [PubMed] [Google Scholar]
- 23.Hardt J, Seyfried S, Weiß C et al. . A pilot single-centre randomized trial assessing safety and efficacy of lateral pararectus abdominis compared with transrectus abdominis muscle stoma placement in patients with temporary loop ileostomies: the PATRASTOM trial. Colorectal Dis 2016; : O81–O90. [DOI] [PubMed] [Google Scholar]
- 24.Jänes A, Cengiz Y, Israelsson LA. Preventing parastomal hernia with a prosthetic mesh: a 5-year follow-up of a randomized study. World J Surg 2009; : 118–121. [DOI] [PubMed] [Google Scholar]
- 25.Law WL, Chu KW, Choi HK. Randomized clinical trial comparing loop ileostomy and loop transverse colostomy for faecal diversion following total mesorectal excision. Br J Surg 2002; : 704–708. [DOI] [PubMed] [Google Scholar]
- 26.López-Cano M, Lozoya-Trujillo R, Quiroga S, et al. Use of a prosthetic mesh to prevent parastomal hernia during laparoscopic abdominoperineal resection: a randomized controlled trial. Hernia 2012; : 661–667. [DOI] [PubMed] [Google Scholar]
- 27.Patil V, Vijayakumar A, Ajitha MB, Kumar LS. Comparison between tube ileostomy and loop ileostomy as a diversion procedure. ISRN Surg 2012; 547523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roed-Petersen K, Andersen B, Baden H, Burcharth F. Morbidity after immediate and delayed opening of sigmoid end colostomy: a randomised trial. Eur J Surg 1992; : 495–497. [PubMed] [Google Scholar]
- 29.Salum M, Wexner SD, Nogueras JJ et al. . Does sodium hyaluronate- and carboxymethylcellulose-based bioresorbable membrane (Seprafilm) decrease operative time for loop ileostomy closure? Tech Coloproctol 2006; : 187–190. [DOI] [PubMed] [Google Scholar]
- 30.Serra-Aracil X, Bombardo-Junca J, Moreno-Matias et al. . Randomized, controlled, prospective trial of the use of a mesh to prevent parastomal hernia. Ann Surg 2009; : 583–587. [DOI] [PubMed] [Google Scholar]
- 31.Speirs M, Leung E, Hughes D et al. . Ileostomy rod – is it a bridge too far? Colorectal Dis 2006; : 484–487. [DOI] [PubMed] [Google Scholar]
- 32.Tang CL, Seow-Choen F, Fook-Chong S, Eu KW. Bioresorbable adhesion barrier facilitates early closure of the defunctioning ileostomy after rectal excision: a prospective, randomized trial. Dis Colon Rectum 2003; : 1,200–1,207. [DOI] [PubMed] [Google Scholar]
- 33.Thoker M, Wani I, Parray FQ et al. . Role of diversion ileostomy in low rectal cancer: a randomized controlled trial. Int J Surg 2014; : 945–951. [DOI] [PubMed] [Google Scholar]
- 34.Vierimaa M, Klintrup K, Biancari F et al. . Prospective, randomised study on the use of a prosthetic mesh for prevention of parastomal hernia of permanent colostomy. Dis Colon Rectum 2015; : 943–949. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez RP, Merlotti GJ, Holevar MR. Colostomy in penetrating colon injury: is it necessary? J Trauma 1996; : 271–275. [DOI] [PubMed] [Google Scholar]
- 36.Gooszen AW, Geelkerken RH, Hermans J et al. . Quality of life with a temporary stoma: ileostomy vs colostomy. Dis Colon Rectum 2000; : 650–655. [DOI] [PubMed] [Google Scholar]
- 37.Jänes A, Cengiz Y, Israelsson LA. Preventing parastomal hernia with a prosthetic mesh. Arch Surg 2004; : 1,356–1,358. [DOI] [PubMed] [Google Scholar]
- 38.Jänes A, Cengiz Y, Israelsson LA. Randomized clinical trial of the use of a prosthetic mesh to prevent parastomal hernia. Br J Surg 2004; : 280–282. [DOI] [PubMed] [Google Scholar]
- 39.Munn Z, Moola S, Lisy K, Riitano D. The Systematic Review of Prevalence and Incidence Data. Adelaide: Joanna Briggs Institute; 2014. [Google Scholar]
- 40.Carne PW, Robertson GM, Frizelle F. Parastomal hernia. Br J Surg 2003; : 784–793. [DOI] [PubMed] [Google Scholar]
- 41.Tam KW, Wei PL, Kuo LJ, Wu CH. Systematic review of the use of a mesh to prevent parastomal hernia. World J Surg 2010; : 2,723–2,729. [DOI] [PubMed] [Google Scholar]
- 42.Shabbir J, Chaudhary BN, Dawson R. A systematic review on the use of prophylactic mesh during primary stoma formation to prevent parastomal hernia formation. Colorectal Dis 2012; : 931–936. [DOI] [PubMed] [Google Scholar]
- 43.Salem L, Flum DR. Primary anastomosis or Hartmann’s procedure for patients with diverticular peritonitis? A systematic review. Dis Colon Rectum 2004; : 1,953–1,964. [DOI] [PubMed] [Google Scholar]
- 44.Rondelli F, Reboldi P, Rulli A et al. . Loop ileostomy versus loop colostomy for fecal diversion after colorectal or coloanal anastomosis: a meta-analysis. Int J Colorectal Dis 2009; : 479–488. [DOI] [PubMed] [Google Scholar]
- 45.Caricato M, Ausania F, Ripetti V et al. . Retrospective analysis of long-term defunctioning stoma complications after colorectal surgery. Colorectal Dis 2007; : 559–561. [DOI] [PubMed] [Google Scholar]
- 46.Chen F, Stuart M. The morbidity of defunctioning stoma. Aust N Z J Surg 1996; : 218–221. [DOI] [PubMed] [Google Scholar]
- 47.Leenen LP, Kuypers JH. Some factors influencing the outcome of stoma surgery. Dis Colon Rectum 1989; : 500–504. [DOI] [PubMed] [Google Scholar]
- 48.Leong AP, Londono-Schimmer EE, Phillips RK. Life-table analysis of stomal complications following ileostomy. Br J Surg 1994; : 727–729. [DOI] [PubMed] [Google Scholar]
- 49.Londono-Schimmer EE, Leong AP, Phillips RK. Life table analysis of stomal complications following colostomy. Dis Colon Rectum 1994; : 916–920. [DOI] [PubMed] [Google Scholar]
- 50.Pearl RK, Prasad ML, Orsay CP et al. . Early local complications from intestinal stomas intestinal stomas. Arch Surg 1985; : 1,145–1,147. [DOI] [PubMed] [Google Scholar]
- 51.Porter JA, Salvati EP, Rubin RJ, Eisenstat TE. Complications of colostomies. Dis Colon Rectum 1989; : 299–303. [DOI] [PubMed] [Google Scholar]
- 52.Wara P, Sørensen K, Berg V. Proximal fecal diversion: review of ten years’ experience. Dis Colon Rectum 1981; : 114–119. [DOI] [PubMed] [Google Scholar]
- 53.Phillips RK, Ritchie JK, Hawley PR. Proctocolectomy and ileostomy for ulcerative colitis: the longer term story. J R Soc Med 1989; : 386–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarkut P, Dundar HZ, Tirnova I et al. . Is stoma care effective in terms of morbidity in complicated ileostomies? Int J Gen Med 2015; : 243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubin MS, Schoetz DJ, Matthews JB. Parastomal hernia. Is stoma relocation superior to fascial repair? Arch Surg 1994; : 413–418. [DOI] [PubMed] [Google Scholar]
- 56.Thyø A, Emmertsen KJ, Pinkney TD, et al. The colostomy impact score: development and validation of a patient reported outcome measure for rectal cancer patients with a permanent colostomy. A population-based study. Colorectal Dis 2017; : O25–O33. [DOI] [PubMed] [Google Scholar]
- 57.Kirkham JJ, Davis K, Altman DG et al. . Core Outcome Set-STAndards for Development: the COS-STAD recommendations. PLoS Med 2017; : e1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]