Abstract
Recently, gaseous signaling molecules, such as carbon monoxide (CO), nitric oxide (NO), and hydrogen sulfide (H2S), which were previously considered to be highly toxic, have been of increasing interest due to their beneficial effects at low concentrations. These so-called gasotransmitters affect many cellular processes, such as apoptosis, proliferation, cytoprotection, oxygen sensing, ATP synthesis, and cellular respiration. It is thought that mitochondria, specifically their respiratory complexes, constitute an important target for these gases. On the other hand, increasing evidence of a cytoprotective role for mitochondrial potassium channels provides motivation for the analysis of the role of gasotransmitters in the regulation of channel function. A number of potassium channels have been shown to exhibit activity within the inner mitochondrial membrane, including ATP-sensitive potassium channels, Ca2+-activated potassium channels, voltage-gated Kv potassium channels, and TWIK-related acid-sensitive K+ channel 3 (TASK-3). The effects of these channels include the regulation of mitochondrial respiration and membrane potential. Additionally, they may modulate the synthesis of reactive oxygen species within mitochondria. The opening of mitochondrial potassium channels is believed to induce cytoprotection, while channel inhibition may facilitate cell death. The molecular mechanisms underlying the action of gasotransmitters are complex. In this review, we focus on the molecular mechanisms underlying the action of H2S, NO, and CO on potassium channels present within mitochondria.
Keywords: mitochondria, potassium channels, KATP channel, BKCa channel, gasotransmitters, carbon monoxide, nitric oxide, hydrogen sulfide, heme
1. Introduction
For a long period of time, gases such as carbon monoxide (CO), hydrogen sulfide (H2S), and nitric oxide (NO) were often considered to be toxic. However, much attention has only been paid to NO due to the fundamental role this gas plays in cardiovascular physiology. Although these gases often have pleiotropic targets, ion channels are probably one of the most important objects with which they interact. Hence, in this review, we describe the regulatory activities of gas molecules towards the class of potassium channels that are present in the inner mitochondrial membrane.
It is not feasible to directly record the activity of potassium channels in mitochondria due to the size of this organelle in comparison to the size of the recording pipette. Even the use of mitoplasts, spherical objects derived by the osmotic downshock of mitochondria stripped of its outer membrane leaving the inner membrane intact, in whole-mitoplast mode is challenging. Therefore, most of the data on mitochondrial ion channels were obtained from excised inner mitochondria membrane patches and require freshly isolated mitochondria. In addition, due to low success rate of such experiments in several studies model cell lines were used for which high expression of mitochondrial channels was established (as indicated in the text).
Potassium channels, including ATP-sensitive potassium channel (mitoKATP), large-conductance calcium-activated potassium channel (mitoBKCa) [1,2], voltage-gated potassium channels (mitoKv1.3 and mitoKv7.4), and TWIK-related acid-sensitive K+ channel 3 (mitoTASK-3), are present in the inner mitochondrial membrane [3]. They affect the integrity of mitochondrial inner membranes, leading to the regulation of energy-transducing processes and the synthesis of reactive oxygen species (ROS). Hence, these channels play an important role in cytoprotection [4,5,6] and constitute promising targets for cancer treatments [7,8,9]. It is important to mention that mitochondrial potassium channels are also present in plants [10] and simple organisms [11,12].
Mitochondrial potassium channels are modulated by inhibitors (channel blockers), such as glibenclamide and 5-hydroxydecanoic acid (5-HD), in the case of mitoKATP channels [13], or charybdotoxin, in the case of mitoBKCa channels [14]. Mitochondrial potassium channels are activated by potassium channel openers such as diazoxide (mitoKATP channels) [13] or NS1619 (mitoBKCa channels) [15]. In principle, all drugs acting on mitochondrial potassium channels have also been previously found to regulate plasma membrane potassium channels.
In this paper, we discuss the interactions of mitochondrial potassium channels with gasotransmitters such as nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S). We attempt to summarize the current gaps in our understanding of the nature of these interactions that may serve as future prospects for further study.
2. Carbon Monoxide
Carbon monoxide has been rediscovered in recent years as a vital mediator in the cell that regulates a wide variety of biochemical reactions. Fundamental to its function in both normal physiological processes and pathologies is its proficiency to regulate a number of ion channels, including members of the calcium-activated K+ (BKCa) [16], voltage-activated K+ (Kv) [17] and Ca2+ channels [18], as well as ligand-gated P2X receptors [19]. The regulatory mechanisms by which CO modulates ion channels are still largely unexplained and stay somewhat dubious. However, the available structure-function studies suggest that a very limited range of protein cofactors confers indirect or direct sensitivity to CO, of a specific ion channel, and indicate that the redox state could play a crucial role in the integrated reaction. Irrespectively of the detailed mechanism by which ion channels are modulated by CO, its endogenous generation is physiologically important, and CO is currently being explored as a potential therapeutic [20].
2.1. Physiological Sources of Carbon Monoxide-Hemoxygenases
Historically, CO has been known to be toxic at high levels (>500 ppm). High CO concentrations cause hypoxemia via competitive binding, with an affinity that is >200-fold higher than that of oxygen, to hemoglobin in its binding sites for oxygen, with resulting formation of carboxyhemoglobin (CO-Hb) [21].
Endogenous CO is formed by oxidative decomposition of heme by heme oxygenases (HOs). In the presence of O2 and reduced nicotinamide adenine dinucleotide phosphate (NADPH), HOs catalyze the first, rate-limiting step in heme degradation, which is the breakdown of heme to equimolar amounts of biliverdin-IXα, iron, and CO [22].
Heme, which is a complex of iron and protoporphyrin IX, is an essential prosthetic group for a number of enzymes involved in the transport of oxygen (hemoglobin) and electrons (NADPH oxidase), as well as in other enzymes such as catalase and peroxidase [23]. However, heme functions not only as a prosthetic group within various proteins but has also been shown to function as a signaling molecule that regulates the functions of proteins, as described below.
Free heme released from intracellular heme-containing proteins causes cell damage and has been shown to have deleterious effects in several pathologies [24]. Heme levels have been found to be significantly increased in the cytosol and mitochondria of the failing heart [25]. The enzymatic reduction of biliverdin by biliverdin reductase produces bilirubin; both biliverdin and bilirubin are powerful antioxidants that scavenge ROS as part of a recycling mechanism [26]. Biliverdin reductase has also been found in mitochondria [27].
The role of free iron is complex. On one hand, at physiological levels, it plays a crucial role in cytoprotective processes [28], including ferritin-mediated processes that protect against mitochondrial dysfunction and oxidative stress [29]. On the other hand, there is evidence that iron produced from heme by HO may act as a pro-oxidant in lipid peroxidation and other reactions utilizing the Haber-Weiss cycle [30].
The high hydrophobicity of heme may promote deleterious iron-dependent reactions leading to the generation of reactive oxygen species (ROS) and membrane lipid peroxidation, thereby disrupting the cellular membranes of several organelles, including mitochondria [31]. Therefore, the levels of heme are tightly regulated via a balance of synthesis and degradation, and cells have developed heme detoxification systems [32]. In mammals, HOs are the only enzymes known to degrade heme, and therefore, they play a critical role in heme and iron homeostasis and are considered to be crucial components of the stress response and defenses against oxidative stress [33]. Three different isoforms of HO have been identified in mammals: inducible HO-1 [34]; constitutive HO-2 [35]; and catalytically non-active HO-3 [36]. HO-1 and HO-2 exhibit approximately 45% sequence identity.
HO-1 is induced by a variety of stimuli, including the presence of free heme, oxidative stress, and UV radiation [37,38,39]. HO-1 is localized to various intracellular compartments, including nuclei and mitochondria [27]. Mitochondria are hubs not only for heme but also for the synthesis of Fe-S cluster cofactors [40]; localization of the HO-1 protein to mitochondria suggests that it plays roles in regulation of turnover of heme proteins and iron in mitochondria, which may be important for protection against conditions, such as hemorrhage or ischemia/reperfusion, that have been found to involve the increased production of ROS. Consequently, increased expression of HO-1 may be observed in inflammatory and cardiovascular diseases [41,42].
A number of studies have demonstrated the beneficial role of HO-1 in mitochondria. Bindu et al. [43] showed that inducing oxidative stress by treating gastric mucosal cells with the non-steroidal anti-inflammatory drug indomethacin resulted in the up-regulation of HO-1 expression and the translocation of HO-1 to mitochondria. The presence of HO-1 in mitochondria has been linked to the inhibition of apoptosis during injury. In another study, it was shown that HO-1 was translocated to mitochondria and had increased activity in a primary culture of human small airway epithelial cells exposed to cigarette smoke extract, suggesting a protective effect of HO-1 against ROS [44].
However, not all the effects of HO-1 that were observed in mitochondria were beneficial. When HO-1 was specifically targeted to renal epithelial cell mitochondria [45], it mitigated hypoxia-mediated mitochondrial injury and cell death, but the selective advantages of its long-term expression were canceled out by its negative influence on the synthesis of mitochondrial proteins that contain heme as a cofactor. This conclusion was in line with the research of Bansal et al. [46], which showed that the overexpression of mitochondrial-targeted HO-1 resulted not only in heme degradation and diminished activity of heme-containing cytochrome c oxidase (CcO) but also induced the increased production of ROS and increased autophagy. Overall, these studies suggest that the level of mitochondrial-targeted HO-1 must be precisely controlled to achieve a balance between its beneficial and deleterious effects.
It has been suggested that HO-2 has different functions than HO-1 in the brain, based on differences in their patterns of expression. HO-1 has been shown to protect cultured cortical astrocytes, but not neurons, from oxidative stress after exposure to hemoglobin [47], whereas HO-2 has been shown to protect against apoptotic cell death in both neuronal cultures and in vivo models of ischemic injury [48]. HO-2 plays a unique role in oxygen sensing and responses to hypoxic conditions in mammalian cells [49]. There are three Cys-Pro signatures, called heme regulatory motifs (HRMs) in HO-2, which are not present in HO-1 [50]. The C-terminal HRM is a thiol/disulfide redox switch that modulates the affinity of the enzyme for heme [50] by responding to redox status of the cell, thereby integrating heme homeostasis with CO signaling and redox regulatory processes related to cellular metabolism. The oxygen sensor activity of HO-2 is involved in the regulation of the activity of the plasmalemmal BKCa channels within the carotid body, as described below. However, the possibility of localized functioning of HO-2 in mitochondria should also be noted; in one study, immunofluorescence staining of pulmonary arterial smooth muscle cells with various subcellular structural markers found that HO-2 was localized not only to the endoplasmic reticulum, but approximately 20% of the enzyme was located also in the mitochondria [51].
Although CO gas is easily diffusible and is able to reach all cellular compartments, the presence of HO-1 in mitochondria and its localized effects indicate that the interplay of CO with heme and possibly other products of HO activity, including biliverdin and free iron, is physiologically meaningful.
2.2. Signal Transduction via Heme-CO Interaction
Several bacterial nickel-dependent metalloenzymes are able to bind CO in their metal clusters, [52] but in mammalian cells, it is believed that the transduction of CO by proteins requires the presence of reduced heme iron (Fe2+). The first identified molecular target of CO was hemoglobin, but heme is also present in a number of electron transfer proteins and redox enzymes.
CO signaling pathways have still not been entirely elucidated. One of these pathways directly links the modulation of soluble guanylate cyclase (sGC) with the production of cyclic guanine monophosphate (cGMP) and the activation of protein kinase G (PKG) in processes involved in neurotransmission, vasodilation, and the inhibition of platelet aggregation [53,54,55,56]. CO-mediated activation of sGC is dependent on the presence of heme in its modulatory domain [57].
A few studies have provided evidence of the presence of sGC in the mitochondrial matrix. It was previously shown that the acceleration of cGMP production in cardiac mitochondria stimulates cytochrome c release in a manner that is independent of the permeability transition that results in apoptosis [58,59]. It has also been shown that protein kinase G-dependent opening of mitoBKCa channels plays a critical role in cardioprotection induced by sildenafil [60].
2.3. Regulation of BKCa Channels by CO
One of the best-characterized effectors of CO is the BKCa channel. It is known that CO activates this channel via direct and indirect mechanisms (Figure 1).
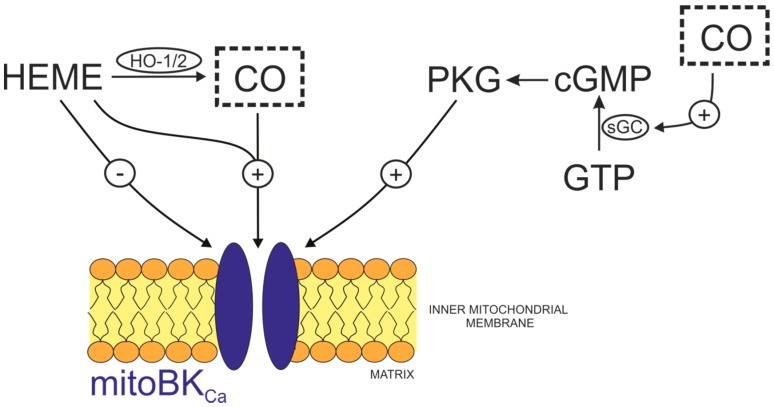
Figure 1.
Regulation of the mitochondrial large-conductance calcium-activated potassium channel (mitoBKCa) activity by CO. The activity of the channel is inhibited (−) by heme alone but could be stimulated (+) in the presence of heme (HEME, Fe-protoporphyrin IX) and CO (gasotransmitter shown in the dotted box) (a product of the activity of HOs). Independently, CO stimulates sGC/cGMP/PKG pathway leading to channel phosphorylation and activation. Abbreviations: CO, carbon monoxide; HO, heme oxygenase; PKG, cGMP-dependent protein kinase; sGC, soluble guanylate cyclase.
The indirect mechanism involves the phosphorylation of BKCa by PKG at specific serines (Ser855, Ser869, and Ser1072 in human BKCa ortolog Slo1), which increases the probability of BKCa assuming an open conformation [61,62,63]. Because CO stimulates sGC, leading to the activation of PKG, it is apparent that CO promotes PKG-mediated phosphorylation of BKCa channels and increases the channels’ activity. This mechanism appears to be valid also for mitoBKCa channels [64].
The direct mechanism involves the bimodal regulation of the BKCa channel via the interaction of CO with its bound heme [16]. Specifically, a segment that links regulators of K+ conductance (RCK) domains, RCK1 and RCK2, has been suspected to bind heme, based on the sequence homology of the short motif within this linker to the heme binding motif (HBM) containing the sequence CXXCH (X is any amino acid) that is present in cytochrome c [16]. Tang et al. [65] demonstrated that FeIII-heme (hemin) is able to block the BKCa channels with high affinity by binding to the HBM, since replacement of the Cys or His residues with Ser or Arg, respectively, abolished the sensitivity of the BKCa channel to hemin. Interestingly, cytochrome c covalently binds heme c via [66], while the noncovalent, reversible binding of heme b occurs within the BKCa channels [16]. The 23-residue peptide known as the heme-binding domain (HBD), which encompasses the HBM, preserves its heme- or hemin-binding properties [16,65]. It was demonstrated, using this model system, that heme, but not hemin, is able to bind CO [16]. Using a series of patch-clamp experiments, Jaggar et al. [16] demonstrated that BKCa channels that were inhibited by heme, but not hemin, could be reactivated by CO. The heme-CO complex exhibited stimulatory properties, since the channels were more active in the presence of heme-CO than in the control conditions. Thus, the substrate (heme) and the product (CO) of heme degradation by HOs regulated channel activity in such a way that an increase in the activity of HOs resulted in the activation of BKCa channels. In addition, using co-immunoprecipitation experiments, it was shown that HO-2 formed a complex with the BKCa channel by binding to the HBD, which provided evidence of a direct regulatory mechanism [67]. Within the CXXCH motif, the histidine residue serves as the axial heme ligand, while the cysteines comprise a thiol/disulfide redox switch that regulates the affinity of the HBM for heme and CO. The dithiol state was demonstrated to bind hemin (Kd = 210 nM) 14-fold more tightly than the disulfide state [68].
The thiol/disulfide redox switch within the BKCa channel and the corresponding thiol/disulfide redox switch within HO-2 are involved in hypoxic responses in the carotid body [69]. Carotid bodies are arterial chemoreceptors that sense changes in blood oxygen and CO levels and respond to hypoxia by secreting acetylcholine, dopamine, and ATP to increase the rate and depth of ventilation [69]. The hypoxia response element within the carotid body consists of glomus cells. BKCa channels on the plasma membranes of the glomus cells are inhibited when the O2 supply becomes compromised. Under these conditions, HO-2 activity is low due to the reduction of the cysteines within the HRM, which leads to lower levels of CO, higher levels of heme, and the binding of heme to the reduced form of the HBD in BKCa channels. This leads to BKCa inhibition, cell depolarization, Ca2+ influx, and transmitter release [67]. On the other hand, under normoxic conditions, CO generated by heme oxygenase-2 (HO-2) during heme degradation activates the BKCa channels, leading to cell hyperpolarization. This is a mechanism by which the BKCa channel responds in a prompt and reversible manner to alterations in the redox state of the cell, specifically as it shifts between hypoxia and normoxia [68].
An analogous mechanism, possibly resulting from the localization of both HO-2 and mitoBKCa inside mitochondria, could operate to control the redox state of mitochondria, which is of great importance. However, there is a lack of studies investigating this topic. In the only study, the effects of CORM-401 on glycolysis and respiration of mitochondria in intact human endothelial cell line EA.hy926 were studied [70]. It was found that CORM-401 treatment results in an increase in the oxygen consumption rate (OCR), inhibited glycolysis, decreased ATP-turnover, and increased proton leakage. Interestingly, the blockade of mitoBKCa with paxilline abolished the increase in OCR induced by CORM-401. Using patch-clamp experiments, the authors of this study showed that CORM-401 activated mitoBKCa channels. These effects were not observed for inactive CORM (iCORM) that was generated using a mix of MnSO4 and the CORM-401 ligand DTC. Therefore, it was argued that the observed effects specifically resulted from the CO released from the donor [70]. However, one must be cautious when interpreting experiments that involve CORMs due to a plethora of side effects that accompany the use of these drugs (see Section 2.5).
2.4. KATP Channels
Activation of mitoKATP has long been implicated in the protection of the heart against ischemia/reperfusion injury [13]. Abrogation of cardioprotection mediated by CORMs (CORM-2 and CORM-3) by the KATP channel inhibitors 5-hydroxydecanoic acid [71] or glibenclamide [72] provided indirect evidence that mitoKATP could be a target of CO. However, as was noted above, the action of CO requires the presence of heme as the receptor. Only recently was it discovered that cardiac plasmalemmal KATP channels are regulated by heme [73]. These channels are hetero-octameric complexes containing four pore-forming K+ channel subunits (Kir6.2) and four regulatory subunits (SUR2A) [74]. The modulation of the activity of this channel is important for the protective response of cardiac muscle to oxidative stress [75]. Curiously, it was found that a CXXHX16H motif in a cytoplasmic sulphonylurea receptor subunit within the channel is responsible for heme binding. In the presence of 500 nM hemin, a moderate (1.6-fold) increase in KATP receptor currents was observed [73]. A more dramatic, several-fold increase in the activation of KATP was observed in the presence of gaseous CO [76].
Unfortunately, there is no definitive evidence that identifies the proteins that constitute mitoKATP.
A non-conventional short SUR2 splice variant approximately 55 kDa in size, known as SUR2A-55, was found predominantly within mitochondrial membrane fractions [77]. However, this variant does not contain the full-length SUR2A heme binding motif. Moreover, there is no consensus regarding the identity of the pore-forming subunit of mitoKATP. Zhou et al. [78] used immunoelectron microscopy and found that Kir6.1 was mainly localized to the mitochondria, while Kir6.2 was located mainly within the endoplasmic reticulum with a minor fraction in the mitochondria. Similar results were observed upon the heterologous expression of Kir6.1-GFP [79]. On the other hand, other studies, including those utilizing cell fractionation, have excluded the presence of Kir6.1 or Kir6.2 in the mitochondria [80,81]. Recently, a splice variant of Kir1.1 lacking the first 19 N-terminal amino acids (designated Kir1.1b or ROMK2) was found to be located in mitochondria of H9c2 cell line and probably constitutes the pore-forming subunit of mitoKATP [82]. There are no data regarding the regulation of mitoKATP or Kir1.1 by gases, including CO.
2.5. Pharmacological CO Donors—CORMs
The experimental and clinical use of CO gas is difficult and can be dangerous. Therefore, the development of so-called CO-releasing molecules (CORMs) as prodrugs for the administration of CO in vitro, in tissue culture, and in living organisms (potentially as a medicine) resulted in a significant surge in the number of studies on this topic. CORMs offer the possibility of a safe and controllable release of CO at low amounts that is triggered by the presence of light, ligands, and enzymes, among other factors, with the ultimate goal of utilizing its therapeutic potential [83,84,85,86,87].
Despite the fact that a number of different CORM molecules are available, to date most studies have been conducted using various types of ruthenium-containing carbonyl complexes such as tricabonyldichlororuthenium(II) dimer (CORM-2) or [Ru(CO)3Cl(glycinate)] (CORM-3) [88]. Heme is generally considered to be the prime target of CO released from CORMs. However, it is not readily apparent whether or not any effect of a CORM is due to CO gas, the CORM itself, or one or more of its breakdown products. In one study that utilized heme-deficient bacteria, it was found that the response to CORM-3 could not be attributed to the classical biochemical targets of CO [89]. In another study, it was shown using crystallography that [Ru(CO)3Cl2(1,3-thiazole)] forms lysozyme-Ru adducts and that mono-carbonyl Ru species [Ru(H2O)4(CO)] were covalently bound to surface histidine and aspartates [90].
In several studies, it was shown that BKCa channels are strongly activated by CORM-2 or CORM-3 [91,92,93,94] and that different regions that are unrelated to the heme binding motif (HBM) were implicated in this phenomenon [95,96]. Specifically, the stimulatory action of CORM-2 on BKCa channels required the presence of an aspartic acid and two histidine residues (365 and 394) in the cytoplasmic RCK1 domain [96]. This effect persisted in conditions preventive for binding between CO and heme in other proteins. Only much later it was found that these histidines form Ru(CO) adducts, and the effects of CORM-2 were abolished in the presence of an excess of free histidine [97]. The authors of this study also uncovered the histidine-dependent action of CORM-2 on other voltage-gated potassium channels, such as Kv1.5 and Kv11.1, in a similar manner to that observed in Kv2.1 channels [98]. The off-site effects seen with CORM-2/CORM-3 were not observed for iron-containing CORM-S1 or manganese-containing CORM-EDE1 [97]. In contrast to the strong stimulatory effects of CORM-2, gaseous CO exerted only negligible effects on BKCa channels in a manner consistent with the targeting of heme by CO, which is only non-covalently and transiently bound to these channels [99] and occupies its binding sites probably only partially under experimental conditions.
The side effects of CORMs are unfortunately not restricted to ruthenium-based compounds. Manganese-containing CORM-401 stimulates respiration, depolarizes the cytoplasmic membrane in a manner similar to uncouplers, and elicits the loss of intracellular potassium in E. coli cells [100]. These properties could explain the increase of the oxygen consumption rate (OCR) in intact mitochondria [70] in the manner independent from the activation of mitoBKCa. Therefore, it appears that there is a need to design and test other CO donors, including nonmetallic CORMs, e.g., CORM-A1 [101], to examine their impact on mitochondrial physiology. In addition, all of the effects reported to date should be verified with the use of gaseous CO [97].
3. Hydrogen Sulfide
Hydrogen sulfide (H2S), although considered to be a toxic gas for many years, was recently recognized to be an important molecule that, at low concentrations, plays an important role in processes such as inflammation, vasculature development [102,103], apoptosis [104], and the preservation of mitochondrial functioning [105,106].
3.1. Physiological Sources of Hydrogen Sulfide
Both enzymatic and non-enzymatic pathways contribute to the endogenous production of H2S. The following enzymes are involved in the generation of H2S from l-cysteine: cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST)/cysteine aminotransferase (CAT) [107]. In addition, it was shown that H2S can also be produced from d-cysteine by the 3-MST/DAO (d-amino acid oxidase) pathway in the kidney and the cerebellum [108]. CBS and CSE are distributed only in the cytoplasm [109], while CAT and 3-MST are found in both the cytoplasm and mitochondria, but primarily within mitochondria [110], and DAO is present in peroxisomes [111]. Although CSE is present only in the cytoplasm of vascular smooth muscle cells (SMCs) at rest, it can be translocated to the mitochondria [109] via the addition of the calcium ionophore A23187, thapsigargin, or tunicamycin to the SMCs. The increase in the amount of H2S in a mitochondrial fraction derived from A23187-treated SMCs was found to be CSE-related [109].
3.2. Pharmacological Donors of H2S
It is also possible to deliver H2S from exogenous sources, including H2S releasing molecules. The most popular of these are inorganic sulfide salts such as sodium sulfide (Na2S) and sodium hydrogen sulfide (NaHS) [112]. Although the positive effects of sulfide salts have been reported in relation to cardioprotection [113] and protection against inflammation [114,115], they release H2S rapidly and spontaneously, which impedes control of its concentration. Numerous other H2S-releasing molecules exist and each of them has both advantages and disadvantages, but they will not be addressed in this review [116]. Because we are focused here on the action of gas signaling molecules on mitochondrial potassium channels, we would like to briefly mention the novel mitochondrial-targeted H2S donors. AP39 and AP123 are mitochondrial slow-release H2S donors, which appear to be much safer than sulfide salts because they release concentrations that exert positive effects at a much lower level than that tolerated in vitro. AP39 and AP123 also have a beneficial effect on cellular bioenergetics by increasing the electron transfer rate of complex III of the respiratory chain [117].
3.3. H2S Regulation of Heme Proteins
The mechanisms underlying the action of H2S, which is complex and complicated, include redox reactions, the formation of persulfides with the -SH groups of cysteines and sulfide-metal interactions in heme proteins [118]. A few targets of H2S will be discussed below, with an emphasis on targets within mitochondria.
H2S reacts with the metallic centers of heme proteins. Coolman et al. [119], using a functional model of the oxygen-reducing sites within cytochrome c oxidase, showed that H2S acts as a competitive inhibitor of cytochrome c oxidase at high concentrations via its reversible binding to FeII heme, thus preventing binding of the enzyme substrate (O2) to the reduced FeIICuI active site. At low concentrations, inhibitory effects of H2S on cytochrome c oxidase are not observed, due to the low affinity of H2S for FeII heme [119]. In addition to the inhibitory effects of H2S on cytochrome c oxidase, it was found that H2S (100 nM–1 µM) supports mitochondrial electron transport in cells isolated from rat liver [120]. H2S also increases OCR-linked respiratory capacity and ATP turnover in the presence of the Krebs-cycle-derived electron donor succinate. In addition, electron transport and production of ATP was stimulated by incubation of mitochondria from rat liver with low concentrations of 3-mercaptopyruvate (3-MP), which is a substrate for mitochondrial H2S production. Similar observations have been made based on mitochondria from cultured murine hepatoma cells [121]. H2S produced by 3-MST provides electrons to sulfide quinone oxidoreductase, which in turn transfers electrons to the mitochondrial electron transport chain and increases ATP production.
The above results indicate that H2S produced within the cell plays a physiological role in bioenergetics of the cell.
3.4. H2S Regulation via Protein S-Sulfhydration
Another mechanism by which H2S modifies the activity of proteins is S-sulfhydration (persulfidation) via the posttranslational modification of cysteine residues (RSH) into persulfides (RSSH) [122]. The ATP synthase (complex V) is one of the main proteins located within the inner membrane of mitochondria that undergoes S-sulfhydration [123]. This modification increases the enzymatic activity of ATP synthase in an NaHS concentration-dependent (10 nM–100 nM) manner. It was found that S-sulfhydration of the α subunit of ATP synthase (ATP5A1) occurs at Cys244 and Cys294. It is significant that the basal S-sulfhydration of ATP synthase is observed in vivo in wild-type mice and is induced by CSE-produced H2S, as this indicates the physiological importance of S-sulfhydration [123]. It is important to note that S-sulfhydration does not occur via the direct interaction of H2S and the -SH group of cysteine, because the sulfurs present in both molecules are typically at lowest i.e., (−2) oxidation state. However, there are other possible ways for the S-sulfhydration of cysteine residues to occur. For example, H2S can react with sulfenic acid (a product of -SH oxidation), S-nitrosated cysteines, and cysteine disulfides to form -SSH groups [124]. In addition, it has been shown that polysulfides can also take part in the S-sulfhydration of cysteine residues in vitro and in cell culture [125].
3.5. Potassium Channels as Targets for H2S
Hydrogen sulfide has various effects on the activity of different potassium channels within the plasma membrane (Figure 2) [126].
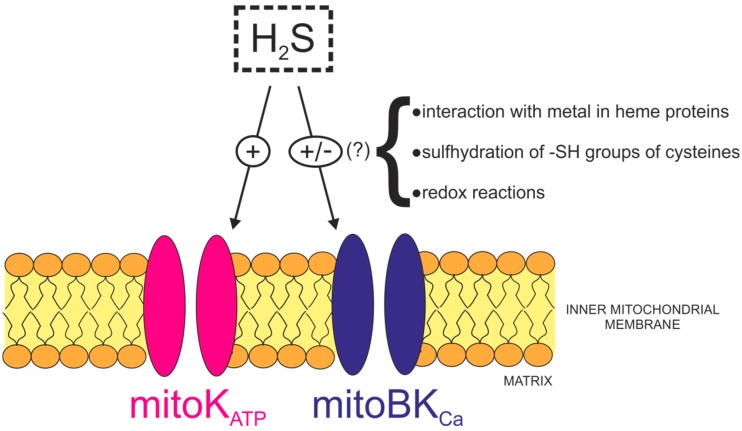
Figure 2.
Regulation of mitochondrial potassium channels, mitochondrial large-conductance calcium-activated potassium channel (mitoBKCa, activation (+) or inhibition (−)), and ATP-sensitive potassium channel (mitoKATP, activation (+)) by H2S (gasotransmitter shown in the dotted box). Modulation of the activity of the mitoBKCa channel by H2S, inferred from regulation of its plasmalemmal counterpart, could result from multiple, unknown (?) mechanisms (bracket).
Mustafa et al. [127] showed in 2011 that the Cys43 in the Kir6.1 subunit of the KATP channel is a major target of H2S-mediated S-sulfhydration (NaHS was used as a donor) in HEK293 cells, which resulted in channel activation. Interestingly, an increase in the activity of ATP-sensitive potassium channels caused by S-sulfhydration was accompanied by reduced binding of ATP and enhanced binding of PIP2 to Kir6.1. It is possible that the S-sulfhydration of Cys43 in Kir6.1 impedes the binding of ATP to the channel due to electrostatic or spatial changes, which could result, in turn, in increased access of PIP2 to its binding site [127]. It was recently shown that H2S affects the activity of other Kir channels, Kir2 and Kir3, in a manner opposite to that of Kir6.1 mentioned above [128]. Specifically, the use of NaHS as an H2S donor resulted in the inhibition of Kir3.2 channels in Xenopus oocytes and Kir3.2 channels in CHO-K1 cells.
Tang et al. [129] revealed a direct effect of both exogenous H2S, in the form of an H2S-containing solution, and endogenous H2S on the activity of KATP channels in vascular smooth muscle cells. It was also shown that inward KATP currents and the probability of the opening of a single KATP channel was increased after exposure to H2S. To demonstrate the effects of endogenous H2S on the activity of KATP channels, various inhibitors of CSE and CBS were used, which resulted in a decrease of the whole-cell KATP currents in a manner independent on the activity of the cGMP signaling pathway [129].
In contrast, less is known about the regulation of the activity of potassium channels in the inner membrane of mitochondria by H2S. In 2016, Testai et al. [130] showed that 4-carboxyphenyl isothiocyanate (4CPI), which is an H2S donor, significantly improved the recovery of several parameters of myocardial function after ischemia and hindered the extent of tissue injury. Pre-treatment of rats with a selective blocker of the mitoKATP channel, 5-hydroxydecanoic acid (5-HD), lead to an abolition of the cytoprotective effects of 4CPI, which suggested that mitoKATP could be one of the targets of H2S donated by 4CPI. Additionally, the addition of 4CPI to isolated rat heart mitochondria caused depolarization of the mitochondrial membrane potential, and this effect was abrogated by ATP, which is a physiological blocker of the mitoKATP channel. A similar effect was observed in the action of NaHS on rat cardiomyocytes, where exposure to a H2S donor resulted in a reduction in the myocardial infarct size, which was in turn impaired by 5-HD [131]. From these experiments, it was inferred that H2S opened mitoKATP channels. Unfortunately, there are no electrophysiological data regarding the regulation of mitoKATP channels by H2S.
Similarly, nothing is known about the regulation of the activity of mitoBKCa channels by H2S. However, it is known that H2S regulates the activity of BKCa channels in the plasma membrane [132,133,134]. The effects of H2S on BKCa are equivocal and may vary in different tissues. The open probability (Popen) and mean open time of a single BKCa channel in rat GH3 pituitary tumor cells was increased after NaHS exposure [132]. This effect was concentration- and voltage-dependent but also Ca2+-independent. In addition, an increase in the activity of the BKCa channel was found to be transient and reversible. Sitdikova et al. [132] also showed that the effects of NaHS on the activity of BKCa channels resulted from the reduction of sulfhydryl groups located on the cytoplasmic side of the channel by NaHS and found that H2S regulation is dependent on BKCa channel phosphorylation [133]. On the other hand, the activity of BKCa channels in HEK 293 cells was inhibited by NaHS in a dose-dependent manner [134].
It can be assumed that the mitoBKCa channels may be modulated by H2S in a similar manner to the BKCa channels within the plasma membrane, but the effects of H2S on these channels are still unknown. Because it is known that H2S interacts with heme proteins and that HBM is present in BKCa channels, a question arises as to whether or not H2S can impact the activity of these channels by interacting with heme bound to the HBM in a manner similar to the action of CO on BKCa channels [16].
4. Nitric Oxide
4.1. The Role and Physiological Sources of Nitric Oxide
Nitric oxide (NO) is a key signaling molecule in the cardiovascular system [135]. During ischemia, NO synthesis is increased in the heart to provide cardioprotection. NO is produced in vivo by nitric oxide synthase (NOS) via the conversion of l-arginine to citrulline [136]. NO plays a role in mitochondrial biogenesis and regulates mitochondrial turnover in the vasculature [137]. Interestingly, the alpha isoform of neuronal NOS-1 has been identified as a mitochondrial NOS (mtNOS) [138,139]. NO produced in mitochondria has an important regulatory impact on cellular metabolism via its reversible inhibition of cytochrome c oxidase [140]. Nitric oxide also reacts with mitochondrial superoxide anions to produce the potent oxidative species peroxynitrite [141], which hinders mitochondrial activities [142].
It is well known that activators of mitoKATP channels, such as diazoxide, can depolarize mitochondria [143]. However, it was found, unexpectedly, that diazoxide increased the phosphorylation of neuronal NOS at positive regulatory serine, 1417 but also decreased NOS phosphorylation at a negative regulatory Ser847, and, as a consequence, increased NO production in cultured neurons [144]. These observations functionally link mitochondrial channels with NO.
4.2. Regulation of Mitochondrial KATP Channels by Nitric Oxide
The cardioprotective properties of nitric oxide suggest that NO may interact with mitochondrial potassium channels (Figure 3).
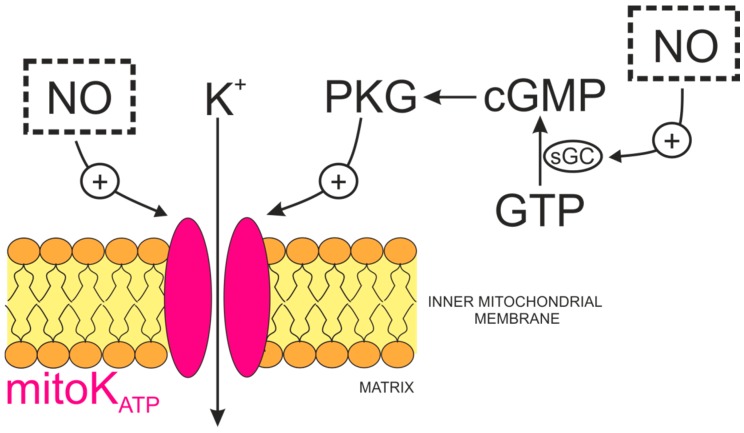
Figure 3.
Regulation of the ATP-sensitive potassium channel (mitoKATP) activity by NO. The activity of the channel is stimulated (+) directly by NO (gasotransmitter shown in the dotted box). Independently, NO could stimulate sGC/cGMP/PKG pathway leading to channel phosphorylation and activation. Abbreviations: PKG, cGMP-dependent protein kinase; sGC, soluble guanylate cyclase.
By measuring mitochondrial redox potential and using this as an index of mitoKATP channel opening in rabbit ventricular myocytes, it was shown that NO may activate mitoKATP channels [145]. The NO donor S-nitroso-N-acetyl-DL-penicillamine (SNAP) activated mitoKATP without stimulating sarcolemmal KATP channels, since its effects were blocked specifically by 5-hydroxydecanoate (5-HD) and NO scavengers. Because many effects of NO are mediated by the cGMP-dependent pathway, it was determined whether or not NO-induced activation of mitoKATP was dependent on 8Br-cGMP. The negative results of this experiment suggested that mitoKATP are directly activated by NO. Interestingly, when activated by the potassium channel opener, diazoxide, mitoKATP channels appeared to be more susceptible to the potentiating effects of NO than channels that were closed [145]. Unambiguous evidence for the direct activation of mitoKATP by NO was obtained by reconstituting cardiac mitoKATP channels into lipid bilayers [146], whereupon the mitoKATP channels were activated by exogenous NO donors. Single channel activity was inhibited by the mitoKATP blockers 5-HD or glibenclamide [146]. In contrast, mitoKATP channel activity that was measured using the patch-clamp technique was inhibited by NO in human T-lymphocyte (Jurkat) cells [147]. Such differences could result from differences in the molecular mechanisms underlying the action of NO on mitoKATP channel proteins. Unfortunately, there are no mechanistic studies on mitochondrial channels that exist that could answer such questions. However, such differences could be elucidated via studies performed on plasmalemmal KATP channels. For example, cell-attached recordings of KATP currents in rat large DRG neurons showed that the KATP channels were stimulated by NO by decreasing their sensitivity to block by intracellular ATP [148]. This effect was also present after application of sGC and PKG inhibitors, indicating that the sGC/cGMP/PKG signaling pathway was not responsible for this phenomenon. This activation remained intact in inside-out patches and was reversed by dithiotreitol (DTT) and prohibited by a thiol-alkylating agent, NEM. These findings indicated that NO activates KATP channels via the direct S-nitrosylation of cysteine residues. During additional experiments in which the currents produced by recombinant SUR1/Kir6.2 channels expressed in COS7 cells were measured, proved that activation mediated by NO involves its interaction with residues in the NBD1 of the SUR1 subunit [148].
On the other hand, NO may also regulate mitoKATP channels indirectly via sGC/cGMP/PKG pathway [149]. For instance, in other studies that were performed in transfected HEK293 cells and in cardiomyocytes isolated from rabbits or genetically modified mice, exogenous exposure to the NO donor NOC-18 in cell-attached mode resulted in an increase of the single-channel activity of Kir6.2/SUR2A, which was abolished by exposure to a selective PKG inhibitor, KT5823 [150]. This sGC-dependent mechanism may likely be involved in the regulation of mitoBKCa channels in cardiomyocytes [64].
5. Final Remarks
In this paper, we have described our current understanding of the interactions of CO, H2S, and NO with mitochondrial potassium channels. Even so, our knowledge of these complex interactions is limited to phenomenology and is lacking molecular mechanisms. Identification of the molecular identity of mitochondrial potassium channels will increase insight into the interactions of gas molecules with mitochondrial channel proteins.
The interacting molecules of mitochondrial potassium channels are probably not limited to CO, H2S, and NO. The noble gas anesthetic, xenon, has been shown to protect the myocardium from ischemia/reperfusion injury [151], and this protection could possibly be mediated by the preservation of myocardial mitochondria and the opening of mitoKATP channels [152]. Additionally, sulfur dioxide (SO2) can also be generated endogenously in mammals and is a physiological endothelium-derived relaxing factor [153]. Moreover, the protective effect of SO2 on myocardial ischemia/reperfusion has been previously observed [154]. The vasodilatory effect of SO2 is related to the opening of KATP and BKCa channels in the plasma membrane [153]. This suggests that the regulation of mitochondrial potassium channels by SO2 is also possible. Other gaseous molecules, including ammonia (NH3), carbon dioxide (CO2), hydrogen gas (H2), and methane (CH4) have also been suggested to possibly serve as signaling molecules [155].
Increased understanding of the regulation of mitochondrial potassium channels by gas molecules will not only lead to increased knowledge of intracellular ion channels, but may also contribute to the future application of these substances and their donor molecules to cytoprotective treatment.
Author Contributions
A.W. prepared figures; P.K., A.W. and A.S. wrote the manuscript.
Funding
The studies described in this paper were supported by the Polish National Science Centre grants No 2015/17/B/NZ1/02496, 2015/19/B/NZ1/02794, and by the Nencki Institute. We are grateful to Monika Zochowska for technical support. We apologize to authors whose work was not cited due to space limitations.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Smith C.O., Nehrke K., Brookes P.S. The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection. Biochem. J. 2017;474:2067–2094. doi: 10.1042/BCJ20160623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krabbendam I.E., Honrath B., Culmsee C., Dolga A.M. Mitochondrial Ca(2+)-activated K(+) channels and their role in cell life and death pathways. Cell Calcium. 2018;69:101–111. doi: 10.1016/j.ceca.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Szabo I., Zoratti M. Mitochondrial channels: Ion fluxes and more. Physiol. Rev. 2014;94:519–608. doi: 10.1152/physrev.00021.2013. [DOI] [PubMed] [Google Scholar]
- 4.Wang L., Zhu Q.L., Wang G.Z., Deng T.Z., Chen R., Liu M.H., Wang S.W. The protective roles of mitochondrial ATP-sensitive potassium channels during hypoxia-ischemia-reperfusion in brain. Neurosci. Lett. 2011;491:63–67. doi: 10.1016/j.neulet.2010.12.065. [DOI] [PubMed] [Google Scholar]
- 5.Xu W., Liu Y., Wang S., McDonald T., Van Eyk J.E., Sidor A., O’Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 6.Murata M., Akao M., O’Rourke B., Marbán E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca(2+) overload during simulated ischemia and reperfusion: Possible mechanism of cardioprotection. Circ. Res. 2001;89:891–898. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- 7.Leanza L., Henry B., Sassi N., Zoratti M., Chandy K.G., Gulbins E., Szabò I. Inhibitors of mitochondrial Kv1.3 channels induce Bax/Bak-independent death of cancer cells. EMBO Mol. Med. 2012;4:577–593. doi: 10.1002/emmm.201200235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leanza L., Trentin L., Becker K.A., Frezzato F., Zoratti M., Semenzato G., Szabò I. Clofazimine, Psora-4 and PAP-1, inhibitors of the potassium channel Kv1.3, as a new and selective therapeutic strategy in chronic lymphocytic leukemia. Leukemia. 2013;27:1782–1785. doi: 10.1038/leu.2013.56. [DOI] [PubMed] [Google Scholar]
- 9.Quast S.A., Berger A., Buttstadt N., Friebel K., Schonherr R., Eberle J. General sensitization of melanoma cells for TRAIL-induced apoptosis by the potassium channel inhibitor TRAM-34 depends on release of SMAC. PLoS ONE. 2013;7:e39290. doi: 10.1371/journal.pone.0039290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matkovic K., Koszela-Piotrowska I., Jarmuszkiewicz W., Szewczyk A. Ion conductance pathways in potato tuber (Solanum tuberosum) inner mitochondrial membrane. Biochim. Biophys. Acta. 2011;1807:275–285. doi: 10.1016/j.bbabio.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Kicinska A., Swida A., Bednarczyk P., Koszela-Piotrowska I., Choma K., Dolowy K., Szewczyk A., Jarmuszkiewicz W. ATP-sensitive potassium channel in mitochondria of the eukaryotic microorganism Acanthamoeba castellanii. J. Biol. Chem. 2007;282:17433–17441. doi: 10.1074/jbc.M701496200. [DOI] [PubMed] [Google Scholar]
- 12.Laskowski M., Kicinska A., Szewczyk A., Jarmuszkiewicz W. Mitochondrial large-conductance potassium channel from Dictyostelium discoideum. Int. J. Biochem. Cell Biol. 2015;60:167–175. doi: 10.1016/j.biocel.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Garlid K.D., Paucek P., Yarov-Yarovoy V., Murray H.N., Darbenzio R.B., D’Alonzo A.J., Lodge N.J., Smith M.A., Grover G.J. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels: Possible mechanism of cardioprotection. Circ. Res. 1997;81:1072–1082. doi: 10.1161/01.RES.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 14.Siemen D., Loupatatzis C., Borecky J., Gulbins E., Lang F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem. Biophys. Res. Commun. 1999;257:549–554. doi: 10.1006/bbrc.1999.0496. [DOI] [PubMed] [Google Scholar]
- 15.Skalska J., Piwońska M., Wyroba E., Surmacz L., Wieczorek R., Koszela-Piotrowska I., Zielińska J., Bednarczyk P., Dołowy K., Wilczynski G.M., et al. A novel potassium channel in skeletal muscle mitochondria. Biochim. Biophys. Acta. 2008;1777:651–659. doi: 10.1016/j.bbabio.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Jaggar J.H., Li A.L., Parfenova H., Liu J.X., Umstot E.S., Dopico A.M., Leffler C.W. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ. Res. 2005;97:805–812. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dallas M.L., Boyle J.P., Milligan C.J., Sayer R., Kerrigan T.L., McKinstry C., Lu P., Mankouri J., Harris M., Scragg J.L., et al. Carbon monoxide protects against oxidant-induced apoptosis via inhibition of Kv2.1. FASEB J. 2011;25:1519–1530. doi: 10.1096/fj.10-173450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duckles H., Al-Owais M.M., Elies J., Johnson E., Boycott H.E., Dallas M.L., Porter K.E., Boyle J.P., Scragg J.L., Peers C. T-Type Ca2+ channel regulation by CO: A mechanism for control of cell proliferation. Adv. Exp. Med. Biol. 2015;860:291–300. doi: 10.1007/978-3-319-18440-1_33. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson W.J., Gadeberg H.C., Harrison A.W., Allen N.D., Riccardi D., Kemp P.J. Carbon monoxide is a rapid modulator of recombinant and native P2X(2) ligand-gated ion channels. Br. J. Pharmacol. 2009;158:862–871. doi: 10.1111/j.1476-5381.2009.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson W.J., Kemp P.J. Carbon monoxide: An emerging regulator of ion channels. J. Physiol. 2011;589:3055–3062. doi: 10.1113/jphysiol.2011.206706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Von Burg R. Carbon monoxide. J. Appl. Toxicol. 1999;19:379–386. doi: 10.1002/(SICI)1099-1263(199909/10)19:5<379::AID-JAT563>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Ryter S.W., Choi A.M. Heme oxygenase-1/carbon monoxide: From metabolism to molecular therapy. Am. J. Respir. Cell Mol. Biol. 2009;41:251–260. doi: 10.1165/rcmb.2009-0170TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poulos T.L. Heme enzyme structure and function. Chem. Rev. 2014;114:3919–3962. doi: 10.1021/cr400415k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiabrando D., Vinchi F., Fiorito V., Mercurio S., Tolosano E. Heme in pathophysiology: A matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharm. 2014;5:61. doi: 10.3389/fphar.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawicki K.T., Shang M., Wu R.X., Chang H.C., Khechaduri A., Sato T., Kamide C., Liu T., Prasad S.V.N., Ardehali H. Increased heme levels in the heart lead to exacerbated ischemic injury. J. Am. Heart Assoc. 2015;4:e002272. doi: 10.1161/JAHA.115.002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barañano D.E., Rao M., Ferris C.D., Snyder S.H. Biliverdin reductase: A major physiologic cytoprotectant. Proc. Natl. Acad. Sci. USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Converso D.P., Taille C., Carreras M.C., Jaitovich A., Poderoso J.J., Boczkowski J. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J. 2006;20:1236–1238. doi: 10.1096/fj.05-4204fje. [DOI] [PubMed] [Google Scholar]
- 28.Ragsdale S.W. Metals and their scaffolds to promote difficult enzymatic reactions. Chem. Rev. 2006;106:3317–3337. doi: 10.1021/cr0503153. [DOI] [PubMed] [Google Scholar]
- 29.MacKenzie E.L., Ray P.D., Tsuji Y. Role and regulation of ferritin H in rotenone-mediated mitochondrial oxidative stress. Free Radic. Biol. Med. 2008;44:1762–1771. doi: 10.1016/j.freeradbiomed.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryter S.W., Tyrrell R.M. The heme synthesis and degradation pathways: Role in oxidant sensitivity—Heme oxygenase has both pro- and antioxidant properties. Free Radic. Biol. Med. 2000;28:289–309. doi: 10.1016/S0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 31.Landar A., Zmijewski J.W., Dickinson D.A., Le Goffe C., Johnson M.S., Milne G.L., Zanoni G., Vidari G., Morrow J.D., Darley-Usmar V.M. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1777–H1787. doi: 10.1152/ajpheart.01087.2005. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S., Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005;157:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Keyse S.M., Applegate L.A., Tromvoukis Y., Tyrrell R.M. Oxidant stress leads to transcriptional activation of the human hem oxygenase gene in cultured skin fibroblasts. Mol. Cell. Biol. 1990;10:4967–4969. doi: 10.1128/MCB.10.9.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paschen W., Uto A., Djuricic B., Schmitt J. Hemeoxygenase expression after reversible ischemia of rat-brain. Neurosci. Lett. 1994;180:5–8. doi: 10.1016/0304-3940(94)90900-8. [DOI] [PubMed] [Google Scholar]
- 35.Maines M.D., Trakshel G.M., Kutty R.K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J. Biol. Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- 36.Hayashi S., Omata Y., Sakamoto H., Higashimoto Y., Hara T., Sagara Y., Noguchi M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004;336:241–250. doi: 10.1016/j.gene.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Beckman J.D., Chen C.S., Nguyen J., Thayanithy V., Subramanian S., Steer C.J., Vercellotti G.M. Regulation of heme oxygenase-1 protein expression by miR-377 in combination with miR-217. J. Biol. Chem. 2011;286:3194–3202. doi: 10.1074/jbc.M110.148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Applegate L.A., Luscher P., Tyrrell R.M. Induction of heme oxygenase—A general response to oxidant stress in cultured-mammalian-cells. Cancer Res. 1991;51:974–978. [PubMed] [Google Scholar]
- 39.Keyse S.M., Tyrrell R.M. Heme oxygenase is the major 32-kDa stress protein-induced in human-skin fibroblasts by UVA radiation, hydrogen-peroxide, and sodium arsenite. Proc. Natl. Acad. Sci. USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barupala D.P., Dzul S.P., Riggs-Gelasco P.J., Stemmler T.L. Synthesis, delivery and regulation of eukaryotic heme and Fe-S cluster cofactors. Arch. Biochem. Biophys. 2016;592:60–75. doi: 10.1016/j.abb.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pae H.O., Chung H.T. Heme oxygenase-1: Its therapeutic roles in inflammatory diseases. Immune Netw. 2009;9:12–19. doi: 10.4110/in.2009.9.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan K.H., Ng M.K.C., Stocker R. Haem oxygenase-I and cardiovascular disease: mechanisms and therapeutic potential. Clin. Sci. 2011;120:493–504. doi: 10.1042/CS20100508. [DOI] [PubMed] [Google Scholar]
- 43.Bindu S., Pal C., Dey S., Goyal M., Alam A., Iqbal M.S., Dutta S., Sarkar S., Kumar R., Maity P., et al. Translocation of heme oxygenase-1 to mitochondria is a novel cytoprotective mechanism against non-steroidal anti-inflammatory drug-induced mitochondrial oxidative stress, apoptosis, and gastric mucosal injury. J. Biol. Chem. 2011;286:39387–39402. doi: 10.1074/jbc.M111.279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slebos D.J., Ryter S.W., van der Toorn M., Liu F., Guo F., Baty C.J., Karlsson J.M., Watkins S.C., Kim H.P., Wang X., et al. Mitochondrial localization and function of heme oxygenase-7 in cigarette smoke-induced cell death. Am. J. Respir. Cell Mol. Biol. 2007;36:409–417. doi: 10.1165/rcmb.2006-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Bolisetty S., Traylor A., Zarjou A., Johnson M.S., Benavides G.A., Ricart K., Boddu R., Moore R.D., Landar A., Barnes S., et al. Mitochondria-targeted heme oxygenase-1 decreases oxidative stress in renal epithelial cells. Am. J. Physiol. Ren. Physiol. 2013;305:F255–F264. doi: 10.1152/ajprenal.00160.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bansal S., Biswas G., Avadhani N.G. Mitochondria-targeted heme oxygenase-1 induces oxidative stress and mitochondrial dysfunction in macrophages, kidney fibroblasts and in chronic alcohol hepatotoxicity. Redox Biol. 2014;2:273–283. doi: 10.1016/j.redox.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regan R.F., Guo Y.P., Kumar N. Heme oxygenase-1 induction protects murine cortical astrocytes from hemoglobin toxicity. Neurosci. Lett. 2000;282:1–4. doi: 10.1016/S0304-3940(00)00817-X. [DOI] [PubMed] [Google Scholar]
- 48.Dore S., Goto S., Sampei K., Blackshaw S., Hester L.D., Ingi T., Sawa A., Traystman R.J., Koehler R.C., Snyder S.H. Heme oxygenase-2 acts to prevent neuronal death in brain cultures and following transient cerebral ischemia. Neuroscience. 2000;99:587–592. doi: 10.1016/S0306-4522(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 49.Yi L., Jenkins P.M., Leichert L.I., Jakob U., Martens J.R., Ragsdale S.W. Heme regulatory motifs in heme oxygenase-2 form a thiol/disulfide redox switch that responds to the cellular redox state. J. Biol. Chem. 2009;284:20556–20561. doi: 10.1074/jbc.M109.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi L., Ragsdale S.W. Evidence that the heme regulatory motifs in heme oxygenase-2 serve as a thiol/isulfide redox switch regulating heme binding. J. Biol. Chem. 2007;282:21056–21067. doi: 10.1074/jbc.M700664200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roth M., Rupp M., Hofmann S., Mittal M., Fuchs B., Sommer N., Parajuli N., Quanz K., Schubert D., Dony E., et al. Heme oxygenase-2 and large-conductance Ca(2+)-activated K(+) channels lung vascular effects of hypoxia. Am. J. Respir. Crit. Care Med. 2009;180:353–364. doi: 10.1164/rccm.200806-848OC. [DOI] [PubMed] [Google Scholar]
- 52.Boer J.L., Mulrooney S.B., Hausinger R.P. Nickel-dependent metalloenzymes. Arch. Biochem. Biophys. 2014;544:142–152. doi: 10.1016/j.abb.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruginsk S.G., Mecawi A.D., da Silva M.P., Reis W.L., Coletti R., de Lima J.B.M., Elias L.L.K., Antunes-Rodrigues J. Gaseous modulators in the control of the hypothalamic neurohypophyseal system. Physiology. 2015;30:127–138. doi: 10.1152/physiol.00040.2014. [DOI] [PubMed] [Google Scholar]
- 54.Durante W., Schafer A.I. Carbon monoxide and vascular cell function. Int. J. Mol. Med. 1998;2:255–262. doi: 10.3892/ijmm.2.3.255. [DOI] [PubMed] [Google Scholar]
- 55.Ryter S.W., Alam J., Choi A.M.K. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 56.Morita T., Perrella M.A., Lee M.E., Kourembanas S. Smooth-muscle cell-derived carbon-monoxide is a regulator of vascular cGMP. Proc. Natl. Acad. Sci. USA. 1995;92:1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brune B., Schmidt K.U., Ullrich V. Activation of soluble guanylate-cyclase by carbon-monoxide and inhibition by superoxide anion. Eur. J. Biochem. 1990;192:683–688. doi: 10.1111/j.1432-1033.1990.tb19276.x. [DOI] [PubMed] [Google Scholar]
- 58.Seya K., Motomura S., Furukawa K. Cardiac mitochondrial cGMP stimulates cytochrome c release. Clin. Sci. 2007;112:113–121. doi: 10.1042/CS20060144. [DOI] [PubMed] [Google Scholar]
- 59.Seya K., Ono K., Fujisawa S., Okumura K., Motomura S., Furukawa K. Cytosolic Ca2+-induced apoptosis in rat cardiomyocytes via mitochondrial NO-cGMP-protein kinase G pathway. J. Pharmacol. Exp. Ther. 2013;344:77–84. doi: 10.1124/jpet.112.198176. [DOI] [PubMed] [Google Scholar]
- 60.Behmenburg F., Dorsch M., Huhn R., Mally D., Heinen A., Hollmann M.W., Berger M.M. Impact of mitochondrial Ca2+-sensitive potassium (mBK(Ca)) channels in sildenafil-induced cardioprotection in rats. PLoS ONE. 2015;10:e0144737. doi: 10.1371/journal.pone.0144737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nara M., Dhulipala P.D.K., Ji G.J., Kamasani U.R., Wang Y.X., Matalon S., Kotlikoff M.I. Guanylyl cyclase stimulatory coupling to K-Ca channels. Am. J. Physiol. Cell Physiol. 2000;279:C1938–C1945. doi: 10.1152/ajpcell.2000.279.6.C1938. [DOI] [PubMed] [Google Scholar]
- 62.Nara M., Dhulipala P.D.K., Kotlikoff M.I. cGMP-dependent protein kinase (PKG) modulation of maxi-K channels expressed in Xenopus oocytes. Am. J. Respir. Crit. Care Med. 1999;159:A723. [Google Scholar]
- 63.Fukao M., Mason H.S., Britton F.C., Kenyon J.L., Horowitz B., Keef K.D. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J. Biol. Chem. 1999;274:10927–10935. doi: 10.1074/jbc.274.16.10927. [DOI] [PubMed] [Google Scholar]
- 64.Frankenreiter S., Bednarczyk P., Kniess A., Bork N.I., Straubinger J., Koprowski P., Wrzosek A., Mohr E., Logan A., Murphy M.P., et al. cGMP-elevating compounds and ischemic conditioning provide cardioprotection against ischemia and reperfusion injury via cardiomyocyte-specific BKCa channels. Circulation. 2017;136:2337–2355. doi: 10.1161/CIRCULATIONAHA.117.028723. [DOI] [PubMed] [Google Scholar]
- 65.Tang X.D., Xu R., Reynolds M.F., Garcia M.L., Heinemann S.H., Hoshi T. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature. 2003;425:531–535. doi: 10.1038/nature02003. [DOI] [PubMed] [Google Scholar]
- 66.Bowman S.E.J., Bren K.L. The chemistry and biochemistry of heme c: Functional bases for covalent attachment. Nat. Prod. Rep. 2008;25:1118–1130. doi: 10.1039/b717196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams S.E.J., Wootton P., Mason H.S., Bould J., Iles D.E., Riccardi D., Peers C., Kemp P.J. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306:2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- 68.Yi L., Morgan J.T., Ragsdale S.W. Identification of a thiol/disulfide redox switch in the human BK channel that controls its affinity for heme and CO. J. Biol. Chem. 2010;285:20117–20127. doi: 10.1074/jbc.M110.116483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang M., Zhong H., Vollmer C., Nurse C.A. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J. Physiol. 2000;525:143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaczara P., Motterlini R., Rosen G.M., Augustynek B., Bednarczyk P., Szewczyk A., Foresti R., Chlopicki S. Carbon monoxide released by CORM-401 uncouples mitochondrial respiration and inhibits glycolysis in endothelial cells: A role for mitoBKCa channels. Biochim. Biophys. Acta. 2015;1847:1297–1309. doi: 10.1016/j.bbabio.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Clark J.E., Naughton P., Shurey S., Green C.J., Johnson T.R., Mann B.E., Foresti R., Motterlini R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ. Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 72.Soni H., Patel P., Rath A.C., Jain M., Mehta A.A. Cardioprotective effect with carbon monoxide releasing molecule-2 (CORM-2) in isolated perfused rat heart: Role of coronary endothelium and underlying mechanism. Vasc. Pharmacol. 2010;53:68–76. doi: 10.1016/j.vph.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 73.Burton M.J., Kapetanaki S.M., Chernova T., Jamieson A.G., Dorlet P., Santolini J., Moody P.C.E., Mitcheson J.S., Davies N.W., Schmid R., et al. A heme-binding domain controls regulation of ATP-dependent potassium channels. Proc. Natl. Acad. Sci. USA. 2016;113:3785–3790. doi: 10.1073/pnas.1600211113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 75.Zingman L.V., Hodgson D.M., Bast P.H., Kane G.C., Perez-Terzic C., Gumina R.J., Pucar D., Bienengraeber M., Dzeja P.P., Miki T., et al. Kir6.2 is required for adaptation to stress. Proc. Natl. Acad. Sci. USA. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kapetanaki S.M., Burton M.J., Basran J., Uragami C., Moody P.C.E., Mitcheson J.S., Schmid R., Davies N.W., Dorlet P., Vos M.H., et al. A mechanism for CO regulation of ion channels. Nat. Commun. 2018;9:907. doi: 10.1038/s41467-018-03291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye B., Kroboth S.L., Pu J.L., Sims J.J., Aggarwal N.T., McNally E.M., Makielski J.C., Shi N.Q. Molecular identification and functional characterization of a mitochondrial sulfonylurea receptor 2 splice variant generated by intraexonic splicing. Circ. Res. 2009;105:1083–1093. doi: 10.1161/CIRCRESAHA.109.195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou M., Tanaka O., Sekiguchi M., He H.J., Yasuoka Y., Itoh H., Kawahara K., Abe H. ATP-sensitive K+-channel subunits on the mitochondria and endoplasmic reticulum of rat cardiomyocytes. J. Histochem. Cytochem. 2005;53:1491–1500. doi: 10.1369/jhc.5A6736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ng K.E., Schwarzer S., Duchen M.R., Tinker A. The intracellular localization and function of the ATP-sensitive K+ channel subunit Kir6.1. J. Membr. Biol. 2010;234:137–147. doi: 10.1007/s00232-010-9241-x. [DOI] [PubMed] [Google Scholar]
- 80.Kuniyasu A., Kaneko K., Kawahara K., Nakayama H. Molecular assembly and subcellular distribution of ATP-sensitive potassium channel proteins in rat hearts. FEBS Lett. 2003;552:259–263. doi: 10.1016/S0014-5793(03)00936-0. [DOI] [PubMed] [Google Scholar]
- 81.Foster D.B., Rucker J.J., Marban E. Is Kir6.1 a subunit of mitoK(ATP)? Biochem. Biophys. Res. Commun. 2008;366:649–656. doi: 10.1016/j.bbrc.2007.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Foster D.B., Ho A.S., Rucker J., Garlid A.O., Chen L., Sidor A., Garlid K.D., O’Rourke B. Mitochondrial ROMK channel is a molecular component of mitoK(ATP) Circ. Res. 2012;111:446–454. doi: 10.1161/CIRCRESAHA.112.266445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kottelat E., Zobi F. Visible light-activated photoCORMs. Inorganics. 2017;5:24. doi: 10.3390/inorganics5020024. [DOI] [Google Scholar]
- 84.Kottelat E., Ruggi A., Zobi F. Red-light activated photoCORMs of Mn(I) species bearing electron deficient 2,2′-azopyridines. Dalton Trans. 2016;45:6920–6927. doi: 10.1039/C6DT00858E. [DOI] [PubMed] [Google Scholar]
- 85.Ruggi A., Zobi F. Quantum-CORMs: Quantum dot sensitized CO releasing molecules. Dalton Trans. 2015;44:10928–10931. doi: 10.1039/C5DT01681A. [DOI] [PubMed] [Google Scholar]
- 86.Zobi F. CO and CO-releasing molecules in medicinal chemistry. Future Med. Chem. 2013;5:175–188. doi: 10.4155/fmc.12.196. [DOI] [PubMed] [Google Scholar]
- 87.Zobi F., Quaroni L., Santoro G., Zlateva T., Blacque O., Sarafimov B., Schaub M.C., Bogdanova A.Y. Live-fibroblast IR imaging of a cytoprotective photoCORM activated with visible light. J. Med. Chem. 2013;56:6719–6731. doi: 10.1021/jm400527k. [DOI] [PubMed] [Google Scholar]
- 88.Ling K., Men F., Wang W.C., Zhou Y.Q., Zhang H.W., Ye D.W. Carbon monoxide and its controlled release: Therapeutic application, detection, and development of carbon monoxide releasing molecules (CORMs) J. Med. Chem. 2018;61:2611–2635. doi: 10.1021/acs.jmedchem.6b01153. [DOI] [PubMed] [Google Scholar]
- 89.Wilson J.L., Wareham L.K., McLean S., Begg R., Greaves S., Mann B.E., Sanguinetti G., Poole R.K. CO-releasing molecules have nonheme targets in bacteria: Transcriptomic, mathematical modeling and biochemical analyses of CORM-3 Ru(CO)(3)Cl(glycinate) actions on a heme-deficient mutant of Escherichia coli. Antioxid. Redox Signal. 2015;23:148–162. doi: 10.1089/ars.2014.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Santos M.F.A., Seixas J.D., Coelho A.C., Mukhopadhyay A., Reis P.M., Romao M.J., Romao C.C., Santos-Silva T. New insights into the chemistry of fac- Ru(CO)(3) (2+) fragments in biologically relevant conditions: The CO releasing activity of Ru(CO)(3)Cl-2(1,3-thiazole), and the X-ray crystal structure of its adduct with lysozyme. J. Inorg. Biochem. 2012;117:285–291. doi: 10.1016/j.jinorgbio.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 91.Riddle M.A., Walker B.R. Regulation of endothelial BK channels by heme oxygenase-derived carbon monoxide and caveolin-1. Am. J. Physiol. Cell Physiol. 2012;303:C92–C101. doi: 10.1152/ajpcell.00356.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dong D.L., Zhang Y., Lin D.H., Chen J., Patschan S., Goligorsky M.S., Nasjletti A., Yang B.F., Wang W.H. Carbon monoxide stimulates the Ca2+-activated big conductance K channels in cultured human endothelial cells. Hypertension. 2007;50:643–651. doi: 10.1161/HYPERTENSIONAHA.107.096057. [DOI] [PubMed] [Google Scholar]
- 93.Wang Z.J., Yue P., Lin D.H., Wang W.H. Carbon monoxide stimulates Ca2+-dependent big-conductance K channels in the cortical collecting duct. Am. J. Physiol. Ren. Physiol. 2013;304:F543–F552. doi: 10.1152/ajprenal.00530.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao H.C., Liu B.L., Zhang Q.Y., Wu X.A., Yu Y., Jesse F.F., Li L.M. Carbon monoxide augments electrical signaling in cultured neural networks of hippocampal neurons partly through activation of BKCa channels. Acta Biochim. Biophys. Sin. 2015;47:383–389. doi: 10.1093/abbs/gmv017. [DOI] [PubMed] [Google Scholar]
- 95.Williams S.E., Brazier S.P., Baban N., Telezhkin V., Muller C.T., Riccardi D., Kemp P.J. A structural motif in the C-terminal tail of slo1 confers carbon monoxide sensitivity to human BK(Ca) channels. Pflug. Arch. Eur. J. Physiol. 2008;456:561–572. doi: 10.1007/s00424-007-0439-4. [DOI] [PubMed] [Google Scholar]
- 96.Hou S.W., Xu R., Heinemann S.H., Hoshi T. The RCK1 high-affinity Ca2+ sensor confers carbon monoxide sensitivity to Slo1 BK channels. Proc. Natl. Acad. Sci. USA. 2008;105:4039–4043. doi: 10.1073/pnas.0800304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gessner G., Sahoo N., Swain S.M., Hirth G., Schonherr R., Mede R., Westerhausen M., Brewitz H.H., Heimer P., Imhof D., et al. CO-independent modification of K+ channels by tricarbonyldichlororuthenium(II) dimer (CORM-2) Eur. J. Pharmacol. 2017;815:33–41. doi: 10.1016/j.ejphar.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jara-Oseguera A., Ishida I.G., Rangel-Yescas G.E., Espinosa-Jalapa N., Perez-Guzman J.A., Elias-Vinas D., Le Lagadec R., Rosenbaum T., Islas L.D. Uncoupling charge movement from channel opening in voltage-gated potassium channels by ruthenium complexes. J. Biol. Chem. 2011;286:16414–16425. doi: 10.1074/jbc.M110.198010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horrigan F.T., Heinemann S.H., Hoshi T. Heme regulates allosteric activation of the Slo1 BK channel. J. Gen. Physiol. 2005;126:7–21. doi: 10.1085/jgp.200509262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wareham L.K., McLean S., Begg R., Rana N., Ali S., Kendall J.J., Sanguinetti G., Mann B.E., Poole R.K. The broad-spectrum antimicrobial potential of [Mn(CO)4(S2CNMe(CH2CO2H))], a water-soluble CO-Releasing Molecule (CORM-401): Intracellular accumulation, transcriptomic and statistical analyses, and membrane polarization. Antioxid. Redox Signal. 2018;28:1286–1308. doi: 10.1089/ars.2017.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abeyrathna N., Washington K., Bashur C., Liao Y. Nonmetallic carbon monoxide releasing molecules (CORMs) Org. Biomol. Chem. 2017;15:8692–8699. doi: 10.1039/C7OB01674C. [DOI] [PubMed] [Google Scholar]
- 102.Winyard M.W., Paul G. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert Rev. Clin. Pharmacol. 2011;4:13–32. doi: 10.1586/ecp.10.134. [DOI] [PubMed] [Google Scholar]
- 103.Dunn W.R., Alexander S.P., Ralevic V., Roberts R.E. Effects of hydrogen sulphide in smooth muscle. Pharmacol. Ther. 2016;158:101–113. doi: 10.1016/j.pharmthera.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 104.Wu D., Hu Q., Liu X., Pan L., Xiong Q., Zhu Y.Z. Hydrogen sulfide protects against apoptosis under oxidative stress through SIRT1 pathway in H9c2 cardiomyocytes. Nitric Oxide. 2015;46:204–212. doi: 10.1016/j.niox.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 105.Elrod J.W., Calvert J.W., Morrison J., Doeller J.E., Kraus D.W., Tao L., Jiao X., Scalia R., Kiss L., Szabo C., et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang X., Wang Q., Guo W., Zhu Y.Z. Hydrogen sulfide attenuates cardiac dysfunction in a rat model of heart failure: A mechanism through cardiac mitochondrial protection. Biosci. Rep. 2011;31:87–98. doi: 10.1042/BSR20100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maclean K., Kraus J. Hydrogen sulfide production and metabolism in mammalian tissues. Signal Transduct. Gasotransmitters. 2004:275–292. doi: 10.1007/978-1-59259-806-9. [DOI] [Google Scholar]
- 108.Shibuya N., Koike S., Tanaka M., Ishigami-Yuasa M., Kimura Y., Ogasawara Y., Fukui K., Nagahara N., Kimura H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013;4:1366. doi: 10.1038/ncomms2371. [DOI] [PubMed] [Google Scholar]
- 109.Fu M., Zhang W., Wu L., Yang G., Li H., Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA. 2012;109:2943–2948. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shibuya N., Tanaka M., Yoshida M., Ogasawara Y., Togawa T., Ishii K., Kimura H. 3-mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 111.Gould S.J., Keller G.A., Subramani S. Identification of peroxisomal targeting signals located at the carboxy terminus of four peroxisomal proteins. J. Cell Biol. 1988;107:897–905. doi: 10.1083/jcb.107.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Powell C.R., Dillon K.M., Matson J.B. A review of hydrogen sulfide (H(2)S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2017;149:110–123. doi: 10.1016/j.bcp.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sodha N.R., Clements R.T., Feng J., Liu Y., Bianchi C., Horvath E.M., Szabo C., Sellke F.W. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur. J. Cardio Thorac. Surg. 2008;33:906–913. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kloesch B., Liszt M., Steiner G., Broll J. Inhibitors of p38 and ERK1/2 MAPkinase and hydrogen sulphide block constitutive and IL-1beta-induced IL-6 and IL-8 expression in the human chondrocyte cell line C-28/I2. Rheumatol. Int. 2012;32:729–736. doi: 10.1007/s00296-010-1682-0. [DOI] [PubMed] [Google Scholar]
- 115.Esechie A., Kiss L., Olah G., Horvath E.M., Hawkins H., Szabo C., Traber D.L. Protective effect of hydrogen sulfide in a murine model of acute lung injury induced by combined burn and smoke inhalation. Clin. Sci. 2008;115:91–97. doi: 10.1042/CS20080021. [DOI] [PubMed] [Google Scholar]
- 116.Zhao Y., Biggs T.D., Xian M. Hydrogen sulfide (H2S) releasing agents: Chemistry and biological applications. Chem. Commun. 2014;50:11788–11805. doi: 10.1039/C4CC00968A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gerő D., Torregrossa R., Perry A., Waters A., Le-Trionnaire S., Whatmore J.L., Wood M., Whiteman M. The novel mitochondria-targeted hydrogen sulfide (H2S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol. Res. 2016;113:186–198. doi: 10.1016/j.phrs.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kimura H. Hydrogen sulfide and polysulfides as signaling molecules. Proc. Jpn. Acad. Ser. B. 2015;91:131–159. doi: 10.2183/pjab.91.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Collman J.P., Ghosh S., Dey A., Decréau R.A. Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc. Natl. Acad. Sci. USA. 2009;106:22090–22095. doi: 10.1073/pnas.0904082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Modis K., Coletta C., Erdelyi K., Papapetropoulos A., Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27:601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- 121.Paul B.D., Snyder S.H. H2S: A novel gasotransmitter that signals by sulfhydration. Trends Biochem. Sci. 2015;40:687–700. doi: 10.1016/j.tibs.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mustafa A.K., Gadalla M.M., Sen N., Kim S., Mu W., Gazi S.K., Barrow R.K., Yang G., Wang R., Snyder S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Módis K., Ju Y., Ahmad A., Untereiner A.A., Altaany Z., Wu L., Szabo C., Wang R. S-sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol. Res. 2016;113:116–124. doi: 10.1016/j.phrs.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang D., Du J., Tang C., Huang Y., Jin H. H2S-induced sulfhydration: Biological function and detection methodology. Front. Pharmacol. 2017;8:608. doi: 10.3389/fphar.2017.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Greiner R., Pálinkás Z., Bäsell K., Becher D., Antelmann H., Nagy P., Dick T.P. Polysulfides link H2S to protein thiol oxidation. Antioxid. Redox Signal. 2013;19:1749–1765. doi: 10.1089/ars.2012.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhong G.Z. Hydrogen sulfide—A potent multichannel anti-arrhythmic drug. J. Cardiovasc. Dis. Res. 2010;1:37–39. doi: 10.4103/0975-3583.59984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mustafa A.K., Sikka G., Gazi S.K., Steppan J., Jung S.M., Bhunia A.K., Barodka V.M., Gazi F.K., Barrow R.K., Wang R., et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ha J., Xu Y., Kawano T., Hendon T., Baki L., Garai S., Papapetropoulos A., Thakur G.A., Plant L.D., Logothetis D.E. Hydrogen sulfide inhibits Kir2 and Kir3 channels by decreasing sensitivity to the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) J. Biol. Chem. 2018;293:3546–3561. doi: 10.1074/jbc.RA117.001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tang G., Wu L., Liang W., Wang R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol. Pharmacol. 2005;68:1757–1764. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- 130.Testai L., Marino A., Piano I., Brancaleone V., Tomita K., Di Cesare Mannelli L., Martelli A., Citi V., Breschi M.C., Levi R., et al. The novel H2S-donor 4-carboxyphenyl isothiocyanate promotes cardioprotective effects against ischemia/reperfusion injury through activation of mitoKATP channels and reduction of oxidative stress. Pharmacol. Res. 2016;113:290–299. doi: 10.1016/j.phrs.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 131.Sivarajah A., Collino M., Yasin M., Benetti E., Gallicchio M., Mazzon E., Cuzzocrea S., Fantozzi R., Thiemermann C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock. 2009;31:267–274. doi: 10.1097/SHK.0b013e318180ff89. [DOI] [PubMed] [Google Scholar]
- 132.Sitdikova G.F., Weiger T.M., Hermann A. Hydrogen sulfide increases calcium-activated potassium (BK) channel activity of rat pituitary tumor cells. Pflug. Arch. Eur. J. Physiol. 2010;459:389–397. doi: 10.1007/s00424-009-0737-0. [DOI] [PubMed] [Google Scholar]
- 133.Sitdikova G.F., Fuchs R., Kainz V., Weiger T.M., Hermann A. Phosphorylation of BK channels modulates the sensitivity to hydrogen sulfide (H2S) Front. Physiol. 2014;5:431. doi: 10.3389/fphys.2014.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Telezhkin V., Brazier S.P., Cayzac S., Muller C.T., Riccardi D., Kemp P.J. Hydrogen sulfide inhibits human BK(Ca) channels. Adv. Exp. Med. Biol. 2009;648:65–72. doi: 10.1007/978-90-481-2259-2_7. [DOI] [PubMed] [Google Scholar]
- 135.Ahmad A., Dempsey S.K., Daneva Z., Azam M., Li N., Li P.L., Ritter J.K. Role of nitric oxide in the cardiovascular and renal systems. Int. J. Mol. Sci. 2018;19:2605. doi: 10.3390/ijms19092605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Förstermann U., Sessa W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miller M.W., Knaub L.A., Olivera-Fragoso L.F., Keller A.C., Balasubramaniam V., Watson P.A., Reusch J.E. Nitric oxide regulates vascular adaptive mitochondrial dynamics. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H1624–H1633. doi: 10.1152/ajpheart.00987.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elfering S.L., Sarkela T.M., Giulivi C. Biochemistry of mitochondrial nitric-oxide synthase. J. Biol. Chem. 2002;277:38079–38086. doi: 10.1074/jbc.M205256200. [DOI] [PubMed] [Google Scholar]
- 139.Haynes V., Elfering S., Traaseth N., Giulivi C. Mitochondrial nitric-oxide synthase: enzyme expression, characterization, and regulation. J. Bioenerg. Biomembr. 2004;36:341–346. doi: 10.1023/B:JOBB.0000041765.27145.08. [DOI] [PubMed] [Google Scholar]
- 140.Brown G.C. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995;369:136–139. doi: 10.1016/0014-5793(95)00763-Y. [DOI] [PubMed] [Google Scholar]
- 141.Packer M.A., Porteous C.M., Murphy M.P. Superoxide production by mitochondria in the presence of nitric oxide forms peroxynitrite. Biochem. Mol. Biol. Int. 1996;40:527–534. doi: 10.1080/15216549600201103. [DOI] [PubMed] [Google Scholar]
- 142.Radi R., Rodriguez M., Castro L., Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch. Biochem. Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 143.Holmuhamedov E.L., Wang L., Terzic A. ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria. J. Physiol. 1999;519:347–360. doi: 10.1111/j.1469-7793.1999.0347m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Katakam P.V., Dutta S., Sure V.N., Grovenburg S.M., Gordon A.O., Peterson N.R., Rutkai I., Busija D.W. Depolarization of mitochondria in neurons promotes activation of nitric oxide synthase and generation of nitric oxide. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H1097–H1106. doi: 10.1152/ajpheart.00759.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sasaki N., Sato T., Ohler A., O’Rourke B., Marban E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation. 2000;101:439–445. doi: 10.1161/01.CIR.101.4.439. [DOI] [PubMed] [Google Scholar]
- 146.Ljubkovic M., Shi Y., Cheng Q., Bosnjak Z., Jiang M.T. Cardiac mitochondrial ATP-sensitive potassium channel is activated by nitric oxide in vitro. FEBS Lett. 2007;581:4255–4259. doi: 10.1016/j.febslet.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 147.Dahlem Y.A., Horn T.F., Buntinas L., Gonoi T., Wolf G., Siemen D. The human mitochondrial KATP channel is modulated by calcium and nitric oxide: A patch-clamp approach. Biochim. Biophys. Acta. 2004;1656:46–56. doi: 10.1016/j.bbabio.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 148.Kawano T., Zoga V., Kimura M., Liang M.Y., Wu H.E., Gemes G., McCallum J.B., Kwok W.M., Hogan Q.H., Sarantopoulos C.D. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: action by direct S-nitrosylation. Mol. Pain. 2009;5:12. doi: 10.1186/1744-8069-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sánchez-Duarte E., Trujillo X., Huerta M., Ortiz-Mesina M., Cortés-Rojo C., Manzo-Ávalos S., Saavedra-Molina A., Montoya-Pérez R. Mitochondrial KATP channels in skeletal muscle are protein kinases C and G and nitric oxide synthase involved in the fatigue process. Open Access Anim. Physiol. 2012;4:21–28. doi: 10.2147/OAAP.S34818. [DOI] [Google Scholar]
- 150.Zhang D.M., Chai Y.P., Erickson J.R., Brown J.H., Bers D.M., Lin Y.F. Intracellular signalling mechanism responsible for modulation of sarcolemmal ATP sensitive potassium channels by nitric oxide in ventricular cardiomyocytes. J. Physiol. 2014;592:971–990. doi: 10.1113/jphysiol.2013.264697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mio Y., Shim Y.H., Richards E., Bosnjak Z.J., Pagel P.S., Bienengraeber M. Xenon preconditioning: The role of prosurvival signaling, mitochondrial permeability transition and bioenergetics in rats. Anesth. Analg. 2009;108:858–866. doi: 10.1213/ane.0b013e318192a520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li Q., Lian C., Zhou R., Li T., Xiang X., Liu B. Pretreatment with xenon protected immature rabbit heart from ischaemia/reperfusion injury by opening of the mitoKATP channel. Heart Lung Circ. 2013;22:276–283. doi: 10.1016/j.hlc.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 153.Wang X.B., Cui H., Du J.B. Sulfur dioxide: A physiologic endothelium derived relaxing factor. Histol. Histopathol. 2017;32:21–26. doi: 10.14670/HH-11-801. [DOI] [PubMed] [Google Scholar]
- 154.Wang X.B., Du J.B., Cui H. Signal pathways involved in the biological effects of sulfur dioxide. Eur. J. Pharmacol. 2015;764:94–99. doi: 10.1016/j.ejphar.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 155.Wang R. Gasotransmitters: Growing pains and joys. Trends Biochem. Sci. 2014;39:227–232. doi: 10.1016/j.tibs.2014.03.003. [DOI] [PubMed] [Google Scholar]