Abstract
A series of novel bivalent β-carboline derivatives were designed and synthesized, and in vitro cytotoxicity, cell apoptosis, and DNA-binding affinity were evaluated. The cytotoxic results demonstrated that most bivalent β-carboline derivatives exhibited stronger cytotoxicity than the corresponding monomer against the five selected tumor cell lines (A549, SGC-7901, Hela, SMMC-7721, and MCF-7), indicating that the dimerization at the C3 position could enhance the antitumor activity of β-carbolines. Among the derivatives tested, 4B, 6i, 4D, and 6u displayed considerable cytotoxicity against A549 cell line. Furthermore, 4B, 6i, 4D, and 6u induced cell apoptosis in a dose-dependent manner, and caused cell cycle arrest at the S and G2/M phases. Moreover, the levels of cytochrome C in mitochondria, and the expressions of bcl-2 protein, decreased after treatment with β-carbolines, which indicated that 6i and 6u could induce mitochondria-mediated apoptosis. In addition, the results of UV-visible spectral, thermal denaturation, and molecular docking studies revealed that 4B, 6i, 4D, and 6u could bind to DNA mainly by intercalation.
Keywords: bivalent β-carbolines, antitumor, apoptosis, DNA-binding affinity, bcl-2
1. Introduction
β-carboline alkaloids, originally isolated from Peganum harmala, are a class of natural and synthetic indole alkaloids with a tricyclic pyrido[3,4-b]indole ring system [1,2]. β-carbolines have multiple biological and pharmacological properties [2,3,4], of which antitumor activity is the most widely studied [5,6,7,8]. Previous studies have shown that β-carbolines exerted their antitumor activity mainly through intercalating into DNA [7,9,10,11,12,13], inducing apoptosis, and inhibiting topoisomerase I and II (Top I and II) [13,14,15], cyclin-dependent kinases (CDKs) [16,17], mitogen-activated protein kinase (MAPK) [18], and I-Kappa-B kinase (IKK) [19]. Particularly, parent carbolines can be inserted into DNA, which can further cause cell apoptosis [20,21]. The interaction between β-carboline alkaloids and DNA could cause changes in DNA conformation, which further affects DNA replication, transcription, and repair [22,23]. Furthermore, the existing studies have demonstrated that β-carboline could induce HepG2 cells apoptosis by down-regulating bcl-2 expression [24].
DNA-targeted antitumor drugs, such as anthracyclines, acridines, and quinones, which exert antitumor activity through DNA insertion, are considered to be one of the most effective drugs in clinical applications [25]. These drugs exert antitumor activity mainly through non-covalent and covalent interactions with the minor groove, major groove, or base pairs (intercalation) of the DNA double helix. The discovery of antitumor drugs has focused on the development of new DNA-intercalating scaffolds, such as β-carboline derivatives [7]. Therefore, we expect to discover and develop new antitumor agents by modifying these bioactive scaffolds with appropriate substituents.
Previous structure-activity relationships (SARs) have shown that the introduction of appropriate substituents at the C1, C3, and N9 positions of the β-carboline scaffolds could enhance antitumor activity and DNA-binding affinity [5,7,10,26,27]. It has also been reported that phenyl and heterocyclic substituted at the C1 and C3 positions of β-carbolines, respectively, have exhibited potential antitumor activity [12,28,29,30]. The introduction of methyl and benzyl substituents at the N9 position of β-carbolines could enhance the DNA affinity [10]. Further studies have demonstrated that dimerization of small molecules by appropriate linkers could significantly improve the DNA-binding affinity. It has been found that the dimerized compounds could bind to DNA by a bis-intercalation mode, causing pronounced changes in DNA structure [31,32]. Bivalent β-carbolines linked at the C6 position or N9 position have been synthesized, and were found to be potential anti-Alzheimer agents [33]. In addition, the synthesis and evaluation of bivalent β-carbolines linked by 3–10 methylene units at the N9 position as antitumor agents have also been reported [34]. Our group has synthesized 25 bivalent β-carbolines modified at the C1 (-CH3), C7 (-OCH3), and N9 positions (-CH3 and -CH2Ph2), and dimerized at the C2 position, and the results showed that the dimerization could significantly increase antitumor activity of β-carbolines. Moreover, compounds 4A, 6b, 6d, and 6e, which exhibit good antitumor activity, have been reported [5,35]. However, the SARs of the antitumor activity in vitro of bivalent β-carbolines linked at the C3 position were rarely reported.
In this study, we designed and synthesized a series of novel β-carboline derivatives modified at the N9 position and linked at the C3 position, and further investigated the antitumor activity and DNA-binding affinity in vitro. We expected to discover novel β-carboline derivatives with promising antitumor activity and DNA-binding affinity.
2. Results
2.1. Chemistry
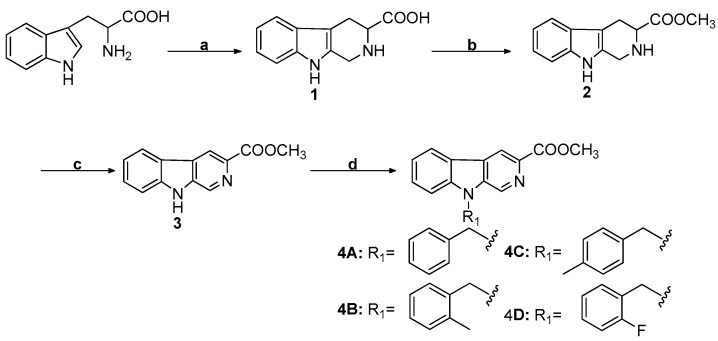
Using L-tryptophan as a raw material, N9 substituted β-carboline-3-carboxylic acid methyl esters (monomers, 4A, 4B, 4C, and 4D) were synthesized mainly through the Pictet–Spengler (P–S) reaction, esterification reaction, and 9-substitution reaction, as previously described [5,36]. The N9 substituted β-carboline-3-carboxylic acid methyl esters prepared in the above steps were hydrolyzed and then reacted with dibromoalkane to obtain a series of bivalent β-carboline derivatives (dimers, 6a-6f, 6g-6l, 6m-6r, and 6s-6x) [37]. The synthetic routes and reaction conditions of β-carboline monomers (4A, 4B, 4C, and 4D) and dimers (6a-6f, 6g-6l, 6m-6r, and 6s-6x) are shown in Scheme 1 and Scheme 2, respectively. In this study, we synthesized 3 novel monomers and 21 novel dimers, and evaluated their antitumor activity.
Scheme 1.
Synthesis of N9-substituted β-carboline-3-carboxylic acid methyl esters (4A, 4B, 4C, and 4D). Reagents and conditions: (a) H+, HCHO, room temperature (r.t.); (b) SOCl2, CH3OH, reflux; (c) Pd/C, xylene, reflux; (d) DMF, NaH, r.t., R1-Br.
Scheme 2.
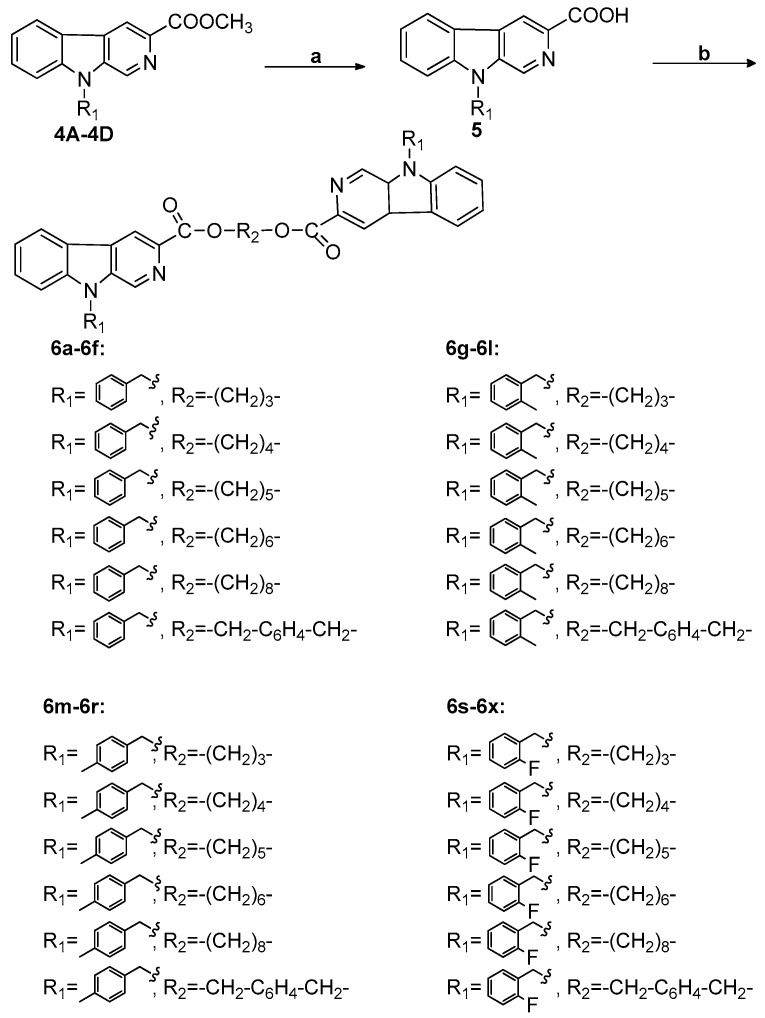
Synthesis of bivalent β-carboline-3-carboxylic acid derivatives (6a-6f, 6g-6l, 6m-6r, and 6s-6x). Reagents and conditions: (a) THF/CH3OH, OH–, r.t.; (b) DMF, K2CO3, BrR2Br, heat.
2.2. In Vitro Cytotoxicity Assay
The antitumor activity of novel β-carboline derivatives modified at the N9 position and linked at the C3 position have been evaluated in vitro against A549, Hela, SGC-7901, SMMC-7721, MCF-7, and MRC5 cell lines using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, with the results expressed as IC50 Values. Previous reports have evidenced that β-carbolines substituted with an alkoxycarbonyl or carboxyl at the C3 position, and with a short alkyl or benzyl at the N9 position, exhibited more significant antitumor activities against Hela cells [5]. In the present research (Table 1), for all the cell lines tested, only the β-carboline monomer substituted with o-fluorobenzyl (4D) at N9 position displayed hihest antitumor activity. For A549 cell line, among all the substituents at the N9 position, it was found that the antitumor activity of p-methylbenzyl (4C, 28.06 μM) substituent was better than that of the o-methylbenzyl (4B, 39.20 μM) substituent. Next, the o-fluorobenzyl side chain with the lowest IC50 value (4D, 13.94 μM) was considered to be the most beneficial to increase antitumor activity at the N9 position. But the antitumor activities of bivalent β-carboline derivatives (dimer, 6i, 6.93 μM; 6u, 5.61 μM) were apparently better than that of the corresponding monomer (4B, 39.20 μM; 4D, 13.94 μM), indicating that the dimerization was an effective modification for improving the antitumor activity of β-carbolines. The IC50 values of bivalent β-carboline derivatives were relatively lower when the linkers were short in length and odd in carbon numbers (6s, 6.12 μM; 6u, 5.61 μM). These results indicated that the introduction of o-fluorobenzyl at the N9 position and the dimerization at the C3 position could significantly increase the antitumor activity of β-carbolines, and they have better antitumor activity when the length of the linkers was four to six methylene units.
Table 1.
The IC50 values (μM) of β-carboline monomers (4A, 4B, 4C, and 4D) and bivalent β-carboline derivatives (6a-6f, 6g-6l, 6m-6r, and 6s-6x) against A549, SGC-7901, Hela, SMMC-7721, MCF-7, and MRC5 cell lines.
| Compounds | Substituents | IC50 (μM) Mean ± SD a | ||||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | A549 b | SGC-7901 b | Hela b | SMMC-7721 b | MCF-7 b | MRC5 | |
| 4A |
|
- | >80 | >80 | >80 | >80 | >80 | 35.60 ± 0.30 |
| 4B |
|
- | 39.20 ± 1.18 | 42.31 ± 2.13 | 31.28 ± 2.33 | >80 | >80 | 51.78 ± 0.10 |
| 4C |
|
- | 28.06 ± 0.13 | 47.98 ± 0.09 | 42.55 ± 0.98 | >80 | >80 | 35.35 ± 0.30 |
| 4D |
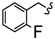
|
- | 13.94 ± 0.07 | 17.84 ± 0.66 | 26.36 ± 1.33 | 35.61 ± 1.33 | 53.92 ± 2.02 | 35.38 ± 0.01 |
| 6a |
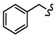
|
-(CH2)3- | 24.27 ± 0.12 | 17.76 ± 0.17 | 17.89 ± 0.03 | 25.73 ± 0.32 | 73.45 ± 0.88 | 32.57 ± 0.10 |
| 6b |
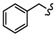
|
-(CH2)4- | 12.26 ± 0.04 | 15.48 ± 0.32 | 18.22 ± 0.52 | 66.53 ± 0.31 | 70.88 ± 2.33 | 64.44 ± 0.09 |
| 6c |
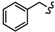
|
-(CH2)5- | 17.65 ± 0.09 | 18.72 ± 0.07 | 18.15 ± 0.03 | 55.15 ± 0.03 | 76.85 ± 3.01 | 39.34 ± 0.11 |
| 6d |
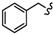
|
-(CH2)6- | 40.03 ± 0.15 | 58.41 ± 0.72 | 32.36 ± 0.02 | 33.49 ± 0.01 | 75.47 ± 2.32 | 66.99 ± 0.10 |
| 6e |
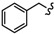
|
-(CH2)8- | 50.32 ± 3.15 | 69.97 ± 0.13 | 53.97 ± 0.56 | 75.36 ± 0.89 | >80 | >100 |
| 6f |
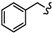
|
-CH2-C6H4-CH2- | 29.04 ± 0.06 | 51.24 ± 3.22 | 66.3 ± 1.02 | 57.35 ± 1.13 | >80 | >100 |
| 6g |
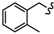
|
-(CH2)3- | 18.33 ± 0.28 | 29.42 ± 1.03 | 17.99 ± 0.15 | 73.49 ± 8.06 | 53.14 ± 2.28 | 32.61 ± 0.10 |
| 6h |
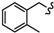
|
-(CH2)4- | 8.09 ± 0.25 | 17.75 ± 0.93 | 45.75 ± 0.55 | 24.71 ± 0.5 | 76.73 ± 5.0 | 50.78 ± 0.09 |
| 6i |
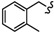
|
-(CH2)5- | 6.93 ± 0.33 | 7.18 ± 0.01 | 9.88 ± 0.04 | 14.22 ± 0.28 | 59.29 ± 5.86 | 37.99 ± 0.10 |
| 6j |
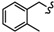
|
-(CH2)6- | 12.18 ± 0.06 | 26.26 ± 1.03 | 18.05 ± 1.03 | 16.68 ± 0.03 | 64.49 ± 3.31 | 41.0 ± 0.07 |
| 6k |
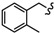
|
-(CH2)8- | 37.36 ± 2.08 | 35.00 ± 2.09 | 36.69 ± 2.02 | 33.93 ± 0.52 | 44.93 ± 2.99 | 65.85 ± 0.07 |
| 6l |
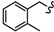
|
-CH2-C6H4-CH2- | 30.13 ± 0.37 | 39.26 ± 1.22 | 59.68 ± 1.52 | 69.66 ± 0.30 | 70.76 ± 2.02 | 37.66 ± 0.13 |
| 6m |
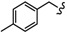
|
-(CH2)3- | 19.84 ± 0.04 | 18.41 ± 0.31 | 17.95 ± 0.02 | 33.32 ± 0.03 | 28.43 ± 0.28 | 49.05 ± 0.12 |
| 6n |
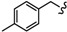
|
-(CH2)4- | 13.36 ± 0.35 | 37.79 ± 1.02 | 27.96 ± 0.99 | 42.72 ± 2.18 | 49.06 ± 2.23 | 26.39 ± 0.01 |
| 6o |
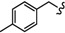
|
-(CH2)5- | 11.23 ± 0.61 | 17.18 ± 0.03 | 18.76 ± 0.46 | 36.62 ± 0.12 | 49.37 ± 1.65 | 31.28±0.07 |
| 6p |
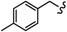
|
-(CH2)6- | 19.28 ± 0.02 | 17.84 ± 0.22 | 15.66 ± 0.33 | 26.75 ± 0.51 | 55.04 ± 2.22 | 47.60 ± 0.02 |
| 6q |
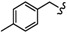
|
-(CH2)8- | 23.65 ± 0.05 | 35.78 ± 0.82 | 45.21 ± 1.66 | 29.82 ± 0.93 | 43.05 ± 2.01 | 42.06 ± 0.09 |
| 6r |
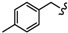
|
-CH2-C6H4-CH2- | 46.61 ± 0.03 | 65.93 ± 1.18 | 58.25 ± 2.98 | >80 | 68.26 ± 1.25 | 56.11 ± 0.01 |
| 6s |
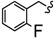
|
-(CH2)3- | 6.12 ± 0.03 | 8.06 ± 0.08 | 13.77 ± 0.11 | 14.71 ± 0.08 | 25.20 ± 1.12 | 22.82 ± 0.02 |
| 6t |
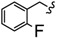
|
-(CH2)4- | 12.39 ± 0.59 | 9.01 ± 0.19 | 15.20 ± 0.32 | 18.26 ± 0.18 | 25.69 ± 1.21 | >100 |
| 6u |
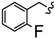
|
-(CH2)5- | 5.61 ± 0.04 | 5.86 ± 0.08 | 14.63 ± 0.36 | 16.68 ± 0.11 | 36.03 ± 2.99 | 39.89 ± 0.05 |
| 6v |
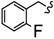
|
-(CH2)6- | 7.95 ± 0.06 | 8.60 ± 0.13 | 22.85 ± 1.36 | 24.65 ± 0.06 | 42.41 ± 2.08 | 67.75 ± 0.11 |
| 6w |
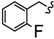
|
-(CH2)8- | 16.54 ± 0.33 | 29.98 ± 0.07 | 31.34 ± 1.88 | 44.57 ± 0.02 | 76.32 ± 3.36 | 60.78 ± 0.16 |
| 6x |
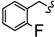
|
-CH2-C6H4-CH2- | 34.27 ± 0.05 | 45.00 ± 0.16 | 39.61 ± 2.59 | >80 | >80 | 63.28 ± 0.10 |
| 5-FU c | C4H3FN2O2 | 15.39 ± 0.09 | >80 | >80 | 52.32 ± 2.06 | 57.69 ± 1.39 | 16.33 ± 0.30 | |
a IC50 was the concentration when cell viability was reduced by 50% after treatment with β-carbolines for 48 h, and all data were the mean values of three independent experiments. b A549: lung carcinoma, SGC-7901: gastric carcinoma, Hela: cervical carcinoma, SMMC-7721: liver carcinoma, MCF-7: breast carcinoma, MRC5: normal lung cell. c 5-FU (5-fluorouracil, which could insert into DNA, interfering with DNA synthesis) was used as a positive control.
Moreover, most compounds exhibited low cytotoxicities against the MRC5 cell line. Because of the modest cytotoxicity of 6i (6.93 μM) and 6u (5.61 μM) against the A549 cell line, and in order to compare the antitumor mechanisms between monomers and dimers, 4B, 6i, 4D, and 6u, the A549 cells were selected to do the further investigation.
2.3. The Morphological Observation of Cell Apoptosis
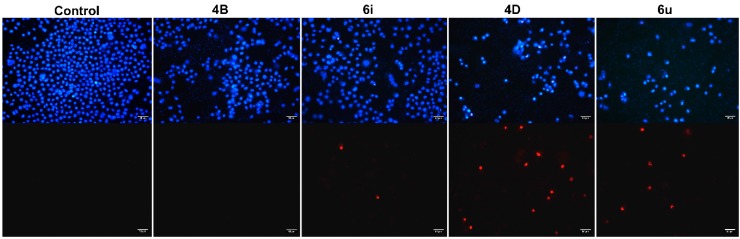
Apoptotic morphology is one of the most intuitive and reliable ways to determine whether cells are apoptotic [38]. The typical features of apoptosis include membrane blebbing, cell contraction, chromatin condensation, and formation of apoptotic bodies [39]. To identify the typical features of apoptosis, we performed Hoechst 33342/propidium iodide (PI) dual staining assay. As shown in Figure 1, A549 cells in the control group were evenly dyed weak blue with clear edge. However, after incubation with 6i, 4D, and 6u for 48 h, the cell density decreased significantly, and the cells were stained with strong blue fluorescence and strong red fluorescence, and shrinkage, condensation, or fragmentation was observed (Figure 1). These results indicated that the second series of dimer (6i) and the fourth series (containing fluorine atom) of monomer (4D) and dimer (6u) could induce cell apoptosis and further cell necrosis, while the second series of monomer (4B) could only induce cell apoptosis in a dose-dependent manner.
Figure 1.
Morphological observation was performed by Hoechst 33342/propidium iodide (PI) dual staining. A549 cells were treated with 4B, 6i, 4D, and 6u at 8 μM for 48 h, and then stained with Hoechst 33342 and PI at 37 °C in dark conditions. The stained cells were observed under a fluorescence microscope (Leica DM6B, Leica, Nussloch, Germany). Hoechst 33342 was used to stain the living and apoptotic nuclei, and emitted blue fluorescence (upper). PI was used to stain the dead nuclei and emitted red fluorescence (lower).
2.4. Cell Cycle Assay
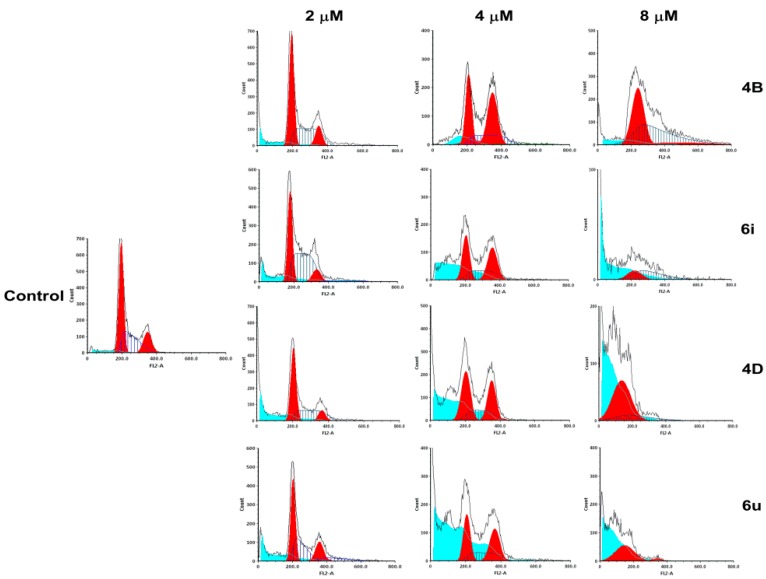
In order to determine the cell cycle arrest induced by β-carbolines, we performed propidium iodide (PI) staining using flow cytometry. As shown in Figure 2 and Table 2, when the concentration of 4B, 6i, 4D, and 6u was 4 μM, the percentages of cells in the G2/M phase increased to 46.96%, 42.40%, 35.46%, and 40.34% (control group 12.25%), respectively, indicating that 4B, 6i, 4D, and 6u could induce cell cycle arrest at the G2/M phase in A549 cells. At 8 μM, the percentages of cells in the S phase of 4B and 6i increased to 45.81% and 53.30% (control group 27.6%), respectively, indicating that 4B and 6i could exert antitumor activity by causing S and G2/M phase arrest. At 8 μM of 4D and 6u, the percentages of cells in the G1 phase increased to 88.01% and 81.62% (control group 60.15%), respectively, indicating that they could exert antitumor activity by causing G1 and G2/M phase arrest. Moreover, at 8 μM, the percentages of cells in sub-G1 by 4B, 6i, 4D, and 6u increased to 4.15%, 21.32%, 46.29%, and 44.92% (control group 1.78%), respectively, indicating that they could also induce apoptosis.
Figure 2.
The cell cycle was detected by flow cytometry using PI single staining. A549 cells were treated with 4B, 6i, 4D, and 6u at 2, 4, and 8 μM for 48 h, and then stained with PI for 30 min at 37 °C. The proportions of cells containing different DNA content were analyzed by FCS Express 5.0 (De Novo Software, Thornhill, Canada).
Table 2.
The percentage of cell cycles of A549 cells at indicated compound concentrations (4B, 6i, 4D, and 6u at 2, 4, and 8 μM).
| Compounds | Concentration (μM) |
Percentage of Cell Cycles (%) | |||
|---|---|---|---|---|---|
| SubG1 | G1 | S | G2/M | ||
| 4B | 2 | 6.56 | 59.38 | 25.16 | 15.46 |
| 4 | 10.49 | 43.06 | 9.98 | 46.96 | |
| 8 | 4.15 | 52.09 | 45.81 | 2.1 | |
| 6i | 2 | 6.86 | 57.38 | 32.08 | 10.54 |
| 4 | 26.33 | 45.19 | 12.41 | 42.40 | |
| 8 | 21.32 | 46.64 | 53.30 | 0.06 | |
| 4D | 2 | 6.41 | 56.89 | 24.17 | 18.94 |
| 4 | 39.03 | 47.21 | 17.33 | 35.46 | |
| 8 | 46.29 | 88.01 | 11.69 | 0.3 | |
| 6u | 2 | 6.84 | 56.24 | 23.79 | 19.97 |
| 4 | 46.25 | 47.06 | 12.60 | 40.34 | |
| 8 | 44.92 | 81.62 | 0.08 | 18.30 | |
| Control | 1% DMSO | 1.78 | 60.15 | 27.6 | 12.25 |
2.5. Cell Apoptosis Assay
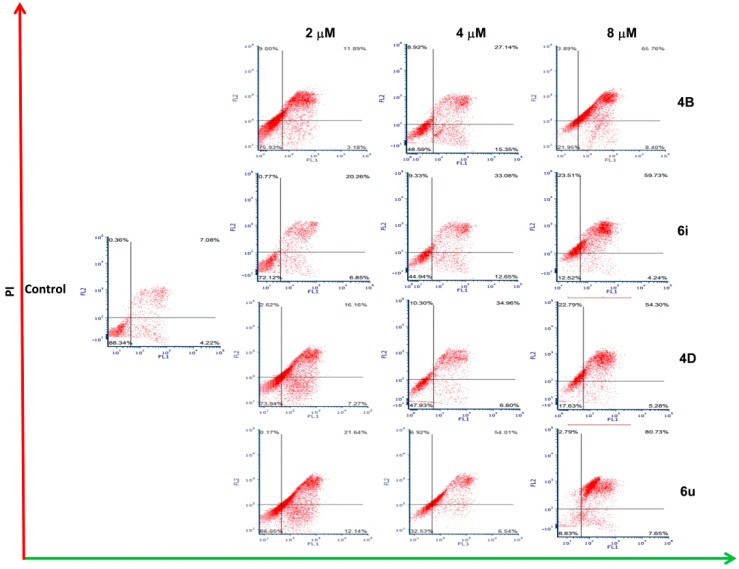
To better understand the apoptosis induced by β-carboline derivatives, we further performed Annexin V-FITC/PI dual staining assay using flow cytometry. The transfer of phosphatidylserine from the cell inner membrane to the cell outer membrane is considered as a potential marker of cell apoptosis. Moreover, Annexin V is a cellular protein which selectively binds phosphatidylserine [40,41]. As shown in Figure 3 and Table 3, after treatment with 8 μM of 4B, 6i, 4D, and 6u for 48 h, the percentages of early apoptosis were 8.40%, 4.24%, 5.28%, and 7.65%, respectively. And the percentages of late apoptosis or necrosis were 69.65%, 83.24%, 77.09%, and 83.52%, respectively, indicating that 4B, 6i, 4D, and 6u could induce significant apoptosis compared with the control group (early apoptosis: 4.22%; late apoptosis or necrosis: 7.44%). To sum up, the results of cell cycle and apoptosis experiments demonstrated that 4B, 6i, 4D, and 6u could exert antitumor activity by inducing cell cycle arrest at the S or G2/M phase and significant cell apoptosis.
Figure 3.
The cell apoptosis was detected by flow cytometry using Annexin V-FITC/PI dual staining. A549 cells were treated with 4B, 6i, 4D, and 6u at 2, 4, and 8 μM for 48 h, and then stained with Annexin V-FITC and PI for 15 min at 37 °C. The proportions of the living cells, the early apoptosis cells, and the late apoptotic cells were analyzed by FCS Express 5.0.
Table 3.
The percentage of cell apoptosis of A549 cells at indicated compound concentrations (4B, 6i, 4D, and 6u at 2, 4, and 8 μM).
| Comp. | Concentration (μM) |
Percentage of Cell Apoptosis (%) | ||
|---|---|---|---|---|
| Normal Living Cell | Early Apoptosis | Late Apoptosis and Necrosis | ||
| 4B | 2 | 75.93 | 3.18 | 20.89 |
| 4 | 48.59 | 15.35 | 36.06 | |
| 8 | 21.95 | 8.40 | 69.65 | |
| 6i | 2 | 72.12 | 6.85 | 21.03 |
| 4 | 44.94 | 12.65 | 42.41 | |
| 8 | 12.52 | 4.24 | 83.24 | |
| 4D | 2 | 73.94 | 7.27 | 18.78 |
| 4 | 47.93 | 6.80 | 45.26 | |
| 8 | 17.63 | 5.28 | 77.09 | |
| 6u | 2 | 66.05 | 12.14 | 21.81 |
| 4 | 32.53 | 6.54 | 60.93 | |
| 8 | 8.83 | 7.65 | 83.52 | |
| Control | 1% DMSO | 88.34 | 4.22 | 7.44 |
2.6. The Detection of Apoptosis-Related Protein (Cytochrome C, bcl-2)
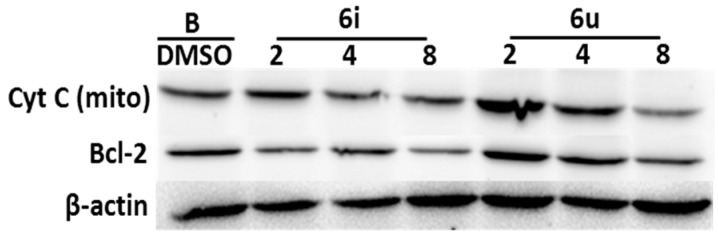
A large number of studies have found that the release of cytochrome C (Cyt C) from mitochondria to cytoplasm, and the decrease in expression of bcl-2 protein, are important features of apoptosis [42,43]. As shown in Figure 4, the levels of Cyt C (in mitochondria) and the expression of bcl-2 protein decreased in a dose-dependent manner after treatment with 6i and 6u. The results demonstrated that 6i and 6u could induce mitochondria-mediated apoptosis.
Figure 4.
Western blot analysis of cytochrome C and bcl-2 proteins. A549 cells were treated with with 6i and 6u at 2, 4, and 8 μM for 48 h. β-actin was used as an internal control.
2.7. DNA Binding Studies
The binding mode and binding strength of β-carboline derivatives (4B, 6i, 4D, and 6u) to DNA were studied through UV-visible spectral, thermal denaturation, and molecular docking studies.
2.7.1. UV-Visible Spectral Study
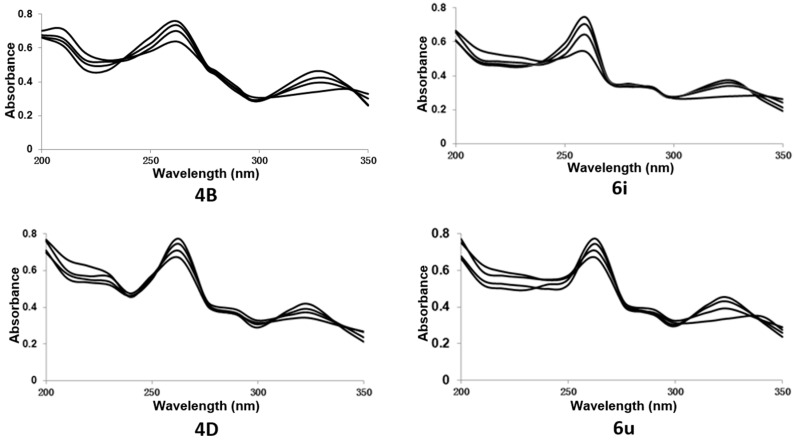
In order to reveal the binding mode of β-carboline derivatives to DNA, the UV absorption spectra of the interactions of β-carbolines (4B, 6i, 4D, and 6u) with calf thymus DNA (CT DNA) were investigated (Figure 5). The absorbance at 325 nm of 4B, 6i, 4D, and 6u was gradually decreased with red shift (4B-15 nm, 6i-18 nm, and 6u-20 nm), indicating that 4B, 6i, and 6u could bind to DNA by intercalation. Among them, the intercalation ability of 4D was very weak (may be a reasonable experimental error), and it may interact with DNA through other intermolecular forces.
Figure 5.
UV-visible spectroscopy study of β-carbolines with calf thymus (CT) DNA. UV-visible absorption titrations were done by adding 200 nM CT DNA solution each time to the quartz cuvette containing 10 μM 4B, 6i, 4D, and 6u. The absorption spectra were recorded from 200 nm to 350 nm.
2.7.2. Thermal Denaturation Study
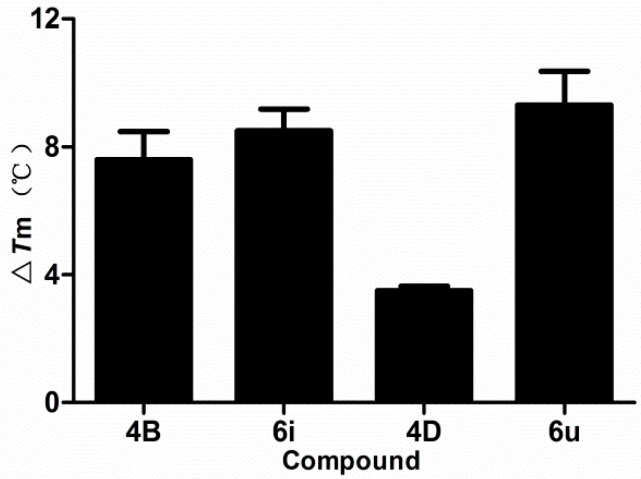
The binding affinity of β-carboline derivatives (4B, 6i, 4D, and 6u) to DNA were investigated through thermal denaturation experiments. Small molecules interact with DNA through the intercalation mode, which makes the DNA double helix more stable, thereby increasing the melting temperature (Tm) [44]. Therefore, the thermal denaturation experiment provides a simple and effective method for detecting DNA-binding affinity. As shown in Figure 6, the ΔTm values of 4B, 6i, 4D, and 6u were 7.5, 8.5, 3.5, and 9.0 °C, respectively, indicating that they could bind to DNA by intercalation, and the binding strength of 4B, 6i, and 6u was greater than that of 4D. Thus, thermal denaturation results indicated that 4B, 6i, 4D, and 6u could exhibit significant DNA-binding affinity.
Figure 6.
Thermal denaturation study of the complexes of β-carbolines (4B, 6i, 4D, and 6u) and CT-DNA. Melting temperatures were measured in PBS-EDTA buffer (1 mM Na2HPO4, 0.1 mM EDTA, PH 7.4) with a β-carboline (4B, 6i, 4D, and 6u)/DNA ratio of 0.5. ΔTm = (ΔTm4B,6i,4D, or 6u + DNA − ΔTmDNA).
2.7.3. Molecular Docking Study
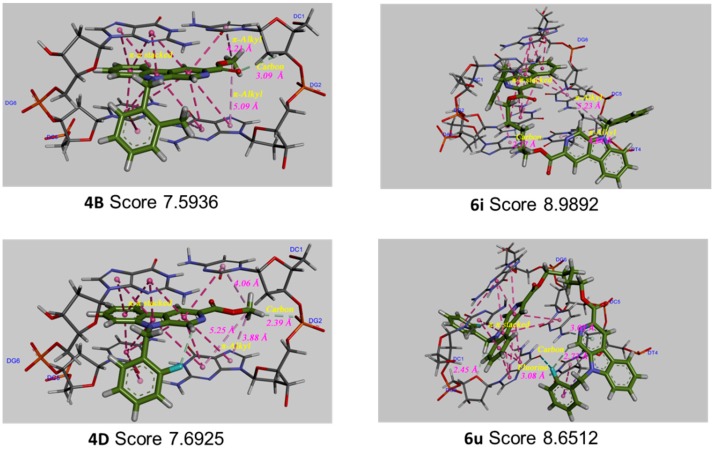
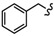
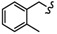
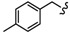
In order to further predict the binding mode of β-carboline to DNA, the Surflex-Dock program in the Sybyl-X 2.0 package (Tripos, Princeton, NJ, USA) was used to perform molecular docking studies between β-carboline derivatives and DNA, to explore possible mechanisms of antitumor activity. The structures and docking scores of the DNA-β-carboline complex are shown in Figure 7. The total scores of 4B, 6i, 4D, and 6u were 7.5936, 8.9892, 7.6925, and 8.6512, respectively, indicating that 4B, 6i, 4D, and 6u could well insert into the DNA. Moreover, the scores of dimers (6i and 6u) were higher than that of monomers (4B and 4D), indicating that the dimers have stronger DNA-binding ability than the corresponding monomers, thereby exerting better antitumor activity. In addition, the planar structure of β-carbolines (4B, 6i, and 4D) could interact with DNA through π-π stacking, the carbon-hydrogen bond (non-classical hydrogen bond), and the π-alkyl bond (hydrophobic force). The presence of the fluorine bond between DNA and 6u might support its higher antitumor activity.
Figure 7.
Three-dimensional conformations of the complexes of β-carbolines (4B, 6i, 4D, and 6u) docked with DNA.
3. Discussion
β-carboline alkaloids are a class of natural and synthetic indole alkaloids with a wide range of pharmacological activities, including antitumor, antiviral, antiparasitic, and antibacterial activities [5,6]. Particularly, previous studies have shown that β-carboline alkaloids exerted antitumor activity through insertion into DNA [10,11,12], inducing apoptosis [13,45], inhibiting CDKs [16], and topoisomerase I and II activity [13,15]. The substitution at the C3 and N9 positions of β-carbolines could significantly increase their DNA insertion ability [7]. Moreover, the existing studies have found that dimerization of small molecules could significantly increase [26].
DNA binding affinity [31,32]. Therefore, designing and synthesizing DNA-targeted β-carboline derivatives is an important way to find potentially effective antitumor drugs. Here, we synthesized a series of bivalent β-carboline derivatives modified at the N9 position and dimerized at the C3 position, and evaluated their antitumor activity and DNA binding affinity in vitro. The IC50 values of 4B, 6i, 4D, and 6u against A549 were 39.20, 6.39, 13.94, and 5.61 μM, respectively.
Previous studies have shown that the antitumor activity of β-carboline could be significantly improved with benzyl substituted at the N9 position and dimerized at the C3 position [5,35]. In this study, the monomers exhibited good-to-strong antitumor activity with the tendency of o-fluorobenzyl > p-methylbenzyl > o-methylbenzyl > benzyl. Furthermore, for the dimers of o-methylbenzyl substituted at the N9 position, most dimers were generally more active than the corresponding monomers, following the tendency of 5 > 4 > 6 > 3 > 8 methylene units. For the dimers of o-fluorobenzyl substituted at the N9 position, most dimers were generally more active than the corresponding monomers, following the tendency of 5 > 3 > 6 > 4 > 8 methylene units. These results indicated that N9-benzyl substitution and C3-dimerizition with 5–6 methylene units were favorable for increasing antitumor activity, which was consistent with the results of previous studies.
Previous studies have shown that cell cycle arrest and cell apoptosis are the main mechanisms used by β-carboline derivatives as antitumor drugs [4,46]. Our previous studies have shown that the introduction of substituents (a methyl or benzyl group) at the N9 position and the dimerization of β-carbolines could significantly increase the antitumor activity, and could cause cell cycle arrest at the S or G2/M phase and induce apoptosis in a dose-dependent manner [36,37]. In the present study, we found that the cell cycle arrested at the S and G2/M phases with sub G1 peaks, and induced apoptosis after treatment with 4B, 6i, 4D, and 6u. Furthermore, the reduction of cytochrome C (Cyt C) in mitochondria, and the decrease in expression of bcl-2 protein, are the typical features of mitochondria-mediated apoptosis [47]. Additionally, the results of western blot showed that the levels of Cyt C (in mitochondria) and the expression of bcl-2 protein decreased in a dose-dependent manner after incubation with 6i and 6u. Taken together, these results revealed that 6i and 6u could induce cell apoptosis through a mitochondria-mediated pathway.
To date, DNA intercalators have been effective antitumor drugs in clinical application. After the drugs are inserted into DNA, they can cause conformational changes of the double helix, which disrupts DNA replication, transcription, and repair [23]. The antitumor activity of a large number of natural and synthetic β-carboline derivatives that could interact with DNA by intercalation have been discovered and evaluated. Furthermore, the interactions between these derivatives (4B, 6i, 4D, and 6u) and DNA were investigated by UV-visible spectroscopy, thermal denaturation, and molecular modeling studies. The absorbance of 4B, 6i, 4D, and 6u decreased with red shift, and the ΔTm values of 4B, 6i, 4D, and 6u were 7.5, 8.5, 3.5, and 9.0 °C, respectively, indicating that 4B, 6i, and 6u could stably bind to DNA by intercalation, which is in agreement with the results of molecular docking studies.
In conclusion, a series of novel bivalent β-carboline-3-carboxylic acid derivatives were synthesized, and antitumor activity and DNA-binding affinity were preliminarily evaluated in vitro. The SARs studies revealed that the introduction of appropriate substituents at the N9 position and dimerization at the C3 position of β-carbolines could significantly increase the antitumor activity against A549, Hela, SGC-7901, SMMC-7721, and MCF-7 cell lines. Furthermore, 4B, 6i, 4D, and 6u could further induce tumor cell cycle arrest and apoptosis, and could interact with DNA through intercalation. In summary, further studies of 4B, 6i, 4D, and 6u could be performed to develop potential DNA-targeted agents in clinical applications.
4. Experimental Section
4.1. Materials and Methods
All chemicals and solvents used in the synthesis were purchased from suppliers. They were dried and purified when needed. Melting points were determined on a capillary melting apparatus and were uncorrected (SGW X-4, Shanghai Shenguang Instrument Co., Ltd., Shanghai, China). The 1H-NMR and 13C-NMR spectra were recorded on a Bruker Avance III at 500 MHz using tetra methyl silane (TMS) as the internal standard. The column chromatography and analytical thin layer chromatography (TLC) were performed with silica gel (100–200 mesh) and silica gel GF254 (Qingdao Marine Chemical Company, Qingdao, China).
All cell lines (A549, SGC-7901, Hela, SMMC-7721, and MCF-7) were purchased from the American Type Culture Collection (Manassas, VA, USA), and were cultured in Dulbecco’s modified Eagle’s medium (DMEM, HycloneLaboratories Inc., Logan, Utah, USA) supplemented with 10% fetal bovine serum (FBS, Gibco&Invitrogen, Carlsbad, CA, USA) at 37 °C and 5% CO2.
4.2. Chemistry
4.2.1. General Synthesis Procedure for N9 Substituted β-Carboline-3-carboxylic Acid Methyl Esters (4A, 4B, 4C, and 4D)
Using L-tryptophan as the raw material, β-carboline monomers (4A, 4B, 4C, and 4D) were synthesized through the Pictet–Spengler reaction (P–S reaction), esterification reaction, oxidation reaction, and N9 substitution reaction, as previously described [5,26,36]. Reaction routes and conditions are shown in Scheme 1.
4.2.2. General Reaction of Synthesis of Bivalent β-Carboline Derivatives (6a-6f, 6g-6l, 6m-6r, and 6s-6x)
The N9 substituted β-carboline-3-carboxylic acid methyl esters (4A, 4B, 4C, and 4D) prepared in the above steps were hydrolyzed and reacted with dibromoalkane to obtain a series of bivalent β-carboline-3-carboxylic acid derivatives (6a-6f, 6g-6l, 6m-6r, and 6s-6x), as previously described [34,35,37,48]. Reaction routes and conditions are shown in Scheme 2.
4.3. MTT Assay
The cytotoxic activities of β-carboline-3-carboxylic acid derivatives were investigated by the MTT method, as previously described [49]. Cells (1 × 104 cells per well) seeded into 96-well plates (JET, Guangzhou, China) were incubated with DMEM containing various concentrations (2.5, 5, 10, 20, 40, and 80 μM) of β-carbolines (4A, 4B, 4C, 4D, 6a-6f, 6g-6l, 6m-6r, and 6s-6x) for 48 h at 37 °C and 5% CO2. Then, 10 µL of 5 mg/mL MTT reagent dissolved in PBS (Phosphate buffered saline) was added per well, and plates were incubated at 37 °C for 4 h. The medium was carefully removed and 100 µL dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. The absorbance was measured at 490 nm with a microplate reader (ThermoFisher Scientific Inc., Waltham, MA, USA). The β-carbolines were dissolved in DMSO as stock solutions (10 mM) at −20 °C, and diluted in the final experimental solution using DMEM before use. A549 (lung carcinoma), SGC-7901 (gastric carcinoma), Hela (cervical carcinoma), SMMC-7721 (liver carcinoma), and MCF-7 (breast carcinoma) cell lines were used for the antitumor activity screening. The IC50 values were the mean values of three independent experiments.
4.4. Hoechst 33342/PI Dual Staining Assay
The morphological observation of apoptosis was performed by Hoechst 33342 and propidium iodide (PI) dual staining according to the manufacturer’s instructions. A549 cells (2 × 104 cells per well) seeded into 24-well plates (JET, Guangzhou, China) were incubated with DMEM containing 4 μM of β-carbolines (4B, 6i, 4D, and 6u) for 48 h at 37 °C and 5% CO2. Then, the cells were stained by Hoechst 33342 and PI at 37 °C for 30 min in dark conditions. Finally, cells were observed and photographed with a fluorescence microscope (LeicaDM6B, Leica, Nussloch, Germany).
4.5. Cell Cycle Assay
The cell cycle was determined by PI single staining. A549 cells (1 × 106 cells per well) seeded into 6-well plates (JET, Guangzhou, China) were incubated with DMEM containing various concentrations (2, 4, and 8 μM) of β-carbolines (4B, 6i, 4D, and 6u) for 48 h at 37 °C and 5% CO2. The cells were harvested, washed with pre-cooled PBS (4 °C), and fixed with pre-cooled 90% ethanol (4 °C). Then, the cells were incubated with 20 μL RNAse (30 μg/mL) and 20 μL PI (50 μg/mL) (Sigma Aldrich, Saint Louis, MO, USA) for 30 min at 37 °C. The samples were analyzed using flow cytometry (BD FACSCalibur, San Jose, CA, USA).
4.6. Cell Apoptosis Assay
The cell apoptosis was evaluated using Annexin V-FITC/PI dual staining (Beyotime, China). A549 cells (1 × 106 cells per well) seeded into 6-well plates (JET, Guangzhou, China) were incubated with DMEM containing various concentrations (2, 4, and 8 μM) of β-carbolines (4B, 6i, 4D, and 6u) for 48 h at 37 °C and 5% CO2. The cells in the supernatant and adherent were collected and washed with PBS, and then the cells were stained with Annexin V-FITC and PI for 20 min at r.t. The samples were analyzed using flow cytometry (Becton Dickinson, San Jose, CA, USA).
4.7. Western Blot Assay
The level of cytochrome C (Cyt C) in mitochondria, and the expression of bcl-2 protein, were evaluated by western blot. A549 cells (1 × 106 cells per well) seeded into 6-well plates (JET, Guangzhou, China) were incubated with DMEM containing various concentrations (2, 4, and 8 μM) of β-carbolines (6i and 6u) for 48 h at 37 °C and 5% CO2. Then, the cells were lysed with RIPA lysis buffer for 30 min on ice (containing 1% PMSF), centrifuged at 12,000× g for 5 min at 4 °C, and then the supernatants were transferred to 1.5 mL EP tubes. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted on polyvinylidene difluoride (PVDF) membranes (Millipore, Burlington, MA, USA), as previously described [50]. The membranes were blocked for 2 h at r.t. with 5% non-fat milk in TBST buffer (20 mM Tris-HCl,150 mM NaCl and 0.05% Tween-20, pH7.4), and then incubated with the mouse anti-bcl-2 monoclonal antibody (Sigma, Saint Louis, MO, USA), anti-cytochrome C monoclonal antibody (Beyotime, Haimen, China), and anti-β-actin antibody (1:4000 dilution; Abcam, Cambridge, MA, USA) overnight at 4 °C, and finally incubated with the peroxidase conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at 37 °C for 1 h. Immunostained proteins were visualized using ECL reagent according to the manufacturer’s instructions (Pierce, Rockford IL, CA, USA). β-actin was used as an internal control.
4.8. UV-Visible Spectral Study
UV-visible spectroscopy was carried out to determine the binding mode of β-carboline derivatives to DNA (CT DNA) using UV-2500 spectrophotometer (Shimadzu, Kyoto, Japan) at 25 °C. Stock solutions of 10 mM β-carboline derivatives were dissolved in DMSO, and 10 μM CT DNA was prepared in 100 mM KBPES buffer (30 mM Potassium Phosphate with 100 mM KCl, pH 7.0) [13]. UV-visible absorption titrations were performed by adding 200 nM CT DNA solution to the quartz cuvette containing approximately 10 μM derivative solutions. UV-Visible absorption spectra were recorded from 200 nm to 350 nm.
4.9. Thermal Denaturation Study
The DNA-binding modes of the β-carboline derivatives (4B, 6i, 4D, and 6u) with CT DNA were examined by DNA thermal denaturation experiments, as previously described [10]. The solutions containing complexes of CT DNA and 4B, 6i, 4D, or 6u were heated using a thermostatic water bath, and the absorbance was measured in 1 °C steps from 30 °C to 95 °C at 260 nm (At) by a UV-Vis spectrophotometer (A is the absorbance of the solution at 260 nm at different temperatures, t is the temperature). The experiments were performed in PE buffer (pH = 7.4) with a β-carboline/DNA ratio of 0.5. The ΔTm value of DNA alone is 56.8 °C. ΔTm = ΔTm(DNA + 4B, 6i, 4D, or 6u) − ΔTm(DNA alone).
4.10. Molecular Modeling Study
Molecular docking software (Sybyl-X 2.0 Tripos, Princeton, NJ, USA) and Discovery Studio 2017 client (BIOVIA, San Diego, CA, USA) was used to investigate the interaction of 4B, 6i, 4D, and 6u with DNA. The DNA was retrieved from the Protein Data Bank (PDB entry code: 1Z3F). The crystal structure of the DNA molecule was prepared by adding all the hydrogen atoms, and the charge was added using the AMBER7 FF99 method. The structures of 4B, 6i, 4D, and 6u were drawn in the Sybyl-X 2.0 package (Tripos, Princeton, NJ, USA). Hydrogen atoms were added, and then the energy was optimized using the Tripos force field and the Gasteiger–Hückel method. The Surflex-Dock scoring function is a weighted sum of non-linear functions based on the binding affinities of DNA-ligand complexes and their crystallographic structures.
Acknowledgments
Chengbao Wang, from the college of veterinary medicine in the university provided some technical support and materials in the biochemical experiments. H.W., an undergraduate from the innovative experimental college in the university participated in some experiments within the present research.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/19/10/3179/s1.
Author Contributions
J.W. conceived and designed the experiments; H.G., N.L., J.D., Y.X. performed the experiments; H.G. analyzed data and drafted the manuscript; S.W. edited and revised the manuscript. All authors approved the final version of the paper.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No. 81773603), the Open Foundation of Key Laboratory of Synthetic and National Foundation Molecule Chemistry of the Ministry of Education (Northwest University, China), the State Key Laboratory of Drug Research (Grant No. SIMM1705KF-09), the National Training Program on Undergraduate Innovation and Entrepreneurship (Grant No. 201803018, Northwest A&F University, China).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bluefarb S.M. Peganum harmala. Int. J. Dermatol. 1980;19:535. doi: 10.1111/j.1365-4362.1980.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 2.Allen J.R.F., Holmstedt B.R. The simple β-carboline alkaloids. Phytochemistry. 1980;19:1573–1582. doi: 10.1016/S0031-9422(00)83773-5. [DOI] [Google Scholar]
- 3.Dai J., Dan W., Li N., Wang J. Computer-aided drug discovery: Novel 3,9-disubstituted eudistomin U derivatives as potent antibacterial agents. Eur. J. Med. Chem. 2018;157:333–338. doi: 10.1016/j.ejmech.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Dai J., Dan W., Schneider U., Wang J. β-Carboline alkaloid monomers and dimers: Occurrence, structural diversity, and biological activities. Eur. J. Med. Chem. 2018;157:622–656. doi: 10.1016/j.ejmech.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Cao R., Chen Q., Hou X., Chen H., Guan H., Ma Y., Peng W., Xu A. Synthesis, acute toxicities, and antitumor effects of novel 9-substituted β-carboline derivatives. Bioorg. Med. Chem. 2004;12:4613–4623. doi: 10.1016/j.bmc.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Cao R., Chen H., Peng W., Ma Y., Hou X., Guan H., Liu X., Xu A. Design, synthesis and in vitro and in vivo antitumor activities of novel β-carboline derivatives. Eur. J. Med. Chem. 2005;40:991–1001. doi: 10.1016/j.ejmech.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Guan H., Chen H., Peng W., Ma Y., Cao R., Liu X., Xu A. Design of β-carboline derivatives as DNA-targeting antitumor agents. Eur. J. Med. Chem. 2006;41:1167–1179. doi: 10.1016/j.ejmech.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Shen Y.-C., Chang Y.-T., Lin C.-L., Liaw C.-C., Kuo Y.H., Tu L.-C., Yeh S.F., Chern J.-W. Synthesis of 1-Substituted Carbazolyl-1,2,3,4-tetrahydro-and Carbazolyl-3,4-dihydro-β-carboline Analogs as Potential Antitumor Agents. Mar. Drugs. 2011;9:256–277. doi: 10.3390/md9020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taira Z., Kanzawa S., Dohara C., Ishida S., Matsumoto M., Sakiya Y. Intercalation of Six β-Carboline Deriyatives into DNA. Jpn. J. Toxicol. Environ. Health. 1997;42:83–91. doi: 10.1248/jhs1956.43.83. [DOI] [Google Scholar]
- 10.Cao R.H., Peng W.L., Chen H.S., Ma Y., Liu X.D., Hou X.R., Guan H.J., Xu A.L. DNA binding properties of 9-substituted harmine derivatives. Biochem. Biophys. Res. Commun. 2005;338:1557–1563. doi: 10.1016/j.bbrc.2005.10.121. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z.Y., Cao R.H., Yu L.A., Shi B.X., Sun J., Guo L.A., Ma Q., Yi W., Song X.A., Song H.C. Synthesis, cytotoxic activities and DNA binding properties of beta-carboline derivatives. Eur. J. Med. Chem. 2010;45:4740–4745. doi: 10.1016/j.ejmech.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 12.Shankaraiah N., Siraj K.P., Nekkanti S., Srinivasulu V., Sharma P., Senwar K.R., Sathish M., Vishnuvardhan M.V.P.S., Ramakrishna S., Jadala C., et al. DNA-binding affinity and anticancer activity of β-carboline–chalcone conjugates as potential DNA intercalators: Molecular modelling and synthesis. Bioorg. Chem. 2015;59:130–139. doi: 10.1016/j.bioorg.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Kamal A., Sathish M., Nayak V.L., Srinivasulu V., Kavitha B., Tangella Y., Thummuri D., Bagul C., Shankaraiah N., Nagesh N. Design and synthesis of dithiocarbamate linked β-carboline derivatives: DNA topoisomerase II inhibition with DNA binding and apoptosis inducing ability. Bioorg. Med. Chem. 2015;23:5511–5526. doi: 10.1016/j.bmc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 14.Deveau A.M., Labroli M.A., Dieckhaus C.M., Barthen M.T., Smith K.S., Macdonald T.L. The Synthesis of Amino-Acid Functionalized β-Carbolines as Topoisomerase II Inhibitors. Bioorg. Med. Chem. Lett. 2001;11:1251–1255. doi: 10.1016/S0960-894X(01)00136-6. [DOI] [PubMed] [Google Scholar]
- 15.Sathish M., Kavitha B., Nayak V.L., Tangella Y., Ajitha A., Nekkanti S., Alarifi A., Shankaraiah N., Nagesh N., Kamal A. Synthesis of podophyllotoxin linked β-carboline congeners as potential anticancer agents and DNA topoisomerase II inhibitors. Eur. J. Med. Chem. 2018;144:557–571. doi: 10.1016/j.ejmech.2017.12.055. [DOI] [PubMed] [Google Scholar]
- 16.Li Y., Liang F.S., Jiang W., Yu F.S., Cao R.H., Ma Q.H., Da X.Y., Jiang J.D., Wang Y.C., So S. DH334, a beta-carboline anti-cancer drug, inhibits the CDK activity of budding yeast. Cancer Biol. Ther. 2007;6:1193–1199. doi: 10.4161/cbt.6.8.4382. [DOI] [PubMed] [Google Scholar]
- 17.He L., Liao S.-Y., Tan C.-P., Ye R.-R., Xu Y.-W., Zhao M., Ji L.-N., Mao Z.-W. Ruthenium–Arene–β-Carboline Complexes as Potent Inhibitors of Cyclin-Dependent Kinase 1: Synthesis, Characterization and Anticancer Mechanism Studies. Chem. Eur. J. 2013;19:12152–12160. doi: 10.1002/chem.201301389. [DOI] [PubMed] [Google Scholar]
- 18.Trujillo J.I., Meyers M.J., Anderson D.R., Hegde S., Mahoney M.W., Vernier W.F., Buchler I.P., Wu K.K., Yang S., Hartmann S.J., et al. Novel tetrahydro-β-carboline-1-carboxylic acids as inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2) Bioorg. Med. Chem. Lett. 2007;17:4657–4663. doi: 10.1016/j.bmcl.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 19.Castro A.C., Dang L.C., Soucy F., Grenier L., Mazdiyasni H., Hottelet M., Parent L., Pien C., Palombella V., Adams J. Novel IKK inhibitors: Beta-carbolines. Bioorg. Med. Chem. Lett. 2003;13:2419–2422. doi: 10.1016/S0960-894X(03)00408-6. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi K., Nagao M., Sugimura T. Interactions of norharman and harman with DNA. Nucleic Acids Res. 1977;4:3679–3685. doi: 10.1093/nar/4.11.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uezono T., Maruyama W., Matsubara K., Naoi M., Shimizu K., Saito O., Ogawa K., Mizukami H., Hayase N., Shiono H. Norharman, an indoleamine-derived β-carboline, but not Trp-P-2, a γ-carboline, induces apoptotic cell death in human neuroblastoma SH-SY5Y cells. J. Neural Transm. 2001;108:943–953. doi: 10.1007/s007020170014. [DOI] [PubMed] [Google Scholar]
- 22.Taira Z., Kanzawa S., Dohara C., Ishida S., Matsumoto M., Sakiya Y. Intercalation of Six β-Carboline Derivatives into DNA. Eisei kagaku. 1997;43:83–91. doi: 10.1248/jhs1956.43.83. [DOI] [Google Scholar]
- 23.Xiao S., Lin W., Wang C., Yang M. Synthesis and biological evaluation of DNA targeting flexible side-chain substituted β-carboline derivatives. Bioorg. Med. Chem. Lett. 2001;11:437–441. doi: 10.1016/S0960-894X(00)00679-X. [DOI] [PubMed] [Google Scholar]
- 24.Chen Q., Chao R., Chen H., Hou X., Yan H., Zhou S., Peng W., Xu A. Antitumor and neurotoxic effects of novel harmine derivatives and structure-activity relationship analysis. Int. J. Cancer. 2005;114:675–682. doi: 10.1002/ijc.20703. [DOI] [PubMed] [Google Scholar]
- 25.Georgi M., Adriana B., Margarita K. Novel Approaches Towards Development of Non-Classical Platinum-Based Antineoplastic Agents: Design of Platinum Complexes Characterized by an Alternative DNA-Binding Pattern and/or Tumor-Targeted Cytotoxicity. Curr. Med. Chem. 2005;12:2177–2191. doi: 10.2174/0929867054864877. [DOI] [PubMed] [Google Scholar]
- 26.Cao R.H., Peng W.L., Chen H.S., Hou X.R., Guan H.J., Chen Q., Ma Y., Xu A.L. Synthesis and in vitro cytotoxic evaluation of 1,3-bisubstituted and 1,3,9-trisubstituted beta-carboline derivatives. Eur. J. Med. Chem. 2005;40:249–257. doi: 10.1016/j.ejmech.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z.Y., Cao R.H., Shi B.X., Guo L., Sun J., Ma Q., Fan W.X., Song H.C. Synthesis and biological evaluation of 1,9-disubstituted beta-carbolines as potent DNA intercalating and cytotoxic agents. Eur. J. Med. Chem. 2011;46:5127–5137. doi: 10.1016/j.ejmech.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Formagio A.S.N., Tonin L.T.D., Foglio M.A., Madjarof C., de Carvalho J.E., da Costa W.F., Cardoso F.P., Sarragiotto M.H. Synthesis and antitumoral activity of novel 3-(2-substituted-1,3,4-oxadiazol-5-yl) and 3-(5-substituted-1,2,4-triazol-3-yl) β-carboline derivatives. Bioorg. Med. Chem. 2008;16:9660–9667. doi: 10.1016/j.bmc.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Savariz F.C., Foglio M.A., de Carvalho J.E., Ruiz A.L., Duarte M.C., da Rosa M.F., Meyer E., Sarragiotto M.H. Synthesis and evaluation of new beta-carboline-3-(4-benzylidene)-4H-oxazol-5-one derivatives as antitumor agents. Molecules. 2012;17:6100–6113. doi: 10.3390/molecules17056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankaraiah N., Nekkanti S., Chudasama K.J., Senwar K.R., Sharma P., Jeengar M.K., Naidu V.G.M., Srinivasulu V., Srinivasulu G., Kamal A. Design, synthesis and anticancer evaluation of tetrahydro-β-carboline-hydantoin hybrids. Bioorg. Med. Chem. Lett. 2014;24:5413–5417. doi: 10.1016/j.bmcl.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 31.Gaugain B., Barbet J., Capelle N., Roques B.P., Le Pecq J.B., Le Bret M. DNA Bifunctional Intercalators. 2. Fluorescence Properties and DNA Binding Interaction of an Ethidium Homodimer and an Acridine Ethidium Heterodimer. Biochemistry. 1978;17:5078–5088. doi: 10.1021/bi00617a002. [DOI] [PubMed] [Google Scholar]
- 32.Capelle N., Barbet J., Dessen P., Blanquet S., Roques B.P., Le Pecq J.B. Deoxyribonucleic acid bifunctional intercalators: Kinetic investigation of the binding of several acridine dimers to deoxyribonucleic acid. Biochemistry. 1979;18:3354–3362. doi: 10.1021/bi00582a023. [DOI] [PubMed] [Google Scholar]
- 33.Rook Y., Schmidtke K.-U., Gaube F., Schepmann D., Wünsch B., Heilmann J., Lehmann J., Winckler T. Bivalent β-Carbolines as Potential Multitarget Anti-Alzheimer Agents. J. Med. Chem. 2010;53:3611–3617. doi: 10.1021/jm1000024. [DOI] [PubMed] [Google Scholar]
- 34.Shi B., Cao R., Fan W., Guo L., Ma Q., Chen X., Zhang G., Qiu L., Song H. Design, synthesis and in vitro and in vivo antitumor activities of novel bivalent β-carbolines. Eur. J. Med. Chem. 2013;60:10–22. doi: 10.1016/j.ejmech.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 35.Wu Q.F., Bai Z.S., Ma Q., Fan W.X., Guo L., Zhang G.X., Qiu L.Q., Yu H.J., Shao G., Cao R.H. Synthesis and biological evaluation of novel bivalent beta-carbolines as potential antitumor agents. MedChemComm. 2014;5:953–957. doi: 10.1039/C4MD00098F. [DOI] [Google Scholar]
- 36.Du H., Tian S., Chen J., Gu H., Li N., Wang J. Synthesis and biological evaluation of N9-substituted harmine derivatives as potential anticancer agents. Bioorg. Med. Chem. Lett. 2016;26:4015–4019. doi: 10.1016/j.bmcl.2016.06.087. [DOI] [PubMed] [Google Scholar]
- 37.Du H., Gu H., Li N., Wang J. Synthesis and biological evaluation of bivalent β-carbolines as potential anticancer agents. MedChemComm. 2016;7:636–645. doi: 10.1039/C5MD00581G. [DOI] [Google Scholar]
- 38.Kerr J.F.R. Morphological criteria for identifying apoptosis. Cell Biol. Lab. Handb. 1994;1:319–329. [Google Scholar]
- 39.Escrich L., Grau N., Meseguer M., Pellicer A., Escribá M.-J. Morphologic indicators predict the stage of chromatin condensation of human germinal vesicle oocytes recovered from stimulated cycles. Fertil. Steril. 2010;93:2557–2564. doi: 10.1016/j.fertnstert.2009.05.077. [DOI] [PubMed] [Google Scholar]
- 40.Weir N.M., Selvendiran K., Kutala V.K., Tong L., Vishwanath S., Rajaram M., Tridandapani S., Anant S., Kuppusamy P. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating akt and p38 mAPK. Cancer Biol. Ther. 2007;6:178–184. doi: 10.4161/cbt.6.2.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wlodkowic D., Skommer J., Darzynkiewicz Z. Cytometry in cell necrobiology revisited. Recent advances and new vistas. Cytom. Part A. 2010;77:591–606. doi: 10.1002/cyto.a.20889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray S.K., Fidan M., Nowak M.W., Wilford G.G., Hogan E.L., Banik N.L. Oxidative stress and Ca2+ influx upregulate calpain and induce apoptosis in PC12 cells. Brain Res. 2000;852:326–334. doi: 10.1016/S0006-8993(99)02148-4. [DOI] [PubMed] [Google Scholar]
- 43.Chiu W.-T., Chang H.-A., Lin Y.-H., Lin Y.-S., Chang H.-T., Lin H.-H., Huang S.-C., Tang M.-J., Shen M.-R. Bcl-2 regulates store-operated Ca2+ entry to modulate ER stress-induced apoptosis. Cell Death Discov. 2018;4:37–50. doi: 10.1038/s41420-018-0039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva M.M., Savariz F.C., Silva-Júnior E.F., de Aquino T.M., Sarragiotto M.H., Santos J.C.C., Figueiredo I.M. Interaction of β-Carbolines with DNA: Spectroscopic Studies, Correlation with Biological Activity and Molecular Docking. J. Braz. Chem. Soc. 2016;27:1558–1568. doi: 10.5935/0103-5053.20160035. [DOI] [Google Scholar]
- 45.Zhang J., Zhang Z., Shu B., Cui G., Zhong G. Cytotoxic and Apoptotic Activity of the Novel Harmine Derivative ZC-14 in Sf9 Cells. Int. J. Mol. Sci. 2018;19:811. doi: 10.3390/ijms19030811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao R.H., Peng W.L., Wang Z.H., Xu A.L. beta-Carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007;14:479–500. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X.-F., Sun R.-Q., Jia Y.-F., Chen Q., Tu R.-F., Li K.-K., Zhang X.-D., Du R.-L., Cao R.-H. Synthesis and mechanisms of action of novel harmine derivatives as potential antitumor agents. Sci. Rep. 2016;6:33204–33219. doi: 10.1038/srep33204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W., Zhang G., Guo L., Fan W., Ma Q., Zhang X., Du R., Cao R. Synthesis and biological evaluation of novel alkyl diamine linked bivalent β-carbolines as angiogenesis inhibitors. Eur. J. Med. Chem. 2016;124:249–261. doi: 10.1016/j.ejmech.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 49.Van Meerloo J., Kaspers G.J.L., Cloos J. Cell Sensitivity Assays: The MTT Assay. In: Cree I.A., editor. Cancer Cell Culture: Methods and Protocols. Humana Press; New York, NY, USA: 2011. pp. 237–245. (Series 731). [DOI] [PubMed] [Google Scholar]
- 50.Chen Q., Shao X., Ling P., Liu F., Shao H., Ma A., Wu J., Zhang W., Liu F., Han G., et al. Low molecular weight xanthan gum suppresses oxidative stress-induced apoptosis in rabbit chondrocytes. Carbohydr. Polym. 2017;169:255–263. doi: 10.1016/j.carbpol.2017.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.