Abstract
A series of 1,3,4-trisubstituted-1,2,3-triazolium bromide salts were prepared by efficient two-step sequences of azide-alkyne cycloaddition and benzylic substitution. The antimicrobial activity of each triazolium salt and correlating triazole precursor was evaluated using a minimum inhibitory concentration (MIC) assay. MIC activities as low as 1 μM against Gram-positive bacteria, 8 μM against Gram-negative bacteria and 4 μM against fungi were observed for salt analogs, while neutral triazoles were inactive. Analogs representing selective and broad-spectrum antimicrobial activity were each identified. MIC structure-activity relationships observed within this motif indicate that the presence of cationic charge and balance of overall hydrophobicity are strongly impactful, while benzyl vs. aryl substituent identity and variation of substituent regiochemistry are not.
Keywords: Antibacterial, antifungal, benzylation, cycloaddition, organic salt
Quaternary ammonium compounds (QACs) are an exemplary class of widely utilized, fully synthetic antibiotics.1 Representative analogs such as benzalkonium chloride have been used as antiseptics for nearly a century. While such compounds take structural inspiration from natural products such as berberine,2 norspermidine3 and cationic antimicrobial peptides,4–8 the fully synthetic nature of QACs permit relatively simple structural variations that are necessary to complete the structure-activity relationship (SAR) profiling studies needed for optimizing therapeutic properties. The cationic nitrogen atoms defining of this class of antimicrobial compounds include benzylic, aliphatic and aromatic ammonium centers. Among the heterocycles representing aromatic ammonium analogs are pyridinium,9 imidazolium10,11 and 1,2,4-triazolium10,12 units.
While modification of simple hydrophobic groups around central ammonium center(s) in a small molecule motif may seem relatively simplistic from a structural diversity perspective, particularly in comparison to the frequently elaborate structures represented by natural product antibiotics, this general synthetic approach has been successfully utilized to prepare many effective and widely used QAC compounds and remains an active area of contemporary research.1,13,14 Analogs of the 1,3,4-trisubstituted-1,2,3-triazolium salt motif have been shown to display ionic liquid, 15,16 catalytic,17 antileishmanial, 18 and antimalarial 10 properties. Surprisingly, although 1,2,4-triazolium salts have been demonstrated as effective heterocyclic components of QAC compounds10,12 and examples of antimicrobial 1,2,3-triazolium-functionalized biopolymers have been recently reported,19–21 studies describing the general antimicrobial properties of aliphatic 1,2,3-triazolium salts themselves are lacking.
With the recent advent of the “click chemistry” era, founded upon the Sharpless-Meldal copper-catalyzed azide-alkyne coupling (CuAAC) reaction first reported in 2002,22,23 the efficient preparation of 1,2,3-triazole compounds with diverse peripheral functionality is now commonplace.24,25 Hence, preparing QAC analogs deriving from this heterocyclic motif and defining general SAR antimicrobial trends stands as an attractive target for developing new antimicrobial compounds. As an initial effort to define general SAR profiles for this class of molecular organic salts, the synthesis and antimicrobial evaluation of amphiphilic 1,3,4-trisubstituted-1,2,3-triazolium bromides with systematic variations in hydrophobicity, regiochemistry and substituent attachment is presented herein.
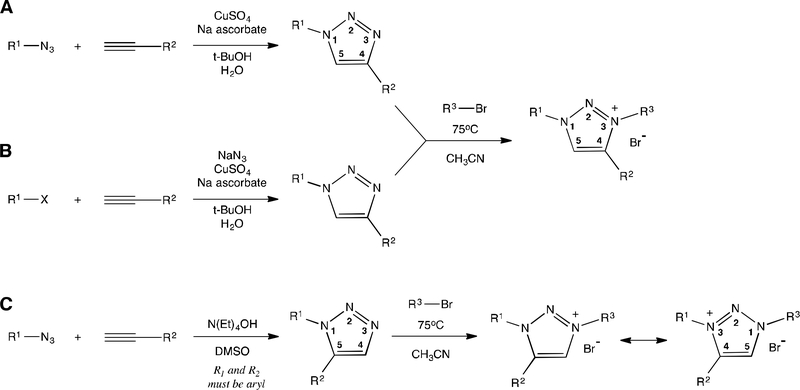
A representative family of 1,4-disubstituted-1,2,3-triazole and 1,3,4-trisubstituted1,2,3-triazolium compounds was prepared using the three established reaction sequences illustrated in Figure 1. Those 1,4-disubstituted-1,2,3-triazole analogs with aryl substitution at the N1 position were prepared from direct copper-catalyzed alkyne-azide cycloaddition (CuAAC)24 (method A) between commercially available alkynes and aryl azides prepared using the Sandmeyer reaction.26 Those 1,4-disubstituted-1,2,3-triazole analogs with N1 benzylic substitution were prepared using a tandem CuAAC method,15,27 where commercially available benzyl bromide and sodium azide were used to prepare the organic azide reactant in the presence of the CuAAC reactant and catalyst (method B), achieving a two-step reaction sequence in a one-pot reaction. The 1,5-disubstituted-1,2,3-triazole analog was prepared from base-catalyzed conditions28 (method C) that promote 1,5regioselective acetylide-alkyne cycloaddition using commercially available alkyne and prepared aryl azide reactants.
Figure 1.
Direct (A, C) and tandem (B) two-step methods to prepare 1,3,4-trisubstituted1,2,3-triazolium salts. The numbering scheme used for substituent identification is shown.
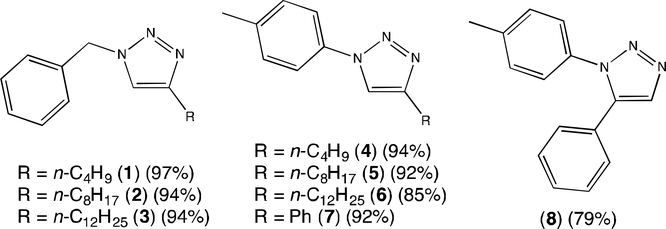
Each 1,2,3-triazole analog was successfully converted to a 1,3,4-trisubstituted-1,2,3-triazolium salt by substitution reaction at the N3 position,15,17 resulting in organic salts with quaternized ammonium centers. The majority of these quaternization reactions proceeded in high yield when an excess of benzyl bromide or 4-tert-butylbenzyl bromide reactant was used, with the exception of 7 having bulky C4 phenyl substitution that significantly reduced benzylation yields due to steric inhibition. Following evaporation of reaction solvent, pure triazolium bromide salts were isolated from a hexane trituration that removed unreacted neutral organics with minimal loss of ionic salt products. Hence, the target compounds in this study were accessible in good yield using only 2–3 overall reaction steps.
The antimicrobial properties of these triazole compounds and triazolium salts were evaluated using microdilution minimum inhibitory concentration assays as described in the CLSI protocol for bacteria29 and yeasts.30 BSL1 strains of Gram-positive bacteria (Bacillus subtilis, Staphylococcus epidermidis), Gram-negative bacteria (Escherichia coli, Enterobacter aerogenes) and yeast (Candida albicans, Saccharomyces cerevisiae) were selected as model organisms for the desired general antimicrobial profiling. Assays were run in triplicate and minimum inhibitory concentration (MIC) values were defined by observing the optical transparency of the most dilute well suppressing microbial growth. Two commercial QAC class antibiotics, cetylpyridinium chloride and benzalkonium chloride, were used as controls.
Assay results are summarized in Table 1. Among this limited family of compounds, examples of analogs displaying both selective and broad-spectrum antimicrobial activity were observed. While it was unsurprising that as representatives of the QAC structural motif these 1,3,4-trisubstituted-1,2,3-triazolium bromide salts did display antibiotic properties, the significant influence that simple substituent variations had on both selectivity and overall antimicrobial activity among this group of analogs was unexpected. None of the neutral 1,2,3-triazole compounds 1–8 showed measurable MIC values, and charged analogs 9, 12, 15, 21 and 23 possessing minimal hydrophobicity among the salts studied also lacked measurable MIC activity.
Table 1.
Survey of Triazolium Salt Antimicrobial Activity
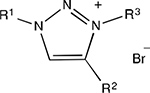 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Analog | Yield* | Minimum Inhibitory Concentration (μM)** | |||||||||
| R1 = | R2 = | R3 = | B.S. | S.E. | E.C. | E.A. | C.A. | S.C. | |||
| 9 | CH2Ph | n-C4H9 | CH2Ph | 96% | >250 | >250 | >250 | >250 | >250 | >250 | |
| 10 | CH2Ph | n-C8H17 | CH2Ph | 98% | 8 | 8 | >250 | >250 | 250 | 250 | |
| 11 | CH2Ph | n-C12H25 | CH2Ph | 95% | 2 | 4 | 16 | 16 | 16 | 16 | |
| 12 | CH2Ph | n-C4H9 | CH2Ph(4-tBu) | 98% | 8 | 8 | >250 | >250 | 250 | 250 | |
| 13 | CH2Ph | n-C8H17 | CH2Ph(4-tBu) | 98% | 1 | 2 | 31 | 31 | 8 | 16 | |
| 14 | CH2Ph | n-C12H25 | CH2Ph(4-tBu) | 95% | 2 | 2 | 250 | 250 | 8 | 16 | |
| 15 | Ph(4-CH3) | n-C4H9 | CH2Ph | 93% | >250 | >250 | >250 | >250 | >250 | >250 | |
| 16 | Ph(4-CH3) | n-C8H17 | CH2Ph | 89% | 4 | 8 | >250 | >250 | 250 | 250 | |
| 17 | Ph(4-CH3) | n-C12H25 | CH2Ph | 97% | 1 | 2 | 8 | 8 | 8 | 8 | |
| 18 | Ph(4-CH3) | n-C4H9 | CH2Ph(4-tBu) | 93% | 4 | 4 | 250 | >250 | 250 | 250 | |
| 19 | Ph(4-CH3) | n-C8H17 | CH2Ph(4-tBu) | 95% | 2 | 2 | 16 | 16 | 4 | 8 | |
| 20 | Ph(4-CH3) | n-C12H25 | CH2Ph(4-tBu) | 97% | 2 | 2 | 250 | 250 | 4 | 4 | |
| 21 | Ph(4-CH3) | Ph | CH2Ph | 48% | >250 | >250 | >250 | >250 | >250 | >250 | |
| 22 | Ph(4-CH3) | Ph | CH2Ph(4-tBu) | 49% | 8 | 8 | >250 | >250 | >250 | >250 | |
| 23 | CH2Ph | Ph | Ph(4-CH3) | 94% | >250 | >250 | >250 | >250 | >250 | >250 | |
| 24 | CH2Ph(4-tBu) | Ph | Ph(4-CH3) | 93% | 16 | 16 | >250 | >250 | >250 | >250 | |
| Cetylpyridinium chloride | 1 | 0.2 | 25 | 16 | 4 | 1 | |||||
| Benzalkonium chloride | 4 | 1 | 62 | 62 | 62 | 12 | |||||
Isolated yields indicated.
Concentrations surveyed: Initial range was 250, 125, 62, 31, 16, 8, 4 and 2 μM, repeated at 10x dilution for potent analogs.
B.S. = Bacillus subtilis (ATCC 6051); S.E. = Staphylococcus epidermidis (ATCC 14990); E.C. = Escherichia coli (ATCC 25922); E.A. = Enterobacter aerogenes (ATCC 13048); C.A. = Candida albicans (ATCC 90028); S.C. = Saccharomyces cerevisiae (ATCC 9763).
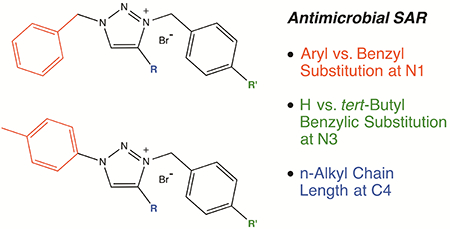
Analogs in the series possessing a moderate amount of peripheral hydrophobicity were observed to exert the most selective antimicrobial activity. 16 and 18 displayed greater than 50-fold selectivity towards the Gram-positive bacteria tested relative to the Gram-negative bacteria and fungi, with 10, 22 and 24 also showing such selectivity at slightly lower discrepancy. Analogs representing the maximum amount of hydrophobicity in the series, 14 and 20, showed expanded bioactivity towards both Gram-positive bacteria and fungi but not Gram-negative bacteria. An increased degree of broad-spectrum antimicrobial activity against all three classes of pathogens was observed for 11, 13, 17 and 19, which possessed more hydrophobicity than the Gram-positive selective analogs (10, 16, 18) but less hydrophobicity than those analogs that discriminated against Gramnegative bacteria (14, 20).
MIC potency for this family of triazolium salts was observed as low as 1 μM (0.5 μg/ml) against Gram-positive bacteria, 4 μM (2 μg/ml) against fungi and 8 μM (4 μg/ml) against Gram-negative bacteria. In comparison to the QAC controls cetylpyridinium chloride and benzalkonium chloride, these MIC values were slightly more potent against Gram-negative bacteria and slightly less potent against Gram-positive bacteria and fungus under the conditions assayed. Benzyl vs. aryl substituent identity at the N1 position was insignificant, as the MIC results of 9–14 largely mirrored 15–20. Regiochemistry of diaryl attachment was also observed to be inconsequential, with the regioisomer pairs 21/23 and 22/24 displaying analogous potency against the set of organisms tested.
Evaluation of this data set enables preliminary structure-activity relationship trends to be defined for this family of molecular 1,3,4-trisubstituted-1,2,3-triazolium bromide salts: (1) the cationic nature of the 1,2,3-triazolium salt is necessary for antimicrobial potency, as none of the neutral 1,2,3-triazole analogs are significantly active (MIC ≥250 μM for each); (2) increasing hydrophobicity at the N3 and C4 positions generally leads to increased potency against Gram-positive bacteria and fungi, while sufficient but not excessive hydrophobicity is ideal for maximum potency against Gram-negative bacteria; (3) no significant differences in potency are observed between aryl and benzyl substitution at the N1 position; and (4) no significant differences in potency are observed for regioisomer analogs with varying 1,4- vs. 1,5-diaryl substitution.
With the dominant driving force for potency being cationic amphiphilic properties, it is hypothesized that this class of molecules exerts antimicrobial activity via interaction with and destabilization of the cell membranes of target organisms.31,32 Against both Gram-positive bacteria and fungi, antimicrobial activity correlated with increasing hydrophobicity within the range of analogs examined. In contrast, against Gram-negative bacteria maximum potency was observed for analogs possessing a combination of moderate hydrophobicity at the triazole N3 and C4 positions relative to those displaying broad-spectrum effects. A decrease in QAC toxicity towards Gram-negative bacteria relative to Gram-positive bacteria is commonly observed13 due to the presence of their outer membrane, which in this case may be preventing the larger sized triazolium analogs from reaching and destabilizing the inner membrane of such organisms.
This investigation demonstrates that simple 1,3,4-trisubstituted-1,2,3-triazolium bromide salts display significant antimicrobial activity against model Gram-positive bacteria, Gram-negative bacteria and fungi in a substituent-dependent manner and establishes a preliminary SAR profile for this class of molecular organic salts. Each analog in this study was prepared efficiently from a sequence of cycloaddition and substitution reaction steps. Analogs representing both selective and broad-spectrum activity were each identified among this family of compounds. While this limited set of analogs allowed a general antibiotic SAR to be defined, a more comprehensive survey of substituents is warranted. Future studies will attempt to optimize both the potency and selectivity of bioactivity by embracing the facile synthetic variation of substituents at the 1-, 3- and 4- positions, as well as examine the antimicrobial properties of select analogs against drugresistant and clinically-relevant pathogen strains.
Supplementary Material
Figure 2.
Identities of the neutral 1,2,3-triazole analogs in this study. Isolated yields are noted.
Highlights.
Aliphatic 1,3,4-trisubstituted-1,2,3-triazolium salts show antimicrobial properties
Analogs representing selective and broad-spectrum antimicrobial activity identified
Potency strongly influenced by hydrophobicity; regiochemistry less impactful
Triazolium salts prepared efficiently using click and benzylation reaction steps
Acknowledgments
This publication was made possible by grants from the National Institute for General Medical Science (NIGMS) (5P20GM103427), a component of the National Institutes of Health (NIH), and its contents are the sole responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Minbiole KPC, Jennings MC, Ator LE, et al. From antimicrobial activity to mechanism of resistance: the multifaceted role of simple quaternary ammonium compounds in bacterial eradication. Tetrahedron. 2016;72(25):3559–3566. doi: 10.1016/j.tet.2016.01.014. [DOI] [Google Scholar]
- 2.Čerňáková M, Košťálová D. Antimicrobial activity of berberine—a constituent of Mahonia aquifolium. Folia Microbiol (Praha). 2002;47(4):375–378. doi: 10.1007/BF02818693. [DOI] [PubMed] [Google Scholar]
- 3.Böttcher T, Kolodkin-Gal I, Kolter R, Losick R, Clardy J. Synthesis and activity of biomimetic biofilm disruptors. J Am Chem Soc. 2013;135(8):2927–2930. doi: 10.1021/ja3120955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechara C, Sagan S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013;587(12):1693–1702. doi: 10.1016/j.febslet.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Lopez S, Kim HS, Choi EC, et al. Antibacterial agents based on the cyclic D,L-alpha-peptide architecture. Nature. 2001;412(6845):452–455. doi: 10.1038/35086601. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher JT, Finlay JA, Callow ME, Callow JA, Ghadiri MR. A Combinatorial Approach to the Discovery of Biocidal Six-Residue Cyclic d,l-α-Peptides Against the Bacteria Methicillin-Resistant Staphylococcus aureus (MRSA) and E. coli and the Biofouling Algae Ulva linza and Navicula perminuta. Chem - A Eur J. 2007;13(14):4008–4013. doi: 10.1002/chem.200601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saar K, Lindgren M, Hansen M, et al. Cell-penetrating peptides: A comparative membrane toxicity study. Anal Biochem. 2005;345(1):55–65. doi: 10.1016/j.ab.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Powers JPS, Hancock REW. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24(11):1681–1691. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Forman ME, Jennings MC, Wuest WM, Minbiole KPC. Building a Better Quaternary Ammonium Compound (QAC): Branched Tetracationic Antiseptic Amphiphiles. ChemMedChem. 2016;11(13):1401–1405. doi: 10.1002/cmdc.201600176. [DOI] [PubMed] [Google Scholar]
- 10.Vlahakis JZ, Mitu S, Roman G, Rodriguez EP, Crandall IE, Szarek WA. The anti-malarial activity of bivalent imidazolium salts. Bioorganic Med Chem. 2011;19(21):6525–6542. doi: 10.1016/j.bmc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Crandall IE, Zhao B, Vlahakis JZ, Szarek WA. The interaction of imidazole-, imidazolium-, and tetrazolium-containing compounds with DNA. Bioorganic Med Chem Lett. 2013;23(5):1522–1528. doi: 10.1016/j.bmcl.2012.11.106. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y, Lu Y-H, Gan L-L, et al. Synthesis, Antibacterial and Antifungal Activities of Novel 1,2,4-Triazolium Derivatives. Arch Pharm (Weinheim). 2009;342(7):386–393. doi: 10.1002/ardp.200800221. [DOI] [PubMed] [Google Scholar]
- 13.Jennings MC, Minbiole KPC, Wuest WM. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect Dis. 2016;1(7):288–303. doi: 10.1021/acsinfecdis.5b00047. [DOI] [PubMed] [Google Scholar]
- 14.Jennings MC, Buttaro BA, Minbiole KPC, Wuest WM. Bioorganic Investigation of Multicationic Antimicrobials to Combat QAC-Resistant Staphylococcus aureus. ACS Infect Dis. 2016;1(7):304–309. doi: 10.1021/acsinfecdis.5b00032. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher JT, Keeney ME, Walz SE. 1-Allyl- and 1-benzyl-3-methyl-1,2,3-triazolium salts via tandem click transformations. Synthesis (Stuttg). 2010;(19):3339–3345. doi: 10.1055/s-0030-1257909. [DOI] [Google Scholar]
- 16.Yan F, Lartey M, Jariwala K, et al. Toward a materials genome approach for ionic liquids: Synthesis guided by ab initio property maps. J Phys Chem B. 2014;118(47):13609–13620. doi: 10.1021/jp506972w. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly KF, Petronilho A, Albrecht M. Application of 1,2,3-triazolylidenes as versatile NHC-type ligands: synthesis, properties, and application in catalysis and beyond. Chem Commun. 2013;49(12):1145–1159. doi: 10.1039/C2CC37881G. [DOI] [PubMed] [Google Scholar]
- 18.Stroppa PHF, Antinarelli LMR, Carmo AML, Gameiro J, Coimbra ES, da Silva AD. Effect of 1,2,3-triazole salts, non-classical bioisosteres of miltefosine, on Leishmania amazonensis. Bioorganic Med Chem. 2017;25(12):3034–3045. doi: 10.1016/j.bmc.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 19.Tan W, Zhang J, Luan F, et al. Synthesis, characterization, and antifungal evaluation of novel 1,2,3-triazolium-functionalized starch derivative. Int J Biol Macromol. 2017;101:845–851. doi: 10.1016/j.ijbiomac.2017.03.171. [DOI] [PubMed] [Google Scholar]
- 20.Tan W, Li Q, Dong F, et al. Novel cationic chitosan derivative bearing 1,2,3-triazolium and pyridinium: Synthesis, characterization, and antifungal property. Carbohydr Polym. 2018;182(October 2017):180–187. doi: 10.1016/j.carbpol.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Shyam R, Charbonnel N, Job A, et al. 1,2,3-Triazolium-Based Cationic Amphipathic Peptoid Oligomers Mimicking Antimicrobial Helical Peptides. ChemMedChem. 2018;13(15):1513–1516. doi: 10.1002/cmdc.201800273. [DOI] [PubMed] [Google Scholar]
- 22.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew Chemie Int Ed 2002;41(14):2596–2599. doi:. [DOI] [PubMed] [Google Scholar]
- 23.Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67(9):3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 24.Meldal M, Tornøe CW. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem Rev. 2008;108(8):2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 25.Totobenazara J, Burke AJ. New click-chemistry methods for 1,2,3-triazoles synthesis: Recent advances and applications. Tetrahedron Lett. 2015;56(22):2853–2859. doi: 10.1016/j.tetlet.2015.03.136. [DOI] [Google Scholar]
- 26.Brase S, Gil C, Knepper K, Zimmermann V. Organic azides: An exploding diversity of a unique class of compounds. Angew Chemie - Int Ed 2005;44(33):5188–5240. doi: 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]
- 27.Indapurkar A, Henriksen B, Tolman J, Fletcher J. Evaluation of triazole-chelated lanthanides as chemically stabile bioimaging agents. J Pharm Sci. 2013;102(8). doi: 10.1002/jps.23616. [DOI] [PubMed] [Google Scholar]
- 28.Kwok SW, Fotsing JR, Fraser RJ, Rodionov VO, Fokin VV. Transition-Metal-Free Catalytic Synthesis of 1,5-Diaryl-1,2,3-triazoles. Org Lett. 2010;12(19):4217–4219. doi: 10.1021/ol101568d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically - Tenth Edition. CLSI document M07-A10. Wayne, PA: Clinical and Laboratory Standards Insititute; 2015. Vol 35; 2015. [Google Scholar]
- 30.CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard - Third Edition. CLSI document M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 31.Denyer SP. Mechanisms of action of antibacterial biocides. Int Biodeterior Biodegrad. 1995;36(3–4):227–245. doi: 10.1016/0964-8305(96)00015-7. [DOI] [Google Scholar]
- 32.Vieira DB, Carmona-Ribeiro AM. Cationic lipids and surfactants as antifungal agents: Mode of action. J Antimicrob Chemother. 2006;58(4):760–767. doi: 10.1093/jac/dkl312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.