Abstract
Objective.
To define the learning curve for transoral endoscopic thyroidectomy via the vestibular approach (TOETVA).
Study Design.
Case series with planned data collection.
Setting.
Tertiary care academic hospital.
Subjects and Methods.
Included patients were those who met the 2015 American Thyroid Association guidelines for lobectomy and our group’s previously documented indications for TOETVA. Operative time (incision to closure) was used as a surrogate for procedural proficiency and plotted as a function of case number to determine a learning curve. A simple moving average of operative time was then calculated, with the proficiency case defined as the case number where the slope of this curve changed. Demographic/characteristic data, outcomes, and complications were compared between the skill acquisition period (case 1 to proficiency case) and the proficiency period (remaining cases). A linear regression model was then used to calculate and compare the slopes of the skill acquisition and proficiency periods in the “operative time versus case number” plot.
Results.
Thirty cases were attempted, with a procedural success rate of 29 of 30 (94%) and no incidence of permanent mental nerve or recurrent laryngeal nerve injury. The proficiency case was case 11. There was a statistically significant difference between the skill acquisition and proficiency periods in slopes of the linear regressions (−16.7 vs −0.3, respectively; P< .001) and median operative times (191 vs 119 minutes, P < .001). There was no difference in demographics, procedural success rate, or complication rate between the periods.
Conclusions.
The learning curve for TOETVA was 11 cases for the surgeon evaluated in this series.
Keywords: remote-access thyroidectomy, transoral thyroidectomy, surgical skill acquisition
Over the last several years, there has been a growing interest from patients and surgeons alike to avoid anterior cervical neck scarring in thyroid surgery.1 Although the transcervical approach has been safely and effectively used since its description >100 years ago, it can result in conspicuous neck scarring.2 Moreover, studies demonstrated that this scarring can negatively affect patient quality of life, regardless of the perceived severity of the scar.3,4 As such, many remote-access techniques have been described, some of which are robotic assisted. These include approaches to the central neck via the axilla, the breast, and a retroauricular incision, among others.5–7 Despite the potential quality-of-life benefits that these procedures may provide, there has not been broad adoption by high-volume thyroid surgeons. One potential contributing factor may be the lengthy learning curves of these robotic-assisted techniques, with some studies noting them to be on the order of 35 to 50 cases.6,8–11 This prolonged time to proficiency and the ancillary resources and equipment needed create significant barriers to widespread integration of these approaches to the thyroid.
More recently, there has been growing literature in regard to the transoral vestibular approach to the thyroid, where the central neck compartment can be accessed via a midline gingivobuccal incision without a cutaneous scar. While initially described as a robotic-assisted technique, the majority of cases completed to date have been via an endoscopic approach.12–22 Early experience with the endoscopic technique has been encouraging, with an excellent safety profile and favorable outcomes in North American and international case series 16,17,21,23. As more surgeons attempt to adopt the technique given its early success, it is important to standardize training for the procedure and prevent avoidable complications. Defining the learning curve for the procedure can help provide a framework to guide the development of such teaching curriculums. Likewise, understanding the number of cases needed to reach proficiency is valuable on an institutional level, as hospitals consider devoting resources to integrate this new procedure within their armamentarium. As early adopters of the transoral endoscopic thyroidectomy vestibular approach (TOETVA), we aim to define the learning curve for the procedure and begin to address these knowledge gaps. We also describe modifications to existing methodology that can be broadly used to define surgeon skill acquisition in the adoption of new procedures.
Methods
After Institutional Review Board approval from the Johns Hopkins School of Medicine, data were prospectively collected for all patients undergoing TOETVA. For the purposes of this study, all cases of TOETVA for thyroid lobectomy were examined as performed by a high-volume endocrine surgeon (J.O.R.) at our institution between September 2016 and March 2018. Cases of TOETVA for total thyroidectomy were excluded from analysis to maintain homogeneity of the data, as the majority of completed cases during this period were lobectomies. Of note, this otolaryngologist–head and neck surgeon was fellowship trained in head and neck endocrine surgery, with no significant experience or training with laparoscopic surgery. Included patients met the 2015 guidelines of the American Thyroid Association for thyroid lobectomy and were offered TOETVA based on indications that we previously described.24,25 Written informed consent for TOETVA was then obtained, with the risks, benefits, and alternatives discussed (including other surgical approaches), noting the possibility of conversion to the transcervical approach. Demographics, nodule characteristics, pathology, and complications were collected and reviewed for all patients. Our surgical technique for TOETVA was previously described.17 Neural integrity monitoring was utilized for all cases, as is customary for transcervical and transoral thyroid procedures at our institution.
For the purposes of defining a learning curve, operative time was used as a surrogate for procedural proficiency. Operative time was defined as the time of incision to the time of skin closure and was plotted as a function of case number. A simple moving average (SMA) of order 3 was then calculated for the series, in a method similar to prior literature describing the learning curves for remote-access approaches to the thyroid.8-10 Use of the moving average method has been advocated, as it can filter individual variations and accentuate data trends, resulting in “smoothing” of temporally dependent data.26 For our purposes, it can account for patient characteristics, such as Hashimoto’s thyroiditis, that may result in extended operative time of any particular case. Previous studies subsequently defined the case number after which the slope of the SMA changed or plateaued as the point of proficiency (proficiency case). We elected to further analyze the data with a linear regression model. The slope of the linear regression of case 1 through the proficiency case (skill acquisition period) was compared with the slope of the linear regression of the remainder of cases (proficiency period) in the “operative time versus case number” plot. Demographic and characteristic data for the subgroups were then compared with the t test for continuous parametric variables and Fisher’s exact test for categorical variables. Similarly, complications— including incidence of permanent recurrent laryngeal nerve (RLN) and mental nerve (MN) injury (defined as symptoms lasting > 3 months)—were recorded and compared between the periods. Finally, operative times between the periods were compared with the Mann-Whitney U test, as they were assumed to be nonparametric. Statistical analysis was completed in Stata Statistical Software 15 (StataCorp LLC, College Station, Texas) with an alpha of 0.05 for statistical significance.
Results
Thyroid lobectomy via TOETVA was attempted in 29 patients. One patient underwent left thyroid lobectomy, followed by completion thyroidectomy; as such, 30 lobectomies were attempted via TOETVA, with 29 of 30 cases being completed via the intended approach. The 15th attempted case was converted to the transcervical approach after bleeding was encountered at the superior pole that could not be controlled with transoral access. The converted case was completed without RLN/MN injury or other complications in 123 total minutes from intraoral incision to cervical incision closure. The operative time of this converted case was excluded in determining the learning curve and comparing median operative times between the skill acquisition and proficiency periods. Included patients had a mean ± SD age of 41 ± 12 years with a mean body mass index of 26.9 ± 6.5 kg/m2. Of 30 cases, 19 (63%) were right-sided procedures; 27 (90%) were women; and the median index nodule size was 3.3 cm (range, 0.8-7.1 cm). There was no statistically significant difference in these data between the skill acquisition and proficiency periods (Table 1). Postoperative pathology was benign in 19 cases; noninvasive follicular thyroid neoplasm with papillary like nuclear features occurred in 2 cases and papillary thyroid carcinoma in 7 cases; and 1 patient who underwent lobectomy, followed by completion thyroidectomy, had minimally invasive Hurthle cell carcinoma.27 Of note, the patient with Hurthle cell carcinoma elected to undergo completion thyroidectomy (via TOETVA) for her own peace of mind after consultation with her endocrinologist, although it was not required per the 2015 guidelines of the American Thyroid Association.
Table 1.
Patient Characteristics.
| Mean ± SD or n (%) |
||||
|---|---|---|---|---|
| Full Series (N = 30) | Skill Acquisition Period (n = 11) | Proficiency Period (n = 19) | P Valuea | |
| Age, y | 41 ± 12 | 43 ± 15 | 39 ± 10 | .43 |
| Female | 27 (90) | 9 (82) | 18 (95) | .53 |
| Body mass index, kg/m2 | 26.9 ± 6.5 | 27.4 ± 6.6 | 26.2 ± 6.8 | .64 |
| Index nodule size, cmb | 3.3 [0.8-7.1] | 3.6 [1.2-5.1] | 3.3 [0.8-7.1] | .94 |
| Right sided | 19 (63) | 7 (64) | 12 (63) | .99 |
Comparison between skill acquisition and proficiency periods.
Median [range].
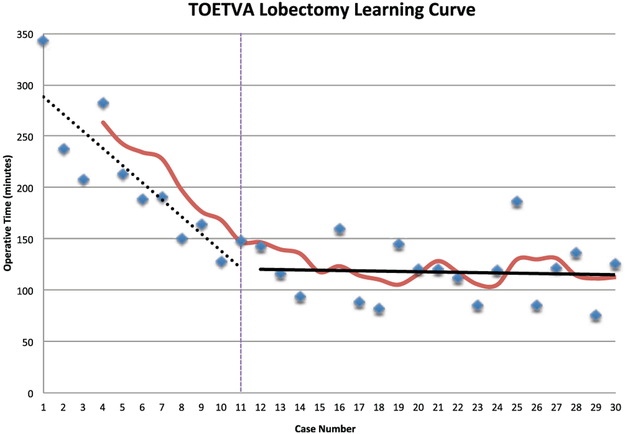
The median operative time for the series was 136 minutes (range, 76-343), with 21 of 30 cases discharged on the same day of surgery. There was no significant difference in operative time based on laterality of the procedure, with medians of 145 versus 127 minutes for right- and left-sided cases, respectively (P = .96). There was no incidence of permanent MN or RLN injury or hematoma/seroma. One patient had temporary RLN injury with resolution of voice complaints and evidence of bilateral true vocal fold motion on fiberoptic examination within 2 months of the initial procedure. The first patient in the series had a surgical drain placed, which was removed at the first postoperative visit. The remainder of patients in the series did not undergo drain placement. The slope of the SMA of the “operative time versus case number” plot appeared to change at case number 11; hence, it was evaluated as the proficiency case as described earlier. The slope of the linear regression of the skill acquisition period (cases 1-11) was −16.7 (95% CI, −25.2 to −8.1), while the slope of the linear regression of the proficiency period was −0.3 (95% CI, −2.9 to 2.3). These slopes were significantly different from each other (P < .001), with cases in the proficiency period being completed on average 87.3 minutes faster than cases in the skill acquisition period as evaluated by this model. Additionally, the slope of the linear regression of the proficiency period was not different from zero (P = .81; Figure 1). Analysis between the skill acquisition and proficiency periods also revealed significant differences in median operative time (191 vs 119 minutes, respectively; P < .001) and rate of same-day discharge (36% vs 89%, P = .004). There was, however, no statistically significant difference in rate of completion of the intended approach or complication rate between the periods (Table 2).
Figure 1.
The learning curve for the transoral endoscopic thyroidectomy vestibular approach (TOETVA). The simple moving average curve is depicted in red, which was used to define the proficiency case, as seen by the vertical line at case 11. The slope of the skill acquisition period (cases 1-11) is depicted by the dashed black line, while the slope of the proficiency period (cases 12-30) is denoted by the solid black line. These slopes are significantly different from each other (P < .001).
Table 2.
Operative Outcomes.
| Patients, n (%) |
||||
|---|---|---|---|---|
| Full Series (N = 30) | Skill Acquisition Period (n = 11) | Proficiency Period (n = 19) | P Valuea | |
| Completion of intended approach | 29 (97) | 11 (100) | 18 (95) | .99 |
| Operative time, minb | 136 [76-343] | 191 [128-343] | 119 [76-186] | <.001 |
| Permanent RLN/MN injury | 0 | 0 | 0 | — |
| Drain placement | 1 | 1 | 0 | .37 |
| Same day discharge | 21 (70) | 4 (36) | 17 (89) | .004 |
Abbreviations: MN, mental nerve; RLN, recurrent laryngeal nerve.
Comparison between skill acquisition and proficiency periods. Bold indicates P <.05.
Median [range].
Discussion
The learning curve for thyroid lobectomy via TOETVA was 11 cases for the surgeon evaluated in this series. Importantly, although cases of total thyroidectomy via TOETVA were excluded from analysis as previously described, all such cases were completed after the calculated proficiency case. As such, experience gained from these procedures would not be expected to significantly undermine the methodology used to calculate the learning curve for TOETVA lobectomy as outlined. After definition of the proficiency case via visual inspection of the SMA curve,8-10 linear regression analysis was used to confirm validity of the distinction between the study periods. During the skill acquisition period, the slope of the linear regression was −16.7, which was significantly different from zero (P = .002). This can be interpreted as each subsequent case being performed on average 16.7 minutes faster than the prior one during this period. Conversely, the slope of the linear regression of the proficiency period was not significantly different from zero (−0.3, P = .81), demonstrating a true plateau in operative time and therefore skill acquisition, suggesting that the learning curve for the procedure had been met. The slopes of the regressions of the 2 periods were also significantly different (P < .001), with cases in the proficiency period being performed on average 87.3 minutes faster. Additionally, there was a significant difference in median operative time (P < .001), further validating the distinction between periods in the study.
When examining the learning curve for any procedure there are many potential factors that can confound analysis and the subsequent appropriate definition of the number of cases required to reach proficiency. These may include a surgeon’s prior experience, the presence of heterogeneous cases/complexity, and appropriately balancing the use of patient-centered outcomes (eg, the presence and severity of complications and how they may affect quality of life) with analysis of more tangible surgical process outcomes such as operative time. In this study we attempted to account for these confounders by commenting on potential differences in demographic/characteristic data as well as complications such as RLN/MN injury between the skill acquisition and proficiency periods. Similarly, we only examined outcomes on TOETVA lobectomies performed by a single surgeon. These were purposeful actions in an effort to maintain and demonstrate homogeneity of the data analyzed while examining both patient-centered and surgical process outcomes simultaneously. Moreover, as previously described, the use of the SMA method in determining learning curve can also help account for inherent variations in case complexity that are not evident based on review of demographic/characteristic data. Importantly, in our series, there was no difference in demographic/characteristic data between the skill acquisition and proficiency periods. This suggests that differences in operative time and therefore proficiency were a result of increased procedural experience rather than variations in potential confounders, such as nodule size, body mass index, or laterality, which may make an individual case more or less challenging. Moreover, the lack of statistically significant differences in other outcome measures, including rate of completion of the intended procedure and permanent MN/RLN injury, supports the use of operative time as a surrogate for operative proficiency in our series. The difference in the rate of same-day discharge between the skill acquisition and proficiency periods (36% vs 89%, P = .004) was likely due to institutional experience with the procedure, as there was no difference in rate of drain placement between periods and no incidence of hematoma/seroma in the series. Given that this series evaluated thyroid lobectomies, these are the main factors that would typically lead to overnight observation in our practice.
The learning curve of 11 cases for this study is consistent with the estimated learning curve of 7 to 10 cases for TOETVA as anecdotally noted in the largest-volume series for the procedure to date, by Anuwong et al.21 Of note, this estimate was based on the assumption of prior laparoscopic surgery experience. Although Anuwong et al did not statistically define a learning curve, the similarity to our findings may suggest that prior laparoscopic experience does not have a significant impact on the individualized learning curves for TOETVA. As a growing number of high-volume thyroid surgeons are otolaryngology–head and neck surgery trained, this is important to consider, as some authors postulated that the lack of laparoscopic experience is a potential barrier to adopting TOETVA within our field.28,29 Our findings in this study do not necessarily support that notion. Evaluation of individual surgeons’ learning curves for TOETVA as stratified per prior laparoscopic experience will be invaluable in further defining this. Moreover, it will help determine if individualized teaching curriculums are needed or if a learning curve of approximately 10 to 12 cases can be used to broadly provide guidelines for graduated learning and independence with the procedure. Additionally, given the endoscopic nature of the procedure, TOETVA may lend itself better to the development of high-fidelity surgical simulators with accompanying competency-based objective measures, an area of relative deficiency in transcervical thyroid training.
The learning curve for TOETVA in our series was substantially shorter than the learning curves described for other remote-access approaches to the thyroid.6,8-11 With a learning curve on the order of 35 to 50 cases and the necessity of a costly surgical robot, many of the prior-described remote-access approaches to the thyroid did not gain widespread support, especially in the United States.1,6,8-11 Conversely, the learning curve for TOETVA appears to be relatively short, and the procedure can be performed with laparoscopic instrumentation available at most institutions. If evaluation of other surgeons’ learning curves with TOETVA supports these findings, these characteristics and safety profile demonstrate the potential for large-scale institutional adoption. Importantly, we aimed to define the number of cases needed to reach proficiency and not mastery with TOETVA. As our experience with the procedure increases, we anticipate a second change in the slope of the learning curve, with thyroid lobectomy via TOETVA ultimately being performed in <100 minutes on a routine basis.
Conclusion
The learning curve for TOETVA was found to be 11 cases for the surgeon evaluated in this series. This is considerably shorter than the learning curves previously described for other remote-access approaches to the thyroid. Moreover, the lack of prior laparoscopic experience did not drastically affect the time to proficiency for the procedure based on prior estimates of the learning curve for TOETVA.
Acknowledgments
Funding source: None.
Footnotes
Disclosures
Competing interests: Ralph P. Tufano, consultant for Medtronics and Hemostatix.
Sponsorships: None.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- 1.Berber E, Bernet V, Fahey TJ 3rd, et al. American Thyroid Association statement on remote-access thyroid surgery. Thyroid. 2016;26:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halsted WS IV. (I) The excision of both lobes of the thyroid gland for the cure of Graves’s disease. (II) The preliminary ligation of the thyroid arteries and of the inferior in preference to the superior artery. Ann Surg. 1913;58:178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Best AR, Shipchandler TZ, Cordes SR. Midcervical scar satisfaction in thyroidectomy patients. Laryngoscope. 2017;127: 1247–1252. [DOI] [PubMed] [Google Scholar]
- 4.Choi Y, Lee JH, Kim YH, et al. Impact of postthyroidectomy scar on the quality of life of thyroid cancer patients. Ann Dermatol. 2014;26:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: patient selection and technical considerations. Surg Laparosc Endosc Percutan Tech. 2011;21:237–242. [DOI] [PubMed] [Google Scholar]
- 6.Kandil EH, Noureldine SI, Yao L, Slakey DP. Robotic transaxillary thyroidectomy: an examination of the first one hundred cases. J Am Coll Surg. 2012;214:558–564. [DOI] [PubMed] [Google Scholar]
- 7.Jackson NR, Yao L, Tufano RP, Kandil EH. Safety of robotic thyroidectomy approaches: meta-analysis and systematic review. Head Neck. 2014;36:137–143. [DOI] [PubMed] [Google Scholar]
- 8.Kang SW, Lee SC, Lee SH, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery. 2009;146:1048–1055. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Yun JH, Nam KH, Soh EY, Chung WY. The learning curve for robotic thyroidectomy: a multicenter study. Ann Surg Oncol. 2011;18:226–232. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Yun JH, Choi UJ, Kang SW, Jeong JJ, Chung WY. Robotic versus endoscopic thyroidectomy for thyroid cancers: a multi-institutional analysis of early postoperative outcomes and surgical learning curves. J Oncol. 2012;2012:734541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim WW, Jung JH, Park HY. The learning curve for robotic thyroidectomy using a bilateral axillo-breast approach from the 100 cases. Surg Laparosc Endosc Percutan Tech. 2015;25:412–416. [DOI] [PubMed] [Google Scholar]
- 12.Richmon JD, Holsinger FC, Kandil E, Moore MW, Garcia JA, Tufano RP. Transoral robotic-assisted thyroidectomy with central neck dissection: preclinical cadaver feasibility study and proposed surgical technique. J Robot Surg. 2011;5:279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richmon JD, Pattani KM, Benhidjeb T, Tufano RP. Transoral robotic-assisted thyroidectomy: a preclinical feasibility study in 2 cadavers. Head Neck. 2011;33:330–333. [DOI] [PubMed] [Google Scholar]
- 14.Clark JH, Kim HY, Richmon JD. Transoral robotic thyroid surgery. Gland Surg. 2015;4:429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razavi CR, Fondong A, Tufano RP, Russell JO. Central neck dissection via the transoral approach. Ann Thyroid. 2017;2(5):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anuwong A Transoral endoscopic thyroidectomy vestibular approach: a series of the first 60 human cases. World J Surg. 2016;40:491–497. [DOI] [PubMed] [Google Scholar]
- 17.Russell JO, Clark J, Noureldine SI, et al. Transoral thyroidectomy and parathyroidectomy—a North American series of robotic and endoscopic transoral approaches to the central neck. Oral Oncol. 2017;71:75–80. [DOI] [PubMed] [Google Scholar]
- 18.Richmon JD, Kim HY. Transoral robotic thyroidectomy (TORT): procedures and outcomes. Gland Surg. 2017;6:285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HY, Chai YJ, Dionigi G, Anuwong A, Richmon JD. Transoral robotic thyroidectomy: lessons learned from an initial consecutive series of 24 patients. Surg Endosc. 2018;32: 688–694. [DOI] [PubMed] [Google Scholar]
- 20.Shan L, Liu J. A systemic review of transoral thyroidectomy. Surg Laparosc Endosc Percutan Tech. 2018;28:135–138. [DOI] [PubMed] [Google Scholar]
- 21.Anuwong A, Ketwong K, Jitpratoom P, Sasanakietkul T, Duh QY. Safety and outcomes of the transoral endoscopic thyroidectomy vestibular approach. JAMA Surg. 2018;153:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dionigi G, Bacuzzi A, Lavazza M, et al. Transoral endoscopic thyroidectomy: preliminary experience in Italy. Updates Surg. 2017;69:225–234. [DOI] [PubMed] [Google Scholar]
- 23.Jitpratoom P, Ketwong K, Sasanakietkul T, Anuwong A. Transoral endoscopic thyroidectomy vestibular approach (TOETVA) for Graves’ disease: a comparison of surgical results with open thyroidectomy. Gland Surg. 2016;5:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razavi CR, Russell JO. ndications and contraindications to transoral thyroidectomy. Ann Thyroid. 2017;2(5):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diggle P Time Series: A Biostatistical Introduction. Oxford, England: Oxford University Press; 1990. [Google Scholar]
- 27.Razavi CR, Tufano RP, Russell JO. Completion thyroidectomy via the transoral endoscopic vestibular approach. Gland Surg. 2018;7(suppl). doi: 10.21037/gs.2018.02.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsden JD, Johnson AP, Cocks HC, Watkinson JC. Who performs thyroid surgery: a review of current otolaryngological practice. Clin Otolaryngol Allied Sci. 2002;27:304–309. [DOI] [PubMed] [Google Scholar]
- 29.Craig WL, Ramsay CR, Fielding S, Krukowski ZH. A crossspecialty survey to assess the application of risk stratified surgery for differentiated thyroid cancer in the UK. Ann R Coll Surg Engl. 2014;96:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]