Abstract
The photochemistry of 1,2-dihydro-1,2-azaborinine derivatives was studied under matrix isolation conditions and in solution. Photoisomerization occurs exclusively to the Dewar valence isomers upon irradiation with UV light (> 280 nm) with high quantum yield (46%). Further photolysis with UV light (254 nm) results in the formation of cyclobutadiene and an iminoborane derivative. The thermal electrocyclic ring-opening reaction of the Dewar valence isomer back to the 1,2-dihydro-1-tert-butyldimethylsilyl-2-mesityl-1,2-azaborinine has an activation barrier of (27.0 ± 1.2) kcalmol−1. In the presence of the Wilkinson catalyst, the ring opening occurs rapidly and exothermically (ΔH = (−48 ± 1) kcalmol−1) at room temperature.
Keywords: boron–nitrogen heterocycles, electrocyclic reactions, photoisomerization, sustainable chemistry, valence isomerization
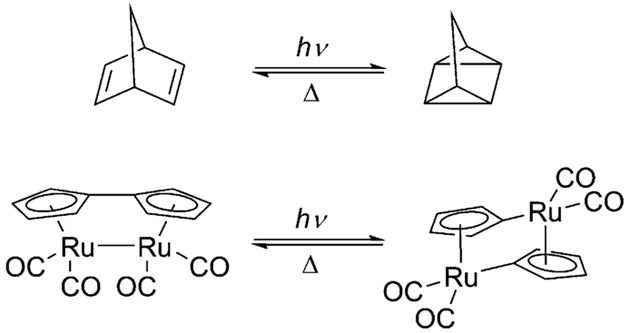
Solar energy as a renewable energy technology is an important part of the solution of the increasing world energy demand and limited fossil energy sources. The development of technologies for storing solar energy is, however, challenging. A promising technology is molecular solar thermal systems (MOST),[1] in which photons induce photoisomerization to a high-energy species that has a sufficiently high activation barrier for the reversible reaction and releases thermal energy only on demand (e.g., in the presence of a catalyst). Beside E/Z isomerization of CC or NN double bonds[2] and photodimerization of anthracene,[3] several valence isomer pairs, such as norbornadiene/quadricyclane,[1g,4] the fulvalene diruthenium system,[5] and hexamethyl-benzene (HMB), which undergoes photoisomerization to hexamethyl Dewar benzene (HMDB) and hexamethylpris-mane,[6] are considered as potential MOST systems (Scheme 1). Herein we present a new valence isomer pair as a candidate for the storage of solar energy that is based on the photoisomerization of 1,2-dihydro-1,2-azaborinine (1) to its Dewar valence isomer 2.
Scheme 1.
Valence isomer pairs studied in the context of energy storage.
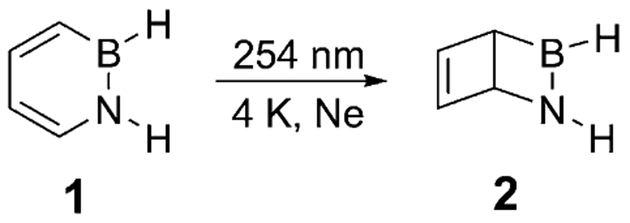
The replacement of two adjacent carbon atoms in benzene by the isoelectronic BN unit leads to 1,2-dihydro-1,2-azaborinines, heterocyclic compounds that have in common with benzene a considerable degree of aromaticity.[7] The polarity of the BN unit modifies the electronic properties,[8] and this heterocycle is therefore of interest in biomedical research[9] and materials science.[10] First syntheses of substituted 1,2-dihydro-1,2-azaborinines were reported by Dewar and Marr[11] and White[12] in the 1960s. Further progress was made by the research groups of Ashe,[13] Perepichka,[10i] Yamaguchi,[10a] Liu,[7a,14] and Braunschweig.[10d,15] Liu and coworkers developed the first synthesis of the parent compound 1,2-dihydro-1,2-azaborinine (1).[7a] The photochemistry of 1,2-dihydro-1,2-azaborinine (1) under matrix isolation conditions was investigated by our group (Scheme 2).[16] The irradiation of 1 isolated in a neon matrix with light of wavelength 254 nm led to full conversion exclusively to the Dewar isomer 2.
Scheme 2.
Photoisomerization of 1,2-dihydro-1,2-azaborinine (1) to the Dewar valence isomer (2).
We also explored the thermal ring-opening reaction of 2 by computational chemistry techniques.[17] Beside the classical conrotatory and disrotatory transition states, two further stepwise ring-opening pathways were identified that were found to be lower in energy than the conrotatory ring opening. With an activation barrier of 22.2 kcalmol−1 [CCSD- (T)/cc-pVQZ//CCSD(T)/TZ2P], the lifetime of 2 should be long enough to allow its detection in solution. It was also found computationally that the polarity of the BN unit would lead to very facile dimerization or oligomerization of 2.[17a]
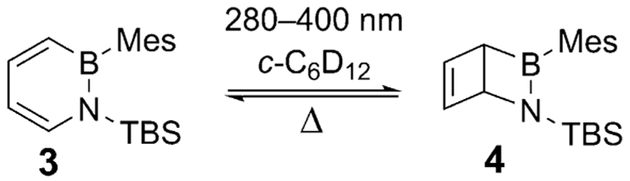
Encouraged by the finding of a sizable barrier for thermal ring opening of 2, we investigated the photochemistry of 1,2-dihydro-1,2-azaborinines in solution. We reasoned that oligomerization of the aminoborane moiety could be suppressed by kinetic stabilization by bulky substituents at B and N. Herein we report the photoconversion of 1,2-dihydro-1-tertbutyldimethylsilyl-2-mesityl-1,2-azaborinine[10e] (3) into the corresponding Dewar valence isomer 4 in solution in cyclohexane at room temperature (Scheme 3). To our surprise, 4 turned out to be stable for weeks at room temperature under inert conditions and was converted back into 3 only slowly.
Scheme 3.
Photoisomerization of 1,2-dihydro-1-tert-butyldimethylsilyl-2-mesityl-1,2-azaborinine (3) to the Dewar isomer (4).
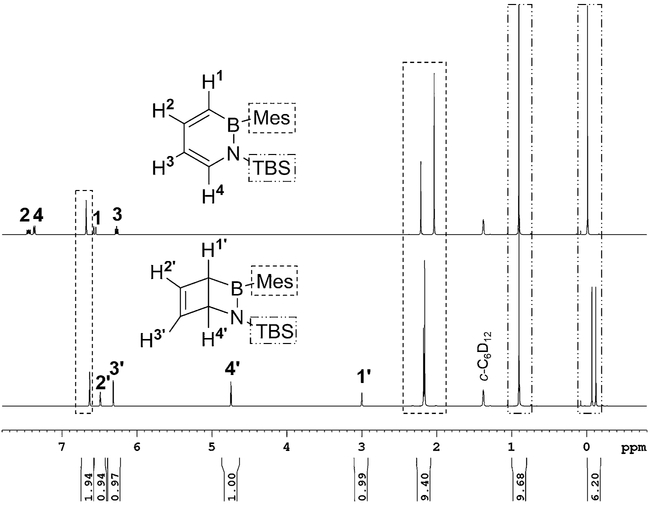
We irradiated a solution of 3 in deuterated cyclohexane (c-C6D12) in a J. Young quartz tube with 280–400 nm light to follow the reaction progress by NMR spectroscopy. The NMR spectra showed decreasing signals of 3 and new increasing signals of 4 without the formation of any by-products. After extended irradiation, full conversion into 4 was observed. The quantum yield of the transformation was determined to be0.46 0.08 (see the Supporting Information for details). The NMR signals of 4 were unambiguously assigned through 2D NMR spectroscopy experiments and comparison with DFT computations of chemical shifts at the B3LYP/6–311 + G** level of theory (see Tables S2 and S4 in the Supporting Information). For example, the boron NMR spectrum showed a new signal at 52.9 ppm (c-C6D12) in accordance with the computed downfield shift of 13.0 ppm as compared to the signal of 3 at 40.3 ppm (c-C6D12). The bridgehead protons were observed at 3.00 and 4.75 ppm (Figure 1), as expected for non-aromatic methine protons, and showed the corresponding coupling in the COSY NMR spectrum (see Figure S9 in the Supporting Information). The two methyl groups of the TBS (tert-butyldimethylsilyl) substituent are diastereotopic owing to the adjacent bridgehead stereocenter.
Figure 1.
1H NMR spectra in c-C6D12 of 1,2-dihydro-1-tert-butyldimethylsilyl-2-mesityl-1,2-azaborinine (3, top trace) and of its Dewar valence isomer (4) after irradiation with 280–400 nm light (bottom trace).
The Dewar isomer 4 could be isolated after the photoreaction. The compound was obtained as a colorless oil in all attempted syntheses, and it was not possible to grow crystals suitable for single-crystal X-ray analysis despite a considerable number of attempts using various techniques, including the crystalline sponge method[18] and the in situ crystallization technique (see Figure S23 for a computed structure).
We also investigated the thermal ring opening of Dewar isomer 4 back to the starting material 3 by NMR spectroscopy. Owing to its high thermal stability, deuterated 1,1,2,2-tetrachloroethane ([D2]TCE) was chosen as the solvent. The 1H NMR spectrum only showed the growth of signals due to 3, without any formation of by-products. The half-life at 100°C was around 25 min. The kinetics of ring opening were studied by NMR spectroscopy in [D2]TCE over a rather narrow temperature range (358–373 K). Arrhenius treatment of the first-order reaction data gave an activation energy (Ea) of (27.0 ± 1.2) kcalmol−1 and a preexponential factor of logA = (12.5 ± 0.7)s−1.
The experimentally determined activation energy is much higher than the barrier computed previously for the ring opening of 2.[17a] As these data were obtained with coupled cluster theory, the “gold standard” of quantum chemistry, it was worthwhile investigating the origin of the difference. As 4 is much too large for computational treatment at the coupled cluster level, we reinvestigated the ring opening of 2 using density functional theory (DFT) at the B3LYP/6–311 + G** level. Gratifyingly, the DFT results are in very good agreement with those of the computationally significantly more involved CCSD(T) method (see Table S10) reported earlier.[17a] Hence, we investigated the ring opening of 4 at the B3LYP/6–311 + G** level. In the presence of the bulky substituents, the thermal ring-opening reaction is no longer stepwise, but rather concerted with a strongly distorted transition state (see Figure S24), as evidenced by computation of the intrinsic reaction coordinate. The lowest energy barrier of 26.1 kcalmol−1 obtained is in very good agreement with experiment and shows that the bulky substituents not only stabilize the Dewar isomer with respect to oligomerization, but also retard ring opening. This is probably due to the increased steric repulsion of the adjacent bulky groups in the planar geometrical arrangement in 3, as the computed heat of reaction is lowered by more than 10 kcalmol−1 for the isomerization of 4→3 versus 2→1 (see Table S10).
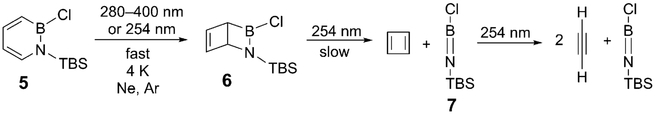
We also investigated the photochemistry of the related chloro derivative 1,2-dihydro-1-tert-butyldimethylsilyl-2-chloro-1,2-azaborinine (5) and found that it also underwent photoisomerization in solution. The corresponding Dewar isomer 6 was also obtained as an oil; however, it was quite readily hydrolyzed by trace amounts of water. As 5 is a thermal precursor to a BN aryne studied by matrix isolation techniques,[19] we also investigated its photochemistry in an argon matrix. Photolysis of 5 with 280–400 nm irradiation quickly led to the formation of the Dewar valence isomer, based on the comparison of experimental and computed IR spectra, as the only photo product. UV spectra showed the disappearance of 5 owing to photolysis at 280–400 nm and the formation of a new band at 215 nm (see Figure S21) that can be assigned to 6 as based on the behavior deduced by IR spectroscopy. A weak absorption (S0→S1) was computed for 6 at 216 nm (f = 0.013) at the TD-B3LYP/6–311 + G** level of theory.
Irradiation of 5 with 254 nm light also quickly resulted in photoisomerization to 6, but upon extended photolysis at 254 nm, the Dewar isomer slowly underwent cycloreversion to the corresponding iminoborane 7 and cyclobutadiene. The latter underwent further cycloreversion to two acetylene molecules upon prolonged irradiation (Scheme 4).[20] The IR signals of 7 could be assigned by comparison with a computed spectrum [B3LYP/6–311 + G**] and showed the natural ratio of 10B and 11B isotopes (see Figure S22).
Scheme 4.
Photochemistry of 1,2-dihydro-1-tert-butyldimethylsilyl-2-chloro-1,2-azaborinine (5) under matrix isolation conditions.
We previously observed that the parent Dewar isomer 2 also underwent a slow photodecomposition reaction, and hence reinvestigated this reaction in light of the results described above. Indeed, prolonged irradiation of the parent systems (both the B−H/N−H and B−D/N−D isotopologues were investigated) resulted in the formation of cyclobutadiene and acetylene. However, the corresponding parent iminoboranes HBNH and DBND could not be observed unambiguously, as the degree of photoisomerization and photodecomposition was lower for the parent system.
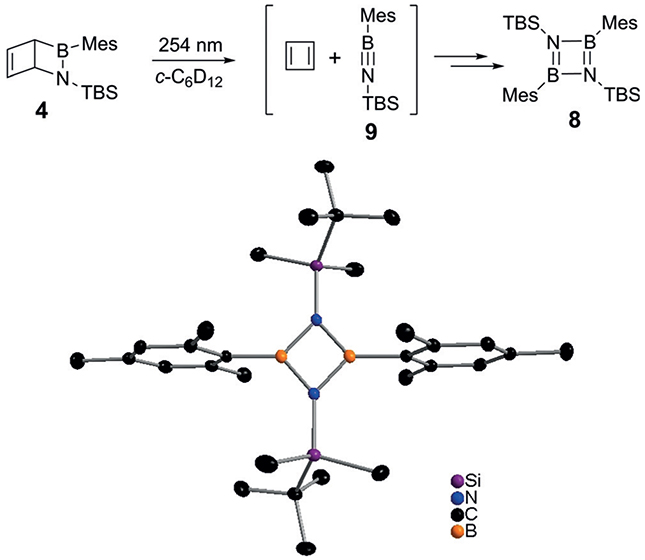
Motivated by the matrix isolation study, we explored the photochemistry of 4 in solution. The NMR spectra after irradiation with 254 nm light showed the disappearance of 4 and formation of new compounds, including 1,3,2,4-diazadiboretidine 8 (Scheme 5), which is stable under conventional conditions and can be purified by column chomatography. A single crystal suitable for X-ray crystallography was obtained by slow evaporation of n-pentane (Scheme 5).[21] The 11B NMR spectrum showed a signal at 17.4 ppm after irradiation that was in the typical range for N-silyl-substituted iminoboranes (16–22 ppm).[21b,22] The computed chemical shift of 17.2 ppm supports the assignment of the intermediate as iminoborane 9. Other photo products could not be identified.
Scheme 5.
Cycloreversion of the Dewar valence isomer of 1,2-dihydro-1-tert-butyldimethylsilyl-2-mesityl-1,2-azaborinine (4) leads to the formation of 8.
These results show that 1,2-dihydro-1,2-azaborinines can undergo a stepwise photoinduced cycloreversion to the acetylene and iminoborane building blocks. The fragmentation discovered in this study is formally the reversion of azaborinine synthesis from iminoborane and acetylenes reported by Braunschweig et al.[23]
Finally, we screened a variety of catalysts to promote the ring-opening reaction at room temperature. In an initial survey of metal catalysts we found that the Wilkinson catalyst was uniquely effective, furnishing cleanly the 1,2-dihydro-1,2-azaborinine 3 from 4 in less than 1 h at room temperature at 3 mol% catalyst loading (see Table S11). More detailed screening of rhodium-based catalysts revealed [Rh(C2H4)2Cl]2 as an equally effective catalyst (see Table S11). On the other hand, cyclooctadiene- or norbornadiene-containing neutral and cationic RhI complexes as well as the RhIII complex [Rh(Cp*)Cl2]2 were not suitable ring-opening catalysts (see Table S11). To determine the energy stored in the photo-generated strained Dewar compound 4, we measured the heat of the ring-opening reaction in a reaction calorimeter. Thus, integration of the heat-flow curve of the ring-opening reaction catalyzed by the Wilkinson catalyst (3 mol%) gave consistently ΔH = (−48 ± 1) kcalmol−1.[24] This value com pares favorably to those of other known MOST systems (e.g., ΔH = −21 kcal mol−1for the norbornadiene/quadricy-clane system;[25] ΔH = −20 kcalmol−1 for the fulvalene diruthenium system),[5d] and agrees with our DFT computations (see Table S10).
In summary, we have demonstrated the isolation of the Dewar valence isomer of two 1,2-dihydro-1,2-azaborinines in solution on a preparative scale. Irradiation of a substituted 1,2-dihydro-1,2-azaborinine in solution in cyclohexane led to full conversion with high quantum yield ((46 ± 8)%) exclusively into the Dewar valence isomer, which was stable for weeks at room temperature under inert conditions. Further irradiation with shorter wavelength UV light led to cycloreversion of the Dewar valence isomer to the corresponding iminoborane and cyclobutadiene. The substituted Dewar isomer is kinetically stable towards thermal electrocyclic ring opening which has an activation energy of (27.0 ± 1.2) kcalmol−1. The thermal ring-opening reaction can be catalyzed efficiently by the Wilkinson catalyst. The energy stored in the strained bicyclic Dewar valence isomer has been determined by calorimetric measurement to be (48 ± 1) kcalmol−1. This study provides a proof of concept of the potential utility of 1,2-dihydro-1,2-azaborinine valence isomer pairs in molecular solar thermal system applications. Current efforts are directed toward tuning the absorption profile of our system to the visible range.
Supplementary Material
Acknowledgements
The research in Tübingen was supported by the Deutsche Forschungsgemeinschaft. The computations were performed on the BwForCluster JUSTUS. We acknowledge support by the state of Baden-Württemberg through bwHPC and the German Research Foundation (DFG) through grant no. INST 40/467–1 FUGG. We thank Dr. Klaus Merz for attempting in situ crystallization, and Prof. Dr. Günter Gauglitz and Prof. Dr. Suning Wang for helpful discussions concerning the determination of the quantum yield. This research was supported in part by the National Institutes of Health NIGMS (R01-GM094541). S.-Y.L. thanks the Humboldt Foundation for the Friedrich Wilhelm Bessel Research Award.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/anie.201712683.
Contributor Information
Klara Edel, Institut für Organische Chemie, Universität Tübingen Auf der Morgenstelle 18, 72076 Tübingen (Germany).
Xinyu Yang, Department of Chemistry, Boston College Chestnut Hill, MA 02467-3860 (USA) liusd−bc.edu.
Jacob S. A. Ishibashi, Department of Chemistry, Boston College Chestnut Hill, MA 02467-3860 (USA) liusd−bc.edu
Ashley N. Lamm, Department of Chemistry and Biochemistry, University of Oregon Eugene, OR 97403-1253 (USA)
Cäcilia Maichle-Mössmer, Institut für Anorganische Chemie, Universität Tübingen Auf der Morgenstelle 18, 72076 Tübingen (Germany).
Zachary X. Giustra, Department of Chemistry, Boston College Chestnut Hill, MA 02467-3860 (USA) liusd−bc.edu
Shih-Yuan Liu, Department of Chemistry, Boston College Chestnut Hill, MA 02467-3860 (USA) liusd−bc.edu); Department of Chemistry and Biochemistry, University of Oregon Eugene, OR 97403-1253 (USA).
Holger F. Bettinger, Institut für Organische Chemie, Universität Tübingen Auf der Morgenstelle 18, 72076 Tübingen (Germany).
References
- [1].a) Lennartson A, Moth-Poulsen K in Molecular Devices for Solar Energy Conversion and Storage (Eds.: Tian H, Boschloo G, Hagfeldt A), Springer, Singapore, 2018, pp. 327–352; [Google Scholar]; b) Lennartson A, Roffey A, Moth-Poulsen K, Tetrahedron Lett. 2015, 56, 1457–1465; [Google Scholar]; c) Kucharski TJ, Tian Y, Akbulatov S, Boulatov R, Energy Environ. Sci 2011, 4, 4449–4472; [Google Scholar]; d) Jorner K, Dreos A, Emanuelsson R, Bakouri O. El, Galvan IF, Börjesson K, Feixas F, Lindh R, Zietz B, Moth-Poulsen K, Ottosson H, J. Mater. Chem. A 2017, 5, 12369–12378; [Google Scholar]; e) Dreos A, Börjesson K, Wang Z, Roffey A, Norwood Z, Kushnir D, Moth-Poulsen K, Energy Environ. Sci 2017, 10, 728–734; [Google Scholar]; f) Börjesson K, Lennartson A, Moth-Poulsen K, ACS Sustainable Chem. Eng 2013, 1, 585–590; [Google Scholar]; g) Dubonosov DA,Bren AV, Chernoivanov VA, Russ. Chem. Rev 2002, 71, 917–927. [Google Scholar]
- [2].a) Caia V, Cum G, Gallo R, Mancini V, Pitoni E, Tetrahedron Lett. 1983, 24, 3903–3904; [Google Scholar]; b) Bastianelli C, Caia V, Cum G, Gallo R, Mancini V, J. Chem. Soc. Perkin Trans. 2 1991, 679–683; [Google Scholar]; c Kolpak AM, Grossman JC, Nano Lett. 2011, 11, 3156–3162; [DOI] [PubMed] [Google Scholar]; d) Feng Y, Liu H, Luo W, Liu E, Zhao N, Yoshino K, Feng W, Sci. Rep 2013, 3, 3260; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Kucharski TJ, Ferralis N, Kolpak AM, Zheng JO, Nocera DG, Grossman JC, Nat. Chem 2014, 6, 441–447. [DOI] [PubMed] [Google Scholar]
- [3].Stein G, Isr. J. Chem 1975, 14, 213–225. [Google Scholar]
- [4].a) Gray V, Lennartson A, Ratanalert P, Börjesson K, Moth-Poulsen K, Chem. Commun 2014, 50, 5330–5332; [DOI] [PubMed] [Google Scholar]; b) Yoshida Z-I, J. Photochem 1985, 29, 27–40. [Google Scholar]
- [5].a) Moth-Poulsen K, Coso D, Börjesson K, Vinokurov N, Meier SK, Majumdar A, Vollhardt KPC, Segalman RA, Energy Environ. Sci 2012, 5, 8534–8537; [Google Scholar]; b) Börjesson K, Lennartson A, Moth-Poulsen K, J. Fluorine Chem. 2014, 161, 24–28; [Google Scholar]; c) Börjesson K, Dzebo D, Albinsson B, Moth-Poulsen K, J. Mater. Chem. A 2013, 1, 8521–8524; [Google Scholar]; d) Kanai Y, Srinivasan V, Meier SK, Vollhardt KPC, Grossman JC, Angew. Chem. Int. Ed 2010, 49, 8926–8929; Angew. Chem. 2010, 122, 9110–9113; [DOI] [PubMed] [Google Scholar]; e) Harpham MR, Nguyen SC, Hou Z, Grossman JC, Harris CB, Mara MW, Stickrath AB, Kanai Y, Kolpak AM, Lee D, Liu D-J, Lomont JP, Moth-Poulsen K, Vinokurov N, Chen LX, Vollhardt KPC, Angew. Chem. Int. Ed 2012, 51, 7692–7696; Angew. Chem. 2012, 124, 7812 – 7816. [DOI] [PubMed] [Google Scholar]
- [6].a) Schaefer W, Hellmann H, Angew. Chem. Int. Ed. Engl 1967, 6, 518–525; Angew. Chem. 1967, 79, 566 – 573; [Google Scholar]; b) Oth JFM, Recl. Trav. Chim. Pays-Bas 1968, 87, 1185–1195; [Google Scholar]; c) Hogeveen H, Volger HC, Chem. Commun 1967, 1133–1134; [Google Scholar]; d) Adam W, Chang JC, Int. J. Chem. Kinet 1969, 1, 487–492. [Google Scholar]
- [7].a) Marwitz AJV, Matus MH, Zakharov LN, Dixon DA, Liu S-Y, Angew. Chem. Int. Ed 2009, 48, 973–977; Angew. Chem. 2009, 121, 991 – 995; [DOI] [PubMed] [Google Scholar]; b) Abbey ER, Zakharov LN, Liu S-Y, J. Am. Chem. Soc 2008, 130, 7250–7252. [DOI] [PubMed] [Google Scholar]
- [8].a) Liu Z, Marder TB, Angew. Chem. Int. Ed 2008, 47, 242–244; Angew. Chem. 2008, 120, 248 – 250; [DOI] [PubMed] [Google Scholar]; b) Bosdet MJD,Piers WE, Can. J. Chem 2009, 87, 8–29; [Google Scholar]; c) Campbell PG,Marwitz AJV, Liu S-Y, Angew. Chem. Int. Ed 2012, 51, 6074–6092; Angew. Chem. 2012, 124, 6178 – 6197; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wang X-Y, Wang J-Y, Pei J, Chem. Eur. J 2015, 21, 3528–3539. [DOI] [PubMed] [Google Scholar]
- [9].a) Liu L, Marwitz AJV, Matthews BW, Liu S-Y, Angew. Chem. Int. Ed 2009, 48, 6817–6819; Angew. Chem. 2009, 121, 6949 – 6951; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Knack DH, Marshall JL, Harlow GP, Dudzik A, Szaleniec M, Liu S-Y, Heider J, Angew. Chem. Int. Ed 2013, 52, 2599–2601; Angew. Chem. 2013, 125, 2660 – 2662; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lee H, Fischer M, Shoichet BK, Liu S-Y, J. Am. Chem. Soc 2016, 138, 12021–12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) Taniguchi T, Yamaguchi S, Organometallics 2010, 29, 5732–5735; [Google Scholar]; b) Marwitz AJV, Jenkins JT, Zakharov LN, Liu S-Y, Angew. Chem. Int. Ed 2010, 49, 7444–7447; Angew. Chem. 2010, 122, 7606 – 7609; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Marwitz AJV, Lamm AN, Zakharov LN, Vasiliu M, Dixon DA, Liu S-Y, Chem. Sci 2012, 3, 825–829; [Google Scholar]; d) Braunschweig H, Hçrl C, Mailänder L, Radacki K, Wahler J, Chem. Eur. J 2014, 20, 9858–9861; [DOI] [PubMed] [Google Scholar]; e) Baggett AW, Vasiliu M, Li B, Dixon DA, Liu S-Y, J. Am. Chem. Soc 2015, 137, 5536–5541; [DOI] [PubMed] [Google Scholar]; f) Saif M, Widom JR, Xu S, Abbey ER, Liu S-Y, Marcus AH, J. Phys. Chem. B 2015, 119, 7985–7993; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Baggett AW, Guo F, Li B, Liu S-Y, Jäkle F, Angew. Chem. Int. Ed 2015, 54, 11191–11195; Angew. Chem. 2015, 127, 11343 – 11347; [DOI] [PubMed] [Google Scholar]; h) Murphy CJ, Miller DP, Simpson S, Baggett A, Pronschinske A, Liriano ML, Therrien AJ, Enders A, Liu S-Y, Zurek E, Sykes ECH, J. Phys. Chem. C 2016, 120, 6020–6030; [Google Scholar]; i) Lepeltier M, Lukoyanova O,Jacobson A, Jeeva S, Perepichka DF, Chem. Commun 2010, 46, 7007–7009. [DOI] [PubMed] [Google Scholar]
- [11].Dewar MJS, Marr PA, J. Am. Chem. Soc 1962, 84, 3782–3782. [Google Scholar]
- [12].White DG, J. Am. Chem. Soc 1963, 85, 3634–3636. [Google Scholar]
- [13].a) Ashe AJ, Fang X, Org. Lett 2000, 2, 2089–2091; [DOI] [PubMed] [Google Scholar]; b) Ashe AJ, Fang X, Fang X, Kampf JW, Organometallics 2001, 20, 5413–5418. [Google Scholar]
- [14].Abbey ER, Lamm AN, Baggett AW, Zakharov LN, Liu S-Y, J. Am. Chem. Soc 2013, 135, 12908–12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].a) Braunschweig H, Gackstatter A, Kupfer T, Scheller T, Hupp F, Damme A, Arnold N, Ewing WC, Chem. Sci 2015, 6, 3461–3465; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Braunschweig H, Celik MA, Hupp F, Krummenacher I, Mailänder L, Angew. Chem. Int. Ed 2015, 54, 6347–6351; Angew. Chem. 2015, 127, 6445 – 6449. [DOI] [PubMed] [Google Scholar]
- [16].Brough SA, Lamm AN, Liu S-Y, Bettinger HF, Angew. Chem. Int. Ed 2012, 51, 10880–10883; Angew. Chem. 2012, 124, 11038 – 11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].a) Bettinger HF, Hauler O, Beilstein J. Org. Chem 2013, 9, 761–766; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Su M-D, Chem. Eur. J 2013, 19, 9663–9667; [DOI] [PubMed] [Google Scholar]; c) Kim J, Moon J, Lim JS, ChemPhysChem 2015, 16, 1670–1675. [DOI] [PubMed] [Google Scholar]
- [18].a) Inokuma Y, Yoshioka S, Ariyoshi J, Arai T, Hitora Y, Takada K, Matsunaga S, Rissanen K, Fujita M, Nature 2013, 495, 461–466; [DOI] [PubMed] [Google Scholar]; b) Hoshino M, Khutia A, Xing H, Inokuma Y, Fujita M, IUCrJ 2016, 3, 139–151; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Waldhart W, Mankad NP, Santarsiero BD, Org. Lett 2016, 18, 6112–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Edel K, Brough SA, Lamm AN, Liu S-Y, Bettinger HF, Angew. Chem. Int. Ed 2015, 54, 7819–7822; Angew. Chem. 2015, 127, 7930 – 7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chapman OL, McIntosh CL, Pacansky J, J. Am. Chem. Soc 1973, 95, 614–617. [Google Scholar]
- [21].a) Compound 8 crystallizes in the monoclinic space group P21/c with two molecules in a unit cell. The alternate BN distances of 1.460 and 1.466 − of the nearly planar rhombic ring with angles of 98.05(9)° at the boron atoms and 81.94(9)° at the nitrogen atoms are consistent with literature data;; Paetzold P, Adv. Inorg. Chem 1987, 31, 123–170. [Google Scholar]
- [22].a) Haase M, Klingebiel U, Angew. Chem. Int. Ed. Engl 1985, 24, 324–324; Angew. Chem. 1985, 97, 335 – 336; [Google Scholar]; b) Elter G, Neuhaus M, Meller A, Schmidt-Bäse D, J. Organomet. Chem 1990, 381, 299–313; [Google Scholar]; c) Nçth H, Angew. Chem. Int. Ed. Engl 1988, 27, 1603–1623; Angew. Chem. 1988, 100, 1664 – 1684. [Google Scholar]
- [23].a) Braunschweig H, Damme A, Jimenez-Halla JOC, Pfaffinger B, Radacki K, Wolf J, Angew. Chem. Int. Ed 2012, 51, 10034–10037; Angew. Chem. 2012, 124, 10177 – 10180; [DOI] [PubMed] [Google Scholar]; b) Braunschweig H, Geetharani K, Jimenez-Halla JOC, Schäfer M, Angew. Chem. Int. Ed 2014, 53, 3500–3504; Angew. Chem. 2014, 126, 3568 – 3572. [DOI] [PubMed] [Google Scholar]
- [24].Tyutyulkov NN, Polansky OE, Fabian J, Naturforsch Z. A 1975, 30, 1308–1310. [Google Scholar]
- [25].An X-W, Xie Y-D, Thermochim. Acta 1993, 220, 17–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.