Abstract
Restraint stress is a psychosocial stressor that suppresses reproductive status, including LH pulsatile secretion, but the neuroendocrine mechamisms underlying this inhibition remains unclear. Reproductive neural populations upstream of gonadotropin releasing hormone (GnRH) neurons, such as kisspeptin, neurokinin B, and RFRP-3 (GnIH) neurons, are possible targets for psychosocial stress to inhibit LH pulses, but this has not been well-examined, especially in mice in which prior technical limitations prevented assessment of in vivo LH pulse secretion dynamics. Here we examined whether one-time acute restraint stress alters in vivo LH pulsatility and reproductive neural populations in male mice, and what the time-course is for such alterations. We found that endogenous LH pulses in castrated male mice are robustly and rapidly suppressed by one-time, acute restraint stress, with suppression observed as quickly as 12–18 min. This rapid LH suppression parallels with increased in vivo corticosterone levels within 15 min of restraint stress. Although Kiss1, Tac2, and Rfrp gene expression in the hypothalamus did not significantly change after 90 or 180 min restraint stress, arcuate Kiss1 neural activation was sigmnifciantly decreased after 180 min. Interestingly, hypothalamic Rfrp neuronal activation was strongly increased at early times after restraint stress initiation, but was attenuated to levels lower than controls by 180 min of restraint stress. Thus, the male neuroendocrine reproductive axis is quite sensitive to short-term stress exposure, with significantly decreased pulsatile LH secretion and increased hypothalamic Rfrp neuronal activation occurring rapidly, within minutes, and decreased Kiss1 neuronal activation also occurring after longer stress durations.
Keywords: Rfrp, GnIH, Kiss1, kisspeptin, restraint, stress, corticosterone
Introduction
Stress disturbs many physiological processes, including reproduction. While multiple types of stressors, including psychosocial stress, have been demonstrated to decrease reproductive hormone secretion in many species, including humans, the specific mechanism(s) underlying this decrease in hormonal output remain unknown (Demura, et al. 1989; Fenster, et al. 1997; Lopez-Calderon, et al. 1991; Mann and Orr 1990; Rivier and Rivest 1991). One common animal model of psychosocial stress is restraint (or immobilization) stress. Previous studies demonstrated that restraint stress decreases mean plasma LH levels in rats, sheep, and monkeys (Chen, et al. 1996; Kirby, et al. 2009; Li, et al. 2006; Tilbrook, et al. 2000, 2002; Wagenmaker, et al. 2009). Furthermore, repeated restraint stress has been shown to disrupt LH pulses in ovariectomized female rats (Li, et al. 2004). Similar studies in mice have historically been lacking because, until just recently, it was not technically possible to measure endogenous pulsatile LH secretion in mice due to their small size and assay limitations. With new advances permitting measurement of murine pulsatile LH, we recently reported that in gonadectomized (GDX) female mice, acute exposure to restraint stress decreased both mean and pulsatile LH secretion (Yang, et al. 2017). However, to date, the underlying mechanisms involved in the restraint stress-induced suppression of LH have not been fully elucidated, especially at the level of the brain. In the hypothalamus, pulsatile GnRH secretion coordinates the downstream pulsatile release of LH from the pituitary, and measurement of LH pulses is often used as a proxy for functional output of GnRH secretion. Several reports suggest that restraint stress suppresses frequency of LH pulses, suggesting that some stress effects must be occurring at the level of the brain to influence the GnRH “pulse generator”, but how this occurs is unknown (Kinsey-Jones, et al. 2009; Li et al. 2004; Orr and Mann 1990; Yang et al. 2017). Additional findings have noted that restraint stress also suppresses the amplitude of LH pulses, as well as basal LH levels, which could reflect effects at the level of the brain, pituitary, or both (Handa, et al. 1994; Tilbrook, et al. 1999).
Alteration of GnRH pulse frequency by stress could possibly occur through direct effects at the level of GnRH neurons or upstream, through GnRH-regulating neural circuits, such as kisspeptin or RFRP-3 neurons. Kisspeptin neurons of the arcuate nucleus (ARC) have recently been implicated as part of the GnRH pulse generator mechanisms and project to GnRH neurons to synchronize bursts of electrical activity and Ca++ influxes, leading to pulsatile GnRH secretion (Clarkson, et al. 2017; Goodman, et al. 2013; Lehman, et al. 2010). In female mice, acute restraint stress decreased ARC Kiss1 neural activation at 45 min, 90 min, and 180 min following the start of restraint exposure (Yang et al. 2017), but had no effect on Kiss1 gene expression at any of these time points. However, in female rats, ARC Kiss1 mRNA expression was decreased when measured 6 hr after restraint stress initiation (Kinsey-Jones et al. 2009). Together, these data suggest that restraint stress may inhibit GnRH pulse generation in female rodents, at least in part, by inhibiting ARC Kiss1 neurons. Whether this occurs in males too, and if so, to what degree, is unknown. ARC kisspeptin neurons are also known as KNDy neurons for their colocalization of kisspeptin, neurokinin B (NKB; encoded by Tac2 gene), and dynorphin, with NKB serving as a key positive regulator of kisspeptin secretion to trigger GnRH pulses (Lehman et al. 2010). Whether alteration of NKB is involved in psychosocial stress inhibition is not yet studied.
RFRP-3 (the mammalian homologue of gonadotropin-inhibiting hormone (GnIH)), is synthesized exclusively in and around the dorsal-medial nucleus (DMN) of the hypothalamus and is inhibitory to the reproductive axis (Clarke, et al. 2008; George, et al. 2017; Johnson, et al. 2007; Kriegsfeld, et al. 2006; Osugi, et al. 2004; Poling, et al. 2012). In mammals, these inhibitory actions occur in the brain (not the pituitary) through pathways that still remain to be elucidated (Anderson, et al. 2009; Ducret, et al. 2010). Previous studies in rats demonstrated that a 3 hr restraint stress increased neural Rfrp expression (Kirby et al. 2009). Another study in female rats showed that LPS-induced immunological stress increased Rfrp levels after 6 hr (Iwasa, et al. 2014). Together, these few data suggest that restraint stress may suppress GnRH pulsatile secretion through upstream neural circuits that include RFRP-3 neurons, but additional studies are needed to confirm and test this possibility, especially in mice, and to link such changes to altered LH secretion.
The current study examines whether a one-time, acute (90 min) restraint stress exposure significantly alters in vivo endogenous pulsatile LH secretion and/or reproductive neural factors in male mice. Changes in in vivo circualting corticosterone (CORT) levels were also measured over the course of restraint stress to correlate with any possible changes in pulsatile LH secretion and to track the changes in timing of each measure. Furthermore, to determine if stimulatory and inhibitory neural regulators of GnRH neurons are altered by restraint stress in males, hypothalamic Kiss, Rfrp, and Tac2 (NKB) gene expression was measured via single-label in situ hybridization (ISH). Lastly, cfos induction, a common marker of recent neuron activation, was also assessed specifically in hypothalamic Kiss1 and Rfrp neurons via double-label ISH.
Materials and Methods
Animals
Adult male C57BL6 mice (8–9 weeks old) were pair housed under a 12 hr light/dark cycle (lights off at 1800) with food and water available ad libitum. Prior to experimentation, all males were bilaterally gonadectomized (GDX) for 4 (Experiment #1), 3.5 (Experiment #2), or 2 (Experiment #3) weeks prior to day of experimentation to increase circulating LH levels and to remove inter-individual differences in circulating gonadal sex steroids, which are known to alter stress responsiveness. In brief, male mice were anesthetized with isoflurane (4%) and kept on a sterile, heated surgical plane. A small invision was made at the ventral midline of the skin and underlying muscle, and each testes was clamped with a hemostat prior to excision with scissors. The muscle wall was then sutured using absorbable sutures and the skin cut closed using sterile surgical wound clips. An analgesic (buprenorphine) was administred subcutaneously the day of GDX and one day-post GDX. All experiments were conducted at the University of California, San Diego under the approval of the Institutional Animal Care and Use Committee.
Experiment #1:
Effects of short-term restraint on in vivo LH pulsatility in male mice
Effects of psychosocial stress or restraint on in vivo LH pulses in male mice have not been previously determined. To determine if short-term restraint stress affects LH pulses at all in GDX males, and if so, how fast and to what degree, blood LH was measured every 6 min during the 90 min pre- and 90 min post-stress periods. GDX male mice were allowed to recover from surgery for 1 week before daily handling was initiated to habituate the mice to the tail-tip bleeding procedure used for collecting blood for LH measurement. Mice were briefly handled daily for 3 weeks prior to the day of serial bleeding, to habituate them to handling during tail tip bleeding on the actual experimental day. All handling, bleeding, and stress induction (restraint) was conducted in the morning between 0900 h and 1200 h to avoid normal circadian elevations in CORT levels that rise later in the day/evening. After 3 weeks of handling acclimation, on the day of the serial bleeding experiment, the mice were separated into a control (non-stressed) group (n=10) and a stress group (n=10). For the initial 90 min of the experiment (pre-stress period), all mice were freely awake and kept in their pair-housed cages. The tail tip of each animal was cut and serial bleeds were collected every 6 minutes. We previously reported LH pulses in OVX female mice sampled at 5 min intervals (Yang et al. 2017). For the current experiment, we increased sampling interval to 6 min to decrease impacts of stress of bleeding and to permit for a greater number of mice to be bled simultaneously each day, while still being frequent enough to detect each LH pulse. For each sample, 3 μl of whole blood was taken from the tail and mixed with 57 μl of assay buffer, and then placed on ice until storage at −20C. Following the initial 90 min baseline bleeding period, stress mice were immediately placed into Broome rodent restraint devices (Harvard Apparatus, Holliston, MA), isolated into new cages, and then continued to be bled every 6 min for the next 90 min (the stress period). By contrast, control mice were kept pair-housed in their home cages and continued to be bled every 6 min for the same 90 min period without any restraint stress exposure.
Serial blood samples collected every 6 min were measured for LH levels by the University of Virginia Ligand Assay Core using an ultra-sensitive murine LH ELISA. The intra- and inter-assay % CVs were 2.3% and <7%, respectively. Functional sensitivity was 0.320 ng/mL. Values are reported as ng/ml of whole blood. Serial blood LH measures were analyzed for endogenous LH pulses. An LH value was determined to be a pulse if the value had a >20% increase from one of the two previous points and LH subsequently decreased by >10% in the subsequent point. For identified LH pulses, the following parameters were calculated: 1) pulse frequency (# pulses/90 min); 2) interpulse interval, defined as the time between 2 pulses. In the restraint group, some mice did not have at least two LH pulses during the 90 min stress period. In cases of only a single pulse, the interpulse interval was defined as the time from the single observed pulse until the end of the stress period. In cases of no identifiable pulses, the interpulse interval was defined to be 90 min (i.e., the duration of the stress period); 3) pulse amplitude, defined to be the difference between a pulse peak value and a preceding nadir, the lowest value of the 2 preceding values from a peak. In the restraint group, animals that did not show any peaks during the post period (the stress period) were excluded from the pulse amplitude calculations; 4) pulse peak, defined as the zenith LH value of each identified pulse; 5) basal LH levels, defined as the average LH of all the nadirs. In addition to these pulse measures, we also calculated overall mean LH, the average of all the LH values for an animal during either the baseline (pre-stress) or stress periods.
Experiment 2:
Time course of acute restraint stress effects on circulating corticosterone in male mice
To determine if CORT is secreted and elevated in a time course that matches the observed suppression in LH in Experiment 2, we assed CORT levels in male mice exposed to acute restraint stress. Adult GDX male mice were allowed to recover 1 week before daily handling to habituate animals to tail bleeding procedure to measure circulating CORT levels. After 2.5 weeks of daily handling, mice were separated into control (n=7) and restraint (n=7) groups. The tail tip of each mouse was cut and 7 ul of whole blood was collected at: −10, 0, 5, 15, 30, 45, 60, and 90 min, with time 0 representing the time of restraint stress initiation (isolated to new cages and placed in Broome rodent restraint devices). Control mice were not exposed to stress and were kept pair-housed in their home cages throughout the serial bleeding procedure.
For measurement of serum CORT, 7 ul of whole blood was pipetted into a Drummond Microcap capillary tube (Drummond Scientific Company, Broomall, PA). Each tube was sealed off using Critoseal capillary tube sealant (Leica, Buffalo Grove, IL) and allowed to clot at room temperature for 2 hr prior to centrifugation (3000 rpm x 15 min) to collect blood serum. Blood serum was stored at −20 C for measurement of CORT using DetectX Corticosterone EIA kit (Arbor Assays, Ann Arbor, MI). The assay sensitivity was 18.6 pg/mL. The inter- and intra-assay %CVs were 4.5% and 6.6%, respectively.
Experiment #3:
Restraint stress effects on reproductive neural populations in male mice
To determine what upstream neuroendocrine mechanisms are altered by acute restraint stress in male mice, we examined gene expression of key stimulatory or inhibitory neuroendocrine regulators of GnRH neurons and proposed players in GnRH pulse generation. Adult GDX males were allowed to recover for 2 weeks following castration before the experiment. On the day of the experiment, males were separated into control (0 min), 45 min, 90 min, and 180 min stress groups (n=10–11/group). As in earlier experiments, this experiment was conducted in the morning between 0900 and 1200 h to avoid circadian rises in baseline CORT levels that normally occur later in the day/evening. Male mice in the stress groups were isolated into new cages, and immediately placed into Broome rodent restraint devices for either 45 min, 90 min, or 180 min. Following stress, mice were immediately anesthetized with isoflurane and blood collected via retro-orbital sampling prior to rapid decapitation. Blood serum was isolated and stored at −20 C for later measurement of CORT levels to confirm that the restraint paradigm properly induced stress, as indicated by increased circulating CORT levels. Blood sera were assayed using DetectX Corticosterone EIA kit (Arbor Assays, Ann Arbor, MI), similar to Experiment #2. Brains were immediately frozen on dry ice before being stored at −80 C to use for in situ hybridization assays.
Single and double-label In Situ Hybridization (ISH)
Brains collected from stressed and control mice from Experiment #3 were used for ISH analyses. Frozen brains were sectioned into 5 sets of 20 uM sections on a cryostat and thaw mounted on Superfrost-plus slides that were stored at −80 C until the assay. Single-label ISH for ARC Kiss1 or Tac2 (NKB) expression or for DMN Rfrp expression were each performed on 1 complete set of brain sections spanning the entire anterior-to-caudal hypothalamus (no subregions were quantified). Double-label ISH for cfos+Kiss1 and cfos+Rfrp were performed on an additional set of brain sections in order to assess neuronal activation. Both single- and double-label ISH assays were performed using well-published protocols and validated P33 or DIG-labeled riboprobes, as previously reported (Poling, et al. 2017; Semaan and Kauffman 2015; Stephens, et al. 2016; Stephens, et al. 2017). After ISH slides were developed and coverslipped, a custom automated image processing and quantification software program (Dr. Don Clifton, University of Washington) was used to analyze ISH slides under computer-assisted microscopy, by an investigator blinded to treatment groups. For single-label experiemnts, the software counted the number of ISH silver grain clusters and the number of silver grains over each cell (giving a semiquantitative index of mRNA expression per cell). Cells are considered positive when the number of silver grains in a cluster exceeded the background by 3-fold. For double-label assays, all red fluorescent DIG-containing cells were identified under fluorescent microscopy and the software was used to quantify silver grains overlaying each DIG cell. The software also objectively sets threshold and calculates background and then uses this to calculate signal-to-background ratios of the silver grains for each cell. A cell was considered to be double-labeled if its signal-to-background ratio was >4.
Statistics
All data were analyzed in GraphPad Prism 6 (La Jolla, CA). In Experiment #1, data were analyzed as a two-way ANOVA (time x group) followed by post-hoc Bonferroni’s multiple comparisons test. In Experiment #2, data were analyzed as a two-way repeated measures ANOVA (time x group), followed by post-hoc Bonferroni’s multiple comparisons test. In Experiment #3, data were analyzed as a one-way ANOVA (group), followed by post-hoc Bonferroni’s multiple comparison test. All data are expressed as mean ± standard error. p<0.05 was considered to be statistically significant.
Results
Experiment 1
Acute restraint stress potently decreases LH pulsatility in GDX male mice
To determine if short-term restraint stress affects LH pulses at all in GDX males, blood LH was measured every 6 min for 90 min periods before and during stress exposure. Figure 1 shows representative LH values during the collection period for 3 no stress control males (Fig. 1A-C) and 3 stress males (Fig. 1D-F). In both groups, notable rapid pulsatile LH secretion was clearly detected during the baseline (no stress) period, with no significant differences in pulse frequency or any other LH measure between control and stress groups during this time (Fig 2), with pulses occurring, on average, once every ~18–20 min (as expected given the GDX status). In control males, there were no differences in any LH parameter between the baseline period and subsequent stress period (Figs. 1, 2). By contrast, male mice exposed to acute restraint stress during the second 90 min period displayed significant and rapid decreases in LH pulsatile secretion, with notable alterations in multiple pulse parameters (Fig 1, 2). The number of detectable pulses (pulse frequency) was significantly decreased by ~50% in stress mice during the 90 min stress period (F(1,23) = 6.754, p<0.05; Fig 2A) while interpulse interval was correspondingly nearly doubled by stress (F(1,23) = 1.115, p<0.05; Fig. 2B). Interestingly, although there were fewer pulses, there was an increase in pulse amplitude during restraint stress (F(1,22)=7.341, p<0.05; Fig 2C), though mean pulse peak levels (i.e., the zenith of a pulse) were significantly lower during restraint stress (F(1,22)=4.649, p<0.05; Fig 2D). Acute restraint stress also markedly decreased basal LH levels by ~50% (F(1,21)=28.39, p<0.05; Fig. 2E).
Figure 1.
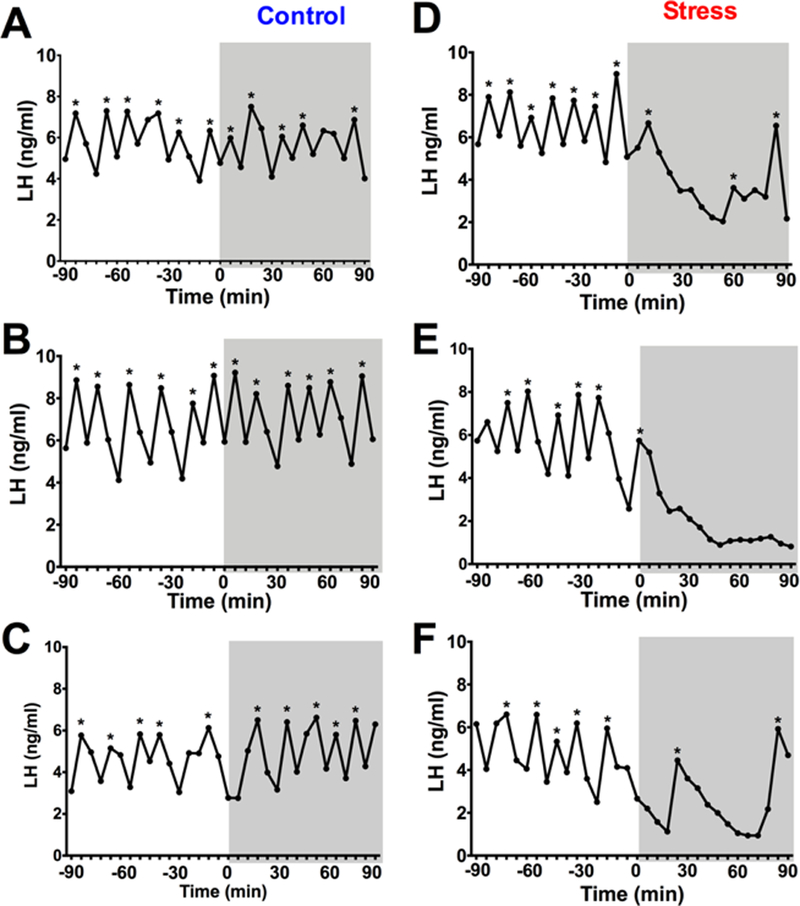
Restraint stress inhibits pulsatile LH in gonadectomized (GDX) male mice. (A-C) represent three individual control mice and (D-F) represent three individual stress mice. Each graph represents an individual male mouse with blood collected every 6 min for 180 min. The initial 90 min period (−90 to 0 min) represent the pre-stress (baseline) period and the gray boxes (time 0 to 90 min) represent the post (stress) period. Pulse peaks are indicated by *.
Figure 2.
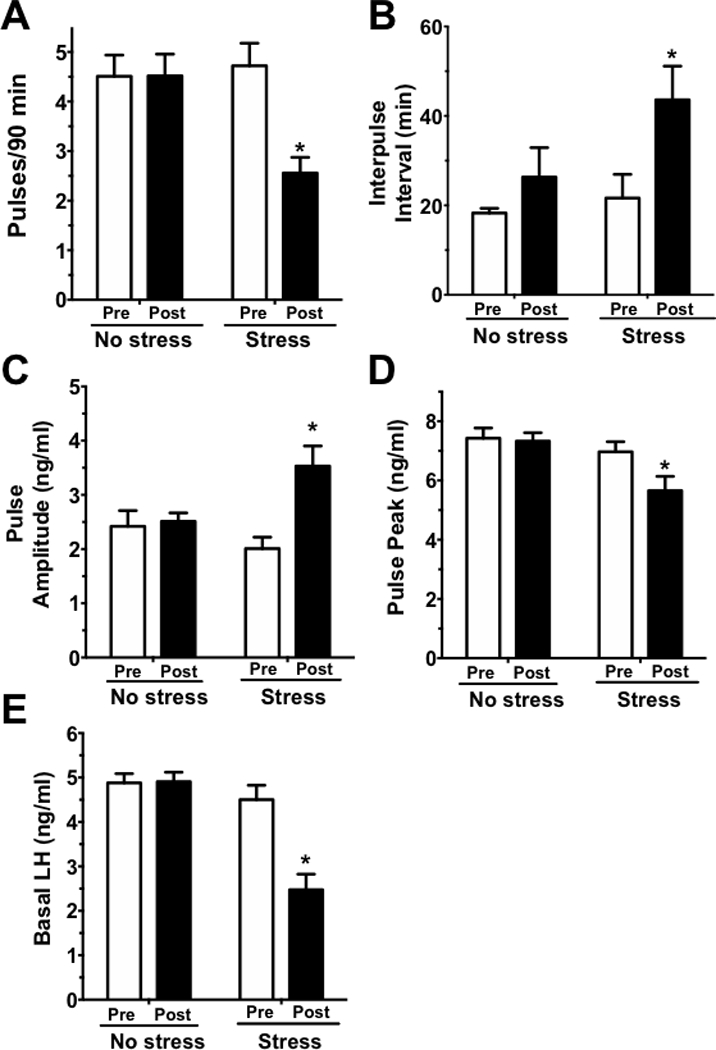
LH pulse parameters in GDX no stress (control) and stress male mice. The following LH parameters were analyzed: (A) pulse frequency, shown as pulses per 90 min, (B) interpulse interval, (C) pulse amplitude, (D) pulse peak levels, and (E) basal LH levels. White bars are the pre-period (baseline) of blood collection (−90 to 0 min) and the black bars are the post-period (stress) of blood collection (0 to 90 min). *, p<0.05 versus baseline (pre-stress) value.
Acute restraint stress rapidly decreases LH within minutes in male mice
Mean LH levels were calculated for the baseline and stress periods for each group. Mean LH levels were similar between the two groups during the baseline period but were significantly reduced in the stress group during the 90 min stress period (F(1,23)=22.81, p<0.05; Fig 3A). To assess the specific time course by which restraint stress lowers LH in male mice, we also analyzed mean LH levels at the 2 baseline time points just before stress exposure and the 4 time points right after initiation of stress. Mean LH was significantly lower in the stress group by 12 minutes following the start of restraint and similarly low at 18 and 24 min after the start of stress (F(6,138)=9.310, p<0.05; Fig 3B), indicating a very rapid inhibitory effect of restraint on the GnRH/LH secretion machinery.
Figure 3.
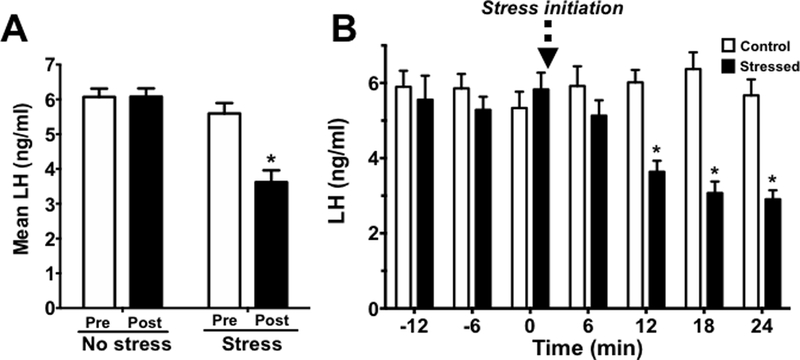
(A) Mean LH measured pre-stress (baseline; white bars) and post-stress initiation (black bars) in no stress (control) and stressed male mice. (B) Detailed time-course of LH changes after stress exposure, measured at 6 min intervals to determine how soon LH decreased following initiation of restraint stress. Mean blood LH levels before (−12 to 0) and after (0 to 24) restraint stress. White bars are control mice and black bars are restraint stressed mice. *p<0.05.
Experiment 2
Corticosterone levels rise within 15 minutes and peak by 30 minutes in acutely stressed male mice
This experiment determined the time-course of CORT secretion in restrained male mice. Before any stress exposure (at time −10 min and 0 min), both control and restraint groups showed low, non-stress levels of CORT that were not different from each other, indicating that tail tip blood collection did not significantly stress the mice (Figure 4). At 5 min after restraint initiation, CORT was not yet significantly elevated. However, by 15 min, CORT was significantly elevated, with restraint mice showing ~3-fold higher levels of CORT versus control mice at this time (F(7,91)=18.82, p<0.05, Figure 4). CORT levels in restrained mice increased further at 30 min and stayed at this elevated level for the remainder of the stress exposure (90 min) (Figure 4).
Figure 4.
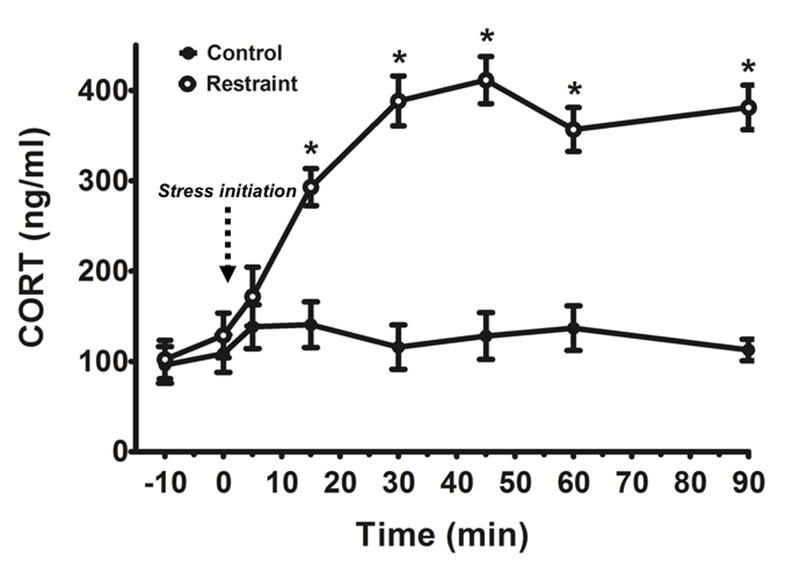
CORT secretion time-course in non-stressed control (closed, black circles) and acutely restrained male mice (open, white circles). Time 0 min represents time of restraint stress initiation in the restraint mice. *p<0.05 control vs. restraint mice, within time point.
Experiment 3
Acute restraint stress in male mice has no effect on Kiss1, Tac2, or Rfrp gene expression
To determine what upstream neuroendocrine mechanisms are altered by acute restraint stress in male mice, we examined gene expression of key stimulatory or inhibitory neuroendocrine regulators of GnRH neurons or GnRH secretion. As expected, serum CORT levels were low in no stress control males, but markedly elevated >5-fold in males that were restraint stressed (Table 1). Brains from these males were first analyzed for Kiss1 and Tac2 mRNA levels in the ARC, as kisspeptin and NKB in this area are both implicated as critical stimulators in the GnRH pulse generation mechanism. There was no significant difference in ARC Kiss1 levels between no stress control male mice and any of the restraint stress groups (Fig. 5A-B). Similarly, there was no difference between control or stressed males in Tac2 levels in the ARC (Fig. 5C-D).
Table 1.
Blood sera CORT levels in GDX male mice measured following restraint stress.
| Group | Mean ± SEM (ng/ml) | Range (ng/ml) |
|---|---|---|
| No stress (control) | 66.0 ± 15.3 | 10.5 – 129.8 |
| 45 min restraint | 614.8 ± 16.7* | 509.5 – 699.8 |
| 90 min restraint | 492.0 ± 20.2* | 383.5 – 565.1 |
| 180 min restraint | 545.0 ± 20.6* | 492.3 – 676.1 |
p<0.05, compared to no stress (control) group.
Figure 5.
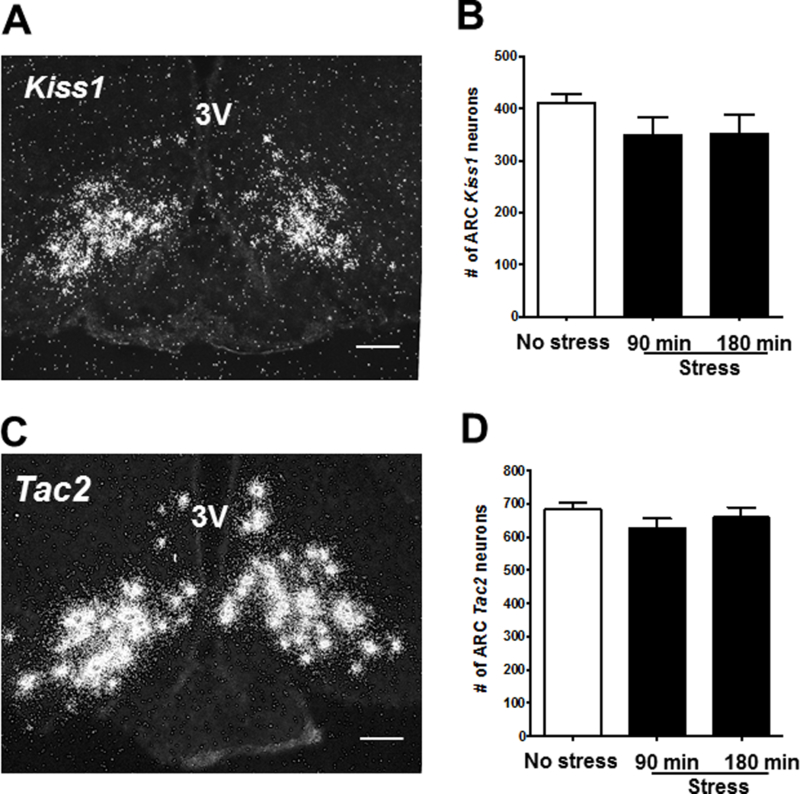
Acute restraint stress has no effect on arcuate Kiss1 or Tac2 cell number in male mice. (A) Representative image of single-label ISH for arcuate Kiss1 in a no stress control mouse. (B) Mean (± SEM) Kiss1 cell number in no-stress control (white bar) and stressed groups (90 and 180 min; black bars). (C) Representative images of single-label ISH for Tac2 in a no stress control mouse. (D) Mean (± SEM) Tac2 cell number in no stress control (white bar) and stressed groups (90 and 180 min; black bars). *p<0.05 versus no stress controls. 3V, third ventricle; ARC, arcuate. Scale bar = 100μm.
We next assessed Rfrp expression levels in the DMN, as RFRP-3 has been implicated as an inhibitor of reproductive hormone secretion and a possible mediator of stress signaling in mammals. Moreover, our previous study in GDX female mice observed a significant increase in Rfrp expression after 180 min of restraint. Here, we did not detect a significant change in Rfrp expression in GDX male mice after 90 min of stress (Fig 6). Although Rfrp levels after 180 min of restraint were ~ 20% higher than no stress controls, this did not reach statistical significance with the Bonferroni’s post-hoc test (Fig 6A-B).
Figure 6.
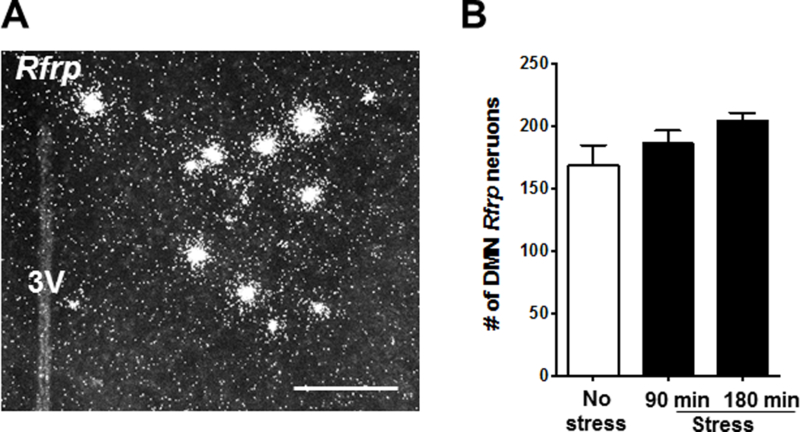
Restraint stress does not change Rfrp coexpression in male mice. (A) Representative image of single-label ISH for Rfrp in the DMN of a no stress control male. (B) Mean (± SEM) Rfrp cell number in no-stress control (white bar) and stressed groups (90 and 180 min; black bars). *p<0.05 versus no stress controls. 3V, third ventricle; DMN, dorsal medial nucleus. Scale bar = 100μm.
Acute restraint stress in males decreases ARC Kiss1 neuron activation
Neuropeptide gene expression may take longer to show changes than neuronal activation and firing. While Kiss1, Tac2, and Rfrp mRNA levels in GDX male mice did not change significantly in the first 3 h with acute restraint stress, it is possible that activation and firing of these reproductive neurons is altered during this time. We hypothesized that stress would inhibit LH levels in part by lowering ARC Kiss1/Tac2 neuron firing, which would be reflected by reduced cfos mRNA induction in these neurons. We therefore used double-label ISH to measure Kiss1+cfos mRNA coexpression (a common measure of neuronal activation) in control and restraint stressed males. Surprisingly, restraint stress had no effect on neuronal activation of ARC Kiss1 neurons of male mice at either 45 or 90 min (Fig 7), even though LH is already reduced at these times (Figures 1, 3). However, by 180 min of restraint stress, there was a significant 33% decrease in the level of Kiss1 neuronal activation (F(3,28)=2.549, p<0.05; Fig 7).
Figure 7.
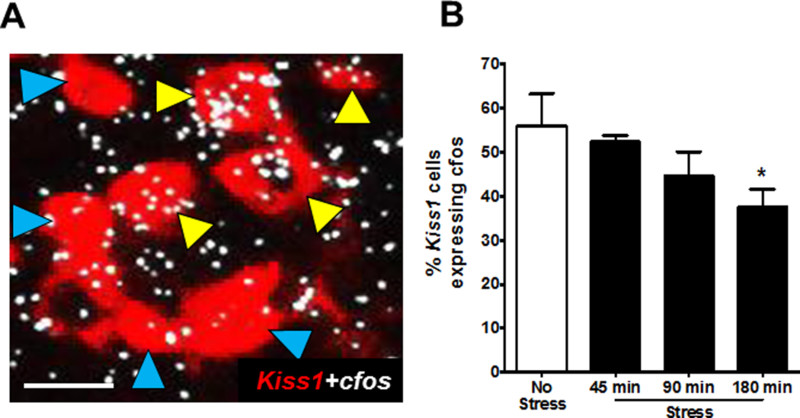
Arcuate Kiss1 neural activation in stress vs no stress control male mice. (A) Representative images of Kiss1 (red fluorescence) and cfos mRNA (silver grains) gene expression in a no stress control male. Yellow arrowheads represent coexpression of Kiss1+cfos; blue arrowheads denote Kiss1 neurons not coexpressing cfos mRNA. (B) Quantification of coexpression of Kiss1 and cfos mRNA in the arcuate of male mice. *p<0.05, compared to no stress controls. Scale bar = 25μm.
Acute restraint stress in males rapidly increases hypothalamic Rfrp neuron activation
Separate from possible effects on kisspeptin neurons, we hypothesized that acute stress may also lower LH pulsatility in part by increasing inhibitory RFRP-3 secretion onto GnRH neurons. We therefore used double-label ISH to measure Rfrp+cfos mRNA coexpression to determine if restraint stress increased the activation of this inhibitory neuroendocrine cell population. Indeed, after 45 min restraint stress, the degree of cfos mRNA induction in Rfrp neurons was largely and significantly increased by ~60% (p<0.05; Fig 8). Interestingly, cfos mRNA levels in Rfrp neurons decreased back down to control levels after 90 min of restraint stress (Fig 8) and then further decreased to levels significantly lower than controls by 180 min of restraint (F(3,28)=35.34, p<0.05; Fig 8).
Figure 8.
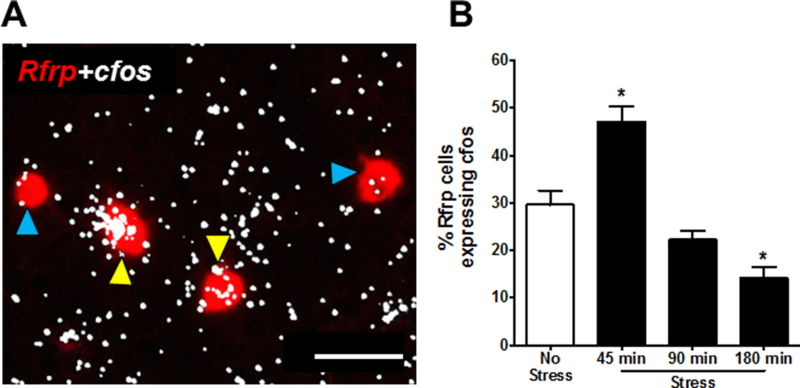
DMN Rfrp neural activation in stress vs no stress control male mice. (A) Representative images of Rfrp (red fluorescence) and cfos mRNA (silver grains) gene expression in a no stress control male. Yellow arrowheads represent coexpression of Rfrp+cfos mRNA; blue arrowheads denote Rfrp neurons not coexpressing cfos mRNA. (B) Quantification of coexpression of Rfrp and cfos mRNA in the DMN of male mice. *p<0.05, compared to no stress controls. Scale bar = 50μm.
Discussion
The current study demonstrates that a one-time, acute psychosocial stress can rapidly and potently inhibit endogenous LH pulsatilile secretion in male mice. We detected clear, rapid, high amplitude LH pulses (approx. every 18–20 min in controls) in our male mice during non-stress conditions, with a high pulse frequency as expected due to their GDX status (i.e., lack of sex steroid negative feedback). Impressively, a one-time, acute restraint stress exposure quickly suppresses LH pulses to ~50% of their normal non-stress frequency. While restraint stress suppressed LH pulses in all mice sampled, there was variability in the patterns of LH suppression during stress. As shown in the representative graphs for the restraint stress group (Fig 1D-F), some males showed a complete repression of LH pulsatile secretion, with lower basal levels and no pulses detected during the stress period. Other stressed males showed a reduced number of pulses with a longer interpulse interval, usually accompanied by lower basal LH levels as well. When this occurred, the peak LH value of the pulse (i.e., the zenith) in stressed mice was lower than non-stressed conditions, indicating that stressed males were not able to secrete as much total LH per individual pulse. While many psychosocial stress studies in rodents have previously been done in females, our present findings indicate that male rodents’ GnRH/LH secretion is also strongly affected, in a very rapid manner, by acute psychosocial stress exposure (Chen, et al. 1992; Li et al. 2006; Li et al. 2004; Viau and Meaney 1991; Yang et al. 2017).
To determine what reproductive neural pathways might be involved in the stress-induced suppression of LH pulsatility in males, we examined hypothalamic Kiss1/Tac2 and Rfrp neurons, which are stimulatory and inhibitory to GnRH secretion, respectively. Acute restraint had no notable effect on either Kiss1, Tac2, or Rfrp gene expression in male mice, up to 180 min of stress exposure. Thus, acute restraint stress does not alter kisspeptin, NKB, or RFRP-3 synthesis in male mice, at least within the first 3 hours of stress. It is likely that longer times > 3 hr are needed to observe decreases in expression for these neuropeptide genes. Indeed, previous studies in female rodents have shown that stress decreases ARC Kiss1 expression when examined 6 hr following stress exposure (Iwasa et al. 2014; Kinsey-Jones et al. 2009), supporting the idea that kisspeptin synthesis is eventually lowered by stress exposure. As far as we are aware, Tac2 expression has not yet been reported in other rodent restraint studies. Another study in male rats found that acute restraint stress for 3 hr increased Rfrp expression, similar to a pattern observed in female mice 3 hr after restraint (Kirby et al. 2009; Yang et al. 2017). In the male mice in our present study, Rfrp expression levels were similarly trending higher at 3 h but this did not reach statistical significance; it is possible Rfrp in males would be statistically elevated if measured a little later (e.g., at 3.5 or 4 h).
Although changes in neuropeptide gene expression may take longer to occur, additional measures of neuron functioning are likely to be altered more quickly. To determine if neural activation of ARC Kiss1 or DMN Rfrp neurons in male mice was regulated by acute restraint stress, we examined coexpression of Kiss1+cfos mRNA and Rfrp+cfos mRNA at several time points. Temporally, the data collected on cfos mRNA expression at 45, 90, and 180 min of restraint stress represent changes in neural activation occurring prior to each of those specific times (based on cfos mRNA induction timing, discussed more below) (Haas, et al. 1991). Surprisingly, despite the ability of restraint stress to rapidly and robustly lower LH pulsatility during the entire 90 min stress period, ARC Kiss1 neuronal activation was not reduced at either 45 min or 90 min of restraint exposure in male mice. However, by 180 min of restraint, there was a significant reduction in Kiss1 neuronal activation, indicating that longer durations of restraint stress eventually suppresses Kiss1 neural activation. In contrast, Rfrp neuron activation in males markedly increased by ~60% when measured at 45 min of restraint stress. This increase was abrogated after 90 min of restraint stress and, interestingly, was further reduced to levels below that of no stress controls after 180 min of restraint stress. The mechanisms and implications for the reduced Rfrp neuron activation at 180 min are not immediately clear. Collectively, these data suggest that, in GDX male mice, Kiss1 neural activation is impacted more slowly by acute restraint stress than Rfrp neural activation.
Experiment #1 determined that mean LH in males was unchanged at 6 min and decreased by 12 and 18 min. Given this rapid effect of restraint stress on LH pulses, any upstream regulators mediating these secretion changes would be expected to be altered by approximately 12–18 min. Rfrp neurons displayed elevated neuronal activation (cfos expression) at 45 min post-stress induction, suggesting they may be involved in the observed decreases in LH. Unlike cFos protein which takes ~60–80 minutes to be synthesized and fully detected (usually by IHC), cfos mRNA is induced quite rapidly, typically being first detected at low-to-medium levels at 15 min after the activation event, peaking at 30 min, remaining elevated at 45 min, and falling again to lower levels by 60 min or later (Gronowski and Rotwein 1995; Haas et al. 1991; Hamamura, et al. 1992; Imaki, et al. 1992; Weaver and Reppert 1995). Thus, elevated cfos mRNA expression observed at a given time point usually indicates an activation of those neurons somewhere betwee 15 to 45 min beforehand (Haas et al. 1991). Therefore, our present finding of elevated Rfrp neuronal activation in stressed males at 45 min after restraint initiation indicates that those Rfrp neurons were activated at some point(s) between 0 and 30 min after the initial stress exposure. Exactly when Rfrp neurons first become activated cannot be determined from our present results and remains unknown; future time-course studies examining several earlier time points after stress (e.g., 10 min, 20 min, 30 min) can address this issue. In contrast, the lack of changes in ARC Kiss1 neural activation at 45 and 90 min suggests that inhibition of KNDy neurons may not be responsible for the rapid decreases in LH during the first 45 min or so of restraint stress exposure, at least in males. However, eventual decreased Kiss1 neuronal activation, as observed later at 180 min, may underlie suppression of LH at later durations of restraint stress. Interestingly, 180 min is the same time-point when Rfrp neurons are no longer showing enhanced activation (but, rather, display reduced activation levels). This may indicate that longer/later stress exposures lower LH pulsatility by dampening Kiss1 neuron activation (i.e., decreasing a stimulatory signal) while very early stress exposures lower LH pulsatility by enhancing Rfrp neuronal activation (i.e., increasing an inhibitory signal). Whether or not a similar pattern of Rfrp or Kiss1 neuron activation also holds true for other types of stressors remains to be determined, but will be important for assessing the generalizability of these findings.
We demonstrate that stress rapidly inhibits the male reproductive neuroendocrine axis, including in vivo LH pulses, but the factors causing this supporession remain unknown. Adrenal CORT may be one of the stress signaling factors that induces the observed reproductive suppression. Our time-course experiment determined that in vivo CORT levels in stressed males were not increased at 5 min but were increased by 15 min of restraint, matching nicely the elevated LH levels at 6 min but significantly suppressed LH by 12–18 min. Although correlative, these data suggest that CORT may be an important candidate to induce this LH suppression by 12–18 min of stress initiation. In mice, the primary CORT receptor is glucocorticoid receptor (GR), which is expressed in multiple brain regions, including in kisspeptin (Takumi, et al. 2012) and RFRP-3 neurons (Kauffman, unpublished). Current studies in our lab will determine if CORT signaling causes the observed restraint stress-induced suppression of LH pulses and/or alterations of Kiss1 and Rfrp neurons, combining the use of transgenic mouse lines and mouse tail tip bleeding. Of note, CORT levels of control, non-stress mice are typically <125 ng/ml and in our serial bleeding experiment remained stable and at this low “baseline” level throughout, suggesting that the serial bleeding procedure is not sufficient on it’s own to induce high stress hormones or to inhibit the reproductive axis.
In summary, we show for the first time that acute restraint stress rapidly and robustly inhibits endogenous LH pulsatility in male mice. Moreover, both Kiss1 and Rfrp neural activation were altered by restraint stress, being decreased and increased, respectively. However, due to the observed rapidity of LH pulse disruption, which happens within minutes of being restrained, diminished ARC Kiss1 neuron activation, which does not occur until later, does not seem to underlie the initial suppression of LH in restraint stressed males. By contrast, Rfrp neurons show a more rapid enhancement in neuron activation that more closely mirriored temporal changes in CORT and LH. Thus, Rfrp neurons may be an important candidate for mediating the observed stress effects during early exposure to an acute stressor. Future studies in our lab are assessing this possibility as well as the specific mechanism(s) by which restraint stress modifies Rfrp and Kiss1 neuron activation and LH pulsatility.
Acknowledgements
The authors thank Dr. Kellie Breen Church for her advice with tail tip pulse bleeding and Michael J Kreismen for his help with analysis of LH pulses.
Acknowledgements/Funding: This work was supported by NSF IOS-1457226, NIH R01 HD090161, and NIH P50 HD012303. J.A.Y. also supported by NIH T32 HD007203.
Footnotes
Disclosure statement: The authors have nothing to disclose.
Declaration of interest: The authors have no conflict of interest to disclose.
References
- Anderson GM, Relf HL, Rizwan MZ & Evans JJ 2009. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology 150 1834–1840. [DOI] [PubMed] [Google Scholar]
- Chen MD, O’Byrne KT, Chiappini SE, Hotchkiss J & Knobil E 1992. Hypoglycemic ‘stress’ and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: role of the ovary. Neuroendocrinology 56 666–673. [DOI] [PubMed] [Google Scholar]
- Chen MD, Ordog T, O’Byrne KT, Goldsmith JR, Connaughton MA & Knobil E 1996. The insulin hypoglycemia-induced inhibition of gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: roles of vasopressin and corticotropin-releasing factor. Endocrinology 137 2012–2021. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, et al. 2008. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology 149 5811–5821. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, et al. 2017. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A 114 E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demura R, Suzuki T, Nakamura S, Komatsu H, Odagiri E & Demura H 1989. Effect of immobilization stress on testosterone and inhibin in male rats. J Androl 10 210–213. [DOI] [PubMed] [Google Scholar]
- Ducret E, Gaidamaka G & Herbison AE 2010. Electrical and morphological characteristics of anteroventral periventricular nucleus kisspeptin and other neurons in the female mouse. Endocrinology 151 2223–2232. [DOI] [PubMed] [Google Scholar]
- Fenster L, Katz DF, Wyrobek AJ, Pieper C, Rempel DM, Oman D & Swan SH 1997. Effects of psychological stress on human semen quality. J Androl 18 194–202. [PubMed] [Google Scholar]
- George JT, Hendrikse M, Veldhuis JD, Clarke IJ, Anderson RA & Millar RP 2017. Effect of gonadotropin-inhibitory hormone on luteinizing hormone secretion in humans. Clin Endocrinol (Oxf) 86 731–738. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM & Lehman MN 2013. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology 154 4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowski AM & Rotwein P 1995. Rapid changes in gene expression after in vivo growth hormone treatment. Endocrinology 136 4741–4748. [DOI] [PubMed] [Google Scholar]
- Haas CA, Reddington M & Kreutzberg GW 1991. Calcitonin Gene-related Peptide Stimulates the Induction of c-fos Gene Expression in Rat Astrocyte Cultures. Eur J Neurosci 3 708–712. [DOI] [PubMed] [Google Scholar]
- Hamamura M, Nunez DJ, Leng G, Emson PC & Kiyama H 1992. c-fos may code for a common transcription factor within the hypothalamic neural circuits involved in osmoregulation. Brain Res 572 42–51. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE & O’Keefe JA 1994. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav 28 464–476. [DOI] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Hotta M & Demura H 1992. Early induction of c-fos precedes increased expression of corticotropin-releasing factor messenger ribonucleic acid in the paraventricular nucleus after immobilization stress. Endocrinology 131 240–246. [DOI] [PubMed] [Google Scholar]
- Iwasa T, Matsuzaki T, Tungalagsuvd A, Munkhzaya M, Kawami T, Niki H, Kato T, Kuwahara A, Uemura H, Yasui T, et al. 2014. Hypothalamic Kiss1 and RFRP gene expressions are changed by a high dose of lipopolysaccharide in female rats. Horm Behav 66 309–316. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Tsutsui K & Fraley GS 2007. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav 51 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, Milligan SR, Lightman SL & O’Byrne KT 2009. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol 21 20–29. [DOI] [PubMed] [Google Scholar]
- Kirby ED, Geraghty AC, Ubuka T, Bentley GE & Kaufer D 2009. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci U S A 106 11324–11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K & Silver R 2006. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A 103 2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Coolen LM & Goodman RL 2010. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151 3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Bowe JE, Kinsey-Jones JS, Brain SD, Lightman SL & O’Byrne KT 2006. Differential role of corticotrophin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. J Neuroendocrinol 18 602–610. [DOI] [PubMed] [Google Scholar]
- Li XF, Edward J, Mitchell JC, Shao B, Bowes JE, Coen CW, Lightman SL & O’Byrne KT 2004. Differential effects of repeated restraint stress on pulsatile lutenizing hormone secretion in female Fischer, Lewis and Wistar rats. J Neuroendocrinol 16 620–627. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon A, Ariznavarreta C, Gonzalez-Quijano MI, Tresguerres JA & Calderon MD 1991. Stress induced changes in testis function. J Steroid Biochem Mol Biol 40 473–479. [DOI] [PubMed] [Google Scholar]
- Mann DR & Orr TE 1990. Effect of restraint stress on gonadal proopiomelanocortin peptides and the pituitary-testicular axis in rats. Life Sci 46 1601–1609. [DOI] [PubMed] [Google Scholar]
- Orr TE & Mann DR 1990. Effects of restraint stress on plasma LH and testosterone concentrations, Leydig cell LH/hCG receptors, and in vitro testicular steroidogenesis in adult rats. Horm Behav 24 324–341. [DOI] [PubMed] [Google Scholar]
- Osugi T, Ukena K, Bentley GE, O’Brien S, Moore IT, Wingfield JC & Tsutsui K 2004. Gonadotropin-inhibitory hormone in Gambel’s white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J Endocrinol 182 33–42. [DOI] [PubMed] [Google Scholar]
- Poling MC, Kim J, Dhamija S & Kauffman AS 2012. Development, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology 153 1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling MC, Luo EY & Kauffman AS 2017. Sex Differences in Steroid Receptor Coexpression and Circadian-Timed Activation of Kisspeptin and RFRP-3 Neurons May Contribute to the Sexually Dimorphic Basis of the LH Surge. Endocrinology 158 3565–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C & Rivest S 1991. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod 45 523–532. [DOI] [PubMed] [Google Scholar]
- Semaan SJ & Kauffman AS 2015. Daily successive changes in reproductive gene expression and neuronal activation in the brains of pubertal female mice. Mol Cell Endocrinol 401 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens SB, Chahal N, Munaganuru N, Parra RA & Kauffman AS 2016. Estrogen Stimulation of Kiss1 Expression in the Medial Amygdala Involves Estrogen Receptor-alpha But Not Estrogen Receptor-beta. Endocrinology 157 4021–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens SBZ, Rouse ML, Tolson KP, Liaw RB, Parra RA, Chahal N & Kauffman AS 2017. Effects of Selective Deletion of Tyrosine Hydroxylase from Kisspeptin Cells on Puberty and Reproduction in Male and Female Mice. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi K, Iijima N, Higo S & Ozawa H 2012. Immunohistochemical analysis of the colocalization of corticotropin-releasing hormone receptor and glucocorticoid receptor in kisspeptin neurons in the hypothalamus of female rats. Neurosci Lett 531 40–45. [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Canny BJ, Serapiglia MD, Ambrose TJ & Clarke IJ 1999. Suppression of the secretion of luteinizing hormone due to isolation/restraint stress in gonadectomised rams and ewes is influenced by sex steroids. J Endocrinol 160 469–481. [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Turner AI & Clarke IJ 2000. Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod 5 105–113. [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Turner AI & Clarke IJ 2002. Stress and reproduction: central mechanisms and sex differences in non-rodent species. Stress 5 83–100. [DOI] [PubMed] [Google Scholar]
- Viau V & Meaney MJ 1991. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology 129 2503–2511. [DOI] [PubMed] [Google Scholar]
- Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ & Karsch FJ 2009. Psychosocial stress inhibits amplitude of gonadotropin-releasing hormone pulses independent of cortisol action on the type II glucocorticoid receptor. Endocrinology 150 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DR & Reppert SM 1995. Definition of the developmental transition from dopaminergic to photic regulation of c-fos gene expression in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res 33 136–148. [DOI] [PubMed] [Google Scholar]
- Yang JA, Song CI, Hughes JK, Kreisman MJ, Parra RA, Haisenleder DJ, Kauffman AS & Breen KM 2017. Acute Psychosocial Stress Inhibits LH Pulsatility and Kiss1 Neuronal Activation in Female Mice. Endocrinology 158 3716–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]


