Abstract
A stereocontrolled synthesis of the C1–C9 segment of the marine natural product peloruside A is described. The key steps involve Sharpless’s catalytic asymmetric dihydroxylation reaction, a chelation-controlled reduction of chiral β-alkoxy ketones to elaborate the syn-1,3-diol functionality and a ring-closing olefin metathesis of a homoallylic alcohol-derived acrylate ester to form an α,β-unsaturated δ-lactone.
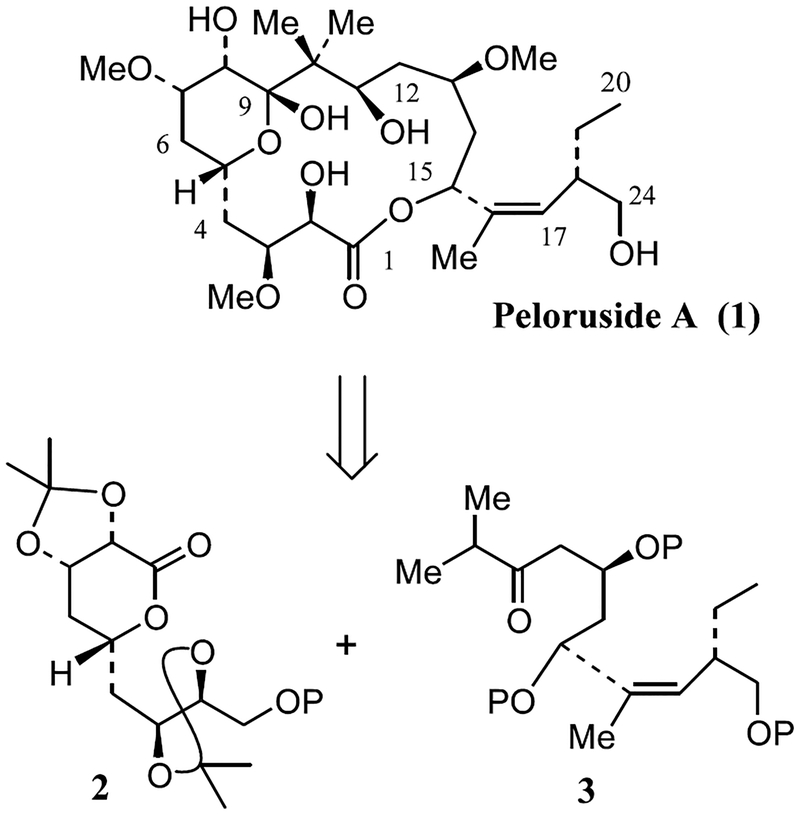
Macrocyclic marine natural products continue to be a rich source for potent antitumor agents with unique structural features.1 However, in many instances scarcity of natural abundance has hindered subsequent in-depth biological studies. Peloruside A, a 16-membered macrolide was recently isolated from the New Zealand marine sponge Mycale hentscheli.2 It displayed potent cytotoxicity against P388 murine leukemia cells at 10 ng/mL. It induces biochemical changes consistent with apoptosis in a number of cultured mammalian cell lines.3a More recently, it has been shown that peloruside A exhibits microtubule-stabilizing activity and arrests cells in the G2-M phase of the cell cycle similar to paclitaxel.3b Peloruside A has structural similarity to epothilones which are undergoing clinical trials.4 Peloruside A contains ten stereogenic centers and its structure and relative stereochemistry have been elucidated by extensive NMR studies.2 As part of our continuing interest in the chemistry and biology of complex natural products with potent antimitotic properties,5 we became intrigued by the unique structural features of peloruside A along with its significant anti-tumor properties. Moreover, scarcity of its supply has precluded its in-depth biological evaluation. Thus far, Paterson and co-workers have only reported the synthesis of various fragments of peloruside A.6 Herein, we describe a convenient enantioselective synthesis of the C1–C9 segment of peloruside A where all five stereo-genic centers have been constructed by asymmetric synthesis.
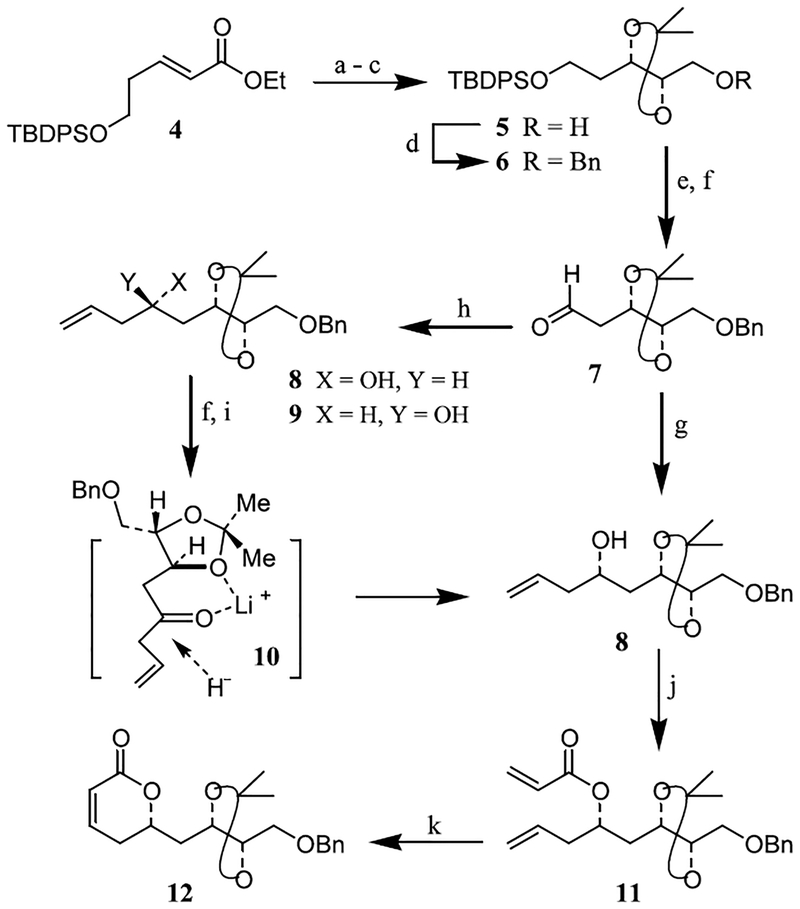
As depicted in Figure 1, our synthetic strategy to peloruside A is convergent and involves the assembly of fragments 2 (C1–C9 segment) and 3 (C10–C24 segment) by an aldol reaction and subsequent macrolactonization between the C1-carboxylic acid and C15-hydroxyl group. The synthesis of the C1–C9 segment commenced with the preparation of α,β-unsaturated ester 4 using known procedures.7 As shown in Scheme 1, ester 4 was transformed into optically active alcohol 5 by a three-step sequence involving: (1) Sharpless asymmetric dihydroxylation8 reaction with AD mix-α in the presence of methanesulfonamide in a mixture (1:1) of t-BuOH and H2O at 0°C provided the corresponding diol in 90% ee;9 (2) exposure of the resulting diol to dimethoxypropane in the presence of a catalytic amount of PPTS to form the isopropylidene derivative; and (3) reduction of the ester with lithium borohydride.
Figure 1.
Scheme 1.
Reagents and conditons: (a) AD mix-α, CH3SO2NH2, tBuOH–H2O (1:1), 0°C, 36 h, (89%); (b) Me2C(OMe)2, PPTS (cat.), Me2CO, 23°C, 5 h (92%); (c) LiBH4, THF, 0°C, 2 h (97%); (d) NaH, BnBr, DMF, 23°C, 4 h (69%); (e) nBu4N+F−, THF, 23°C, 2 h (99%); (f) Dess–Martin, NaHCO3, CH2Cl2, 23°C, 2 h; (g) CH2=CHCH2B[(−)-Ipc]2, THF, −78°C, 3 h (71%); (h) CH2=CHCH2MgBr, Et2O, 0°C, 1 h (74%); (i) LiAlH4, LiI, Et2O, −78°C, 30 min (87%); (j) CH2=CHCOCl, Et3N, CH2Cl2, 0°C, 1 h (62%); (k) Cl2(PCy3)2Ru=CHPh, CH2Cl2, 40°C, 14 h (90%).
The overall procedure is very convenient and provided desired optically active alcohol 5 in 80% yield (three steps) after silica gel chromatography { = −9.62 (c 0.52, CHCl3)}. Protection of alcohol 5 as a benzyl ether provided 6 in multigram quantities. Removal of the TBDPS group followed by Dess–Martin oxidation10 of the resulting alcohol in CH2Cl2 in the presence of NaHCO3 for 2 h provided aldehyde 7 in 87% yield.
To install the 1,3-diol functionality selectively, aldehyde 7 was subjected to Brown’s asymmetric allylboration protocol with allyldiisopinocampheylborane to provide homoallylic alcohol 8 diastereoselectively in 71% yield.11 A diastereomeric ratio of 83:17 was determined by 1H and 13C NMR analysis. In an alternative procedure, aldehyde 7 was also converted to alcohol 8 diastereoselectively using chelation-controlled reduction as the key step. Thus, treatment of 7 with allylmagnesium bromide provided a diastereomeric mixture (syn:anti=42:58 by 1H NMR analysis) of alcohols 8 and 9 in 74% yield. The mixture of alcohols 8 and 9 was oxidized by Dess–Martin periodinane10 to give the corresponding β-ketone in 88% yield. A chelation-controlled reduction by LAH in the presence of LiI at −78°C in ether provided alcohol 8 diastereoselectively (syn:anti=91:9 by 1H NMR analysis) in 87% yield.12 The observed diastereoselectivity can be rationalized by stereochemical model 10 in which, due to the presence of the gem-dimethyl group on the β-face, the carbonyl reduction proceeded from the less hindered α-face providing 8 selectively. This three-step sequence is operationally simple and provided convenient access to desired alcohol 8. Our next plan was to form an α,β-unsaturated δ-lactone and then elaborate the 1,2-diol functionality at C7 and C8 stereoselectively. For the synthesis of the corresponding α,β-unsaturated δ-lac-tone, we utilized the ring-closing olefin metathesis protocol described by us recently.13 As shown, alcohol 8 was reacted with acryloyl chloride and Et3N in CH2Cl2 at 0°C to afford acrylate ester 11. Acrylate ester 11, upon exposure to a catalytic amount of first generation commercial Grubbs’s catalyst14 (10 mol%) in CH2Cl2 at 40°C for 14 h, provided α,β-unsaturated δ-lactone 12 in 90% yield after silica gel chromatography.
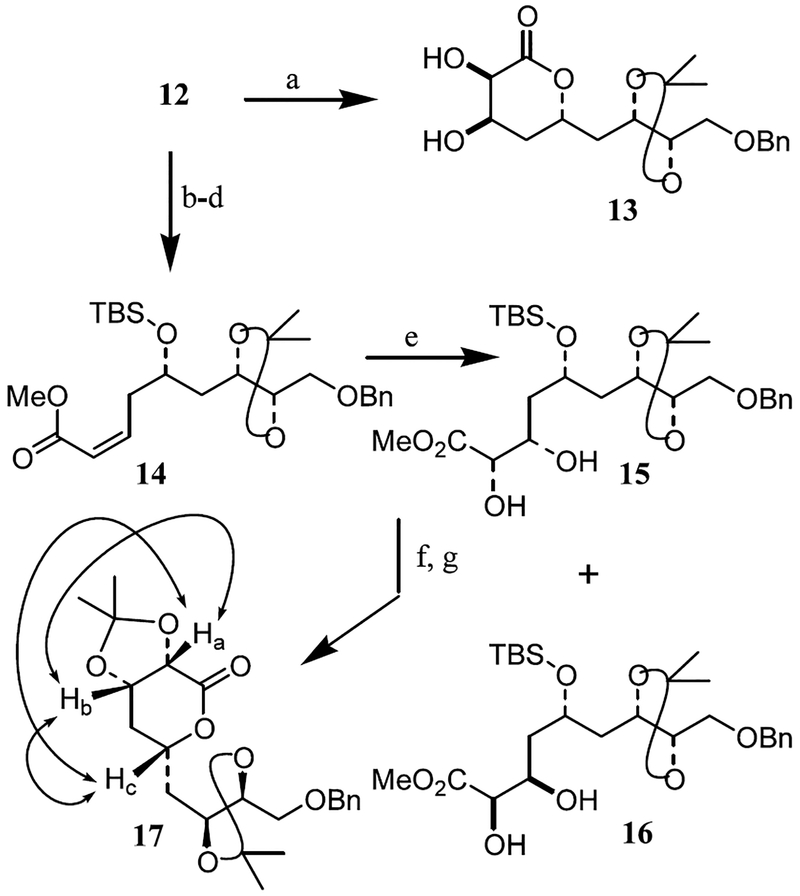
To append the C7 and C8-1,2-diol functionality, we attempted catalytic osmylation of α,β-unsaturated δ-lactone 12 in aqueous acetone (Scheme 2). This has however, provided undesired diol 13 as a single diastereomer in 67% yield. The depicted stereochemistry was assigned based upon NOESY experiments. Sharpless’s asymmetric dihydroxylation8 of 12 with AD mix-β did not proceed even after prolonged (24 h) reaction time at 23°C. This prompted us to investigate asymmetric dihydroxylation of the corresponding open chain α,β-unsaturated ester. Thus, saponification of lactone 12 with aqueous sodium hydroxide was followed by protection of the resulting hydroxy acid as the corresponding TBDMS protected derivative.15 Esterification of the resulting acid with diazomethane afforded methyl ester 14 in 80% yield over three steps. This cis-α,β-unsaturated ester was then subjected to asymmetric dihydroxylation with AD mix-β at 0°C for 72 h. This afforded a mixture of diastereomeric cis-1,2-diols in 72% yield with the major product (15) being the desired diastereomer (diastereomeric ratio 91:9 was determined by 1H and 13C NMR analysis). The diastereomers were separated by silica gel chromatography. The depicted stereochemistry of 15 and 16 are based upon subsequent stereochemical assignment of lactone 17. It should be noted that catalytic osmylation of 14 at 23°C for 6 h afforded the cis-1,2-diols 15 and 16 as a 1:1 mixture of diastereomers in 85% yield. Diol 15 was converted to δ-lactone 17 as follows. Treatment of 15 with nBu4N+F− in THF resulted in removal of TBDMS group and concomitant lactonization. Protection of the diol functionality with dimethoxypropane and a catalytic amount of PPTS furnished isopropylidene derivative 17 in 63% over two steps.16 Stereochemical assignment of 17 was based upon NOESY experiments. The spatial proximity of the protons Ha (δ 4.24 ppm), Hb (δ 4.58 ppm) and Hc (δ 4.44 ppm) is clearly evident in the NOESY spectrum. Either derivative of 15 or 17 is now suitable for the synthesis of peloruside A.
Scheme 2.
Reagents and conditons: (a) OsO4 (cat.), NMO, Me2CO:H2O (7:1), 23°C, 12 h (72%); (b) NaOH, THF–H2O, 0°C, 14 h; (c) TBDMSCl, imidazole, DMF, 23°C, 18 h; (d) CH2N2, Et2O, 0°C, 0.5 h (80% for three steps); (e) AD mix-β, CH3SO2NH2, tBuOH–H2O (1:1), 0°C, 72 h (72%); (f) nBu4N+ F−, THF, 23°C, 3 h; (g) Me2C(OMe)2, PPTS (cat.), Me2CO, 23°C, 24 h (63% for two steps).
In summary, a stereocontrolled synthesis of the C1–C9 fragment of peloruside A has been achieved. The key steps are the Sharpless asymmetric dihydroxylation reaction, Grubbs’ ring-closing olefin metathesis and a chelation-controlled reduction of a chiral β-alkoxy ketone to install the syn-1,3-diol functionality stereoselectively. Further work toward the total synthesis of peloruside A is in progress.
Acknowledgements
This research work was supported in part by Merck Research Laboratories and the National Institutes of Health.
References
- 1.Harris CR; Danishefsky SJ J. Org. Chem 1999, 64, 8434. [Google Scholar]
- 2.West LM; Northcote PT J. Org. Chem 2000, 65, 445. [DOI] [PubMed] [Google Scholar]
- 3.(a) Hood KA; Backstrom BT; West LM; North-cote PT; Berridge MV; Miller JH Anti-Cancer Drug Design 2001, 16, 155; [PubMed] [Google Scholar]; (b) Hood KA; West LM; Rouwe B; Northcote PT; Berridge MV; St. Wakefield J; Miller JH Cancer Res 2002, 62, 3356. [PubMed] [Google Scholar]
- 4.Bollag DM; McQueney PA; Zhu J; Hensens O; Koupal L; Liesch J; Goetz M; Lazarides E; Woods CM Cancer Res 1995, 55, 2325. [PubMed] [Google Scholar]
- 5.(a) Ghosh AK; Wang Y J. Am. Chem. Soc 2002, 122, 11027; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh AK; Wang Y; Kim JT J. Org. Chem 2001, 66, 8973; [DOI] [PubMed] [Google Scholar]; (c) Pryor DE; O’Brate A; Bilcer G; Diaz JF; Wang Y; Kabaki M; Jung MK; Andreu JM; Ghosh AK; Giannakakou P; Hamel E Biochemistry 2002, 41, 9109. [DOI] [PubMed] [Google Scholar]
- 6.Paterson I; Di Francesco ME; Kuhn T Org. Lett 2003, 5, 599. [DOI] [PubMed] [Google Scholar]
- 7.Nacro K; Baltas M; Gorrichon L Tetrahedron 1999, 55, 14013. [Google Scholar]
- 8.For an excellent review, please see: Kolb HC; Van-Nieuwenhze MS; Sharpless KB Chem. Rev 1994, 94, 2483. [Google Scholar]
- 9.Optical purity of the corresponding diol (90% ee) was determined by chiral HPLC analysis (5% 2-propanol/hexane, flow rate 1 mL/min, Rt=5.3 min for the minor isomer and Rt=6.8 min for the major isomer) using Daicel Chiracel OD column.
- 10.Merger SD; Schreiber SL J. Org. Chem 1994, 59, 7549. [Google Scholar]
- 11.(a) Jadhav PK; Bhat KS; Perumaal T; Brown HC J. Org. Chem 1986, 51, 432; [Google Scholar]; (b) Racgerlaa US; Brown HC J. Org. Chem 1991, 56, 401. [Google Scholar]
- 12.(a) Ghosh AK; Lei H J. Org. Chem 2002, 67, 8783; [DOI] [PubMed] [Google Scholar]; (b) Mori Y; Kuhara M; Takeuchi A; Suzuki M Tetrahedron Lett 1988, 29, 5419. [Google Scholar]
- 13.(a) Ghosh AK; Cappiello J; Shin D Tetrahedron Lett 1998, 39, 4651; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh AK; Liu C Chem. Commun 1999, 1743; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nicolaou KC; Rodriguez RM; Mitchell HJ; van Delft FL Angew. Chem., Int. Ed. Engl 1998, 37, 1874. [Google Scholar]
- 14.For an excellent review, see: Grubbs RH; Chang S Tetrahedron 1998, 54, 4413. [Google Scholar]
- 15.(a) Ghosh AK; Shin D; Mathivanan P Chem. Com-mun 1999, 1025; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh AK; McKee SP; Thompson WJ; Darke PL; Zugay JC J. Org. Chem 1993, 58, 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.All new compounds gave satisfactory spectroscopic and analytical results. Lactone 17: =+ 43.28 (c 0.67, CHCl3); IR (thin film) 1758 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.38–7.30 (m, 5H), 4.60–4.51 (m, 3H), 4.44 (m, 1H), 4.24 (d, J=7.7 Hz, 1H), 4.0 (m, 1H), 3.88 (m, 1H),3.67 (dd, J=9.9, 5 Hz, 1H), 3.54 (dd, J=9.9, 5.7 Hz, 1H), 2.47 (ddd, J=14.2, 8.1, 1.4 Hz, 1H), 2.08 (ddd, J=14.3, 7.6, 6.2 Hz, 1H), 1.89 (ddd, J=14.3, 6.1, 4.8 Hz, 1H), 1.72 (ddd, J=14.2, 12.1, 8.0), 1.50 (s, 3H), 1.40 (s, 3H), 1.38 (s, 6H); 13C NMR (125 MHz): δ 170.6, 138.1, 128.9, 128.3 (2C), 112.1, 109.5, 80.1, 75.3, 74.1, 73.1, 72.9,72.2, 70.8, 38.6, 34.8, 27.6, 27.3 (2C), 25.7; HRMS (FAB) m/z calcd for C22H51O7 (M++H): 407.2070; found: 407.2071.