INTRODUCTION
Chronic pancreatitis (CP) is a low prevalence disease.1–3 In 2006, there were approximately 50 cases of definite CP per 100,000 population in Olmsted County, MN 3, translating to a total of 150,000–200,000 cases in the US population. Clinical features of CP are highly variable and include minimal, or no symptoms of debilitating pain repeated episodes of acute pancreatitis, pancreatic exocrine and endocrine insufficiency and pancreatic cancer. CP profoundly affects the quality of life, which can be worse than other chronic conditions and cancers.4
Natural history studies for CP originated mainly from centers outside the U.S.5−9,10,11 conducted during the 1960–1990’s and consisted primarily of males with alcoholic CP. Only one large retrospective longitudinal cohort study has been conducted in the US for patients seen at the Mayo Clinic from 1976–1982.12 While these data provide general insights into disease evolution, it is difficult to predict the probability of outcomes or disease progression in individual patients. Few data exist on the risk of progression in patients with recurrent acute pancreatitis, or in the early-stage disease when definitive morphological features of CP are not evident. There are no longitudinal prospective cohort studies of CP in the US.
In the past two decades, new knowledge has broadened the etiologic profile of CP to highlight contributions from genetic13, autoimmune14 and environmental (smoking)15 factors. Improvement in imaging techniques has enabled better recognition of morphological and functional changes in the pancreas16. The clinical significance of Type 3c diabetes (Type 3c DM) in patients with diagnosed or undiagnosed pancreatic disease is increasingly recognized.17,18 The impact of these developments on the natural history of CP are unknown.
The evaluation of chronic abdominal pain costs an estimated $30 billion in healthcare and lost wages annually19. Patients with suspected or definite CP comprise a significant fraction of these patients. While diagnosing moderate-severe CP is often straightforward, detection of early-stage CP remains difficult due to the absence of reliable morphologic and functional diagnostic methods. Biopsy of the pancreas is not usually performed as it may not provide a definite diagnosis and entails a risk of biopsy-related pancreatitis. Patients often undergo an exhaustive array of costly studies (endoscopic, radiologic) with their attendant risks. Pancreatic function testing (PFT) is usually performed as a clinical test in patients with chronic abdominal pain or suspected CP to assess for the presence of early-stage disease, but this practice varies between centers20–22, and limited data suggests a high negative predictive value of PFT, however, it is cumbersome to perform, has low positive predictive value (~50%)23, and has not gained widespread use (<20 centers in the US).
Since treatment options for definite CP are limited, patients with early-stage CP or at high-risk of developing CP are ideally suited for interventions (e.g., anti-inflammatory or anti-fibrotic medications) to prevent the development of definite CP and its associated morbidity. It will be desirable to have a pract, fast and cost-efficient test(s) to exclude CP with high certainty, to reliably rule-in early-stage CP or help predict disease progression in these patients, to identify patients suitable for intervention (medication, surgery, etc.) and to monitor their effects to slow or reverse disease progression.
ETIOLOGY
The etiology of CP is determined after a thorough patient investigation considering all known risk factors, including alcohol consumption and smoking, as well as laboratory values (triglyceride levels; Ca2+ levels for ruling out elevated primary hyperparathyroidism (PHPT); carbohydrate-deficient transferrin (CDT)/phosphatidylethanol levels, and family medical history.
The most common risk factor for CP is alcohol abuse, with a logarithmic risk increase, although the type of alcohol consumed is irrelevant.24 The amount and duration of alcohol consumption required to develop CP have not been unequivocally defined. Some authors suggest at least 80 g/day for at least six years would be a threshold for developing chronic pancreatitis. Smoking is probably an independent risk factor, and smoking cessation is advisable for CP patients 25
Autoimmune pancreatitis (AIP) should be ruled out following current consensus guidelines and when no other etiology can be found in patients. Please see Nima Hafezi-Nejad, Vikesh K. Singh, Christopher Fung, et al. article “Magnetic Resonance Imaging of Autoimmune Pancreatitis,” in this issue for information on typical imaging and clinical findings of AIP.
Cholecystolithiasis and choledocholithiasis are not considered independent risk factors for the development of CP. Whether anatomic anomalies such as pancreas divisum increase the CP risk is still a matter of debate; however, with additional risk factors, pancreas divisum might lead to CP development. If no etiological factor can be identified, genetic screening for predisposing variants can be offered.
Genetic factors also contribute to CP development. The most important genetic risk factors are variants in cationic trypsinogen (PRSS1), serine protease inhibitor Kazal-type 1 (SPINK1) and carboxypeptidase A1 (CPA1). Further genetic susceptibility genes are cystic fibrosis transmembrane conductance regulator (CFTR), chymotrypsinogen C (CTRC) and carboxyesterlipase (CEL).13
CLINICAL FEATURES
Abdominal pain is a dominant feature of chronic pancreatitis. The pain is typically epigastric, often radiates to the back, is occasionally associated with nausea and vomiting, and may be partially relieved by sitting upright or leaning forward. The pain is usually worse 15 to 30 minutes after eating. Early in the course of chronic pancreatitis, the pain may occur in discrete attacks; as the condition progresses, the pain tends to become more continuous.
The pain in chronic pancreatitis varies among patients. This pattern was illustrated in a prospective cohort of 207 patients with alcoholic CP in which two typical pain patterns were observed.26 The first was characterized by episodes of pain (usually lasting less than ten days) with pain-free intervals lasting from months to more than a year. The second pattern was characterized by prolonged periods of daily pain or clusters of severe pain exacerbations often requiring repeated hospitalizations. Also, although abdominal pain is the most consistent finding in patients with chronic pancreatitis, it may be absent in some cases. In one series, for example, 20 percent of patients with chronic pancreatitis presented with evidence of pancreatic exocrine or endocrine dysfunction in the absence of pain.12
Patients with severe pancreatic exocrine dysfunction cannot correctly digest complex foods or absorb partially digested breakdown products. Nevertheless, clinically significant protein and fat deficiencies do not occur until over 90 percent of pancreatic function is lost.27
Steatorrhea usually occurs before protein deficiencies since lipolytic activity decreases faster than proteolysis.28 The clinical manifestations of fat malabsorption include loose, greasy, foul-smelling stools that are difficult to flush. Malabsorption of the fat-soluble vitamins (A, D, E, and K) and vitamin B12 may also occur, although clinically symptomatic vitamin deficiency is rare.29
Glucose intolerance occurs with some frequency in chronic pancreatitis, but overt diabetes mellitus usually occurs late in the course of the disease. Patients with the calcifying CP, particularly those who develop them early, may develop diabetes more frequently than those with the non-calcifying CP.30 Diabetes is also more likely to occur in patients with a family history of type 1 or type 2 diabetes; this observation suggests a role for an underlying decrease in pancreatic reserve or insulin responsiveness. Pancreatic surgery (including drainage or pancreaticoduodenectomy) does not appear to increase the risk of diabetes. Exceptions include distal pancreatectomy and significant pancreatic resection in the setting of extensive pancreatic fibrosis.30 Diabetes which develops in patients with CP is usually insulin-requiring. However, it is different from typical type 1 diabetes in that the pancreatic alpha cells, which produce glucagon, are also affected; as a result, there is an increased risk of hypoglycemia, both treatment-related and spontaneous. Diabetic ketoacidosis and nephropathy are rare; neuropathy and retinopathy occur more frequently.28
HISTOPATHOLOGY
Histologically, the two most common features of CP are the loss of acinar tissue (atrophy) and fibrosis. The fibrosis may surround the lobules (perilobular or interlobular fibrosis) or extend into the lobules of acinar tissue (intralobular fibrosis).31 Chronic inflammatory infiltrate may be present, but this feature is highly variable and disappears late in the course of CP. A diagnosis of CP may be made by atrophy and fibrosis in the absence of other changes. Chronic pancreatitis can be a patchy or localized process with regional involvement. This feature is best understood by considering the mechanisms of pathogenesis, in particular, the necrosis-fibrosis hypothesis, which posits that CP develops as a result of multiple episodes of AP with necrosis and scarring. This process may be patchy at first, progressing to a diffuse pattern after multiple episodes. This is commonly considered to be the mechanism in alcoholic CP, paraduodenal CP, and likely hereditary pancreatitis. On the other hand, duct obstruction can lead to progressive fibrosis and loss of acinar tissue that may be localized or segmental, as in the presence of an obstructing neoplasm, or may be diffuse as is characteristic of cystic fibrosis.
IMAGING STUDIES
Imaging studies that may be useful in chronic pancreatitis include plain abdominal films, transabdominal ultrasound (US), CT scan, magnetic resonance imaging (MRI) combined with MR Cholangiopancreatography (MRCP), Endoscopic Retrograde Cholangiopancreatography (ERCP), and endoscopic ultrasound (EUS).
Calcifications within the pancreatic duct are present on plain film in approximately 30 percent of patients with chronic pancreatitis. Calcium deposition is most common with alcoholic pancreatitis, but can also be seen in the hereditary and tropical forms of the disorder; it is rare in idiopathic pancreatitis.
Transabdominal ultrasonography, CT scan, and MRI/MRCP may show ductal dilatation, enlargement of the pancreas, calcifications, and post-inflammatory fluid collections adjacent to the gland. The sensitivity and specificity of US for the diagnosis of chronic pancreatitis are 60 to 70 percent and 80 to 90 percent, respectively.32 The corresponding values for CT scanning are 75 to 90 and 85 percent, respectively.33 Most common CT imaging features of CP are listed in Table 1. 33
Table 1.
Imaging features of CP observed by CT.
| CT Features of CP | Incidence |
|---|---|
| Ectatic pancreatic duct | 68% |
| Atrophy | 54% |
| Calcifications | 50% |
| Fluid collections | 30% |
| Focal pancreatic enlargement | 30% |
| Biliary ductal dilatation | 29% |
| Alterations in peri-pancreatic fat | 16% |
| Others | Contiguous organ invasion, large cavities, focal acute pancreatitis, intraductal filling defects, disconnected/disrupted pancreatic duct. |
MRCP is becoming the diagnostic test of choice since MRI/MRCP is a more sensitive imaging tool for the diagnosis of CP by evaluating both parenchymal and ductal changes. Most common findings of CP seen by MRI and MRCP are listed in Table 2. Ductal abnormalities are very specific and reliable MRI signs of CP, however, signal intensity changes either by T1-weighted gradient echo or T1 mapping may precede ductal abnormalities and detect early CP.34–38} One study investigated the association between the bicarbonate level of the pancreatic juice and the T1-weighted gradient echo signal and reported a significant direct correlation. The signal intensity ratio of 1.2 yielded sensitivity of 77% and specificity of 83% for detection of pancreatic exocrine dysfunction (AUC 0.89).34 This imaging finding can be very helpful information to the clinician who is evaluating a patient whose symptoms are suspected of CP but has normal ductal findings.
Table 2.
Features of CP seen by MRI/MRCP with or without secretin.
| MRI and MRCP Features of CP | |
|---|---|
| Main pancreatic duct and side branches | Strictures Ductal filling defects Ectatic side branches Ductal contour irregularity Disconnected/disrupted main pancreatic duct Congenital anomalies (e.g., pancreas divisum) |
| Parenchyma | Atrophy Steatosis |
| Fluid collections | Walled off necrosis vs pseudocyst |
| Secretin MRCP specific findings | Increase in diameter of main pancreatic duct Decreased duodenal filling by the pancreatic juice |
| T1 signal change | Decrease T1 signal in pre-contrast phase |
| Biliary system | Dilatation/strictures |
| Duodenum | Obstruction/stricture |
MRCP can also be performed by utilizing the hormone secretin, which stimulates a normal pancreas to secrete a significant amount of fluid while transiently increasing the tone of the sphincter of Oddi. Transient increase in the diameter of the duct improves the depiction of the anatomy, which can be useful in cases where detailed evaluation of the pancreatic duct is most desired in patients with the suspected pancreatic disease. 39,40 Improved visualization of the ductal anatomy can be important in differentiating side-branch IPMNs from other cystic neoplasms, diagnosis and classification of chronic pancreatitis, disconnected pancreatic duct syndrome and ductal anomalies such as anomalous pancreaticobiliary junction and pancreas divisum. In the post-pancreatectomy patients, stimulation by secretin can give information about the patency of the pancreatico-enteric anastomosis. Duodenal filling during the post-secretin phase of the MRCP can estimate the excretory reserve of the pancreas.41 It is expected that with increasing severity of CP there will be a decrease in the number of acinar cells and the fluid output, which can be detected with S-MRCP. Current consensus is that duodenal filling during secretin MRCP does not help to evaluate the grade of severity of CP, because a substantial number of patients with severe CP may still have a normal duodenal filling.
Diffusion-weighted MRI measures the restriction of free water molecules in the gland. The more fibrosis there is, the more likely there will be less diffusion of water molecules (which is measured as apparent diffusion coefficient). The apparent diffusion coefficient value is expected to be lower in patients with pancreatic fibrosis than in normal patients. Exploiting this idea, one can evaluate the gland using diffusion MRI after IV secretin stimulation and enhance the sensitivity to depict subtle abnormalities in diffusion restriction and separate normal patients from those with early CP.42
MR Elastography (MRE) has been shown to be a reliable marker of hepatic fibrosis in patients with the chronic liver disease. While there are no controlled data evaluating this technique in patients with CP, there is room for optimism as recent data demonstrated the feasibility of using MRE to determine pancreatic stiffness in healthy volunteers. Reproducible stiffness measurements were noted throughout the pancreas, with imaging parameters and equipment different than that used for liver imaging. Preliminary data suggest that pancreatic MRE can provide promising and reproducible stiffness measurements throughout the pancreas, potentially allowing for assessment of pancreatic fibrosis. 43
ERCP had been utilized to identify ductal abnormalities or obstructions, to clarify ductal anatomy before surgical intervention, and to confirm the patency of postsurgical anastomoses, including pancreatico-jejunostomies.44 Guidelines published by the American Society for Gastrointestinal Endoscopy (ASGE) in 2006, recommend that ERCP should be reserved for patients in whom the diagnosis remains unclear after pancreatic function testing (PFT) or other non-invasive (CT or MRI) or less invasive imaging studies (EUS) have been performed.45 Characteristic beading of the main pancreatic duct and ectatic side branches is diagnostic of chronic pancreatitis. The Cambridge classification has divided patients into normal, equivocal, mild, moderate and severe CP categories based upon ductal changes on ERCP.46,47 Today, ERCP is rarely used for diagnostic purposes.
Endoscopic ultrasonography may be as sensitive as ERCP or pancreatic function testing, but requires a highly skilled gastroenterologist to perform48 and multicenter studies showed the inter-observer agreement to be less than optimal for the diagnosis of CP.49 The most predictive endosonographic feature is the presence of stones. Other suggestive features include visible side branches, cysts, lobularity, an irregular main pancreatic duct, hyperechoic foci and strands, dilation of the main pancreatic duct, and hyperechoic margins of the main pancreatic duct. Many endosonographers consider the presence of four or more of these features to be highly suggestive of chronic pancreatitis.48
Several invasive and noninvasive pancreatic function tests are available for the diagnosis of pancreatic insufficiency, which can be classified as direct or indirect. Direct tests involve the stimulation of the pancreas through the administration of a meal or hormonal secretagogues, after which duodenal fluid is collected and analyzed to quantify normal pancreatic secretory content (i.e., enzymes, and bicarbonate). Only a few specialized centers perform these tests. Their main role is in the diagnosis of early chronic pancreatitis in patients with compatible clinical features but without characteristic radiographic findings. The estimated sensitivity and specificity of secretin pancreatic function testing in diagnosing chronic pancreatitis are 82% and 86%, respectively.23 Indirect tests measure the consequences of pancreatic insufficiency and are more widely available. However, they depend upon the consequences of pancreatic maldigestion, which are not apparent until normal enzyme secretory output has declined by more than 90 percent. Thus, they are insensitive to early pancreatic insufficiency.
LABORATORY
Serum concentrations of amylase and lipase may be slightly elevated in patients with chronic pancreatitis. However, but these enzymes are more commonly normal for the following reasons: CP is a patchy, focal disease, leading to a minimal increase in pancreatic enzymes within the blood and there is frequently significant fibrosis, resulting in a decreased abundance of these enzymes within the pancreas. Thus, serum measurements of amylase or lipase should be reserved only for the diagnosis of acute pancreatitis and not chronic pancreatitis where they are neither diagnostic nor prognostic. It is not unusual that a patient with elevated amylase or lipase values <3 times the upper limit of normal is labeled as having chronic pancreatitis when, in fact, these are non-diagnostic.
The complete blood count, electrolytes, and liver function tests are typically normal. Elevations of serum bilirubin and alkaline phosphatase suggest compression of the intrapancreatic portion of the bile duct by edema, fibrosis, or pancreatic cancer. Markers of chronic autoimmune pancreatitis include an elevated ESR, IgG4, rheumatoid factor, ANA, and anti-smooth muscle antibody titer.
Steatorrhea should no longer be diagnosed qualitatively by Sudan staining of feces since it is nonspecific. A 72-hour quantitative fecal fat determination is the gold standard. The quantitative test is usually performed over 72 hours; excretion of more than 7 g of fat per day is diagnostic of malabsorption, although patients with steatorrhea often have values greater than 10 g/day. In the proper clinical setting (e.g., in a patient with typical symptoms of abdominal pain), confirmation of increased fecal fat excretion may be sufficient to diagnose chronic pancreatitis.
Given the cumbersome nature of the 72-hour fecal fat test, measurement of fecal elastase can be helpful for evaluating pancreatic exocrine dysfunction, and it is considered the test of choice. Among pancreatic function tests, fecal elastase measurement is the most sensitive and specific, especially in the early phases of pancreatic insufficiency. Also, its values are independent of pancreatic enzyme replacement therapy and require only a single random stool sample. According to unpublished data from the manufacturer, values less than 200 mcg/g are suggestive of pancreatic insufficiency (sensitivity and specificity of 93 percent).50
GENETIC TESTING
In the past few years, genetic mutations have been associated with chronic pancreatitis. These genes include the CFTR gene responsible for cystic fibrosis, SPINK-1, which encodes for trypsin inhibitor, and the PRSS-1 gene linked to hereditary pancreatitis. In a study where extensive sequencing of the CFTR gene was performed in conjunction with functional analyses, 44 percent of patients with idiopathic chronic pancreatitis were found to have at least one variant in the CFTR gene which was associated with CFTR dysfunction.51 The fact that 22 percent of healthy controls had at least one variant in that study combined with the data that over 2000 variants (i.e., not disease proven mutations and thus of unknown significance) have been detected in the CFTR gene by extensive sequencing, indicates that CF genotyping should not be performed routinely to diagnose a patient with chronic pancreatitis. Alternatively, sweat chloride testing may be of benefit since it assesses CFTR function and does not rely on full gene sequencing.52 Up to 10 percent of patients will have abnormal results, which should prompt further investigation of occult male infertility or lung disease and may warrant professional genetic counseling. SPINK-1 mutations are present in 23 percent of patients with chronic pancreatitis but are seen in 2 percent of healthy individuals.53 In conjunction with the finding that homozygous mutations can be found in healthy individuals, testing for this gene is presently not of diagnostic or therapeutic benefit and hence not recommended. PRSS-1 mutations can be diagnostic of hereditary pancreatitis which can present with recurrent acute episodes of pancreatitis and progress to the chronic form.
CLASSIFICATION
CP has been classified into different forms (calcifying, obstructive, autoimmune and groove). These classifications are based on clinical features, morphological characteristics and response to treatment. In calcifying CP, for example, perilobular fibrosis and acinar destruction with infiltration of acute and chronic inflammatory cells are present. Obstructive CP develops as a secondary complication due to an area of obstruction with dilatation of the pancreatic duct proximal to the stenosis, atrophy of acinar cells and fibrosis. Finally, groove pancreatitis affects the groove between the pancreatic head, duodenum and the bile duct.
Classification systems are of great importance for guiding management strategies, since treatment strategies cannot rely solely on the type and degree of morphological changes in the pancreas, but need to include clinical, functional and imaging findings. So far no globally accepted classification system has been established. Classification systems for CP are:
Cambridge Classification
Manchester classification
ABC classification
M-ANNHEIM
TIGAR-O
Rosemont classification
There is no MRI/MRCP, CT or US-specific classification criteria for CP. Radiologists are interpreting MRCP often time use Cambridge classification which was designed for ERCP more than three decades ago. 46 There is a need for a new staging system for CP, specifically designed for CT, MRI, and MRCP combining the ductal and the parenchymal changes secondary to pancreatic fibrosis. American Pancreatic Association released a morphology characterization imaging guide for the current imaging modalities (Table 3). 47
Table 3.
Cambridge classification adapted for findings seen on MRCP, CT, and US. American Pancreatic Association Practice Guidelines, 2014. 47
| Cambridge Classification | MRCP/ERCP findings | US/CT/MR findings |
|---|---|---|
| 0 Normal |
no abnormal signs | no abnormal signs |
| I Equivocal |
<3 abnormal branches | one of the following: - dilated main pancreatic duct (2 – 4mm) - slight gland enlargement - heterogeneous parenchyma - small cavities (<10 mm) - irregular ducts - focal pancreatitis - increased echogenicity of main duct wall - irregular head/body contour |
| II Mild |
3 or more abnormal branches | ≥ 2 of the following: - dilated main duct (2–4 mm) - gland enlargement - heterogeneous parenchyma - small cavities (<10 mm) - irregular ducts - focal AP - increased echogenicity of main duct wall - irregular head/body contour |
| III Moderate |
>3 abnormal side branches and abnormal main duct | Same as above |
| IV Severe |
all above and 1 or more of - large cavity >10mm - intraductal filling defects - duct obstruction (stricture) - duct dilatation or irregularity |
Above changes and 1 or more of: - large cavity >10mm - gland enlargement - intraductal filling defects/calculi - duct obstruction/stricture/ or gross irregularity |
The Manchester classification system uses imaging modalities and clinical signs of CP.54 The degree of severity is mostly influenced by the presence of exocrine and endocrine insufficiency or the presence of complications, while imaging findings are of minor importance. The ABC classification recommends similar findings to the Manchester classification system. 55,56 The Rosemont classification was developed to diagnose CP using EUS.57
Two major classification systems have been established to help assess risk factors in the development of CP: TIGAR-O and M-ANNHEIM and are helpful in guiding providers as to when to initiate testing for CP. Etiological factors in the M-ANNHEIM system are; alcohol consumption; nutrition; hereditary factors; ductal factors; immunology; miscellaneous and rare metabolic disorders (e.g., hypercalcemia, hyperparathyroidism, chronic renal failure, drugs, toxins.58 The M-ANNHEIM system includes the stage, severity and clinical findings of CP and offers a severity index. Different guidelines recommend using the TIGAR-O classification. This system comprises six etiologic groups: toxic/metabolic, idiopathic, genetic, autoimmune, recurrent acute pancreatitis, and obstructive groups. 59
DIFFERENTIAL DIAGNOSIS
Pancreatic cancer is the primary diagnosis that must be considered in patients suspected of having chronic pancreatitis. An endoscopic sampling of the pancreatic juice might be necessary to differentiate CP from the main or mixed-type intra-ductal papillary mucinous neoplasm. Acute pancreatitis may also be difficult to distinguish from chronic pancreatitis in some patients.
There are data to suggest that chronic pancreatitis is associated with an increased risk of developing pancreatic carcinoma.60,61 In a report from the International Pancreatitis Study Group, 2015 patients with chronic pancreatitis were followed for a mean of 7.4 years.60 A total of 56 pancreatic cancers were identified. The expected number of cases of cancer calculated from country-specific incidence data and adjusted for age and sex was 2.13, yielding a standardized incidence ratio (the ratio of observed to expected cases) of 26.3.
Findings suggestive of possible pancreatic cancer in a patient thought or known to have chronic pancreatitis include older age, the absence of a history of alcohol use, weight loss, a protracted flare of symptoms, and the onset of significant constitutional symptoms. Supporting data for malignancy include a pancreatic duct stricture greater than 10 mm in length on ERCP.62 Markers such as CA 19–9, and carcinoembryonic antigen (CEA) is helpful if abnormal, but normal values do not rule out pancreatic cancer.
Radiologists sometimes encounter lesions that show focal enlargement or distortion of the normal contour of the pancreas while still lacking pathognomonic features of pancreatic carcinomas. In such cases, a small percentage of patients with such focal enlargements of the pancreas will have a conventional pancreatic carcinoma, while a small percentage of the patients may have an inflammatory pancreatic mass (IPM). Despite these histories that suggest the presence of chronic pancreatitis, one may not usually be certain whether a mass appearing at the pancreas is related to IPM or cancer. The duct-penetrating sign on MRCP images (a smoothly stenotic or normal main pancreatic duct penetrating a mass) was seen more frequently in IPM than in pancreatic cancer (Figure 1).63
Figure 1.
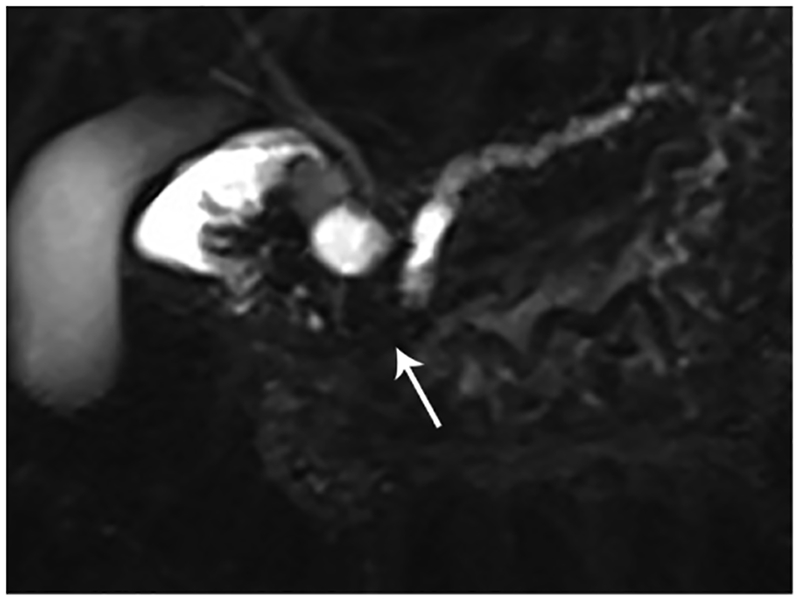
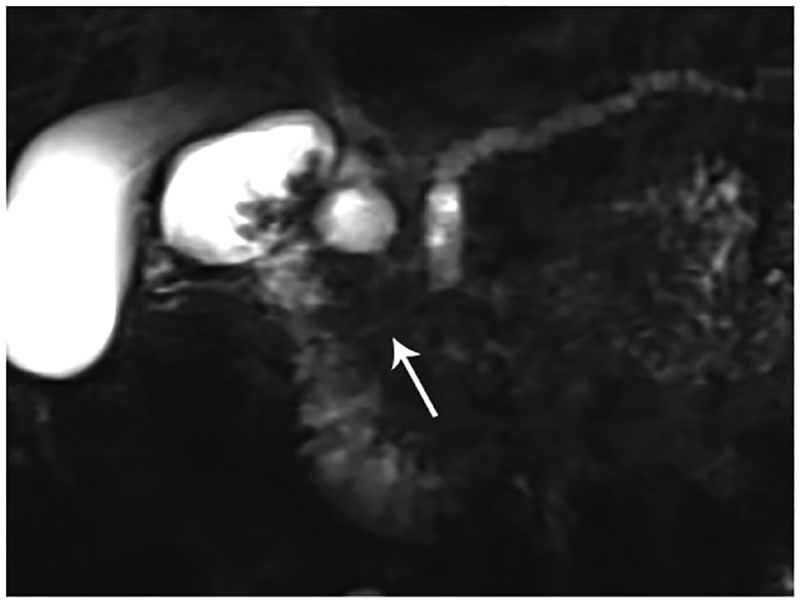
Penetrating duct sign. (A) Coronal MRCP image in a 49-year-old patient with abdominal pain. There is obstruction of the pancreatic duct in the pancreatic head (arrow). Differential diagnosis includes CP given the history, however, also concerning for pancreatic cancer. (B) Coronal MRCP image obtained after administration of the secretin. The PD became visible (arrow) following stimulation of the pancreas with secretin. There is a smoothly narrowed PD from the level of obstruction to the sphincter compatible with penetrating duct sign. This finding favors a benign etiology of obstruction rather than malignancy.
Paraduodenal pancreatitis, also known as groove pancreatitis, is a rare form of chronic pancreatitis that masquerades as pancreatic adenocarcinoma affecting the pancreaticoduodenal groove, a potential space between the head of the pancreas, duodenum, and common bile duct (Figure 2). Imaging findings of groove pancreatitis often overlap with primary duodenal, ampullary, or pancreatic neoplasms, which often results in a diagnostic challenge.64 Also, paraduodenal pancreatitis can be mistaken for cystic pancreatic lesions, especially when there is involvement of the duodenal wall. Preoperative recognition of this entity is essential to avoid unnecessary procedures, although surgery, such as pancreaticoduodenectomy, may still be required to relieve obstructive symptoms.
Figure 2.
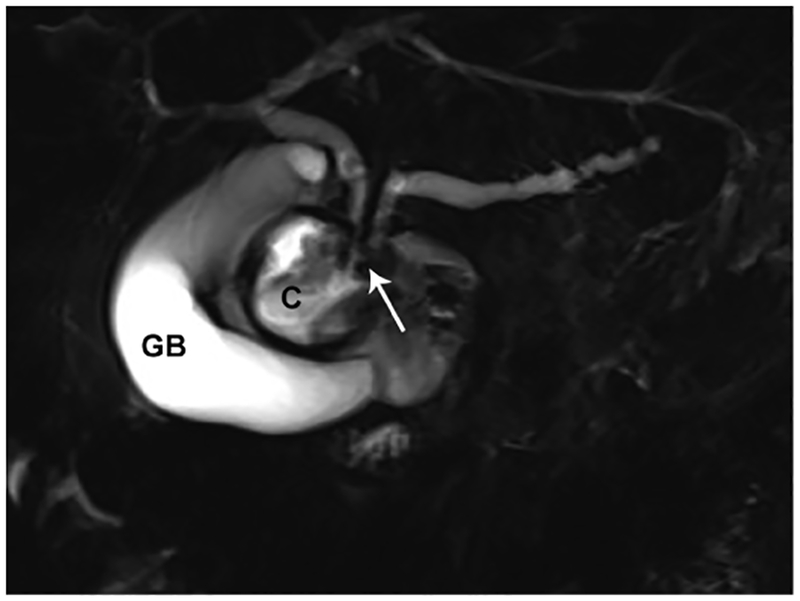
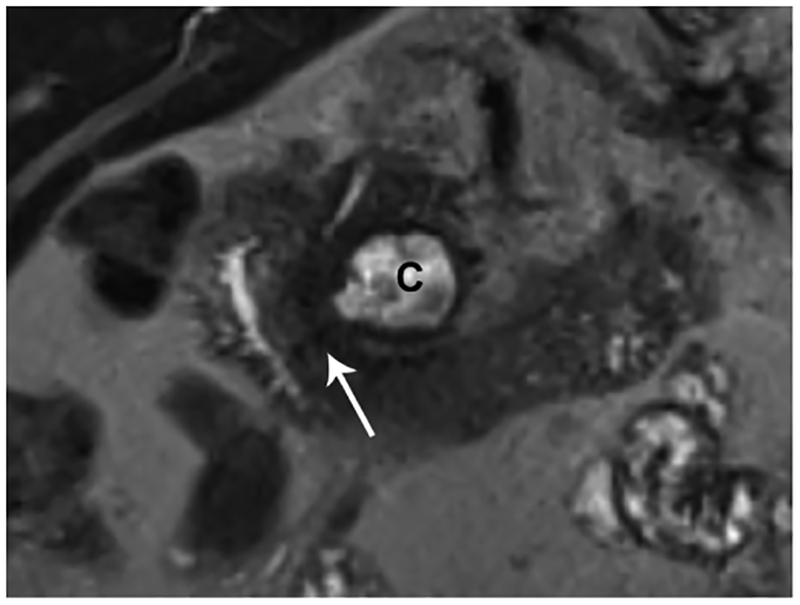
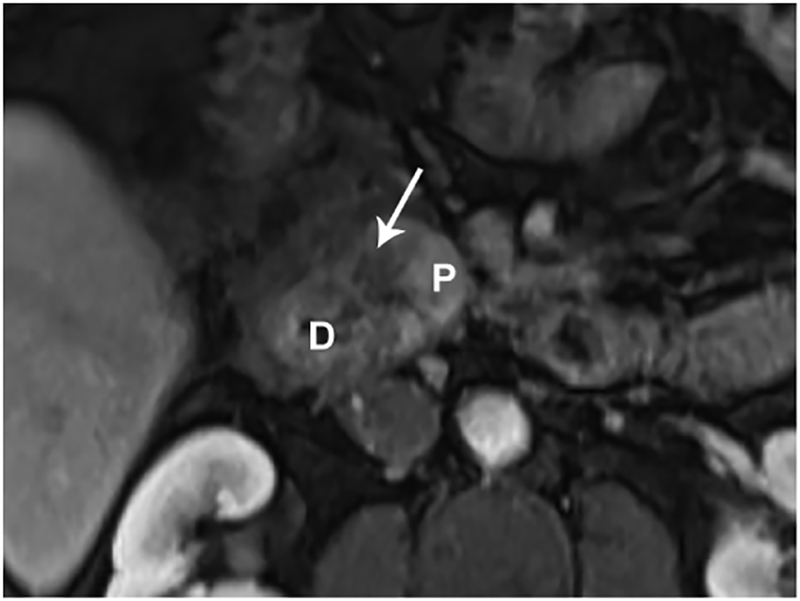
Paraduodenal/groove pancreatitis. (A) Coronal MRCP image in a 36-year-old male with a history of alcohol abuse. There is obstruction of both the PD and the biliary tree in the region of the pancreaticoduodenal junction (arrow). The patient presented with acute pancreatitis and developed a post-inflammatory cyst (C). (B) A coronal T2-weighted image shows a relatively T2 hypointense tissue (arrow) causing stricture of the PD. (C) Axial T1-weighted image after contrast administration is shown. There is a hypoenhancing lesion (arrow) corresponding to T2 hypointense soft tissue in the pancreaticoduodenal junction concerning for necrotizing pancreatitis and possibly a malignancy. There are acute inflammatory changes in and around the duodenum and pancreatic head in addition to the history of acute on CP. Combination of the clinical and imaging findings favor a non-malignant etiology such as paraduodenal, also called groove CP. (G= gallbladder; C= post-inflammatory cyst; D=duodenum; P=pancreas)
COMPLICATIONS
There are several potential complications of chronic pancreatitis which require active surveillance by clinicians, including diabetes, exocrine pancreatic insufficiency, metabolic bone disease, and pancreatic cancer (Table 4). 65 Most common complications of CP are endocrine/exocrine insufficiencies, and metabolic bone disorder are not diagnosed by imaging studies. Those seen by the cross-sectional imaging include but not limited to; post-inflammatory cyst formation, bile duct or duodenal obstruction, pancreatic ascites or pleural effusion, splenic vein thrombosis, and pancreatic cancer.5 Patients may also develop acute attacks of pancreatitis, particularly alcoholics who continue drinking.
Table 4.
Common complications of CP.
| Complications of Chronic Pancreatitis | |
|---|---|
| Endocrine insufficiency (T3cDM) | Up to 80% |
| Exocrine insufficiency | 30–80% |
| Metabolic bone disease | 66% |
| Splenic vein thrombosis | 10–20% |
| Biliary obstruction | Up to 25% |
| Pancreatic cancer | 4% |
| Duodenal stricture | 1% |
CONCLUSION
The diagnosis of CP can be challenging since biopsy of the pancreas is not performed, and laboratory studies and imaging procedures may be normal during the early stage of the disease. The diagnosis of the advanced CP is confirmed if there are calcifications within the pancreas on plain abdominal films or computed tomography (CT) scan, an abnormal pancreatogram or an abnormal secretin pancreatic function test in subtle cases of early pancreatitis. Identification of early stage CP and its treatment may delay or prevent morbidity secondary to CP. In settings where MRI/MRCP is available and of high quality, it may be the imaging test of choice, allowing for assessment of ductal changes and potentially obviating the need for an invasive procedure. Stimulation of the pancreas using intravenous secretin may improve the diagnostic accuracy in the detection of ductal and parenchymal abnormalities seen in CP. T1 signal intensity changes in the pancreatic parenchyma may precede ductal abnormalities and may detect early CP. Ultrasound and CT are best for the late findings of CP but are limited in the diagnosis of early or mild pancreatitis. Contrast-enhanced CT scan can rule out other causes of pain that mimic CP and is helpful for the diagnosis and complications of CP. There is a need for an EUS-like MRI staging system for CP, combining the ductal findings with the parenchymal changes.
Key Points.
MRI, CT, and EUS are the best imaging methods for establishing a diagnosis of CP. ERCP is reserved for therapeutic purposes.
The diagnosis of chronic pancreatitis remains challenging in early stages of the disease. T1 signal intensity changes of the parenchyma may precede ductal abnormalities and detect early CP.
The use of secretin increases the diagnostic potential of MRCP in the evaluation of patients with known or suspected CP.
There is a need for an MRI/MRCP based diagnostic criteria for CP, combining the ductal findings with the parenchymal changes secondary to fibrosis.
Genetic discoveries are rapidly uncovering new susceptibility factors. Knowledge of gene and gene-environment interactions may translate into new diagnostic and treatment paradigms.
Synopsis.
Diagnosis of CP requires a complete medical history and clinical investigations, including imaging technologies and function tests. MRI/MRCP is the preferred diagnostic tool for detection of ductal and parenchymal changes in CP patients. Ductal changes may not be present in the initial phase of the CP therefore early diagnosis remains challenging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Temel Tirkes receives support from National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number U01DK108323. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Hirota M, Shimosegawa T, Masamune A, et al. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology. 2014;14(6):490–496. [DOI] [PubMed] [Google Scholar]
- 2.Levy P, Dominguez-Munoz E, Imrie C, Lohr M, Maisonneuve P. Epidemiology of chronic pancreatitis: burden of the disease and consequences. United European Gastroenterol J. 2014;2(5):345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol. 2011;106(12):2192–2199. [DOI] [PubMed] [Google Scholar]
- 4.Amann ST, Yadav D, Barmada MM, et al. Physical and mental quality of life in chronic pancreatitis: a case-control study from the North American Pancreatitis Study 2 cohort. Pancreas. 2013;42(2):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86(5 Pt 1):820–828. [PubMed] [Google Scholar]
- 6.Ammann RW, Buehler H, Muench R, Freiburghaus AW, Siegenthaler W. Differences in the natural history of idiopathic (nonalcoholic) and alcoholic chronic pancreatitis. A comparative long-term study of 287 patients. Pancreas. 1987;2(4):368–377. [DOI] [PubMed] [Google Scholar]
- 7.Cavallini G, Frulloni L, Pederzoli P, et al. Long-term follow-up of patients with chronic pancreatitis in Italy. Scand J Gastroenterol. 1998;33(8):880–889. [DOI] [PubMed] [Google Scholar]
- 8.Lankisch PG, Lohr-Happe A, Otto J, Creutzfeldt W. Natural course in chronic pancreatitis. Pain, exocrine and endocrine pancreatic insufficiency and prognosis of the disease. Digestion. 1993;54(3):148–155. [DOI] [PubMed] [Google Scholar]
- 9.Maisonneuve P, Lowenfels AB, Mullhaupt B, et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005;54(4):510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dani R, Mott CB, Guarita DR, Nogueira CE. Epidemiology and etiology of chronic pancreatitis in Brazil: a tale of two cities. Pancreas. 1990;5(4):474–478. [DOI] [PubMed] [Google Scholar]
- 11.Marks IN, Bank S, Louw JH. Chronic pancreatitis in the Western Cape. Digestion. 1973;9(5):447–453. [DOI] [PubMed] [Google Scholar]
- 12.Layer P, Yamamoto H, Kalthoff L, Clain JE, Bakken LJ, DiMagno EP. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994;107(5):1481–1487. [DOI] [PubMed] [Google Scholar]
- 13.Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology. 2013;144(6):1292–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart PA, Zen Y, Chari ST. Recent Advances in Autoimmune Pancreatitis. Gastroenterology. 2015;149(1):39–51. [DOI] [PubMed] [Google Scholar]
- 15.Greer JB, Thrower E, Yadav D. Epidemiologic and Mechanistic Associations Between Smoking and Pancreatitis. Curr Treat Options Gastroenterol. 2015;13(3):332–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akisik MF, Jennings SG, Aisen AM, et al. MRCP in patient care: a prospective survey of gastroenterologists. AJR Am J Roentgenol. 2013;201(3):573–577. [DOI] [PubMed] [Google Scholar]
- 17.Andersen DK. The practical importance of recognizing pancreatogenic or type 3c diabetes. Diabetes Metab Res Rev. 2012;28(4):326–328. [DOI] [PubMed] [Google Scholar]
- 18.Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes Metab Res Rev. 2012;28(4):338–342. [DOI] [PubMed] [Google Scholar]
- 19.Hulisz D. The burden of illness of irritable bowel syndrome: current challenges and hope for the future. J Manag Care Pharm. 2004;10(4):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas. 2005;31(1):63–68. [DOI] [PubMed] [Google Scholar]
- 21.Conwell DL, Wu BU. Chronic pancreatitis: making the diagnosis. Clin Gastroenterol Hepatol. 2012;10(10):1088–1095. [DOI] [PubMed] [Google Scholar]
- 22.Conwell DL, Zuccaro G Jr., Vargo JJ, et al. An endoscopic pancreatic function test with synthetic porcine secretin for the evaluation of chronic abdominal pain and suspected chronic pancreatitis. Gastrointest Endosc. 2003;57(1):37–40. [DOI] [PubMed] [Google Scholar]
- 23.Ketwaroo G, Brown A, Young B, et al. Defining the accuracy of secretin pancreatic function testing in patients with suspected early chronic pancreatitis. Am J Gastroenterol. 2013;108(8):1360–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Tamakoshi A, Hayakawa T, Ogawa M, Ohno Y, Research Committee on Intractable Pancreatic D. Associations of alcohol drinking and nutrient intake with chronic pancreatitis: findings from a case-control study in Japan. Am J Gastroenterol. 2001;96(9):2622–2627. [DOI] [PubMed] [Google Scholar]
- 25.Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci. 1999;44(7):1303–1311. [DOI] [PubMed] [Google Scholar]
- 26.Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology. 1999;116(5):1132–1140. [DOI] [PubMed] [Google Scholar]
- 27.DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288(16):813–815. [DOI] [PubMed] [Google Scholar]
- 28.Mergener K, Baillie J. Chronic pancreatitis. Lancet. 1997;350(9088):1379–1385. [DOI] [PubMed] [Google Scholar]
- 29.Toskes PP, Hansell J, Cerda J, Deren JJ. Vitamin B 12 malabsorption in chronic pancreatic insufficiency. N Engl J Med. 1971;284(12):627–632. [DOI] [PubMed] [Google Scholar]
- 30.Malka D, Hammel P, Sauvanet A, et al. Risk factors for diabetes mellitus in chronic pancreatitis. Gastroenterology. 2000;119(5):1324–1332. [DOI] [PubMed] [Google Scholar]
- 31.Kloppel G Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol. 2007;20 Suppl 1:S113–131. [DOI] [PubMed] [Google Scholar]
- 32.Bolondi L, Li Bassi S, Gaiani S, Barbara L. Sonography of chronic pancreatitis. Radiol Clin North Am. 1989;27(4):815–833. [PubMed] [Google Scholar]
- 33.Luetmer PH, Stephens DH, Ward EM. Chronic pancreatitis: reassessment with current CT. Radiology. 1989;171(2):353–357. [DOI] [PubMed] [Google Scholar]
- 34.Tirkes T, Fogel EL, Sherman S, et al. Detection of exocrine dysfunction by MRI in patients with early chronic pancreatitis. Abdom Radiol (NY). 2017;42(2):544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tirkes T, Lin C, Fogel EL, Sherman SS, Wang Q, Sandrasegaran K. T1 mapping for diagnosis of mild chronic pancreatitis. J Magn Reson Imaging. 2017;45(4):1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balci C MRI assessment of chronic pancreatitis. Diagn Interv Radiol. 2011;17(3):249–254. [DOI] [PubMed] [Google Scholar]
- 37.Balci NC, Smith A, Momtahen AJ, et al. MRI and S-MRCP findings in patients with suspected chronic pancreatitis: correlation with endoscopic pancreatic function testing (ePFT). J Magn Reson Imaging. 2010;31(3):601–606. [DOI] [PubMed] [Google Scholar]
- 38.Choueiri NE, Balci NC, Alkaade S, Burton FR. Advanced imaging of chronic pancreatitis. Curr Gastroenterol Rep. 2010;12(2):114–120. [DOI] [PubMed] [Google Scholar]
- 39.Hellerhoff KJ, Helmberger H 3rd, Rosch T, Settles MR, Link TM, Rummeny EJ Dynamic MR pancreatography after secretin administration: image quality and diagnostic accuracy. AJR Am J Roentgenol. 2002;179(1):121–129. [DOI] [PubMed] [Google Scholar]
- 40.Sherman S, Freeman ML, Tarnasky PR, et al. Administration of secretin (RG1068) increases the sensitivity of detection of duct abnormalities by magnetic resonance cholangiopancreatography in patients with pancreatitis. Gastroenterology. 2014;147(3):646–654 e642. [DOI] [PubMed] [Google Scholar]
- 41.Cappeliez O, Delhaye M, Deviere J, et al. Chronic pancreatitis: evaluation of pancreatic exocrine function with MR pancreatography after secretin stimulation. Radiology. 2000;215(2):358–364. [DOI] [PubMed] [Google Scholar]
- 42.Akisik MF, Aisen AM, Sandrasegaran K, et al. Assessment of chronic pancreatitis: utility of diffusion-weighted MR imaging with secretin enhancement. Radiology. 2009;250(1):103–109. [DOI] [PubMed] [Google Scholar]
- 43.Shi Y, Glaser KJ, Venkatesh SK, Ben-Abraham EI, Ehman RL. Feasibility of using 3D MR elastography to determine pancreatic stiffness in healthy volunteers. J Magn Reson Imaging. 2015;41(2):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NIH state-of-the-science statement on endoscopic retrograde cholangiopancreatography (ERCP) for diagnosis and therapy. NIH Consens State Sci Statements. 2002;19(1):1–26. [PubMed] [Google Scholar]
- 45.Adler DG, Lichtenstein D, Baron TH, et al. The role of endoscopy in patients with chronic pancreatitis. Gastrointest Endosc. 2006;63(7):933–937. [DOI] [PubMed] [Google Scholar]
- 46.Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25(7):756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conwell DL, Lee LS, Yadav D, et al. American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: evidence-based report on diagnostic guidelines. Pancreas. 2014;43(8):1143–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc. 2001;53(3):294–299. [DOI] [PubMed] [Google Scholar]
- 49.Stevens T, Lopez R, Adler DG, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc. 2010;71(3):519–526. [DOI] [PubMed] [Google Scholar]
- 50.Keim V, Teich N, Moessner J. Clinical value of a new fecal elastase test for detection of chronic pancreatitis. Clin Lab. 2003;49(5–6):209–215. [PubMed] [Google Scholar]
- 51.Bishop MD, Freedman SD, Zielenski J, et al. The cystic fibrosis transmembrane conductance regulator gene and ion channel function in patients with idiopathic pancreatitis. Hum Genet. 2005;118(3–4):372–381. [DOI] [PubMed] [Google Scholar]
- 52.Ooi CY, Gonska T, Durie PR, Freedman SD. Genetic testing in pancreatitis. Gastroenterology. 2010;138(7):2202–2206, 2206 e2201. [DOI] [PubMed] [Google Scholar]
- 53.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25(2):213–216. [DOI] [PubMed] [Google Scholar]
- 54.Bagul A, Siriwardena AK. Evaluation of the Manchester classification system for chronic pancreatitis. JOP. 2006;7(4):390–396. [PubMed] [Google Scholar]
- 55.Bank S, Singh P, Pooran N. Proposal for a new grading system for chronic pancreatitis: the ABC system. J Clin Gastroenterol. 2002;35(1):3–4. [DOI] [PubMed] [Google Scholar]
- 56.Buchler MW, Martignoni ME, Friess H, Malfertheiner P. A proposal for a new clinical classification of chronic pancreatitis. BMC Gastroenterol. 2009;9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2009;69(7):1251–1261. [DOI] [PubMed] [Google Scholar]
- 58.Schneider A, Lohr JM, Singer MV. The M-ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol. 2007;42(2):101–119. [DOI] [PubMed] [Google Scholar]
- 59.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120(3):682–707. [DOI] [PubMed] [Google Scholar]
- 60.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89(6):442–446. [DOI] [PubMed] [Google Scholar]
- 61.Bang UC, Benfield T, Hyldstrup L, Bendtsen F, Beck Jensen JE. Mortality, cancer, and comorbidities associated with chronic pancreatitis: a Danish nationwide matched-cohort study. Gastroenterology. 2014;146(4):989–994. [DOI] [PubMed] [Google Scholar]
- 62.Shemesh E, Czerniak A, Nass S, Klein E. Role of endoscopic retrograde cholangiopancreatography in differentiating pancreatic cancer coexisting with chronic pancreatitis. Cancer. 1990;65(4):893–896. [DOI] [PubMed] [Google Scholar]
- 63.Ichikawa T, Sou H, Araki T, et al. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology. 2001;221(1):107–116. [DOI] [PubMed] [Google Scholar]
- 64.Mittal PK, Harri P, Nandwana S, et al. Paraduodenal pancreatitis: benign and malignant mimics at MRI. Abdom Radiol (NY). 2017;42(11):2652–2674. [DOI] [PubMed] [Google Scholar]
- 65.Ramsey ML, Conwell DL, Hart PA. Complications of Chronic Pancreatitis. Dig Dis Sci. 2017;62(7):1745–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]


