Abiraterone acetate plus prednisone in addition to androgen-deprivation therapy (ADT) was efficacious versus ADT alone in Japanese men with metastatic hormone-naïve prostate cancer. No new safety concerns were identified.
Keywords: abiraterone, hormone-naïve prostate cancer, Japan, metastatic
Abstract
Objectives
To evaluate the efficacy and safety of abiraterone acetate plus prednisone (AAP) plus androgen-deprivation therapy (ADT) in Japanese subgroup with newly diagnosed, metastatic hormone-naïve prostate cancer (mHNPC) from Phase 3, randomized, global LATITUDE study.
Methods
Men with mHNPC having ≥2 of 3 high-risk factors (Gleason score ≥8, ≥3 bone lesions or measurable visceral metastases) randomly received abiraterone acetate 1000-mg+ prednisone 5-mg+ADT (AAP group) or ADT+Placebos (Placebo group). Coprimary endpoints were overall survival (OS) and radiographic progression-free survival (rPFS).
Results
Of total 1199 patients in the LATITUDE study, 70 (5.8%) were Japanese (n = 35 each in the AAP and placebo group). After a median follow-up of 35.02 months (range: 2.5–42.3), median OS was not reached in both AAP group and placebo group (HR: 0.635; 95% CI, 0.152–2.659) and the median length of rPFS was not reached in the AAP group and was 22 months in the placebo group (HR:0.219; 95% CI, 0.086–0.560). The most frequently reported adverse events (>20% in either group) in the Japanese subgroup were hypertension, nasopharyngitis, weight increased, hypokalemia, hot flush, back pain, hyperglycemia, ALT and AST elevation. The incidence of Grade 3 or 4 adverse events was 65.7% (23/35) in the AAP group and 20% (7/35) in the placebo group. The efficacy and safety findings of Japanese subgroup were consistent with that of the overall study population.
Conclusion
Treatment with AAP plus ADT has shown a positive risk–benefit balance and may serve as a new treatment option to improve the prognosis of Japanese mHNPC patients with high-risk features.
Introduction
The incidence of prostate cancer is less common in Japan when compared with global population, however, aging and western lifestyle have contributed to a gradual increase in the incidence and mortality from prostate cancer in Japanese population in the recent years (1–3). Men with prostate cancer in Japan are most commonly diagnosed with high-risk characteristics and advanced-stage disease than those in most other developed countries, presumably because of lack of early detection by prostate-specific antigen (PSA) screening (4). Patients with newly diagnosed metastatic hormone-naïve prostate cancer (mHNPC), particularly with high-risk features, have a poor prognosis (5). In Japan, mHNPC accounts for ~10% of newly diagnosed prostate cancers and castration using androgen-deprivation therapy (ADT) has remained the first choice of treatment (6–8). The ADT includes bilateral orchiectomy or luteinizing hormone-releasing hormone (LH-RH) analogs, with or without first generation androgen-receptor inhibitors. Although there is an initial clinical benefit with such treatments, most patients eventually become resistant and progress to metastatic castration-resistant prostate cancer (mCRPC) around 1–2 years, despite preserving a low testosterone level (9). Novel agents with different mechanisms of action that preserve low androgen levels by further decreasing androgen production or inhibiting the androgen receptor function were later developed to provide additional benefits. A combination of docetaxel and ADT has shown to improve overall survival (OS) benefit in several randomized studies conducted in patients with mHNPC, especially with a high metastatic burden (10–13). However, patient disease status, comorbidities and patient preferences limit the use of docetaxel. There are currently limited treatment options for the mHNPC in Japan, despite the development of new therapeutic options for mCRPC.
Abiraterone acetate (AA) is the prodrug of abiraterone that blocks the androgen biosynthesis by specifically inhibiting the enzyme cytochrome P-450c17. AA plus prednisone (AAP) along with ADT significantly prolonged OS and was shown to provide other clinical benefits in patients with mCRPC in both pre- and post-chemotherapy setting in the global population (14–18) and in the Japanese population (19–22). This treatment is currently approved for mCRPC in >100 countries, including Japan (23), based on the efficacy and safety findings observed in Phase 2 and 3 randomized studies. The addition of AAP to ADT has also shown a reduction in tumor burden in men with high-risk, localized prostate cancer who received neoadjuvant therapy, which suggests its plausible role in inhibiting the extragonadal androgen biosynthesis before it progresses to castration resistance in mHNPC patients (24,25). A Phase 3, multinational, double-blind, placebo-controlled study (LATITUDE) was recently conducted in patients with newly diagnosed mHNPC to evaluate the efficacy and safety of AAP in addition to ADT versus ADT alone. Findings from LATITUDE showed that the addition of AAP to ADT significantly prolonged OS and rPFS compared with ADT alone in men with newly diagnosed mHNPC (26). The OS benefit demonstrated with AAP in LATITUDE was confirmed in a recently reported meta-analysis (27). The present subgroup analysis of LATITUDE study was performed to evaluate the clinical benefits of combining AAP with ADT in Japanese men with newly diagnosed mHNPC.
Methods
The respective Institutional Review Board approved the study protocol. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Clinical Practices and applicable regulatory requirements. All patients provided written informed consent before enrollment.
Patients
Men aged ≥18 years with newly diagnosed (≤3 months before randomization), pathologically confirmed prostate cancer without neuroendocrine differentiation or small-cell histologic features and with an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 to 2 (on a 5-point scale, with higher numbers indicating greater disability) were eligible for enrollment. Additionally, patients with high-risk, mHNPC, were enrolled only if they fulfilled 2 of the 3 following high-risk factors: (1) a Gleason score of ≥8, (2) presence of ≥3 bone lesions by positive bone scans and (3) the presence of measurable visceral metastasis on CT or MRI scan (according to Response Evaluation Criteria In Solid Tumors [RECIST] version 1.1 criteria).
Patients with a medical condition that would contraindicate prednisone use or require >5 mg/day of systemic prednisone treatment; significant cardiac, adrenal or liver dysfunction; a significant laboratory abnormality; a malignancy other than prostate cancer or non-melanoma skin cancer within 5 years; previous pharmacotherapy, radiation therapy or surgery for metastatic prostate cancer (with the exception of ≤3 months of ADT or one course of palliative radiation or surgical therapy to treat symptoms associated with metastatic disease) were excluded.
Study design
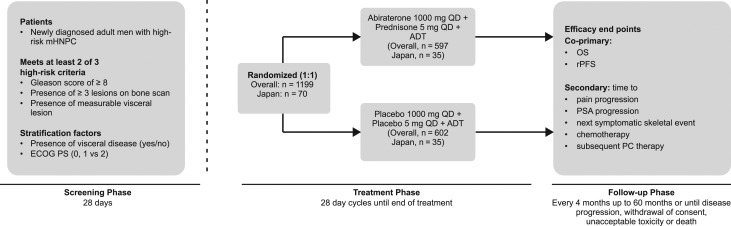
This was a subgroup analysis of a double-blind, placebo-controlled, Phase 3 study (LATITUDE, NCT01715285); conducted at 235 sites in 34 countries, including Japan. The detailed methodology of the primary study has been published elsewhere (26). Briefly, eligible patients were stratified according to the presence or absence of measurable visceral disease and ECOG performance-status score (0 or 1 vs 2) and randomized (1:1) to receive ADT and AA (1000 mg orally once daily, 4 × 250 mg tablets) and prednisone (5 mg daily) (AAP group) or ADT and respective placebos (placebo group), at least 1 h before or 2 h after a meal (Fig. 1). The ADT utilized were LH-RH analogs, or anti-androgens for up to 3 months prior to randomization, but were allowed for only 2 weeks after Cycle 1; or orchiectomy ≤3 months from randomization. Patients without surgical castration received ongoing ADT to maintain serum testosterone levels less than 50 ng/dl.
Figure 1.
Study design. ADT, androgen-deprivation therapy; ECOG, Eastern Cooperative Oncology Group; mHNPC, metastatic hormone-naïve prostate cancer; OS, overall survival; PC, prostate cancer therapy; PSA, prostate-specific antigen; rPFS, radiographic progression-free survival; QD, once daily.
Efficacy endpoints
The coprimary efficacy endpoints were OS (time from randomization to death from any cause) and radiographic progression-free survival (rPFS; time from randomization to the occurrence of radiographic progression or death from any cause). Secondary efficacy endpoints were time to pain progression (defined as time from randomization to the first date a patient experiences an increase of >30% from baseline in the worst pain category on the Brief Pain Inventory–Short Form [BPI-SF] as observed at two consecutive evaluations performed >4 weeks apart), time to PSA progression (on the basis of Prostate Cancer Working Group 2 [PCWG2] criteria), time to next skeletal-related event (a clinical or pathological fracture, spinal cord compression, palliative radiation to bone, or surgery involving bone), time to initiation of chemotherapy, and time to the next therapy for prostate cancer.
Efficacy assessments
Radiographic progression on bone scanning was assessed based on PCWG2 and progression of soft tissue lesions was evaluated by CT or MRI based on RECIST 1.1; every 4 months from week 16. Survival status and subsequent chemotherapy for prostate cancer were assessed at regular follow-up intervals (of 4 months) up to 60 months or until patient’s death, lost to follow-up, withdrawal of consent or study termination. Serum PSA evaluation was performed at baseline, once a month in the first year, then every 2 months until end of treatment.
Safety assessments
Safety was evaluated based on AEs, vital sign measurements and clinical laboratory tests (hematology, serum chemistry, and liver function tests). AE severity grade was assessed according to the National Cancer Institute (NCI)-Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0.
Statistical methods
Statistical analyses for the overall study population are described in the primary publication (26). First interim OS analysis and single rPFS analysis was planned after observing ~50% (426 events) of the total required death events in overall patients in the study. An Independent Data Monitoring Committee evaluated the data. The overall level of significance for the study was 0.05, with 0.049 allocated in the testing of OS, and 0.001 allocated in the testing of rPFS. Efficacy endpoints were analyzed using intent-to-treat (ITT) population that included all the patients randomized into the study. The safety analysis set comprised all patients who received at least one dose of study medication. An unstratified analysis was conducted for coprimary and secondary endpoints in the Japanese subgroup. Kaplan Meier estimates were used for the analysis of OS and rPFS. A non-stratified Cox regression model was used to estimate the hazard ratios (HR) and the associated 95% confidence interval (CI). Consistency in the efficacy and safety results between the Japanese subgroup and overall population were assessed.
Results
Patients
The data reported here are based on the clinical cut-off date (31 October 2016) for analysis of rPFS and the first interim analysis of OS. Of total 1199 patients in the LATITUDE study, 70 (5.8%) were Japanese and were randomized (1:1) to the AAP or placebo group (n = 35 each), Fig. 1. All baseline characteristics of Japanese population were mostly consistent with the overall population and were similar between two groups; except for higher mean baseline serum PSA observed in the AAP group versus placebo and slight differences in the disease locations and Gleason scores between Japanese population and overall population (Table 1). The median age of Japanese patients was 70 years (range: 51–82 years). The median time that the Japanese patients received the treatment was longer (AAP group: 30.98 [range: 1.4–43.0] months; placebo: 18.4 [range: 3.1–39.5] months) than the global population (AAP group: 23.98 [range: 0.1–43.0] months; placebo: 14.28 [range: 0.7–42.6] months). At the time of cut-off date, in the Japanese subgroup, 14/35 (40%) patients in the AAP group and 28/35 (80%) patients in the placebo group discontinued the study, with progressive disease being the primary reason for discontinuation (9/14 in the AAP group, 19/28 in the placebo group).
Table 1.
Demographics and baseline characteristics in the Japanese subgroup versus overall population (ITT population)
| Japanese subgroup | Overall population | |||
|---|---|---|---|---|
| AAP (n = 35) | Placebo (n = 35) | AAP (n = 597) | Placebo (n = 602) | |
| Age, years, median (range) | 70.0 (57–82) | 70 (51–80) | 68.0 (38–89) | 67.0 (33–92) |
| Time from initial diagnosis to first dose, months, mean (SD) | 1.6 (0.69) | 1.5 (0.65) | 1.8 (0.73) | 1.9 (0.75) |
| Disease location, n (%) | 35 | 35 | 596 | 600 |
| Bone | 31 (88.6) | 33 (94.3) | 580 (97.3) | 585 (97.5) |
| Node | 19 (54.3) | 18 (51.4) | 283 (47.5) | 287 (47.8) |
| Prostate mass | 15 (42.9) | 16 (45.7) | 151 (25.3) | 154 (25.7) |
| Lungs | 6 (17.1) | 10 (28.6) | 73 (12.2) | 72 (12.0) |
| Liver | 1 (2.9) | 2 (5.7) | 32 (5.4) | 30 (5.0) |
| Viscera | 1 (2.9) | 0 | 18 (3.0) | 13 (2.2) |
| Gleason score at initial diagnosis, n (%) | ||||
| <7 | 0 | 0 | 4 (0.7) | 1 (0.2) |
| 7 | 1 (2.9) | 1 (2.9) | 9 (1.5) | 15 (2.5) |
| 8 | 11 (31.4) | 11 (31.4) | 267 (44.7) | 281 (46.7) |
| 9 | 21 (60.0) | 21 (60.0) | 280 (46.9) | 264 (43.9) |
| 10 | 2 (5.7) | 2 (5.7) | 37 (6.2) | 41 (6.8) |
| Bone lesions at screening, n (%) | ||||
| 0 | 1 (2.9) | 2 (5.7) | 6 (1.0) | 7 (1.2) |
| 1–2 | 1 (2.9) | 3 (8.6) | 5 (0.8) | 10 (1.7) |
| 3–10 | 14 (40.0) | 12 (34.3) | 202 (33.8) | 208 (34.6) |
| 11–20 | 5 (14.3) | 4 (11.4) | 109 (18.3) | 97 (16.1) |
| >20 | 14 (40.0) | 14 (40.0) | 275 (46.1) | 280 (46.5) |
| Patients with high risk at screening | ||||
| GS≥8 + ≥3 bone lesions | 32 (91.4) | 29 (82.9) | 573 (96.0) | 569 (94.7) |
| GS≥8 + measurable visceral | 4 (11.4) | 9 (25.7) | 82 (13.7) | 169 (14.1) |
| ≥3 bone lesions + measurable visceral | 3 (8.6) | 5 (14.3) | 84 (14.1) | 169 (14.1) |
| GS≥8 + ≥3 bone lesions + measurable visceral | 2 (5.7) | 4 (11.4) | 71 (11.9) | 141 (11.8) |
| Baseline serum PSA, ng/ml, | ||||
| Mean (SD) | 432.3 (1098.11) | 182.6 (363.95) | 263.2 (791.44) | 201.7 (647.81) |
| Median (range) | 54.79 (0.1; 5163.9) | 27.61 (0.2; 1813.8) | 25.43 (0.0; 8775.9) | 23.05 (0.1; 8889.6) |
| Baseline hemoglobin, g/l | ||||
| Mean (SD) | 132.3 (13.67) | 130.8 (15.63) | 130.5 (16.96) | 131.6 (17.43) |
| Median (range) | 132.00 (94.0; 156.0) | 133.00 (99.0; 157.0) | 132.0 (90.0; 175.0) | 133.0 (89.0; 174.0) |
| Baseline lactate dehydrogenase (U/l) | ||||
| Mean (SD) | 180.3 (32.05) | 185.4 (32.92) | 199.3 (133.11) | 193.6 (104.22) |
| Median (range) | 175.0 (127; 264) | 180.0 (119; 264) | 177.0 (73; 2634) | 176.0 (67; 1444) |
| Total treatment duration (months), median range | 30.98 (1.4;43.0) | 18.4 (3.1; 39.5) | 23.98 (0.1;43.0) | 14.28 (0.7;42.6) |
| Patient who received subsequent therapy | 12 (34.3) | 27 (77.1) | 191 (32.0) | 322 (53.5) |
| Patients who received life-prolonging therapy | 9 (25.7) | 23 (65.7) | 125 (20.9) | 246 (40.9) |
| Docetaxel | 8 (22.9) | 16 (45.7) | 106 (17.8) | 187 (31.1) |
| Enzalutamide | 2 (5.7) | 18 (51.4) | 30 (5.0) | 76 (12.6) |
| Cabazitaxel | 0 | 2 (5.7) | 11 (1.8) | 30 (5.0) |
| Radium-223 | 0 | 1 (2.9) | 11 (1.8) | 27 (4.5) |
| Abiraterone acetate + prednisone | 0 | 5 (14.3) | 10 (1.7) | 53 (8.8) |
| Patients who received previous prostate cancer therapy | 34 | 30 | 560 | 560 |
| Surgery | 0 | 0 | 22 (3.7) | 23 (3.8) |
| Radiotherapy | 0 | 0 | 19 (3.2) | 26 (4.3) |
| Hormonal | 34 (97.1) | 30 (85.7) | 559 (93.6) | 558 (92.7) |
| GnRH analoga | 29 (82.9) | 21 (60.0) | 449 (75.2) | 450 (74.8) |
| Orchiectomy | 1 (2.9) | 3 (8.6) | 73 (12.2) | 71 (11.8) |
| Anti-androgensb | 27 (77.1) | 26 (74.3) | 373 (62.5) | 371 (61.6) |
| Otherc | 1 (2.9) | 1 (2.9) | 7 (1.2) | 10 (1.7) |
BPI-SF, Brief Pain Inventory-Short Form; ECOG, Eastern Cooperative Oncology Group; GS, Gleason score; PSA, prostate-specific antigen.
aAgonist or antagonist;
bAll anti-androgens were first generation antiandrogens (e.g. bicalutamide, nilutamide, flutamide, cyproterone acetate);
cInclude estrogen and glucocorticoid.
Coprimary efficacy endpoints
Overall survival
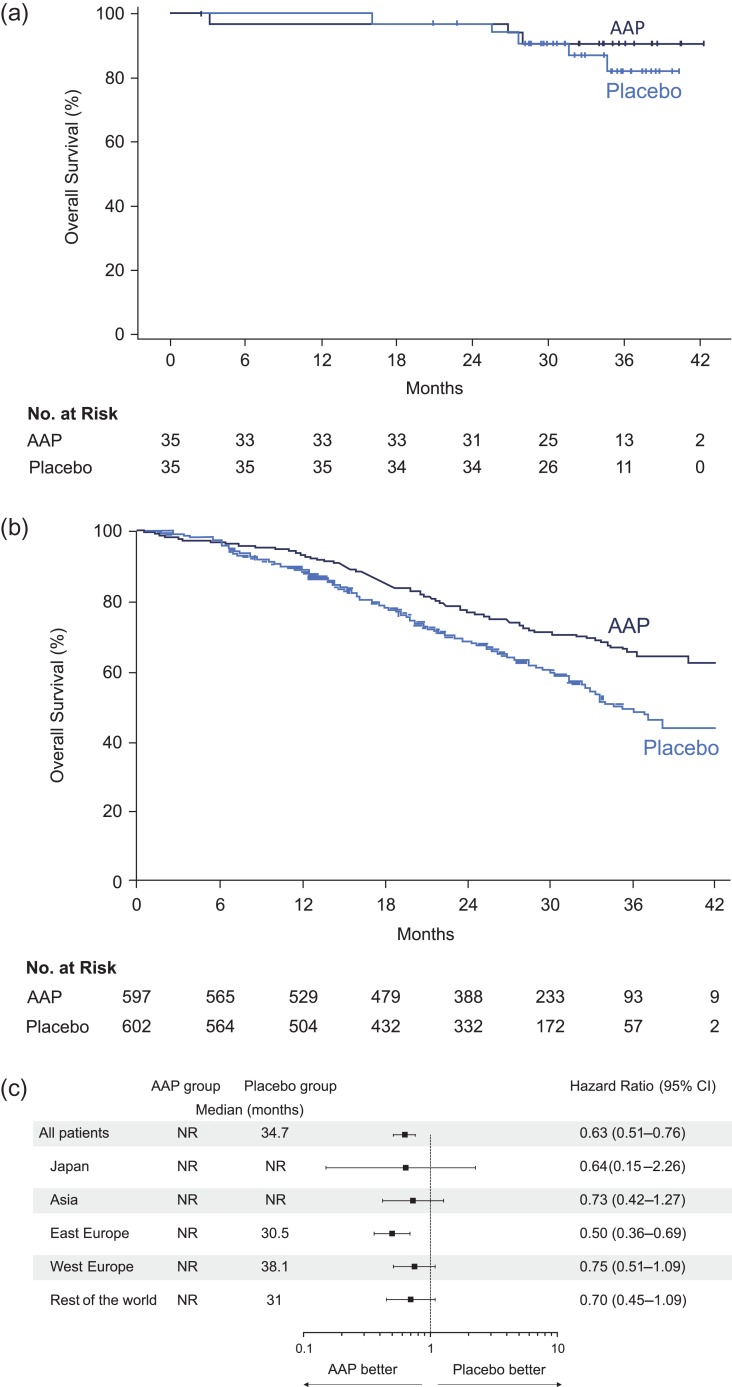
At the time of clinical cut-off, eight deaths (AAP: n = 3, placebo: n = 5) were reported in Japanese subgroup, with a median follow-up of 35.02 months (range: 2.5; 42.3). The median OS was not reached in both AAP group and placebo group in Japanese subgroup (Fig. 2a). The rate of OS in Japanese patients was higher among the AAP group compared with placebo (HR: 0.64; 95% CI: 0.15–2.66) and was comparable to the overall population (HR: 0.62; 95% CI: 0.51–0.76; P < 0.0001) (Fig. 2a and 2b). Furthermore, the forest plots suggested a favorable treatment effect of AAP on overall survival regardless of race and ethnicity (Fig. 2c).
Figure 2.
(a) Kaplan Meier estimate of overall survival in Japanese subgroup. (b) Kaplan Meier estimate of overall survival in overall population (ITT population). (c) Forest plots of treatment effect on overall survival within racial and ethnic subgroups.
Radiographic progression-free survival
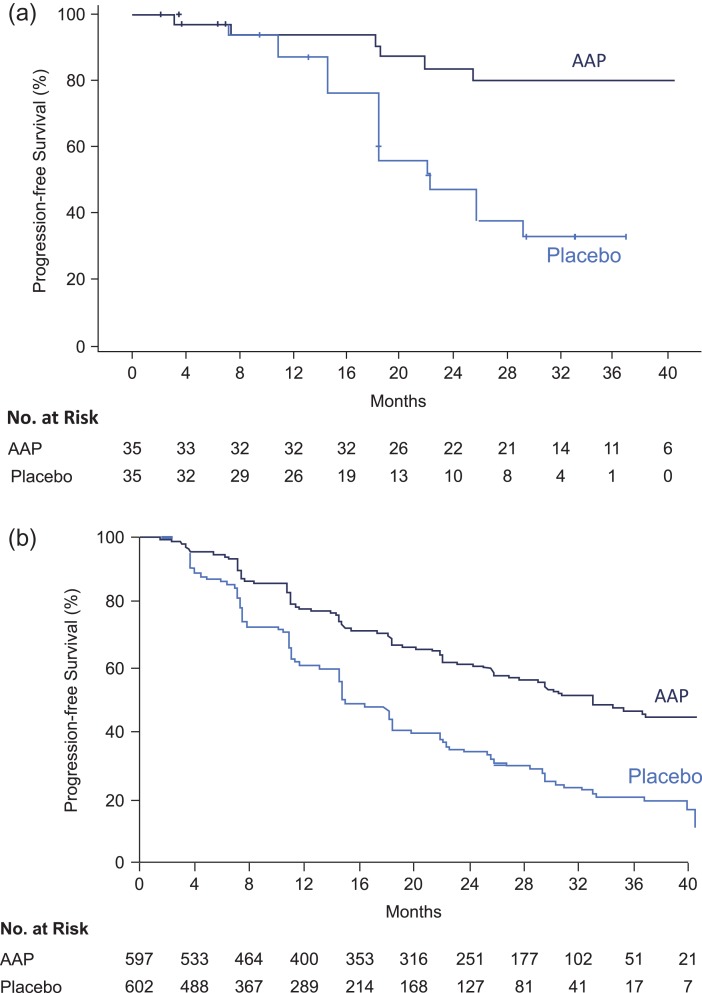
At the time of analysis, 23 patients (AAP: n = 6, placebo: n = 17) had radiographic progression or death in the Japanese subgroup. The median PFS was not reached in the AAP group and was 22 months in the placebo group. A reduced risk of rPFS in the Japanese patients was observed in the AAP group versus placebo (HR: 0.22; 95% CI: 0.09–0.56), which was consistent with the overall population (HR = 0.47; 95% CI: 0.39, 0.55; P < 0.0001) (Fig. 3a and 3b).
Figure 3.
(a) Kaplan Meier estimate of radiographic progression-free survival in Japanese subgroup. (b) Kaplan Meier estimate of radiographic progression-free survival in overall population (ITT population).
Secondary efficacy endpoints
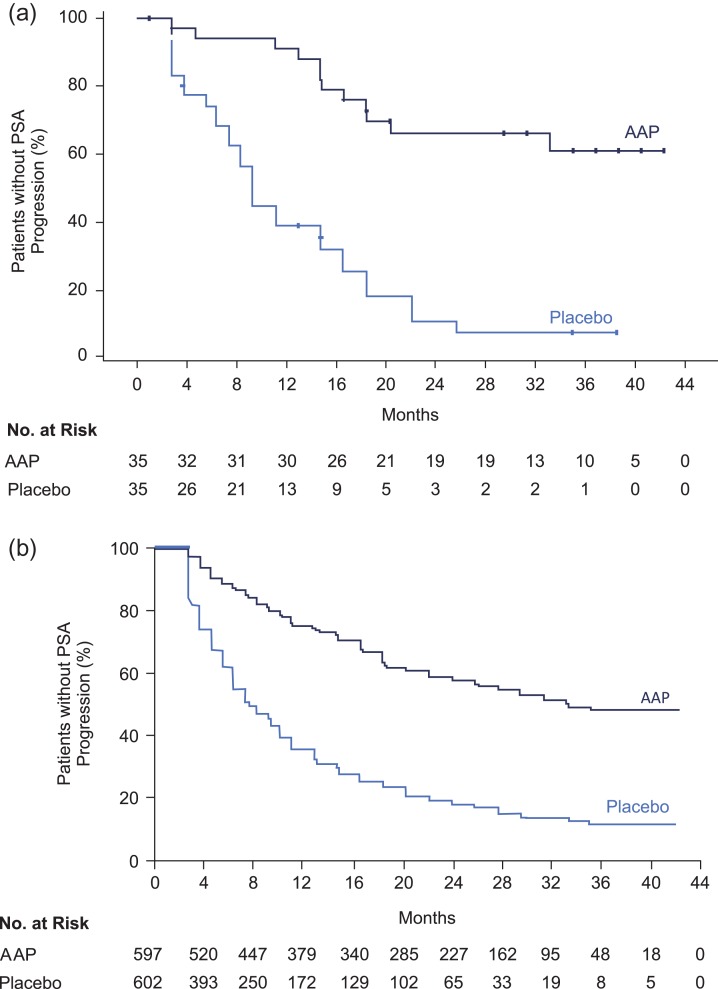
In the Japanese subpopulation, treatment with AAP delayed the time to pain progression (HR, 0.68; 95% CI: 0.35, 1.33), PSA progression (HR, 0.19; 95% CI: 0.09, 0.38; Fig. 4a and 4b), time to initiation of chemotherapy (HR, 0.43; 95% CI, 0.18–1.01) and time for the subsequent prostate cancer therapy (HR, 0.28; 95% CI, 0.14–0.55) when compared with placebo (Table 2). However, the AAP treatment did not reduce the risk of skeletal-related events (HR, 2.41; 95% CI: 0.82–7.05). In the overall population, there was a significantly improved outcome in all secondary endpoints in the AAP group versus placebo (Table 2).
Figure 4.
(a) Kaplan Meier estimate of time to PSA progression in Japanese subgroup. (b) Kaplan Meier estimate of time to PSA progression in overall population (ITT population).
Table 2.
Secondary efficacy endpoints in the Japanese subgroup versus overall population (ITT population)
| Endpoints (months) | Japanese subgroup | Overall population | ||||
|---|---|---|---|---|---|---|
| AAP (N = 35) | Placebo (N = 35) | Hazard ratio (95% CI) | AAP (N = 597) | Placebo (N = 602) | Hazard ratio (95% CI); P value | |
| Median time to pain progression | NR | 10.15 | 0.68 (0.35,1.33) | NR | 16.6 | 0.70 (0.58;0.83); P < 0.0001 |
| Median time to PSA progression | NR | 9.26 | 0.19 (0.09,0.38) | 33.2 | 7.4 | 0.30 (0.26;0.35); P < 0.0001 |
| Median time to next skeletal-related event | NR | NR | 2.41 (0.82,7.06) | NR | NR | 0.70 (0.54;0.92); P = 0.0086 |
| Median time to chemotherapy | NR | 35.55 | 0.43 (0.18,1.02) | NR | 38.9 | 0.44 (0.35;0.56); P < 0.0001 |
| Median time to subsequent prostate cancer therapy | NR | 18.56 | 0.28 (0.14,0.56) | NR | 21.6 | 0.42 (0.35;0.50); P < 0.0001 |
AAP, abiraterone acetate plus prednisone; NR, not reached; PSA, prostate-specific antigen.
Overall, the treatment effect of AAP in Japanese subgroup, in terms of coprimary endpoints and most of the secondary efficacy endpoints, was consistent with that of the overall population.
Safety
The overall incidence of AEs occurred at similar frequencies between AAP group and placebo group in the Japanese subgroup (both 97% [34/35]) as well as in the overall population (both 93%) (Table 3). The most frequently reported AEs (>20% in either AAP or placebo group) in the Japanese subgroup were hypertension (51.4% [18/35] vs 22.9% [8/35]), nasopharyngitis (37.1% [13/35] vs 31.4% [11/35]), weight increased (both 34.3% [12/35]), hypokalemia (34.3% [12/35] vs 0%), hot flush (both 31.4% [11/35]), back pain (28.6% [10/35] vs 20% [7/35]), hyperglycemia (22.9% [8/35] vs 14.3% [5/35]), ALT (22.9% [8/35] vs 31.4% [11/35]) and AST elevation (22.9% [8/35] vs 28.6% [10/35]) (Table 4). In the overall population, the most common AEs (≥20% in either AAP or placebo group) were hypertension (37% vs 22%), hypokalemia (20% vs 4%) and back pain (18% vs 20%). In the Japanese subgroup, CTCAE Grade 3 or 4 AEs were identified in 65.7% (23/35) of patients in the AAP group and 20% (7/35) in the placebo group (Table 3); in the overall population, 63% (374/597) were in the AAP group and 48% (287/602) in the placebo. The incidence of serious AEs in the Japanese subgroup was 17.1% (6/35) in the AAP group and 8.6% (3/35) in the placebo group; however, in the overall patients, the incidence between the AAP group and placebo group was similar (27.6% vs 24.3%, respectively). The AEs leading to treatment discontinuation were reported in 5.7% (2/35) of patients in the AAP group and 11.4% (4/35) in the placebo group in Japanese subgroup and 10.1% (61/597) of patients in the AAP group and in 12.2% (73/602) in the placebo in overall patients.
Table 3.
Summary of adverse events in the Japanese subgroup versus overall population (safety population)
| N (%) | Japanese subgroup | Overall population | ||
|---|---|---|---|---|
| AAP (N = 35) | Placebo (N = 35) | AAP (N = 597) | Placebo (N = 602) | |
| Any AE | 34 (97.1) | 34 (97.1) | 558 (93.5) | 557 (92.5) |
| Grade 3 or 4 AEs | 23 (65.7) | 7 (20.0) | 374 (62.6) | 287 (47.7) |
| Any serious AEs | 6 (17.1) | 3 (8.6) | 165 (27.6) | 146 (24.3) |
| Any AE leading to treatment discontinuation | 2 (5.7) | 4 (11.4) | 73 (12.2) | 61 (10.1) |
| AE leading to death | 1 (2.9) | 0 | 28 (4.7) | 24 (4.0) |
| AEs of special interest | ||||
| Grade 3 or 4 mineralocorticoid-related AEs | ||||
| Hypertension | 12 (34.3) | 2 (5.7) | 126 (21.1) | 63 (10.5) |
| Hypokalemia | 4 (11.4) | 0 | 62 (10.4) | 8 (1.3) |
| Fluid retention/edema | 0 | 0 | 5 (0.8) | 0 |
| Grade 3 hepatotoxicity | 3 (8.6) | 1 (2.9) | 46 (7.7) | 20 (3.3) |
AAP, abiraterone acetate plus prednisone; AE, adverse event.
Table 4.
Summary of most common adverse events in the Japanese subgroup (safety population)
| Most common adverse eventsan (%) | AAP (N = 35) | Placebo (N = 35) | ||||
|---|---|---|---|---|---|---|
| All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | |
| Hypertension | 18 (51.4) | 12 (34.3) | 0 | 8 (22.9) | 2 (5.7) | 0 |
| Nasopharyngitis | 13 (37.1) | 0 | 0 | 11 (31.4) | 0 | 0 |
| Hypokalemia | 12 (34.3) | 3 (8.6) | 1 (2.9) | 0 | 0 | 0 |
| Weight gain | 12 (34.3) | 0 | 0 | 12 (34.3) | 2 (5.7) | 0 |
| Hot flush | 11 (31.4) | 0 | 0 | 11 (31.4) | 0 | 0 |
| Back pain | 10 (28.6) | 0 | 0 | 7 (20.0) | 0 | 0 |
| Hyperglycemia | 8 (22.9) | 4 (11.4) | 0 | 5 (14.3) | 1 (2.9) | 0 |
| ALT increased | 8 (22.9) | 1 (2.9) | 0 | 11 (31.4) | 1 (2.9) | 0 |
| AST increased | 8 (22.9) | 1 (2.9) | 0 | 10 (28.6) | 1 (2.9) | 0 |
| Insomnia | 5 (14.3) | 0 | 0 | 3 (8.6) | 0 | 0 |
| Rib fracture | 5 (14.3) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Constipation | 4 (11.4) | 0 | 0 | 4 (11.4) | 4 (11.4) | 0 |
| Dental caries | 4 (11.4) | 1 (2.9) | 0 | 4 (11.4) | 2 (5.7) | 0 |
| Diarrhea | 4 (11.4) | 1 (2.9) | 0 | 4 (11.4) | 2 (5.7) | 0 |
| Vomiting | 4 (11.4) | 0 | 0 | 4 (11.4) | 1 (2.9) | 0 |
| Hematuria | 4 (11.4) | 0 | 0 | 0 | 0 | 0 |
| Hyperbilirubinemia | 4 (11.4) | 0 | 0 | 0 | 0 | 0 |
AAP, abiraterone acetate plus prednisone; AEs, adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
aAdverse events reported by CTCAE toxicity grades.
Incidence of AEs of special interest, mineralocorticoid-related AEs such as hypertension and hypokalemia and corticosteroid AE (Grade 3 or 4) such as hyperglycemia (Grade 3), in the Japanese subgroup was higher in the AAP group versus the placebo group (Table 3 and 4). In the overall population, a similar trend was observed only for Grade 3 or 4 hypertension and hypokalemia events (Table 3). Fluid retention, edema and cardiac disorders (Grade 3 or 4) were not reported in the Japanese subgroup; however, in the overall population, five (0.8%) patients reported edema. Grade 3 hepatotoxicity (including ALT increased, AST increased and hepatic function abnormal) in the Japanese subgroup was reported in 8.6% (3/35) patients in AAP group versus 2.9% (1/35) patients in the placebo; in the overall population, the incidence was 7.7% (46/597) in the AAP group versus 3.3% (20/602) in the placebo.
In the Japanese subgroup, one patient in the AAP group died due to AE of cerebral hemorrhage. In the overall population, death was reported in 28/597 (4.7%) in the AAP and 24/602 (4.0%) in the placebo; the most common reason being cardiac disorders (AAP group: 10/597 [1.7%] and placebo group: 6/602 [1.0%]).
Discussion
In this subgroup analysis of the global, Phase 3 study (LATITUDE study), the addition of AAP to ADT improved coprimary outcomes, OS and rPFS, compared with ADT alone in Japanese men with newly diagnosed mHNPC. The combination of AAP plus ADT versus placebo plus ADT also prolonged time to PSA progression, along with most of the secondary endpoints. There were no notable safety concerns identified in Japanese patients. The overall efficacy and safety findings were in line with those observed in the global population for AAP treatment (26).
The observed 36% lower relative risk of death (HR, 0.64; 95% CI: 0.15–2.66) and 78% lower relative risk of rPFS (HR: 0.22; 95% CI: 0.09–0.56), support the addition of AAP to ADT versus ADT alone in the Japanese patients with newly diagnosed mHNPC, who had at least two high-risk features. Favorable clinical outcomes were observed with AAP plus ADT despite 40.9% of patients in the placebo group and 20.9% in the AAP group receiving subsequent life-prolonging therapy, mostly docetaxel and enzalutamide (Table 1). In Japanese subgroup, survival benefits showed a similar trend to that of the overall population although 65.7% of the patients in the placebo and 25.7% in the AAP group, received subsequent life-prolonging therapy.
Most of the results for the secondary efficacy endpoints of time to pain progression, PSA progression, time to initiation of chemotherapy and subsequent prostate cancer therapy in Japanese subpopulation showed consistency with the results in overall patients, supporting the AAP treatment (HR < 1.0).
The higher risk for time to next skeletal-related event in the AAP group versus the placebo in the Japanese subgroup contrasted with the findings observed in the overall population. Of five patients who experienced rib fracture in the AAP group, three patients experienced rib fracture within 6 months from the initiation of AAP treatment; and two patients reported no events related to progression of disease or symptoms. These differences in outcomes could be attributed to several factors such as relatively few events and a small number of patients in this subgroup analysis. The role of differences in ethnicity and treatment environment influencing the treatment outcomes after hormonal therapy, especially between Japanese and Western patients with prostate cancer has been previously elucidated in retrospective studies (28–30). Findings from these studies suggest that Japanese patients showed better prognosis in terms of OS rate compared with the Western populations. The precise mechanism behind these differences is unclear, but it has been argued that Japanese patients show a greater sensitivity to hormonal therapy (30,31). Moreover, the Japanese population has a higher life expectancy than Westerners regardless of the disease state, and show a favorable prognosis of OS in breast cancer, renal cancer and non-small cell lung cancer (32–34).
Consistency in the OS outcome benefit was observed between patients receiving ADT monotherapy here and a prospective study (ZAPCA) involving Japanese men with mHNPC treated with ‘combined androgen blockade’ (CAB; bicalutamide + LH–RH agonist), despite the differences in CAB and ADT modalities (35). Available evidence suggests a possible modest survival benefit of CAB over ADT monotherapy in the treatment of metastatic prostate cancer, however some controversy still exist (36). It is also unclear if this slight benefit is advantageous as CAB showed decline in quality of life and tolerability in few studies (37,38). Additionally, in a subgroup analysis of a prospective study conducted in Japanese patients, CAB treatment did not indicate superior benefits over ADT monotherapy in the D2 setting (39). Therefore, there is currently a need for novel agents for effective management of mHSPC (40) and the present study findings suggest that potential role of AAP combined with ADT in ‘high-risk’ mHSPC.
The safety findings observed with the AAP group in this subgroup were consistent with that of the overall study population as well as with the previous studies conducted in mCRPC patients, reporting an increased incidence of the mineralocorticoid-related AEs of hypertension and hypokalemia (14–17,19,20). The frequency of Grade 3 hypertension and hypokalemia in the AAP group in overall population of LATITUDE study was higher than the previous global AAP studies (COU-AA-301 and COU-AA-302) involving mCRPC patients (14,17). However, the overall relative risk of hypertension (all grades) in the global LATITUDE study of AA with 5 mg/day prednisone + ADT (1.6 [95% CI, 1.4–1.9]) was similar to that in previous studies of 10 mg/day prednisone (COU-AA-301:1.4 [95% CI, 0.9–2.0]; COU-AA-302:1.6 [95% CI, 1.2–2.1]) (41). The potential contributing factors for the higher incidence of Grade 3 or 4 hypertension in LATITUDE versus previous studies (COU-AA-301 and -302) may include longer treatment exposure (24 months vs 8 and 13.8 months), lower prednisone dose (5 vs 10 mg/day); and use of stringent grading with CTCAE version 4.0 versus 3.0. For instance, some Grade 2 type of hypertension events in previous studies were categorized as Grade 3 in LATITUDE study. The incidence of Grade 3 hypertension, hypokalemia and hyperglycemia occurring in the AAP group was higher in the Japanese subpopulation than the overall population. It should also be noted that longer treatment exposure in Japanese subpopulation versus the overall population (34 months vs 24 months) could have contributed to the high incidence of Grade 3 AEs in this subgroup. Overall, Grade 3 hypertension, hypokalemia and hyperglycemia events were shown to be manageable in Japanese subpopulation, underscoring the need for more appropriate and timely management of AEs, especially steroid-related AEs.
In the global LATITUDE study, in addition to the survival benefits observed, AAP plus ADT has shown clinical benefits in overall patient-reported outcomes based on consistent improvement in progression of pain, fatigue, prostate cancer symptoms, functional status and overall health-related quality of life (42).
In summary, treatment with AAP and ADT resulted in improved efficacy outcomes in terms of OS and rPFS in the Japanese patients with newly diagnosed mHNPC. AAP treatment showed an acceptable safety profile in the Japanese population. The efficacy and safety results of Japanese subgroup were consistent with those of overall population of LATITUDE study. Treatment with AAP showed a positive risk–benefit balance and may serve as a new therapeutic option to improve the prognosis of Japanese men with mHNPC having high-risk characteristics.
Acknowledgements
The authors thank the study participants without whom this study would not have been accomplished. Writing assistance was provided by Ramji Narayanan, M Pharm, ISMPP CMPPTM (SIRO Clinpharm Pvt. Ltd, Thane, India), funded by Janssen Research & Development, LLC and additional editorial support was provided by Namit Ghildyal, PhD (Janssen Research & Development, LLC). This study was funded by Janssen Research & Development, LLC.
Conflict of interest statement
Satoshi Fukasawa received research funding from Janssen. Hiroyoshi Suzuki received research funding from Astellas, Takeda, Daiichi-Sankyo, Pfizer, Bayer, Nihon-Kayaku, Taiho; and personal fee or honoraria from Takeda, Astrazeneca, Janssen, Bayer, Daiichi-Sankyo, Astellas and Sanofi. Nobuaki Matsubara received research funding from Janssen and Sanofi. Karim Fizazi received personal fee or honoraria from Janssen, Sanofi, Astellas, Takeda, Merck and Amgen. Kentaro Enjo, Kazushiro Kawaguchi, and Hidehisa Noguchi are employees of Janssen Pharmaceutical K.K., Japan. Namphuong Tran and Mary Todd are employees of Janssen Research & Development, LLC, USA.
Registration: This study is registered at clinicaltrials.gov: NCT01715285
Previous presentation: Poster presented at 2018 Genitourinary Cancers Symposium, 8–10 February 2018, San Francisco, CA.
References
- 1.Prostate cancer estimated incidence: mortality and prevalence worldwide in 2012. IARC Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2. Chen R, Ren S, Yiu MK, et al. . Prostate cancer in Asia: a collaborative report. Asian J Urol 2014;1:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ito K. Prostate cancer in Asian men. Nat Rev Urol 2014;11:197–212. [DOI] [PubMed] [Google Scholar]
- 4. Cooperberg MR, Hinotsu S, Namiki M, et al. . Risk assessment among prostate cancer patients receiving primary androgen deprivation therapy. J Clin Oncol 2009;27:4306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gravis G, Boher JM, Chen YH, et al. . Burden of metastatic castrate naive prostate cancer patients, to identify men more likely to benefit from early docetaxel: further analyses of CHAARTED and GETUG-AFU15 Studies. Eur Urol 2018;73:847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanaka N, Fujimoto K, Hirayama A, Yoneda T, Yoshida K, Hirao Y. Trends of the primary therapy for patients with prostate cancer in Nara uro-oncological research group (NUORG): a comparison between the CaPSURE data and the NUORG data. Jpn J Clin Oncol 2010;40:588–92. [DOI] [PubMed] [Google Scholar]
- 7. Onozawa M, Hinotsu S, Tsukamoto T, et al. . Recent trends in the initial therapy for newly diagnosed prostate cancer in Japan. Jpn J Clin Oncol 2014;44:969–81. [DOI] [PubMed] [Google Scholar]
- 8. Fujimoto H, Nakanishi H, Miki T, et al. . Oncological outcomes of the prostate cancer patients registered in 2004: report from the Cancer Registration Committee of the JUA. Int J Urol 2011;18:876–81. [DOI] [PubMed] [Google Scholar]
- 9. Fitzpatrick JM, Bellmunt J, Fizazi K, et al. . Optimal management of metastatic castration-resistant prostate cancer: highlights from a European Expert Consensus Panel. Eur J Cancer 2014;50:1617–27. [DOI] [PubMed] [Google Scholar]
- 10. Sweeney CJ, Chen YH, Carducci M, et al. . Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015;373:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. James ND, Sydes MR, Clarke NW, et al. . Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387:1163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gravis G, Fizazi K, Joly F, et al. . Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol 2013;14:149–58. [DOI] [PubMed] [Google Scholar]
- 13. Gravis G, Boher JM, Joly F, et al. . Androgen Deprivation Therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized Phase 3 GETUG-AFU15 Trial. Eur Urol 2016;70:256–62. [DOI] [PubMed] [Google Scholar]
- 14. de Bono JS, Logothetis CJ, Molina A, et al. . Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fizazi K, Scher HI, Molina A, et al. . Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012;13:983–92. [DOI] [PubMed] [Google Scholar]
- 16. Ryan CJ, Smith MR, de Bono JS, et al. . Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan CJ, Smith MR, Fizazi K, et al. . Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16:152–60. [DOI] [PubMed] [Google Scholar]
- 18. Rathkopf DE, Smith MR, de Bono JS, et al. . Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol 2014;66:815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsubara N, Uemura H, Satoh T, et al. . A Phase 2 trial of abiraterone acetate in Japanese men with metastatic castration-resistant prostate cancer and without prior chemotherapy (JPN-201 Study). Jpn J Clin Oncol 2014;44:1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagai T, Naiki T, Iida K, et al. . Early abiraterone acetate treatment is beneficial in Japanese castration-resistant prostate cancer after failure of primary combined androgen blockade. Prostate Int 2017;6:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsubara N, Uemura H, Fukui I, et al. . Phase-1 study of abiraterone acetate in chemotherapy-naive Japanese patients with castration-resistant prostate cancer. Cancer Sci 2014;105:1313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Satoh T, Uemura H, Tanabe K, et al. . A phase 2 study of abiraterone acetate in Japanese men with metastatic castration-resistant prostate cancer who had received docetaxel-based chemotherapy. Jpn J Clin Oncol 2014;44:1206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abiraterone Acetate. Updated on 2015-07-29. Available from: https://www.pharmacodia.com/yaodu/html/v1/chemicals/d5c186983b52c4551ee00f72316c6eaa.html#JP.
- 24. Taplin ME, Montgomery B, Logothetis CJ, et al. . Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol 2014;32:3705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Efstathiou E, Li W, Gormley M, et al. . Biological heterogeneity in localized high-risk prostate cancer (LHRPC) from a study of neoadjuvant abiraterone acetate plus leuprolide acetate (LHRHa) versus LHRHa. J Clin Oncol 2015;33:5005. [Google Scholar]
- 26. Fizazi K, Tran N, Fein L, et al. . Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017;377:352–60. [DOI] [PubMed] [Google Scholar]
- 27. Rydzewska LHM, Burdett S, Vale CL, et al. . Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis. Eur J Cancer 2017;84:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fukagai T, Namiki TS, Carlile RG, Yoshida H, Namiki M. Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU Int 2006;97:1190–3. [DOI] [PubMed] [Google Scholar]
- 29. Namiki M, Ueno S, Kitagawa Y, Fukagai T, Akaza H. Effectiveness and adverse effects of hormonal therapy for prostate cancer: Japanese experience and perspective. Asian J Androl 2012;14:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Namiki M, Akaza H, Lee SE, et al. . Prostate Cancer Working Group report. Jpn J Clin Oncol 2010;40:i70–5. [DOI] [PubMed] [Google Scholar]
- 31. Fukagai T, Shimada M, Yoshida H, Namiki T, Carlile RG. Clinical-pathological comparison of clinical prostate cancer between Japanese Americans in Hawaii and Japanese living in Japan. Int J Androl 2000;23:43–4. [DOI] [PubMed] [Google Scholar]
- 32. Zhou W, Christiani DC. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer 2011;30:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Naito S, Tomita Y, Rha SY, et al. . Kidney Cancer Working Group Report. Jpn J Clin Oncol 2010;40:i51–6. [DOI] [PubMed] [Google Scholar]
- 34. Maskarinec G, Sen C, Koga K, Conroy SM. Ethnic differences in breast cancer survival: status and determinants. Women’s Health (Lond Engl) 2011;7:677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kamba T, Kamoto T, Maruo S, et al. . A phase III multicenter, randomized, controlled study of combined androgen blockade with versus without zoledronic acid in prostate cancer patients with metastatic bone disease: results of the ZAPCA trial. Int J Clin Oncol 2017;22:166–73. [DOI] [PubMed] [Google Scholar]
- 36. Moul JW. Twenty years of controversy surrounding combined androgen blockade for advanced prostate cancer. Cancer 2009;115:3376–8. [DOI] [PubMed] [Google Scholar]
- 37. Lukka H, Waldron T, Klotz L, Winquist E, Trachtenberg J. Maximal androgen blockade for the treatment of metastatic prostate cancer – a systematic review. Curr Oncol 2006;13:81–93. [PMC free article] [PubMed] [Google Scholar]
- 38. Arai Y, Akaza H, Deguchi T, et al. . Evaluation of quality of life in patients with previously untreated advanced prostate cancer receiving maximum androgen blockade therapy or LHRHa monotherapy: a multicenter, randomized, double-blind, comparative study. J Cancer Res Clin Oncol 2008;134:1385–96. [DOI] [PubMed] [Google Scholar]
- 39. Akaza H, Hinotsu S, Usami M, et al. . Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer 2009;115:3437–45. [DOI] [PubMed] [Google Scholar]
- 40. Gillessen S, Attard G, Beer TM, et al. . Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol 2018;73:178–211. [DOI] [PubMed] [Google Scholar]
- 41. Fizazi K, Tran N, Fein L, et al. Abiraterone Acetate Plus Prednisone 5 mg QD in Metastatic Castration-Naïve Prostate Cancer: Detailed Safety Analyses From the LATITUDE Phase 3 Trial. Poster presented at the 2018 Genitourinary Cancers Symposium, February 8–10, 2018, San Francisco, CA [Abstract 182]. 2018.
- 42. Chi KN, Protheroe A, Rodriguez-Antolin A, et al. . Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol 2018;19:194–206. [DOI] [PubMed] [Google Scholar]