Abstract
While orthostatic hypotension (OH) is often considered a contraindication to blood pressure (BP) treatment, evidence is lacking. We examined the effect of BP goal or initial medication choice on OH in AASK (African American Study of Kidney Disease), a 2×3 factorial trial. African Americans with chronic kidney disease (CKD) attributed to hypertension were randomly assigned one of two BP goals: intensive (mean arterial pressure [MAP]≤92mmHg) or standard (MAP 102–107mmHg), and one of three initial medications (ramipril, metoprolol, amlodipine). Postural changes in systolic BP (SBP), diastolic BP (DBP), or heart rate (HR) were determined after 2:45minutes of standing. OH was assessed each visit and defined using the consensus definition (drop in SBP≥20mmHg or DBP≥10mmHg). Median follow-up was 4-years. Outcomes were congestive heart failure, stroke, nonfatal cardiovascular disease (CVD), fatal CVD, any CVD (composite of preceding events), and all-cause mortality. There were 1,094 participants (mean age 54.5±10.7yrs; 38.8% female; OH was assessed at 52,864 visits). Mean seated SBP, DBP, and HR were 150.3±23.9mmHg, 95.5±14.2mmHg, and 72.0±12.6bpm. A more intensive BP goal did not alter the distributions of standing BP and was not associated with OH, but metoprolol was associated with systolic OH compared to ramipril (OR 1.68; 95%CI:1.15,2.46) and amlodipine (OR 1.94;95%CI:1.09,3.44). While consensus OH was associated with stroke (HR 5.01;95%CI:1.80,13.92), nonfatal CVD (HR 2.28;95%CI:1.21,4.30), and any CVD event (HR 2.12;95%CI:1.12,3.98), neither BP goal or medication altered this risk. Concerns about causing OH or its CVD consequences should not deter a lower BP goal among adults with CKD attributed to hypertension.
Keywords: trial, orthostatic hypotension, hypertension treatment, blood pressure, ramipril, metoprolol, amlodipine
Orthostatic hypotension (OH) is a predictor of syncope, stroke, cardiovascular disease (CVD), and early mortality.1–6 It is also an important risk factor for falls in both middle-aged adults1 and older adults with hypertension.7 OH has been associated with both blood pressure (BP) treatment8 and specific classes of anti-hypertension agents, such as alpha-1-blockers,9 diuretics,10,11 and beta-blockers.8,9,12 These concerns have led to cautionary warnings in recent national guidelines regarding the initiation of antihypertensive therapy in adults with a prior history of falls.13 Similar warnings have been voiced by other professional societies14,15 despite secondary analyses of BP trials demonstrating that more intensive BP goals are not associated with risk of OH.16,17 Furthermore, several studies have shown that the chronic treatment of hypertension with certain medications, especially calcium channel blockers and angiotensin converting enzyme inhibitors (ACEi), is associated with a reduction in OH or falls.18–20
AASK examined the effects of two mean arterial pressure (MAP) goals (102 to 107 mm Hg or ≤92 mm Hg) and three initial medication therapies (ramipril, metoprolol, or amlodipine) on kidney disease progression in African American adults with chronic kidney disease attributed to hypertension.21 Throughout the trial, participants were assessed for OH and followed for the development of CVD and mortality. During the trial phase of AASK, a lower BP goal had no impact on mortality or kidney disease progression, but ramipril was more effective than metoprolol or amlodipine in slowing decline in kidney function.22 However, the effects of these interventions on OH have not been reported.
In this secondary analysis of the trial phase of AASK, we had the following objectives: (1) determine the impact of BP treatment goal on OH and postural change in BP and heart rate (HR), (2) determine the effect of initial antihypertensive medication on OH and postural change in BP and HR, and (3) evaluate whether the relationship between postural change in BP or HR and CVD events or mortality was similar among subjects assigned different treatment goals or initial BP agents. We hypothesized that BP goal and choice of agent would not affect postural change in OH and would not influence the relationship between OH and CVD events.
Methods
Requests for data, analytic methods, and study materials should be directed to study authors.
Study Participants
As reported previously, AASK participants were self-reported African Americans, aged 18–70 years, with chronic kidney disease attributed to hypertension (glomerular filtration rate of 20 to 65 ml/min and DBP >95 mm Hg). Adults with diabetes (i.e. a fasting glucose >140 mg per deciliter, a random glucose >200 mg per deciliter, or diabetes treatment), a urinary protein-to-creatinine ratio of >2.5, malignant hypertension in the preceding 6 months, secondary hypertension, or heart failure were excluded from this study. Participants were recruited from 21 academic centers primarily based on case finding from medical chart reviews. Adults with treated hypertension were required to meet qualifying BP criteria at only one clinic visit, while untreated adults with hypertension were required to meet qualifying BP criteria during each of two consecutive clinic visits. The mean number of years with hypertension was over 10 years.23
Study Design
The AASK trial enrolled participants between February 1995 and September 1998. In this 2 × 3 factorial trial, 1,094 participants were randomized to one of two BP goals: intensive BP control (MAP ≤92 mm Hg) or standard control (MAP of 102–107 mm Hg). Participants were also randomized to one of three initial medication therapies: metoprolol (a sustained-release beta blocker), ramipril (an angiotensin-converting-enzyme inhibitor or ACEi), or amlodipine (a dihydropyridine calcium-channel blocker). As a result, it was possible to be randomized to 1 of 6 intervention assignments (intensive-ramipril, intensive-metoprolol, intensive-amlodipine, standard-ramipril, standard-metoprolol, or standard-amlodipine). To achieve assigned BP goals, the assigned medication was maximized first (based on tolerance and safety thresholds). If further therapy was necessary, other antihypertensive medications (furosemide, doxazosin, clonidine, and hydralazine or minoxidil) were sequentially added. Follow-up for the trial ended September 30th, 2001.21 The primary outcome of AASK included any of the following criteria: (1) a confirmed, 50%- reduction in glomerular filtration rate or a reduction by 25 mL/min per 1.73 m2 from baseline; (2) end stage renal disease (ESRD), defined as need for renal replacement therapy; or (3) death.
Orthostatic Hypotension
Seated and standing vital signs (SBP, DBP, & HR) were measured during pre-randomization and follow-up visits in research clinics. As a result, participants could have OH detected before or after allocation to the different treatment options. Pre-randomization visits were comprised of both screening visits and BP medication titration visits. There were, on average, 3–4 pre-randomization visits per participant. Follow-up visits occurred monthly for the first 6 months and then every 2 months for the trial duration.24 Follow-up visits were intermittently more frequent as needed for closer BP monitoring or medication titration. Participants were asked to refrain from exercise, caffeine, and smoking at least 30 minutes prior to and until completion of each BP measurement. Participants sat quietly for 5 minutes with their feet flat in an upright position. Then with one palm turned upward, radial pulses were counted via palpation for 30 seconds and multiplied by 2 to determine HR over a full minute. Afterward, three BP assessments were performed by certified, trained, observers using a Hawksley random zero sphygmomanometer. The mean seated, resting BP was determined based on the second two of three measurements.22
The participant was next asked to stand quietly for 2 minutes. The observer then raised the participant’s arm for 15 seconds and placed their arm on an adjacent table. HR was palpated for the ensuing 30 seconds and multiplied by 2 to convert to beats per minute. At 2 minutes and 45 seconds, the observer performed a single standing BP assessment.
Postural change in SBP, DBP, or HR from the seated to standing position was determined by taking the standing measure and subtracting the mean seated measure for SBP, DBP or HR. OH was defined using thresholds based on the original consensus definition, i.e. a decrease in either systolic or diastolic BP of at least 20 or 10 mm Hg, respectively.25,26 We also examined orthostatic tachycardia based on an increase in HR of at least 20 beats per minute (bpm), using a clinical definition.27 OH was defined by its individual components as well as using each of two composite definitions: the consensus definition (either SBP or DBP) or a clinical definition based on either OH or orthostatic tachycardia (met at least one of the three criteria based on SBP, DBP, or HR).
Clinical Events
During the trial, participants were followed for death as well as hospitalizations for CVD (e.g. myocardial infarction, stroke, heart failure, and revascularization). All hospitalizations were reviewed for CVD events based on a prespecified protocol by the Cardiovascular Outcomes Committee (blinded to randomization assignment). See Supplement Figure S1 for the original adjudication form. Events were reviewed by 1–3 committee members. In the present study, events were considered definite or probable if at least 1 (in cases of 1 or 2 adjudicators) or 2 (in cases of 3 adjudicators) determined that the event met the qualifying criteria. CV outcomes of interest were: (1) CHF, (2) stroke, (3) nonfatal CVD, (4) fatal CVD, and (5) any CVD event.
CHF was defined as hospitalization for CHF or a questionable CHF hospitalization (1 of 3 adjudicators) with use of related therapy, namely, an ionotropic agent, vasodilator, ACE inhibitor, increased dose of diuretic, ultrafiltration, or dialysis. Stroke was defined by clinical report of a permanent neurological deficit lasting at least 24 hours in duration, or a questionable clinical report (1 of 3 adjudicators) with either hospitalization related to stroke or confirmation by radiographic imaging. Nonfatal CVD events were based on a clinical report of a myocardial infarction or a cardiac revascularization procedure without evidence of death. Fatal CVD was determined based on chart review of all deaths by the adjudication committee. Any CVD event was defined as any CHF, stroke, nonfatal CVD, or fatal CVD events based on the definitions above. In addition, we included any events that met the trial’s CVD criteria for a secondary outcome, a tertiary outcome, or a CVD hospitalization (see item 11 in Supplement Figure S1). We also examined all-cause mortality (mortality from any cause). As ESRD was one of the primary outcomes of the AASK trial, follow-up ended with ESRD (except for mortality). Participant hospitalizations were not tracked post-ESRD.
Other covariates
Body mass index (BMI) was determined from standardized measurements of height and weight. Creatinine and glucose were measured in serum collected prior to randomization using standard assays.21 Baseline SBP, DBP, or HR was the first recorded value for each participant who was enrolled in the study.
Statistical Analysis
Study population characteristics were described using means (SD) and proportions, overall and by the two BP goals and by the three initial medications. Kernel density plots were used to model the distribution of SBP, DBP, or HR in seated or standing positions as well as the distribution of postural change between position (standing minus seated measures) by baseline values (pre-randomization visits) and BP goal as well as by initial antihypertensive medication.
Generalized estimating equations (normal family, identity link, exchangeable correlation structure) with a robust variance estimator were used to determine the effect of BP goal (intensive vs standard) or initial medication assignment (metoprolol versus ramipril, amlodipine versus ramipril, or metoprolol versus amlodipine) on SBP, DBP, or HR in seated or standing positions as well as on postural change, i.e. standing minus seated measures. Generalized estimating equations (binomial family, logit link, exchangeable correlation structure) were also used to examine the effect of BP goal (intensive vs standard) or initial medication assignment (metoprolol versus ramipril, amlodipine versus ramipril, or metoprolol versus amlodipine) on OH defined by postural change in SBP, DBP, or HR alone, OH defined by the consensus definition (postural change in SBP or DBP), or OH defined by a composite definition (postural change in SBP, DBP, or HR). In the models above, the multiple follow-up visits were treated equally regardless of post-randomization timing. Both BP goal and initial antihypertensive medication assignments were included in these models. Note that all GEE models use a Huber/White/sandwich estimator of variance that does not rely on correct specification of the within-group correlation matrix allowing for conservative and yet valid variance estimation.28
We modeled the association of postural change in SBP, DBP, or HR with CVD outcomes (CHF, stroke, nonfatal CVD, fatal CVD, any CVD) or all-cause mortality using Cox proportional hazards models adjusted for age and sex. Models of BP goal were further adjusted for initial medication, while models of initial medication were further adjusted for BP goal. Analyses involving the overall population were adjusted for both BP goal and initial medication. Postural change was treated as a time-varying covariate, varying at each follow-up visit. In cases of missing visits, the prior BP measurement was carried forward. The association between postural change in SBP, DBP, and HR were compared overall, across BP goals, and across initial medication assignments. Interaction terms were used to determine whether the associations differed by BP group or initial antihypertensive medication (metoprolol versus ramipril, amlodipine versus ramipril, or metoprolol versus amlodipine). We also compared F-statistics via Wald tests to evaluate the addition of all initial antihypertensive medication interaction terms overall. OH definitions were only examined overall (versus by BP goal or medication assignment) in sensitivity analyses, due to limited numbers of events between treatment groups.
All analyses were conducted with STATA version 14.0 (Stata Corporation, College Station, TX, USA).
Results
Population Characteristics
Of the 1,094 trial participants, mean age was 54.5 yr (SD, 10.7) and 38.8% were women (Table 1). The mean SBP was 150.3 (SD, 23.9) mm Hg, DBP was 95.5 (14.2), and HR was 72.0 (SD, 12.6). Mean BMI was 30.6 kg/m2 (SD, 6.6), mean serum creatinine was 2.0 (SD, 0.7) mg/dL, and serum glucose was 95.0 (SD, 18.5) mg/dL. Population characteristics were similar across randomized groups. Three participants dropped out prior to the first follow-up visit, and there was one death before the first follow-up visit. The 1,090 participants with follow-up visits participated in 3,946 pre-randomization visits and 48,918 post-randomization visits. During pre-randomization visits, 35 participants had OH based on SBP (detected in 43 visits), 30 had OH based on DBP (35 visits), 108 had orthostatic tachycardia based on HR (136 visits), 58 had OH based on the consensus definition (136 visits), and 156 had OH based on any one of the three criteria (203 visits). Similarly, during post-randomization visits there were 258 participants with OH based on SBP (672 visits), 216 with OH based on DBP (459 visits), 581 with orthostatic tachycardia based on HR (1,743 visits), 344 with OH based on the consensus definition (935 visits), and 709 with OH based on any one of the 3 criteria (2,621 visits).
Table 1.
Characteristics of participants enrolled in the AASK trial (N = 1,094), mean (SD) or %
| Blood Pressure Goal |
Initial Medication |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Overall, N = 1,094 | Intensive Goal, N = 540 | Standard Goal, N = 554 | Ramipril, N = 436 | Metoprolol, N = 441 | Amlodipine, N = 217 |
| Age, yr | 54.5 (10.7) | 54.4 (10.9) | 54.5 (10.4) | 54.2 (10.9) | 54.8 (10.4) | 54.3 (10.7) |
| Female, % | 38.8 | 38.1 | 39.5 | 38.8 | 38.5 | 39.6 |
| Mean SBP, mm Hg | 150.3 (23.9) | 151.6 (25.0) | 149.0 (22.6) | 151.0 (23.3) | 149.8 (23.7) | 150.0 (25.3) |
| Mean DBP, mm Hg | 95.5 (14.2) | 96.3 (14.7) | 94.8 (13.7) | 96.0 (14.5) | 95.0 (14.0) | 95.7 (14.1) |
| Mean heart rate, mm Hg | 72.0 (12.6) | 72.3 (12.4) | 71.8 (12.8) | 72.3 (11.7) | 71.6 (12.8) | 72.3 (13.8) |
| Body mass index, kg/m2 | 30.6 (6.6) | 30.5 (6.7) | 30.6 (6.5) | 30.6 (6.3) | 30.9 (6.9) | 29.8 (6.3) |
| Serum creatinine, mg/dL | 2.0 (0.7) | 2.0 (0.7) | 2.0 (0.7) | 2.0 (0.7) | 2.0 (0.7) | 2.1 (0.8) |
| Serum glucose, mg/dL | 95.0 (18.5) | 95.5 (19.4) | 94.5 (17.5) | 94.5 (17.7) | 96.0 (18.8) | 93.8 (19.2) |
| Total number of visits, N | 52,877 | 26,810 | 26,067 | 21,395 | 21,396 | 10,086 |
| Pre-randomization N of visits per participant, mean (SD) | 3.6 (1.2) | 3.6 (1.2) | 3.6 (1.3) | 3.6 (1.1) | 3.7 (1.2) | 3.6 (1.5) |
| Post-randomization N of visits per participant, mean (SD) | 44.9 (21.2) | 46.3 (21.4) | 43.5 (21.0) | 45.6 (22.8) | 44.8 (20.1) | 43.5 (20.2) |
Note: These characteristics values represent the earliest visit of participants enrolled in the study (almost always the screening visit). Participants had on average 3.6 visits (screening visits or blood pressure medication titration visits) during the period preceding randomization.
Absolute Levels of Postural Change in SBP, DBP, or HR
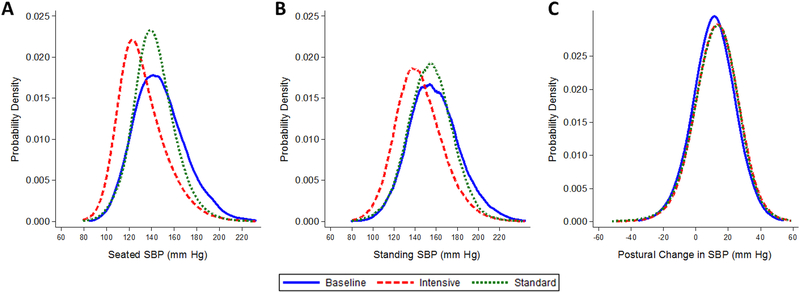
We examined the distribution of SBP measured during pre-randomization visits and by BP goal measured during post-randomization visits (Figure 1A-C). There was a leftward (downward) shift in the distribution of SBP from pre-randomization with a greater shift observed among those assigned the intensive BP goal. Similar shifts in SBP distributions were observed after 2:45 minutes of standing. The postural change between standing and seated SBP measurements was nearly identical pre-randomization and during follow-up in the intensive and standard BP goal groups. Findings were similar for DBP and HR with virtually identical differences in postural change during pre-randomization visits and during follow-up in the intensive and standard BP groups (Supplement Figure S2A-C & S3A-C).
Figure 1.
Kernel density plots of the distributions of (A) seated systolic blood pressure (mm Hg), (B) standing systolic blood pressure, and (C) postural change between standing and seated systolic blood pressure (mm Hg) at baseline (i.e. pre-randomization) (solid line) and during follow-up in participants assigned the intensive blood pressure goal (mean arterial pressure or MAP ≤92 mm Hg) (dash line) and in participants assigned the standard BP goal (MAP between 102–107 mm Hg) (dotted line). There were 3,946 baseline visits, 24,844 post-randomization visits among participants assigned the intensive BP goal, and 24,074 post-randomization visits among participants assigned the standard BP goal.
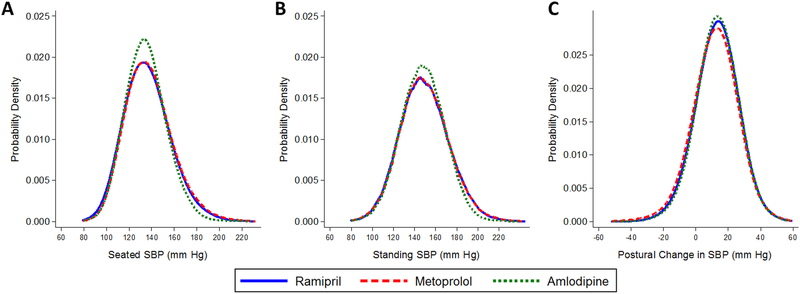
Distributions of SBP measured during follow-up were examined by the three randomized initial medication assignments (Figure 2A-C). Both seated and standing SBP measures were similar across medication classes. This was also observed for DBP and HR (Supplement Figure S4A-C and S5A-C).
Figure 2.
Kernel density plots of the distribution of (A) average seated systolic blood pressure (mm Hg), (B) standing systolic blood pressure, and (C) the difference between standing and seated systolic blood pressure (mm Hg) measured during follow-up visits according to initial antihypertensive medication: ramipril (solid line), metoprolol (dash line), or amlodipine (dotted line). There were 19,843 post-randomization visits among participants assigned ramipril, 19,774 post-randomization visits among participants assigned metoprolol, and 9,301 post-randomization visits among participants assigned amlodipine.
We examined the effect of treatment goal on the difference in postural change for seated and standing SBP, DBP, and HR during follow-up (Supplement Table S1). Compared to the standard goal, the intensive goal further lowered seated SBP by −10.28 mm Hg and standing SBP by −9.97 mm Hg, with no significant difference between the two measures (Δ = 0.31 mm Hg; 95% CI: −0.49, 1.12). Similarly, compared to the standard group, the intensive goal further lowered seated DBP by −6.46 mm Hg and standing DBP by −5.84 mm Hg with a significant difference (Δ = 0.61 mm Hg; 95% CI: 0.02, 1.20). There was no difference in HR (seated, standing, or the difference) between participants assigned to either intensive or standard BP goal.
Metoprolol was associated with a small drop in SBP after standing compared with ramipril (−1.11 mm Hg; 95% CI: −2.02,−0.20), but there was no difference in corresponding postural changes in SBP between metoprolol and amlodipine and between amlodipine and ramipril (Supplement Table S1). There was no difference in DBP upon standing between metoprolol, ramipril, or amlodipine. In contrast, there were greater drops in HR after standing from metoprolol versus ramipril (−1.50 bpm; 95% CI: −1.87,−1.14) and versus amlodipine (−1.30 bpm; 95% CI: −1.74,−0.85). Postural HR change did not differ between amlodipine and ramipril.
Relationships of Orthostatic Hypotension with Blood Pressure Goal and Medication Assignment
Intensive versus standard BP goal was not associated with any of the individual components of OH nor composite definitions (Table 2). Conversely, metoprolol versus ramipril was associated with higher odds of OH based on SBP (OR 1.71; 95% CI: 1.15, 2.53) and consensus OH (OR 1.58; 95% CI: 1.14, 2.20). Metoprolol was also associated with higher odds of OH based on SBP versus amlodipine (OR 1.88; 95% CI: 1.04, 3.14). As expected, metoprolol was inversely associated with orthostatic tachycardia.
Table 2.
Odds of orthostatic hypotension or orthostatic tachycardia by contrasts in blood pressure goal and initial medication, N = 1,090 (number of post-randomization visits was 48,918)
| OR (95% CI) |
|||||
|---|---|---|---|---|---|
| Contrast | Drop in SBP of ≥20 mm Hg, N = 715 visits | Drop in DBP of ≥10 mm Hg, N= 494 visits | Rise in HR of ≥20 beats per minute, N = 1,879 visits | Consensus Definition: Either BP Criteria, N = 1,005 visits | Any Criteria (SBP, DBP, or HR), N = 2,824 visits |
| Intensive vs Standard | 0.84 (0.58, 1.22) | 0.89 (0.60, 1.30) | 1.10 (0.90, 1.35) | 0.93 (0.68, 1.28) | 1.05 (0.88, 1.26) |
| Metoprolol vs Ramipril | 1.71 (1.15, 2.53)* | 1.43 (0.96, 2.15) | 0.44 (0.35, 0.55)* | 1.58 (1.14, 2.20)* | 0.72 (0.59, 0.88)* |
| Amlodipine vs Ramipril | 0.91 (0.50, 1.65) | 1.26 (0.74, 2.14) | 0.96 (0.74, 1.23) | 1.05 (0.65, 1.68) | 0.97 (0.76, 1.23) |
| Metoprolol vs Amlodipine | 1.88 (1.04, 3.41)* | 1.14 (0.65, 1.99) | 0.46 (0.35, 0.60)* | 1.51 (0.93, 2.44) | 0.74 (0.58, 0.96) |
Note: Participants were assigned to one of six interventions: intensive-ramipril, intensive-metoprolol, intensive-amlodipine, standard-ramipril, standard-metoprolol, or standard-amlodipine. Models were adjusted for initial medication in the blood pressure goal comparisons, and adjusted for blood pressure goal in the initial medication comparisons.
P < 0.05
Postural Change in SBP, DBP, or HR and Clinical Events
The median follow-up time for each outcome was 4 years. Overall, for each 10 mm Hg drop in SBP after standing, there was a higher risk of stroke (HR 1.38; 95% CI: 1.14, 1.66), nonfatal CVD (HR 1.23; 95% CI: 1.13, 1.34), and any CVD (HR 1.21; 95% CI: 1.11, 1.32) (Table 3). We observed a borderline non-significant interaction between intensive and standard BP goals with regards to drop in SBP after standing and stroke (HR was 1.71 intensive vs 1.17 standard; P = 0.054). For each 10 mm Hg drop in DBP after standing, there was a higher risk of nonfatal CVD (HR 1.17; 95% CI: 1.04, 1.30) and any CVD (HR 1.14; 95% CI: 1.02, 1.27). For each 10 bpm increase in HR after standing, there was a higher risk of all-cause mortality (HR 1.32; 95% CI: 1.04, 1.67). Similar to SBP above, there were no significant interactions between BP treatment goal and outcomes for postural change in DBP or HR. In a sensitivity analysis examining OH components in the overall study population, OH based on SBP, DBP, or the consensus definition for OH (based on SBP or DBP) were strongly associated with 4–5 times the risk of stroke (Supplement Table S2). OH based on SBP or the consensus definition for OH (based on SBP or DBP) were also associated with nonfatal CVD and any CVD. The composite definition of OH or orthostatic tachycardia was associated with nonfatal CVD. None of the definitions were associated with CHF, fatal CVD, or all-cause mortality.
Table 3.
Association of postural change in systolic blood pressure, diastolic blood pressure, or heart rate with clinical events overall and by blood pressure goal assignment
| Outcome | HR (95% CI) |
|||
|---|---|---|---|---|
| Overall, N = 1090* | Intensive, N = 537* | Standard, N = 553* | P-interaction | |
| Congestive heart failure, 56/1088 | ||||
| SBP per −10 mm Hg | 1.16 (0.95, 1.42) | 1.22 (0.91, 1.64) | 1.12 (0.85, 1.47) | 0.66 |
| DBP per −10 mm Hg | 1.07 (0.82, 1.39) | 1.05 (0.73, 1.52) | 1.08 (0.75, 1.56) | 0.95 |
| HR per 10 bpm | 1.01 (0.70, 1.46) | 0.97 (0.58, 1.62) | 1.04 (0.63, 1.73) | 0.82 |
| Stroke, 56/1090 | ||||
| SBP per −10 mm Hg | 1.38 (1.14, 1.66)† | 1.71 (1.30, 2.24)† | 1.17 (0.91, 1.52) | 0.054 |
| DBP per −10 mm Hg | 1.23 (0.96, 1.59) | 1.56 (1.07, 2.28)† | 1.04 (0.73, 1.48) | 0.12 |
| HR per 10 bpm | 1.11 (0.78, 1.59) | 0.83 (0.51, 1.34) | 1.36 (0.92, 2.03) | 0.15 |
| Nonfatal CVD, 286/1078 | ||||
| SBP per −10 mm Hg | 1.23 (1.13, 1.34)† | 1.25 (1.11, 1.42)† | 1.21 (1.07, 1.37)† | 0.69 |
| DBP per −10 mm Hg | 1.17 (1.04, 1.30)† | 1.09 (0.94, 1.28) | 1.25 (1.06, 1.47)† | 0.28 |
| HR per 10 bpm | 1.06 (0.90, 1.24) | 1.01 (0.81, 1.26) | 1.11 (0.88, 1.39) | 0.73 |
| Fatal CVD, 30/1090 | ||||
| SBP per −10 mm Hg | 0.99 (0.75, 1.31) | 0.85 (0.58, 1.25) | 1.13 (0.76, 1.67) | 0.33 |
| DBP per −10 mm Hg | 0.94 (0.66, 1.34) | 0.70 (0.43, 1.12) | 1.30 (0.77, 2.17) | 0.09 |
| HR per 10 bpm | 1.19 (0.78, 1.82) | 1.36 (0.70, 2.64) | 1.03 (0.56, 1.88) | 0.59 |
| Any CVD (combined classification or any), 308/1078 | ||||
| SBP per −10 mm Hg | 1.21 (1.11, 1.32)† | 1.22 (1.08, 1.37)† | 1.21 (1.07, 1.36)† | 0.88 |
| DBP per −10 mm Hg | 1.14 (1.02, 1.27)† | 1.04 (0.89, 1.21) | 1.27 (1.08, 1.49)† | 0.08 |
| HR per 10 bpm | 1.07 (0.92, 1.24) | 1.02 (0.82, 1.26) | 1.11 (0.90, 1.37) | 0.69 |
| Mortality (from any cause), 95/1090 | ||||
| SBP per −10 mm Hg | 1.10 (0.95, 1.28) | 1.12 (0.89, 1.43) | 1.09 (0.89, 1.32) | 0.75 |
| DBP per −10 mm Hg | 1.16 (0.95, 1.41) | 1.07 (0.79, 1.45) | 1.23 (0.95, 1.59) | 0.55 |
| HR per 10 bpm | 1.32 (1.04, 1.67)† | 1.43 (0.95, 2.15) | 1.26 (0.94, 1.68) | 0.67 |
Overall models were adjusted for age, sex, blood pressure goal, and initial medication. Models within strata of BP goal were adjusted for initial medication.
Of the original 1094 randomized, 4 did not attend any follow-up visits. Denominators vary based on nonfatal events that occurred prior to follow-up visits.
P < 0.05
Note: CVD represents cardiovascular disease.
We evaluated whether postural change in SBP, DBP, or HR were associated with clinical events by initial medication (ramipril, metoprolol, amlodipine) and whether there was effect modification (Supplement Table S3). In general, there was little difference between initial medications.
Discussion
In this trial of African American adults with chronic kidney disease attributed to hypertension, the intensive BP goal did not affect postural change in SBP, DBP, or HR. Use of metoprolol as an initial medication compared with either ramipril or amlodipine increased the odds of having OH defined by SBP, and reduced the odds of OH by HR, although the absolute effects of metoprolol on SBP and HR were quite small. While postural changes in SBP or DBP were associated with CVD events, BP goal or initial medication had minimal impact on these associations.
BP treatment has been associated with OH in some observational studies,8,29 while others report no association.7,18,30 Recently, trials of treatment goal have not shown that more aggressive BP treatment increases risk of OH.16,17 In a secondary analysis of the ACCORD trial, a SBP goal of <120 mm Hg versus <140 mm Hg was not associated with OH (OR 0.93; 95% CI: 0.80, 1.07). Similarly, in the SPRINT trial, a SBP goal of <120 mm Hg versus <140 mm Hg actually lowered the risk of OH (HR 0.88; P = 0.01), despite increasing the risk of hypotensive episodes and syncope.17 Our study similarly demonstrates that BP goal (MAP ≤92 mm Hg versus MAP of 102–107 mm Hg) had no effect on OH after 2:45 minutes of standing. It is possible that BP treatment impacts severity of drop in BP immediately after standing as reported by others;12 however, AASK was not designed to address this issue. It should also be noted that some guidelines define OH as a standing SBP < 90 mm Hg. While this was not a focus of the current report, the leftward shift in the distribution of standing SBP in the intensive group, was consistent with a higher prevalence of SBP < 90 mm Hg.
In this study, metoprolol compared with ramipril or amlodipine was associated with higher odds of having OH based on definitions that included SBP (but not heart rate). This is consistent with prior literature on beta blockers and OH.8,9,12 It should be noted that the association between postural change in SBP, DBP, or HR with CVD events did not differ among those with metoprolol compared to ramipril or amlodipine. Beta-blockers reduce the heart’s ability to augment HR and cardiac output in response to gravity-induced shifts in intravascular volume, causing greater drops in BP upon standing.31 The fact that metoprolol, and more broadly beta-blockers as a class, influence postural changes in SBP and HR in differing directions is important for the identification of OH in clinical practice.
Despite the many studies showing a relationship between OH and CVD events3,5,6,32–35 as well as CVD mortality,6,33,36,37 several studies do not support this association.38–41 In fact, while the ACCORD trial found OH to be associated with death and heart failure, it was not associated with atherosclerotic events.16 Despite the limited number of CVD events in AASK, our study demonstrated significant associations between postural change in SBP and DBP with nonfatal CVD or any CVD event. Further, postural change in SBP was significantly associated with a higher risk of stroke. However, we did not find significant associations with mortality, fatal CVD events, or CHF.
Our study has several limitations. First, this trial was limited to African American adults with chronic kidney disease attributed to hypertension. Most were middle age. Adults with other diseases associated with OH, e.g. Parkinson’s disease or diabetes, were not enrolled in this study. Also, it should be noted that many participants were obese, which may affect generalizability. Second, subtypes of cardiovascular events (e.g. fatal CVD, stroke, or CHF) were few, which limited power to study the association of OH with clinical outcomes. Third, the OH protocol relied on a single measurement performed 2:45 minutes after participants went from seated (versus supine) to standing. Details related to delays in the standing procedure, the number of measurement attempts, or the actual time that measurements resulted were not recorded. This could contribute to missed OH. Recent studies have demonstrated value from early measurements of OH initiated immediately after standing.2,42–45 Furthermore, measuring OH from a seated position may have blunted the full gravitational effect of posture change and possibly reduced our ability to detect OH, resulting in misclassification. Fourth, we did not have details related to falls or syncopal events, both at enrollment or during follow-up, which are important outcomes related to OH. As a result, we are unable to comment as to the effects of BP goal on these important clinical outcomes based on this study. These events could represent a disadvantage to more intensive treatment if intensive therapy were found to cause these events. Fifth, this study was concluded in 2001. However, the data while old still are extremely relevant given renewed interest in lower BP goals and the common use of most medications, particularly ramipril and amlodipine. Finally, the association between OH with CVD is derived from an observational analysis with the potential for confounding.
This study has several strengths. First, the randomized design of the AASK study allowed us to evaluate whether treatment goal or initial BP medication caused OH or altered the association of postural change in SBP, DBP, or HR with clinical events. Second, our study included 4 years of follow-up and an adjudication process, which minimized misclassification of events. Third, OH measurements were obtained via a standardized protocol with rigorously trained staff, minimizing imprecision and bias. Last, the many visits in this study resulted in 52,864 OH assessments, including measurements before and during follow-up, which allowed for OH to vary over the follow-up period in our models. This large number of repeat measurements is rarely available in other datasets.
Perspectives
This study has important implications. Our study extends prior reports by showing that a more intensive BP goal does not cause OH after ~3 minutes. This finding is especially important given that the recent ACC/AHA guidelines recommend a more intensive BP goal in many patients with hypertension.13 Second, this study illustrates how the presence of OH may differ by initial BP medication (in this case metoprolol); importantly, the relationship of OH to CVD effects did not differ by initial medication.
In conclusion, findings from our study should further mitigate concerns that an intensive BP goal might cause OH. Replication of our study is warranted in other populations, e.g. older persons, non-African-Americans, persons with diabetes, and persons without CKD.
Supplementary Material
What Is New?
A lower blood pressure treatment goal does not increase risk of orthostatic hypotension. Initial use of beta blockers to lower blood pressure does increase orthostatic hypotension without affecting long-term cardiovascular sequelae.
What Is Relevant?
There are concerns about adverse effects of lower blood pressure treatment goals. Current guidelines consider orthostatic hypotension a reason to down-titrate antihypertensive therapy.
Summary
Concerns that blood pressure treatment might cause orthostatic hypotension or its cardiovascular consequences should not deter a lower blood pressure goal among adults with kidney disease attributed to hypertension.
ACKNOWLEDGMENTS
Sources of Funding: SPJ is supported by a NIH/ NHLBI 7K23HL135273–02. The original AASK study was supported by grants to each clinical center and the coordinating center from the National Institute of Diabetes and Digestive and Kidney Diseases; by the Office of Research in Minority Health (now the National Center on Minority Health and Health Disparities); by institutional grants from the National Institutes of Health (M01 RR-00080, M01 RR-00071, M0100032, P20-RR11145, M01 RR00827, M01 RR00052, 2P20 RR11104, RR029887, and DK 2818–02); by King Pharmaceuticals, which provided monetary support and antihypertensive medications to each clinical center; and by Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn, which donated antihypertensive medications. The authors thank the staff and participants of the AASK study for their important contributions.
This research was also supported by grants R01 AG041785 and R01 AG025037 to Dr. Lipsitz from the National Institute on Aging. Dr. Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife, Boston, MA.
Abbreviations used:
- AASK
African American Study of Kidney Disease and Hypertension
- OH
orthostatic hypotension
- BP
blood pressure
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- HR
heart rate or hazard ratio
- CVD
cardiovascular disease
- CHF
congestive heart failure
- OR
odds ratio
- CI
confidence interval
Footnotes
DISCLOSURE
The authors declare that there is no conflict of interest associated with this manuscript.
This trial is registered at clinicaltrials.gov, number: NCT01206062
References
- 1.Juraschek SP, Daya N, Appel LJ, Miller ER, Windham BG, Pompeii L, Griswold ME, Kucharska-Newton A, Selvin E. Orthostatic Hypotension in Middle-Age and Risk of Falls. Am J Hypertens. 2017;30:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juraschek SP, Daya N, Rawlings AM, Appel LJ, Miller ER, Windham BG, Griswold ME, Heiss G, Selvin E. Association of History of Dizziness and Long-term Adverse Outcomes With Early vs Later Orthostatic Hypotension Assessment Times in Middle-aged Adults. JAMA Intern Med 2017;177:1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19:508–519. [DOI] [PubMed] [Google Scholar]
- 4.Yatsuya H, Folsom AR, Alonso A, Gottesman RF, Rose KM. Postural changes in blood pressure and incidence of ischemic stroke subtypes: the ARIC study. Hypertension. 2011;57:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J. 2010;31:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juraschek SP, Daya N, Appel LJ, Miller ER, McEvoy JW, Matsushita K, Ballantyne CM, Selvin E. Orthostatic Hypotension and Risk of Clinical and Subclinical Cardiovascular Disease in Middle‐Aged Adults. Journal of the American Heart Association. 2018;7:e008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamaruzzaman S, Watt H, Carson C, Ebrahim S. The association between orthostatic hypotension and medication use in the British Women’s Heart and Health Study. Age Ageing. 2010;39:51–56. [DOI] [PubMed] [Google Scholar]
- 9.Svetkey LP, Brobyn R, Deedwania P, Graham R, Morganroth J, Klotman P. Double-blind comparison of doxazosin, nadolol and placebo in patients with mild-to-moderate hypertension. Curr Ther Res. 1988;43:969–978. [Google Scholar]
- 10.Heseltine D, Bramble MG. Loop diuretics cause less postural hypotension than thiazide diuretics in the frail elderly. Curr Med Res Opin. 1988;11:232–235. [DOI] [PubMed] [Google Scholar]
- 11.Myers MG, Kearns PM, Kennedy DS, Fisher RH. Postural hypotension and diuretic therapy in the elderly. Can Med Assoc J. 1978;119:581–585. [PMC free article] [PubMed] [Google Scholar]
- 12.Canney M, O’Connell MDL, Murphy CM, O’Leary N, Little MA, O’Seaghdha CM, Kenny RA. Single Agent Antihypertensive Therapy and Orthostatic Blood Pressure Behaviour in Older Adults Using Beat-to-Beat Measurements: The Irish Longitudinal Study on Ageing. PLoS ONE. 2016;11:e0146156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 14.KDIGO. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease [Internet] 2013. [cited 2017 Dec 11]; Available from: http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf [DOI] [PubMed]
- 15.Moyer VA US Preventive Services Task Force. Prevention of falls in community-dwelling older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:197–204. [DOI] [PubMed] [Google Scholar]
- 16.Fleg JL, Evans GW, Margolis KL, Barzilay J, Basile JN, Bigger JT, Cutler JA, Grimm R, Pedley C, Peterson K, Pop-Busui R, Sperl-Hillen J, Cushman WC. Orthostatic Hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) Blood Pressure Trial: Prevalence, Incidence, and Prognostic Significance. Hypertension. 2016;68:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuo K, Mikami H, Ogihara T, Tuck ML. Changes in frequency of orthostatic hypotension in elderly hypertensive patients under medications. American Journal of Hypertension. 1996;9:263–268. [DOI] [PubMed] [Google Scholar]
- 19.Lipsitz LA, Habtemariam D, Gagnon M, Iloputaife I, Sorond F, Tchalla AE, Dantoine TF, Travison TG. Reexamining the Effect of Antihypertensive Medications on Falls in Old Age. Hypertension. 2015;66:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahlaee HR, Latt MD, Schneider CR. Association Between Chronic or Acute Use of Antihypertensive Class of Medications and Falls in Older Adults. A Systematic Review and Meta-Analysis. Am J Hypertens. 2018;31:467–479. [DOI] [PubMed] [Google Scholar]
- 21.Appel LJ, Wright JT, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X, AASK Collaborative Research Group. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright JT, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG, African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. [DOI] [PubMed] [Google Scholar]
- 23.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, Douglas M, Dowie D, Faulkner M, Gabriel A, Gassman J, Greene T, Hall Y, Hebert L, Hiremath L, Jamerson K, Johnson CJ, Kopple J, Kusek J, Lash J, Lea J, Lewis JB, Lipkowitz M, Massry S, Middleton J, Miller ER, Norris K, O’Connor D, Ojo A, Phillips RA, Pogue V, Rahman M, Randall OS, Rostand S, Schulman G, Smith W, Thornley-Brown D, Tisher CC, Toto RD, Wright JT, Xu S, African American Study of Kidney Disease and Hypertension (AASK) Study Group . Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285:2719–2728. [DOI] [PubMed] [Google Scholar]
- 24.Juraschek SP, Miller ER, Appel LJ. Orthostatic Hypotension and Symptoms in the AASK Trial. Am J Hypertens. 2018;31:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. [DOI] [PubMed] [Google Scholar]
- 26.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy The Consensus Committee of the American Autonomic Society and the American Academy of Neurology; Neurology. 1996;46:1470. [DOI] [PubMed] [Google Scholar]
- 27.Emergency Nurses Association. Clinical Practice Guideline: Orthostatic Vital Signs. 2015;
- 28.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 29.Fotherby MD, Potter JF. Orthostatic hypotension and anti-hypertensive therapy in the elderly. Postgraduate Medical Journal. 1994;70:878–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fedorowski A, Burri P, Melander O. Orthostatic hypotension in genetically related hypertensive and normotensive individuals. J Hypertens. 2009;27:976–982. [DOI] [PubMed] [Google Scholar]
- 31.Ricci F, De Caterina R, Fedorowski A. Orthostatic Hypotension: Epidemiology, Prognosis, and Treatment. J Am Coll Cardiol. 2015;66:848–860. [DOI] [PubMed] [Google Scholar]
- 32.Fan X-H, Wang Y, Sun K, Zhang W, Wang H, Wu H, Zhang H, Zhou X, Hui R. Disorders of orthostatic blood pressure response are associated with cardiovascular disease and target organ damage in hypertensive patients. Am J Hypertens. 2010;23:829–837. [DOI] [PubMed] [Google Scholar]
- 33.Verwoert GC, Mattace-Raso FUS, Hofman A, Heeringa J, Stricker BHC, Breteler MMB, Witteman JCM. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc. 2008;56:1816–1820. [DOI] [PubMed] [Google Scholar]
- 34.Fedorowski A, Hedblad B, Melander O. Early postural blood pressure response and cause-specific mortality among middle-aged adults. Eur J Epidemiol. 2011;26:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose KM, Tyroler HA, Nardo CJ, Arnett DK, Light KC, Rosamond W, Sharrett AR, Szklo M. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens. 2000;13:571–578. [DOI] [PubMed] [Google Scholar]
- 36.Rose KM, Eigenbrodt ML, Biga RL, Couper DJ, Light KC, Sharrett AR, Heiss G. Orthostatic hypotension predicts mortality in middle-aged adults: the Atherosclerosis Risk In Communities (ARIC) Study. Circulation. 2006;114:630–636. [DOI] [PubMed] [Google Scholar]
- 37.Ricci F, Fedorowski A, Radico F, Romanello M, Tatasciore A, Di Nicola M, Zimarino M, De Caterina R. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J. 2015;36:1609–1617. [DOI] [PubMed] [Google Scholar]
- 38.Veronese N, De Rui M, Bolzetta F, Zambon S, Corti MC, Baggio G, Toffanello ED, Maggi S, Crepaldi G, Perissinotto E, Manzato E, Sergi G. Orthostatic Changes in Blood Pressure and Mortality in the Elderly: The Pro.V.A Study. Am J Hypertens. 2015;28:1248–1256. [DOI] [PubMed] [Google Scholar]
- 39.Hossain M, Ooi WL, Lipsitz LA. Intra-individual postural blood pressure variability and stroke in elderly nursing home residents. J Clin Epidemiol. 2001;54:488–494. [DOI] [PubMed] [Google Scholar]
- 40.Fedorowski A, Wahlstrand B, Hedner T, Melander O. Systolic and diastolic component of orthostatic hypotension and cardiovascular events in hypertensive patients: the Captopril Prevention Project. J Hypertens. 2014;32:75–81. [DOI] [PubMed] [Google Scholar]
- 41.Chou R-H, Liu C-J, Chao T-F, Chen S-J, Tuan T-C, Chen T-J, Chen S-A. Association between orthostatic hypotension, mortality, and cardiovascular disease in Asians. Int J Cardiol. 2015;195:40–44. [DOI] [PubMed] [Google Scholar]
- 42.Räihä I, Luutonen S, Piha J, Seppänen A, Toikka T, Sourander L. Prevalence, predisposing factors, and prognostic importance of postural hypotension. Arch Intern Med. 1995;155:930–935. [DOI] [PubMed] [Google Scholar]
- 43.Maurer MS, Cohen S, Cheng H. The degree and timing of orthostatic blood pressure changes in relation to falls in nursing home residents. J Am Med Dir Assoc. 2004;5:233–238. [DOI] [PubMed] [Google Scholar]
- 44.Braam EAJE, Verbakel D, Adiyaman A, Thien T Orthostatic hypotension: revision of the definition is needed. J Hypertens. 2009;27:2119–2120; author reply 2120. [DOI] [PubMed] [Google Scholar]
- 45.Romero-Ortuno R, Cogan L, Foran T, Kenny RA, Fan CW. Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people. J Am Geriatr Soc. 2011;59:655–665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.