Abstract
We report the case of a 64-year-old woman, presenting with pleuritic chest pain and weight loss. She had a previous history of breast malignancy and no clear risk factors for tuberculosis (TB). Initial investigations showed a right-sided pleural effusion and pleural thickening suggestive of malignancy, which would have been in keeping with the clinical presentation. Initial pleural biopsy showed features suggestive of possible TB infection, though no growth on cultures. A repeat biopsy was negative on initial microscopy, but was culture positive for Mycobacterium tuberculosis, also identifying isoniazid resistance. This case highlights that TB remains an important differential even in the absence of classical risk factors, and illustrates the diagnostic challenges it poses. It also highlights the value of culture positivity in identification of drug resistance and facilitation of appropriate treatment.
Keywords: tb and other respiratory infections, drugs: respiratory system, tuberculosis
Background
Mycobacterium tuberculosis (MTB) in the UK is normally considered as a differential diagnosis for patients with particular risk factors, including originating from or travel to a high prevalence setting, known tuberculosis (TB) exposure, homelessness and immunodeficiency. This case is important as it shows that TB, and specifically extrapulmonary TB, can be contracted in the UK with none of the ‘classical’ risk factors. The case highlights the natural course of tuberculous pleural effusions, namely to spontaneously resolve only for the disease to recur at a later date, as well as demonstrating the importance of making every effort to obtain a tissue and microbiological diagnosis given the increasing prevalence of drug-resistant disease.
Case presentation
A 64-year-old female accounts manager presented with a 6-month history of right-sided pleuritic chest pain and 19kg weight loss. Associated symptoms included anorexia, fatigue and feeling generally unwell. She had no fevers or night sweats, and no dental issues. Prior to this, she recalled a 2-month history of a productive cough, which was treated with three courses of oral antibiotics with subsequent resolution.
She had never smoked and had no significant passive smoking exposure. She lived in a flat with her family and pet dog, and only drank alcohol socially. She was born in the UK, with no known TB contacts and no personal history of TB. She was not immunosuppressed. She had not travelled to TB endemic countries.
Her medical history included breast cancer in 2008, treated with chemoradiotherapy, surgical resection and lymph node clearance. She had lymphoedema secondary to axillary lymph node clearance; a dilated cardiomyopathy (possibly due to her chemotherapy) and atrial fibrillation (she was on rivaroxaban). She had no significant family history. An initial chest radiograph showed a moderate-to-large right-sided pleural effusion (figure 1).
Figure 1.
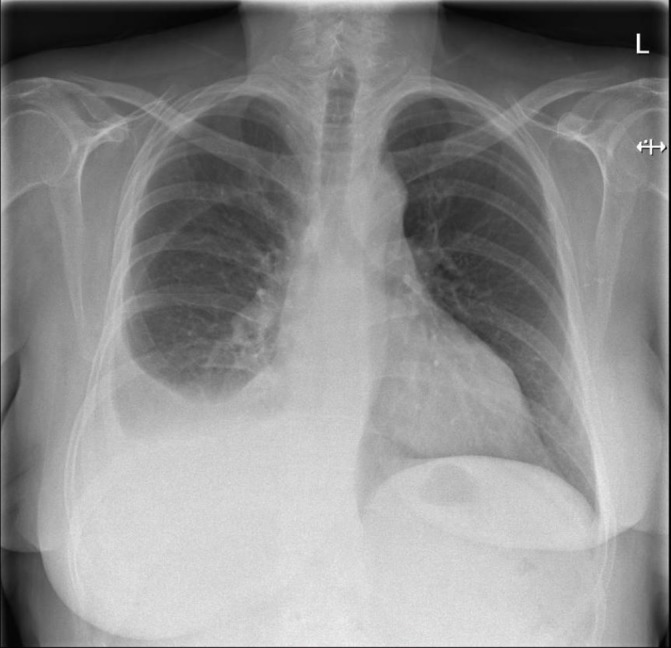
Initial chest X-ray (29 March 2017), moderate/large right-sided effusion.
A subsequent CT scan 6 weeks later revealed right-sided pleural thickening and some volume loss in the right hemithorax. Interestingly, by this time, the CT also showed that the effusion had largely resolved (figure 2), with a maximum depth of 27 mm, which was not deemed amenable to needle sampling. Her chest examination at this time was unremarkable, and there was no evidence of clubbing or lymphadenopathy.
Figure 2.
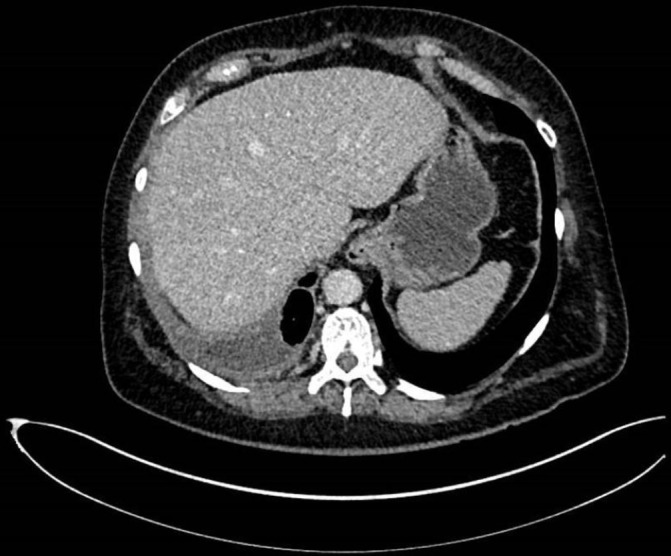
CT (12 May 2017): right pleural thickening and volume loss in the right hemithorax. Small right pleural effusion and necrotic right peribronchial lymph node.
A positron emission tomography (PET) scan (figure 3) was arranged, which showed moderate fludeoxyglucose (FDG) avidity in the right-sided pleural thickening and which was felt to be a good target for biopsy.
Figure 3.
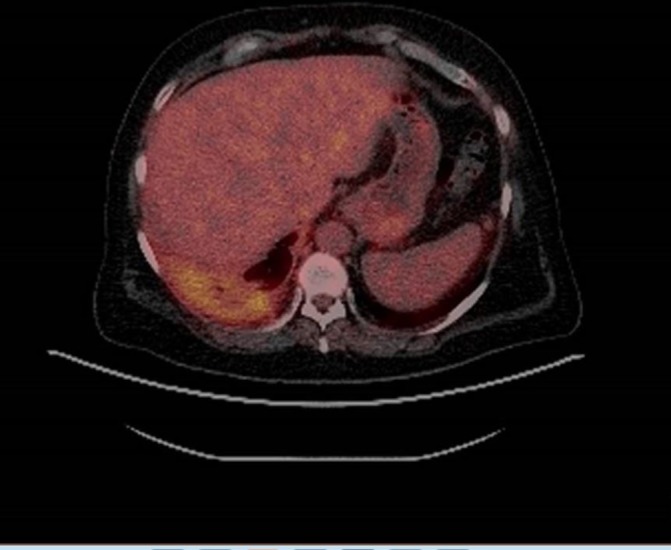
Positron emission tomography (27 June 2017): right pleural thickening with moderate-level metabolic activity.
An ultrasound-guided biopsy of the FDG-avid portion of the pleura was arranged, which revealed necrotising granulomas and no evidence of malignancy. Given her medical history, the majority of samples were sent for histological assessment. The single core sent for TB PCR and TB microscopy and culture was smear negative, PCR negative and culture negative.
A 3-month interval CT scan (figure 4) showed further resolution of the effusion, with ongoing diffuse pleural thickening. Given the lack of diagnostic certainty from initial samples, in addition to a strongly reactive TB Elispot test, a further ultrasound-guided pleural biopsy was arranged; by this time, there was no pleural fluid remaining. This sample was smear negative but was culture positive for MTB at 36 days.
Figure 4.
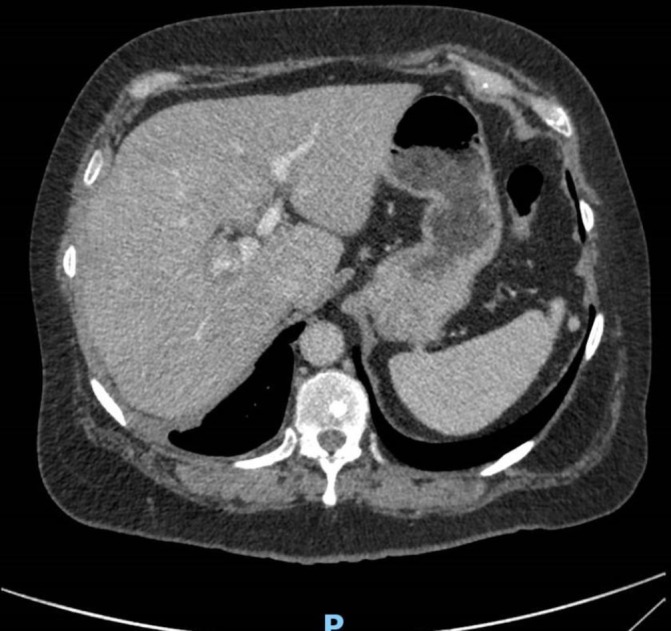
CT (17 August 2018) resolution of the right pleural effusion. Ongoing diffuse pleural thickening and an area of folded lung at the right lung base.
Investigations
Laboratory tests
HIV, hepatitis C virus and hepatitis B virus all negative.
TB Elispot: reactive.
Full blood count and renal profile unremarkable with inflammatory markers in normal range.
Liver function tests: unremarkable.
First pleural fluid/biopsy: multiple granulomata with multinucleated giant cells. Focal necrosis was seen. The appearances are those of necrotising granulomatous inflammation. There is no evidence of malignancy. Acid fast bacilli or fungal organisms are not identified on special stains (Ziehl-Neelsen and Grocott).
Second pleural biopsy: mild chronic inflammatory infiltrate composed of lymphocytes and plasma cells. No granuloma or necrosis is seen. Ziehl-Neelsen stain for acid fast bacilli is negative; MTB grown after 36 days, isoniazid resistant.
Imaging
Initial chest radiograph (figure 1): moderate/large right-sided effusion.
CT (12 May 2017) (figure 2): right pleural thickening and volume loss in the right hemithorax. Small right pleural effusion and necrotic right peribronchial lymph node. Appearances are concerning for malignancy. (note that figure 2 shows only pleural thickening, with the other findings evident in slices at others levels, not shown here).
PET (27 June 2017) (figure 3): right pleural thickening with moderate-level metabolic activity. Persistent pleural thickening extending along mediastinal surfaces is concerning. The posterior basal pleura would be a good target for biopsy.
CT (14 August 2017) (figure 4): resolution of the right pleural effusion. Ongoing diffuse pleural thickening and an area of folded lung at the right lung base.
Differential diagnosis
The primary differential in this case was felt to be malignancy, including metastatic disease from a new primary, possible recurrence of her breast malignancy or primary pleural malignancy including mesothelioma. Other potential differentials included infections such as TB, the sequelae of an empyema due to other pathogens or a non-malignant pleural fibroma.
Treatment
Following isolation of MTB, the patient was started on rifampicin, isoniazid, pyrazinamide, ethambutol and pyridoxine. Public Health England was notified and screening of close contacts was arranged.
Disc sensitivity testing later identified isoniazid resistance, and the patient’s medications were altered accordingly. She was also switched from rivaroxaban to warfarin due to potential drug interactions (although warfarin may also interact with rifampicin, this can be monitored with appropriate medication dose adjustments applied). Her treatment has been complicated by a transient rise in her alanine aminotransferase, but she is now in the process of reintroducing her antituberculous medications.
Outcome and follow-up
She is still on medication, and being regularly seen in the TB clinic. Her chest X-ray appearances continue to improve with resolution of the right-sided pleural changes.
Family screened: no evidence of active TB.
Discussion
TB is the second deadliest communicable disease.1 Extrapulmonary TB is the presenting issue in approximately 25% of adults globally, with lymph nodes and the pleura being the most common sites of disease.2 In the UK, over 50% of TB cases had evidence of extrapulmonary disease.3 Consequently, TB is one of the most common causes of pleural exudates globally. Treatment is becoming more complicated as the incidence of drug-resistant TB increases.1 Although cases of pleural TB such as this one are not uncommon, this case highlights some valuable learning points for clinicians in low incidence settings.
There were 453 cases of pleural TB reported in England during 2016, representing 8% of all TB cases where the site was identified3; however, it is not clear how many people had isolated pleural TB from these published data. Globally, 3%–5% of patients with TB have pleural disease,4 though this can be as high as 30% in high prevalence settings.5 Until recently, effusions related to TB were largely thought to be an immunological phenomenon; however, current diagnostic techniques frequently enable the isolation of TB from the effusion and pleura, suggesting that effusions are commonly the result of paucibacillary mycobacterial infections of the pleural space.6 TB pleural effusions often spontaneously resolve, leaving a thickened pleura7; however, up approximately two-third of patients will go on to develop active TB.8 Given this, there is a window of opportunity to diagnose these patients while they have features consistent with pleural TB, and as such a high index of suspicion is needed.
As was the case with the patient we discussed, pleural effusions due to TB are often moderately sized, right sided and unilateral.9 Interestingly, our patient showed no lung parenchymal disease on cross-sectional imaging, which is found in over 80% of pleural TB cases.10 In such cases, induced sputum is an important and often underutilised diagnostic approach,2 and bronchoscopy can also be considered. The gold standard for diagnosis remains identification of MTB in the pleural fluid, sputum or pleura.2 However, pleural TB often presents a diagnostic challenge, with positive pleural fluid culture in only 40% of cases.11 Pleural biopsy is more likely to be diagnostic, which with can have diagnostic yields reaching 90%.12 Unfortunately, the sensitivity of PCR testing is still low in pleural TB.13
A further test that can be considered is adenosine deaminase (ADA) in the pleural fluid. This can be used to rule out TB as it has a high negative predictive value in low prevalence settings.14 In high prevalence settings, ADA is frequently used to support a diagnosis of TB and initiate treatment when clinical suspicion is high but confirmatory tests have been negative.2 However, ADA is not available in our hospital, and would not have changed our management in this patient. The reactive TB Elispot does not differentiate between active and latent infection, but the positive result in our patient certainly contributed to further investigations. We would always however interpret a negative TB Elispot with caution given a false-negative rate in our laboratory of up to 15%.
Patient’s perspective.
What were your thoughts when you initially developed symptoms?
I thought it was cancer coming back, maybe lung cancer. That’s what I was worried about. When it came back as TB, it was a relief and shock. I’d never even given it a thought that it could be TB. That was something that an older generation could get.
How are things going now?
Getting a bit fed up, due to lots of blood tests. My liver function has been going off, so I’ve been having lots of tests. That’s getting me down a bit.
Do you have a message for medical staff?
I think it’s been great in terms of treatment, the staff have been keeping a good eye on me. I think it’s a surprise that TB is common again. I wasn’t too upset about losing 3 stone, it was my sons wedding, so that was good.
Learning points.
Tuberculosis (TB) remains an important cause of pleural disease in the UK.
Always consider pleural TB in the differential of patients with an exudative effusion.
Tuberculous effusions are often self-limiting even with ongoing infection.
Drug-resistant TB is a real issue, even in low incidence settings, making resistance testing highly important, and empirical treatment more risky.
TB should be considered even in the absence of classical risk factors and in immunocompetent individuals.
Footnotes
Contributors: OMK, MR and CR were all involved directly with the patients care. All authors contributed to planning the article. KEJP drafted the article, with OMK, MR and CR making significant comments and contributions to multiple drafts. All authors have agreed on the final draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.WHO. Global Tuberculosis Report. 2016. http://www.who.int/tb/publications/global_report/en/
- 2.Vorster MJ, Allwood BW, Diacon AH, et al. . Tuberculous pleural effusions: advances and controversies. J Thorac Dis 2015;7:981–91. 10.3978/j.issn.2072-1439.2015.02.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public Health England (PHE) 2017. Tuberculosis in England. 2017. Report. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/654152/TB_Annual_Report_2017.pdf (accessed 31 Jan 2018).
- 4.Baumann MH, Nolan R, Petrini M, et al. . Pleural tuberculosis in the United States: incidence and drug resistance. Chest 2007;131:1125–32. 10.1378/chest.06-2352 [DOI] [PubMed] [Google Scholar]
- 5.Diacon AH, Van de Wal BW, Wyser C, et al. . Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J 2003;22:589–91. 10.1183/09031936.03.00017103a [DOI] [PubMed] [Google Scholar]
- 6.Ruan SY, Chuang YC, Wang JY, et al. . Revisiting tuberculous pleurisy: pleural fluid characteristics and diagnostic yield of mycobacterial culture in an endemic area. Thorax 2012;67:822–7. 10.1136/thoraxjnl-2011-201363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonmezoglu Y, Turna A, Cevik A, et al. . Factors affecting morbidity in chronic tuberculous empyema. Thorac Cardiovasc Surg 2008;56:99–102. 10.1055/s-2007-965301 [DOI] [PubMed] [Google Scholar]
- 8.Roper WH, Waring JJ. Primary serofibrinous pleural effusion in military personnel. Am Rev Tuberc 1955;71:616–34. [DOI] [PubMed] [Google Scholar]
- 9.Valdés L, Alvarez D, San José E, et al. . Tuberculous pleurisy: a study of 254 patients. Arch Intern Med 1998;158:2017–21. [DOI] [PubMed] [Google Scholar]
- 10.Ko JM, Park HJ, Kim CH. Pulmonary changes of pleural TB: up-to-date CT imaging. Chest 2014;146:1604–11. 10.1378/chest.14-0196 [DOI] [PubMed] [Google Scholar]
- 11.Light RW. Update on tuberculous pleural effusion. Respirology 201015:451–8. 10.1111/j.1440-1843.2010.01723.x [DOI] [PubMed] [Google Scholar]
- 12.Koegelenberg CF, Irusen EM, von Groote-Bidlingmaier F, et al. . The utility of ultrasound-guided thoracentesis and pleural biopsy in undiagnosed pleural exudates. Thorax 2015;70:995–7. 10.1136/thoraxjnl-2014-206567 [DOI] [PubMed] [Google Scholar]
- 13.Trajman A, da Silva Santos Kleiz de Oliveira EF, Bastos ML, et al. . Accuracy of polimerase chain reaction for the diagnosis of pleural tuberculosis. Respir Med 2014;108:918–23. 10.1016/j.rmed.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 14.Skouras VS, Kalomenidis I. Pleural fluid tests to diagnose tuberculous pleuritis. Curr Opin Pulm Med 2016;22:367–77. 10.1097/MCP.0000000000000277 [DOI] [PubMed] [Google Scholar]


