Abstract
Objective
Mutations affecting contractile-related proteins in the extracellular matrix (ECM), microfibrils, or vascular smooth muscle cells (VSMCs) can predispose the aorta to aneurysms. We reported previously that the low-density lipoprotein receptor-related protein 1 (LRP1) maintains vessel wall integrity, and smooth muscle LRP1-deficient (smLRP1−/−) mice exhibited aortic dilatation. The current study focused on defining the mechanisms by which LRP1 regulates vessel wall function and integrity.
Approach and Results
Isometric contraction assays demonstrated that vasoreactivity of LRP1-deficient aortic rings was significantly attenuated when stimulated with vasoconstrictors, including phenylephrine, thromboxane receptor agonist U-46619, increased potassium, and L-type Ca2+ channel ligand FPL-64176. Quantitative proteomics revealed proteins involved in actin polymerization and contraction were significantly downregulated in aortas of smLRP1−/− mice. However, studies with calyculin A indicated that while aortic muscle from smLRP1−/− mice can contract in response to calyculin A, a role for LRP1 in regulating the contractile machinery is not revealed. Furthermore, intracellular calcium imaging experiments identified defects in calcium release in response to a ryanodine receptor agonist in smLRP1−/− aortic rings and cultured VSMCs.
Conclusions
These results identify a critical role for LRP1 in modulating VSMC contraction by regulating calcium signaling events that potentially protects against aneurysm development.
Keywords: LRP1, aortic aneurysm, calcium signaling, cytoskeletal dynamics
Introduction
Aortic aneurysms and dissections are often undiagnosed but can be fatal. The absence of effective medical therapies is due to our incomplete understanding of aortic disease development. Both disruption of vascular smooth muscle cell (VSMC) contractile function and aberrant extracellular matrix (ECM) synthesis and fragmentation contribute to aortic disease.1,2
VSMCs express a unique repertoire of contractile, and ion channels proteins, and signaling molecules that are required for contractile function.3 Unlike skeletal or cardiac myocytes that are terminally differentiated, VSMCs retain remarkable plasticity4 and can undergo ‘phenotypic switching’ between contractile and synthetic state (reviewed in5,6). The phenotypic state of VSMCs plays a critical role in blood vessel development and homeostasis. This plasticity may contribute to major vascular disease states, such as aortic aneurysm and dissections,7,8 atherosclerosis, and hypertension (reviewed in 6).
Phenotypic switching of VSMCs during vascular disease states is triggered by stimuli received from the local microenvironment.9,10,8Numerous genes have been identified as causing thoracic aortic aneurysm11,12 and highlight the importance of maintaining appropriate interactions between VSMCs and medial ECM. These interactions include those between VSMCs and elastin fibers, which are critical for mechanotransduction, a process in which smooth muscle cells (SMCs) convert mechanical stimuli into biochemical signals.
The actin-myosin contractile apparatus mediates the force generation responsible for VSMC contraction (reviewed in 13,14). This process requires an increase in cytosolic concentrations of calcium ions (Ca2+), which signaling pathways induce by promoting influx from extracellular sources or releasing from intracellular stores (reviewed in15). Extracellular Ca2+ influx is mediated by ion channels located in the plasma membrane. The most prominent is an L-type voltage-dependent Ca2+ channel (Cav1.2).16 The largest pool of Ca2+ in VSMCs is located in the sarcoplasmic reticulum, which releases Ca2+ through inositol 1,4,5-trisphosphate and ryanodine receptors (RyR). These two mechanisms tightly control intracellular Ca2+ levels to balance vasoconstriction versus vasorelaxation of VSMCs.17,18 Defects in Ca2+ signaling and VSMC contraction also predispose individuals to aortic aneurysm formation.19
Our studies have identified the low-density lipoprotein receptor-related protein 1 (LRP1) as a major VSMC receptor with a crucial role in maintaining vessel wall integrity.20 However, the molecular mechanism(s) by which LRP1 functions in vessel wall homeostasis is not well understood. LRP1 is a large endocytic protein with a major function of trafficking its ligands into the cell and mediating their lysosomal degradation (reviewed in21,22). Genome wide association studies have revealed that variants in the LRP1 gene are associated with vascular diseases, including aneurysms.23–26 In mice, genetic deletion of LRP1 in smooth muscle cells (smLRP1−/−) results in development of spontaneous thoracic aneurysms, as well as progressive aortic root growth, aberrant thickening of the aortic media, and fragmentation and disarray of elastic fibers.20,27 In addition, smLRP1−/− mice showed a significant increase in collagen deposition, a potential consequence of increased TGF-β signaling,28,29 and excessive accumulation of connective tissue growth factor,20 an LRP1 ligand30 and key mediator of fibrosis.31 In the current study, we identify an essential role for VSMC LRP1 in controlling contractility by regulating Ca2+ signaling events important for actin polymerization and cytoskeletal dynamics. This newly identified function of LRP1 is likely pivotal for its remarkable ability to modulate vascular homeostasis, which if disrupted, leads to aneurysm formation.
Materials and Methods
The authors believe all supporting data are available within the published article. Additional data inquiries should be directed to the corresponding author. Details of the major resources can be found in the online-only Data Supplement42,44,45,46.
Animals
Animal studies were approved by the Institutional Animal Care and Use Committee of the University of Maryland, School of Medicine and the University of Kentucky. All mice were weaned at 3 weeks of age, maintained on a 12-hour light/12-hour dark cycle, fed a standard rodent diet (4% wt/wt fat; Harlan Teklad), and given water ad libitum. Embryonic deletion of lrp1 in VSMC was achieved by crossing transgenic mice expressing Cre recombinase under the control of a SM22 SMC specific promoter with mice expressing loxP sites flanking the lrp1 gene. After extensive backcrossing, the resulting offspring, lrp1flox/flox, SM22-Cre−/− (LRP1+/+ or wild-type) and lrp1flox/flox, SM22-Cre+/− (smLRP1−/−), were used in experimental studies with wild-type littermates serving as controls. All studies were performed on male mice. Since young mice (10-day-old and 15-week-old) were analyzed in the current study we chose to use only male sex. Genetic studies show that male sex is associated with more severe and earlier onset of symptoms. Studies on animal mouse models of thoracic aortic aneurysm either did not specify or exclusively used male mice (reviewed in 32).
Postnatal deletion of lrp1 in VSMC was achieved by using transgenic smooth muscle actin SMA-Cre-ERT2 mice33 (provided generously by Dr. Pierre Chambon at IGBMC, France), where tamoxifen-inducible Cre is fused with a modified estrogen receptor ligand binding domain (ERT2) and under the control of a SMC-specific SMA promoter. lrp1flox/flox transgenic mice were crossed with SMA-Cre-ERT2 mice to obtain lrp1flox/flox, SMA-Cre-ERT2 mice. LRP1 deletion following tamoxifen injection (smaLRP1−/−) was performed as follows: Tamoxifen (Sigma-Aldrich) was dissolved in 100% ethanol for a stock concentration of 200 mg/mL. The tamoxifen stock was diluted in corn oil for a 20 mg/mL working concentration and stored protected from light at 4°C for the duration of the injections. lrp1flox/flox, SMA-Cre-ERT2 mice at 7 weeks of age were administered 50 μL tamoxifen/corn oil solution via intraperitoneal injection once every 24 hours for five consecutive days. lrp1flox/flox, SMA-Cre-ERT2 littermates injected with only ethanol/corn oil (vehicle-induced) were used as controls. Tamoxifen- or vehicle-injected mice were used in experiments at 15 weeks of age.
qRT-PCR
Total RNA from wild-type and smLRP1−/− descending thoracic aorta (DTAs) was isolated using TRIzol™ Reagent (Invitrogen) as directed by the manufacturer. Total RNA (1 μg) was then used to synthesize cDNA using the RT2 First Strand Kit (Qiagen). Real-time PCR was performed on a 7900HT Sequence Detection System (Applied Biosystems) using the RT2 SYBR Green ROX qPCR Mastermix (Qiagen) and RT2 Profiler™ Mouse Hypertension PCR Array (Qiagen). Relative gene expression data were analyzed using the 2−ΔΔCt method. Gapdh was used as for data normalization.
Immunoblotting
DTAs were dissected from wild-type and smLRP1−/− mice, and the perivascular adipose tissue and adventitia were removed. Aortic tissue was extracted as detailed in the online-only Data Supplement. Equal amounts of tissue homogenates were separated on a Novex™ 4–12% Tris-Glycine Mini Protein Gel (Invitrogen) and electrophoretically transferred to a nitrocellulose membrane (Thermo Scientific). The membrane was blocked with 3% Blotting-Grade Blocker (Bio-Rad) and incubated with the following primary antibodies overnight at 4°C: Cav1.2 calcium channel at 2 μg/mL (MAB13170; EMD Millipore), PKG at 1:1000 (ADI-KAP-PK005-D; Enzo Life Sciences), filamin A at 1:1000 (MAB1678; EMD Millipore), smooth muscle myosin heavy chain 11 at 1:1000 (ab53219; Abcam), myosin light chain kinase at 1:1000 (EP1458Y, Abcam) anti-LRP1 antibody [2629] at 2.5 μg/mL, α-Smooth Muscle Actin at 1:200 (1A4, Sigma-Aldrich), GAPDH (14C10) at 1:1000 (2118; Cell Signaling Technology), and Hsp90A.1 at 1.5:1000 (PA5–49672; Invitrogen). The membrane was washed three times with 0.05% Tween 20 (Sigma-Aldrich) in tris-buffered saline (TBS-T), and antibody binding to the membrane was detected with IRDye® 800CW Donkey anti-mouse IgG or 680RD donkey anti-rabbit IgG secondary antibody (LI-COR Biosciences) at a concentration of 1:5000. The membrane was then washed three times with TBS-T and imaged using a LI-COR Odyssey Infrared Imaging System. Protein abundance was quantified by densitometry using ImageJ (NIH) and normalized to GAPDH or Hsp90.
Aortic ring isometric contraction assay
DTAs from wild-type, smLRP1−/−, and smaLRP1−/− mice were dissected and placed in a tissue culture dish containing Krebs solution (125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, 1 mM MgCl2, 25 mM NaHCO3, pH 7.4). Aortic rings were prepared and equilibrated as detailed in the online-only Data Supplement. The following stimuli were tested: 120 mM KCl (Sigma-Aldrich), phenylephrine (PE; Sigma-Aldrich), 1 μM U-46619 (Sigma-Aldrich), 1 μM FPL 64176 (Sigma-Aldrich), 0.3 μM calyculin A (Sigma-Aldrich), and 1 mM 4-chloro-m-cresol (4-CmC; Sigma-Aldrich). After each stimulus, aortic rings were washed and re-equilibrated for 15–30 minutes before application of the next stimulus. Force measurements were acquired and recorded using LabChart Pro (ADInstruments). For each stimulus, aortic rings were normalized to their respective baseline force measurement recorded immediately before addition of the stimulus. Aortic ring isometric contraction assays using PE are presented as means ± SEM with 95% confidence intervals indicated.
Global quantification of protein expression
DTAs were dissected from 10-day-old wild-type and smLRP1−/− mice, and PVAT and adventitia were removed. For each experiment, 5 mm long aortic sections from 4 wild-type and 4 smLRP1−/− mice were pooled based on genotype and subjected to differential extraction.34 The final extract was then processed for proteomics analysis using the filter aided sample prep (FASP) method.35 Peptides were isotopically labeled by reductive alkylation, fractionated,36 and analyzed by rpLC-MS/MS using a 2 hour separation gradient for each fraction. Raw data from the four biological replicates were processed using MaxQuant (v1.5.5.1)37 against a SwissProt mouse database (v2017_06). Initial precursor mass tolerance was set to 30 p.p.m. and an MS/MS mass tolerance of 0.7 Da with Match Between Runs enabled. Search results were filtered with a false discovery rate of 0.01 with a minimum of two peptides quantified. Median normalization and statistical analyses were performed in the R programming language (v3.4.0).38 Protein differential expression was assessed using empirical Bayes moderated t-tests implemented in the limma (v3.32.2)39 R package and controlled for multiple hypothesis testing using the q-value (v2.8.0)40 R package. Differentially expressed proteins were considered significant with a q-value ≤ 0.05 and fold change ≥ 2 in either direction. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE41 partner repository with the dataset identifier PXD007544.
Immunofluorescence
Freshly dissected DTAs from wild-type, smLRP1−/−, tamoxifen-, and vehicle-induced smaLRP1 mice were embedded in Tissue-Plus® O.C.T. Compound (Scigen 4583), frozen using 2-methylbutane (Sigma M-32631) on dry ice, and transferred to dry ice before storage at −80°C. Frozen tissue blocks were cut into 10 μm sections and mounted on glass slides. Mounted sections were fixed in 100% ethanol (Pharmco-AAPER 111000200) for 10 minutes, washed three times with phosphate buffered saline (PBS), followed by 3 washes with PBS, 1% BSA, 0.2% Tween 20 (wash buffer) in a humidified chamber. Sections were then blocked with goat serum (Sigma G-9023) at 1:50 in wash buffer for 30 minutes. After blocking, sections were incubated with rabbit anti-LRP1 antibody [2629] at a concentration of 10 μg/mL for 90 minutes. Sections were then washed 3 times with wash buffer and incubated with secondary antibody goat anti-rabbit IgG Alexa Fluor® 488 (Invitrogen A-11008) at a concentration of 1:200 for one hour, protected from light. Sections were washed three times with wash buffer, followed by three washes with PBS, and mounted using Vectashield Antifade mounting medium with DAPI (Vector Laboratories H-1200). All steps were performed at room temperature, and all incubation steps and blocking were performed in a humidified chamber. Fluorescent images were captured using a Carl Zeiss LSM 510 META NLO confocal microscope with an EC Plan Neofluar® 40×/1.3 Oil DIC M27 objective lens. Images were acquired using Zeiss LSM 510 software and subsequently processed using Zeiss ZEN 2011 (Blue Edition) software.
Surface plasmon resonance (SPR)
Binding of the voltage-gated Ca2+ (Cav) channel α2δ−1 subunit (OPCA01496; Aviva Systems Biology) to LRP1 was assessed using a Biacore 3000 optical biosensor system (GE Healthcare Life Sciences). Full length LRP1, purified from placenta, was coupled to a Sensor Chip CM5 (BR-1003–99; GE Healthcare Life Sciences) using an Amine Coupling Kit (BR-1000–50; GE Healthcare Life Sciences). Binding of various concentrations of ligand was tested as detailed in the online-only Data Supplement.
Intracellular calcium imaging by fluorescent confocal microscopy
DTAs from wild-type and smLRP1−/− mice were dissected and placed in a tissue culture dish containing 112 mM NaCl, 25.7 mM NaHCO3, 4.9 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KHPO4, 11.5 mM glucose, 10 mM HEPES, pH 7.4 (modified Krebs buffer) aerated with 100% O2. Aortic rings were prepared for imaging as detailed in the online-only Data Supplement. Changes in intracellular Ca2+ concentrations to various stimuli were imaged and recorded at room temperature using a Carl Zeiss LSM 5 LIVE confocal system mounted on an Axiovert 200 M inverted microscope equipped with a gravity-fed semi-local perfusion apparatus (AutoMate Scientific, Inc.). Excitation for rhod-2 was provided by the 532 nm line of a 50 mW diode laser at 2%, and emitted light was collected at > 550 nm. Individual rings were imaged with a 10×/0.3 NA objective lens. Multiple rings were analyzed from each animal, and multiple animals were analyzed for each genotype. Details of image recordings and analysis are included in the online-only Data Supplement.
Isolation and culture of mouse aortic SMCs (mAoSMCs)
Female mice were used for all in vitro experiments as sex does not matter in vitro. DTAs were dissected from multiple 19–25-week-old wild-type and smLRP1−/− mice, placed in Dulbecco’s phosphate-buffered saline (DPBS; Corning) supplemented with 1% antibiotic-antimycotic solution (A/A; Corning), and kept on wet ice. Aortas were pooled based on genotype. The perivascular adipose tissue (PVAT) and adventitia was removed from each aorta, and aortas were cut open longitudinally and gently scraped on the lumen side to remove endothelial cells. Aortas were then incubated in Dulbecco’s modification of Eagle’s medium (DMEM; Corning), 20% fetal bovine serum (FBS; Sigma-Aldrich), 1% A/A overnight at 37°C, 5% CO2. The following day, aortas were washed with DPBS and digested with 300 U/mL collagenase, type 2 (Worthington Biochemical Corp.) and 0.7 U/mL elastase (Worthington Biochemical Corp.) in DMEM, 1% A/A for one hour at 37°C, 5% CO2. After digestion, aortas were triturated to obtain a single cell suspension and cells were centrifuged at 1,200 rpm for 7 minutes. Cells were resuspended and cultured in Clonetics™ SmGM-2™ growth medium (Lonza), 10% FBS on 0.1% gelatin (Sigma-Aldrich) coated tissue culture dishes. Cultures were maintained at 37°C, 5% CO2 in a humidified atmosphere.
Cell proliferation assay
mAoSMCs from DTAs were isolated and cultured as described above and used in proliferation assays at passage 2. Cells were trypsinized and resuspended at 2 × 105 cells/mL in Clonetics™ SmGM-2™ growth medium containing 10% FBS. Cells were then seeded at 4.5 × 104 cells/well (225 μL of the cell suspension) in a 48-well tissue culture plate pre-coated with 0.1% gelatin and incubated at 37°C, 5% CO2 (0-hour timepoint). At each subsequent interval, 25 μL of PrestoBlue™ Cell Viability Reagent (Invitrogen) was added to each well and incubated at 37°C, 5% CO2 for 20 minutes. Fluorescence intensity was read using a Tecan GENios™ Pro plate reader (excitation 550 nm, emission 612 nm, gain 30). After each fluorescent reading, the media/dye solution was removed from each well and replenished with 225 μL of fresh SmGM-2™ growth medium, 10% FBS. Fluorescence intensity was read at 4, 16, 24, 48, 72, and 96 hours, and cells were maintained at 37°C, 5% CO2 in a humidified atmosphere between timepoints. Each well was normalized to its respective fluorescence intensity at the 4-hour timepoint.
Intracellular calcium imaging of isolated mAoSMCs by fluorescent confocal microscopy
DTAs were dissected from multiple 8–18-week-old male and female wild-type and smLRP1−/− mice. mAoSMCs were isolated as described above from individual DTAs and cultured in Vascular Smooth Muscle Cell Growth Medium (ATCC®), 10% FBS on 0.1% gelatin (Sigma-Aldrich) coated 35 mm glass bottom culture dishes (MatTek Corporation). Cultures were maintained at 37°C, 5% CO2 in a humidified atmosphere until imaging by confocal microscopy at passage 1–2. Growth medium was aspirated from each dish and mAoSMCs were incubated in Krebs buffer with 1 μM rhod-2-AM (Life Technologies) for 30 minutes at room temperature protected from light. After 30 minutes, the buffer-dye solution was aspirated from the culture dish and dye loaded mAoSMCs were incubated in Krebs buffer for an additional 30 minutes at room temperature protected from light. Resting steady-state fluorescence levels (F0) were imaged and recorded for 3–9 cells per dish at room temperature using a Fluoview 500 confocal system mounted on an Olympus IX71 inverted microscope and viewed with a 60×/1.20 NA water immersion objective. Fibers were excited at 532 nm, and the fluorescence emitted above 550 nm was detected. Images were analyzed as detailed in the online-only Data Supplement.
FlexStation intracellular calcium assays of isolated mAoSMCs
DTAs were dissected from multiple 8–19-week-old male and female wild-type and smLRP1−/− mice. mAoSMCs from DTAs were isolated and cultured as described above in Clonetics™ SmGM-2™ growth medium (Lonza), 10% FBS and used in FlexStation intracellular calcium assays at passage 3. Cells were seeded at 4 × 103 cells/well in a 96-well clear bottom, black tissue culture plate (Corning) pre-coated with 0.1% gelatin and incubated at 37°C, 5% CO2 in a humidified atmosphere for approximately 48 hours. After 48 hours, the growth medium was removed from the adherent cell cultures and cells were loaded with fluo-4 NW indicator dye as per manufacturer’s instructions (Molecular Probes F36206). Tissue culture plates were incubated at 37°C for 30 minutes, then at room temperature for an additional 30 minutes protected from light. Baseline fluorescence (F0) was measured (excitation at 485 nm, emission at 538 nm) at room temperature using a FlexStation 3 Benchtop Multi-Mode Microplate Reader (Molecular Devices) and Softmax® Pro 5.4.6 software (Molecular Devices). Changes in intracellular [Ca2+] upon stimulation with 1 mM 4-CmC were measured and recorded using the automated fluidics capabilities of the FlexStation 3 microplate reader. Measurements were recorded immediately following 4-CmC addition at 2 second intervals for a total of 20 minutes. Each sample (i.e. “n”) represents mAoSMCs isolated and combined from one male and one female mouse, and each replicate represents one well of the 96-well plate.
The change in fluorescence normalized to the baseline fluorescence (ΔF/F0) was calculated for each timepoint, and replicate ΔF/F0 values (12–16) were averaged for each sample. Sample ΔF/F0 values were then combined to obtain average ΔF/F0 values for each genotype. Average ΔF/F0 values for each genotype was adjusted by subtracting the respective zero timepoint ΔF/F0 value (i.e. initial timepoint y = 0) and adjusted ΔF/F0 values were plotted as a function of time. The data was fit to a single exponential curve with a drift component to account for non-specific fluorescence (GraphPad Prism 7.0 software).
Scanning electron microscopy (SEM)
Wild-type and smLRP1−/− mice were euthanized, flushed, and dissected as described above for TEM. Samples were fixed in 2% paraformaldehyde, 2.5% glutaraldehyde, 2 mM CaCl2 in 0.1 M PIPES buffer overnight at 4°C then conductively stained following a method described by Deerinck et al.47 and as described in the online-only Data Supplement.
Transmission electron microscopy (TEM)
Wild-type and smLRP1−/− mice were euthanized by carbon dioxide asphyxiation, and the whole system was flushed with Dulbecco’s phosphate-buffered saline (DPBS; Corning) by cardiac perfusion. DTAs were then dissected, fixed in 3% glutaraldehyde, 0.1 M sodium cacodylate (pH 7.4), and stained sequentially with osmium tetroxide, tannic acid, and uranyl acetate. Aortas were dehydrated through a graded methanol series, infiltrated with Epon, embedded in pure Epon, and polymerized. Aortic sections (60 nm) were counterstained with uranyl acetate and lead citrate, and images were captured using a FEI Tecnai T12 transmission electron microscope and Advanced Microscopy Techniques (AMT) CCD camera system. Several grids were examined for each aorta.
Histology and vessel morphometry
Serial paraffin embedded sections (5 μm) of DTAs from wild-type, smLRP1−/−, and smaLRP1−/− mice were subjected to hematoxylin and eosin (H&E), Masson’s trichrome, and elastic Van Gieson (EVG) staining. Morphometric measurements were performed using EVOS FL Auto Imaging System software (Invitrogen), and all measurements were performed by operators blinded to sample identification.
Statistical analyses
Summary data are represented as mean ± SEM when data had normal distributions and as medians when data distributions were less well defined. Statistical significance was assessed using parametric two sample t-test, two-way ANOVA followed by Bonferroni posthoc tests or non-parametric Mann-Whitney rank-sum test for unpaired data sets. A p-value ≤ 0.05 was set as the threshold for significance.
Results
smLRP1 deficiency resulted in reduced expression of vasoregulatory genes in the DTA.
A significant decrease in mean arterial blood pressure has been reported in mid-aged smLRP1−/− mice due to both lower diastolic and systolic pressures.20,48 Vascular resistance and blood pressure are regulated by vascular reactivity and tone which are affected by VSMC signaling, including Ca2+ signaling. It has also been revealed that an increase in intracellular Ca2+ initiates VSMC contraction, differentiation, and proliferation,49 which suggests that Ca2+ may be a crucial molecule involved in stretch-induced VSMC differentiation. To identify a possible role of LRP1 in regulating vascular tone, we examined mRNA abundance by quantitative RT-PCR of selected genes involved in biological pathways regulating blood vessel constriction and dilation (Table I, in the online-only Data Supplement). These analyses revealed that in the absence of smLRP1, mRNA levels for α−1D adrenergic receptor (adra1d, p = 0.01) (Fig. 1A), a member of the G-protein coupled receptors (GPCR), and calcium voltage-gated channel subunit α1 C (cacna1c, p = 0.036) (Fig. 1B) were attenuated compared to wild-type levels. L-type Ca2+ channel (Cav1.2) protein expression levels were quantified by immunoblotting (Fig. 1C), The mean value of abundance of Cav1.2 antigen in smLRP1−/− mice was trending toward decreased values when compared to wild-type mice, although the immunoblot results did not reach statistical significance (Fig. 1D, p = 0.07).
Fig. 1. LRP1 deficiency attenuated vasoreactivity of the DTA.
Fold change in (A) adra1d and (B) cacna1c mRNAs in DTAs of smLRP1−/− mice compared to WT mice quantified by qRT-PCR (n = 3). (C) Immunoblot analyses of aortic extracts from WT and smLRP1−/− mice using anti-Cav1.2 IgG (n = 4). (D) Immunoblot results in (C) were quantified by densitometry using NIH ImageJ software and normalized to GAPDH (p < 0.07). (E-I) Aortic rings prepared from WT and smLRP1−/− mice were mounted on tungsten wires and attached to a differential capacitor force transducer. Aortic rings were then incubated with phenylephrine (PE; E, n = 12), U-46619 (F, n = 12), KCl (G, n = 12), FPL-64176 (H, n = 4), or calyculin A (I, n = 4) and force generation was recorded. The data represent mean ± SEM of results. 95% confidence intervals are indicated by blue dashed lines, **p < 0.05, ***p < 0.0001.
LRP1 deficiency resulted in aberrant SMC contraction.
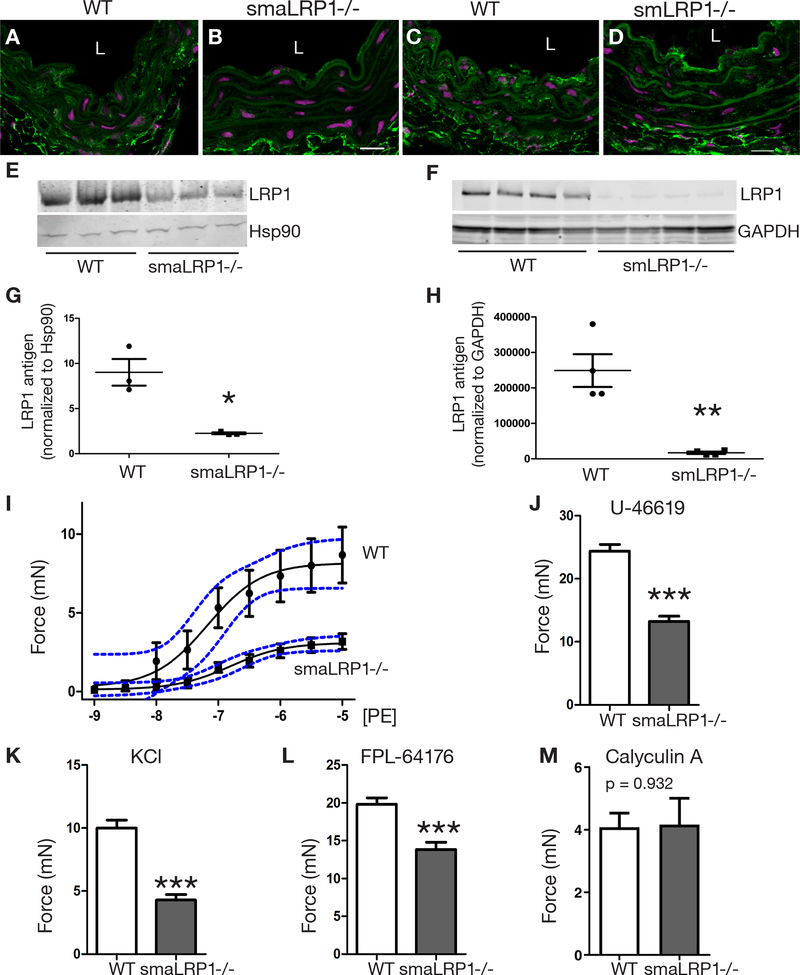
To evaluate the functional effects of LRP1 deficiency in VSMCs, in vitro vascular contractility was assessed in segments of the DTA by measuring contractile force under isometric conditions. In aortic ring isometric contraction assays, GPCR-induced vasoconstriction responses to two agonists were investigated: 1) phenylephrine (PE), an α1-adrenergic receptor agonist, and 2) U-46619, a thromboxane receptor agonist which typically elicits an increase in intracellular Ca2+ and Ca2+ sensitization of contraction. LRP1-deficient aortic rings completely failed to contract in response to PE stimulation (Fig. 1E, blue dashed lines: 95% confidence intervals). In addition, U-46619-mediated contractile force was diminished by 50% compared to wild-type aortas (Fig. 1F, p < 0.0001). We also analyzed potassium (120 mM KCl)-induced vasoconstriction, which occurs through a receptor-independent mechanism involving SMC depolarization. Consistent with results obtained with PE, LRP1-deficient aortic rings failed to contract to increased KCl concentrations (Fig. 1G, p < 0.0001). These results are in agreement with previously reported PE and KCl responses in segments of aorta isolated from smLRP1−/− mice.50 In addition, Cav1.2 mediated contraction was determined by stimulation with the Ca2+ channel ligand FPL 64176. FPL 64176-induced vascular contraction was significantly reduced (27%, p = 0.007) in aortic rings isolated from smLRP1−/− mice compared to aortic rings isolated from wild-type mice (Fig. 1H). However, when aortic segments of smLRP1−/− mice were incubated with calyculin A, a protein phosphatase inhibitor that prevents dephosphorylation of 20-kDa light chain of myosin (MLC20) (reviewed in51), contractile responses were elevated in LRP1-deficient aortas compared to the aortas from wild-type mice (Fig. 1I, p = 0.003). These results reveal that while aortic muscle from smLRP1−/− mice can contract in response to calyculin A, a role for LRP1 in regulating the contractile machinery is not revealed.
smLRP1 regulated expression of actin-associated and cytoskeletal proteins.
The three major cytoskeletal filaments, microfilaments (actin), intermediate filaments, and microtubules, collectively define and maintain cell shape and structure and are key to important cellular events, including cell division, movement, and vesicular transport.52 DTAs isolated from 15-week-old adult wild-type and smLRP1−/− mice were analyzed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Numerous changes were observed in smLRP1−/− aortas in VSMC cytoskeleton and its interaction with the medial ECM. In the vessels from wild-type mice, through attachments to dense bodies in the cytoplasm, and dense plaques on the membrane, VSMC actin cytoskeleton (Fig. IA, white asterisk in the online-only Data Supplement) forms connections with ECM molecules, including collagen fibers (white arrowhead) and elastic lamina (el), were extensive (Fig. IA in the online-only Data Supplement). Furthermore, synthetic organelles were less abundant cytoplasmic components (Fig. IA in the online-only Data Supplement). The medial ECM of wild-type mice also showed numerous and highly organized collagen bundles with striations (Fig. IA, C, white arrowheads in the online-only Data Supplement). In contrast, aortic media of smLRP1−/− displayed highly disorganized ECM with unbundled, randomly dispersed collagen (Fig. IB, D, white arrowheads in the online-only Data Supplement) and disrupted elastic lamina (el).
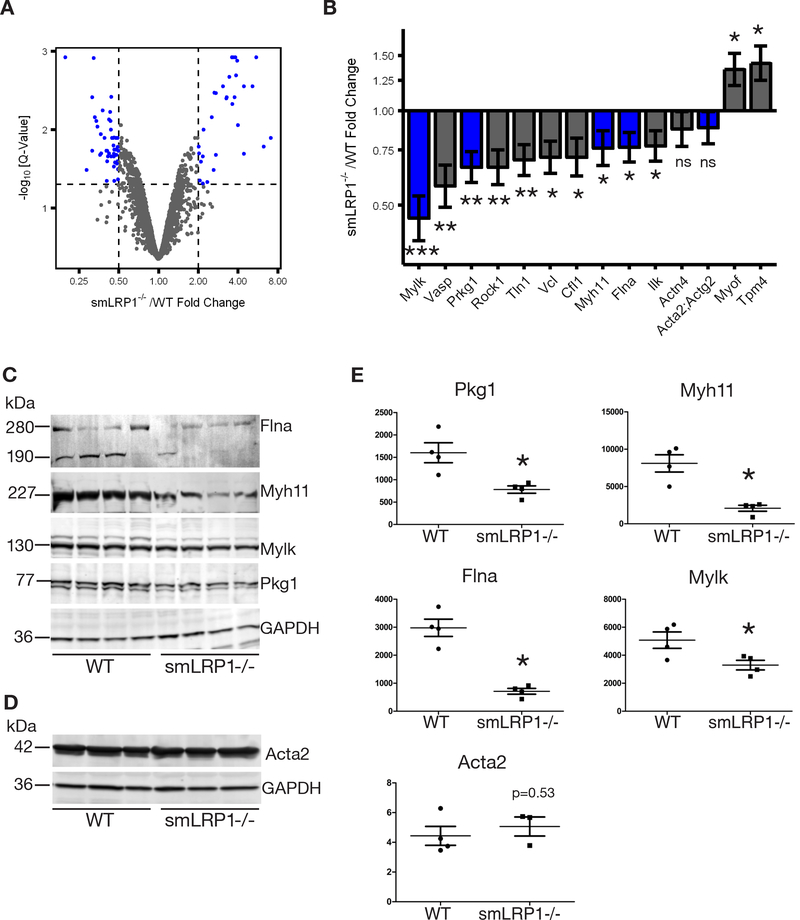
To identify potential mechanisms by which VSMC LRP1 regulates actin cytoskeleton interaction with adhesion molecules to form connections with the ECM and the effects of cytoskeletal remodeling on vascular contractility, we used quantitative proteomics to identify differentially expressed proteins in DTAs (Fig. 2A). Our quantitative proteomics data identified 245 differentially regulated proteins in smLRP1−/− mice (represented as blue dots in Fig. 2A). This included, a considerable number that have fundamental roles in actin polymerization and VSMC contraction (Fig. 2B, Table II in the online-only Data Supplement). Several members of the intracellular elastin-contractile unit, which is fundamental in linking and transmitting forces from elastin fibers to VSMCs,53 were significantly down-regulated in smLRP1−/− mice (Fig. 2B). A multiprotein integrin associated complex provides a link between VSMCs and the ECM to enable mechanotransduction.53 Protein expression for several members of this complex were attenuated in smLRP1−/− mice, including filamin A (Flna, 0.76-fold, p = 0.022), integrin linked kinase (Ilk, 0.77-fold, p = 0.026), vinculin (Vcl, 0.71-fold, p = 0.011), talin-1 (Tln1, 0.7-fold, p = 0.007), and α-actinin 4 (Actn4, 0.88-fold, p = 0.25). Protein expression of actin depolymerizing factor cofilin (Cfl, 0.71-fold, p = 0.022) and actin polymerizing protein vasodilator stimulated phosphoprotein (Vasp, 0.58-fold, p = 0.004) were significantly downregulated in smLRP1−/− mice (Fig. 2B). Proteins modulating the contractile process, including myosin light chain kinase (Mylk, 0.45-fold, p < 0.001), a type I cGMP-dependent protein kinase (Prkg1, 0.66-fold, p = 0.0032), SMC-specific myosin heavy chain (Myh11, 0.76-fold, p = 0.036), and Rho-associated protein kinase-1 (Rock1, 0.66-fold, p = 0.0059), were also significantly downregulated in smLRP1−/− mice. Abundance of tropomyosin (Tpm4, 1.42-fold, p = 0.012), which associates with actin to provide structural stability and filament function, and myoferlin (Myof, 1.35-fold, p = 0.018), an important protein in the repair of plasma membrane injury, were significantly upregulated. However, abundance of SMC actin (α-actin; Acta2 and γ-actin; Actg2) did not change. Immunoblot analyses of DTA extracts from 10-day-old wild-type and smLRP1−/− mice confirmed our proteomics data analyses showing significant attenuation in the expression of four actin-associated proteins, Flna (both full length 280 kDa and cleavage product 190 kDa were analyzed), Myh11, Mylk, and Pkg1, and no change in the abundance of SMC α-actin, Acta2 (Fig. 2D, E).
Fig. 2. LRP1 expression modulated VSMC phenotype.
(A) A volcano plot showing the -log10 q-values (y-axis) versus fold changes (x-axis) for each protein. (B) Fold changes in actin-associated proteins in smLRP1−/− mice relative to WT were quantified by mass-spectrometry (*p < 0.05, **p < 0.005, ***p < 0.0005, n = 3). Blue bars indicate proteins that were confirmed by immunoblot analyses. Immunoblot analyses of aortic extracts from smLRP1−/− and WT using anti-Flna, anti-Myh11, anti-Mylk, anti-Pkg1, anti-GAPDH IgGs (C, n = 4) and anti-Acta2 and anti-GAPDH IgGs (D, n =3). Specific protein bands are indicated to the left of the panel by a line and their calculated molecular weights. Quantification of immunoblot results in (E) using NIH ImageJ software and normalized to GAPDH (*p < 0.05).
LRP1 expression in adult SMCs was required for maintaining a contractile phenotype.
The contractile defect noted in smLRP1−/− mice might arise from a vascular ECM that is improperly formed during development. Alternatively, LRP1 may be required for VSMC contraction and may alter intracellular signaling events. To test these two possibilities, we generated a mouse model containing an inducible Cre enzyme expressed in SMCs to allow ablation of lrp1 following tamoxifen injection. This ensures normal development of contractile SMCs and a functioning vascular ECM prior to temporally controlled ablation of LRP1. For these experiments, lrp1flox/flox transgenic mice were bred with mice expressing Cre recombinase fused with a modified estrogen receptor ligand binding domain (ERT2) under the control of an inducible SMC actin promoter (SMA-Cre-ERT2) to obtain lrp1flox/flox, SMA-Cre-ERT2 mice. Attenuation of LRP1 expression in 15-week-old mice following tamoxifen injection (smaLRP1−/−) was confirmed in DTAs by immunofluorescence and western blot analyses (Fig. 3A-B, E, G). After confirming partial deletion of LRP1 (75% knockdown) in VSMCs, we analyzed possible vessel wall remodeling in smaLRP1−/− mice in comparison to wild-type mice by histomorphometry (Fig. IIA, B in the online-only Data Supplement). At 15 weeks of age, the DTA walls of smaLRP1−/− mice had no significant thickening (Fig. IIC in the online-only Data Supplement). The contractile function of aortic segments from smaLRP1−/− mice was analyzed using the same aortic ring isometric contraction assays as the embryonic smLRP1 deficient tissue in which the same GPCR-induced vasoconstriction responses by two agonists were investigated: PE and U-46619. We detected significantly reduced contraction responses for both agonists in smaLRP1−/− aortic rings compared to wild-type rings (Fig. 3I, J; 60% PE response, blue dashed lines: 95% confidence intervals and 40% U-46619 response, p < 0.0001, respectively). High KCl concentrations also resulted in a significantly reduced contraction (Fig. 3K, 55% response, p < 0.0001). FPL 64176-induced vascular contraction was also significantly reduced in smaLRP1−/− aortic rings, similar to the effect we observed in smLRP1−/− aortic rings (Fig. 3L, 30% response, p = 0.0004). When aortic segments of smaLRP1−/− mice were incubated with calyculin A, we detected a contractile response in LRP1-deficient aortas that was indistinguishable from contractile response of the aortas from wild-type mice (Fig. 3M, p = 0.932). This contractile response is similar to the effect we observed in smLRP1−/− aortic rings, supporting our finding that LRP1 deficiency impacts regulation of Ca2+ signaling events upstream of myosin phosphorylation by MYLK. Even though SMA-Cre-ERT2 knockdown (75%) was not as effective as SM22-Cre knockdown (90%) (Fig. 3C, D, F, H), residual levels of LRP1 protein in VSMCs of DTAs was not sufficient to rescue the defective contractile phenotype observed. This data further emphasizes the importance of LRP1 expression in maintaining the contractile function of SMCs, and that defective formation of the vascular ECM during development does not account for the contractile defect.
Fig. 3. Conditional deletion of LRP1 in adult VSMCs attenuated aortic vasoreactivity.
(A-D) Loss of LRP1 expression in medial VSMCs, but not adventitial fibroblasts after tamoxifen administration of (A, C) lrp1flox/flox (WT), (B) SMA-Cre−/+-ERT2 (smaLRP1−/−) and (D)sm22-Cre−/+ (smLRP1−/−) mice. Scale bars, 20 μm. L: lumen. (E) Immunoblot analysis of WT and smaLRP1−/− aortic medial tissue extracts after tamoxifen administration using anti-LRP1 or anti-Hsp90 IgGs (n = 3). (F) Immunoblot analysis of WT and smLRP1−/− aortic medial extracts using anti-LRP1 or anti-GAPDH IgGs (n=4). (G-H) Quantification of immunoblots in (E-F) used NIH ImageJ software and was normalized to Hsp90 (*p = 0.01) or GAPDH (**p = 0.003). (I-M) Isometric contraction assays were performed on aortic rings isolated from WT (vehicle-induced) mice and tamoxifen-induced smaLRP1−/− mice. Aortic rigs were incubated with phenylephrine (PE; I, n = 8), U-46619 (J, n = 8), KCl (K, n = 8), FPL-64176 (L, n = 8) or calyculin A (M, n = 3) and force generation was recorded. The data represent mean ± SEM. Confidence intervals (95%) are indicated by blue dashed lines, ***p < 0.001.
Voltage-gated Ca2+ channel auxiliary subunit alpha2delta-1 (α2δ−1) is a novel LRP1 ligand.
The auxiliary α2δ subunits of voltage-gated Ca2+ channels are extracellular membrane associated proteins that function as a chaperone to increase the expression of functional voltage-gated Ca2+ channels at the plasma membrane and increase Ca2+ currents.54,55 α2δ−1 is required for Cav1.2 membrane expression and smooth muscle cell contractility.56 Recent work from Kadurin and colleagues demonstrated that LRP1 influenced trafficking of the α2δ−1 subunit, and therefore plasma membrane expression of Cav1.2 and Ca2+ currents.57 We support this study further by showing that the α2δ−1 subunit binds directly to purified LRP1 using surface plasmon resonance (SPR) experiments. The results revealed a concentration-dependent binding of α2δ−1 to the LRP1-coated sensor chip (Fig. 4A). To determine the affinity of the interaction, the association data were fit to a pseudo-first order process to obtain Req values for each concentration of α2δ−1. Req values were then plotted as function of α2δ−1 total concentration from which a KD value of 48 ± 24 nmol/L was obtained by non-linear regression analysis (Fig. 4B). LRP1 ligand binding, similar to LDL receptor binding, requires Ca2+.58,59 Therefore, we repeated the SPR experiment in the presence of EDTA to confirm the specificity of α2δ−1 subunit binding to LRP1. We detected complete inhibition of α2δ−1 subunit binding to LRP1 in the presence of EDTA (Fig. 4C).
Fig. 4. α2δ−1 is a novel LRP1 ligand.
(A) Binding of increasing concentrations of α2δ−1 (9.4, 18.8, 37.5, 75, and 150 nmol/L) to LRP1 immobilized on the surface of a Biacore sensor chip. (B) Req, determined from fitting the association data to a pseudo-first order process in (A), were replotted vs. α2δ−1 concentrations, and the KD was determined by non-linear regression analysis. (C) The binding of 75 nM α2δ−1 was measured in the presence of Ca2+ or 3 mM EDTA.
SMC LRP1 modulated ryanodine receptor responses.
The plasticity that allows VSMCs to adapt to ever-changing environmental cues and regulate their phenotype also allows subtle, but significant changes in their Ca2+ sensitivity.15 It is well documented that changes in intracellular Ca2+ concentration is central to contractile activation of VSMCs.60 This signal can originate either from the extracellular environment or from intracellular stores. Ca2+-dependent relaxation is mediated by Ca2+ release events through ryanodine receptor (RyR) channels in the sarcoplasmic reticulum. In arterial smooth muscle, local Ca2+ release events, or “Ca2+ sparks”, have been suggested to oppose myogenic vasoconstriction and to influence vasorelaxation by activating co-localized Ca2+-activated potassium channels.17
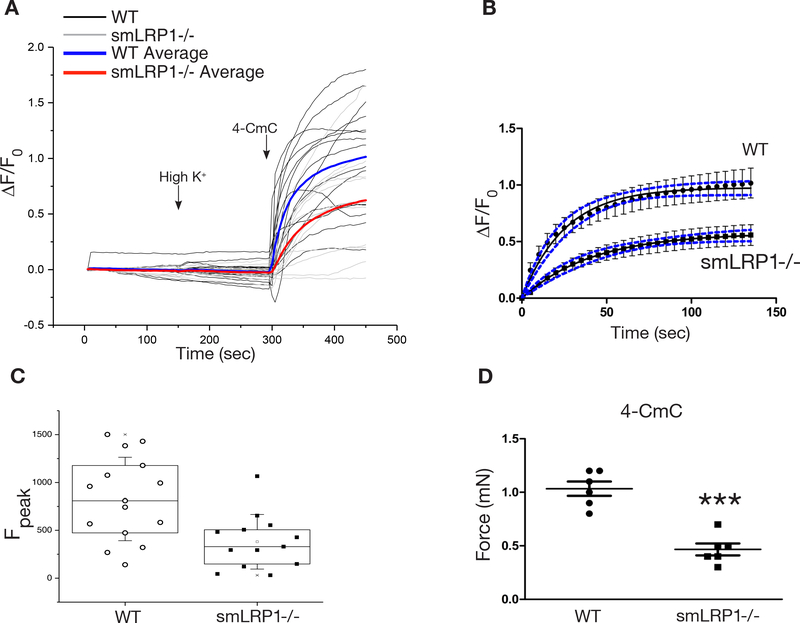
To provide molecular information on the attenuated vascular contraction observed in LRP1-deficient aortic rings, intracellular Ca2+-dependent vasoactivity was examined by a commonly used RyR activator, 4-chloro-m-cresol (4-CmC). Experiments were performed on freshly isolated DTA rings from wild-type and smLRP1−/− mice. Aortic rings were first incubated in high K+ concentrations (100 mM KCl) and then to 4-CmC. Aortic rings isolated from neither wild-type nor smLRP1−/− mice responded to high K+ (Fig. 5A), and this is likely due to the possibility of already depolarized therefore unresponsive VSMCs. However, aortic rings isolated from wild-type mice responded to 4-CmC with a rapid release of Ca2+ and contraction was detected by fluorescence confocal microscopy (Fig. 5A). In addition, the rate of Ca2+ release was significantly slower in smLRP1-deficient aortic rings (Fig. 5B, Movie I) (k = 0.039 ± 0.005 s−1 for WT and k = 0.024 ± 0.004 s−1 for smLRP1−/− mice). In the absence of smLRP1, the Ca2+ released upon 4-CmC incubation was significantly attenuated, and peak magnitudes of the 4-CmC-induced Ca2+ transients were significantly lower than wild-type aortic rings (Fig. 5C). To evaluate vasoconstriction responses upon 4-CmC incubation under isometric conditions, we utilized aortic ring contraction assays as described previously. 4-CmC-induced vascular contraction was significantly reduced in aortic rings isolated from smLRP1−/− mice compared to aortic rings isolated from wild-type mice (Fig. 5D, 45% response, p < 0.0001), supporting the attenuated Ca2+ release detected by fluorescent confocal microscopy experiments. These data reveal a novel and essential role for LRP1 in modulating RyR sensitivity in VSMCs and regulating intracellular Ca2+ levels, which in turn mediates VSMC contraction.
Fig. 5. LRP1 mediated ryanodine receptor responses in aortic VSMCs.
Aortic rings from adult WT and smLRP1−/− mice were loaded with a fluorescent Ca2+ indicator dye rhod-2 AM. 4-CmC-induced Ca2+ release and contractions were imaged and recorded utilizing fluorescent confocal microscopy. (A) Fluorescence emissions were recorded during high potassium (100 mM) perfusion and 4-CmC (1 mM) application (WT n = 15, smLRP1−/− n = 13). Addition of KCl and 4-CmC are marked with arrows. (B) Rate of Ca2+ release in aortic rings upon 4-CmC induction. Data from (A) were fitted to a first order process using non-linear regression analysis. The data points represent mean ± SEM, while the black curves show the best-fit line. Confidence intervals (95%) are indicated by blue dashed lines. (C) Summary of 4-CmC-induced peak intracellular Ca2+ concentrations in WT and smLRP1−/− aortic rings. (D) Comparison of force measurements from isometric contraction assays performed on aortic rings challenged with 4-CmC (n = 6). ***p < 0.0001.
LRP1-deficient primary VSMCs recapitulates functional defects of smLRP1−/− DTAs.
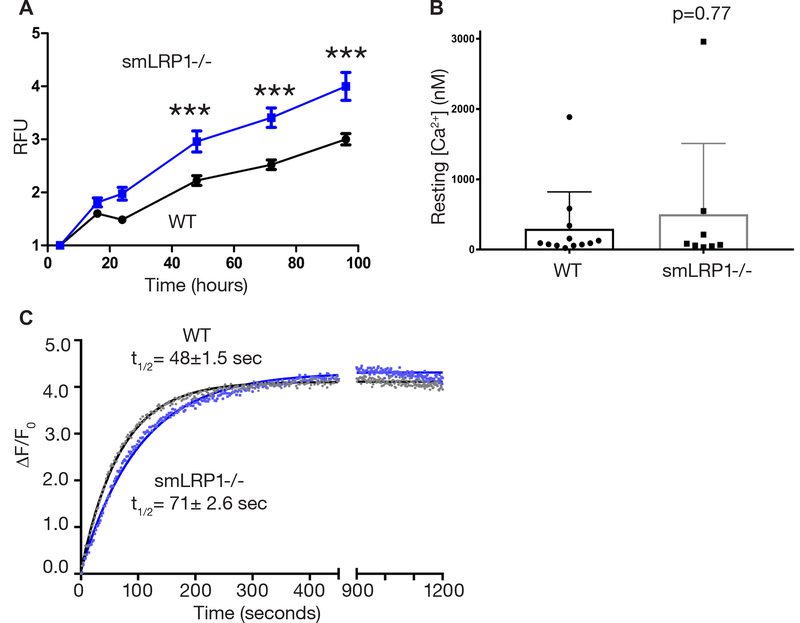
We used primary VSMCs isolated from wild-type and smLRP1−/− mice DTA to support our data showing the requirement of LRP1 for contractile function. In vitro experiments conducted with primary cells cultured in conditions conducive for a contractile phenotype revealed that LRP1-deficient VSMCs had a higher proliferation rate compared to wild-type VSMCs (Fig. 6A) indicative of a less contractile and more synthetic phenotype.
Fig. 6. VSMCs from smLRP1−/− mice exhibited an enhanced proliferative synthetic state and altered Ca2+ signaling.
(A) Proliferation curves of VSMCs isolated from WT and smLRP1−/− DTAs and cultured in contractile media. Statistical tests were by two-way ANOVA, with Bonferroni posttests (***p < 0.001, n = 8). RFU: Relative fluorescence units. (B) Resting steady state Ca2+ concentrations of WT and smLRP1−/− VSMCs. Statistical tests by Mann-Whitney test. (C) Activation of RyR by incubation with 1 mM 4-CmC in WT and smLRP1−/− VSMCs. Changes in [Ca2+] was detected using the FlexStation 3 plate reader and fluorescent fluo-4 NW calcium assay. Half-life (t1/2) values are within 95% confidence intervals.
Regulation of the intracellular Ca2+ concentration in VSMCs is critical for controlling vascular tone as Ca2+ are ubiquitous intracellular second messengers that modulate contractile activity and numerous other cellular processes. Therefore, we investigated intracellular Ca2+ concentrations at rest in primary isolated VSMCs. Intracellular Ca2+ levels in single VSMCs from wild-type and smLRP1−/− mice were measured using the Ca2+ indicator rhod-2 and the ‘Fmax’ equation.43 Resting calcium concentrations [Ca2+] were not significantly different between wild-type and smLRP1−/− VMSCs (wild-type cells: mean [Ca2+] = 296 nM, median [Ca2+] = 91 nM, min = 21 nM, max = 1884 nM, n = 12 from 5 mice vs. smLRP1−/− cells: mean [Ca2+] = 501 nM, median [Ca2+] = 75 nM, min = 33 nM, max 2958 nM, n = 8 from 3 mice; p = 0.77 Mann-Whitney test). We also investigated changes in intracellular Ca2+ concentrations of these primary VSMCs in response to RyR activator, 4-CmC. A fast and robust Ca2+ release response was detected upon 4-CmC treatment in wild-type VSMCs (t1/2 = 48 ± 1.5; Fig. 6C) using the fluorescence-based fluo-4 NW Ca2+ assay kit (Molecular Probes). Ca2+ release was well described by a first order process with a rate constant of 0.014±0.0001 s−1 (t1/2 = 48 ± 1.5 Fig. 6C). However, Ca2+ release in LRP1-deficient VSMCs was attenuated as the rate were slower (k = 0.009 ± 0.0001 s−1) indicated by longer half-life (t1/2 = 71± 2.6 sec, Fig. 6C). These data demonstrate that similar to the aortic rings, primary isolated VSMCs from smLRP1−/− mice have attenuated Ca2+ release upon RyR activation.
Discussion
We used mice with SMC-specific lrp1 deletion at distinct developmental stages to reveal a role for LRP1 in maintaining vascular homeostasis. Our results indicate that in the absence of SMC LRP1, VSMCs fail to respond to vasoactivators which we propose is due to a defect in Ca2+ signaling. This proposal is supported by several observations. First, our results reveal that the abundance of anchoring proteins, such as those linking SMC-specific α-actin to cytoplasmic dense bodies (e.g. talin and filamin A), and contractile proteins (e.g. myosin heavy chain and myosin light chain kinase) were significantly attenuated in smLRP1−/− aortas. Likewise, vascular contractile function was significantly attenuated in smLRP1−/− aortas. Interestingly, we observed an elevated contractile response in LRP1-deficient aortas compared to aortas from wild-type mice upon calyculin A incubation of aortic segments. This may be due to an increase in contraction by calyculin A which counteracts a defect in the contractile machinery. A similar effect was also reported by Chen et al. where calyculin A incubation resulted in attenuating TNFα−induced downregulation of MYLK activity of IKK2 but not blockage of the classic MYLK pathway with an intracellular Ca2+ chelator.61 Increased contractile force upon calyculin A incubation has also been reported in a model of hypertension, which is associated with an increase in mono- and di-phosphorylation of myosin regulatory light chain. 62 Calyculin A may also unmask basal activities of different Ser/Thr kinases other than MYLK which may induce actin polymerization as reported in Leung et al. 63 In the absence of additional experiments, while aortic muscle from smLRP1−/− mice can contract in response to calyculin A, a role for LRP1 in regulating the contractile machinery is not revealed and requires further investigation.
Second, we observed that LRP1 binds tightly to the voltage-gated Ca2+ channel auxiliary subunit α2δ−1. Interestingly, a recent study proposed a plausible and exciting mechanism involving the endocytic function of LRP1. Kadurin et al.57 demonstrated that the auxiliary α2δ−1 subunit of voltage-gated Ca2+ channels co-immunoprecipitates with LRP1. Furthermore, overexpression of an LRP1 mini-receptor affected activation of these Ca2+ channels, possibly by regulating α2δ−1 subunit trafficking. Since α2δ−1 is essential for plasma membrane expression of voltage-gated Ca2+ channels in VSMCs, dysregulation of α2δ−1 trafficking can directly block these channels and inhibit surface expression, leading to vasodilation.56 We confirmed that purified LRP1 binds directly to α2δ−1 with high affinity, and these studies identify α2δ−1 as a novel LRP1 ligand. Through regulation of α2δ−1 plasma membrane expression and modulation of RyR responses, LRP1 may play key roles in excitation-contraction coupling and maintenance of Ca2+ homeostasis. Further studies are necessary to reveal the exact molecular mechanism by which LRP1 modulates VSMC Ca2+ signaling.
Finally, we confirmed that the role of smooth muscle LRP1 in maintaining VSMC contractile function and Ca2+ homeostasis is cell autonomous as isolated primary VSMCs from smLRP1−/− mice retained the attenuated contractile phenotype observed in aortic rings.
Our previous study also reported a remarkable lack of attachment of medial VSMCs to the aortic wall ECM in the absence of LRP1.20 This highly disorganized ECM in vessels from smLRP1−/− mice was confirmed in the present study using scanning and transmission electron microscopy. Thus, LRP1 also contributes directly or indirectly to normal development of SMC-vascular ECM interactions, that are critical for mechanosensing. Mechanisms by which LRP1 regulates ECM assembly are not fully understood at present. This may involve LRP1’s role in trafficking integrin-β1 to the cell surface independent of its endocytic function64 and its involvement in adhesion-deadhesion of cells by mediating integrin and focal adhesion interactions.64–66 In addition, it has been proposed that LRP1 facilitates detachment at the cell trailing edge by mediating internalization of integrin adhesion complexes.66 LRP1 is also involved in mediating integrin-β1 recruitment and subsequent stimulation of integrin-linked kinase (ILK) signaling.67 ILK is essential for actin polymerization and the formation of stress fibers and focal adhesions and, therefore, is known to strengthen integrin-cytoskeleton connections. Finally, LRP1 is known to regulate the extracellular activity of numerous proteases which participate in the degradation of the ECM.
To determine if ECM disruption and disorganization during vascular development contributes to a lack of SMC contractility we observed in adult mice, we conducted experiments in which LRP1 ablation was initiated in 7-week-old mice by a tamoxifen-inducible SMA-Cre and then measured the contractile responses in 15-week-old mice. The contractile response to multiple vasoconstrictors remained significantly attenuated when compared to wild-type mice even though no significant ECM remodeling was detected in smaLRP1−/− mice. These results confirm that the diminished contractile responses in smaLRP1−/− mice are likely due to a requirement for LRP1 expression in ensuring normal Ca2+ signaling.
The defective VSMC contraction observed in smLRP1−/− mice provides mechanistic insight into why these mice develop spontaneous aneurysms, and studies utilizing mouse models and human genetic studies highlight the importance of LRP1 expression in protecting the vasculature from aneurysm formation. Two independent genome wide association studies identified a susceptibility locus for abdominal aortic aneurysm within intron 1 of the LRP1 gene.23,68 In addition to previously reported associations with migraine and ischemic stroke risks,69,70 the latest study shows an association of the same LRP1 SNP variant (rs11172113) with sporadic thoracic aortic dissection.25 Interestingly, Chan et al.71 detected a significant reduction in LRP1 protein abundance in aortas from abdominal aortic aneurysm patients. They demonstrated further that translational repression of LRP1 was caused by microRNA-205, resulting in excess accumulation of matrix metalloprotease 9,72 a protease that directly degrades the ECM and an LRP1 ligand that accumulates in smLRP1−/− mice.73 Recently, exome sequencing identified a missense mutation in the LRP1 gene in two Marfan syndrome families.24 These patient studies are supported by genetic studies in mice, which have confirmed an important role for LRP1 in protecting the vasculature from aneurysm development.20,27,74 As a result of SMC-specific LRP1 deletion, dilatations were identified in the aortic root and descending aorta of aged mice48 and proximal descending aorta of young adult mice.20 smLRP1-deficiency also promoted angiotensin II-induced vascular disease by exacerbating ascending aorta and superior mesenteric artery dilation.75 These studies also revealed several vascular pathologies, including tortuous aorta, aortic root and proximal descending aorta dilatations, extensive fragmentation of elastic fibers, aortic medial thickening, and evidence of increased TGF-β signaling.20,27 Collectively, these studies suggest an important role of LRP1 for vascular development, which is not confined to specific vascular beds, but rather a general role that extends to multiple vasculature segments with different SMC lineages. Overall, our findings unveil a critical role for LRP1 in vascular development and homeostasis in which LRP1 modulates VSMC contraction by regulating calcium signaling events.
Supplementary Material
Highlights.
LRP1 promoted the contractile function of the aortic wall by maintaining appropriate interactions between VSMCs and the ECM and promoting the contractile phenotype of VSMCs
LRP1 bound tightly to the voltage-gated Ca2+ channel auxiliary subunit α2δ−1
LRP1 has a novel role in mediating intracellular Ca2+ levels via regulating Ca2+ efflux through activated RyR.
Acknowledgments:
We would like to thank Elizabeth Smith for histology and the University of Maryland, Baltimore Electron Microscopy Core Imaging Facility (EMCIF) for their expertise. We would also like to thank to Erhan Muratoglu for assistance in figure preparation.
Sources of Funding: This work was supported by grants from the American Heart Association (15SDG24470170, S.C.M.), National Heart, Lung, and Blood Institute, National Institutes of Health (R35 HL135743, D.K.S.; F31 HL131293, D.T.A.; R01 HL133723, A.D.), Marfan Foundation (D.K.S.), National Institute of Environmental Health Sciences, National Institutes of Health (R01 ES024516, Z.Y.), National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (R37 AR055099, M.F.S), and National Cancer Institute, National Institutes of Health (F31 CA213815, W.E.F.). This work also utilized an electron microscopy sample preparation instrument that was purchased with funding from a National Institutes of Health Shared Instrumentation Grant (1S10RR26870–1) awarded to the University of Maryland, Baltimore.
Abbreviations
- Cav1.2
L-type voltage-dependent Ca2+ channel
- DTA
descending thoracic aorta
- ECM
Extracellular matrix
- LRP1
Low-density lipoprotein receptor-related protein 1
- RyR
Ryanodine receptor
- smLRP1−/−
Smooth muscle cell-specific LRP1 deficiency
- smaLRP1−/−
Inducible smooth muscle cell-specific LRP1 deficiency
- VSMC
Vascular smooth muscle cell
Footnotes
Disclosures: None.
References
- 1.Humphrey JD, Milewicz DM, Tellides G, Schwartz MA. Dysfunctional mechanosensing in aneurysms. Science. 2014;344:477–479. doi: 10.1126/Science.1253026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milewicz DM, Trybus KM, Guo D, Sweeney HL, Regalado E, Kamm K, Stull JT. Altered smooth muscle cell force generation as a driver of thoracic aortic aneurysms and dissections. Arterioscler Thromb Vasc Biol. 2016;37:26–34. doi: 10.1161/ATVBAHA.116.303229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. [DOI] [PubMed] [Google Scholar]
- 4.Perry RLS, Rudnicki MA. Molecular mechanisms regulating myogenic determination and differentiation. Front Biosci. 2000;5:D750–67. [DOI] [PubMed] [Google Scholar]
- 5.Gomez D, Owens GK, Berne RM. Smooth muscle cell phenotypic switching in atherosclerosis. 2012;95:156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens GK, Kumar MS, Wamhoff BR. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol Rev. 2004;84:767–801. [DOI] [PubMed] [Google Scholar]
- 7.Ailawadi G, Moehle CW, Pei H, Walton SP, Yang Z, Kron IL, Lau CL, Owens GK. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J Thorac Cardiovasc Surg. 2009;138:1392–1399. doi: 10.1016/j.jtcvs.2009.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphrey JD, Schwartz MA, Tellides G, Milewicz DM. Role of mechanotransduction in vascular biology: Focus on thoracic aortic aneurysms and dissections. Circ Res. 2015;116:1448–1461. doi: 10.1161/CIRCRESAHA.114.304936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez D, Swiatlowska P, Owens GK. Epigenetic control of smooth muscle cell identity and lineage memory. Arterioscler Thromb Vasc Biol. 2015;35:2508–2516. doi: 10.1161/ATVBAHA.115.305044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: Building and repairing blood vessels. Circ Res. 2011;108:365–377. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brownstein AJ, Ziganshin BA, Kuivaniemi H, Simon C, Bale AE, Elefteriades JA. Genes associated with thoracic aortic aneurysm and dissection : an update and clinical implications. Aorta. 2017;5:11–20. doi: 10.12945/j.aorta.2017.17.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milewicz DM, Carlson A a, Regalado ES. Genes Predisposing to Thoracic Aortic Aneurysms and Dissections: Associated Phenotypes, Gene-Specific Management, and Genetic Testing. Cardiol Clin. 2010;28:191–197. doi: 10.1016/j.ccl.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murrell M, Oakes PW, Lenz M, Gardel ML. Forcing cells into shape: the mechanics of actomyosin contractility. Nat Publ Gr. 2015;16:486–498 doi: 10.1038/nrm4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levayer R, Lecuit T. Biomechanical regulation of contractility: Spatial control and dynamics. Trends Cell Biol. 2012;22:61–81. doi: 10.1016/j.tcb.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II : modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. [DOI] [PubMed] [Google Scholar]
- 16.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:555. [DOI] [PubMed] [Google Scholar]
- 17.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. [DOI] [PubMed] [Google Scholar]
- 18.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca 2+ -sensitive ion channels. Cell Calcium. 2003;34:211–229. doi: 10.1016/S0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 19.Milewicz DM, Guo D-C, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet. 2008;9:283–302. doi: 10.1146/annurev.genom.8.080706.092303. [DOI] [PubMed] [Google Scholar]
- 20.Muratoglu SC, Belgrave S, Hampton B, Migliorini M, Coksaygan T, Chen L, Mikhailenko I, Strickland DK. LRP1 protects the vasculature by regulating levels of connective tissue growth factor and HtrA1. Arterioscler Thromb Vasc Biol. 2013;33:2137–2146. doi: 10.1161/ATVBAHA.113.301893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lillis AP, Mikhailenko I, Strickland DK. Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J Thromb Haemost. 2005;3:1884–1893. doi: 10.1111/j.1538-7836.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 22.Herz J, Strickland DK. Multiligand receptors LRP : a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI200113992.Introduction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bown MJ, Jones GT, Harrison SC, Wright BJ, Bumpstead S, et al. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am J Hum Genet. 2011;89:619–627. doi: 10.1016/j.ajhg.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Yu J, Wang K, Wang B, Wang M, Zhang S, Qin S, Yu Z. Exome sequencing identified new mutations in a Marfan syndrome family. Diagn Pathol. 2014;9:25. doi: 10.1186/1746-1596-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo D, Grove ML, Prakash SK, Eriksson P, Hostetler EM, LeMaire SA, Body SC, Shalhub S, Estrera AL, Safi HJ, Regalado ES, Zhou W, Mathis MR, Eagle KA, Yang B, Willer CJ, Boerwinkle E, Milewicz DM. Genetic variants in LRP1 and ULK4 are associated with acute aortic dissections. Am J Hum Genet. 2016;99:1–8. doi: 10.1016/j.ajhg.2016.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galora S, Saracini C, Palombella AM, Pratesi G, Pulli R, Pratesi C, Abbate R, Giusti B. Low-density lipoprotein receptor-related protein 5 gene polymorphisms and genetic susceptibility to abdominal aortic aneurysm. J Vasc Surg. 2013;58:1062–1068.e1. doi: 10.1016/j.jvs.2012.11.092. [DOI] [PubMed] [Google Scholar]
- 27.Zhou L, Takayama Y, Boucher P, Tallquist MD, Herz J. LRP1 regulates architecture of the vascular wall by controlling PDGFRbeta-dependent phosphatidylinositol 3-kinase activation. Schmidt HH, ed. PLoS One. 2009;4:e6922. doi: 10.1371/journal.pone.0006922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi P, Karsenty G, Roberts AB, Roche NS, Sporn MB, de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell. 1988;52:405–414. [DOI] [PubMed] [Google Scholar]
- 29.Schlumberger W, Thie M, Rauterberg J, Robenek H. Collagen synthesis in cultured aortic smooth muscle cells. Modulation by collagen lattice culture, transforming growth factor-beta 1, and epidermal growth factor. Arterioscler Thromb Vasc Biol. 1991;11:1660–1666. doi: 10.1161/01.ATV.11.6.1660. [DOI] [PubMed] [Google Scholar]
- 30.Segarini PR, Nesbitt JE, Li D, Hays LG, Yates JR, Carmichael DF, Yates III JR. The low density lipoprotein receptor-related protein/alpha 2-Macroglobulin receptor is a receptor for connective tissue growth factor. J Biol Chem. 2001;276:40659–40667. doi: 10.1074/jbc.M105180200. [DOI] [PubMed] [Google Scholar]
- 31.Phanish MK, Winn SK, Dockrell MEC. Connective tissue growth factor-(CTGF, CCN2) - A marker, mediator and therapeutic target for renal fibrosis. Nephron - Exp Nephrol. 2010;114:e83–e92. doi: 10.1159/000262316. [DOI] [PubMed] [Google Scholar]
- 32.Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS, Smith JD. Consideration of sex differences in design and reporting of experimental arterial pathology studies-statement from ATVB c council. Arterioscler Thromb Vasc Biol. 2018;38:292–303. doi: 10.1161/ATVBAHA.117.309524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wendling O, Bornert J-M, Chambon P, Metzger D. Efficient temporally-controlled targeted mutagenesis in smooth muscle cells of the adult mouse. Genesis. 2009;47:14–18. doi: 10.1002/dvg.20448. [DOI] [PubMed] [Google Scholar]
- 34.Didangelos A, Yin X, Mandal K, Saje A, Smith A, Xu Q, Jahangiri M, Mayr M. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: a proteomics approach. Mol Cell Proteomics. 2011;10:M111.008128. doi: 10.1074/mcp.M111.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 36.Hao P, Guo T, Li X, Adav SS, Yang J, Wei M, Sze SK. Novel application of electrostatic repulsion-hydrophilic interaction chromatography (ERLIC) in shotgun proteomics: comprehensive profiling of rat kidney proteome. J Proteome Res. 2010;9:3520–3526. doi: 10.1021/pr100037h. [DOI] [PubMed] [Google Scholar]
- 37.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 38.R Core Team R. R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed August 21, 2017.
- 39.Ritchie ME, Phipson B, Wu D Di, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vizcaíno JA, Csordas A, Del-Toro N, Dianes JA, Griss J, Lavidas I, Mayer G, Perez-Riverol Y, Reisinger F, Ternent T, Xu QW, Wang R, Hermjakob H. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016;44:D447–D456. doi: 10.1093/nar/gkv1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia H, Rochefort NL, Chen X, Konnerth A. In vivo two-photon imaging of sensory-evoked dendritic calcium signals in cortical neurons. Nat Protoc. 2011;6:28–35. doi: 10.1038/nprot.2010.169. [DOI] [PubMed] [Google Scholar]
- 43.Maravall M, Mainen ZF, Sabatini BL, Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys J. 2000;78:2655–2667. doi: 10.1016/S0006-3495(00)76809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navedo MF, Takeda Y, Nieves-cintrón M, Molkentin JD, Santana LF. Elevated Ca2+ sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells. Am J Physiol Cell Physiol. 2010;298:211–220. doi: 10.1152/Ajpcell.00267.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du C, MacGowan GA, Farkas DL, Koretsky AP. Calibration of the calcium dissociation constant of Rhod2in the perfused mouse heart using manganese quenching. Cell Calcium. 2001;29:217–227. doi: 10.1054/ceca.2000.0186. [DOI] [PubMed] [Google Scholar]
- 46.Escobar AL, Velez P, Kim AM, Cifuentes F, Fill M, Vergara JL. Kinetic properties of DM-nitrophen and calcium indicators: Rapid transient response to flash photolysis. Pflugers Arch Eur J Physiol. 1997;434:615–631. doi: 10.1007/s004240050444. [DOI] [PubMed] [Google Scholar]
- 47.Deerinck TJ, Bushong EA, Thor A, Ellisman MH. NCMIR - National Center for Microscopy and Imaging Research - NCMIR - National Center for Microscopy and Imaging Research. https://www.ncmir.ucsd.edu/sbem-protocol/. Published 2010. Accessed August 21, 2017. [Google Scholar]
- 48.Basford JE, Koch S, Anjak A, Singh VP, Krause EG, Robbins N, Weintraub NL, Hui DY, Rubinstein J. Smooth muscle LDL receptor-related protein-1 deletion induces aortic insufficiency and promotes vascular cardiomyopathy in mice. PLoS One. 2013;8:1–13. doi: 10.1371/journal.pone.0082026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kudryavtseva O, Aalkjaer C, Matchkov V V. Vascular smooth muscle cell phenotype is defined by Ca2+ dependent transcription factors. FEBS J. 2013;280:5488–5499. doi: 10.1111/febs.12414. [DOI] [PubMed] [Google Scholar]
- 50.Basford JE, Moore ZWQ, Zhou L, Herz J, Hui DY. Smooth muscle LDL receptor-related protein-1 inactivation reduces vascular reactivity and promotes injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2009;29:1772–1778. doi: 10.1161/ATVBAHA.109.194357.Smooth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- 52.Gunning P, O’neill G, Hardeman E. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiol Rev. 2008:1–35. doi: 10.1152/physrev.00001.2007. [DOI] [PubMed] [Google Scholar]
- 53.Karimi A, Milewicz DM. Structure of the elastin-contractile units in the thoracic aorta and how genes that cause thoracic aortic aneurysms and dissections disrupt this structure. Can J Cardiol. 2016;32:26–34. doi: 10.1016/j.cjca.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Petegem F, Clark KA, Chatelain FC, Minor DL Jr., Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dolphin AC. Calcium channel auxiliary α2 δ and β subunits: trafficking and one step beyond. Nat Rev Neurosci. 2012;13:542–555. doi: 10.1038/nrn3311. [DOI] [PubMed] [Google Scholar]
- 56.Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. Smooth muscle cell alpha2delta-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res. 2009;105:948–955. doi: 10.1161/CIRCRESAHA.109.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kadurin I, Rothwell SW, Lana B, Nieto-Rostro M, Dolphin AC. LRP1 influences trafficking of N-type calcium channels via interaction with the auxiliary α2δ−1 subunit. Sci Rep. 2017;7:43802. doi: 10.1038/srep43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fass D, Blacklow S, Kim PS, Berger JM. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature. 1997;388:691–693. doi: 10.1038/41798. [DOI] [PubMed] [Google Scholar]
- 60.Pfitzer G Signal Transduction in Smooth Muscle Invited Review: Regulation of myosin phosphorylation in smooth muscle. J appl Physiol. 2001;91:497–503. [DOI] [PubMed] [Google Scholar]
- 61.Chen M, Ma L, Hall JE, Liu X, Ying Z. Dual regulation of tumor necrosis factor-α on myosin light chain phosphorylation in vascular smooth muscle. Am J Physiol - Hear Circ Physiol. 2015;308:H398–H406. doi: 10.1152/ajpheart.00691.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho YE, Ahn DS, Morgan KG, Lee YH. Enhanced contractility and myosin phosphorylation induced by Ca2+-independent MLCK activity in hypertensive rats. Cardiovasc Res. 2011;91:162–170. doi: 10.1093/cvr/cvr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leung YM, Kwan TK, Kwan CY, Daniel EE. Calyculin A-induced endothelial cell shape changes are independent of [Ca2+]i elevation and may involve actin polymerization. Biochim Biophys Acta - Mol Cell Res. 2002;1589:93–103. doi: 10.1016/S0167-4889(02)00161-1. [DOI] [PubMed] [Google Scholar]
- 64.Salicioni AM, Gaultier A, Brownlee C, Cheezum MK, Gonias SL. Low density lipoprotein receptor-related protein-1 promotes 1 integrin maturation and transport to the cell surface. J Biol Chem. 2004;279:10005–10012. doi: 10.1074/jbc.M306625200. [DOI] [PubMed] [Google Scholar]
- 65.Orr AW, Pedraza CE, Pallero MA, Elzie CA, Goicoechea S, Strickland DK, Murphy-Ullrich JE. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J Cell Biol. 2003;161:1179–1189. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao C, Lawrence DA, Li Y, Von Arnim CAF, Herz J, Su EJ, Makarova A, Hyman BT, Strickland DK, Zhang L. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 2006;25:1860–1870. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu K, Wu C, Mars WM, Liu Y. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor – related protein 1 – mediated integrin signaling. J Clin Invest. 2007;117:3821–3832. doi: 10.1172/JCI32301DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galora S, Saracini C, Pratesi G, Sticchi E, Pulli R, Pratesi C, Abbate R, Giusti B. Association of rs1466535 LRP1 but not rs3019885 SLC30A8 and rs6674171 TDRD10 gene polymorphisms with abdominal aortic aneurysm in Italian patients. J Vasc Surg. 2015;61:787–792. doi: 10.1016/j.jvs.2013.10.090. [DOI] [PubMed] [Google Scholar]
- 69.Harriott AM, Heckman MG, Rayaprolu S, Soto-Ortolaza AI, Diehl NN, Kanekiyo T, Liu C-C, Bu G, Malik R, Cole JW, Meschia JF, Ross OA. Low density lipoprotein receptor related protein 1 and 6 gene variants and ischaemic stroke risk. Eur J Neurol. 2015;22:1235–1241. doi: 10.1111/ene.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chasman DI, Schurks M, Anttila V, de Vries B, Schminke U, Launer LJ, Terwindt GM, van den Maagdenberg AMJM, Fendrich K, Volzke H, Ernst F, Griffiths LR, Buring JE, Kallela M, Freilinger T, Kubisch C, Ridker PM, Palotie A, Ferrari MD, Hoffmann W, Zee RYL, Kurth T Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan CYT, Chan YC, Cheuk BL, Cheng SW. A pilot study on low-density lipoprotein receptor-related protein-1 in Chinese patients with abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2013;46:549–556. [DOI] [PubMed] [Google Scholar]
- 72.Chan CYT, Chan YC, Cheuk BLY, Cheng SWK. Clearance of matrix metalloproteinase-9 is dependent on low-density lipoprotein receptor-related protein-1 expression downregulated by microRNA-205 in human abdominal aortic aneurysm. J Vasc Surg. 2017;65:509–520. doi: 10.1016/j.jvs.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 73.Sakalihasan N, Delvenne P, Nusgens B V, Limet R, Lapière CM. Activated forms of MMP2 and MMP9 in abdominal aortic aneurysms. J Vasc Surg. 1996;24:127–133. [DOI] [PubMed] [Google Scholar]
- 74.Boucher P, Gotthardt M, Li W-P, Anderson RGW, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 75.Davis FM, Rateri DL, Balakrishnan A, Howatt DA, Strickland DK, Muratoglu SC, Haggerty CM, Fornwalt BK, Cassis LA, Daugherty A. Smooth muscle cell deletion of low-density lipoprotein receptor-related protein 1 augments angiotensin II-induced superior mesenteric arterial and ascending aortic aneurysms. Arterioscler Thromb Vasc Biol. 2015;35:155–162. doi: 10.1161/ATVBAHA.114.304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.