Abstract
Effect of temperatures and illumination of temperate winter on photosynthesis and respiration was studied in the psychrophilic microalgae, Koliella antarctica (Trebouxiophyceae). Outdoor and indoor algal cultures were compared. Photosynthetic as well as respiration rates increased as light and temperature increased, until 35 °C, more in outdoor than in indoor cells, in agreement with the calculated Q10 values. K. antarctica showed important strategy mechanisms of adaption to the several temperature and light conditions. These significant photo-acclimation and thermo-acclimation abilities make it possible to cultivate Koliella for different uses, under less expensive outdoor conditions. Therefore, K. antarctica shows important strategy mechanisms of adaption to various temperature and light conditions; moreover, by varying the culture conditions, it is possible to modulate and optimize the growth and accordingly the biomass production. This is a very interesting point since it has been proved that this microalga is a promising potential source of functional ingredients, such as polyunsaturated fatty acids and carotenoids, suitable for industrial purposes.
Keywords: Acclimation, Koliella antarctica, Photobioreactor, Photosynthesis, Psychrophilic microalgae, Respiration
Introduction
In the past decades, given the critical role attributed to the microalgae in biotechnology, medicine, and pharmacology applications, both isolation of new species and cultivation of microalgae for applicative purposes have made remarkable progress.
Today the cultivation of temperate or tropical microalgae is widespread, while the utilisation of polar microalgae for the biotechnological purpose is rather rare, despite some studies prove that psychrophilic microalgae produce valuable metabolites (Kvìderovà et al. 2017).
The Antarctic continent is a hostile environment covering an area of 14 million km2 (Lyon and Mock 2014). It is characterized by extreme temperatures (from + 5 to − 2 °C in the summer, and from − 10 to − 2 °C during the winter). The solar radiation are highly variable in the summer, from < 10 (in the water column under the ice sheers) to 250 µmol photons m−2 s−1 (in the shallow water of the sea) and even less than 1 µmol photons m−2 s−1 in the winter (Arrigo et al. 1998; Thomas and Dieckmann 2002; Rivas et al. 2016). Therefore, temperature and light represent the main stressors for Antarctic algae. Microalgae, the main primary producers in the polar regions (Kvìderovà et al. 2017), develop several adaptive features such as biosynthesis of pigments, polyols, sugars and lipids (Cid-Agüero et al. 2012) to adapt to these severe conditions prevailing in the polar regions of the world. In fact, polynsaturated fatty acids perform a protective role against the adverse effect of high light intensity and UV radiation (Whitelam and Codd 1986; Rezanka et al. 2008). At low temperature, even dim irradiances can saturate photosynthesis causing the production of reactive oxygen species (ROS), key drivers of photooxidation (La Rocca et al. 2014). Therefore, these psychrophilic organisms developed some mechanisms to cope with these harsh and stressful environmental conditions. Several of the microalgal adaptations may provide a useful basis for biotechnological applications. In fact, organisms such as Koliella, adapted to very cold places, show a series of well-usable features in the biotechnology sectors, for example (1) enzymes with high catalytic efficiency at low temperature relative to their mesophilic and thermophilic counterparts; (2) ability to tolerate the increase water viscosity; and (3) competence to maintain their membrane fluidity at low temperature (Varshney et al. 2015). In general, according to Varshney et al. (2015), psychrophiles microalgae can potentially be used for the production of detergents, fine chemicals and in food industry. Besides, in the past years, psychrophilic microalgae have been proposed as bioremediators during the winter season in temperate regions, in fact it has been demonstrated that they could be cultured in wastewater (Feller and Gerday 2003).
Koliella antarctica is a genus of psychrophilic unicellular green alga belonging to the class of Trebouxiophyceae, which exhibits uninucleate cell forming short, unbranched filaments shaped by 2–3 cells (Ferrara et al. 2012). Koliella was isolated, for the first time, from an ice-free water sample collected at a depth of 3 m in the Ross Sea (Antarctica) during the austral summer 1989–1990 (Andreoli et al. 1998).
According to Fogliano et al. (2010), K. antarctica has a high content of polyunsaturated fatty acids (PUFA), mainly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and it is also rich in carotenoids such as lutein and astaxanthin. This microalga is a promising potential source of those functional ingredients primarily used for industrial purposes (Fogliano et al. 2010).
Koliella can be grown in a laboratory at a temperature as low as 2 °C (Andreoli et al. 2000; Ferrara et al. 2012). Besides, the culture of K. antarctica can be maintained over 60 days in the dark showing a rapid recovery of growth after exposure to light (Ferroni et al. 2006); showing that K. antarctica possesses a relevant capacity to acclimate to extreme light conditions. Previous studies confirm that K. antarctica can adapt to different physical environmental parameters such as temperature (from − 2 to 20 °C) and light (from 8 to 60 µmol photons m−2 s−1) (Andreoli et al. 1998; Fogliano et al. 2010).
In the present paper, we investigated and compared the photosynthetic and respiratory performances of outdoor cultures of K. antarctica grown during the temperate winter season in a temperate climate zone with indoor microalgae cultures.
Materials and methods
Strain and culture conditions
Koliella antarctica SAG 2030 (Chlorophyta, Trebouxiophyceae) was isolated from a seawater sample collected in the Terra Nova Bay, Ross Sea, Antarctica (Andreoli et al. 1998). The alga was kept in a laboratory and constantly maintained in 200 mL of the basal nutrient medium at 10 °C under continuous illumination (150 µmol photons m−2 s−1). The basal medium contained: 0.3 g KH2PO4, 0.18 g K2HPO4, 0. 5 g KNO3, 0.1 g NaCl, 0.02 g CaCl2, 0.3 g MgSO4, 0.003 g FeSO4. It was dissolved in 1 L of deionised water and included trace elements (Cu, Mo, Mn, Zn and B) as described by Rigano et al. (1993). The pH of the culture medium was 6.5.
Indoor cultures
The photobioreactor for indoor culture of K. antarctica consisted of a water-jacketed tube of 8 cm diameter and 4 L volume, which was connected to a Frigomix U plus Thermomix UB from BRAUN, adjusted at 10 or 15 ± 0.01 °C as required. The tube was continuously illuminated (fluorescent lamps: Philips TLD 30 W/54, daylight, Naples, Italy), with the incident light being 150 μmol photons m−2 s−1 on each side at the external surface. Photon flux density was measured using a quantum meter model QMSS-SUN (Apogee Instruments Logan, UT). The cell suspension in the tube was vigorously bubbled with air enriched with 2% CO2, at the rate of 80–100 l h−1.
Outdoor cultures
The photobioreactor for outdoor culture of K. antarctica consisted of a tube of 8 cm diameter, 8 L volume, vertically positioned, and located in the open air in Naples, Italy (google maps URL: https://goo.gl/maps/SoGqpWaTqEs). Inside the photobioreactor, both the temperature and the irradiance (μmol photons m−2 s−1) of the culture on an external surface of the photobioreactor, were measured in the morning (9.00 h), at noon (12.00 h) and in the afternoon (15.00 h). The cell suspension in the culture unit was vigorously bubbled with air enriched with 2% CO2, at the rate of about 80–100 l h−1.
Specific growth rates
The growth was monitored by measuring the dry cells weights. Then the biomass concentration (g L−1) was calculated also by measuring optical density at 540 nm in order to produce a standard curve relating the dry weight (DW) of K. antarctica biomass (g L−1) to the optical density. This standard curve was subsequently used to calculate the biomass of individual samples based on their optical density. The calculated biomass was used to construct growth curves from which we obtained maximum specific growth rate (μmax) from the log phase of the growth curves by exponential regression. The productivity was calculated by the equation P = (Xi − X0)/ti, where P = productivity (mg L−1 day−1), X0 = initial biomass density (mg L−1), Xi = biomass density at time i (mg L−1), and ti = time interval (h) between X0 and Xi (Colla et al. 2007). To calculate the dry weight, 25–50 mL of cell suspension were passed through a nylon membrane filter having a pore size of 0.45 μm (HNWP Millipore, Naples). The filter was accurately washed with distilled water, dried at 95 °C in an oven until constant weight was reached and weighed.
Photosynthetic rates
The rate of photosynthesis of K. antarctica’s outdoor- and indoor-cultured cells was measured as O2 exchange in a small glass water-jacketed gas exchange chamber (5 cm high, 2.5 cm diameter), equipped with an oxygen electrode (Orion 97-08) and connected to an EA 920 ion analyser adjusted in O2 mode. Data were registered at 0.5 min intervals. The duration of light treatment depended on the photosynthetic capacity of the microalgae. To measure the photosynthesis, cells were collected by centrifugation from culture during exponential growth, re-suspended to a final concentration of about 2–3 µg Chl mL−1 in a fresh basal medium supplemented with 10 mM NaHCO3, to prevent carbon limitation, and finally transferred to the oxygen electrode chamber at different light intensities supplied by a Braun slide projector. Light intensity was attenuated as required with neutral density glass filters (Schott Italglass, Genova). Photon flux density was measured using a quantum meter with a separate sensor model QMSS-SUN (Apogee Instruments Logan, UT) inside the reaction chamber containing cell suspension for photosynthesis measurement. The medium in the chamber was magnetically stirred. When needed, the cell suspension was bubbled with a stream of N2 to reduce the concentration of dissolved O2. The evolution of photosynthetic oxygen and the consumption of respiratory oxygen were expressed on a total chlorophyll basis. The rate of gross photosynthesis was derived from the algebraic sum of the rate of the net photosynthetic O2 evolution and the rate of respiration.
Photosynthesis vs. irradiance responses were measured at 15 and 25 °C in the photon flux density range of 30–2000 μmol photons m−2 s−1. PB–I responses at the two temperatures were quantified by measuring photosynthetic rates normalized to biomass (PB) versus photon flux density (I), conventionally depicted by PB–I response curves, which are defined by the following function parameters: , the maximum rate of photosynthesis normalized to the Chl concentration; α, the slope of the initial linear portion of the curve PB–I; and Ik, the photon flux density approximating the onset of light-saturated photosynthesis. To calculate the photosynthetic parameters, PB–I curves were developed at each incubation temperature by non-linear regression to the model of Webb et al. (1974).
Curve fitting was performed with SigmaPlot (Version 12).
versus temperature responses were measured at a temperature range of 10–30 °C at the saturating photon flux density. To measure respiration vs. temperature responses, the gas exchange chamber was covered with aluminium foil. The Q10 value (the factor with which the rate increases by raising the temperature to 10 °C) for photosynthesis rate and respiration rate was estimated from the slope of the respective Arrhenius plot.
Chlorophyll content
Cell samples (about 5 mL) were collected by centrifugation (4000×g for 10 min), and the packed cells were treated with 5 mL of N, N-dimethylformamide. The samples were protected by light with a black cloth and refrigerated at 4 °C for 24 h. The total chlorophyll content of the cells was estimated spectrophotometrically at 647 and 664 nm, according to Inskeep’s and Bloom’s (1985) method.
Statistical analysis
One- and two-way variance analyses (ANOVA) were performed using SigmaPlot 12.0. Where ANOVAs indicated significant differences, post hoc comparisons were performed using multiple comparison Tukey HSD tests. If necessary, data were log + 1 (x) transformed before the analysis. The data shown are presented as at least three independent determinations.
Results and discussion
Koliella antarctica is a green microalga living in the extreme Antarctic environment. In the present study, we investigated the possibility to cultivate this psychrophilic microalga outdoor (Naples open air, Italy) during the winter season, despite the relevant differences existing between the mild climate conditions of this Mediterranean region compared to the natural habitat of this organism.
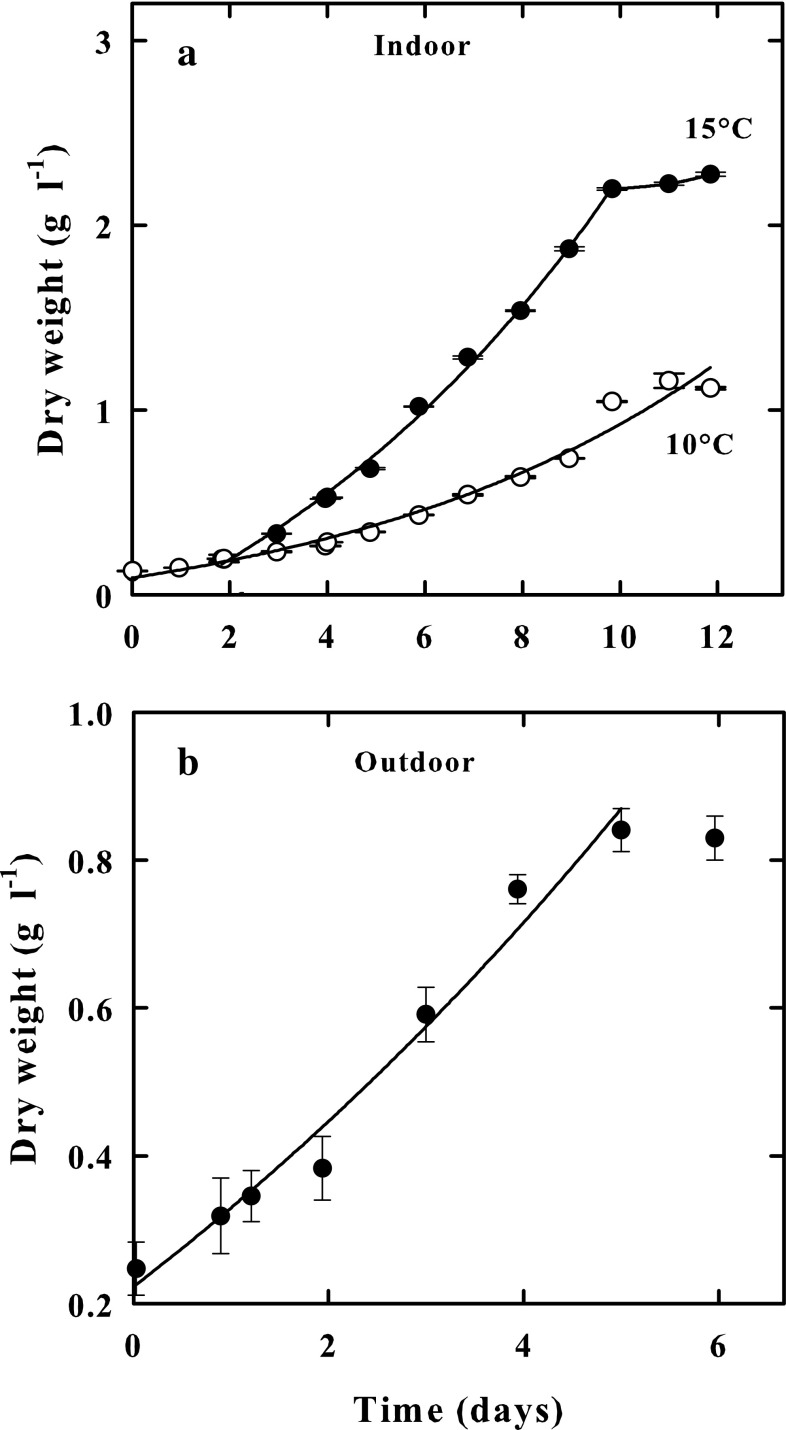
Koliella antarctica grown in the indoor culture systems exhibited after 300 h (about 12 days) of cultivation, a growth rate (µ), measured in exponential phases, of 0.30 d−1 at 10 °C, and 0.60 d−1 at 15 °C. The exposure of the culture to a temperature of 20 °C did not visibly affect it. The cells, indeed, were able to resume growth when exposed to lower temperatures (not shown). Volumetric productivity after 300 h was 1.02 g DW L−1 d−1 at 10 °C and was 2.30 g DW L−1 d−1 a 15 °C (Fig. 1a).
Fig. 1.
a Growth of K. antarctica in indoor cultures expressed as dry weight (g L−1), at 10 °C (Empty circle) and 15 °C (Black circle). b Growth of K. antarctica in an outdoor culture performed during the winter period, expressed as dry weight (g L−1). Data are presented as means of three independent determinations. Bars show ± SE
The microalga was also grown under outdoor conditions in Naples during the winter season between February and March 2016. The Fig. 1b shows the growth of K. antarctica in the outdoor culture. The mean solar irradiance at the surface of culture tube during this period was 125 ± 10.1 µmol photons m−2 s−1 at 9.00 h, 552 ± 31 µmol photons m−2 s−1 at 12 h, and 373 ± 18.4 µmol photons m−2 s−1 at 15.00 h. Irradiance values up to 1600 ± 54.3 µmol photons m−2 s−1 were recorded during sunny days, and values as low as 20 ± 2.5 µmol photons m−2 s−1 during cloudy days. The average temperature inside the photo-bioreactor was 11 ± 3 °C at 9.00 h, increasing to an average value of 16 ± 2.3 °C at 12 h and 20 ± 4.1 °C at 15.00 h. Exceptionally, in the afternoon of a sunny day, when irradiance was 1800 ± 125 μmol photons m−2 s−1, temperature values of 22.5 ± 4 °C were measured inside the culture. Taking into consideration the previously stated climatic conditions, K. antarctica showed a growth of 0.3 d−1 (μmax), measured as an increase in dry weight (g L−1), and a volumetric productivity of 0.12 g DW L−1 d−1. As observed by optical microscopy, the culture was free of undesired algae contaminating the K. antarctica culture during the whole growth period.
This microalga can be easily grown either in highly controlled indoor and outdoor cultures in a Mediterranean region during the winter season, exhibiting a biomass production of 0.18 g DW L−1 d−1, comparable to the one obtained with other microalgae commonly cultured in outdoor photobioreactors. In fact, the productivity of 0.55 g DW L−1 d−1 was reported for Spirulina platensis and Anabaena siamensis (Richmond et al. 1993), 0.37 g DW L−1 d−1 for Nannochloropsis sp. (Quinn et al. 2012), and 0.146 g DW L−1 d−1 for Chlorococcum littorale and 0.266 g DW L−1 d−1 for Chaetoceros calcitrans (Sato et al. 2006).
The possibility to perform Antarctic algae outdoor cultures in a Mediterranean zone appears relevant in this study since the photobioreactors could be used for the production of algal biomass or for wastewater treatment in the winter season, which is not suitable for the growth of mesophilic algae (Vona et al. 2004). This characteristic could further contribute to lower the cost of algal biomass production, by avoiding the additional technological procedure required to prevent the growth of contaminating mesophilic algae. Furthermore, the productivity of both indoor and outdoor cultures of K. antarctica can be certainly increased through the optimization of the photobioreactor technical characteristics.
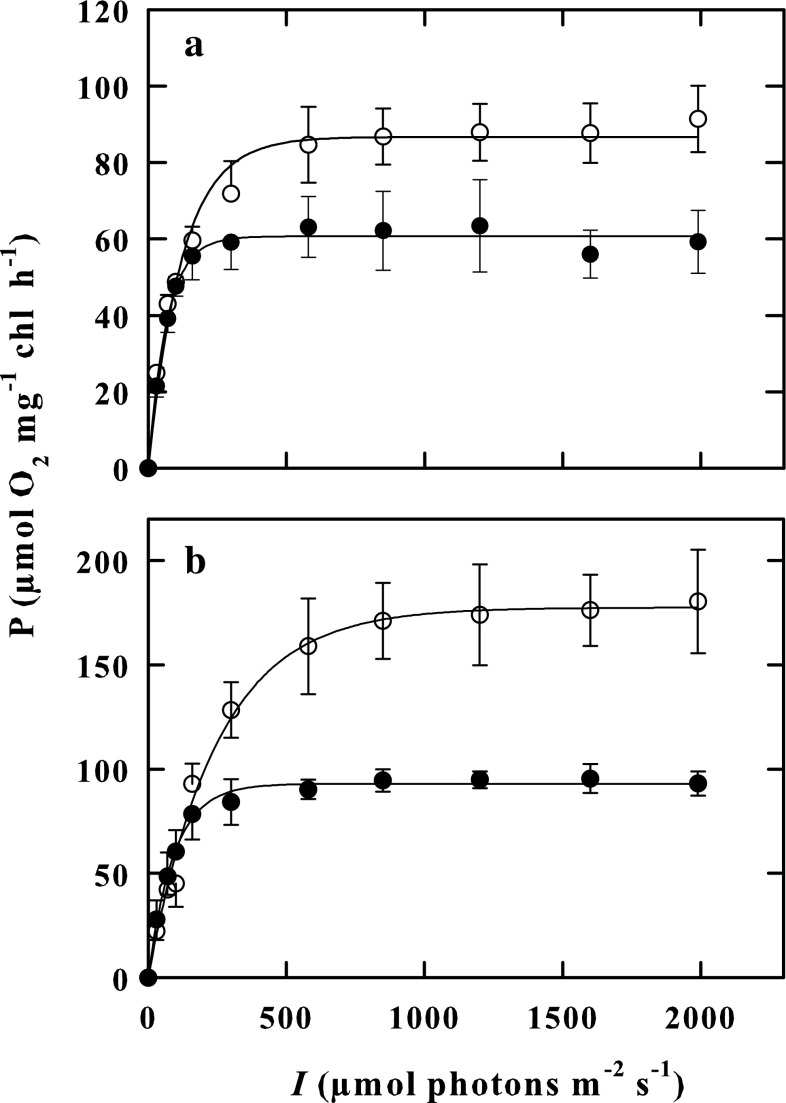
K. antarctica cells indoor-cultured at 10 °C and outdoor-cultured were exposed in a laboratory study to increasing light, in the photon flux density range of 30–2000 μmol photons m−2 s−1, at two fixed temperatures of 15 and 25 °C, and their photosynthetic rates comparatively studied. Neither of the two experimental conditions (out-and indoor cells) showed photo-inhibition phenomena in the exploited lighting range (Fig. 2). The for the outdoor grown cells were higher than those for indoor grown cells either at 15 °C (F = 8.092, P = 0.047) or at 25 °C (F = 16.521, P = 0.015) (Table 1).
Fig. 2.
PB–I curves as measured at 15 °C (a) and 25 °C (b) in indoor-cultured cells (Black circle) and in outdoor-cultured cells (empty circle) of K. antarctica in the photon flux density range of 30–2000 μmol photons m−2 s−1. Vertical bars show ± SE (when larger than the symbol); n = 3. Data are means of different experiments
Table 1.
Photosynthetic rate parameters of indoor and outdoor cultured cells of K. antarctica
| T (°C) | I k | α | ||
|---|---|---|---|---|
| Indoor cultured cells | 15 | 65 ± 4.22 | 95 ± 12.98 | 0.7 ± 0.08 |
| 25 | 93 ± 5.18 | 135 ± 42.40 | 0.83 ± 0.22 | |
| Outdoor cultured cells | 15 | 82 ± 4.48 | 145 ± 8.69 | 0.57 ± 0.05 |
| 25 | 176 ± 19.90 | 297 ± 15.12 | 0.59 ± 0.06 |
Units: (µmol O2 evolved h−1 mg−1 Chl); α, (µmol O2 evolved h−1 mg−1 Chl) (μmol photons m−2 s−1); Ik (μmol photons m−2 s−1). Data represent mean ± SE, n = 3
The function parameter α, the slope of the initial light-limited region of the photosynthesis-irradiance curve, was not significantly different at the two assay temperatures of 15 and 25 °C in both indoor- (F = 0.274, P = 0.628) and outdoor-cultured cells (F = 0.0804, P = 0.791) (Table 1); however, in indoor cells, α values appeared higher than in outdoor cells. In contrast, Ik, the photon flux density, approximating the onset of light-saturated photosynthesis, increased with increasing assay temperature from 15 to 25 °C in outdoor grown cells (F = 75.502, P < 0.001) but not in indoor grown cells (F = 0.805, P = 0.420). Besides, Ik values were higher in outdoor grown cells than in indoor grown cells either at 15 °C (F = 10.217, P = 0.033) or at 25 °C (F = 12.906, P = 0.023) (Table 1).
Temperature responses of gross photosynthesis (net photosynthetic oxygen evolution plus respiratory oxygen consumption) of indoor-grown cells at 10 °C, and of outdoor-grown cells of K. antarctica, were separately measured in the laboratory at five different temperatures of 10, 15, 20, 25 and 30 °C, in the saturating photon flux density range of 1200–1800 μmol photons m−2 s−1. As reported in Table 2, the rate of gross photosynthesis at saturating photon flux () density increased as incubation temperature increased in both outdoor-grown cells and indoor-grown cells (F = 52.159, P < 0.001), although the increase was greater in the former cells than in the latter ones (F = 21.407, P < 0.001). In fact, the photosynthesis rate of outdoor cells concerning indoor-grown cells was higher by 16% at 10 °C, and by 43% at 30 °C.
Table 2.
Effect of various temperatures on gross photosynthesis and respiration in indoor- and outdoor-cultured cells of Koliella antarctica
| Growth conditions | T(°C) | Photosynthetic rate () (μmol O2 evolved mg−1chl h−1) | (%) | Respiratory rate (μmol O2 consumed mg−1chl h−1) | (%) |
|---|---|---|---|---|---|
| Indoor-cultured cells | 10 | 43.9 ± 7.14 | 23 | 6.4 ± 0.60 | 18 |
| 15 | 54.1 ± 3.63 | 28 | 10.0 ± 1.16 | 27 | |
| 20 | 95.5 ± 4.79 | 49 | 13.9 ± 2.37 | 44 | |
| 25 | 106.6 ± 5.69 | 55 | 24.1 ± 3.39 | 66 | |
| 30 | 193.6 ± 13.07 | 100 | 36.5 ± 4.04 | 100 | |
| Outdoor-cultured cells | 10 | 50.9 ± 2.28 | 18 | 4.9 ± 0.94 | 13 |
| 15 | 84.8 ± 13.56 | 31 | 8.3 ± 0.71 | 22 | |
| 20 | 131.2 ± 23.33 | 47 | 13.2 ± 1.55 | 34 | |
| 25 | 182.4 ± 4.51 | 66 | 21.8 ± 1.52 | 57 | |
| 30 | 277.8 ± 58.20 | 100 | 38.4 ± 4.48 | 100 |
Values of photosynthetic and respiratory rate reported are mean ± SE, n = 3
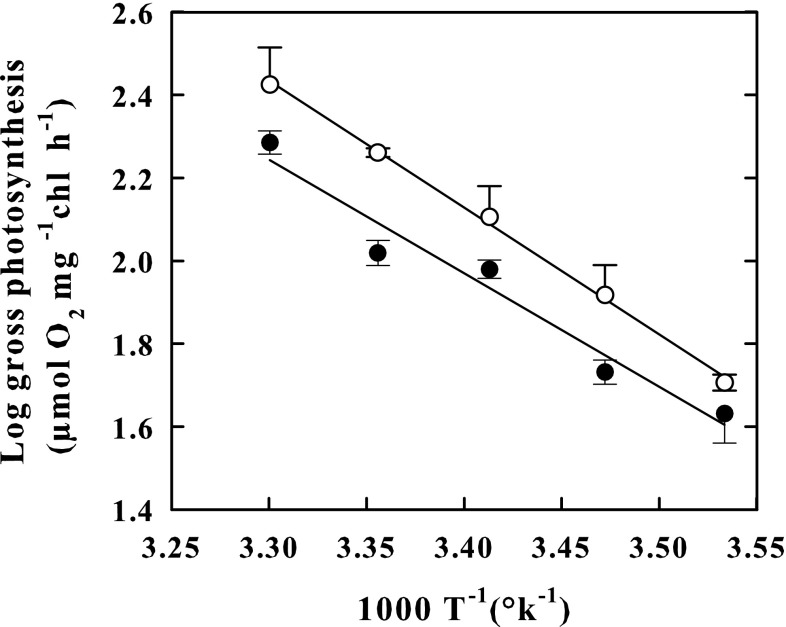
The Arrhenius plot constructed with photosynthesis rates measured at 10, 15, 20, 25 and 30 °C showed linear patterns both in outdoor- (r2 = 0.998) and in indoor-grown cells (r2 = 0.96) (Fig. 4), but exhibited different slopes, increasing with temperature faster in the former cells than in the latter ones. The apparent Q10 value for photosynthesis rate was higher in the outdoor-cultured cells (2.29) than in the indoor-cultured cells (2.07) (Fig. 3). A rise in the temperature to 35 °C produced in both types of cells resulted in a decrease of 50% of the rate of photosynthesis regarding the maximum rate peaking at 30 °C (not shown).
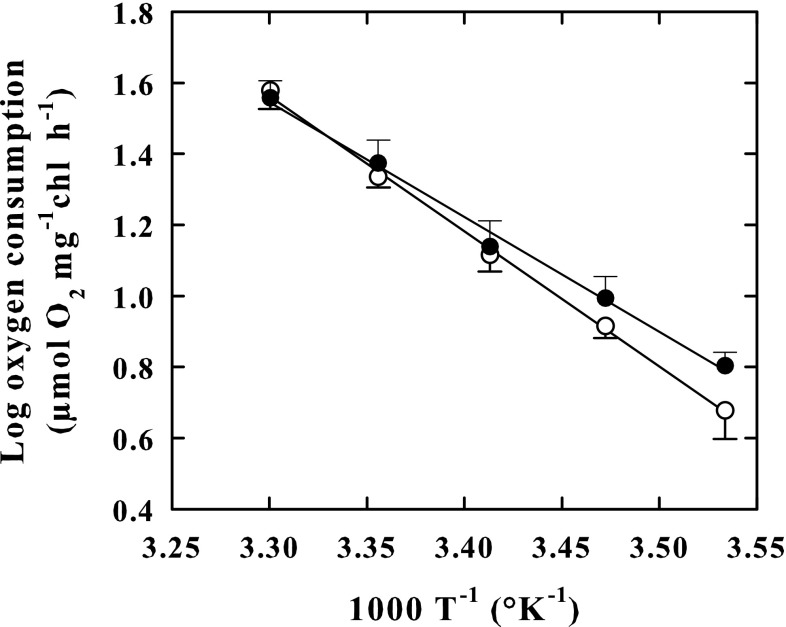
Fig. 4.
Arrhenius plots of respiratory activity in the outdoor-cultured cells (empty circle) and in indoor-cultured cells (Black circle) of K. antarctica. Plots show log10 respiratory rate versus reciprocal of absolute temperature. Vertical bars show ± SE (when larger than the symbol); n = 3. Data are means of different experiments. Apparent Q10 values were 2.79 in outdoor-cultured cells and 2.43 in indoor-cultured cells. The lines were fitted by linear regression
Fig. 3.
Arrhenius plots of photosynthetic activity in the outdoor-cultured cells (empty circle) and in indoor-cultured cells (Black circle) of K. antarctica. Plots show log10 gross photosynthesis versus reciprocal of absolute temperature. Vertical bars show ± SE (when larger than the symbol); n = 3. Data are means of different experiments. Apparent Q10 values were 2.29 in outdoor-cultured cells and 2.07 in indoor-cultured cells. The lines were fitted by linear regression
The reduced biomass productivity of K. antarctica is undoubtedly attributable to the fact that this microalga shares its maximum growth rate at a temperature close to 15 °C, where the photosynthesis rate is 30% only of the maximum rate peaking at 30 °C. The presence of heat sensitive cell structures impeding development of K. antarctica above 15 °C, prevents this alga using its maximum photosynthetic primary productivity for growth and biomass formation. Furthermore, the photosynthesis of K. antarctica are heat stable up to 30 °C (Vona et al. 2004), which represents an adaptation strategy mechanism to increase the primary productivity of the cell, in response to any sudden increase in the environmental temperature level. Moreover, the photosynthetic activity of outdoor-cultured cells of K. antarctica showed, at each temperature, a values higher than that measured in indoor-cultured cells exposed to a constant irradiance of 150 μmol photons m−2 s−1, which indicates that the former cells are acclimated in using more efficiently any increase in light intensity. This conclusion is also supported by the finding that Ik value, the photon flux density approximating the onset of light-saturated photosynthesis, measured in outdoor-cultured cells was substantially higher than in indoor-cultured cells. Similarly, cells of the cyanobacterium Synechocystis aquatilis can assume different Ik values according to the light intensities at which they are grown (Grobbelaar and Kurano 2003). The observation that in outdoor-grown cells either photosynthesis rate increases with temperature faster, or the apparent Q10 value for photosynthesis rate results higher than in the indoor-grown cells is notable. These results suggest that the outdoor-grown cells are acclimated to become more productive, even with increasing light, as noted above, and with increasing temperature. It is evident that these temperature and light acclimation phenomena might enable K. antarctica to improve primary productivity at any increase in temperature and light intensity during the day periods. In this connection, it is relevant that in this alga photoinhibition phenomena were not detected. Photoinhibition is a time-dependent process, which occurs on the same time scale as is needed to produce P/I (photosynthesis-irradiance) curves (Geider and Osborne 1992). In K. antarctica, the PB–I response curves did not exhibit the reduction of rate photosynthesis at light intensity levels due to photoinhibition. According to Platt et al. 1980, the β photoinhibition parameter is equal to zero. The fact that in K. antarctica, the PB-I response curves do not show photoinhibition at any temperature tested, supports the idea that photosynthetic apparatus of this alga is protected against photooxidative damage to PSII, as described in other algae and cyanobacteria (Coles and Jones 2000; Nishiyama et al. 2001; Thomas et al. 2001; Vonshak et al. 2001). It also explains why cells cultured in the outdoor photobioreactor in the open air did not show photoinhibition phenomena even when exposed to full sun irradiation. In addition, according to La Rocca et al. (2014), K. antarctica can activate a strong NPQ (non-photochemical quenching) and dissipate a large fraction of absorbed energy as heat in a few seconds after the exposure to strong illumination.
The respiratory oxygen consumption rate of K. antarctica cells cultured indoor at 10 °C, or outdoor were separately studied at five different temperatures of 10, 15, 20, 25 and 30 °C immediately after the obscuration of the oxygen reaction chamber with aluminium foil.
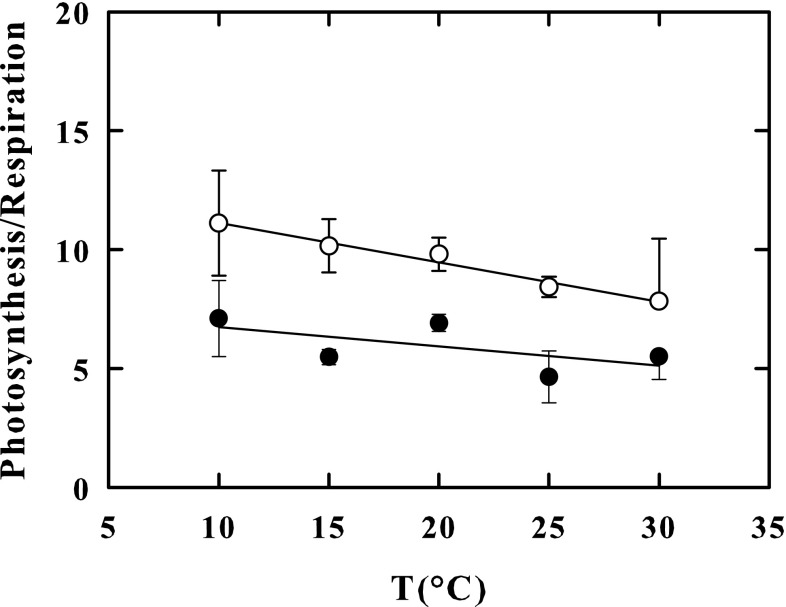
In outdoor-grown-cells, the respiration rate at 10 °Cwas 23% lower than in indoor-grown cells, whereas at 30 °C, it was 5% higher in outdoor-grown cells than in the indoor cells. As a result, cell respiration rates of outdoor cells increased faster with increasing incubation temperature from 10 to 30 °C (F = 80.271, P < 0.001) (Table 2). Accordingly, the Arrhenius plots constructed with respiration rates measured at 10, 15, 20, 25 and 30 °C (Fig. 4), showed linear patterns in both outdoor-grown cells (r2 = 0.998) and indoor-grown cells (r2 = 0.993) but exhibited different slopes. The apparent Q10 value (2.79) calculated from the Arrhenius plot of outdoor-grown cells, was notably greater than the one calculated from the Arrhenius plot of indoor-grown cells (2.43). Consequently, the photosynthesis/respiration ratio (Fig. 5), was higher in outdoor-grown cells than in indoor grown cells (F = 16.770, P < 0.001) over the entire temperature range of 10–30 °C (Fig. 5) but was 1.6-fold at 10 °C and only 1.4-fold at 30 °C, higher in outdoor-grown cells than in indoor grown cells. In both types of cells, an increase in the temperature to 35 °C produced a decrease in the rate of respiration by 50% with respect to the maximum rate peaking at 30 °C (not shown).
Fig. 5.
Photosynthesis/respiration ratio in the outdoor-cultured cells (empty circle) and in indoor-cultured cells (Black circle) of K. antarctica, at 10, 15, 20, 25 and 30 °C. Vertical bars show ± SE (when larger than the symbol); n = 3. Data are means of different experiments. The lines were fitted by linear regression
Unlike photosynthesis, which showed higher rates in outdoor-grown cells than in indoor-grown cells independently of temperature, the respiratory rates of outdoor cells resulted only 5% higher at 30 °C, or even 23% lower at 10 °C, compared to those of indoor-grown cells. Like the photosynthesis, however, the respiration was stable up to 30 °C and decreased at 35 °C, suggesting that in K. antarctica, an adaptation strategy mechanism for this process also exists, certainly to increase metabolic energy production of the cells in response to any increase in temperature. Besides, the apparent Q10 value of respiration was higher in outdoor cultured cells than in indoor cultured cells suggesting that, as with photosynthesis, there are also thermo-acclimation phenomena for respiration. As suggested by Davison (1991), thermo-acclimation of Q10 of respiration might be controlled by changes in the degree of saturation of fatty acids in the mitochondrial membrane.
In conclusion, the present study suggests that the psychrophilic microalga K. antarctica has a large photo- and thermo-acclimation potential. Based on these results, it can grow under a large variety of temperature and light conditions. Therefore, it would be interesting to optimize the cultivation conditions to increase the production of biomass and of natural functional ingredients.
Acknowledgements
The authors are very grateful to Professor Petronia Carillo for critical reading of the manuscript. This work was supported by a Grant from Regione Campania, Law 5/2002, year 2007.
References
- Andreoli C, Lokhrost GM, Mani AM, Scarabel L, Moro I, La Rocca N, Tognetto L. Koliella antarctica sp. nov. (Klebsormidiales) a new marine green microalga from the Ross Sea (Antarctica) Arch Hydrobiol Algol Stud. 1998;90:1–8. [Google Scholar]
- Andreoli C, Moro I, La Rocca N, Dalla Valle L, Masiero L, Rascio N, Dalla Vecchia F. Ecological, physiological, and biomolecular survey on microalgae from Ross sea (Antartica) Ital J Zool. 2000;67(suppl):147–156. doi: 10.1080/11250000009356370. [DOI] [Google Scholar]
- Arrigo KR, Weiss AM, Smith WO. Physical forcing of phytoplankton dynamics in the southwestern Ross Sea. J Geophys Res. 1998;103:1007–1021. doi: 10.1029/97JC02326. [DOI] [Google Scholar]
- Cid-Agüero P, Cárdenas PO, Moreno JD. Growth response of Antarctic snow microalgae cultures belonging to the Chlamidomonadaceae family to the effects of temperature, irradiance and supporting media. An Inst Patagonia (Chile) 2012;40(1):153–156. doi: 10.4067/S0718-686X2012000100019. [DOI] [Google Scholar]
- Coles JF, Jones RC. Effect of temperature on photosynthesis-light response and growth of four phytoplankton species isolated from a tidal freshwater river. J Phycol. 2000;36:7–16. doi: 10.1046/j.1529-8817.2000.98219.x. [DOI] [Google Scholar]
- Colla LM, Oliveira Reinehr C, Reichert C, Vieira Costa JA. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regime. Bioresour Technol. 2007;98:1489–1493. doi: 10.1016/j.biortech.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Davison IR. Environmental effects on algal photosynthesis: temperature. J Phycol. 1991;27:2–8. doi: 10.1111/j.0022-3646.1991.00002.x. [DOI] [Google Scholar]
- Feller G, Gerday C. Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol. 2003;1(3):200–208. doi: 10.1038/nrmicro773. [DOI] [PubMed] [Google Scholar]
- Ferrara M, Guerriero G, Cardi M, Esposito S. Purification and biochemical characterisation of a glucose-6-phosphate dehydrogenase from the psychrophilic green alga Koliella antarctica. Extremophiles. 2012;17(1):53–62. doi: 10.1007/s00792-012-0492-6. [DOI] [PubMed] [Google Scholar]
- Ferroni L, Baldisserotto C, Zennaro V, Soldani C, Fasulo MP, Pancaldi S. Acclimation to darkness in the marine chlorophyte Koliella antarctica cultured under low salinity: hypothesis on its origin in the polar environment. Eur J Phycol. 2006;41(4):1–14. [Google Scholar]
- Fogliano V, Andreoli C, Martello A, Caiazzo M, Lobosco O, Formisano F, Carlino PA, Meca G, Graziani G, Di Martino Rigano V, Vona V, Carfagna S, Rigano C. Functional ingredients produced by culture of Koliella antartica. Aquaculture. 2010;299:115–120. doi: 10.1016/j.aquaculture.2009.11.008. [DOI] [Google Scholar]
- Geider RJ, Osborne BA. Algal photosynthesis, the measurement of algal gas exchange. London: Chapman and Hall; 1992. p. 256. [Google Scholar]
- Grobbelaar JU, Kurano N. Use of photoacclimation in the design of a novel photobioreactor to achieve high yields in algal mass cultivation. J Appl Phycol. 2003;15:121–126. doi: 10.1023/A:1023802820093. [DOI] [Google Scholar]
- Inskeep WP, Bloom PR. Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80% acetone. Plant Physiol. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvìderovà J, Shukla SP, Pushparaj B, Elster J. Perspectives of low-temperature biomass production of polar microalgae and biotechnology expansion into high latitudes. Psychrophiles: From Biodiversity to Biotechnology; 2017. pp. 585–600. [Google Scholar]
- La Rocca N, Sciuto K, Meneghesso A, Moro I, Rascio N, Morosinotto T. Photosynthesis in extreme environments: responses to different light regimes in the Antarctic alga Koliella antarctica. Physiol Plant. 2014;153(4):654–667. doi: 10.1111/ppl.12273. [DOI] [PubMed] [Google Scholar]
- Lyon BR, Mock T. Polar microalgae: new approaches towards understanding adaption to an extreme and changing environment. Biology (Basel) 2014;3(1):56–80. doi: 10.3390/biology3010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 2001;20(20):5587–5594. doi: 10.1093/emboj/20.20.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T, Gallegos CL, Harrison WG. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res. 1980;38:687–701. [Google Scholar]
- Quinn JC, Yates T, Douglas N, Weyer K, Butler J, Bradley TH, Lammers PJ. Nannochloropsis production metrics in a scalable outdoor photobioreactor for commercial applications. Bioresour Technol. 2012;117:164–171. doi: 10.1016/j.biortech.2012.04.073. [DOI] [PubMed] [Google Scholar]
- Rezanka T, Nedbalová L, Sigler K. Unusual medium-chain polyunsaturated fatty acids from the snow alga Chloromonas brevispina. Microbiol Res. 2008;163(4):373–379. doi: 10.1016/j.micres.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Richmond A, Boussiba S, Vonshak A, Kopel R. A new tubular reactor for mass production of microalgae outdoors. J Appl Phycol. 1993;5:327–332. doi: 10.1007/BF02186235. [DOI] [Google Scholar]
- Rigano C, Di Martino Rigano V, Vona V, Esposito S, Di Martino C. Effect of inhibitors on ammonium assimilation in Chlorella sorokiniana in light and darkness. Physiol Plant. 1993;89:602–606. doi: 10.1111/j.1399-3054.1993.tb05221.x. [DOI] [Google Scholar]
- Rivas C, Navarro N, Huovinen P, Gómez I. Photosynthetic UV stress tolerance of the Antarctic snow alga Chlorella sp. modified by enhanced temperature? Revista Chilena de Historia Natural. 2016;89(7):1–9. [Google Scholar]
- Sato T, Usui S, Tsuchiya Y, Kondo Y. Invention of outdoor closed type photobioreactor for microalgae. Energy Convers Manag. 2006;47:791–799. doi: 10.1016/j.enconman.2005.06.010. [DOI] [Google Scholar]
- Thomas DN, Dieckmann GS. Antarctic sea ice—a habitat for extremophiles. Science. 2002;295:641–644. doi: 10.1126/science.1063391. [DOI] [PubMed] [Google Scholar]
- Thomas H, Ougham H, Hortensteiner S. Recent advances in the cell biology of chlorophyll catabolism. Adv Bot Res. 2001;35:1–52. doi: 10.1016/S0065-2296(01)35003-6. [DOI] [Google Scholar]
- Varshney P, Mikulic P, Vonshak A, Beardall J, Wangikar PP. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour Technol. 2015;184:363–372. doi: 10.1016/j.biortech.2014.11.040. [DOI] [PubMed] [Google Scholar]
- Vona V, Di Martino Rigano V, Lobosco O, Carfagna S, Esposito S, Rigano C. Temperature responses of growth, photosynthesis, respiration and NADH: nitrate reductase in the cryophilic and mesophilic algae. New Phytol. 2004;163:325–331. doi: 10.1111/j.1469-8137.2004.01098.x. [DOI] [PubMed] [Google Scholar]
- Vonshak A, Torzillo G, Masojidek J, Boussiba S. Sub-optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneus (Eustigmatophyta) Plant Cell Environ. 2001;24:1113–1118. doi: 10.1046/j.0016-8025.2001.00759.x. [DOI] [Google Scholar]
- Webb WL, Newton M, Starr D. Carbon dioxide exchange of Alnus rubra. Oecologia. 1974;17:281–291. doi: 10.1007/BF00345747. [DOI] [PubMed] [Google Scholar]
- Whitelam GC, Codd GA. Damaging effects of light on microorganisms. In: Herbert RA, Codd GA, editors. Microbes in extreme environments. London: Academic Press; 1986. pp. 129–169. [Google Scholar]