Abstract
Effect of silicon (Si) on the response of strawberry (Fragaria × ananassa var. Parus) plants to arbuscular mycorrhizal fungus (AMF) was studied under growth chamber conditions. Plants were grown in perlite irrigated with nutrient solution without (− Si) or with (+ Si) 3 mmol L−1 Si (~ 84 mg L−1 Si as Na2SiO3) in the absence (− AMF) or presence (+ AMF) of fungus. Dry matter production, root colonization rate, photosynthesis rate and water relation parameters were all improved by both Si and AMF, and the highest amounts were achieved by + Si + AMF treatment. Mycorrhizal effectiveness increased by Si treatment associated with higher Si concentration in the + AMF plants. Leaf concentrations of total soluble and cell wall-bound phenolics were increased by Si accompanied by the enhanced activity of phenylalanine ammonia lyase, but not polyphenol oxidase. Profile of phenolics compound revealed that gallic acid, caffeic acid, epicatechin, chlorogenic acid, ellagic acid and kaempferol increased by both Si and AMF treatments, while p-coumaric acid decreased. In addition to vegetative growth, both treatments improved fruit yield and its quality parameters. Our results showed that Si and AMF acted in a synergistic manner and improved growth and biochemical parameters in strawberry plants. However, the mechanism for Si-mediated increase of mycorrhizal effectiveness is not known, thereby needing further elucidation.
Keywords: Silicon, Mycorrhizal effectiveness, Fragaria × ananassa, Rhizophagus clarus, Rhizophagus intraradices, Glomus versiform
Introduction
Silicon (Si) is the second most abundant element in the earth’s crust, which is not necessary for growth and metabolism of higher plants. Regarding differences among plant species in their capacity to take up Si as well as Si content in tissues, plants are classified as Si accumulators and non-accumulators (Hajiboland 2012; Broadley et al. 2012). It has been shown that Si accumulators need this element for optimum growth and productivity, while for non-accumulator species, Si has been considered a beneficial element alleviating effects of various stresses such as drought, salinity, heat, cold, water lodging and nutrient imbalances (Guntzer et al. 2012; Hajiboland 2012).
Increasing evidence show that even under non-stress conditions, Si supplementation improves plants dry matter production in both accumulator and non-accumulator species. Addition of Si to growth medium increases leaf photosynthetic rate and carbohydrates content, and improves uptake of nutrients in accumulators and non-accumulators (Hajiboland 2012; Etesami and Jeong 2018). In addition to primary metabolism, there are also reports showing Si effect on the phenylpropanoid metabolism, concentrations of phenolic compounds and activity of metabolizing enzymes (Maksimovic et al. 2007; Hajiboland et al. 2017a).
The phenylpropanoid pathway is responsible for synthesis of various secondary metabolites, including phenol esters, coumarins, flavonoids and lignin. All phenylpropanoids are derived from trans-cinnamic acid formed from l-phenylalanine through the activity of phenylalanine ammonia-lyase (PAL) as the key enzyme in biosynthesis of phenolic compounds. Polyphenol oxidase (PPO) oxidizes polyphenols and by hydroxylation of monophenols, is involved in the lignification (Passardi et al. 2005). Peroxidases are involved in a broad range of biochemical processes such as lignin and suberin synthesis, cross-linking of cell wall (CW) components, and defense against pathogens (Passardi et al. 2005).
Association of plants with arbuscular mycorrhizal fungi (AMF) is the most abundant symbiosis between higher plants and fungi, improving plant nutrients uptake, particularly phosphorus and ameliorates effects of stresses such as drought and salinity (Porcel et al. 2012; Hajiboland 2013). Mycorrhizal fungus effectiveness (or plant responsiveness to mycorrhizas) is represented by the difference in growth between plants with and without mycorrhizas as a property of the interaction between plant and fungus species (Janos 2007). Different factors may influence mycorrhizal effectiveness such as plant species and genotype, fungal species and soil conditions (Tawaraya 2003). Among soil chemical factors, effect of soil nutrients, particularly phosphorus availability has been widely investigated while influence of Si on the mycorrhizal effectiveness has not been studied yet.
Strawberry is one of the most popular berry fruits in the world with considerable quantities of phenolic compounds, particularly anthocyanins (Costa et al. 2011). Strawberries fruit is exceptionally rich in phenolic compounds such as flavonols (kaempferol and quercetin derivatives), anthocyanidins, proanthocyanidins, hydrolysable tannins and phenolic acids (Amil-Ruiz et al. 2011; Giampieri et al. 2014; Hajiboland et al. 2017b). The majority of studies on this plant have focused on the phenolic compounds and their antioxidant activity in fruits, while information on the phenolics synthesis and profile in its vegetative tissues is highly limited. In addition, although strawberry has been identified as a Si accumulator species (Miyake and Takahashi 1986), little is known about Si effect on growth, physiology and phenolics metabolism in this species.
Phenolic compounds such as flavonoids have some positive effects on fungal growth parameters e.g. hyphal growth and branching, germination of spores and formation of secondary spores (Steinkellner et al. 2007). Considering the effect of Si on the metabolism of phenolic compounds (Maksimovic et al. 2007; Hajiboland et al. 2017a) and the role of these compounds in facilitating interactions between microorganisms and host (Steinkellner et al. 2007), it is plausible that Si influences plant-AMF interactions via modifying phenolics metabolic pathways. It has been stated that changes in the plant flavonoids metabolism by AMF may play a role in facilitating interactions between the fungus and the host (Mandal et al. 2010).
There are reports on mycorrhization of strawberry plants and improving effects of AMF on plant growth, photosynthesis and number of fruits (Borkowska 2002). However, there are currently no data on how Si affects AMF effectiveness, phenolics metabolism and fruit quality parameters. The main objective of this work was to evaluate the effect of Si on growth, colonization by AMF and its effectiveness in strawberry plants. Considering the importance of phenolic compounds in this species, the phenolics metabolism and profile were particularly emphasized to investigate the mechanism for the Si effect on the plant-AMF interaction.
Materials and methods
Plant and fungus materials
Strawberry (Fragaria × ananassa cv. Paros) plants were provided as rooted daughter plants by the Horticulture Department, Ferdowsi University of Mashhad, Iran.
Isolation of Rhizophagus clarus (formerly, Glomus clarum), Rhizophagus intraradices (formerly, Glomus intraradices) and G. versiform was provided by the Soil Science Department, University of Tabriz, Iran. In a preliminary experiment, all three AMF species were used to inoculate plants and after 8 weeks of growth, biomass and root colonization were recorded. Regarding higher effectiveness of R. clarus, this AMF species was used for the main experiment.
Isolation of AMF species was propagated on clover plants in 3.5 L pots containing sterile sandy loam soil under greenhouse conditions. Rorison’s nutrient solution with 50% phosphorus prepared with deionized water (Merryweather and Fitter 1991) was added to the pots twice a week. After 4 months, top plants were cut off and pot materials containing soil, mycorrhizal roots, hyphae and spores were thoroughly mixed and used as fungal inoculum.
Plants culture and treatments
The young daughter plants with two expanded leaves were transferred to 3 L plastic pots filled with washed perlite, and one plant was cultivated in each pot. Prior to transferring plants to the pots, 60 mg of inoculums were mixed with perlite. The − AMF treatments received the same amount of autoclaved AMF inoculums. The pots were irrigated daily at 85% field capacity after weighing with Hoagland nutrient solution (pH 5.8–6.0) or water at intervals. The total volume of nutrient solution applied to each pot started with 50 mL pot−1 week−1 and reached 250 mL pot−1 week−1 at the fruiting stage.
The concentration of water soluble Si was determined in the used perlite prior to planting. Perlite was watered for 6 weeks at field capacity, then the solution was collected by vacuum filtration and acidified to less than pH 2 with high-purity nitric acid (1004562500, Merck) (Jaiswal 2004). Silicon concentration was determined by atomic absorption spectroscopy (AAS, iCE 3000 Series, AA Spectrometer, Thermo Scientific).
One week after transplanting, Si (Na2SiO3, 307815, Sigma) treatment was started. The Si solution (adjusted to pH 6.1 using HCl) was gradually added to the pots by irrigation within 3 weeks to reach 3 mmol L−1 perlite (~ 84 mg L−1 perlite).
Plants were grown under controlled environmental conditions with a temperature regime of 25°/18 °C day/night, 14/10 h light/dark period, a relative humidity of 30% and at a photon flux density of approximately 400 μmol m−2 s−1.
Harvest of vegetative plants, measurement of gas exchange parameters and colonization rate
Five weeks after starting Si treatments (6 weeks after transfer of daughter plants to the pots), plants were harvested. Before harvest, gas exchange parameters were determined in the attached leaves. Net photosynthesis rate (μmol m−2 s−1), transpiration rate (mmol m−2 s−1) and stomatal conductance (mol m−2 s−1) were determined with a calibrated portable gas exchange system (LCA-4, ADC Bioscientific Ltd., UK). Instant water use efficiency was calculated as the ratio of photosynthesis to transpiration (μmol mmol−1).
At harvest, shoot and roots were separated, washed with distilled water and blotted dry on filter papers. Fresh weight (FW) and after drying at 70 °C for 48 h, dry weight (DW) of samples were determined. Subsamples were taken for biochemical analyses before drying. Silicon concentration was determined by atomic absorption spectroscopy (AAS, iCE 3000 Series, AA Spectrometer, Thermo Scientific) after microwave digestion of plant dried samples (Jaiswal 2004).
To evaluate AMF colonization, fine roots (1 g FW) were cleared in 10% KOH and stained with 0.05% trypan blue in lacto-glycerin (Giovanetti and Mosse 1980). The colonization frequency (%) of roots was determined by the gridline intersect method (Giovanetti and Mosse 1980). The mycorrhizal effectiveness (plant responsiveness to AMF) was calculated according to the following equation using the shoot DW of inoculated (+ AMF) and uninoculated (− AMF) plants (Janos 2007):
Leaf osmotic potential and relative water content
Osmotic potential and relative water content were determined in the second leaves harvested at 1 h after turning on the lights in the growth chamber. For determination of osmotic potential (ψs), the leaves were homogenized in the pre-chilled mortar and pestle, and centrifuged at 4000g for 20 min at 4 °C. The osmotic pressure of the samples was measured by an osmometer (Micro-Osmometer, Herman Roebling Messtechnik, Germany) and the mili-osmol data were recalculated to MPa (Babu et al. 1999). For determination of relative water content (RWC), leaf disks (5 mm diameter) were prepared and after determining FW, they were submerged for 20 h in distilled water. Then, leaf disks were blotted dry gently on a paper towel, and weighed (TW, turgid weight). Dry weight (DW) of samples was determined after drying in oven at 70 °C for 24 h. The obtained data were used for calculation of RWC (%) according to the following equation (Babu et al. 1999):
Biochemical determinations
To determine non-structural carbohydrates, the samples were homogenized in 100 mM phosphate buffer (pH 7.5) at 4 °C. After centrifugation at 12,000g for 15 min, supernatant was used to determine total soluble sugars whereas the pellets were kept for starch analysis according to the method described in Yemm and Willis (1954). An aliquot of the supernatant was mixed with anthrone (319899, Sigma)–sulfuric acid (1007312500, Merck) reagent and incubated for 10 min at 100 °C. After cooling, absorbance was determined at 625 nm. The standard curve was created using glucose (346351, Merck) in the range of 0–50 mg L−1 (Yemm and Willis 1954). To determine the starch content, the pellet was resuspended in a 4:1 (v/v) mixture of 8 N HCl/dimethylsulfoxide (472301, Sigma) to dissolve the starch by agitating for 30 min at 60 °C. After centrifugation, the supernatant was mixed with the iodine–HCl solution and left for 15 min at room temperature before absorbance was determined at 600 nm. Starch (1012531000, Merck) was used for the generation of the standard curve in the range of 0–50 mg L−1 (Yemm and Willis 1954).
To determine the soluble proteins and total free α-amino acids, the samples were homogenized in 100 mM phosphate buffer (pH 6.5) and centrifuged at 12,000g for 15 min (Yemm and Cocking 1955). Total soluble protein was determined using a commercial reagent (B6916, Sigma) and bovine serum albumin (BSA, 821007, Merck) as standard in the range of 0–2.5 mg mL−1. Total free α-amino acid content was assayed using a ninhydrin (1067620010, Merck) colorimetric method. Glycine (8160130250, Merck) was used to produce standard curve in the range of 0–1.0 mmol L−1 (Yemm and Cocking 1955).
Leaf and fruit samples were used for extraction of water soluble and CW-bound phenolics according to the method described elsewhere (Hajiboland et al. 2013). In brief, phenolics were extracted three times in 70% aqueous methanol (1060092500, Merck) at 4 °C in dark and after centrifugation, supernatant was used for determination of water soluble phenolics and the pellet for CW-bound phenolics. The supernatant was dried and extracted with diethyl ether (1009291001, Merck) and after acidification and extraction with ethyl acetate (1007891000, Merck), phenolics were determined after solubilizing in methanol. The pellet was washed sequentially with water and Triton (X100, Sigma), then the phenolics were liberated from the CW with 20 mM NH4-oxalate (70 °C) (1011920250, Merck) and then with 100 mM NaOH (1064691000, Merck) for 24 h. After acidification and extraction with ethyl acetate, CW-bound phenolics were determined after solubilizing in methanol (Hajiboland et al. 2013). The phenolics concentration was determined spectrophotometrically at 750 nm using Folin–Ciocalteu reagent (1090010100, Sigma) and gallic acid (398225, Sigma) as standard in the range of 0–0.5 mg mL−1 (Swain and Hillis 1959).
Phenylalanine ammonia lyase (PAL, EC 4.3.1.5) activity was assayed after extraction in 50 mM sodium borate buffer (pH 7.0), containing 2 mM EDTA (1084211000, Merck), 18 mM 2-mercaptoethanol (M6250, Sigma) and 2% (w/v) insoluble polyvinylpyrrolidone (02286, Sigma). After centrifugation, enzyme extract was incubated with 100 mM borate buffer (pH 8.8) containing 12 mM l-phenylalanine (1072560025, Merck) for 30 min at 30 °C. The absorbance was recorded at 290 nm and the amount of trans-cinnamic acid was calculated using its extinction coefficient of 9630 M−1. Enzyme activity was expressed as the rate of conversion of l-phenylalanine to trans-cinnamic acid mg−1 protein min−1 (Hajiboland et al. 2013). Polyphenol oxidase (PPO, EC 1.14.18.1) was extracted in 200 mM sodium phosphate buffer (pH 6.5). Assay solution consisted of 10 mM pyrogallol (1006120250, Merck) and 200 mM sodium phosphate buffer (pH 6.5) and reaction was started by adding enzyme extract at 30 °C. Change in the absorbance at 334 nm due to oxidation of pyrogallol was followed for 10 min and the activity was expressed as ∆A at 334 nm mg−1 protein min−1 (Hajiboland et al. 2013). The guaiacol (G5502, Sigma) test was used to determine peroxidase (POD, EC 1.11.1.7) activity. The enzyme was extracted by 10 mM phosphate buffer (pH 7.0), and assayed in a solution containing 10 mM phosphate buffer, 5 mM H2O2 and 4 mM guaiacol at 470 nm. The enzyme activity was expressed as enzyme protein required for the formation of 1 μmol tetraguaiacol mg−1 protein min−1 (Hajiboland et al. 2013).
Analysis of phenolic compounds using HPLC
The qualitative and quantitative profiles of phenolic compounds were studied using a HPLC system, Agilent Technologies SL 1200 Series (Waldbronn, Germany) equipped with a binary pump and UV–Vis detector and a reversed-phase Hypersil Gold C18 column (5 µm particle size, 250*4.6 mm). The mobile phase consisted of 1% aqueous acetic acid solution and methanol. Chromatograms were obtained at four different wavelengths (254, 278, 300 and 370 nm), according to absorption maxima of analysed compounds. Each compound was identified by its retention time and by spiking with standards under the same conditions. The identities of constituents were also confirmed with a photodiode array (PDA) detector by comparing to UV spectra of the standards in the wavelength range 220–450 nm (Nour et al. 2013). Standards including gallic acid (398225, Sigma), caffeic acid (C0625, Sigma), epicatechin (68097, Sigma), chlorogenic acid (Y0000569, Sigma), p-coumaric acid (03200595, Sigma), quercetin (Y0001009, Sigma), ellagic acid (14668, Sigma) and kaempferol (96353, Sigma) were purchased from Sigma Chemical Co. (USA). The concentration of standards for creating the calibration curves was in the range of 0-100 µg mL−1. Two independent analyses were performed within each biological replicate.
Harvest at fruiting stage and evaluation of fruit quality parameters
After harvesting vegetative plants, one group of plants was allowed to grow for further 8 weeks until fruiting stage. Fruits (2–4 fruits per pot) were harvested at the full-red stage. After determining FW, the fruits were longitudinally divided into halves to determine DW after being oven-dried at 70 °C for 3 days. The other half was maintained in liquid nitrogen until analysis.
Free radical scavenging activity of the fruit extract was evaluated using DPPH (2,2-diphenyl-1-picrylhydrazyl) (D9132, Sigma) quenching efficacy index at 515 nm (Panico et al. 2009). Ascorbic acid was determined using DNP (dinitrophenylhydrazine) (D199303, Sigma) reagent (DNP-thiourea-copper sulfate) (Klopotek et al. 2005). Titratable acidity of the fruit extract was obtained through titration of 10 ml juice with 0.1 N NaOH to a pH of 8.2–8.3. The results were expressed as citric acid (8187071000, Merck) equivalents (Helrich 1990). Anthocyanins content was determined using a pH differential method at pH 1.0 and pH 4.5 in the methanol/HCl (98:2, v/v) extract (Giusti and Wrolstad 2001).
Statistical analyses
The experimental design was a complete randomized block with 2 × 2 factors and four replications. Four independent pots were considered as four replicates, and data were presented as mean ± SD. Two-way ANOVA was performed for all parameters determined in the main experiment with R. clarus (Table 1). Pairwise Multiple Comparison Procedure was performed by the Tukey’s test (p < 0.05) for identification of significant differences in the mean values among the treatments. The Sigma stat (3.02) was used for statistical analysis.
Table 1.
Results of two way ANOVA analyses (mean square data) with factors including inoculation with arbuscular mycorrhizal fungus, Rhizophagus clarus (AMF) and treatment with silicon (Si) and their interactions
| Parameters | AMF | Si | AMF × Si | Parameters | AMF | Si | AMF × Si |
|---|---|---|---|---|---|---|---|
| Shoot DW | 0.233*** | 0.191*** | 0.041** | PAL activity | 0.004ns | 0.046* | 0.000ns |
| Root DW | 0.203*** | 0.164*** | 0.002ns | POD activity | 0.106ns | 0.168ns | 0.212ns |
| Colonization rate | 5625.0*** | 65.367** | 62.647* | Gallic acid | 138.65*** | 293.27*** | 63.601*** |
| Si concentration (Shoot) | 0.631*** | 10.958*** | 0.148*** | Caffeic acid | 2590.8*** | 31.923*** | 0.040ns |
| Si concentration (Root) | 0.029*** | 0.007*** | 0.001** | Epicatechin | 29.702*** | 69.722*** | 8.123** |
| Photosynthesis | 5.06*** | 2.89*** | 0.160ns | Chlorogenic acid | 461.176*** | 291.556*** | 244.141*** |
| Transpiration | 0.005ns | 0.0002ns | 0.0002ns | p-Coumaric acid | 2.641ns | 20.476*** | 71.826*** |
| Stomatal conductance | 0.559* | 0.146ns | 0.014ns | Quercetin | 14.440*** | 25.503*** | 1.690ns |
| Water use efficiency | 7.810* | 10.890** | 1.243ns | Ellagic acid | 70.560*** | 7.290*** | 0.902ns |
| Osmotic potential | 0.001ns | 0.021*** | 0.009* | Kaempferol | 158.76*** | 1.822* | 4.203** |
| Relative water content | 12.895* | 29.746*** | 27.017*** | Fruit quality | |||
| Soluble proteins | 23.112*** | 28.329*** | 0.114ns | Fruit DW | 0.061** | 0.049** | 0.003ns |
| Free Amino acids | 0.053ns | 0.0001ns | 0.068ns | DPPH assay | 650.25*** | 121.00** | 72.25ns |
| Soluble sugars | 0.311ns | 1.925* | 0.035ns | Ascorbic acid | 0.041** | 0.026* | 0.003 ns |
| Starch | 0.073* | 0.026ns | 0.003ns | Acidity | 0.032** | 0.128*** | 0.022** |
| Soluble phenolics | 38.440*** | 107.122*** | 2.890ns | Soluble phenolics | 4.379*** | 1.856** | 0.238ns |
| CW-bound phenolics | 0.018ns | 0.112*** | 0.006ns | CW-bound phenolics | 0.111** | 0.016ns | 0.007ns |
| PPO activity | 0.000ns | 0.000ns | 0.000*** | Anthocyanins | – | 90.048*** | – |
Effects are presented as ns: non-significant and significant difference: ***p < 0.001, **p < 0.01, *p < 0.05
Results
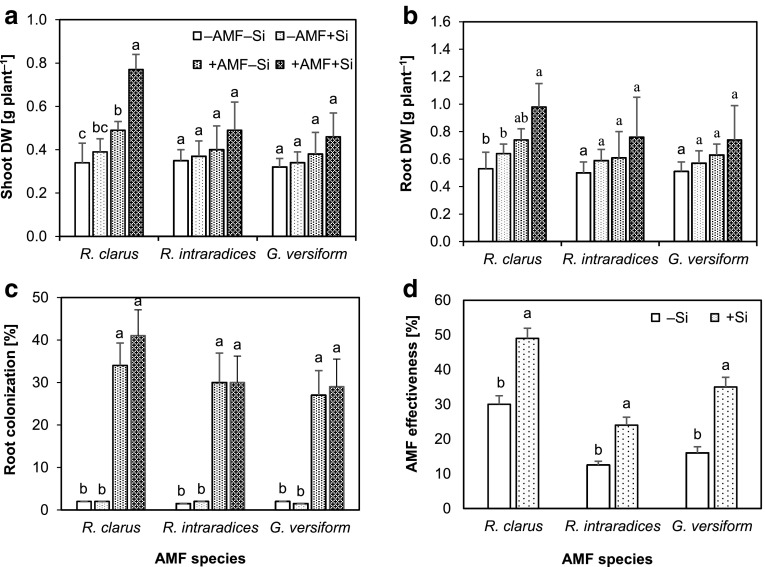
Shoot and root DW was slightly improved by Si in the − AMF plants. Although the effect of inoculation with AMF was higher than that of Si, a significant effect was observed only for shoot DW of plants inoculated with G. clarus. The combination of Si and AMF treatments resulted in higher growth, which was significant in plants inoculated with G. clarus for both shoot and root biomass (Fig. 1a, b).
Fig. 1.
Dry weight (DW) of shoot (a) and roots (b), colonization rate of roots by arbuscular mycorrhizal fungus (AMF) (c) and AMF effectiveness (d) in the strawberry plants grown for eight weeks without (− AMF) or with (+ AMF) inoculation with three AMF species in the absence (− Si) or presence (+ Si) of 3 mmol L−1 Si (as Na2SiO3). Data are mean ± SD. Bars indicated by the same letter are not significantly different (Tukey test, p < 0.05)
Root colonization was approximately 30-40% in the inoculated plants. A low (< 2%) colonization rate observed in the –AMF plants could be derived from the fungal propagules existing in the roots of daughter plants (Fig. 1c). Mycorrhizal effectiveness ranged from 12 to 49% depending on AMF species and Si treatment. In the plants inoculated with G. clarus, the highest AMF effectiveness was observed irrespective of the Si treatment. Si treatment improved mycorrhizal effectiveness that was significant for all three AMF species (Fig. 1d).
According to the results of the two-way ANOVA, both AMF and Si significantly affected growth and colonization parameters in the main experiment conducted with G. clarus. A significant interaction effect of Si and AMF was observed except for root DW (Table 1). Both Si and AMF increased shoot and root DW; however, a significant difference with control (− Si − AMF) plants was observed in the combination of both treatments (+ Si + AMF) (Fig. 2a, b). A low colonization rate was observed in the uninoculated (− AMF) plants similar to that observed in the preliminary experiment. However, effect of Si on the root colonization rate in the + AMF plants was significant in contrast to the result of that experiment (Fig. 2c). Leaf and root Si concentrations were expectedly higher in + Si plants. Inoculation with the AMF significantly increased Si concentration in the roots and leaves of + Si plants. In the –Si plants, significant effect of AMF was observed only in the roots (Fig. 2d).
Fig. 2.
Dry weight (DW) of shoot (a) and roots (b), colonization rate of roots (c) and Si concentration in the leaves and roots (d) of strawberry plants grown for 6 weeks without (− AMF) or with (+ AMF) inoculation with Rhizophagus clarus in the absence (− Si) or presence (+ Si) of 3 mmol L−1 Si (as Na2SiO3). Data are mean ± SD. Bars indicated by the same letter are not significantly different (Tukey test, p < 0.05)
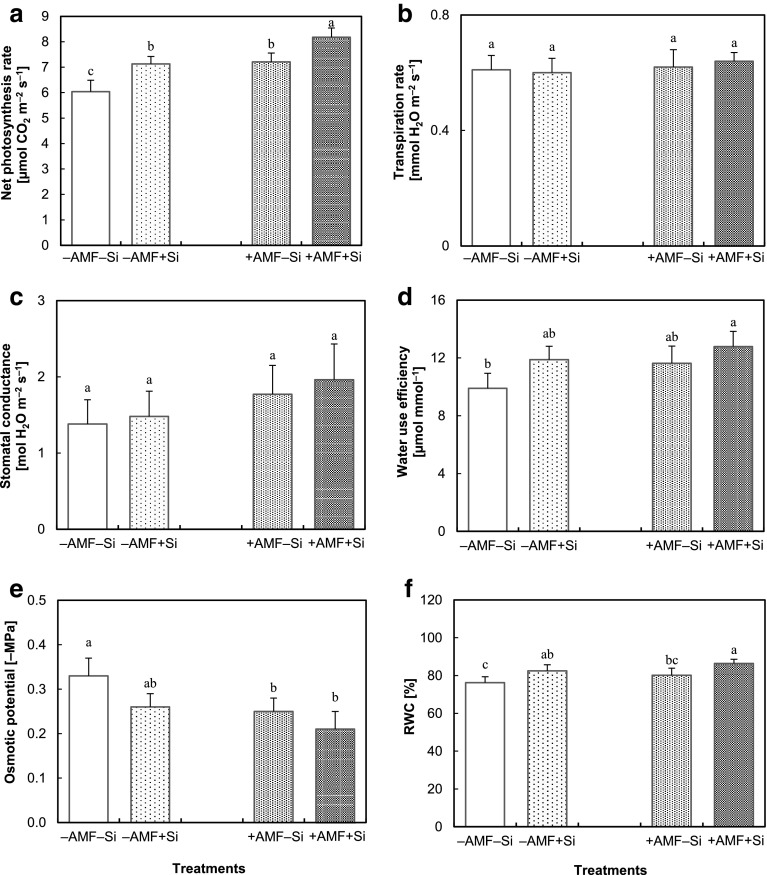
ANOVA results of gas exchange and water relation parameters indicated that stomatal conductance was significantly influenced only by AMF and leaf osmotic potential was influenced only by Si, while photosynthetic rate, WUE and RWC were affected by both treatments. A significant interaction effect of Si and AMF was observed for leaf osmotic potential and RWC (Table 1). Net photosynthesis rate was elevated by both Si and AMF, the highest amount was found in the combination of the two treatments (Fig. 3a). Stomatal conductance (Fig. 3b) was only slightly improved by Si and AMF treatments, while transpiration rate (Fig. 3c) remained almost stable, resulting in significantly higher water use efficiency in the + Si + AMF plants compared to − Si − AMF ones (Fig. 3d). Leaf osmotic potential slightly increased by both treatments (Fig. 3e) and simultaneously, RWC increased by both Si and AMF, particularly under combination of both treatments (Fig. 3f).
Fig. 3.
Gas exchange parameters including net photosynthesis rate (a), transpiration rate (b), stomatal conductance (c) and water relation parameters including water use efficiency (d), osmotic potential (e) and relative water content (RWC) (f) in the leaves of strawberry plants grown for 6 weeks without (− AMF) or with (+ AMF) inoculation with Rhizophagus clarus in the absence (− Si) or presence (+ Si) of 3 mmol L−1 Si (as Na2SiO3). Data are mean ± SD. Bars indicated by the same letter are not significantly different (Tukey test, p < 0.05)
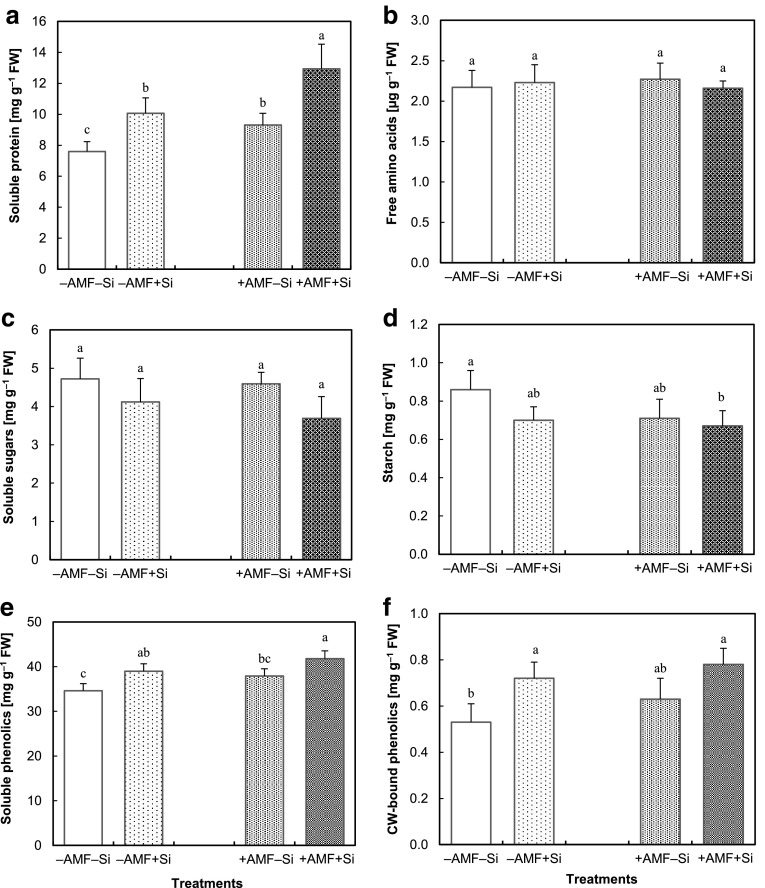
ANOVA results of the metabolite concentrations in the leaves revealed that both treatments influenced total soluble proteins and phenolics without any interaction effect. Concentration of starch was influenced only by AMF, while soluble sugars and CW-bound phenolics contents were affected only by Si, but not the AMF treatment (Table 1). Plants treated with Si as well as those inoculated with AMF had higher protein in the leaves; the highest concentrations was found in the + Si + AMF plants (Fig. 4a). The total free α-amino acid content (Fig. 4b) was not influenced by the applied treatments while soluble sugars and starch concentrations tended to be decreased by both treatments, this effect was significant for starch in the combinative treatment of Si and AMF (Fig. 4c, d). Both soluble and CW-bound phenolics concentrations significantly increased upon Si treatment in the absence or presence of AMF, the latter treatment alone did not influence this parameter significantly (Fig. 4e, f).
Fig. 4.
Concentration of total soluble proteins (a), free α-amino acids (b), soluble sugars (c), starch (d), soluble (e) and cell wall (CW)-bound (f) phenolics in the leaves of strawberry plants grown for 6 weeks without (− AMF) or with (+ AMF) inoculation with Rhizophagus clarus in the absence (− Si) or presence (+ Si) of 3 mmol L−1 Si (as Na2SiO3). Data are mean ± SD. Bars indicated by the same letter are not significantly different (Tukey test, p < 0.05)
A significant interaction effect of Si and AMF was observed for PPO activity (Table 1). Multiple comparison of means showed that activity of PPO significantly decreased by Si treatment in the − AMF plants, while increased in the + AMF ones. PAL activity was influenced only by Si treatment that was significant in the − AMF plants. Activity of POD was not changed significantly either by Si or AMF inoculation (Table 2).
Table 2.
Activity of polyphenol oxidase (PPO), phenylalanine ammonia-lyase (PAL) and peroxidase (POD) in the leaves of strawberry plants grown for 6 weeks without (− AMF) or with (+ AMF) inoculation with Rhizophagus clarus in the absence (− Si) or presence (+ Si) of 3 mmol L−1 Si (as Na2SiO3)
| Treatments | PPO (ΔA334 mg−1 protein h−1) | PAL (nmol mg−1 protein min−1) | POD (µmol mg−1 protein min−1) |
|---|---|---|---|
| − AMF | |||
| − Si | 3.0 ± 0.42a | 0.51 ± 0.04b | 5.47 ± 1.15a |
| + Si | 1.8 ± 0.18c | 0.65 ± 0.06a | 5.81 ± 1.12a |
| + AMF | |||
| − Si | 1.8 ± 0.24c | 0.59 ± 0.05ab | 5.93 ± 1.22a |
| + Si | 2.4 ± 0.36b | 0.66 ± 0.07a | 5.82 ± 1.17a |
Data are mean ± SD. Data in each column indicated by the same letter are not significantly different (Tukey test, p < 0.05)
ANOVA results showed that Si significantly influenced all the analyzed phenolic compounds except for p-coumaric acid that was influenced by Si, but not AMF (Table 1). Similar to the effect of Si, AMF colonization increased phenolics concentrations (except for p-coumaric acid); this effect was significant for caffeic acid, chlorogenic acid, quercetin, ellagic acid and kaempferol (Table 3). The highest concentrations of all analyzed phenolic compounds were found in the combination of both treatments. Both Si and AMF as single treatments decreased concentration of p-coumaric acid, while an increase was observed in the combination of Si and AMF (Table 3).
Table 3.
Composition of phenolic compounds in the soluble fraction of leaves in strawberry plants grown for 6 weeks without (− AMF) or with (+ AMF) inoculation with Rhizophagus clarus in the absence (− Si) or presence (+ Si) of 3 mmol L−1 Si (as Na2SiO3)
| Treatments | Gallic acid (µg mg−1 total phenol) | Caffeic acid (µg mg−1 total phenol) | Epicatechin (µg mg−1 total phenol) | Chlorogenic acid (µg mg−1 total phenol) |
|---|---|---|---|---|
| − AMF | ||||
| − Si | 10.3 ± 1.1c | 9.3 ± 1.2d | 7.9 ± 0.3c | 7.6 ± 1.1c |
| + Si | 14.2 ± 1.3b | 12.6 ± 1.2c | 9.7 ± 0.5b | 8.6 ± 1.5c |
| + AMF | ||||
| − Si | 11.4 ± 1.0c | 34.6 ± 0.9b | 8.5 ± 0.8bc | 10.5 ± 1.0b |
| + Si | 25.8 ± 1.2a | 36.9 ± 1.1a | 14.5 ± 1.3a | 26.3 ± 1.2a |
| Treatments | p-Coumaric acid (µg mg−1 total phenol) | Quercetin (µg mg−1 total phenol) | Ellagic acid (µg mg−1 total phenol) | Kaempferol (µg mg−1 total phenol) |
|---|---|---|---|---|
| − AMF | ||||
| − Si | 11.4 ± 0.9a | 7.2 ± 1.0c | 3.0 ± 0.05d | 2.1 ± 0.10c |
| + Si | 4.5 ± 0.5d | 10.3 ± 1.0ab | 3.9 ± 0.08c | 3.5 ± 0.11b |
| + AMF | ||||
| − Si | 6.1 ± 0.7c | 9.7 ± 1.1b | 6.4 ± 0.7b | 9.3 ± 0.2a |
| + Si | 8.3 ± 0.9b | 11.8 ± 1.0a | 8.4 ± 0.7a | 9.0 ± 0.5a |
Data are mean ± SD. Data in each column indicated by the same letter are not significantly different (Tukey test, p < 0.05)
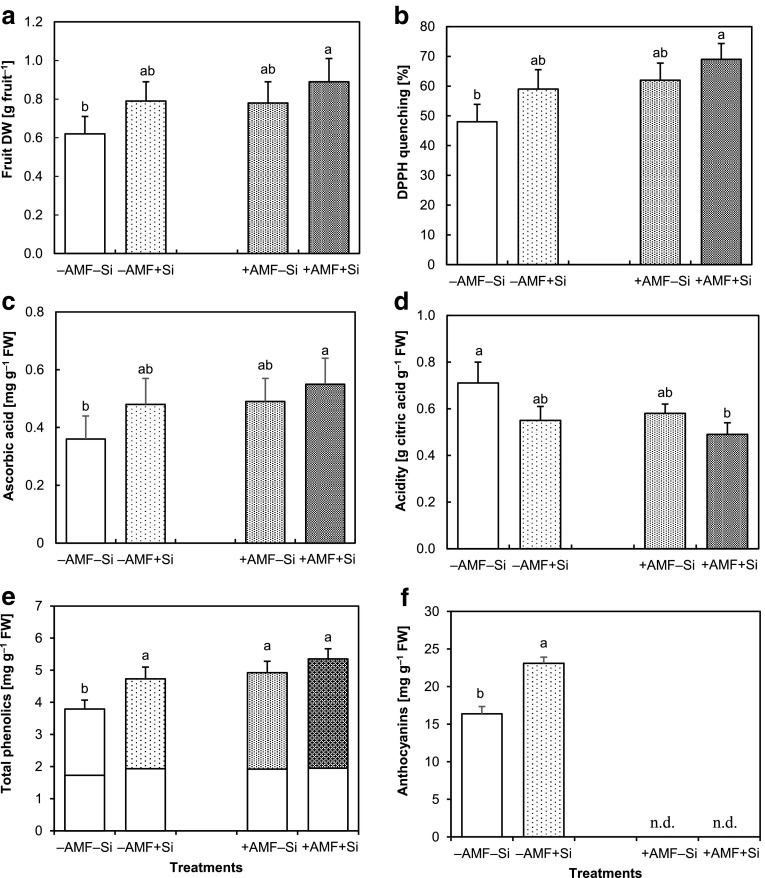
All fruit quality parameters were significantly influenced by Si and AMF treatments except for the CW-bound phenolics that was not affected by Si (Table 1). Fruit weight increased by both Si and AMF treatments, and the highest fruit DW was obtained under combination of both treatments (Fig. 5a). The total antioxidative (DPPH quenching) activity (Fig. 5 b) and concentration of ascorbic acid (Fig. 5c) were similarly increased by applied treatments, and the highest values for both parameters were achieved in + AMF + Si treatment. In contrast, titratable acidity of fruit juice decreased by both treatments and significantly lower amount of juice acidity was observed in the + AMF + Si plants (Fig. 5d). Similar to the vegetative plants, soluble phenolics concentration in the fruits was higher in the single and in the combination of Si and AMF treatments, while CW-bound phenolics concentration was not significantly influenced either by Si or AMF (Fig. 5e). Fruit anthocyanins concentration was increased by Si treatment (Fig. 5f).
Fig. 5.
Dry weight (DW) (a), total antioxidative activity (measured as quenching of DPPH radical) (b), concentration of ascorbic acid (c), titratable acidity (d), concentrations of soluble (e) and cell wall (CW)-bound (f) phenolics in the fruits of strawberry plants grown for 14 weeks without (− AMF) or with (+ AMF) inoculation with Rhizophagus clarus in the absence (− Si) or presence (+ Si) of 3 mmol L−1 Si (as Na2SiO3). Data are mean ± SD. Bars indicated by the same letter are not significantly different (Tukey test, p < 0.05)
Discussion
There are evidences suggesting that Si has an improving effect on plants productivity even under optimum growth conditions in the field (Guntzer et al. 2012). However, mechanisms for higher mycorrhizal effectiveness in Si supplemented plants observed in this work have not been investigated so far and are reported here for the first time.
Effect of Si and AMF on the growth of strawberry plants
Growth promoting effect of Si observed in the strawberry plants of this work was also reported for this species in a solution culture study (Miyake and Takahashi 1986). Growth enhancement by AMF association was accompanied by enhanced Si acquisition. It has been observed that maize plants colonized with AMF and grown in acid soil had higher Si concentration over the non-mycorrhizal plants (Clark and Zeto 2000). Various mechanisms could be involved in improving dry matter production in strawberry plants with Si, AMF and their combination. Increased net photosynthesis rate and protein concentrations, i.e. C and N metabolism, were the most probable mechanisms observed in this work. Mycorrhizal plants have often elevated photosynthesis rate due to higher stomatal conductance (Porcel et al. 2012; Hajiboland 2013). In this study, however, elevation of photosynthesis mainly resulted from non-stomatal mechanisms. Higher contents of leaf chlorophyll and higher activity of photosynthetic enzymes (Guntzer et al. 2012; Hajiboland 2012) may all be involved in the Si-mediated elevation of photosynthesis rate. Contribution of changes in canopy structure and light reflectance observed in graminaceous species (Broadley et al. 2012; Hajiboland 2012) are also the mechanisms deserving further studies in non-graminaceous species such as strawberry.
Improvement of water relation parameters could be another mechanism to enhance plant growth by Si and AMF. Although, in this study, strawberry plants were irrigated sufficiently (85% FC), slight or significant increase of leaf osmotic potential and RWC indicates that + Si + AMF plants were able to retain water in the leaves more efficiently. This efficiency was not the result of lower water loss, but likely higher water uptake capacity of plants. Higher root surface area as could be judged by higher root biomass was at least partly responsible for higher water uptake capacity in + Si + AMF plants. Root hydraulic conductance and water uptake capacity are directly improved via extended extra radical hyphae as well as modifications in the expression of aquaporins (Porcel et al. 2012; Hajiboland 2013). Owing to shallow root system, large leaf area and high water content of fruits, strawberry is extremely sensitive to drought. Both yield and fruit size are reduced when the crop is not irrigated sufficiently (Ghaderi et al. 2015).
Effect of Si and AMF on the quality of fruits
Both Si and AMF treatments improved not only yield, but also the market value of fruits so that the highest values were obtained in the combination of both treatments. Higher antioxidative effect of strawberry juice under applied treatments was linked to higher ascorbic acid and phenolics concentrations that may have resulted from upregulation of synthesis of these metabolites in the fruits. Lower titratable acidity is also regarded a quality parameter since sweet fruits are preferred by consumers (Costa et al. 2011).
Effect of Si and AMF on plants phenolics metabolism and profile
Study of the Si effect on phenolics metabolism in tobacco showed reduction of soluble and CW-bound phenolics concomitant with downregulation of PAL, PPO and soluble POD fraction by Si (Hajiboland et al. 2017a). In contrast, in this work, the strawberry plants showed high phenol fractions and PAL activity in + Si plants irrespective of the inoculation status. The reason for the observed difference between the Si non-accumulator tobacco and strawberry as an accumulator species is unknown. It is tempting to hypothesize that in non-accumulators such as tobacco, Si may hinder C metabolites allocation to the phenylpropanoid pathway through downregulation of metabolizing enzymes, consequently favoring the allocation of fixed C to growth and dry matter production under these conditions (Hajiboland et al. 2017a). In Si accumulator species, however, upregulation of this pathway may have in turn some important ecological functions such as plant defense as observed for cucumber against powdery mildew diseases (Fawe et al. 1998).
In contrast to PAL, PPO activity declined by Si in the strawberry plants of this work. It has been observed that Si stimulates the formation of Si-polyphenol complexes causing phenolic compounds to be less available as substrates for PPO and POD (Maksimovic et al. 2007). In rice leaves, Si binds to the phenol-carbohydrate complexes with their phenol-moieties, but not with the carbohydrate (Inanaga and Okasaka 1995). Formation of Si-phenol complexes, thus, may affect the pool of phenols in plant tissues and reduce lignin formation.
The underlying mechanisms for Si-mediated modification of plant phenolics metabolism are obscure. The changes in the activity of enzymes upon Si treatment were mainly attributed to the post-transcriptional and/or allosteric regulation of enzymes (Van Bockhaven et al. 2012). Although, there is no definitive evidence yet that Si binds to proteins or has direct biochemical functions, in vitro complexation of orthosilicic acid by certain sugars and hydroxyl-amino acids has been documented (Jugdaohsingh et al. 2008). Silicon may influence plant mechanism through hormone signaling. In the leaves challenged with pathogens, Si stimulates the biosynthesis of salicylic and jasmonic acids, and triggers activation of jasmonic acid and ethylene signaling pathways (Van Bockhaven et al. 2012).
Phenolics profile was significantly modified by both Si and AMF treatments and their combination. Concentrations of the majority of phenolic compounds found in the leaves were slightly or significantly increased in + Si plants except for p-coumaric acid. Mycorrhization also modified the levels of all analyzed phenolics in the leaves except for gallic acid and epicatechin. Castellanos-Morales et al. (2010) reported effect of AMF on the phenolics profile in the strawberries fruit as an increase in the concentrations of p-coumaric acid, quercetin and kaempferol, while observed a decrease in the gallic acid and ellagic acid concentrations. Different results with our work for p-coumaric and ellagic acid could be, at least partly, attributed to the different organs (fruits vs. leaves) analyzed in these studies. To our knowledge, except for the work of Castellanos-Morales et al. (2010) and our data reported here, there are no data on the effect of AMF on phenolics profile in the strawberry plants. However, there are several reports on the changes in the pattern of secondary plant compounds by AMF inoculation in other plant species. During establishment of the AMF symbiosis, flavonoids and phenolic compounds are accumulated (Vierheilig 2004). Mycorrhization induced changes at the levels of p-coumaric acid and ferulic acid in the roots of onion (Grandmaison et al. 1993), at the levels of biochanin A, formononetin, genistein and daidzein in the roots of alfalfa (Larose et al. 2002) and at the levels of isoflavone in the leaves of red clover (Khaosaad et al. 2008). The upregulation of phenolics metabolic pathway could be regarded either as a specific host-AMF signaling i.e. for flavonoids (García-garrido and Ocampo 2002) and/or a reflection of plant defense strategy since the response of plants to AMF involves a temporal and spatial activation of defense mechanisms (García-garrido and Ocampo 2002).
Among phenolic compounds detected in the leaves of strawberry, concentration of p-coumaric acid differently responded to the applied treatments. It decreased by both Si and AMF as single treatments, while in contrast to the − AMF plants, Si treatment increased p-coumaric acid concentration in the + AMF one. The same interaction effect of treatments was observed for PPO activity, but its probable significance for AMF effectiveness is obscure. More detailed studies are required to unravel the mechanisms for Si and AMF interaction effect on the phenylpropanoid pathway.
Mechanisms for the positive interactions between Si and AMF
A clear synergistic effect between AMF and Si was observed on the analyzed physiological and biochemical parameters in this work. The most prominent synergistic effects were higher root colonization and greater mycorrizal effectiveness of + Si compared to − Si plants. In addition to increased Si uptake in AMF plants, synergistic effects may be also explained by Si-induced stimulation of root growth, which may promote the AMF colonization, and consequently increase the beneficial effects of AMF in + Si + AMF plants. The elevated photosynthesis rate by Si was likely another mechanism for the synergistic effect of Si on AMF effectiveness. There is a positive correlation between photosynthetic rate and the hyphal absorption capacity (Smith and Read 2008). Mycorhizal association is completely dependent on the organic C supply from their host plant (Smith and Read 2008). A recent report on the effect of Si on mycorrhizal chickpea (Garg and Bhandari 2016) showed an increase in salinity tolerance by both Si and AMF, but a synergistic effect was not detected. The effect of Si on mycorrhizal effectiveness has not been investigated so far, but there are some pieces of evidence on the beneficial effects of Si on nodulated legume species such as doubling the number of infection sites on root hairs, enhanced invasion of Rhizobium and increased symbiotic performance (Nelwamondo and Dakora 1999).
The synergistic effect of Si and AMF could also be ascribed to the Si-mediated modifications in the phenolics metabolism in the host plant. Silicon treatment may modulate different compounds in the host plant, which can influence AMF interaction by host. It has been demonstrated that phenolic compounds such as flavonoids may play a role in facilitating interactions between the fungus and host (Mandal et al. 2010), have some positive effects on fungal growth parameters e.g. hyphal growth and branching, germination of spores (Steinkellner et al. 2007) and formation of secondary spores, and play a role during fungal invasion and arbuscule formation inside the root (Hassan and Mathesius 2012). It is plausible that Si enhances symbiosis formation by modifying the main released phenolics and/or reducing polymerization and lignin synthesis, thereby facilitating AMF formation in the strawberry plants of this work.
Overall, our results demonstrate that Si plays a function in the metabolism of accumulator species not only under stress but also under optimum growth conditions. Silicon-mediated metabolic alterations occurred in parallel with AMF-related modifications in the plants metabolism in a synergistic manner resulting in improving photosynthesis rate and water relation parameters as well as in the modifications detected in the phenolics metabolism.
Acknowledgements
The authors would like to thank Dr G. Neumann (University of Hohenheim, Germany) for providing facility of Si analysis and Dr H.R. Beheshti (Testa Quality Control Laboratory, North-East Food Industrial Technology and Biotechnology Park, Mashhad, Iran) and Dr M.S. Nabavi (Department of Agriculture, Payame Noor University, Tehran, Iran) for their assistance in the HPLC analysis.
Abbreviations
- AMF
Arbuscular mycorrhizal fungus
- CW
Cell wall
- PAL
Phenylalanine ammonia-lyase
- POD
Peroxidase
- PPO
Polyphenol oxidase
- RWC
Relative water content
- Si
Silicon
Authors’ contribution
RH: Project supervisor, responsible for experimental design, interpretation of results, writer of the manuscript. NM: Cultivation of plants, performing all analyses; NA: Project co-supervisor, providing fungal inoculum, involvement in the writing and correction of the paper; ZE: Providing facility and assistance in the HPLC analysis; JF: Providing facility and assistance in the HPLC analysis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Amil-Ruiz F, Blanco-Portales R, Muñoz-Blanco J, Caballero JL. The strawberry plant defense mechanism: a molecular review. Plant Cell Physiol. 2011;52:1873–1903. doi: 10.1093/pcp/pcr136. [DOI] [PubMed] [Google Scholar]
- Babu RC, Pathan MS, Blum A, Nguyen HT. Comparison of measurement methods of osmotic adjustment in rice cultivars. Crop Sci. 1999;39:150–158. doi: 10.2135/cropsci1999.0011183X003900010024x. [DOI] [Google Scholar]
- Borkowska B. Growth and photosynthetic activity of micropropagated strawberry plants inoculated with endomycorrhizal fungi (AMF) and growing under drought stress. Acta Physiol Plant. 2002;24:365–370. doi: 10.1007/s11738-002-0031-7. [DOI] [Google Scholar]
- Broadley M, Brown P, Cakmak I, Ma JF, Rengel Z, Zhao F. Beneficial elements. In: Marschner P, editor. Marschner’s mineral nutrition of higher plants. Oxford: Elsevier; 2012. pp. 249–269. [Google Scholar]
- Castellanos-Morales V, Villegas J, Wendelin S, Vierheilig H, Eder R, Cárdenas-Navarro R. Root colonisation by the arbuscular mycorrhizal fungus Glomus intraradices alters the quality of strawberry fruits (Fragaria × ananassa Duch.) at different nitrogen levels. J Sci Food Agric. 2010;90:1774–1782. doi: 10.1002/jsfa.3998. [DOI] [PubMed] [Google Scholar]
- Clark RB, Zeto SK. Mineral acquisition by arbuscular mycorrhizal plants. J Plant Nutr. 2000;23:867–902. doi: 10.1080/01904160009382068. [DOI] [Google Scholar]
- Costa FB, Duarte PS, Puschmann R, Finger FL. Quality of fresh-cut strawberry. Hortic Bras. 2011;29:477–484. doi: 10.1590/S0102-05362011000400006. [DOI] [Google Scholar]
- Etesami H, Jeong BR. Silicon (Si): review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotox Environ Safe. 2018;147:881–896. doi: 10.1016/j.ecoenv.2017.09.063. [DOI] [PubMed] [Google Scholar]
- Fawe A, Abou-zaid M, Menzies JG, Bélanger RR. Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology. 1998;88:396–401. doi: 10.1094/PHYTO.1998.88.5.396. [DOI] [PubMed] [Google Scholar]
- García-garrido JM, Ocampo JA. Regulation of the plant defense response in arbuscular mycorrhizal symbiosis. J Exp Bot. 2002;53:1377–1386. doi: 10.1093/jexbot/53.373.1377. [DOI] [PubMed] [Google Scholar]
- Garg N, Bhandari P. Silicon nutrition and mycorrhizal inoculations improve growth, nutrient status, K+/Na+ ratio and yield of Cicer arietinum L. genotypes under salinity stress. Plant Growth Regul. 2016;78:371–387. doi: 10.1007/s10725-015-0099-x. [DOI] [Google Scholar]
- Ghaderi N, Normohammadi S, Javadi T. Morpho-physiological responses of strawberry (Fragaria × ananassa) to exogenous salicylic acid application under drought stress. J Agric Sci Technol. 2015;17:167–178. [Google Scholar]
- Giampieri F, Alvarez-Suarez JM, Battino M. Strawberry and human health: effects beyond antioxidant activity. J Agric Food Chem. 2014;62:3867–3876. doi: 10.1021/jf405455n. [DOI] [PubMed] [Google Scholar]
- Giovanetti M, Mosse B. An evaluation of techniques for measuring vesicular–arbuscular mycorrhizal infection in roots. New Phytol. 1980;84:489–500. doi: 10.1111/j.1469-8137.1980.tb04556.x. [DOI] [Google Scholar]
- Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV–visible spectroscopy. In: Wrolstad RE, Acree TE, An H, Decker EA, Pennere MH, Reid DS, Schwartz SJ, Shoemaker CF, Sporns P, editors. Current protocol in food analytical chemistry. New York: Wiley; 2001. pp. F1.2.1–F.1.2.13. [Google Scholar]
- Grandmaison J, Olah GM, Van Calsteren MR, Furlan V. Characterization and localization of plant phenolics likely involved in the pathogen resistance expressed by endomycorrhizal roots. Mycorrhiza. 1993;3:155–164. doi: 10.1007/BF00203609. [DOI] [Google Scholar]
- Guntzer F, Keller C, Meunier JD. Benefits of plant silicon for crops: a review. Agron Sustain Dev. 2012;32:201–213. doi: 10.1007/s13593-011-0039-8. [DOI] [Google Scholar]
- Hajiboland R. Effect of micronutrient deficiencies on plant stress responses. In: Ahmad P, Prasad MNV, editors. Abiotic stress responses in plants. New York: Springer; 2012. pp. 283–329. [Google Scholar]
- Hajiboland R. Role of arbuscular mycorrhiza in amelioration of salinity. In: Ahmad P, Azzoz MM, Prasad MNV, editors. Salt stress in plants, signaling, omics and adaptations. New York: Springer; 2013. pp. 301–354. [Google Scholar]
- Hajiboland R, Bahrami-rad S, Bastani S. Phenolics metabolism in boron-deficient tea (Camellia sinensis) plants. Acta Biol Hung. 2013;64:196–206. doi: 10.1556/ABiol.64.2013.2.6. [DOI] [PubMed] [Google Scholar]
- Hajiboland R, Bahrami-rad S, Poschenrieder C. Silicon modifies both local and systemic responses to mechanical stress in tobacco leaves. Biol Plant. 2017;61:187–191. doi: 10.1007/s10535-016-0633-3. [DOI] [Google Scholar]
- Hajiboland R, Moradtalab N, Eshaghi Z, Feizy J. Effect of silicon supplementation on growth and metabolism of strawberry plants at three developmental stages. N Z J Crop Hortic Sci. 2017;46:144–161. doi: 10.1080/01140671.2017.1373680. [DOI] [Google Scholar]
- Hassan S, Mathesius U. The role of flavonoids in root–rhizosphere signaling: opportunities and challenges for improving plant–microbe interactions. J Exp Bot. 2012;63:3429–3444. doi: 10.1093/jxb/err430. [DOI] [PubMed] [Google Scholar]
- Helrich K. Official methods of analysis. 15. Arlington: Association of Official Analytical Chemists (AOAC); 1990. [Google Scholar]
- Inanaga S, Okasaka A. Calcium and silicon binding compounds in cell walls of rice shoots. Soil Sci Plant Nutr. 1995;4:103–110. doi: 10.1080/00380768.1995.10419563. [DOI] [Google Scholar]
- Jaiswal PC. Soil, plant and water analysis. New Delhi: Kalyani Publishers; 2004. [Google Scholar]
- Janos DP. Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza. 2007;17:75–91. doi: 10.1007/s00572-006-0094-1. [DOI] [PubMed] [Google Scholar]
- Jugdaohsingh R, Kinrade SD, Powell JJ. Is there a biochemical role for silicon? In: Collery P, Maymard I, Thephanides T, Khassanova L, Collery T, editors. Metal ions in biology and medicine. Montrouge: John Libbey Eurotext; 2008. pp. 45–55. [Google Scholar]
- Khaosaad T, Krenn L, Medjakovic S, Ranner A, Lössl A, Nell M, et al. Effect of mycorrhization on the isoflavone concentration and the phytoestrogen activity of red clover. J Plant Physiol. 2008;165:1161–1167. doi: 10.1016/j.jplph.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Klopotek Y, Otto K, Böhm V. Processing strawberries to different products alters contents of vitamin C, total phenolics, total anthocyanins, and antioxidant capacity. J Agric Food Chem. 2005;53:5640–5646. doi: 10.1021/jf047947v. [DOI] [PubMed] [Google Scholar]
- Larose G, Chenevert R, Moutoglis P, Gagne S, Piché Y, Vierheilig H. Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. J Plant Physiol. 2002;159:1329–1339. doi: 10.1078/0176-1617-00896. [DOI] [Google Scholar]
- Maksimovic JD, Bogdanovic J, Maksimovic V, Nikolic M. Silicon modulates the metabolism and utilization of phenolic compounds in cucumber (Cucumis sativus L.) grown at excess manganese. J Plant Nutr Soil Sci. 2007;170:739–744. doi: 10.1002/jpln.200700101. [DOI] [Google Scholar]
- Mandal SM, Chakraborty D, Dey S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav. 2010;5:359–368. doi: 10.4161/psb.5.4.10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merryweather JW, Fitter AH. A modified method for elucidating the structure of the fungal partner in a vesicular ± arbuscular mycorrhiza. Mycol Res. 1991;95:1435–1437. doi: 10.1016/S0953-7562(09)80399-7. [DOI] [Google Scholar]
- Miyake Y, Takahashi E. Effect of silicon on the growth and fruit production of strawberry plants in a solution culture. Soil Sci Plant Nutr. 1986;32:321–326. doi: 10.1080/00380768.1986.10557510. [DOI] [Google Scholar]
- Nelwamondo A, Dakora FD. Silicon promotes nodule formation and nodule function in symbiotic cowpea (Vigna unguiculata) New Phytol. 1999;142:463–467. doi: 10.1046/j.1469-8137.1999.00409.x. [DOI] [Google Scholar]
- Nour V, Trandafir I, Cosmulescu S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J Chromatogr Sci. 2013;51:883–890. doi: 10.1093/chromsci/bms180. [DOI] [PubMed] [Google Scholar]
- Panico AM, Garufi F, Nitto S, Di Mauro R, Longhitano RC, Magrì G, De Guidi G. Antioxidant activity and phenolic content of strawberry genotypes from Fragaria × ananassa. Pharm Biol. 2009;47:203–208. doi: 10.1080/13880200802462337. [DOI] [Google Scholar]
- Passardi F, Cosio C, Penel C, Dunand C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005;24:255–265. doi: 10.1007/s00299-005-0972-6. [DOI] [PubMed] [Google Scholar]
- Porcel R, Aroca R, Ruiz-Lozano JM. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron Sustain Dev. 2012;32:181–200. doi: 10.1007/s13593-011-0029-x. [DOI] [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. San Diego: Academic Press; 2008. [Google Scholar]
- Steinkellner S, Lendzemo V, Langer I, Schweiger P, Khaosaad T, Toussaint JP, Vierheilig H. Flavonoids and strigolactones in root exudates as signals in symbiotic and pathogenic plant-fungus interactions. Molecules. 2007;12:1290–1306. doi: 10.3390/12071290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain T, Hillis EE. The phenolic constituents of Prunus domestica I. The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Tawaraya K. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci Plant Nutr. 2003;49:655–668. doi: 10.1080/00380768.2003.10410323. [DOI] [Google Scholar]
- Van Bockhaven J, De Vleesschauwer D, Höfte M. Towards establishing broad-spectrum disease resistance in plants: silicon leads the way. J Exp Bot. 2012;64:1281–1293. doi: 10.1093/jxb/ers329. [DOI] [PubMed] [Google Scholar]
- Vierheilig H. Regulatory mechanisms during the plant—arbuscular mycorrhizal fungus interaction. Can J Bot. 2004;82:1166–1176. doi: 10.1139/b04-015. [DOI] [Google Scholar]
- Yemm EW, Cocking EC. The determination of amino acids with ninhydrin. Analyst. 1955;80:209–213. doi: 10.1039/an9558000209. [DOI] [Google Scholar]
- Yemm EW, Willis AJ. The estimation of carbohydrates extracts by anthrone. Biochem J. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]