Abstract
The short time response to salt stress was studied in Cakile maritima. Plants were exposed to different salt concentrations (0, 100, 200 and 400 mM NaCl) and harvested after 4, 24, 72 and 168 h of treatment. Before harvesting plants, tissue hydration, osmotic potential, inorganic and organic solute contents, and ornithine-δ-aminotransferase activity were measured. Plants of C. maritima maintained turgor and tissue hydration at low osmotic potential mainly at 400 mM NaCl. The results showed that, in leaves and stems, Na+ content increased significantly after the first 4 h of treatment. However, in roots, the increase of Na+ content remained relatively unchanged with increasing salt. The K+ content decreased sharply at 200 and 400 mM NaCl with treatment duration. This decrease was more pronounced in roots. The content of proline and amino acids increased with increasing salinity and treatment duration. These results indicated that the accumulation of inorganic and organic compounds was a central adaptive mechanism by which C. maritima maintained intracellular ionic balance under saline conditions. However, their percentage contribution to total osmotic adjustment varies from organ to organ; for example, Na+ accumulation mainly contributes in osmotic adjustment of stem tissue (60%). Proline contribution to osmotic adjustment reached 36% in roots. In all organs, proline as well as δ-OAT activity increased with salt concentration and treatment duration. Under normal growth conditions, δ-OAT is mainly involved in the mobilization of nitrogen required for plant growth. However, the highly significant positive correlation between proline and δ-OAT activity under salt-stress conditions suggests that ornithine pathway contributed to proline synthesis.
Keywords: Halophyte, Organic solutes, Osmoregulation, δ-OAT activity, Salt stress
Introduction
Plants encounter a variety of abiotic stresses including salt stress, a major constraint for plant growth (Ashraf et al. 2008). It limits plant development by disruption of various biochemical reactions and physiological processes such as photosynthesis (Athar et al. 2015). The deleterious effects of salinity on plants are associated with various types of stress (1) water deficit stress, related to the sharp decrease in water availability and the greater difficulty of its absorption (2) ion toxicity, that occurs when the accumulation of salts in tissue disrupts metabolic activity, (3) limited nutrient uptake resulting from the antagonism between Na+ and cations and (4) oxidative stress (Munns and Tester 2008). As a result of salinity, imbalances in metabolism and salt-induced symptoms lead to damage of membrane and eventually cellular death.
Maintaining growth of the plant on salt affected land is related to its ability to maintain cellular turgor at low osmotic potential and maintaining low cytosolic Na+ contents with high K+/Na+ ratios. These features help in continuing K+ dependent biochemical processes through osmotic adjustment (Munns and Tester 2008). Osmotic adjustment (OA) in response to salinity stress is a physiological adaptation which has drawn attention during the last years (Li et al. 2017; Nguyen et al. 2017). OA can be accomplished through modes by which Na+ influx is restricted or by its sequestration into the vacuole leading to decrease in toxic concentration of ions in the cytoplasm, or by synthesis of organic compounds generically named compatible solutes or osmolytes (Ashraf 2004; Munns and Tester 2008). Many of these compatible solutes contain nitrogen. These N-containing compounds include glycine betaine, proline and polyamines (Ashraf and Foolad 2007; Mansour and Ali 2017). Hence the nitrogen metabolism is significant under stress conditions.
Proline, an amino acid, which is accumulated in response to diverse types of abiotic stress (Verbruggen and Hermans 2008) is one of such molecules that has several roles. Overproduction and accumulation of proline in salt stressed plants play an important role in tolerance of plants to NaCl by reducing oxidative stresses (Molinari et al. 2007). This metabolite offers a wide range of protective roles including stabilizing of cellular structure and reduction of damage in the photosynthetic apparatus, redox status balance, cytosolic pH maintenance, stabilization of protein structure and through participating in stress signal (Maggio et al. 2002), thereby influencing adaptive responses (Verbruggen and Hermans 2008). Besides its role as an osmoprotectant, it function as a solute to decrease leaf water potential, maximizing water uptake and/or reducing transpiration to maintain cell turgor pressure during conditions associated with salinity and water deficit stress (Ashraf and Foolad 2007; Ashraf and Harris 2004).
In higher plants, two pathways of proline biosynthesis have been demonstrated, one from glutamate, and the other from ornithine (Kishor et al. 2005; Mansour and Ali 2017; Roosens et al. 2002). The role of the glutamate pathway in proline accumulation is well established and has been shown to be the predominant pathway in response to osmotic stress (Mansour and Ali 2017). Concerning ornithine pathway, the ornithine-δ-aminotransferase (δ-OAT) has been shown to participate in proline synthesis. It catalyzes the loss of the ornithine-δ-amino group from ornithine to provide pyrroline -5- carboxylate (P5C) in mitochondria. The product formed is transported into the cytoplasm which is further reduced to proline by P5CR (Roosens et al. 2002). Proline catabolism is catalyzed by the sequential action of two mitochondrial enzymes, proline dehydrogenase (ProDH) and P5C dehydrogenase (P5CDH). ProDH oxidized proline to pyrroline-5-carboxylate (P5C). The P5C is then converted to glutamate semi-aldehyde (GSA) by spontaneous and reversible reaction and then it is oxidized to glutamate by P5CDH (Szabados and Savouré 2010). δ-OAT can be involved in proline catabolism. For example, Sesuvium portulacastrum, (Messedi et al. 2006) showed an increase in δ-OAT activity concomitant with a decrease in proline concentrations in nitrogen deficient plants subjected to salt stress. These reports suggest that this enzyme which is normally involved in proline biosynthesis (Slama et al. 2006) can be also implied in its catabolism upon nitrogen deficiency condition. It has also already been demonstrated using oat knockout mutants of Arabidopsis thaliana that δ-OAT helps in nitrogen recycling from arginine (Funck et al. 2008). However, the authors did not find significant role of δ-OAT in proline biosynthesis.
Cakile maritima (Brassicaceae), known as sea rocket, is annual succulent C3 oilseed halophyte, colonizing exclusively coastal areas, and contributing effectively to the stabilization of sandy beaches. It has been shown through several studies on physiological responses of C. maritima to salinity that this species respond to salt stress by a variety of mechanisms including osmotic adjustment, high selectivity of K+ over Na+ (Debez et al. 2006b; Messedi et al. 2016). However, such stress tolerance responses varied due to intensity and duration of salt stress, and its occurence in combination with other abiotic stress (Woodrow et al. 2017). Under short term high salinity stress, this Brassicacious halophytic plant showed improved salt tolerance due to exogenous application of proline through osmotic adjustment ensured by enhancement in proline accumulation. As described earlier, enhancement in endogenous proline level is cumulative impact of effect of its biosynsthesis induction, reduction in degradation and re-allocation between the cells via specific Pro transport proteins (Mansour and Ali 2017; Szabados and Savouré 2010). In view of all these reports on proline induced salt tolerance in C. maritima and importance of proline biosynthesis via ornithine pathway during salt stress (Mansour and Ali 2017; Roosens et al. 1998; Woodrow et al. 2017; Xue et al. 2009), the present study aimed to investigate the short time effect of salinity on osmotic adjustment of this halophyte. In addition, it also aims to draw the relationship between proline accumulation and δ-OAT activity to dissect the possible role of this pathway in salt tolerance in Cakile maritima.
Materials and methods
Plant material and growth conditions
Seeds of C. maritima were collected in June on the sandy beaches of Raoued (located 20 km north of Tunis). The siliques were decorticated manually and seeds were disinfected with hypochlorite sodium (1%) for a few minutes and rinsed thoroughly with distilled water to remove all traces of chloride. They were then germinated in petri dishes. After 1 week, the seedlings were transferred into hydroponic medium in plastic tubs (5 L) filled with Hewitt’s nutrient solution (Hewitt 1966). The culture was carried out in a greenhouse under natural light conditions with an average day/night temperature of 25/18 °C and a relative humidity of 65/90%. The experimental station is located in Borj Cédria, close to the Mediterranean Sea shore, 30 km south-east of Tunis (10°10′E, 36°48′N; 10 m of altitude), with a mean temperature and annual rainfall of 19.4 °C and 456 mm, respectively. The salt treatment is applied to seedlings 3 weeks after germination. The saline solution is prepared by adding sodium chloride NaCl (0, 100, 200 and 400 mM) to the nutrient medium. Plants were collected 4, 24, 72 and 168 h after the beginning of treatments.
Growth and water status
Fresh weight (FW) and dry weight (DW) of leaves, stems and roots was measured. Tissue water content (WC) was determined using the following equation: .
FW was determined immediately after harvest. DW was obtained after oven drying leaf, stem and root samples at 60 °C for 72 h.
Osmolarity was determined as follows using the method Martinez et al. (2004) by measuring freezing point with a vapor pressure osmometer (Osmomat 030) and converting from mOsmol kg−1 to MPa using the formula: Ψs (MPa) = −C (mOsmol kg−1) × 2.58 × 10−3 according to the Van’t Hoff equation.
Determination of inorganic solutes
Minerals solutes were extracted from different tissues of the plant in HNO3 (0.5%). Na+ and K+ were assayed by flame emission photometry (Corning, UK). The selectivity of K+ over Na+ (SK/Na) was estimated from ion contents as:
Two parameters were used to characterize potassium nutrition: the potassium absorption efficiency (KAE) and potassium use efficiency (KUE) of leaves.
Extraction and assay of amino acids
Plant tissues (100 mg DW) were extracted three times in boiling 50% (v/v) methanol (1:1). Methanol extracts were dried under vacuum and the soluble compounds redissolved in 1 ml distilled water and centrifuged at 10,000×g for 15 min at room temperature. The amino acids analysis was carried out by mixing 20 µL of the supernatant (50% methanol extraction), citrate buffer and ninhydrin reagent. The mixture was incubated at 100 °C for 20 min. After cooling, we added 2 ml of 60% ethanol and assayed the coloured complex at 570 nm relative to a standard curve established with glycine (0-15 mg glycine per tube) (Yemm et al. 1955).
Proline measurement
Free proline content was determined according to Bates et al. (1973). Proline concentration was calculated from a standard curve using 0-20 µM l-proline.
Contribution of solutes to osmotic adjustment
Concentrations of organic solutes as well as inorganic ions were calculated for the water content at full turgor. These concentrations were used to estimate the contribution of each solute to osmotic adjustment. The amount of osmotically active solutes in various tissues, the “osmotic pool”, was estimated by bringing back the concentrations analyzed in osmoticums to the osmolality assayed directly in the tissue (Silveira et al. 2009).
Ornithine-δ-aminotransferase activity
The extraction of enzyme was assayed by the method suggested by da Rocha et al. (2012). Supernatants collected are taken to measure the activity of δ-OAT as described by Kim et al. (1994). In a final volume of 1 ml, the incubation medium contained 50 mM ornithine, 5 mM α-ketoglutarate, 0.05 mM pyridoxal 5-phosphate and 50 mM potassium phosphate buffer, the final pH was 8.0. Reaction was initiated by adding α-ketoglutarate. The incubation was carried out at 37 °C for 20 min, the reaction was stopped by adding 0.3 ml perchloric acid (3 N) and 0.2 ml ninhydrin (2%). After incubation at 100 °C for 5 min in a water bath and centrifugation at 2500 g at ambient temperature for 15 min, the precipitate formed in pink is dissolved in 1.5 ml of absolute ethanol. The assay was performed therefore at 510 nm. It is estimated that the appearance of the reaction product and therefore the activity of this enzyme is the corresponding variation of the optical density of 0.01 at 510 nm to a unit of enzyme activity per mg of protein (Yang et al. 2011).
Statistical analysis
All statistical analyses were performed using the software XLSTAT V2011 (www.xlstat.com). Throughout this work, two types of statistical analyses were performed: a comparison of multiple overages (Tukey’s test) and principal component analysis (PCA).
Results
Plant growth
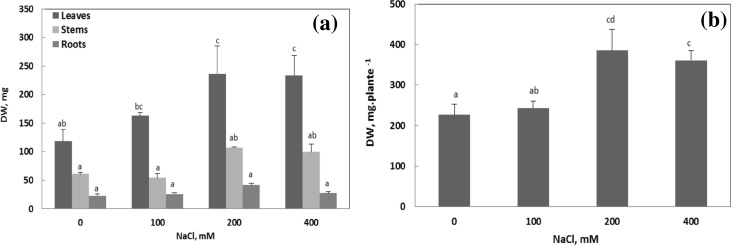
The evolution of growth of Cakile maritima plants subjected to salt was determined after 7 days of treatment (Fig. 1). Results showed that growth of Cakile maritima was stimulated by salt. This is a typical halophile behavior; thus, greater increase in growth was found at 200 and 400 mM NaCl. The same variations were observed for the different organs. The dry weight (DW) of leaves and stems significantly increased in plants exposed to 200 or 400 mM NaCl. Similarly, root DW was significantly increased by 79% in plants treated with 200 mM NaCl.
Fig. 1.
Changes in the growth of the various organs and the whole plant Cakile maritima at the final harvest (7 days) depending on the salinity of the culture medium. a Dry weights of leaves, stems and roots of Cakile maritima. b Whole plant dry weight
Tissue Hydration
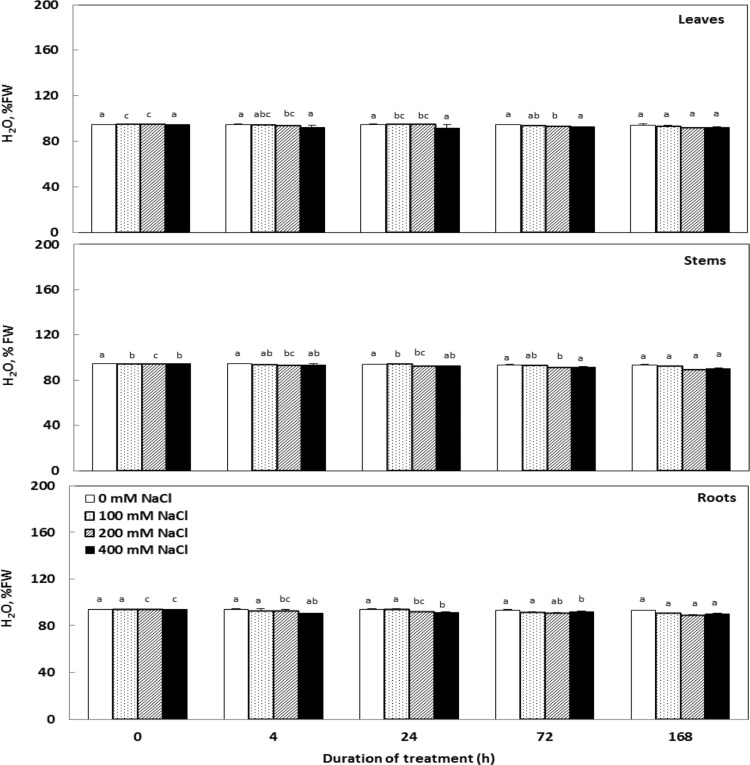
Changes in tissue hydration are shown in Fig. 2. Data showed that tissues hydration did not display any significant reduction in response to a higher range of salinity. This parameter remained particularly high (90% FW) in various organs.
Fig. 2.
Effect of salinity on the hydration of various organs Cakile maritima plants subjected to 4, 24, 72 and 168 h to different concentrations of NaCl (0, 100, 200 and 400 mM). This parameter was expressed as a percentage of the fresh material. Values followed by at least one same letter were not significantly different at P < 0.05
Osmotic potential
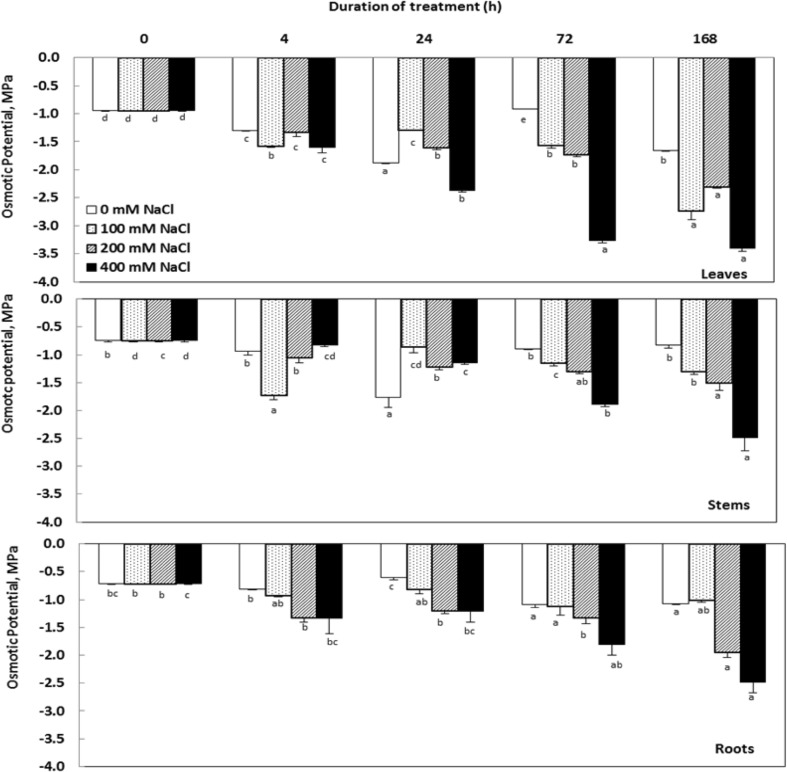
The presence of NaCl in the nutrient solution significantly reduced the values of osmotic potential in different organs of C. maritima. The lowest values were generally observed after 7 days treatment. These changes are more pronounced in the aerial parts for all NaCl concentrations. The values of this parameter were higher in roots compared to those measured in the leaves and stems, generating an important gradient of osmotic potential between these organs (Fig. 3).
Fig. 3.
Variations in osmotic potential organs Cakile maritima plants subjected to 4, 24, 72 and 168 h to different concentrations of NaCl (0, 100, 200 and 400 mM). Values followed by at least one same letter were not significantly different at P < 0.05
Inorganic ions accumulation
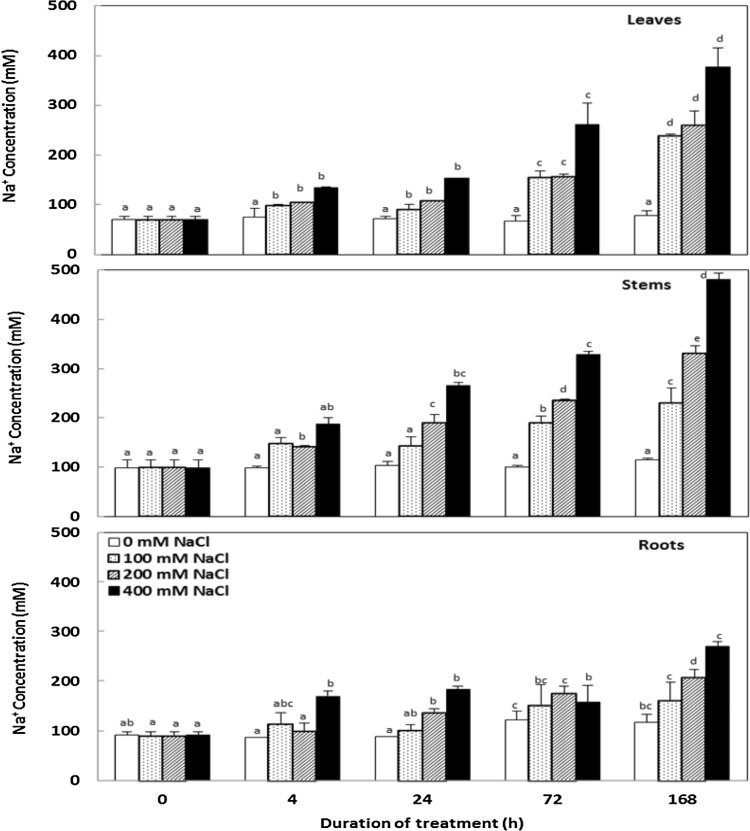
Na+ content in various organs significantly increased from the first 4 h upon salt treatment (Fig. 4). This increase is accentuated with the salinity of the culture medium and the treatment duration. The roots accumulated significantly less Na+ than aerial parts.
Fig. 4.
Changes in the sodium concentrations in organs Cakile maritima plants subjected to 4, 24, 72 and 168 h to different concentrations of NaCl (0, 100, 200 and 400 Mm). Values followed by at least one same letter were not significantly different at P < 0.05
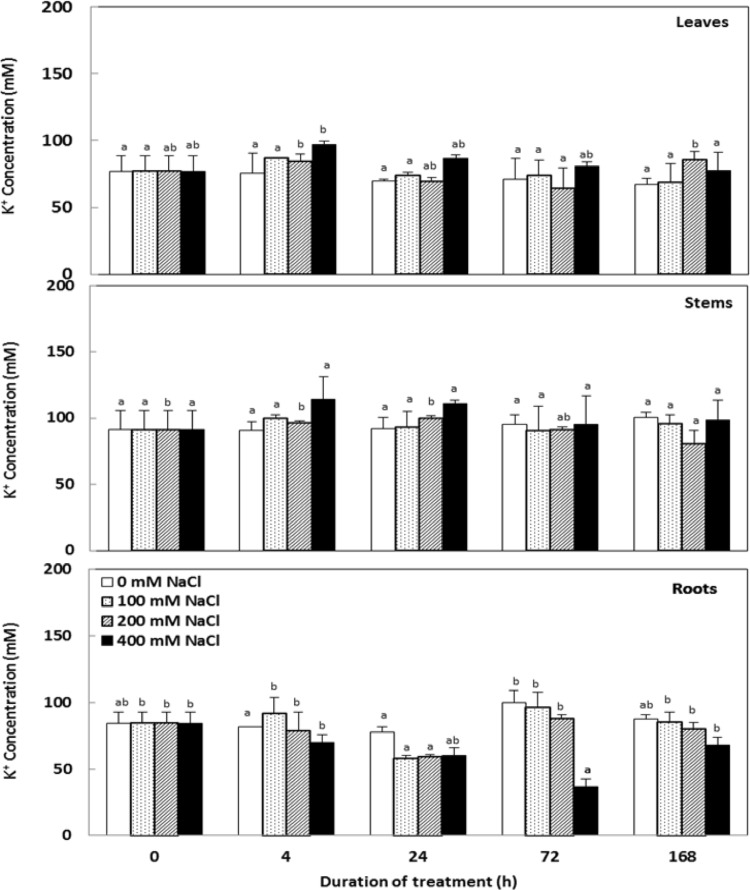
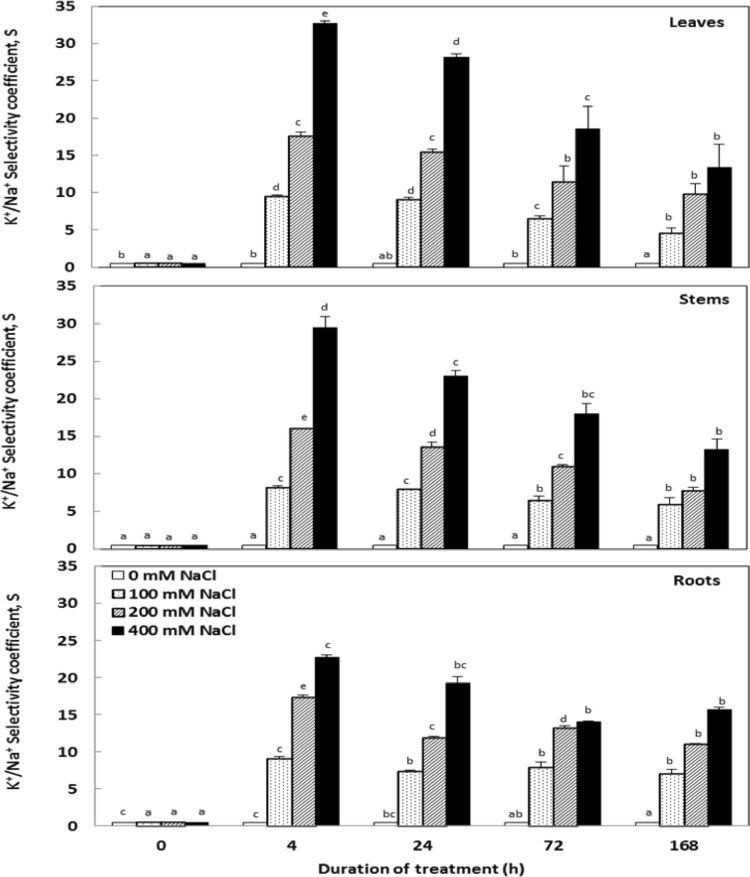
K+ remained higher in aerial parts (Fig. 5) but it decreased significantly in roots especially at higher salt concentrations (200 and 400 mM NaCl) compared to control plants at the end of 7 days. Interestingly, C. maritima showed a pronounced selectivity for K+ uptake over Na+, as reflected by the marked enhance of the K+ versus Na+ selectivity coefficient ratio (S) with salt treatments (Fig. 6). Additionally, our data revealed a significant reduction on potassium absorption efficiency (KAE) which was conversely compensated by an enhancement in its use efficiency (KUE) (Table 1) depending on NaCl concentrations in the medium culture.
Fig. 5.
Variations in the potassium concentrations in organs Cakile maritima plants subjected to 4, 24, 72 and 168 h to different concentrations of NaCl (0, 100, 200 and 400 mM). Values followed by at least one some letter were not significantly different at P < 0.05
Fig. 6.
Variations in the K+/Na+ selectivity coefficient (S) in the tissues of Cakile maritima plants subjected to 4, 24, 72 and 168 h to different concentrations of NaCl (0, 100, 200 and 400 mM). Values followed by at least one same letter are not significantly different at P < 0.05
Table 1.
Potassium absorption efficiency (KAE) and potassium use efficiency (KUE) in Cakile maritima exposed to different NaCl levels for 7 days
| NaCl (mM) | KAE (µmol K+ mg−1 DWroots) | KUE (mg DW µmol−1 K+) |
|---|---|---|
| 0 | 10.666 | 0.807 |
| 100 | 10.513 | 0.899 |
| 200 | 9.900 | 1.115 |
| 400 | 9.478 | 1.256 |
Amino acids
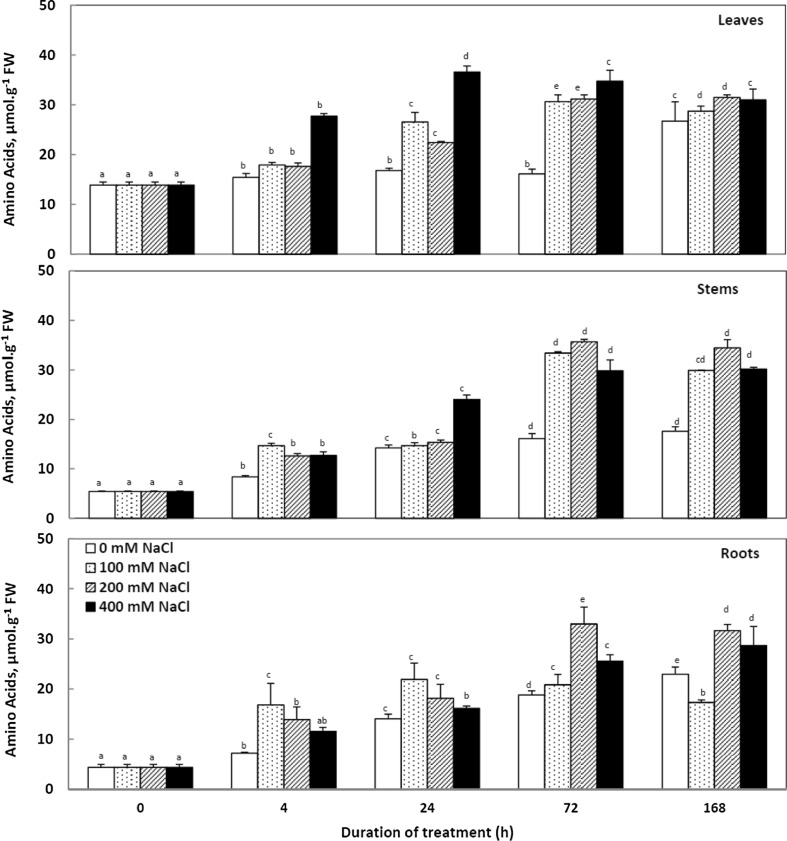
Salinity stress increased amino acids content in different organs compared to control (Fig. 7). This stimulation is distinguishable in leaves since the first 4 h of treatment. At the term of treatment, a reduction in the levels of these compounds is observed. Moreover, we note that at the end of treatment, the amino acids levels in plants grown in 200 mM NaCl are almost three times higher than those measured in control plants.
Fig. 7.
Variations in amino acids in leaves, stems and roots of Cakile maritima plants subjected to 4, 24, 72 and 168 h to different concentrations of NaCl (0, 100, 200 and 400 Mm). Values followed by at least one some letter are not significantly different at P < 0.05
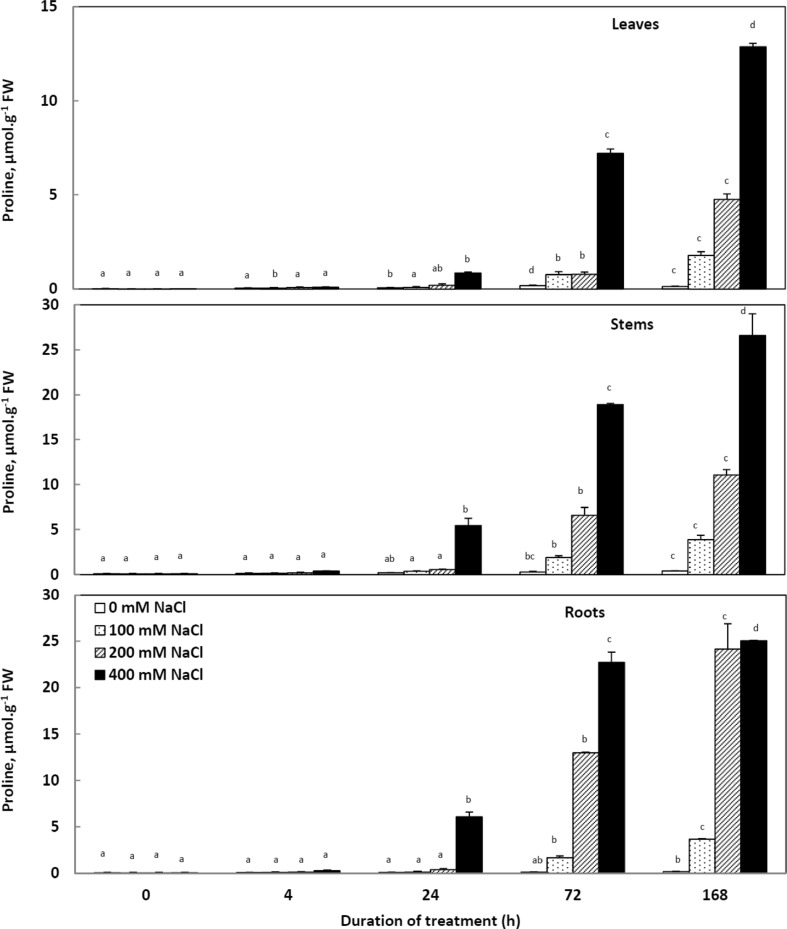
Proline content
In control plants, proline accumulation remained almost unchanged during the whole period of treatment (Fig. 8). Salinity stress induced an increase in proline concentration after 24 h of salt treatment in all organs. Proline concentrations are generally higher in roots and stems than in leaves.
Fig. 8.
Variations of the proline concentrations in the tissues of Cakile maritima plants subjected to 4, 24, 72 and 168 h to different concentrations of NaCl (0, 100, 200 and 400 mM). Values followed by at least one same letter are not significantly different at P < 0.05
Osmotic adjustment contribution
Table 2 shows the relative contribution of the solutes to osmotic adjustment (OAs). Under salt condition, the Na+ contribution to leaf and stems osmolality increased and reached a maximum at 200 and 400 mM NaCl. In contrast, the one from K+, decreased significantly with salinity and time in all organs.
Table 2.
Total “osmotic pool” was estimated by dividing the amount of all analyzed solutes (Na+, K+, amino acids and proline) by the amount of water in the leaf tissue: the contribution of each solute was expressed as a percentage of the total “osmotic pool”. The values are means of three replicates
| Na+ | K+ | Amino acids | Proline | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 400 | 100 | 200 | 400 | 100 | 200 | 400 | 100 | 200 | 400 | |
| Leaves | ||||||||||||
| 0 h | 19.03 | 19.03 | 19.03 | 20.95 | 20.95 | 20.95 | 1.98 | 1.98 | 1.98 | 0.02 | 0.02 | 0.02 |
| 4 h | 16.06 | 20.14 | 21.66 | 14.21 | 16.24 | 15.66 | 2.30 | 1.36 | 1.95 | 0.08 | 0.15 | 0.13 |
| 24 h | 18.21 | 17.29 | 16.60 | 14.66 | 11.10 | 9.40 | 1.91 | 1.88 | 1.52 | 0.19 | 0.34 | 0.66 |
| 72 h | 25.55 | 23.27 | 20.62 | 12.15 | 9.56 | 6.36 | 2.65 | 2.48 | 1.48 | 1.35 | 1.23 | 6.13 |
| 7j | 22.45 | 29.03 | 28.52 | 6.46 | 9.51 | 5.86 | 0.95 | 1.91 | 1.27 | 1.80 | 5.86 | 10.65 |
| Stems | ||||||||||||
| 0 h | 34.25 | 34.25 | 34.25 | 31.76 | 31.76 | 31.76 | 0.99 | 0.99 | 0.99 | 0.38 | 0.38 | 0.38 |
| 4 h | 22.12 | 34.80 | 58.96 | 14.90 | 23.78 | 35.92 | 1.17 | 1.65 | 4.54 | 0.25 | 0.53 | 1.29 |
| 24 h | 43.51 | 40.32 | 60.17 | 25.73 | 21.12 | 25.28 | 2.34 | 1.74 | 2.34 | 1.18 | 1.27 | 13.34 |
| 72 h | 43.11 | 47.39 | 44.90 | 20.36 | 18.28 | 13.04 | 4.04 | 3.86 | 2.22 | 4.62 | 14.26 | 27.97 |
| 7j | 46.17 | 57.01 | 49.80 | 19.19 | 13.89 | 10.19 | 1.77 | 1.90 | 1.73 | 8.42 | 21.11 | 30.33 |
| Roots | ||||||||||||
| 0 h | 33.08 | 33.08 | 33.08 | 30.47 | 30.47 | 30.47 | 2.34 | 2.34 | 2.34 | 0.20 | 0.20 | 0.20 |
| 4 h | 32.13 | 19.66 | 32.62 | 25.73 | 15.34 | 13.45 | 11.20 | 7.34 | 6.37 | 0.28 | 0.29 | 0.59 |
| 24 h | 32.58 | 29.61 | 38.84 | 18.42 | 12.74 | 12.76 | 8.85 | 3.70 | 8.82 | 0.48 | 0.93 | 14.04 |
| 72 h | 35.51 | 34.12 | 22.46 | 20.62 | 17.02 | 5.27 | 6.74 | 12.62 | 6.56 | 4.37 | 27.68 | 34.51 |
| 7j | 41.69 | 27.50 | 22.91 | 23.30 | 10.61 | 7.03 | 9.54 | 18.76 | 11.15 | 10.42 | 35.93 | 28.94 |
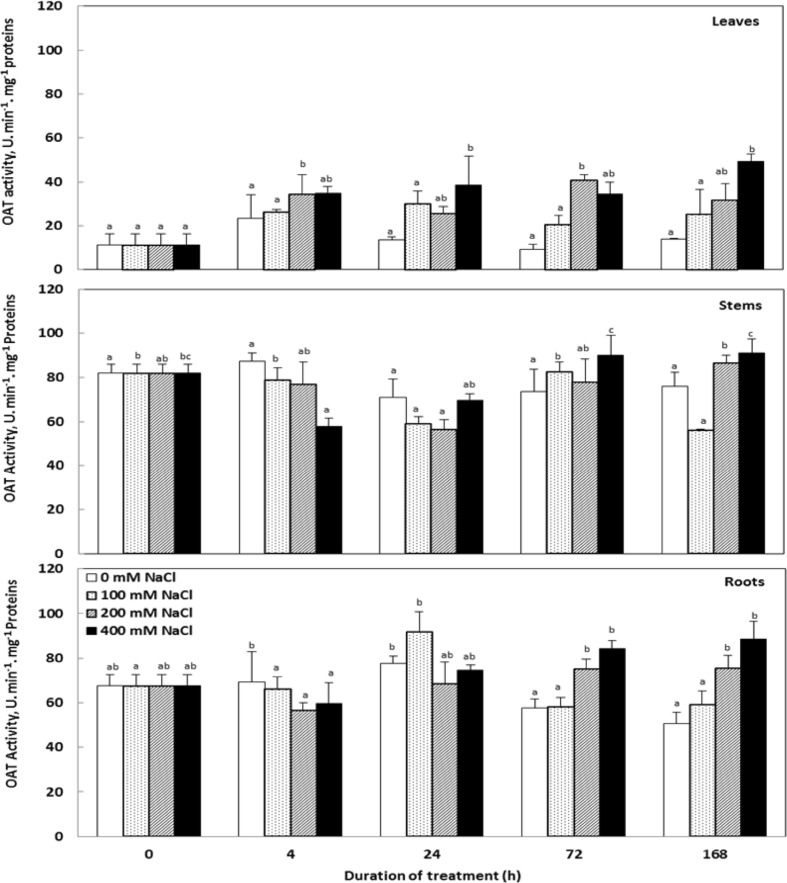
OAT activity
Results (Fig. 9) showed that OAT is active in all organs of C. maritima independently of the absence or presence of salt in culture medium. This activity is more important in stems and roots because increase in OAT activity in stems and roots were 6–7 folds greater than increase in OAT activity in the leaves.
Fig. 9.
Variations in the OAT activity in the tissues of Cakile maritima plants subjected to 4, 24, 72 and 168 h to different concentrations of NaCl (0, 100, 200 and 400 mM). Values followed by at least one same letter are not significantly different at P < 0.05
The treatment of C. maritima plants with salinity led to a significant OAT activity. This increase is a function of the NaCl concentration in the culture medium and the treatment duration. Moreover, there is 2–3 fold increase in OAT activity in leaves, but in stems it is variable and negligible. In roots, only minor increase was observed in higher NaCl concentrations. During the whole stress period, the maximal increase in OAT activity was observed at 400 mM NaCl for 7 days and reached 350, 121 and 176% of the control values in leaves, stems and roots respectively.
PCA analysis
In the PCA analysis performed for two treatments (200 and 400 mM NaCl) and four times (4, 24, 72, 168 h) considering two parameters (proline and OAT activity), we noted that OAT activity and proline accumulation showed a highest correlation coefficient with saline and time of treatments (Table 3).
Table 3.
Correlation matrices (Pearson (n)) and analysis of the correlation between the activity of δ-OAT and proline concentration depending on the salt concentration and time of treatment
| Variables | Proline leaves | Proline stems | Proline roots | OAT leaves | OAT stems | OAT roots |
|---|---|---|---|---|---|---|
| 200 mM NaCl | ||||||
| Proline leaves | 1 | 0.893 | 0.904 | − 0.018 | 0.565 | 0.469 |
| Proline stems | 0.893 | 1 | 0.989 | 0.224 | 0.611 | 0.649 |
| Proline roots | 0.904 | 0.989 | 1 | 0.173 | 0.622 | 0.639 |
| OAT leaves | − 0.018 | 0.224 | 0.173 | 1 | 0.627 | − 0.110 |
| OAT stems | 0.565 | 0.611 | 0.622 | 0.627 | 1 | 0.117 |
| OAT roots | 0.469 | 0.649 | 0.639 | − 0.110 | 0.117 | 1 |
| 400 mM NaCl | ||||||
| Proline leaves | 1 | 0.977 | 0.938 | 0.510 | 0.854 | 0.781 |
| Proline stems | 0.977 | 1 | 0.976 | 0.457 | 0.885 | 0.809 |
| Proline roots | 0.938 | 0.976 | 1 | 0.363 | 0.934 | 0.840 |
| OAT leaves | 0.510 | 0.457 | 0.363 | 1 | 0.359 | 0.411 |
| OAT stems | 0.854 | 0.885 | 0.934 | 0.359 | 1 | 0.802 |
| OAT roots | 0.781 | 0.809 | 0.840 | 0.411 | 0.802 | 1 |
| Variables | Proline feuilles | Proline tiges | Proline racines | OAT feuilles | OAT tiges | OAT racines |
|---|---|---|---|---|---|---|
| 4 h | ||||||
| Proline feuilles | 1 | 0.630 | 0.656 | 0.753 | − 0.505 | − 0.381 |
| Proline tiges | 1 | 0.926 | 0.485 | − 0.857 | − 0.318 | |
| Proline racines | 1 | 0.300 | − 0.919 | − 0.476 | ||
| OAT feuilles | 1 | − 0.168 | − 0.131 | |||
| OAT tiges | 1 | 0.493 | ||||
| OAT racines | 1 | |||||
| 24 h | ||||||
| Proline feuilles | 1 | 0.962 | 0.979 | 0.660 | 0.293 | − 0.323 |
| Proline tiges | 1 | 0.991 | 0.557 | 0.383 | − 0.291 | |
| Proline racines | 1 | 0.618 | 0.403 | − 0.287 | ||
| OAT feuilles | 1 | − 0.100 | − 0.135 | |||
| OAT tiges | 1 | − 0.019 | ||||
| OAT racines | 1 | |||||
| 72 h | ||||||
| Proline feuilles | 1 | 0.963 | 0.866 | 0.438 | 0.554 | 0.759 |
| Proline tiges | 1 | 0.966 | 0.629 | 0.511 | 0.892 | |
| Proline racines | 1 | 0.780 | 0.461 | 0.951 | ||
| OAT feuilles | 1 | 0.349 | 0.800 | |||
| OAT tiges | 1 | 0.364 | ||||
| OAT racines | 1 | |||||
| Variables | Proline leaves | Proline stems | Proline roots | OAT leaves | OAT stems | OAT roots |
|---|---|---|---|---|---|---|
| 168 h | ||||||
| Proline leaves | 1 | 0.992 | 0.820 | 0.888 | 0.660 | 0.883 |
| Proline stems | 1 | 0.844 | 0.898 | 0.651 | 0.872 | |
| Proline roots | 1 | 0.756 | 0.741 | 0.899 | ||
| OAT leaves | 1 | 0.502 | 0.725 | |||
| OAT stems | 1 | 0.606 | ||||
| OAT roots | 1 | |||||
Variables were centered on their means and normalized with a standard deviation of 1
Bold values represent significant correlations at 0.05 level
Discussion
In the present study, Cakile maritima growth was significantly stimulated at 200 and 400 mM NaCl after short term treatment (7 days). These data confirm the halophile character of this plant. Salt induced growth-stimulation under moderate salinity has been previously documented in this species after a long-term exposure to salinity and in many other salt-requiring halophytic species such as Batis maritima (Debez et al. 2006a, 2010). In the same way, while exposing two Atriplex species to a range of salinity (0-1000 mM NaCl) for ten days, Belkheiri and Mulas (2013) found that growth of both Atriplex species increased up to 400 mM NaCl and decreased at higher salinity stress. Likewise, Thellungiella halophila – a close relative to Arabidopsis thaliana can tolerate extreme level of salt stress (up to 500 mM NaCl) and this has been observed in a number of studies (Amtmann 2009; Bressan et al. 2001; Inan et al. 2004). It is suggested that growth stimulation or no adverse impacts on growth were due to accumulation of proline and lower accumulation of Na in the meristem that inhibit growth (Amtmann 2009). However, accumulation of such amount of salts can affect the hormonal balance or osmolyte accumulation which may initiate salt adaptive responses (Albacete et al. 2008; Munns and Tester 2008). In view of these information, Amtmann (2009) suggested that such halophytic plants can be used in studying salt stress adaptive strategies particularly those of proline-related signaling pathways. At higher salt stress which inhibits plant growth, such halophytic plants can be used in identifying hormonal pathways that inhibit growth. Thus, the lower growth of the plant is associated to the end of new leaf expansion (Maggio et al. 2007) and a limited leaf growth by slowing the cellular division (Albacete et al. 2008).
Salt stress in C. maritima led to a slow decrease in tissue hydration which remains insignificant and tissues stay hydrated. Our data corroborate the findings of Lohaus et al. (2000), who observed that no significant differences were found in water spaces between control and salinity in short-term treatment in Zea mays. High salinity also induced a significant decrease in osmotic potential (ψs). The decrease in this parameter is known to contribute to turgor maintenance. In C. maritima, the decline in ψs appears from the first 4 h and lasts up to 24 h of treatment with a concomitant maintenance of tissue hydration when the salt is brought to a moderate dose (100 and 200 Mm NaCl). We also noticed that the values of ψs are remarkably higher in the roots compared to those detected in the leaves, generating a significant osmotic potential gradient between these organs. This gradient is usually observed in halophytes and it is essential to create a driving force in the roots to absorb water from a hypertonic solution and its movement throughout the plant (Glenn and Brown 1998).
Maintenance of tissue hydration as well as the decrease of ψs was concomitant with an increase of the Na+ concentrations. This behavior is consistent with the salt includer character of this plant which enables it to use Na+ for osmotic adjustment and maintenance of the leaf water potential at more negative values than in the nutrient solution ensuring a suitable water supply to the plant. In turn, this high tissue hydration contributes greatly to the dilution of toxic solutes in order to prevent cell damage (Taffouo et al. 2006). On the other hand, this accumulation followed an increasing ion gradient from the roots to the leaves of plants characterizing the inclusive type. The preferential accumulation of this ion in the leaves is consistent with the ability of this species to compartmentalize sodium in the vacuole and its use in place of K+ for osmotic adjustment (OA) (Debez et al. 2006b). Indeed, our results showed the increase of Na+ concentration in C. maritima leaves was concomitant with a significant reduction in K+ uptake represented by the potassium absorption efficiency (KAE) which decreased with increasing NaCl concentrations in the culture medium (Table 1). These results are in agreement with other studies showing that plants growing under salinity conditions suffer from ionic imbalance and nutrient deficiency (Ashraf and Harris 2004; Munns 2005). It is well known that high concentrations of Na+ can inhibit K+ uptake (Munns 2005; Munns and Tester 2008). This is due to their physicochemical similarity, which promotes ionic competition for binding sites on membrane transporters (Munns 2005). However, despite this competition between Na+ and K+ and higher quantity of Na+ in the nutrient solution, the comparison of the ratios of their concentration in the tissues and those in the nutrient solution clearly shows selective absorption of K+ (Fig. 6). In addition, the K+/Na+ selectivity coefficient, a very good indicator of the salt plant tolerance, was enhanced by increased NaCl levels, in all the organs. In addition, this limitation of the absorption of K+ had no effect on C. maritima growth in all salt concentrations used in the present work. Indeed, our finding showed that the potassium use efficiency (KUE) increased with salt at the end of treatment (Table 1). These results are in agreement with those showed by Slama et al. (2008) in Sesuvium portulacastrum subjected to salinity alone or combined with drought stress. These findings provide further evidence concerning the ability of C. maritima to replace K+ by Na+ in non- specific functions as osmotic adjustment by accumulating it in the vacuole (Harrouni et al. 2003). So, K+ would be preserved for the vital functions of the plant, namely activation of enzymes, protein synthesis and photosynthesis, as well as for the transport of solutes in the phloem, although, it plays an important role in expanding cells particularly for young leaves (Olías et al. 2009; Yao et al. 2010). These results suggest that this species, for a short-term treatment, has greater capacity to maintain a favorable cellular environment for growth and other metabolic activities.
The importance of inorganic solutes in Cakile maritima plants exposed to salt stress was highlighted by their relative contribution to the total osmolality; the main inorganic ions involved in OA under salt-free conditions were Na+ and K+. The relative contribution of the Na+ in salt stressed plants reached values of 22; 29 and 29% in leaves, 46, 57 and 50% in stems and 42, 27 and 23% in roots at 100 mM, 200 mM and 400 mM NaCl, respectively in the term of 7 days (short-term treatment) (Table 2). Interestingly, under saline conditions, Na+ was more effective in the osmotic adjustment in all organs. However, the relative contribution of K+ to the total osmolality decreased with increased NaCl levels especially in roots. In halophytes, the involvement of Na+ in OA has been well documented. In Ricinus communis L, Rodrigues et al. (2014) showed that the relative contribution of salt ions reached values of 55, 65 and 69% in leaves and 33, 42 and 58% in roots when plants were respectively subjected to 50 mM, 100 mM and 150 mM NaCl treatments during 15 days. Similar results were reported by Messedi et al. (2016) in C. maritima showing that the contribution of Na+ in OA is 50% at 200 and 400 mM NaCl after 4 weeks of treatment.
According to Martinez et al. (2004), compatible solutes like sugars, glycerol, proline or glycine betaine can also contribute to this process. In our study, the contribution of amino acids to osmotic adjustment remains insignificant by increased concentration of NaCl and the time of treatment. However, a marked increase was found in proline accumulation in leaves, stems and roots of C. maritima during first 24 h of salt stress (Fig. 8). At the end of treatment, proline content increased manifolds in roots of plants grown at 400 mM. Its relative contribution to “osmotic pool” in plants was enhanced with medium salinity and treatment duration. Proline was more effective in the osmotic adjustment in roots. It reached values of 36% in plants subjected to 200 mM NaCl for 7 days (Table 2). These results have been observed in several plant species such as Paspalum vaginatum (Lee et al. 2008). Under these conditions, proline could be considered as an osmoregulator. Our data are in contrast with other showing an insufficient accumulated proline amount, to ensure an osmotic adjustment in rice subjected to salt for 7 days (Demiral and Turkan 2005).
In addition to its two roles as an osmoregulator and osmoprotector, proline may act as nitrogen source in the cell at the lifting of the stress conditions, where the accumulation of this nitrogenous compound could be utilized as a form of N storage (Sanchez et al. 2001). In its biotope, C. maritima grows in a nitrogen-poor medium which is the most limiting element in saline-environments. Under these conditions, the plant remobilizes its nitrogen reserve by the degradation of nitrogenous compounds (da Rocha et al. 2012). In this study, our data showed that the δ-OAT was active in plants grown under normal conditions, but was not concomitant with a significant accumulation of proline where it is almost absent. These results showed that the plant transformed ornithine to pyrroline-5-carboxylate (P5C). According to Rayapati and Stewart (1991), in the absence of stress, and due to its toxicity, this product converted spontaneously to GSA which is rapidly transformed to glutamate as a final product. This later serves as the nitrogen donors (Funck et al. 2008) which are mobilized to leaves to ensure production of biomass. In addition, our results suggest that δ-OAT, normally involved in proline biosynthesis, can be also implied in its catabolism. Indeed, δ-OAT interconverts P5C into ornithine and, therefore plays an important role in degradation of proline to obtain glutamate as a final product (Adams and Frank 1980; Delauney et al. 1993; Funck et al. 2008).
On the other hand, the results presented in this work provides evidence that the accumulation of proline in different organs of C. maritima subjected to salt stress is concomitant to a stimulation of the δ-OAT activity suggesting a close involvement of this enzyme in proline anabolism. This is well illustrated in the stems and roots of the cultivated plants in the presence of 200 and 400 mM NaCl. We used a statistical study expressed by the correlation matrix (Pearson (n)) (Table 3). Our results showed a significant and a positive correlation between the δ-OAT activity and the proline concentration. This correlation increased with increasing NaCl concentrations in the culture medium and reached values of 0.611 and 0.885 in stems, and 0.639 and 0.840 in roots at 200 and 400 mM NaCl, respectively. These data demonstrate the involvement of the δ-OAT in proline biosynthesis. The correlation between these two parameters was also studied as a function of treatment duration. Our results showed that this correlation was strongly positive from the first 4 h of salt treatment in the leaves (r = 0.753) and increased with treatment duration to reach its maximum at the term of treatment, 7 days (0.888). Moreover, this positive correlation is significantly high from 72 h of treatment in the roots (0.951) and after 7 days in the stems (0.651). These results are in disagreement with other studies showing that proline is first synthesized in the roots and then transported into the leaves, since the transporters have been detected in the roots (Ueda et al. 2002). Moreover, in Hordeum vulgare, it has been showed that the transcript level of Hv ProT (proline transporter) was induced in roots at 30 min after 200 mM NaCl treatment. However, the transcript level was very low in leaves and did not increase by salt stress (Ueda et al. 2001). In contrast, we have shown an increase in proline concentration first in the leaves following the correlation between proline and OAT from the first 4 h of treatment. The proline synthesized can be subsequently transported to other organs (stems and roots).
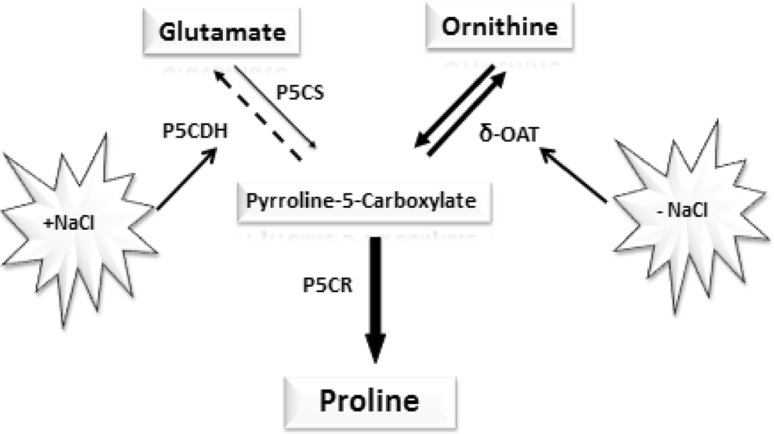
The δ-OAT has been shown to participate in proline biosynthesis in several plants such as those determined in Cashew leaves, submitted to salt (da Rocha et al. 2012), in greenbean plants subjected to a cold stress (Ruiz et al. 2002), in transgenic tobacco, rice plants (Roosens et al. 2002; Wu et al. 2005) and in Medicago truncatula (Armengaud et al. 2004). These results are in agreement with findings in Fragilariospis cylindrus and showed that NaCl induced significant elevation of δ-OAT activity suggesting that the ornithine pathway may be responsible for proline accumulation in response to NaCl treatment (Krell et al. 2007). It has also documented that accumulation of proline in stressed plants depends on operation efficiency of different biosynthetic pathways in different plants. For example, ornithine pathway is predominant in leguminous plant species for biosynthesis and accumulation of proline in stressed plants, whereas grass species accumulated proline via glutamate pathway (Mansour and Ali 2017). While working with Arabidopsis mutants for proline biosynthesis, Funck et al. (2008) reported that proline biosynthesis in Arabidopsis plants was mainly through glutamate pathway and ornithine pathway was not involved. Such differences in operation of different biosynthetic pathways might have been due to differences in nutritional requirement such as nitrogen (N) nutrition (AbdElgawad et al. 2015). Some studies suggested that proline biosynthesis under stress conditions is not only regulated by operation of different proline biosynthetic pathways but also due to intra-cellular proline transport (Gagneul et al. 2007), intra-organ transport (Lehmann et al. 2011), and type of tissue such as root tip (Ueda et al. 2001). Although contribution of glutamate in proline biosynthesis cannot be denied, our results strongly suggested at least a reasonable contribution of ornithine in proline accumulation in stressed leaves. Moreover, the repression of the δ-OAT activity from application of salt extending upto 24 h in stems and roots lead us to propose the involvement of the glutamate pathway in the accumulation of proline. When glutamate stock is depleted, the δ-OAT activity returns again. The latter will be more important by increased NaCl concentration in the culture medium and the time of treatment. This is in agreement with the variations of the accumulated quantity of proline. On the whole, data suggest that the δ-OAT is involved in the metabolism of proline according to the model shown in Fig. 10. Further biochemical and molecular data are needed to check its validity.
Fig. 10.
The proline biosynthesis pathways in Cakile maritima in our culture conditions
Conclusion
The halophytic character of Cakile maritima was particularly associated with the ability to use Na+ in osmotic adjustment and thus, maintain adequate water supply by lowering the osmotic potential. Our results also showed that the presence of salt in the culture medium resulting in synthesis of proline in the leaves and its transport to the stems and roots. Under normal growth conditions, the role of δ-OAT does not seem to be linked to biosynthesis of proline. Rather it is related to the necessity for the plantlets to dispose of a nitrogen source. In contrast, under salt-stress conditions, the δ-OAT activity serves, with the glutamate pathway, to proline synthesis in various organs of Cakile maritima.
Acknowledgements
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research (LR15CBBC02).
Author’s contribution
DH: PhD Scholar conducted the experiment; CA: Professor, Design the experiment and finalize the manuscript; HA: Professor, editing and finalizing manuscript, submission to the Journal on behalf of Drosaf Messedi, handle the manuscript and will responsible for submission of responses to any query; MA: Professor, Editing and finalizing the manuscript; DM: Assistant Professor, conceive and design the experiment, writing the manuscript, the person who won the research grant for this project, corresponding author.
Contributor Information
Habib-ur-Rehman Athar, Email: habibathar@yahoo.com.
Dorsaf Messedi, Email: dorsaf_messedi@yahoo.com.
References
- AbdElgawad H, De Vos D, Zinta G, Domagalska MA, Beemster GTS, Asard H. Grassland species differentially regulate proline concentrations under future climate conditions: an integrated biochemical and modelling approach. New Phytol. 2015;208:354–369. doi: 10.1111/nph.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams E, Frank L. Metabolism of proline and the hydroxyprolines. Annu Rev Biochem. 1980;49:1005–1061. doi: 10.1146/annurev.bi.49.070180.005041. [DOI] [PubMed] [Google Scholar]
- Albacete A, Ghanem ME, Martínez-Andújar C, Acosta M, Sánchez-Bravo J, Martínez V, Lutts S, Dodd IC, Pérez-Alfocea F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot. 2008;59:4119–4131. doi: 10.1093/jxb/ern251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann A. Learning from evolution: Thellungiella generates new knowledge on essential and critical components of abiotic stress tolerance in plants. Mol Plant. 2009;2:3–12. doi: 10.1093/mp/ssn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengaud P, Thiery L, Buhot N, Grenier-de March G, Savoure A. Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol Plant. 2004;120:442–450. doi: 10.1111/j.0031-9317.2004.00251.x. [DOI] [PubMed] [Google Scholar]
- Ashraf M. Some important physiological selection criteria for salt tolerance in plants. Flora Morphol Distrib Funct Ecol Plants. 2004;199:361–376. doi: 10.1078/0367-2530-00165. [DOI] [Google Scholar]
- Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007;59:206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- Ashraf M, Harris PJC. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3–16. doi: 10.1016/j.plantsci.2003.10.024. [DOI] [Google Scholar]
- Ashraf M, Athar HR, Harris PJC, Kwon TR. Some prospective strategies for improving crop salt tolerance. Adv Agron. 2008;97:45–110. doi: 10.1016/S0065-2113(07)00002-8. [DOI] [Google Scholar]
- Athar H-u-R, Zafar ZU, Ashraf M. Glycinebetaine improved photosynthesis in canola under salt stress: evaluation of chlorophyll fluorescence parameters as potential indicators. J Agron Crop Sci. 2015;201:428–442. doi: 10.1111/jac.12120. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Belkheiri O, Mulas M. The effects of salt stress on growth, water relations and ion accumulation in two halophyte Atriplex species. Environ Exp Bot. 2013;86:17–28. doi: 10.1016/j.envexpbot.2011.07.001. [DOI] [Google Scholar]
- Bressan RA, Zhang CQ, Zhang H, Hasegawa PM, Bohnert HJ, Zhu JK. Learning from the Arabidopsis experience. The next gene search paradigm. Plant Physiol. 2001;127:1354–1360. doi: 10.1104/pp.010752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha IM, Vitorello VA, Silva JS, Ferreira-Silva SL, Viegas RA, Silva EN, Silveira JA. Exogenous ornithine is an effective precursor and the delta-ornithine amino transferase pathway contributes to proline accumulation under high N recycling in salt-stressed cashew leaves. J Plant Physiol. 2012;169:41–49. doi: 10.1016/j.jplph.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Debez A, Saadaoui D, Ramani B, Ouerghi Z, Koyro H-W, Huchzermeyer B, Abdelly C. Leaf H+-ATPase activity and photosynthetic capacity of Cakile maritima under increasing salinity. Environ Exp Bot. 2006;57:285–295. doi: 10.1016/j.envexpbot.2005.06.009. [DOI] [Google Scholar]
- Debez A, Taamalli W, Saadaoui D, Ouerghi Z, Zarrouk M, Huchzermeyer B, Abdelly C. Salt effect on growth, photosynthesis, seed yield and oil composition of the potential crop halophyte Cakile maritima. In: Öztürk M, Waisel Y, Khan MA, Görk G, editors. Biosaline agriculture and salinity tolerance in plants. Basel: Birkhäuser; 2006. pp. 55–63. [Google Scholar]
- Debez A, Saadaoui D, Slama I, Huchzermeyer B, Abdelly C. Responses of Batis maritima plants challenged with up to two-fold seawater NaCl salinity. J Plant Nutr Soil Sci/Zeitschrift für Pflanzenernährung und Bodenkunde. 2010;173:291. doi: 10.1002/jpln.200900222. [DOI] [Google Scholar]
- Delauney AJ, Hu CA, Kishor PB, Verma DP. Cloning of ornithine delta-aminotransferase cDNA from Vigna aconitifolia by trans-complementation in Escherichia coli and regulation of proline biosynthesis. J Biol Chem. 1993;268:18673–18678. [PubMed] [Google Scholar]
- Demiral T, Turkan I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot. 2005;53:247–257. doi: 10.1016/j.envexpbot.2004.03.017. [DOI] [Google Scholar]
- Funck D, Stadelhofer B, Koch W. Ornithine-δ-aminotransferase is essential for arginine catabolism but not for proline biosynthesis. BMC Plant Biol. 2008;8:40. doi: 10.1186/1471-2229-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneul D, Aïnouche A, Duhazé C, Lugan R, Larher FR, Bouchereau A. A reassessment of the function of the so-called compatible solutes in the halophytic plumbaginaceae Limonium latifolium. Plant Physiol. 2007;144:1598–1611. doi: 10.1104/pp.107.099820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn E, Brown J. Effects of soil salt levels on the growth and water use efficiency of Atriplex canescens (Chenopodiaceae) varieties in drying soil. Am J Bot. 1998;85:10. doi: 10.2307/2446548. [DOI] [PubMed] [Google Scholar]
- Harrouni MC, Daoud S, Koyro H-W. Effect of seawater irrigation on biomass production and ion composition of seven halophytic species in Morocco. In: Lieth H, Mochtchenko M, editors. Cash crop halophytes: recent studies: 10 years after Al Ain Meeting. Dordrecht: Springer; 2003. pp. 59–70. [Google Scholar]
- Hewitt EJ. Sand and water culture methods used in the study of plant nutrition. 2. Farnham Royal: Commonwealth Agricultural Bureaux; 1966. [Google Scholar]
- Inan G, Zhang Q, Li P, Wang Z, Cao Z, Zhang H, Zhang C, Quist TM, Goodwin SM, Zhu J, Shi H, Damsz B, Charbaji T, Gong Q, Ma S, Fredricksen M, Galbraith DW, Jenks MA, Rhodes D, Hasegawa PM, Bohnert HJ, Joly RJ, Bressan RA, Zhu JK. Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiol. 2004;135:1718–1737. doi: 10.1104/pp.104.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Rho HW, Park JW, Park BH, Kim JS, Lee MW. Assay of ornithine aminotransferase with ninhydrin. Anal Biochem. 1994;223:205–207. doi: 10.1006/abio.1994.1574. [DOI] [PubMed] [Google Scholar]
- Kishor PK, Sangam S, Amrutha R, Laxmi PS, Naidu K, Rao K, Rao S, Reddy K, Theriappan P, Sreenivasulu N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci. 2005;88:424–438. [Google Scholar]
- Krell A, Funck D, Plettner I, John U, Dieckmann G. Regulation of proline metabolism under salt stress in the psychrophilic diatom Fragilariopsis Cylindrus (Bacillariophyceae) J Phycol. 2007;43:753–762. doi: 10.1111/j.1529-8817.2007.00366.x. [DOI] [Google Scholar]
- Lee G, Carrow RN, Duncan RR, Eiteman MA, Rieger MW. Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environ Exp Bot. 2008;63:19–27. doi: 10.1016/j.envexpbot.2007.10.009. [DOI] [Google Scholar]
- Lehmann S, Gumy C, Blatter E, Boeffel S, Fricke W, Rentsch D. In planta function of compatible solute transporters of the AtProT family. J Exp Bot. 2011;62:787–796. doi: 10.1093/jxb/erq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Peng D, Zhang X, Peng Y, Chen M, Ma X, Huang L, Yan Y. Na+ induces the tolerance to water stress in white clover associated with osmotic adjustment and aquaporins-mediated water transport and balance in root and leaf. Environ Exp Bot. 2017;144:11–24. doi: 10.1016/j.envexpbot.2017.09.011. [DOI] [Google Scholar]
- Lohaus G, Hussmann M, Pennewiss K, Schneider H, Zhu JJ, Sattelmacher B. Solute balance of a maize (Zea mays L.) source leaf as affected by salt treatment with special emphasis on phloem retranslocation and ion leaching. J Exp Bot. 2000;51:1721–1732. doi: 10.1093/jexbot/51.351.1721. [DOI] [PubMed] [Google Scholar]
- Maggio A, Miyazaki S, Veronese P, Fujita T, Ibeas JI, Damsz B, Narasimhan ML, Hasegawa PM, Joly RJ, Bressan RA. Does proline accumulation play an active role in stress-induced growth reduction? Plant J. 2002;31:699–712. doi: 10.1046/j.1365-313X.2002.01389.x. [DOI] [PubMed] [Google Scholar]
- Maggio A, Raimondi G, Martino A, De Pascale S. Salt stress response in tomato beyond the salinity tolerance threshold. Environ Exp Bot. 2007;59:276–282. doi: 10.1016/j.envexpbot.2006.02.002. [DOI] [Google Scholar]
- Mansour MMF, Ali EF. Evaluation of proline functions in saline conditions. Phytochemistry. 2017;140:52–68. doi: 10.1016/j.phytochem.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Martinez JP, Lutts S, Schanck A, Bajji M, Kinet JM. Is osmotic adjustment required for water stress resistance in the Mediterranean shrub Atriplex halimus L? J Plant Physiol. 2004;161:1041–1051. doi: 10.1016/j.jplph.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Messedi D, Slama I, Laabidi N, Ghnaya T, Savoure A, Soltani A, Abdelly C. Effect of nitrogen deficiency, salinity and drought on proline metabolism in Sesuvium portulacastrum. In: Öztürk M, Waisel Y, Khan MA, Görk G, editors. Biosaline agriculture and salinity tolerance in plants. Basel: Birkhäuser; 2006. pp. 65–72. [Google Scholar]
- Messedi D, Farhani F, Hamed KB, Trabelsi N, Ksouri R, Athar H-u-R, Abdelly C. Highlighting the mechanisms by which proline can confer tolerance to salt stress in Cakile maritima. Pak J Bot. 2016;48:417–427. [Google Scholar]
- Molinari HBC, Marur CJ, Daros E, de Campos MKF, de Carvalho J, Bespalhok JC, Pereira LFP, Vieira LGE. Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol Plant. 2007;130:218–229. doi: 10.1111/j.1399-3054.2007.00909.x. [DOI] [Google Scholar]
- Munns R. Genes and salt tolerance: bringing them together. New Phytol. 2005;167:645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Meir P, Sack L, Evans JR, Oliveira RS, Ball MC. Leaf water storage increases with salinity and aridity in the mangrove Avicennia marina: integration of leaf structure, osmotic adjustment and access to multiple water sources. Plant Cell Environ. 2017;40:1576–1591. doi: 10.1111/pce.12962. [DOI] [PubMed] [Google Scholar]
- Olías R, Eljakaoui Z, Li J, de Morales PA, Marin-Manzano MC, Pardo JM, Belver A. The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ. 2009;32:904–916. doi: 10.1111/j.1365-3040.2009.01971.x. [DOI] [PubMed] [Google Scholar]
- Rayapati PJ, Stewart CR. Solubilization of a proline dehydrogenase from maize (Zea mays L.) mitochondria. Plant Physiol. 1991;95:787–791. doi: 10.1104/pp.95.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues CRF, Silva EN, da Mata Moura R, dos Anjos DC, Hernandez FFF, Viégas RA. Physiological adjustment to salt stress in R. communis seedlings is associated with a probable mechanism of osmotic adjustment and a reduction in water lost by transpiration. Ind Crops Prod. 2014;54:233–239. doi: 10.1016/j.indcrop.2013.12.041. [DOI] [Google Scholar]
- Roosens NHCJ, Thu TT, Iskandar HM, Jacobs M. Isolation of the ornithine-δ-aminotransferase cDNA and effect of salt stress on its expression in Arabidopsis thaliana. Plant Physiol. 1998;117:263–271. doi: 10.1104/pp.117.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosens NH, Al Bitar F, Loenders K, Angenon G, Jacobs M. Overexpression of ornithine-delta-aminotransferase increases proline biosynthesis and confers osmotolerance in transgenic plants. Mol Breed. 2002;9:73–80. doi: 10.1023/A:1026791932238. [DOI] [Google Scholar]
- Ruiz JM, Sánchez E, García PC, López-Lefebre LR, Rivero RM, Romero L. Proline metabolism and NAD kinase activity in greenbean plants subjected to cold-shock. Phytochemistry. 2002;59:473–478. doi: 10.1016/S0031-9422(01)00481-2. [DOI] [PubMed] [Google Scholar]
- Sanchez E, Lopez-Lefebre LR, Garcia PC, Rivero RM, Ruiz JM, Romero L. Proline metabolism in response to highest nitrogen dosages in green bean plants (Phaseolus vulgaris L. cv. Strike) J Plant Physiol. 2001;158:593–598. doi: 10.1078/0176-1617-00268. [DOI] [Google Scholar]
- Silveira JAG, Araújo SAM, Lima JPMS, Viégas RA. Roots and leaves display contrasting osmotic adjustment mechanisms in response to NaCl-salinity in Atriplex nummularia. Environ Exp Bot. 2009;66:1–8. doi: 10.1016/j.envexpbot.2008.12.015. [DOI] [Google Scholar]
- Slama I, Messedi D, Ghnaya T, Savoure A, Abdelly C. Effects of water deficit on growth and proline metabolism in Sesuvium portulacastrum. Environ Exp Bot. 2006;56:231–238. doi: 10.1016/j.envexpbot.2005.02.007. [DOI] [Google Scholar]
- Slama I, Ghnaya T, Savouré A, Abdelly C. Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation of Sesuvium portulacastrum. C R Biol. 2008;331:442–451. doi: 10.1016/j.crvi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Taffouo V, Kenne M, Fotsop OW, Sameza M, Ndomou M, Amougou A. Salinity effects on growth, ionic distribution and water content in salt-tolerant species: Gossypium hirsutum (Malvaceae) J Cam Acad Sci. 2006;6:167–174. [Google Scholar]
- Ueda A, Shi WM, Sanmiya K, Shono M, Takabe T. Functional analysis of salt-inducible proline transporter of barley roots. Plant Cell Physiol. 2001;42:1282–1289. doi: 10.1093/pcp/pce166. [DOI] [PubMed] [Google Scholar]
- Ueda A, Shi W, Nakamura T, Takabe T. Analysis of salt-inducible genes in barley roots by differential display. J Plant Res. 2002;115:0119–0130. doi: 10.1007/s102650200017. [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- Woodrow P, Ciarmiello LF, Annunziata MG, Pacifico S, Iannuzzi F, Mirto A, D’Amelia L, Dell’Aversana E, Piccolella S, Fuggi A, Carillo P. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol Plant. 2017;159:290–312. doi: 10.1111/ppl.12513. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang Q, Ma Y, Chu C. Isolation and expression analysis of salt up-regulated ESTs in upland rice using PCR-based subtractive suppression hybridization method. Plant Sci. 2005;168:847–853. doi: 10.1016/j.plantsci.2004.10.020. [DOI] [Google Scholar]
- Xue X, Liu A, Hua X. Proline accumulation and transcriptional regulation of proline biothesynthesis and degradation in Brassica napus. BMB Rep. 2009;42:28–34. doi: 10.5483/BMBRep.2009.42.1.028. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhang Y, Wei X, You J, Wang W, Lu J, Shi R. Comparative antioxidative responses and proline metabolism in two wheat cultivars under short term lead stress. Ecotoxicol Environ Saf. 2011;74:733–740. doi: 10.1016/j.ecoenv.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Yao X, Horie T, Xue S, Leung H-Y, Katsuhara M, Brodsky DE, Wu Y, Schroeder JI. Differential sodium and potassium transport selectivities of the rice OsHKT2; 1 and OsHKT2; 2 transporters in plant cells. Plant Physiol. 2010;152:341–355. doi: 10.1104/pp.109.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemm EW, Cocking EC, Ricketts RE. The determination of amino-acids with ninhydrin. Analyst. 1955;80:209–214. doi: 10.1039/an9558000209. [DOI] [Google Scholar]