Abstract
The Nigella sativa pharmacological properties are mainly ascribed to its volatile oil, of which thymoquinone is an important bioactive component. Surprisingly, till date, no standard formulation or thymoquinone rich N. sativa extract is under clinical use probably due to its poor extraction and lesser stability in the already used solvents. In the present investigation solubility, extraction, percent composition and total antioxidant activity from the seeds of N. sativa was explored using five solvents. An HPLC method was standardized in an isocratic system (C-18 column, flow rate of 1.0 ml/min, mobile phase—water:methanol: 30:70, detection wavelength—254 nm, retention time—8.77 min) for quantification of thymoquinone. To further confirm the presence of thymoquinone in the respective extracts absorbance spectra analysis has been carried out and compared with pure thymoquinone. Additionally total antioxidant activity of Nigella sativa extracts has been evaluated using ascorbic acid as standard. Our results showed maximum percentage yield in aqueous extract while methanol having the least yield and the ethanol, benzene and hexane extracts exhibited moderate yields. A linear standard calibration curve of thymoquinone showed R2 as 0.999 and % RSD as 7.166. The HPLC analysis revealed maximum percentage composition of thymoquinone in the benzene extract, whereas in the hexane and methanol extracts the content was less. Aqueous and ethanol extracts displayed insignificant thymoquinone content. Absorbance spectra analysis confirms the presence of thymoquinone peak in the benzene, hexane and methanol extracts while aqueous and ethanol extracts showed minimal absorbance. Maximum total antioxidant activity was observed in the aqueous extract while minimum was observed in the methanolic extract. Weak positive (+ 0.3676) correlation was established between percent composition of thymoquinone and antioxidant activity among different extracts indicating that thymoquinone may not be the only factor for antioxidant activity, but other phytochemicals might also contribute. However, we for the first time demonstrated that the benzene extract of N. sativa has better solubility and percent composition of thymoquinone as compared to other solvents. It can be concluded that the solubility, differential composition of bioactive components among these extracts may have diverse effects on the total antiradical activity. Thus, our study provides insights on optimization and standardization of bioactive rich formulation of N. sativa.
Keywords: Antioxidant, Benzene, Nigella sativa, RP-HPLC, Solvent extraction, Thymoquinone
Introduction
Mother nature has been an ultimate resource for therapeutic proxies for thousands of years and products derived from foliage continues to play crucial role in basic health care of about 80–85% of the present population. However, the proclamation of safety, quality, and effectiveness of herbal products is significant and a parallel concern. The extraction of bioactive constituents and development of standards for plant-based drugs is a demanding assignment that needs novel and inspired approaches.
Among the various medicinal plants the black seed, also known as Nigella sativa Linn. (Ranunculaceae) is an unbelievable herb with a rich historical milieu. It is generally cultivated in the Middle-East, some parts of Europe and Western Asia (India, Pakistan, and Afghanistan). Its seeds oil has been commonly used for the remediation of arthritis, hypercholesterolemia and lung infections in Arab traditional system of medication (Khader et al. 2009). Numerous noteworthy pharmacological properties of Nigella sativa have been reported which include infertility effect, immunomodulatory effects, anti-diabetic, nociceptive effect, anti-histaminic, hypotensive effect, antioxidant, anti-inflammatory, antimicrobial and antitumor (Salem 2005; Iqbal et al. 2015).
Most of these pharmacological properties of the entire seeds or their extracts of N. sativa are mainly ascribed to its volatile oil, of which thymoquinone (2-isopropyl-5-methyl-1, 4-benzoquinone) is the most profuse (30–60%) component (Gali-Muhtasib et al. 2008; Banerjee et al. 2010; Severina et al. 2013; Zubair et al. 2013). A number of studies reported its therapeutic effects such as anti-diabetic, antihistaminic, anti-inflammatory, analgesic, antioxidant and anticancer potential (Woo et al. 2012). Thymoquinone exerts its biological functions mainly by modulating physiological redox state both under pathological and normal conditions where it can act as pro-oxidant and antioxidant, respectively (Woo et al. 2012; Badary et al. 2003; Dergarabetian et al. 2013). Investigation (in vitro and in vivo) of N. sativa also revealed antioxidant and antineoplastic properties. N. sativa also showed an antitumor activity on the human hepatoma cell line (Thabrew et al. 2005).
However, it is important to consider the fact that the diverse biological activities of herbal plants are multifactorial and attributed to the presence of a large milieu of bioactive components. In this context plant’s other bioactive components and secondary metabolites, which are made-up of organic molecules with unique carbon arrangements also played crucial role in many ways (Matsuura et al. 2018). Secondary metabolites are not only required for cells to survive, but it plays a vital character in an interface of the cells with its ambiances, confirming the constant existence of the organism to its biomes (Ncube and Staden 2015). Normally the secondary metabolites are of low molecular weight and its production is specific to cell, tissue and organ. Secondary metabolites generally contain phenolics and tannins, which are majorly responsible for the antioxidant property of the plants (Impei et al. 2015). Gismondi et al. (2017) also showed phenolics of Vitis vinifera as potent source of antioxidants. Thymoquinone ascertained to affect mostly all hallmarks of cancer, and medicines that modulate even one of them ought to be considered as good for clinical trials (Schneider-Stock et al. 2014).
Surprisingly, to date thymoquinone has not been taken in clinical trials, primarily due to poor extraction, lesser stability and content in the already used solvents such as ethanol and methanol etc. (Cardoso et al. 2012; Abdelwahab et al. 2013). The previously testified preparations of thymoquinone evidently share difficulties of burst release and low drug loading (Singh et al. 2013; Ganea et al. 2010; Ravindran et al. 2010; Alam et al. 2012; Odeh et al. 2012), which extremely hinder its in-vivo effectiveness. We also hypothesize that it is not only thymoquinone but other bioactive components and secondary metabolites in N. sativa which may contribute to such a rich and diverse biological therapeutic effects reported by various workers as mentioned above. However, the lack of standard formulation for clinical trials may arise due to differential solubility and percent composition of these compounds in various extracts. Thus, it is crucial to screen the best solvent for extracting thymoquinone in terms of stability, higher content and shelf life and comparing with biological (antioxidant) activity in thymoquinone rich as well as thymoquinone deficient extracts. This may help us to provide insights about the possibility of other bioactive compounds and highlight the need of their identification in future for a complete standardized formulation. This information is also vital, since it may contribute in understanding the physicochemical effects of the medication, which may deliver the basis for the improvement of a healthy dosage method.
A number of workers also investigated the physico-chemical properties of thymoquinone (Raschi et al. 2010; Petrucci et al. 2012; Pagola et al. 2003). Numerous analytical techniques have been used for the determination of thymoquinone such as TLC (Basha et al. 2006), GC (Burits and Bucar 2000; Houghton et al. 1995), HPLC (Ghosheh et al. 1999), HPTLC (Velho-Pereira et al. 2011). In spite of this, a detailed literature inspection showed a lack of stability and solubility studies. In this report, we aimed to study the extraction potential, percent composition, stability and solubility of thymoquinone in five different solvents namely methanol, ethanol, water, benzene and hexane.
The purpose of current investigation was to develop an RP-HPLC method with best extraction solvent for thymoquinone from N. sativa. We also aimed to examine the antioxidant potential of various extracts of N. sativa, to strengthen our notion that differential content of thymoquinone and possibly (other bioactive compounds) and solubility in these extracts will have diverse probable antioxidant activity.
Material method
Plant material
The seeds of N. sativa were provided by National Research Centre on Seed Spices (NRCSP), Tabiji, Ajmer, Rajasthan, India. NRCSP designated this study material as Ajmer Nigella (AN1). The seeds were carefully washed with tap water and then by distilled water. The washed seeds (AN1) were blotted on blotting paper and shade dried at room temperature. Fine powder of dried seeds was obtained using tissue blender. Five types of solvents namely methanol, ethanol, water, benzene and hexane were used to prepare variety of extracts from the sample. The extracts were filtered through Whatman filter paper and filtrate was evaporated till dryness (Khoddami et al. 2013). The dried extracts were dissolved to 1 mg/ml stock solution with methanol and filtered with 0.22 μm syringe filter for RP-HPLC analysis. The volatile constituent of N. sativa was collected in small vials and stored in refrigerator until use.
Reagents
Authentic standards of thymoquinone were purchased from Sigma-Aldrich with certified purity of 99.0% and HPLC-grade methanol (Rankem, RFCL Limited) was used in detection by RP-HPLC.
Instrumentation
RP-HPLC analysis was carried out using a UFLC Shimadzu corporation Kyoto, Japan chromatograph Model with pump Model LC-20AD, and UV–Vis detector Model SPD-20A. The C-18 column (250 × 4.6 mm) was made of stainless steel having matrix of 5 μm particle diameter, (Shiseido, Japan). Data acquisition was performed using Lab Solution Lite software (Shimadzu Corporation Kyoto, Japan).
RP-HPLC conditions
RP-HPLC assays were carried out according to the protocol of Ghada et al. (2013), with minor modifications. RP-HPLC method was used in an isocratic system, flow rate of 1.0 ml/min, mobile phase [water:methanol (30: 70, v/v)], the wavelength detection at 254 nm, with retention time of 8.77 min and all determinations were performed at ambient temperature (25–30 °C). In advance, the mobile phase was again filtered through 0.22 μm disposable syringe Millipore filters followed by degassing by bath sonicator.
Stock standard solutions were prepared by dissolving 10 mg thymoquinone, in 10 ml methanol. Prior to investigation, precautions were taken to make sure that the stability of thymoquinone is retained, as it is light and heat sensitive. Therefore, quickly after preparation, samples for study were transferred to amber coloured volumetric flasks and refrigerated. For calibration curve standard solution of (1 mg/ml) was diluted using mobile phase. The concentration ranges from 0.0312 to 2.0 μg/ml. Each standard solution was injected (20 μl) and data was analyzed and chromatographed under the definite conditions defined formerly. Each standard and unknown was run in triplicate. For confirmation of thymoquinone peak and in order to negate the slight variation of retention time every time the experiment was performed and when unknown sample was injected, the standard was also injected alongside. In addition, thymoquinone standard was injected at increasing concentrations to confirm the retention time and peak of thymoquinone. Peak areas were plotted alongside the corresponding concentrations to draw the calibration graph. A linear relationship was obtained.
Spectrum scanning
Spectrophotometer (Shimadzu UV-1800) is used for absorbance spectrum scanning of thymoquinone. The scanning was done by the protocol of Belete and Dagne (2014) with minor modifications.
Total antioxidant activity
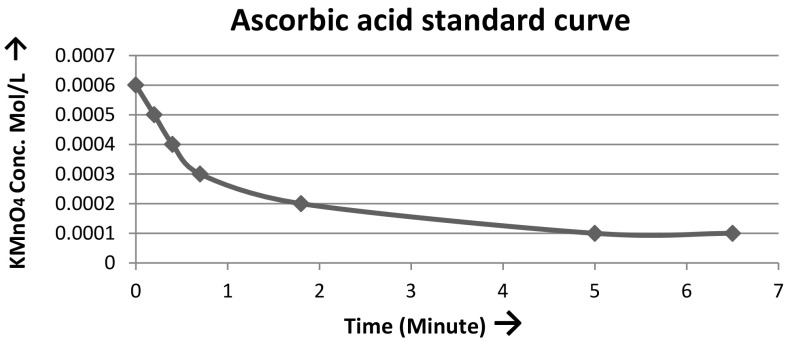
Total antioxidant activity was assayed by the method of Cacig et al. (2005) and Iqbal et al. (2017) with minor modifications. One gram of tissue was homogenized in 4 ml of double distilled water and incubated for 24 h at 4 °C. It was then filtered twice with Whatman No. 1 filter paper and the filtrate collected was stored at 4 °C. Suitable amount of sample was taken in a 3 ml glass cuvette containing the oxidative mixture of 0.18 ml potassium permanganate (0.01 M); 0.42 ml sulfuric acid (2 M) and 2.4 ml distilled water. The decrease in absorbance was measured at 535 nm. Ascorbic acid was taken as standard. Readings were taken in triplicate and the mean activity ± standard deviation was calculated and plotted (Fig. 1).
Fig. 1.
Variation of the potassium permanganate concentration after adding 1 ml of ascorbic acid 0.01 (mmol/ml)
In order to quantitate and compare the antioxidant activities, the following formula was used:
where A50—antioxidant activity expressed, reflected in the time until the sample induces a decrease of the oxidizing agent (potassium permanganate) concentration up to one half, compared against a standard (ascorbic acid) (mmol equivalent standard/gram plant); t plant sample—the time until the sample induces a decrease of the permanganate concentration up to one half (min); t standard—the time until the standard (ascorbic acid) induces a decrease of the permanganate concentration up to one half (min) (0.66 min as seen in standard curve); C standard—standard (ascorbic acid) concentration (mmol/ml) (0.01 mmol/ml); m plant—weight (gram) of the plant sample subjected to extraction (1 g); V plant sample—volume of the plant extract subjected to the analysis (0.1 ml); V standard—volume of the standard subjected to the analysis (1 ml); V extract—volume (ml) of the obtained extract (4 ml).
Statistical analysis
Mean ± standard deviation was calculated and graph was plotted. Correlation was also estimated using MS excel.
Results
Percentage yield of N. sativa extracts prepared from five solvents
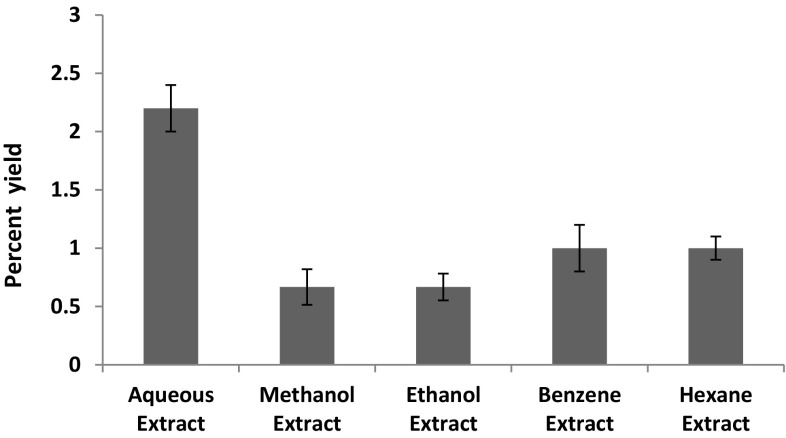
Extracts of N. sativa was prepared using five different solvents to analyze and screen the most suitable solvent for thymoquinone in terms of extract of yield. The percentage yields of these five extracts were calculated as shown in Fig. 2. The aqueous extract showed the maximum yield (2.2 ± 0.2%), whereas the methanol extract have minimum yield (0.633 ± 0.152%). In addition, the ethanol, benzene and hexane extracts have 0.66 ± 0.11, 0.96 ± 0.2 and 1.0 ± 0.1% yields respectively (Fig. 2).
Fig. 2.
Differential percentage yields of extracts in five different solvents
Spectrum scan
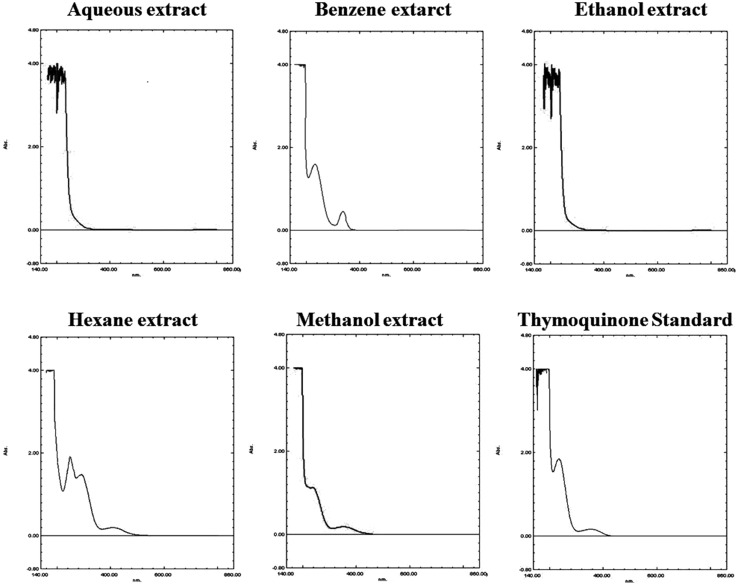
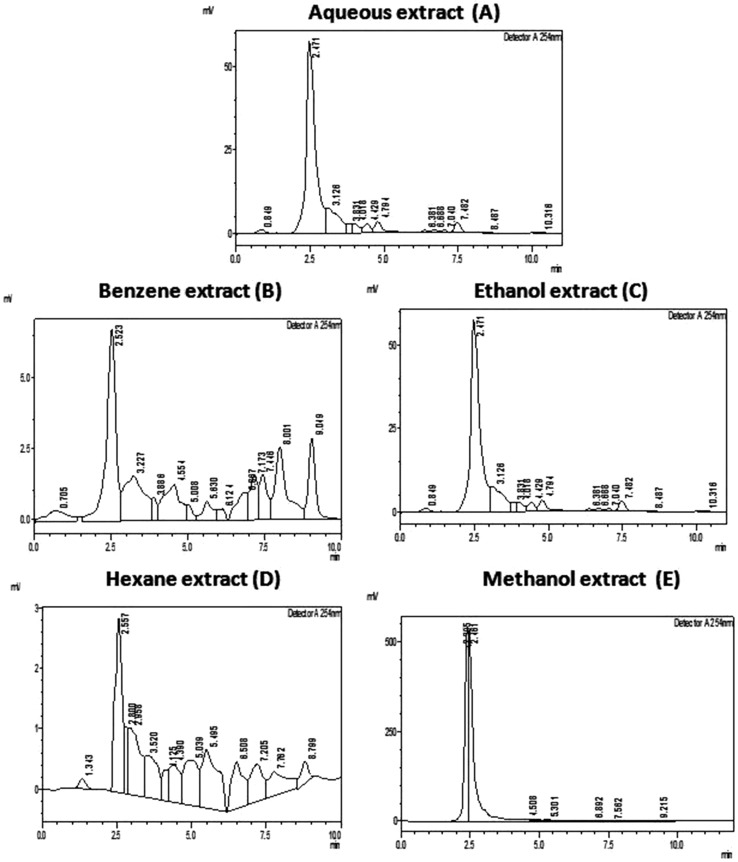
Absorbance spectrum scanning of thymoquinone standard and various extract was done using UV-Visible spectrophotometer in order to determine the peak of thymoquinone standard (maximum absorbance at 254 nm) and to confirm its presence in various extracts. The absorbance spectrum scanning results are shown in Fig. 3, confirms the presence of thymoquinone peak in the benzene, hexane and methanol extracts while aqueous and ethanol extracts showed minimal absorbance.
Fig. 3.
UV-Visible spectrum scan of thymoquinone standard and various extracts using Shimadzu Spectrophotometer
Preparation of linear calibration curve of thymoquinone
Before quantitative analysis of thymoquinone in all the N. sativa extracts by RP-HPLC a linear calibration curve of thymoquinone was prepared using concentration range (0.0312–2.0 μg/ml). The RP-HPLC conditions used are already explained in detail in the method section.
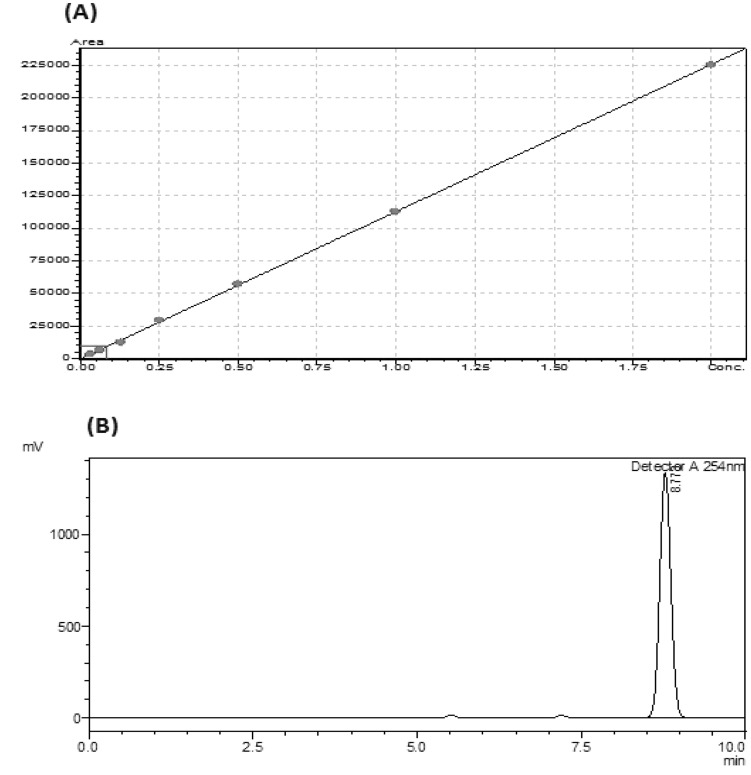
As shown in Fig. 4 the mean coefficient of determination (R2) was 0.999 whereas % RSD was found to be 7.166. Linearity, precision, accuracy, selectivity and robustness of data were taken care for the validation and reproducibility of the method.
Fig. 4.
Thymoquinone standard curve (a) with mean coefficient of determination (R2) = 0.999 using concentration range (0.0312–2.0 μg/ml). Thymoquinone standard peak (b) was detected at retention time of 8.77 min. Data analyzed and acquired using Lab Solution Lite software
Percentage composition of thymoquinone from five N. sativa extracts evaluated by RP-HPLC
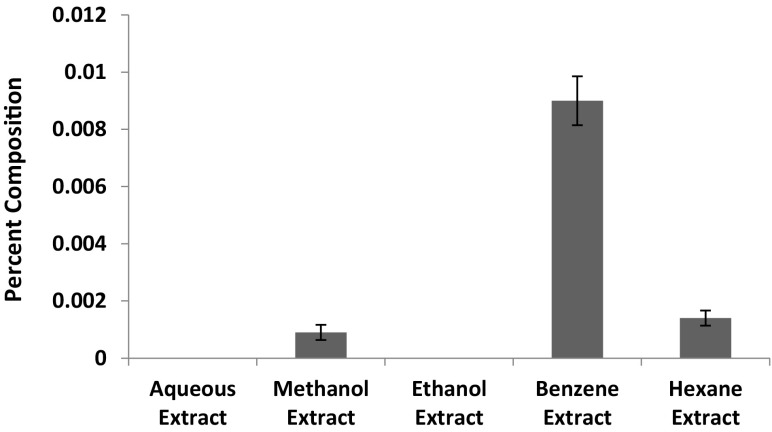
In search of the best solvent for the extraction of thymoquinone from five N. sativa extracts, the sample from each of these extracts were undertaken for quantitative analysis of thymoquinone using standardized RP-HPLC method. As shown in Fig. 5 the benzene extract contains the maximum percentage (0.0090 ± 0.0008) of thymoquinone in comparison to other extracts. The percentage composition of thymoquinone in hexane and methanol extracts is 0.001 ± 0.0002 and 0.0009 ± 0.0002 respectively, whereas in aqueous and ethanol extracts the quantity of thymoquinone was negligible. When the content of thymoquinone was compared among the extracts, it was observed that thymoquinone content is 1.55 folds more in hexane extract as compared to methanol extract while in benzene extract it is 10.0 folds more than methanol extract. The chromatogram of each extract is shown in Fig. 6a–e.
Fig. 5.
Variable percentage composition (content) of thymoquinone in different extracts
Fig. 6.
Chromatogram showing peaks of Thymoquinone in aqueous (a), benzene (b), ethanol (c), hexane (d) and methanol (e) extracts. Data was analyzed and acquired using Lab Solution Lite software
Antioxidant activity
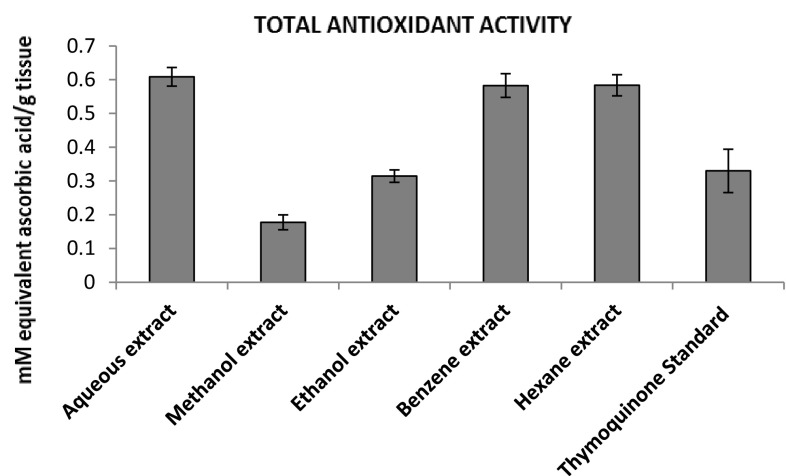
Antioxidant activity is based on the redox reactions between the antioxidant sample and the potassium permanganate in sulfuric acid media, leading to sample discoloration. The total antioxidant activity of different extracts of N. sativa was evaluated as shown in Fig. 7. The results showed maximum total antioxidant activity in the aqueous extract (0.6086± 0.0277 mM equivalent of ascorbic acid/gram of tissue), while minimum was observed in the methanolic extract (0.1776 ± 0.0220 mM equivalent of ascorbic acid/gram of tissue). When correlation was established between percent composition and antioxidant activity among different extracts it was a weak positive (+ 0.3676) correlation.
Fig. 7.
Total antioxidant activity of different extracts of Nigella sativa
Discussion
In the present investigation, we for the first time reported the extraction of thymoquinone from N. sativa seeds using benzene as a stable solvent. We have conducted a detailed comparative study between five solvents (methanol, ethanol, water, benzene and hexane) in terms of extraction potential, yield, better solubility, stability, percent composition of thymoquinone, and total antioxidant activity in these extracts.
This is a unique study in a way that not only it displayed the most suitable solvent for extracting thymoquinone but also compared the yield, solubility, stability and antioxidant activity in thymoquinone rich as well as thymoquinone deficient extracts. The current study has standardized an RP-HPLC method for quantification and detection of thymoquinone using mobile phase of water: methanol (30:70, v/v) at 254 nm, with RT of 8.77 min. Our results showed mean coefficient of determination (R2) as 0.999, whereas, % RSD was found to be 7.166. Detailed schemes were employed for taking care of linearity, precision, accuracy, selectivity and robustness of data so that the validation and reproducibility of the method can be maintained.
Herbal drug formulation’s standardization in terms of quality of raw ingredients, manufacturing methods and composition is necessary to make sure quality and most favorable levels of active principles for their bio-effectiveness. As thymoquinone is a chief phytochemical constituent of N. sativa seeds, a robustic and simple technique was necessary for its standardization and formulation.
We observed different level of yields using five solvents in which the maximum percentage yield was observed in an aqueous extract. The yield study generally indicates a better source for extraction in terms of more amounts of bioactive compounds using a particular solvent. However, other phytochemicals which are soluble in water could be the possible reason for its high yield and consequently percentage yield varied accordingly to solvents. In a study by Rajesh et al. (2013) the phytochemical analysis of aqueous extract of Ocimum sanctum was observed, where the percent yield was determined to compare the chemical constituent with respect to yield of the extract. Therefore, it could be considered that percent yield is an important factor for bioactive content in different extracts. However, this parameter alone cannot be used as a yardstick to claim a better solvent. Other factors like presence of specific bioactive compounds and biological activities should also be considered. Thus we used absorbance analysis (spectrophotometric) and retention time (HPLC) to confirm the presence of thymoquinone in these extracts, so that thymoquinone rich as well as thymoquinone deficient extracts can be identified. The results are in accordance with the report of Belete and Dagne (2014) where the absorbance scanning of thymoquinone also showed maximum at 254 nm.
The HPLC analysis revealed maximum percentage composition of thymoquinone in the benzene extract, whereas in the hexane and methanol extracts the content was less. The lowest percentage composition of thymoquinone was observed in the aqueous extract. This is in accordance to the study of Jumah et al. (2014), where the aqueous solubility and stability of thymoquinone was studied and suggested that the content of thymoquinone may differ as a result of various factors of extraction. In our study the thymoquinone content was 1.55 folds more in the hexane extract as compared to the methanol extract while in the benzene extract it was 10.0 folds more than the methanol extract. This clearly indicates that the benzene extract has the maximum percentage composition of thymoquinone and can be used for the extraction and analyzing purpose. Even the most widely used methanol extract contains lesser amount of thymoquinone. The aqueous and ethanol extracts are not promising in terms of their thymoquinone extraction potential. In spite of this the results should be interpreted very carefully. The lesser content of thymoquinone in the ethanol and methanol is quite unusual as per the available reports (Houghton et al. 1995; El-Tahir et al. 2006). But it is not indicating that thymoquinone is not at all soluble in these extracts, it is only in relative term we are reporting that as compared to benzene the solubility and/or stability of thymoquinone is low. Reports suggest that thymoquinone has been extracted using solvents like ethanol and methanol etc. (Houghton et al. 1995; El-Tahir et al. 2006). However, very little information in regard to stability of the thymoquinone in these preparations or in the course of manufacture and later through the shelf-life, causes a foremost difficulty in drug formulations. It was earlier observed that supercritical fluid extraction method can be used to harvest good quality and quantity extracts from N. sativa which are rich in antioxidants (Rao et al. 2007). Antioxidant screening by RP-HPLC of methanolic extract of some medicinal plants viz. Syzygium cumini also showed phenolic to be the main source of antioxidant (Guleria et al. 2013). Al-Said et al. (2002) had worked on the effect of different precursors such as thymol and menthol on the biosynthesis of thymoquinone. Results reported by Al-Ani (2008) also confirmed thymol production in the callus obtained from the N. sativa leaf explants. Production of thymoquinone in the selected calli of leaf and root which had been grown in the dark was examined. It was studied by Basha et al. (2006) that the determination of thymoquinone spots, attained from the methanolic extract of oil from N. sativa was established by GC/MS, which is basically undistinguishable to thymoquinone standard. The solvents comprised of benzene: isopropyl ether in a ratio of 1:1 and measured at 254 nm. However, the thymoquinone solubility differs in diverse aqueous solvents, and may be overlooked because of stability problems. Studies related to stability of thymoquinone exhibited low profile in all the aqueous solutions with swift deprivation that differ with different solvents (Salmani et al. 2014). Therefore in this context our results are crucial as the content of thymoquinone was maximum in benzene solvent and was stable for longer duration. Our study clearly show a wide range of solubility of thymoquinone in various solvents, but benzene may be preferred over others simply because of higher solubility, stability and higher content of thymoquinone extracted using this solvent. These results are in accordance to a preliminary report of Johnson and Krishnaveni (2012) where benzene, petroleum ether and chloroform extract showed prominent and moderate extraction of phytochemicals using HPLC from Dictyota bartayresiana. The phytochemicals showed the presence of alkaloids, phenolics, steroids, tannins, saponins, glycosides and sugars. However, in case of thymoquinone from N. sativa seeds this is a novel report first of its kind where benzene has been shown as a most suitable solvent for the extraction and stability of thymoquinone along with significant antioxidant activity.
Beyond N. sativa, thymoquinone has been reported in high concentration in other plants such as Monarda fistulosa (Helena et al. 2015). This study estimated thymoquinone content in Monarda didyma and M. fistulosa and reported 0.6 mg TQ/g M. didyma and 1.7 mg TQ/g M. fistulosa respectively and percent extract as 5.2 and 10.2% w/w respectively. However, substantial thymoquinone loss during hydro-distillation was confirmed. The extraction with hexane ensured maximum thymoquinone yields (0.7 and 2.1 mg/g) but its concentration in the extracts was low (2.6 and 3.8%). The comparison between N. sativa and M. didyma or M. fistulosa clearly indicates lesser thymoquinone content in N. sativa.
Maximum total antioxidant activity was observed in the aqueous extract, while minimum was observed in the methanolic extract. Interestingly, in the aqueous extract in spite of negligible presence of thymoquinone it showed significant total antioxidant activity, thus the presence of other bioactive compounds with antioxidant potential cannot be ruled out. In our study, a weak positive (+ 0.3676) correlation was established between percent composition of thymoquinone and antioxidant activity among different extracts indicating that thymoquinone may not be the only factor for antioxidant activity, but other phytochemicals might also contribute. It is reported that the antioxidant potential of thymoquinone may vary depending upon the choice of its extraction procedure or selection of solvent (Ashraf et al. 2011).
At the same time, the thymoquinone rich extracts like benzene and hexane also possess relatively significant antioxidant potential which could be attributed to the presence of thymoquinone. The antioxidant potential of thymoquinone was also revealed by Ismail et al. (2010). They showed that that thymoquinone efficiently enhanced the plasma and liver antioxidant capability and improved the expression of liver antioxidant genes in rats possessing hypercholesterolemism.
Conclusion
In this study, a simple, linear, subtle, accurate and robust RP-HPLC method for the determination of thymoquinone has been established. The method is isocratic with a simple mobile phase, minimal sample preparation and simple, rapid assay procedures. The study revealed that N. sativa possesses good amount of thymoquinone and is a potent antioxidant. Therefore, we recommend that this method can be used for routine investigation of thymoquinone in crude plant samples and phyto-pharmaceuticals. It is observed that depending upon the extraction solvent, amount of thymoquinone varies. Best solvent system was found to be benzene for extraction of thymoquinone from N. sativa. However, in order to identify best solvent, various factors must be taken into consideration simultaneously such as yield, solubility, stability, composition analysis and biological activity. It can be concluded that the solubility, differential composition of bioactive components among these extracts may have diverse effect on the total antiradical activity. Thus, our study provides insights of various factors required for optimization and standardization of bioactive rich formulation of N. sativa. Our study provides insights about the possibility of other bioactive compounds and highlight the need of their identification in future for a complete standardized formulation. We anticipate this work to be helpful in upcoming research on thymoquinone formulations that may accelerate its therapeutic applications. Developing a standardized extract with a stable and higher level of pharmaceutical product by selecting a suitable solvent can be helpful in modern clinical practice.
Conflict of interest
Authors worked for this manuscript declares that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent is not applicable.
References
- Abdelwahab SI, Sheikh BY, Taha MM, How CW, Abdullah R, Yagoub U, El-Sunousi R, Eid EE. Thymoquinone-loaded nanostructured lipid carriers: preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration. Int J Nanomed. 2013;8:2163–2172. doi: 10.2147/IJN.S44108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S, Khan ZI, Mustafa G, Kumar M, Islam F, Bhatnagar A, Ahmad FJ. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: a pharmacoscintigraphic study. Int J Nanomed. 2012;7:5705–5718. doi: 10.2147/IJN.S35329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ani NK. Thymol production from callus culture of Nigella sativa L. Plant Tissue Cult Biotechnol. 2008;18(2):181–185. [Google Scholar]
- Al-Said M, El-Olemy MM, Elhag HM. Biotechnological production of biologically active metabolites by plant cell culture techniques. Riyadh: KACST; 2002. pp. 218–256. [Google Scholar]
- Ashraf SS, Madduri VR, Fatima SK, Shahnaz Q, Al-Marzouqi AH, Irwin S, Chandranath Abdu A. Nigella sativa extract as a potent antioxidant for petrochemical-induced oxidative stress. J Chromatogr Sci. 2011;49:321–326. doi: 10.1093/chrsci/49.4.321. [DOI] [PubMed] [Google Scholar]
- Badary OA, Taha RA, Gamal El-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003;26:87–98. doi: 10.1081/DCT-120020404. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Padhye S, Azmi A, Wang Z, Philip PA, Kucuk O, Sarkar FH, Mohammad RM. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr Cancer. 2010;62:938–946. doi: 10.1080/01635581.2010.509832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha LI, Abou MSR, Aboul-Enein HY. TLC Assay of thymoquinone in black seed oil (Nigella sativa linn) and identification of dithymoquinone and thymol. J Liq Chromatogr. 2006;18(1):105–115. doi: 10.1080/10826079508009224. [DOI] [Google Scholar]
- Belete Y, Dagne E. HPTLC assay of thymoquinone in black seed and black seed oil (Nigella sativa linn) and identification of thymoquinone conversion with UV-Vis. J Drug Deliv Ther. 2014;4(4):1–5. [Google Scholar]
- Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::AID-PTR621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Cacig S, Szabo MR, Lupea AX, Ardelean A. Determination of the antioxidant activity of Ziziphus jujuba and Hydrangea incognita aqueous extracts. Studia Univ Vasile Goldis Ser st Vietii. 2005;15:69–72. [Google Scholar]
- Cardoso T, Galhano CIC, Ferreira Marques MF, da Silva AM. Thymoquinone β-cyclodextrin nanoparticles system: a preliminary study. Spectroscopy. 2012;27:329–336. doi: 10.1155/2012/902486. [DOI] [Google Scholar]
- Dergarabetian EM, Ghattass KI, El-Sitt SB, Al-Mismar RM, El-Baba CO, Itani WS, Melhem NM, El-Hajj HA, Bazarbachi AA, Schneider-Stock R. Thymoquinone induces apoptosis in malignant T-cells via generation of ROS. Front Biosci (Elite Ed) 2013;5:706–719. doi: 10.2741/E651. [DOI] [PubMed] [Google Scholar]
- El-Tahir K, El-Din H, Bakeet DM. The black seed Nigella sativa Linnaeus—a mine for multi cures: a plea for urgent clinical evaluation of its volatile oil. J Taibah Univ Med Sci. 2006;1(1):1–19. [Google Scholar]
- Gali-Muhtasib H, Kuester D, Mawrin C, Bajbouj K, Diestel A, Ocker M, Habold C, Foltzer-Jourdainne C, Schoenfeld P, Peters B. Thymoquinone triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Cancer Res. 2008;68:5609–5618. doi: 10.1158/0008-5472.CAN-08-0884. [DOI] [PubMed] [Google Scholar]
- Ganea GM, Fakayode SO, Losso JN, van Nostrum CF, Sabliov CM, Warner IM. Delivery of phytochemical thymoquinone using molecular micelle modified poly(D, L lactide-coglycolide) (PLGA) nanoparticles. Nanotechnology. 2010;21:1–10. doi: 10.1088/0957-4484/21/28/285104. [DOI] [PubMed] [Google Scholar]
- Ghada MH, Randa A, Abdel S, Rabab MS, Mostafa KM. HPLC–DAD determination of seven antioxidants and caffeine in different phytopharmaceuticals. J Chromatogr Sci. 2013;52:1–7. doi: 10.1093/chromsci/bmt086. [DOI] [PubMed] [Google Scholar]
- Ghosheh OA, Houdi AA, Crooks PA. High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L.) J Pharm Biomed Anal. 1999;19:757–762. doi: 10.1016/S0731-7085(98)00300-8. [DOI] [PubMed] [Google Scholar]
- Gismondi A, Di Marco G, Canuti L, Canini A. Antiradical activity of phenolic metabolites extracted from grapes of white and red Vitis vinifera L. cultivars. Vitis. 2017;56:19–26. [Google Scholar]
- Guleria S, Tiku AK, Singh G, Koul A, Gupta S, Rana S. In vitro antioxidant activity and phenolic contents in methanol extracts from medicinal plants. J Plant Biochem Biotechnol. 2013;22(1):9–15. doi: 10.1007/s13562-012-0105-6. [DOI] [Google Scholar]
- Helena S, Marie S, Martin T. Supercritical CO2 extraction of volatile thymoquinone from Monarda didyma and M. fistulosa herbs. J Supercrit Fluids. 2015 doi: 10.1016/j.supflu.2015.01.004. [DOI] [Google Scholar]
- Houghton PJ, Zarka R, de las Heras B, Hoult JR. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61:33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- Impei S, Gismondi A, Canuti L, Canini A. Metabolic and biological profile of autochthonous Vitis vinifera L. ecotypes. Food Funct. 2015;6(5):1526–1538. doi: 10.1039/C5FO00110B. [DOI] [PubMed] [Google Scholar]
- Iqbal MS, Ansari MI, Ahmad I, Pandey B. Morpho-physiological characterization of seeds and seedlings of Nigella sativa Linn.: study on Indian germplasm. Int Res J Biol Sci. 2015;4(4):38–42. [Google Scholar]
- Iqbal Z, Iqbal MS, Mishra K. Screening of antioxidant property in medicinal plants belonging to the family Apocynaceae. Asian J Pharm Clin Res. 2017;10(12):415–418. doi: 10.22159/ajpcr.2017.v10i12.22303. [DOI] [Google Scholar]
- Ismail M, Al-Naqeep G, Chan KW. Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic Biol Med. 2010;48(5):664–672. doi: 10.1016/j.freeradbiomed.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Krishnaveni E. UV-VIS spectroscopic and HPLC studies on Dictyota bartayresiana lamour. Asian Pac J Trop Biomed. 2012;S5:14-S518. [Google Scholar]
- Jumah MMS, Sajid A, Huixia L, Jianping Z. Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents, pH and light. Molecules. 2014;19(5):5925–5939. doi: 10.3390/molecules19055925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader M, Bresgen N, Eckl PM. In vitro toxicological properties of thymoquinone. Food ChemToxicol. 2009;47(1):129–133. doi: 10.1016/j.fct.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Khoddami A, Meredith AW, Thomas HR. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura HN, Malik S, de Costa F, Yousefzadi M, Mirjalili MH, Arroo R, Bhambra AS, Strnad M, Bonfill M, Fett-Neto AG. Specialized plant metabolism characteristics and impact on target molecule biotechnological production. Mol Biotechnol. 2018;60(2):169–183. doi: 10.1007/s12033-017-0056-1. [DOI] [PubMed] [Google Scholar]
- Ncube B, Staden JV. Tilting plant metabolism for improved metabolite biosynthesis and enhanced human benefit. Molecules. 2015;20:12698–12731. doi: 10.3390/molecules200712698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeh F, Ismail SI, Abu-Dahab R, Mahmoud IS, Bawab A. Thymoquinone in liposomes: a study of loading efficiency and biological activity towards breast cancer. Drug Deliv. 2012;19:317–371. doi: 10.3109/10717544.2012.727500. [DOI] [PubMed] [Google Scholar]
- Pagola S, Benavente A, Raschi A, Romano E, Molina MAA, Stephens PW. Crystal structure determination of thymoquinone by high-resolution X-ray powder diffraction. AAPS PharmSciTech. 2003;5:1–8. doi: 10.1208/pt050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucci R, Marrosu G, Astolfi P, Lupidi G, Greci L. Cyclic voltammetry, spectroelectrochemistry and electron spin resonance as combined tools to study thymoquinone in aprotic medium. Electrochim Acta. 2012;60:230–238. doi: 10.1016/j.electacta.2011.11.055. [DOI] [Google Scholar]
- Rajesh H, Rao SN, Prathima KS, Megha RN, Rejeesh EP, Lovelyn J. Phytochemical analysis of aqueous extract of Ocimum Sanctum Linn. Int J Univers Pharm Bio Sci. 2013;2(2):462–468. [Google Scholar]
- Rao MV, Al-Mazrouqi AH, Fatima-Shad K, Ashraf S, Adem A. Comparative evaluation of SFE and solvent extraction methods on the yield and composition of Black seeds (Nigella sativa) J Liq Chromatgr Relat Technol. 2007;30:2545–2555. doi: 10.1080/10826070701540100. [DOI] [Google Scholar]
- Raschi AB, Romano E, Benavente AM, Ben Altabef A, Tuttolomondo ME. Structural and vibrational analysis of thymoquinone. Spectrochim Acta Part A Mol Biomol Spectrosc. 2010;77:497–505. doi: 10.1016/j.saa.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Ravindran J, Nairb HB, Sunga B, Prasada S, Tekmalb RR, Aggarwala BB. Thymoquinone poly (lactide-co-glycolide) nanoparticles exhibit enhanced anti-proliferative, anti-inflammatory, and chemosensitization potential. Biochem Pharmacol. 2010;79:1640–1647. doi: 10.1016/j.bcp.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5(13–14):1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Salmani JMM, Asghar S, Lv H, Zhou J. Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents, pH and light. Molecules. 2014;19:5925–5939. doi: 10.3390/molecules19055925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Stock R, Fakhoury IH, Zaki AM, El-Baba CO, Gali-Muhtasib HU. Thymoquinone: fifty years of success in the battle against cancer models. Drug Discov Today. 2014;19:18–30. doi: 10.1016/j.drudis.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Severina II, Severin FF, Korshunova GA, Sumbatyan NV, Ilyasova TM, Simonyan RA, Rogov AG, Trendeleva TA, Zvyagilskaya RA, Dugina VB. In search of novel highly active mitochondria-targeted antioxidants: thymoquinone and its cationic derivatives. FEBS Lett. 2013;587:2018–2024. doi: 10.1016/j.febslet.2013.04.043. [DOI] [PubMed] [Google Scholar]
- Singh A, Ahmad I, Akhter S, Jain GK, Iqbal Z, Talegaonkar S, Ahmad FJ. Nanocarrier based formulation of thymoquinone improves oral delivery: stability assessment, in-vitro and in-vivo studies. Colloids Surf B. 2013;102:822–832. doi: 10.1016/j.colsurfb.2012.08.038. [DOI] [PubMed] [Google Scholar]
- Thabrew MI, Mitry RR, Morsy MA, Hughes RD. Cytotoxic effects of a decoction of Nigella sativa, Hemidesmus indicus and Smilax glabra on human hepatoma HepG2 cells. Life Sci. 2005;77:1319–1330. doi: 10.1016/j.lfs.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Velho-Pereira RM, Barhate CR, Kulkarni SR, Jagtap AG. Validated high-performance thin-layer chromatographic method for the quantification of thymoquinone in Nigella Sativa extracts and formulations. Phytochem Anal. 2011;22:367–373. doi: 10.1002/pca.1289. [DOI] [PubMed] [Google Scholar]
- Woo CC, Kumar AP, Sethi G, Tan KHB. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012;83:443–451. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Zubair H, Khan HY, Sohail A, Azim S, Ullah MF, Ahmad A, Sarkar FH, Hadi SM. Redox cycling of endogenous copper by thymoquinone leads to ROS-mediated DNA breakage and consequent cell death: putative anticancer mechanism of antioxidants. Cell Death Dis. 2013;4:660. doi: 10.1038/cddis.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]