Abstract
The seeds of chickpea provide an exceptional source of dietary proteins and is one of the important legumes in both developed and developing countries over the world. The available germplasm of cultivated chickpea is deficient in desired biochemical signatures. To identify new sources of variations for breeding, reduced subsets of germplasm such as mini-core collection can be explored as an effective resource. In the present investigation, mini-core collections consisting of 215 accessions of chickpea were extensively evaluated for tapping biochemical diversity. Analysis included ten biochemical parameters comprising total protein, total free amino acids, phytic acid, tannin, total phenolics, total flavonoids, lectin, DPPH radical scavenging activity, in vitro digestibility of protein and starch. The spectrum of diversity was documented for total protein (4.60–33.90%), total free amino acids (0.092–9.33 mg/g), phytic acid (0.009–4.06 mg/g), tannin (0.232–189.63 mg/g), total phenolics (0.15–0.81 mg/g), total flavonoids (0.04–1.57 mg/g), lectin (0.07–330.32 HU/mg), DPPH radical scavenging activity (26.74–49.11%), in vitro protein digestibility (59.45–76.22%) and in vitro starch digestibility (45.63–298.39 mg of maltose/g). The principal component analysis revealed association of chickpea higher protein content to the lower level of total phenolics and flavonoid contents. The dendrogram obtained by unweighted pair group method using arithmetic average cluster analysis grouped the chickpea accessions into two major clusters. This is the first comprehensive report on biochemical diversity analysed in the mini-core chickpea accessions. The ultimate purpose of conducting such studies was to deliver information on nutritional characteristics for effective breeding programmes. Depending on the objectives of the breeding aforesaid accessions could be employed as a parent.
Electronic supplementary material
The online version of this article (10.1007/s12298-018-0579-3) contains supplementary material, which is available to authorized users.
Keywords: Chickpea, Mini-core collection, Genetic diversity, In vitro protein digestibility, Lectin, DPPH
Introduction
A subclass representing 10% of the overall accessions constitute core collection and signifies the genetic variability of entire germplasm (Frankel 1984). The accessions contained in some of the crop species are several thousand and subsequently challenging for assessment (Upadhyaya et al. 2010). To overcome this, mini-core was developed consisting of 1% of entire collection representing spectrum of diversity. The core and mini-core collections thus stand for diversity of entire collection promoting germplasm utilization and crop improvement enhancement. Mini-core approach consequently provides information of different germplasm that is required for evolving broad-based cultivars (Upadhyaya et al. 2013).
Chickpea (Cicer arietinum L.) belonging to family Fabaceae, is a major food legume in countries across the world. To reduce the malnutrition in developing countries, seeds of chickpea offer cheapest source of protein and high nutrition (Thudi et al. 2017). The functionality of chickpea seed proteins has received attention in recent years, owing to anti-angiotensin-I converting enzyme, anticancer, antidiabetic and anti HIV-1 reverse transcriptase effects (Roy et al. 2010). In our previous report, biochemical characterization of 20 different chickpea seed accessions was carried out to ascertain its nutritional status (Bhagyawant et al. 2015). International crops research institute for semi-arid tropics (ICRISAT), Patancheru (A.P.) India, has been front runner in collecting and maintaining more than 20,000 chickpea accessions from various parts of the world (www.icrisat.org).
Crop improvement programmes are being pursued vigorously. Germplasm with broad genetic variability is a primary need to breed and develop cultivars with superior yield having enhanced nutrients. Being a self-pollinated winter crop, available germplasm of cultivated chickpea shows a low genetic and biochemical variation profile. This therefore, further necessitates the exploitation of supplementary germplasm like mini-core accessions for procuring biochemical traits of nutritional composition. For the chickpea future crop improvement, mini-core collection needs to be targeted.
Chickpea was referred as ‘orphan crop’ until last decade due to restricted genome resources (Varshney et al. 2012). Thereafter, significant accomplishments were made in deployment of genetic resources leading to chickpea crop enhancement (Roorkiwal et al. 2017). Previously chickpea mini-core accessions were assessed for tolerance to soil salinity (Serraj et al. 2004), root traits (Kashiwagi et al. 2005), multiple disease resistance (Pande et al. 2006), variability under different environments (Parameshwarappa et al. 2012), agronomic evaluation for biotic and abiotic stresses (Upadhyaya et al. 2013) and AFLP analysis to identify diverse genetic stocks (Saeed and Darvishzadeh 2016). However, there does not seem to be any clinching evidences in the literature domain about biochemical characterization of mini-core chickpea accessions. At present, chickpea improvement programmes augmenting nutritional quality is the principal concern for chickpea breeders worldwide. Studies on biochemical variations in chickpea mini-core seed germplasm are unknown till date and may provide further breeding guidelines. The interesting facet of the work being representation of 215 mini-core chickpea accessions obtained from ICRISAT employed for analysing biochemical signatures. The information presented here could assist chickpea breeders to select parents for future breeding programmes especially for biofortification.
Materials
Two hundred and fifteen mini-core accessions of chickpea (C. arietinum L.) were used for analysis (Fig. 1), out of which four accessions were Indian national checks. The mature and dry seed material was obtained from International Crops Research Institute for the Semi-arid Tropics (ICRISAT), Patancheru, Hyderabad India, under MTA understanding. Seeds were ground in a grinder and obtained seed powder was first defatted using chilled acetone and air dried. Analysis was performed under ambient conditions of temperature and humidity.
Fig. 1.
Single seed representation of 215 chickpea mini-core accessions
Methods
Extraction and estimation of total protein and total amino acid content
The total protein was extracted by the process of Fan and Sosulski (1974). The chickpea seeds powder (100 mg) was kept overnight in 0.1 N NaOH (25 ml) and centrifuged at 10,000 rpm for 20 min. The obtained supernatant was used for the total protein estimation following the procedure of Lowry et al. (1951).
The total amino acid content was extracted by following the method of Moore and Stein (1948). 80% ethanol (5–10 ml) was added to seed powder (500 mg) and the contents were subsequently centrifuged. The obtained supernatant was pooled to read the intensity of sample at 570 nm.
Seed analysis for antinutritional composition
Extraction of tannins and phytic acid
The tannin was extracted following the procedure of Schandrel (1970) and tannins were estimated as tannic acid acid equivalents. The phytic acid was extracted from the chickpea seeds by the method of Wilcox et al. (2000).
Extraction of total phenolic content (TPC)
Total phenolic contents were extracted and estimated as described by Swain and Hillis (1959). The chickpea seed powder was homogenised with 80% of methanol for 1 h. Filtered supernatant was then mixed by adding Folin-phenol reagent and sodium carbonate, and the absorbance was measured at 650 nm.
Estimation of total flavonoid content
Total flavonoid of methanolic extract of chickpea seeds was determined by Khoo et al. (2013). The extract was dissolved in 2% aluminium chloride and incubated for 30 min at room temperature. The absorbance was measured at 414 nm. The total flavonoid content was estimated as quercetin equivalent (QE).
Extraction of lectin and hemagglutination assay
100 mg dry seed powder defatted twice with acetone (1:2 w/v) was kept in 1000 µl of 10 mM Tris–HCl, containing 150 mM NaCl (pH 7.2). This mixture after stirring for 16 h, was centrifuged at 10,000 rpm for 20 min in cold and the supernatant was collected as a source of lectin.
The hemagglutination activity was determined using trypsinised rabbit erythrocytes. The fresh erythrocytes separated from plasma by centrifugation at 3000 rpm for 4 min at 5–10 °C and washed extensively with phosphate buffer saline (PBS). Finally, 3% suspension was prepared in PBS and the two-fold serial dilution was used to perform hemagglutination tests in microtitre plate (Liener 1962). The erythrocyte suspension was mixed with serially diluted lectin and agglutination was observed at room temperature. The reciprocal of the highest dilution of the lectin that showed complete agglutination (titre) is the unit of hemagglutination activity (U). The specific activity is measured as hemagglutination per mg of protein.
Seed analysis for antioxidant composition
DPPH radical scavenging assay
Following the method of Bersuder et al. (1998), radical scavenging activity was determined. The chickpea seed powder (100 mg) was extracted in 2 ml methanol. 1 ml of supernatant was mixed with 3 ml of 0.1 mM DPPH and incubated for 30 min in dark. The absorbance was measured at 517 nm and ascorbic acid was used as a positive control. DPPH radical scavenging activity was calculated using the equation:
where A blank is the absorbance of the control reaction and A sample is the absorbance of sample.
In vitro protein digestibility
Employing a binary enzyme system that consisted of trypsin (14,600 U/mg) and α-chymotrypsin (48 U/mg), an in vitro protein digestibility was worked out. 5 ml of the enzyme cocktail preserved on ice was added to 50 ml of powdered seed suspension, adjusted to pH 8.0 using 1 M NaOH and kept at 37 °C incubation (Hsu et al. 1977). The change in pH was measured after ten min period (pH10min) and percent in vitro protein digestibility (IVPD) was determined by following equation.
where X = pH of protein suspension after digestion (10 min) with binary enzyme cocktail.
In vitro starch digestibility
Following the method of Singh et al. (1982), in vitro starch digestibility (IVSD) was determined. Briefly, the chickpea flour suspensions was incubated for amylolysis in the presence of pancreatic amylase (1260 U/mg) suspension at 20 °C for 2 h. Thereafter, 2 ml of 3,5-dinitrosalicylic acid reagent was added to terminate the reaction and the mixture was heated for 5 min. The absorbance of the suspension was measured at 550 nm taking maltose as standard. IVSD was expressed as mg of maltose released per gram of sample on a dry weight basis.
Statistical analysis
The one way analysis of variance followed by Dunnett’s correction post hoc analysis using XLSTAT 2013 was carried out to notice significant differences among the all genotypes. The multivariate principal component analysis and the similarity matrix was accessed by unweighted pair group method with arithmetic mean (UPGMA) using NTSYS pc 2.02.
Results and discussion
Chickpea is positioned as an essential pulse crop of the world due to its abundant vital nutritive values and health benefits to human beings. The breeders from all over the world at all times explore elite genotypes which can serve as donor parents. Therefore, the development of new cultivars with high protein and nutritionally balanced contents is an obligatory breeding objective (Kahraman et al. 2017). The nutrients and/or antinutrients propositions are essential parameters for ascertaining seed quality (Roy et al. 2010). Owing to this, nutritional (total proteins, total free amino acids) and antinutritional (tannins, phytic acid, total phenolic and flavonoids content) components of mini-core chickpea accessions were analysed. The values of means for all attributes in seeds comprising of the maximum and minimum along with the values of standard deviation were considered.
Total protein and free amino acid
The protein content of mini-core collected chickpea seeds is assorted in its composition. Total proteins observed to be in a range of 4.60–33.90%. The average content of total protein across all the accessions was observed to be 28.75%. The protein content of ICC-13599, ICC-9848, ICC-9942, ICC-4872, ICC-12968, ICC-2065, ICC-14831, ICC-12947, ICC-7819 and ICC-4533 accessions was observed higher in all 211 accessions. While, lowest protein content was found in ICC-11121 compared to four Indian national checks ICC-4948, ICC-4973, ICC-12968 and ICC-15996. The variation detected may be due to their places of origin and therefore referred as genetic.
Free amino acids were observed in a range of 0.092–9.33 mg/g across all the mini-core accessions. The average of free amino acids was found to be 6.21 mg/g. The maximum free amino acid contents were observed in ICC-1715 (9.33 ± 0.31 mg/g). Out of 211 samples, 10 accessions ICC-1715, ICC-8058, ICC-4567, ICC-15802, ICC-10755, ICC-3946, ICC-6571 ICC-11879, ICC-7441, ICC-9755 contained highest than the average values across all the mini-core seed accessions while minimum was exhibited by ICC-12307 (0.092 ± 0.1 mg/g) compared to four Indian national checks. Our results are consistent with those of Kaur et al. (2014) and Melki et al. (2017) who determined average of 24 mg protein content in chickpea. Consumption of chickpea seed provides crucial nutrients for sustainable life processes (Gupta et al. 2017). For example, human body needs amino acids owing to several biological activities vis-à-vis supports the cell metabolism and repairing of tissue. In addition, they form antibody against combating bacteria and viruses and play vital role in building nucleoproteins of RNA and DNA (Imura and Okada 1998). Seeds of pulses store protein in their development stages therefore mature seeds are enriched with high contents of protein and hence the total free amino acids.
Tannin and phytic acid
The average of tannic acids was found to be 56.64 mg/g across all the accessions. The maximum value was observed in ICC-2072 (189.63 ± 1.17 mg/g). Out of 211 samples, 10 accessions ICC-2072, ICC-16269, ICC-5639, ICC-2210, ICC-2242, ICC-3230, ICC-14098, ICC-2065, ICC-2919, ICC-4872 depicted higher than the average values across all the mini-core seed accessions while minimum was exhibited by ICC-13764 (0.232 ± 0.01 mg/g) compared to four Indian national checks.
The average phytic acid contents was found to be 0.91 mg/g across the mini-core chickpea accessions. The maximum phytic acid was found in ICC-7184 (4.06 ± 0.05 mg/g). Out of 211 samples, ten accessions ICC-7184, ICC-7819, ICC-13219, ICC-2990, ICC-12037, ICC-12947, ICC-11764, ICC-15610, ICC-1422, contained higher phytic acid while minimum was exhibited by ICC-5879 (0.009 ± 0.0 mg/g) compared to four Indian national checks ICC-4948, ICC-4973, ICC-12968, and ICC-15996.
The antinutritional properties of tannins affects the development of stable complexes with protein and vitamins. Tannins bind with the saliva proteins and mucosal membrane of the mouth during food mastication thus reducing digestibility of the proteins and carbohydrates (Vadivel and Janardhanan 2005). Kaur et al. (2014) reported 8.23 mg/g tannin content in chickpea seeds. Tannins provide resistance to enzymes for digestive degradation therefore did not get absorbed and transported to other tissues of the body. However, soaking and some of the thermal processes effectively releases free tannins as one of the means of their removal (Vidal-Valverde et al. 1994). From nutritional point of view, presence of even low levels of tannins is undesirable. In the present studies, total phytic acid content varied from 0.42 to 26.80 mg/g with an average value of 26.80 mg/g. Similar levels of tannin and phytate were observed by different authors (Alajaji and El-Adawy 2006; Xu et al. 2016).
In legumes, phytic acid is the chief storage form of phosphorous. The negatively charged phytate molecule of phytic acid binds to nutritionally essential divalent cations, such as iron, zinc, magnesium and calcium. Subsequently, the insoluble complexes are formed that makes the minerals inaccessible for absorption and utilization. The effects of phytic acid in humans are associated by its interaction with proteins, vitamins and minerals thereby restricting mineral bioavailability and also protein digestibility (Francis et al. 2001). Chickpea seeds are rich in micronutrients like folate, magnesium, zinc and iron also. There exists a significant diversity in iron content from 2.4 to 11 mg/100 g which may be due to genotype differentiation and environmental factors (Tan et al. 2017). Several methods such as germination, fermentation, soaking, dehulling, and cooking have been reported to improve the nutritional quality (Reddy et al. 1982).
Determination of total phenolic content (TPC) and total flavonoid content (TFC)
The TPC analysed by Folin-Ciocalteu is widely ranged across mini-core seeds and are represented in Table 1. In the present study, total phenolic content ranged 0.15–0.81 mg/g in all the accessions. ICC-5135 showed significantly highest (0.81 mg/g) content of total phenols while ICC-11944 revealed lowest (0.15 mg/g). Mini-core seeds of chickpea had significant content of phenolics, in consistent with the Kaur et al. (2014), who reported average phenolic contents of 0.51–1.17 mg/g. These differences attributed may be due to gene pool and origin. The antioxidant activities in grains are mostly attributed to the phenolic compounds (Yao et al. 2010). Compared to other legumes, Peng et al. (2008) observed that the mung bean extracts had the highest total phenolic contents. The phenols play vital role in deactivating metal ions. Moreover, the polyphenols also contribute as scavengers of hydroxyl and peroxyl radicals that control cardiovascular diseases (Luo et al. 2002).
Table 1.
Mini-core collection of chickpea accessions depicting seed composition variability
| S.no. | Chickpea accession | Total protein (%) | Total free amino acid (mg/g) | Phytic acid (mg/g) | Tannic acid (mg/g) | Total phenolics (mg/g) | Total flavonoid (mg/g) | Lectin (HU/mg) | DPPH (% Inhibition) | In vitro protein digestibility (%) | In vitro starch digestibility (mg of maltose/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ICC-12824 | 16.5 | 7.29 ± 0.92 | 0.215 ± 0.02 | 43.82 ± 1.79 | 1.167 ± 0.08 | 0.404 ± 0.01 | 81.26 | 41.21 ± 0.52 | 71.08 ± 0.32 | 158.90 ± 2.11 |
| 2 | ICC-12851 | 21.1 | 7.88 ± 0.57 | 0.149 ± 0.02 | 17.81 ± 0.72 | 1.287 ± 0.12 | 0.649 ± 0.07 | 48.5 | 44.03 ± 0.46 | 70.22 ± 1.33 | 181.09 ± 1.12 |
| 3 | ICC-12866 | 13.9 | 1.73 ± 0.16 | 0.75 ± 0.03 | 29.93 ± 1.43 | 0.41 ± 0.90 | 0.65 ± 0.01 | 22.8 | 46.70 ± 0.69 | 70.48 ± 0.55 | 193.32 ± 4.94 |
| 4 | ICC-12726 | 16.5 | 7.08 ± 0.31 | 0.241 ± 0.02 | 29.64 ± 1.44 | 0.884 ± 0.07 | 0.416 ± 0.02 | 62.06 | 41.21 ± 0.52 | 72.01 ± 1.24 | 181.26 ± 1.50 |
| 5 | ICC-12654 | 12.2 | 8.46 ± 0.07 | 0.161 ± 0.02 | 23.28 ± 0.27 | 1.64 ± 0.01 | 0.751 ± 0.07 | 83.9 | 34.12 ± 1.21 | 70.62 ± 1.89 | 161.11 ± 1.22 |
| 6 | ICC-12537 | 20.2 | 8.79 ± 0.07 | 0.159 ± 0.02 | 24.62 ± 1.08 | 0.97 ± 0.02 | 0.367 ± 0.03 | 101.38 | 47.18 ± 0.91 | 70.84 ± 0.67 | 114.96 ± 3.70 |
| 7 | ICC-16261 | 13.1 | 7.96 ± 0.19 | 0.214 ± 0.01 | 82.89 ± 2.52 | 0.915 ± .03 | 0.597 ± 0.04 | 156.33 | 40.86 ± 1.24 | 71.01 ± 0.12 | 156.11 ± 2.29 |
| 8 | ICC-16269 | 16.5 | 6.92 ± 0.47 | 0.199 ± 0.0 | 186.20 ± 0.63 | 1.204 ± 0.07 | 0.225 ± 0.01 | 124.12 | 43.13 ± 0.44 | 71.26 ± 1.53 | 186.96 ± 2.78 |
| 9 | ICC-16374 | 11.6 | 6.92 ± 0.26 | 0.14 ± 0.02 | 74.75 ± 0.72 | 1.21 ± 0.09 | 0.525 ± 0.05 | 176.55 | 44.09 ± 1.90 | 70.86 ± 0.65 | 168.29 ± 4.12 |
| 10 | ICC-16903 | 18.1 | 5.21 ± 0.26 | 0.208 ± 0.0 | 140.97 ± 0.90 | 1.291 ± 0.11 | 0.251 ± 0.03 | 56.5 | 48.91 ± 0.42 | 72.45 ± 1.46 | 187.66 ± 1.74 |
| 11 | ICC-16796 | 23.9 | 5.38 ± 0.65 | 0.19 ± 0.01 | 78.88 ± 0.63 | 1.476 ± 0.02 | 0.407 ± 0.02 | 85.69 | 39.20 ± 1.10 | 70.20 ± 1.50 | 159.16 ± 2.45 |
| 12 | ICC-16487 | 26.5 | 7.25 ± 0.22 | 0.297 ± 0.01 | 126.84 ± 0.99 | 1.559 ± 0.05 | 0.658 ± 0.04 | 77.28 | 41.82 ± 0.91 | 68.42 ± 1.05 | 212.64 ± 3.14 |
| 13 | ICC-13863 | 22.2 | 6.75 ± 0.38 | 0.237 ± 0.0 | 56.04 ± 1.98 | 1.871 ± 0.05 | 0.787 ± 0.12 | 92.25 | 40.06 ± 0.24 | 70.11 ± 1.22 | 226.11 ± 1.52 |
| 14 | ICC-13892 | 20.4 | 5.13 ± 0.54 | 0.162 ± 0.01 | 88.17 ± 5.67 | 1.218 ± 0.03 | 0.393 ± 0.04 | 100.39 | 43.11 ± 1.04 | 71.76 ± 1.43 | 177.17 ± 2.71 |
| 15 | ICC-14051 | 16.1 | 6.58 ± 0.47 | 0.215 ± 0.01 | 45.67 ± 0.99 | 1.255 ± 0.03 | 0.273 ± 0.04 | 127.20 | 40.17 ± 1.90 | 71.49 ± 0.66 | 161.91 ± 3.52 |
| 16 | ICC-14199 | 17.4 | 9.21 ± 0.31 | 0.226 ± 0.01 | 92.30 ± 2.25 | 1.23 ± 0.08 | 0.515 ± 0.00 | 117.70 | 41.19 ± 1.22 | 70.82 ± 1.92 | 197.62 ± 2.27 |
| 17 | ICC-14077 | 26.1 | 5.42 ± 1.02 | 0.294 ± 0.0 | 139.82 ± 5.58 | 1.454 ± 0.05 | 0.700 ± 0.09 | 313.86 | 37.20 ± 1.36 | 71.04 ± 1.40 | 184.44 ± 3.28 |
| 18 | ICC-14098 | 27.4 | 7.04 ± 0.58 | 0.152 ± 0.01 | 146.76 ± 0.27 | 1.256 ± 0.07 | 1.061 ± 0.04 | 74.7 | 29.12 ± 1.18 | 69.27 ± 1.56 | 176.19 ± 1.74 |
| 19 | ICC-1915 | 25.3 | 6.63 ± 0.01 | 1.18 ± 0.03 | 35.66 ± 1.03 | 0.42 ± 0.90 | 0.42 ± 0.01 | 50.8 | 47.50 ± 0.69 | 70.28 ± 1.08 | 161.71 ± 1.90 |
| 20 | ICC-1923 | 12.4 | 5.75 ± 0.54 | 0.101 ± 0.02 | 141.79 ± 3.51 | 1.814 ± 0.06 | 0.509 ± 0.00 | 330.32 | 49.11 ± 1.12 | 71.49 ± 1.26 | 118.65 ± 1.25 |
| 21 | ICC-2065 | 31.2 | 5.38 ± 0.66 | 0.067 ± 0.01 | 145.93 ± 0.36 | 1.493 ± 0.12 | 0.542 ± 0.07 | 65.64 | 46.06 ± 1.10 | 70.10 ± 1.59 | 202.54 ± 2.48 |
| 22 | ICC-2072 | 23.9 | 5.38 ± 0.81 | 0.02 ± 0.01 | 189.63 ± 1.17 | 1.531 ± 0.01 | 0.890 ± 0.04 | 85.69 | 41.82 ± 1.56 | 68.11 ± 1.23 | 206.15 ± 2.63 |
| 23 | ICC-2210 | 26.5 | 6.96 ± 0.40 | 0.102 ± 0.01 | 179.96 ± 2.97 | 1.441 ± 0.05 | 0.170 ± 0.03 | 77.28 | 40.62 ± 1.48 | 70.04 ± 1.04 | 188.99 ± 3.68 |
| 24 | ICC-2242 | 12.2 | 4.50 ± 0.33 | 0.209 ± 0.02 | 163.36 ± 1.08 | 1.313 ± 0.06 | 0.554 ± 0.05 | 83.9 | 42.51 ± 2.42 | 71.27 ± 1.49 | 171.19 ± 4.25 |
| 25 | ICC-5639 | 28.6 | 3.17 ± 0.59 | 0.046 ± 0.01 | 185.81 ± 4.14 | 1.46 ± 0.03 | 0.443 ± 0.01 | 35.8 | 45.10 ± 2.10 | 70.46 ± 1.61 | 192.29 ± 2.46 |
| 26 | ICC-5845 | 13.7 | 5.17 ± 0.88 | 0.039 ± 0.01 | 122.65 ± 3.15 | 1.688 ± 0.08 | 1.055 ± 0.18 | 149.48 | 42.39 ± 2.22 | 70.19 ± 1.92 | 141.21 ± 1.94 |
| 27 | ICC-5878 | 16.9 | 6.33 ± 0.58 | 0.203 ± 0.01 | 109.41 ± 0.72 | 2.606 ± 0.05 | 0.813 ± 0.05 | 121.18 | 39.10 ± 2.36 | 71.18 ± 1.40 | 164.19 ± 2.41 |
| 28 | ICC-5434 | 11.9 | 7.58 ± 0.07 | 0.11 ± 0.01 | 110.37 ± 0.90 | 1.592 ± 0.03 | 0.510 ± 0.06 | 172.10 | 29.12 ± 2.14 | 69.80 ± 1.44 | 196.41 ± 1.11 |
| 29 | ICC-5504 | 17.1 | 7.96 ± 0.19 | 0.1 ± 0.02 | 112.60 ± 3.51 | 1.457 ± 0.02 | 0.176 ± 0.05 | 119.76 | 46.10 ± 1.29 | 70.20 ± 1.92 | 216.90 ± 3.22 |
| 30 | ICC- 5613 | 6.1 | 2.63 ± 0.08 | 0.67 ± 0.06 | 25.4 ± 1.00 | 0.46 ± 5.23 | 0.42 ± 0.01 | 13.45 | 46.16 ± 1.91 | 69.32 ± 0.81 | 96.66 ± 4.23 |
| 31 | ICC-11664 | 14.4 | 5.67 ± 0.01 | 0.227 ± 0.03 | 107.95 ± 0.99 | 1.766 ± 0.04 | 0.231 ± 0.04 | 142.22 | 35.94 ± 0.35 | 74.24 ± 1.26 | 214.96 ± 1.36 |
| 32 | ICC-11764 | 12.8 | 2.32 ± 0.13 | 1.2 ± 0.05 | 27.4 ± 1.03 | 0.29 ± 0.50 | 0.63 ± 0.01 | 11.79 | 29.7 ± 1.13 | 75.31 ± 0.66 | 123.84 ± 5.38 |
| 33 | ICC-11627 | 26.1 | 6.46 ± 0.69 | 0.104 ± 0.0 | 84.92 ± 2.16 | 1.365 ± 0.06 | 0.125 ± 0.01 | 156.93 | 34.16 ± 1.05 | 69.12 ± 1.21 | 118.67 ± 2.12 |
| 34 | ICC-11879 | 14.6 | 9.63 ± 0.54 | 0.195 ± 0.02 | 48. ± 0.09 | 1.496 ± 0.09 | 0.384 ± 0.12 | 35.06 | 34.23 ± 1.08 | 73.19 ± 1.16 | 132.14 ± 1.77 |
| 35 | ICC-11944 | 18.2 | 4.48 ± 0.11 | 0.83 ± 0.05 | 32.52 ± 2.10 | 0.15 ± 2.32 | 0.69 ± 0.05 | 8.68 | 46.16 ± 0.11 | 71.19 ± 0.83 | 284.6 ± 4.67 |
| 36 | ICC-12028 | 26.4 | 10.08 ± 0.83 | 0.114 ± 0.01 | 24.3 ± 3.33 | 1.747 ± 0.04 | 0.351 ± 0.04 | 19.3 | 47.26 ± 1.54 | 59.45 ± 0.14 | 126.43 ± 2.19 |
| 37 | ICC-8195 | 12.6 | 6.21 ± 0.89 | 0.145 ± 0.01 | 49.87 ± 0.72 | 1.529 ± 0.11 | 1.090 ± 0.01 | 9.6 | 35.72 ± 1.75 | 71.69 ± 1.80 | 204.62 ± 2.60 |
| 38 | ICC-8261 | 28.4 | 11.00 ± 0.13 | 0.115 ± 0.01 | 35.81 ± 1.98 | 1.483 ± 0.07 | 0.510 ± 0.11 | 36.05 | 31.29 ± 2.18 | 72.19 ± 1.06 | 236.67 ± 2.78 |
| 39 | ICC-8318 | 13.3 | 5.92 ± 0.51 | 0.055 ± 0.0 | 27.99 ± 4.41 | 1.267 ± 0.11 | 1.373 ± 0.08 | 76.99 | 39.26 ± 1.07 | 69.40 ± 1.42 | 197.34 ± 2.08 |
| 40 | ICC-8151 | 16.7 | 6.38 ± 0.45 | 0.146 ± 0.01 | 52.1 ± 2.97 | 1.045 ± 0.07 | 0.572 ± 0.02 | 15.3 | 36.22 ± 1.08 | 73.10 ± 0.96 | 168.42 ± 2.44 |
| 41 | ICC-8058 | 13.6 | 9.38 ± 0.50 | 0.168 ± 0.02 | 86.64 ± 5.94 | 1.542 ± 0.14 | 1.569 ± 0.09 | 106.4 | 42.11 ± 1.29 | 70.27 ± 0.63 | 284.6 ± 4.67 |
| 42 | ICC-7867 | 19.1 | 19.38 ± 1.13 | 0.2 ± 0.01 | 89.63 ± 0.45 | 1.248 ± 0.04 | 0.424 ± 0.16 | 6.7 | 45.44 ± 1.71 | 71.64 ± 0.48 | 92.81 ± 2.60 |
| 43 | ICC-7323 | 9.6 | 2.31 ± 0.07 | 1.03 ± 0.15 | 13.96 ± 1.50 | 0.41 ± 1.11 | 0.69 ± 0.02 | 9.21 | 45.53 ± 0.11 | 71.52 ± 0.56 | 263.83 ± 2.77 |
| 44 | ICC-7441 | 18.2 | 9.67 ± 0.26 | 0.308 ± 0.01 | 36.07 ± 1.35 | 1.584 ± 0.03 | 0.172 ± 0.02 | 28.13 | 43.53 ± 1.63 | 70.06 ± 0.32 | 198.19 ± 1.38 |
| 45 | ICC-7554 | 15.6 | 21.21 ± 0.63 | 0.236 ± 0.02 | 108.84 ± 1.26 | 1.933 ± 0.01 | 0.184 ± 0.01 | 16.4 | 44.74 ± 1.60 | 71.33 ± 0.16 | 233.47 ± 2.18 |
| 46 | ICC-7571 | 29.8 | 11.79 ± 0.44 | 0.241 ± 0.02 | 34.12 ± 1.54 | 1.534 ± 0.07 | 0.490 ± 0.04 | 17.18 | 35.32 ± 1.82 | 71.41 ± 0.61 | 214.92 ± 2.46 |
| 47 | ICC-7668 | 14.6 | 2.42 ± 0.11 | 0.78 ± 0.06 | 17.03 ± 0.82 | 0.43 ± 2.23 | 0.54 ± 0.01 | 11.46 | 35.56 ± 0.65 | 72.73 ± 0.56 | 148.96 ± 2.47 |
| 48 | ICC-7819 | 30.5 | 2.61 ± 0.10 | 2.39 ± 0.03 | 22.42 ± 0.90 | 0.43 ± 0.70 | 0.71 ± 0.05 | 8.79 | 33.03 ± 1.28 | 72.36 ± 0.95 | 126.91 ± 3.52 |
| 49 | ICC-3776 | 18.9 | 15.75 ± 0.43 | 0.368 ± 0.01 | 114.76 ± 3.15 | 1.597 ± 0.04 | 0.209 ± 0.00 | 27.08 | 41.74 ± 2.23 | 71.10 ± 0.12 | 117.38 ± 1.82 |
| 50 | ICC-3946 | 14.7 | 9.46 ± 0.52 | 0.225 ± 0.02 | 70.74 ± 4.68 | 1.398 ± 0.03 | 0.187 ± 0.02 | 34.82 | 44.38 ± 2.11 | 71.43 ± 0.46 | 197.17 ± 2.44 |
| 51 | ICC-4182 | 24.8 | 8.13 ± 0.45 | 0.298 ± 0.01 | 50.83 ± 5.04 | 1.212 ± 0.08 | 0.167 ± 0.05 | 58.38 | 35.29 ± 1.82 | 71.12 ± 0.28 | 209.02 ± 1.76 |
| 52 | ICC-4418 | 17.4 | 9.33 ± 0.07 | 0.283 ± 0.01 | 83.40 ± 2.70 | 0.98 ± 0.08 | 0.199 ± 0.03 | 58.85 | 36.68 ± 1.43 | 68.73 ± 0.23 | 182.06 ± 2.27 |
| 53 | ICC-4463 | 29.2 | 10.33 ± 0.07 | 0.249 ± 0.01 | 127.93 ± 6.93 | 1.208 ± 0.03 | 0.184 ± 0.03 | 17.53 | 31.63 ± 1.46 | 70.36 ± 0.95 | 138.01 ± 2.12 |
| 54 | ICC-4495 | 26.2 | 1.84 ± 0.12 | 0.61 ± 0.03 | 18.28 ± 1.50 | 0.34 ± 1.30 | 0.3 ± 0.01 | 24.87 | 28.06 ± 0.90 | 69.44 ± 0.72 | 197.17 ± 4.23 |
| 55 | ICC-13628 | 14.6 | 13.92 ± 1.35 | 0.261 ± 0.02 | 126.78 ± 2.25 | 1.256 ± 0.09 | 0.190 ± 0.01 | 35.06 | 42.34 ± 1.13 | 70.22 ± 0.40 | 127.58 ± 1.50 |
| 56 | ICC-13764 | 17.2 | 10.38 ± 0.45 | 0.259 ± 0.04 | 0.232 ± 0.01 | 1.108 ± 0.06 | 0.321 ± 0.05 | 59.53 | 41.18 ± 2.01 | 70.21 ± 0.18 | 177.07 ± 2.35 |
| 57 | ICC-13816 | 21.5 | 14.17 ± 2.89 | 0.31 ± 0.01 | 108.59 ± 0.54 | 1.343 ± 0.12 | 0.218 ± 0.25 | 5.95 | 39.92 ± 1.26 | 68.11 ± 0.34 | 219.04 ± 1.56 |
| 58 | ICC-13523 | 18.3 | 13.25 ± 0.90 | 0.228 ± 0.01 | 64.76 ± 7.11 | 1.469 ± 0.1 | 0.685 ± 0.04 | 6.99 | 34.60 ± 1.48 | 70.09 ± 0.17 | 189.06 ± 2.17 |
| 59 | ICC-13524 | 26.1 | 7.33 ± 0.76 | 0.314 ± 0.01 | 30.79 ± 6.66 | 1.218 ± 0.02 | 0.436 ± 0.01 | 4.90 | 35.63 ± 1.06 | 70.28 ± 0.18 | 137.01 ± 1.49 |
| 60 | ICC-13599 | 33.9 | 2.21 ± 0.12 | 0.91 ± 0.10 | 15.52 ± 1.12 | 0.44 ± 2.32 | 0.64 ± 0.03 | 37.30 | 38.12 ± 1.05 | 72.91 ± 0.56 | 45.63 ± 0.44 |
| 61 | ICC-12037 | 9.3 | 2.32 ± 0.12 | 1.27 ± 0.06 | 34.96 ± 1.72 | 0.31 ± 0.61 | 0.35 ± 0.01 | 67.35 | 45.63 ± 0.29 | 69.65 ± 0.50 | 196.91 ± 4.28 |
| 62 | ICC-12155 | 15.5 | 2.25 ± 0.13 | 0.243 ± 0.01 | 69.78 ± 2.52 | 1.276 ± 0.05 | 0.104 ± 0.00 | 8.25 | 46.70 ± 1.02 | 69.22 ± 1.41 | 144.26 ± 2.31 |
| 63 | ICC-12299 | 16.9 | 1.83 ± 0.19 | 0.232 ± 0.01 | 106.55 ± 1.98 | 0.973 ± 0.02 | 0.109 ± 0.02 | 15.14 | 36.23 ± 1.43 | 70.14 ± 1.23 | 157.08 ± 2.43 |
| 64 | ICC-12307 | 25.4 | 0.092 ± 0.1 | 0.211 ± 0.01 | 89.18 ± 0.82 | 1.296 ± 0.15 | 0.31 ± 0.01 | 39.09 | 36.54 ± 1.06 | 70.46 ± 0.84 | 179.21 ± 1.73 |
| 65 | ICC-12328 | 20.9 | 1.74 ± 0.13 | 0.49 ± 0.05 | 31.93 ± 1.51 | 0.26 ± 4.63 | 0.7 ± 0.01 | 31.45 | 46.23 ± 0.45 | 69.69 ± 0.83 | 166.66 ± 4.94 |
| 66 | ICC-12492 | 19.2 | 4.46 ± 0.19 | 0.106 ± 0.02 | 67.94 ± 3.51 | 1.074 ± 0.03 | 0.239 ± 0.01 | 6.66 | 41.22 ± 1.01 | 68.25 ± 1.45 | 143.12 ± 2.46 |
| 67 | ICC-13357 | 15.1 | 2.42 ± 0.19 | 0.24 ± 0.02 | 69.78 ± 2.52 | 1.083 ± 0.13 | 0.372 ± 0.04 | 16.95 | 44.70 ± 1.14 | 69.18 ± 1.43 | 185.78 ± 1.27 |
| 68 | ICC-13441 | 16.4 | 1.92 ± 0.19 | 0.191 ± 0.01 | 73.28 ± 0.27 | 1.185 ± 0.01 | 0.251 ± 0.03 | 15.60 | 36.02 ± 1.18 | 70.01 ± 1.42 | 247.04 ± 1.33 |
| 69 | ICC-13461 | 24.2 | 5.71 ± 0.19 | 0.137 ± 0.02 | 139.19 ± 2.52 | 1.054 ± 0.04 | 0.409 ± 0.00 | 10.57 | 34.41 ± 1.46 | 70.29 ± 0.46 | 147.54 ± 1.98 |
| 70 | ICC-16524 | 15.1 | 1.79 ± 0.19 | 0.257 ± 0.01 | 50.38 ± 2.16 | 1.171 ± 0.05 | 0.148 ± 0.04 | 16.95 | 46.23 ± 1.59 | 70.02 ± 0.51 | 189.19 ± 1.01 |
| 71 | ICC-13219 | 7.5 | 4.82 ± 0.10 | 1.46 ± 0.49 | 34.48 ± 0.80 | 0.40 ± 1.13 | 0.53 ± 0.01 | 53.18 | 47.36 ± 2.62 | 68.55 ± 0.77 | 133.32 ± 5.12 |
| 72 | ICC-13283 | 18.4 | 2.75 ± 0.13 | 0.181 ± 0.01 | 41.86 ± 4.95 | 1.306 ± 0.08 | 0.191 ± 0.03 | 13.91 | 40.17 ± 1.21 | 71.16 ± 1.47 | 116.19 ± 1.82 |
| 73 | ICC-12916 | 21.3 | 1.63 ± 0.13 | 0.139 ± 0.01 | 65.71 ± 0.90 | 1.223 ± 0.16 | 0.215 ± 0.00 | 48.07 | 41.40 ± 1.34 | 68.18 ± 0.32 | 147.12 ± 1.40 |
| 74 | ICC-12928 | 7.4 | 2.25 ± 0.13 | 0.183 ± 0.02 | 29.64 ± 4.14 | 1.127 ± 0.07 | 0.115 ± 0.00 | 138.37 | 35.12 ± 1.26 | 70.29 ± 0.18 | 134.26 ± 2.44 |
| 75 | ICC-12947 | 30.8 | 1.91 ± 0.08 | 1.26 ± 0.04 | 31.35 ± 1.10 | 0.48 ± 1.20 | 0.41 ± 0.04 | 11.36 | 34.93 ± 0.4 | 69.39 ± 0.63 | 138.71 ± 1.17 |
| 76 | ICC-13077 | 16.5 | 3.17 ± 0.19 | 0.248 ± 0.01 | 53.31 ± 0.36 | 1.102 ± 0.02 | 0.182 ± 0.01 | 87.7 | 41.54 ± 1.23 | 68.76 ± 0.98 | 164.21 ± 1.14 |
| 77 | ICC-13124 | 20.4 | 10.63 ± 0.63 | 0.16 ± 0.01 | 62.15 ± 2.88 | 1.222 ± 0.1 | 0.130 ± 0.01 | 70.91 | 42.20 ± 1.33 | 70.29 ± 1.29 | 171.04 ± 1.20 |
| 78 | ICC-13187 | 19.4 | 6.83 ± 0.44 | 0.13 ± 0.01 | 62.40 ± 7.29 | 1.365 ± 0.07 | 0.146 ± 0.04 | 74.63 | 37.72 ± 1.17 | 70.02 ± 0.51 | 136.82 ± 1.90 |
| 79 | ICC-14831 | 31.1 | 2.23 ± 0.13 | 0.86 ± 0.02 | 29.56 ± 0.81 | 0.44 ± 0.71 | 0.36 ± 0.01 | 42.27 | 34.45 ± 0.96 | 72.69 ± 0.44 | 131.27 ± 1.60 |
| 80 | ICC-15264 | 11.4 | 14.46 ± 0.40 | 0.13 ± 0.01 | 50.89 ± 1.17 | 1.182 ± 0.1 | 0.188 ± 0.00 | 127.01 | 46.16 ± 1.21 | 70.26 ± 1.24 | 152.14 ± 2.41 |
| 81 | ICC-15294 | 19.3 | 6.79 ± 0.38 | 0.213 ± 0.0 | 31.17 ± 2.25 | 1.188 ± 0.09 | 0.113 ± 0.01 | 75.02 | 41.27 ± 1.94 | 69.17 ± 0.12 | 142.09 ± 1.72 |
| 82 | ICC-15333 | 17.4 | 11.71 ± 0.62 | 0.162 ± 0.02 | 48.98 ± 4.23 | 1.049 ± 0.05 | 0.167 ± 0.02 | 58.8 | 42.30 ± 2.46 | 71.80 ± 0.81 | 155.34 ± 2.30 |
| 83 | ICC-15406 | 27.2 | 8.04 ± 0.26 | 0.172 ± 0.01 | 79.58 ± 0.54 | 0.768 ± 0.07 | 0.376 ± 0.04 | 37.64 | 39.26 ± 1.89 | 69.07 ± 0.64 | 106.01 ± 2.19 |
| 84 | ICC-15435 | 21.5 | 8.13 ± 0.38 | 0.101 ± 0.08 | 49.55 ± 1.80 | 1.3 ± 0.03 | 0.167 ± 0.03 | 33.6 | 34.34 ± 0.49 | 68.11 ± 0.94 | 147.08 ± 1.90 |
| 85 | ICC-9643 | 10.8 | 6.83 ± 0.40 | 0.203 ± 0.1 | 27.93 ± 1.17 | 1.326 ± 0.09 | 0.172 ± 0.00 | 189.62 | 40.52 ± 1.64 | 70.15 ± 1.30 | 122.78 ± 2.29 |
| 86 | ICC-9755 | 14.2 | 9.88 ± 0.25 | 0.289 ± 0.0 | 88.74 ± 2.70 | 1.329 ± 0.04 | 0.157 ± 0.01 | 101.97 | 41.68 ± 1.34 | 70.05 ± 0.10 | 179.45 ± 1.32 |
| 87 | ICC-9848 | 33.7 | 2.10 ± 0.39 | 0.78 ± 0.03 | 35.23 ± 0.73 | 0.52 ± 2.71 | 0.2 ± 0.02 | 18.78 | 30.36 ± 0.94 | 72.40 ± 0.70 | 117.94 ± 1.93 |
| 88 | ICC-9862 | 11.4 | 6.83 ± 0.26 | 0.245 ± 0.0 | 48.92 ± 1.26 | 0.932 ± .11 | 0.096 ± 0.01 | 44.9 | 31.45 ± 0.19 | 69.18 ± 0.29 | 177.24 ± 1.10 |
| 89 | ICC-9895 | 16.2 | 13.04 ± 0.69 | 0.042 ± 0.0 | 50.70 ± 1.98 | 1.298 ± 0.14 | 0.152 ± 0.00 | 31.6 | 40.22 ± 1.24 | 70.11 ± 0.32 | 282.11 ± 2.01 |
| 90 | ICC-9942 | 32.7 | 11.17 ± 0.44 | 0.125 ± 0.0 | 51.02 ± 2.61 | 1.458 ± 0.01 | 0.179 ± 0.00 | 7.82 | 41.91 ± 1.30 | 68.91 ± 0.19 | 148.62 ± 1.29 |
| 91 | ICC-5879 | 22.3 | 9.21 ± 0.47 | 0.009 ± 0.0 | 45.48 ± 6.30 | 1.288 ± 0.1 | 0.516 ± 0.03 | 45.91 | 31.22 ± 2.11 | 71.02 ± 1.03 | 127.14 ± 1.30 |
| 92 | ICC-6263 | 12.8 | 11.17 ± 0.73 | 0.146 ± 0.01 | 86.90 ± 3.87 | 1.31 ± 0.05 | 0.425 ± 0.15 | 80 | 40.52 ± 2.45 | 69.22 ± 1.23 | 202.24 ± 2.01 |
| 93 | ICC-6279 | 16.1 | 9.00 ± 0.45 | 0.188 ± 0.0 | 107.19 ± 2.52 | 1.171 ± 0.05 | 0.257 ± 0.04 | 63.6 | 41.81 ± 1.30 | 70.16 ± 0.11 | 136.28 ± 2.08 |
| 94 | ICC-6293 | 30.2 | 11.00 ± 0.25 | 0.196 ± 0.0 | 34.35 ± 0.81 | 1.32 ± 0.01 | 0.115 ± 0.01 | 67.81 | 32.34 ± 0.94 | 71.28 ± 2.16 | 246.11 ± 1.46 |
| 95 | ICC-6306 | 19.1 | 10.08 ± 0.44 | 0.2 ± 0.01 | 28.24 ± 4.59 | 1.14 ± 0.09 | 0.254 ± 0.00 | 53.61 | 35.05 ± 0.15 | 70.15 ± 0.32 | 195.39 ± 1.68 |
| 96 | ICC-6537 | 13.9 | 10.46 ± 0.26 | 0.187 ± 0.01 | 43.70 ± 1.35 | 1.017 ± 0.02 | 0.373 ± 0.03 | 0.07 | 39.12 ± 1.35 | 71.41 ± 1.04 | 197.11 ± 2.09 |
| 97 | ICC-6877 | 12.6 | 10.38 ± 0.57 | 0.18 ± 0.01 | 35.50 ± 1.08 | 0.942 ± 0.01 | 0.176 ± 0.02 | 20.31 | 40.11 ± 1.19 | 69.18 ± 0.21 | 244.21 ± 3.40 |
| 98 | ICC-7184 | 11.3 | 2.42 ± 0.11 | 4.06 ± 0.05 | 19.51 ± 1.01 | 0.42 ± 0.90 | 0.27 ± 0.02 | 57.73 | 40.21 ± 0.86 | 70.92 ± 0.35 | 109.48 ± 3.79 |
| 99 | ICC-7255 | 22.4 | 7.58 ± 0.44 | 0.187 ± 0.01 | 29.33 ± 1.98 | 1.241 ± 0.09 | 0.457 ± 0.01 | 91.42 | 30.74 ± 1.62 | 69.40 ± 0.92 | 157.44 ± 1.10 |
| 100 | ICC-7272 | 12.6 | 8.25 ± 0.13 | 0.084 ± 0.01 | 70.36 ± 1.26 | 1.27 ± 0.01 | 0.088 ± 0.01 | 40.63 | 44.21 ± 1.73 | 70.27 ± 0.48 | 245.40 ± 2.18 |
| 101 | ICC-7308 | 14.7 | 3.33 ± 0.19 | 0.206 ± 0.01 | 61.07 ± 3.78 | 1.212 ± 0.06 | 0.052 ± 0.00 | 69.65 | 44.31 ± 1.10 | 71.28 ± 2.14 | 186.08 ± 2.09 |
| 102 | ICC-7315 | 25.4 | 10.38 ± 0.25 | 0.175 ± 0.01 | 71.44 ± 1.71 | 1.199 ± 0.02 | 0.339 ± 0.00 | 40.31 | 34.24 ± 0.14 | 70.15 ± 0.35 | 249.41 ± 1.06 |
| 103 | ICC-8855 | 15.2 | 14.21 ± 0.36 | 0.245 ± 0.01 | 28.75 ± 3.69 | 1.379 ± 0.11 | 0.119 ± 0.03 | 134.73 | 41.52 ± 0.18 | 71.24 ± 1.15 | 197.91 ± 1.88 |
| 104 | ICC-8950 | 18.1 | 2.79 ± 0.19 | 0.183 ± 0.01 | 44.66 ± 0.54 | 0.741 ± 0.06 | 0.199 ± 0.01 | 113.14 | 40.10 ± 1.39 | 69.25 ± 0.27 | 197.11 ± 1.09 |
| 105 | ICC-9002 | 14.7 | 3.38 ± 0.25 | 0.185 ± 0.01 | 86.20 ± 2.88 | 1.585 ± 0.03 | 0.179 ± 0.01 | 69.65 | 41.56 ± 1.33 | 70.04 ± 1.51 | 232.01 ± 1.70 |
| 106 | ICC-9137 | 13.5 | 1.43 ± 0.17 | 0.60 ± 0.02 | 25.75 ± 0.91 | 0.42 ± 1.10 | 0.37 ± 0.0 | 24.77 | 45.12 ± 1.55 | 68.34 ± 0.75 | 164.09 ± 2.36 |
| 107 | ICC-9402 | 21.1 | 16.63 ± 0.45 | 0.137 ± 0.01 | 68.83 ± 1.17 | 1.303 ± 0.05 | 0.122 ± 0.02 | 194.12 | 41.22 ± 1.94 | 70.27 ± 0.46 | 189.04 ± 1.93 |
| 108 | ICC-9586 | 13.1 | 3.21 ± 0.44 | 0.089 ± 0.01 | 46.95 ± 2.16 | 1.526 ± 0.17 | 0.143 ± 0.00 | 78.16 | 40.32 ± 1.56 | 71.01 ± 2.22 | 192.12 ± 3.16 |
| 109 | ICC-11121 | 4.6 | 3.93 ± 0.16 | 1.08 ± 0.04 | 8.44 ± 0.61 | 0.30 ± 0.93 | 0.23 ± 0.0 | 17.25 | 28.66 ± 1.00 | 70.87 ± 0.28 | 167.94 ± 1.93 |
| 110 | ICC-11198 | 12.1 | 1.46 ± 0.36 | 0.104 ± 0.01 | 23.98 ± 0.36 | 1.388 ± 0.02 | 0.103 ± 0.01 | 84.6 | 31.64 ± 1.43 | 71.24 ± 1.16 | 262.22 ± 6.01 |
| 111 | ICC-11284 | 13.7 | 1.96 ± 0.52 | 0.107 ± 0.01 | 48.03 ± 4.77 | 1.223 ± 0.01 | 0.124 ± 0.02 | 37.37 | 46.21 ± 1.67 | 69.12 ± 0.26 | 156.42 ± 2.16 |
| 112 | ICC-11378 | 14.5 | 5.75 ± 0.25 | 0.036 ± 0.0 | 33.27 ± 0.72 | 0.94 ± 0.12 | 0.130 ± 0.01 | 14.5 | 43.12 ± 1.44 | 70.14 ± 1.28 | 274.21 ± 1.40 |
| 113 | ICC-11498 | 18.1 | 2.29 ± 0.19 | 0.036 ± 0.01 | 63.30 ± 3.06 | 1.464 ± 0.07 | 0.107 ± 0.03 | 56.57 | 40.30 ± 1.68 | 69.72 ± 0.29 | 196.28 ± 2.26 |
| 114 | ICC-11584 | 19.4 | 1.75 ± 0.38 | 0.08 ± 0.02 | 65.46 ± 3.78 | 1.249 ± 0.03 | 0.170 ± 0.02 | 105.56 | 29.34 ± 1.00 | 71.10 ± 1.01 | 172.01 ± 2.71 |
| 115 | ICC-15868 | 15.7 | 1.71 ± 0.11 | 0.88 ± 0.03 | 37.94 ± 1.60 | 0.72 ± 0.92 | 0.43 ± 0.03 | 41.58 | 43.56 ± 1.32 | 70.94 ± 0.24 | 214.09 ± 7.14 |
| 116 | ICC-15888 | 15.1 | 2.21 ± 0.29 | 0.1 ± 0.0 | 88.30 ± 6.03 | 1.267 ± 0.1 | 0.225 ± 0.04 | 67.81 | 40.26 ± 1.34 | 69.02 ± 0.63 | 267.58 ± 2.38 |
| 117 | ICC-16207 | 19.4 | 3.17 ± 0.31 | 0.077 ± 0.01 | 68.00 ± 2.52 | 1.207 ± 0.07 | 0.055 ± 0.01 | 74.63 | 41.10 ± 1.08 | 72.64 ± 1.31 | 270.36 ± 1.22 |
| 118 | ICC-15618 | 16.7 | 11.00 ± 0.33 | 0.047 ± 0.01 | 88.93 ± 5.67 | 1.46 ± 0.1 | 0.210 ± 0.01 | 61.31 | 26.74 ± 2.19 | 69.72 ± 0.44 | 186.08 ± 2.52 |
| 119 | ICC-15697 | 22.0 | 2.31 ± 0.12 | 0.82 ± 0.54 | 25.35 ± 0.60 | 0.21 ± 0.61 | 0.61 ± 0.02 | 30.14 | 45.02 ± 1.60 | 71.10 ± 1.01 | 87.17 ± 2.34 |
| 120 | ICC-15802 | 11.2 | 9.42 ± 0.19 | 0.027 ± 0.0 | 49.24 ± 1.89 | 1.179 ± 0.05 | 0.331 ± 0.02 | 25.83 | 44.12 ± 2.01 | 70.20 ± 1.23 | 153.34 ± 2.18 |
| 121 | ICC-15606 | 5.1 | 4.40 ± 0.10 | 1.03 ± 0.14 | 12.20 ± 0.80 | 0.18 ± 0.40 | 0.27 ± 0.01 | 15.92 | 29.73 ± 1.62 | 69.02 ± 0.63 | 171.53 ± 3.84 |
| 122 | ICC-15610 | 12.8 | 2.20 ± 0.25 | 1.18 ± 0.05 | 13.44 ± 1.61 | 0.33 ± 1.10 | 0.42 ± 0.02 | 13.22 | 47.03 ± 0.46 | 71.36 ± 1.30 | 197.94 ± 3.63 |
| 123 | ICC-15612 | 15.4 | 11.88 ± 0.54 | 0.047 ± 0.01 | 80.22 ± 3.69 | 1.592 ± 0.04 | 0.361 ± 0.04 | 94.02 | 39.04 ± 1.01 | 68.12 ± 0.34 | 236.10 ± 2.67 |
| 124 | ICC-15510 | 16.2 | 14.18 ± 0.29 | 0.051 ± 0.01 | 88.30 ± 1.98 | 0.948 ± 0.04 | 0.104 ± 0.00 | 89.38 | 45.43 ± 1.44 | 70.62 ± 1.44 | 242.18 ± 3.09 |
| 125 | ICC-15518 | 18.3 | 15.46 ± 0.51 | 0.019 ± 0.0 | 81.49 ± 0.54 | 1.148 ± 0.03 | 0.131 ± 0.03 | 158.25 | 27.83 ± 0.12 | 69.12 ± 0.89 | 174.25 ± 2.16 |
| 126 | ICC-15567 | 22.5 | 10.75 ± 0.54 | 0.107 ± 0.02 | 30.03 ± 5.22 | 0.942 ± 0.06 | 0.385 ± 0.03 | 182.04 | 46.31 ± 0.66 | 71.04 ± 2.92 | 181.36 ± 1.48 |
| 127 | ICC-14402 | 15.5 | 4.11 ± 0.13 | 0.89 ± 0.03 | 32.55 ± 0.82 | 0.24 ± 0.81 | 0.53 ± 0.02 | 11.32 | 44.13 ± 1.24 | 68.53 ± 0.95 | 185.63 ± 1.17 |
| 128 | ICC-14595 | 20.8 | 13.75 ± 1.00 | 0.028 ± 0.0 | 38.87 ± 0.09 | 1.256 ± 0.01 | 0.090 ± 0.04 | 196.92 | 29.33 ± 1.02 | 70.26 ± 1.28 | 262.08 ± 2.19 |
| 129 | ICC-14669 | 24.7 | 7.92 ± 0.01 | 0.076 ± 0.01 | 89.63 ± 3.60 | 0.862 ± 0.05 | 0.488 ± 0.05 | 165.82 | 41.01 ± 1.32 | 68.34 ± 0.96 | 199.49 ± 1.26 |
| 130 | ICC-14778 | 6.1 | 2.43 ± 0.11 | 0.68 ± 0.04 | 15.96 ± 0.53 | 0.49 ± 0.61 | 0.52 ± 0.01 | 14.28 | 48.63 ± 1.50 | 70.75 ± 0.26 | 203.32 ± 3.46 |
| 131 | ICC-14799 | 18.7 | 1.66 ± 0.10 | 4.15 ± 0.12 | 20.73 ± 0.73 | 0.23 ± 1.21 | 0.33 ± 0.0 | 8.93 | 45.70 ± 0.34 | 71.15 ± 1.08 | 204.09 ± 7.10 |
| 132 | ICC-14815 | 14.4 | 2.40 ± 0.10 | 0.88 ± 0.03 | 40.42 ± 1.70 | 0.70 ± 1.23 | 0.41 ± 0.0 | 13.21 | 29.60 ± 0.45 | 76.22 ± 0.31 | 140.50 ± 4.37 |
| 133 | ICC-6571 | 21.2 | 9.54 ± 0.26 | 0.042 ± 0.01 | 44.59 ± 3.06 | 1.093 ± 0.04 | 0.121 ± 0.02 | 48.30 | 35.33 ± 1.02 | 69.36 ± 0.15 | 118.42 ± 1.22 |
| 134 | ICC-6579 | 14.6 | 19.58 ± 0.56 | 0.042 ± 0.0 | 27.42 ± 3.60 | 1.381 ± 0.02 | 0.187 ± 0.06 | 70.13 | 41.03 ± 0.67 | 70.17 ± 1.08 | 219.56 ± 2.27 |
| 135 | ICC-6802 | 16.2 | 10.42 ± 0.31 | 0.128 ± 0.17 | 25.00 ± 0.27 | 1.523 ± 0.11 | 0.306 ± 0.07 | 63.20 | 39.91 ± 2.18 | 68.18 ± 0.96 | 223.99 ± 1.68 |
| 136 | ICC-6811 | 18.4 | 11.75 ± 0.38 | 0.095 ± 0.01 | 21.37 ± 1.35 | 1.155 ± 0.03 | 0.200 ± 0.01 | 55.65 | 41.66 ± 1.47 | 70.16 ± 0.62 | 172.11 ± 1.27 |
| 137 | ICC-6816 | 10.9 | 8.71 ± 0.64 | 0.084 ± 0.02 | 24.94 ± 2.88 | 1.013 ± 0.07 | 0.340 ± 0.05 | 93.94 | 32.30 ± 1.01 | 70.10 ± 1.11 | 190.15 ± 2.18 |
| 138 | ICC-6874 | 20.0 | 1.60 ± 0.09 | 0.77 ± 0.04 | 26.90 ± 0.50 | 0.33 ± 0.31 | 0.13 ± 0.01 | 32.18 | 45.73 ± 0.63 | 71.05 ± 0.51 | 214.09 ± 1.17 |
| 139 | ICC-4533 | 30.2 | 2.40 ± 0.10 | 0.85 ± 0.03 | 20.34 ± 0.72 | 0.46 ± 1.82 | 0.46 ± 0.04 | 45.28 | 32.90 ± 1.14 | 73.01 ± 0.57 | 87.68 ± 1.53 |
| 140 | ICC-4567 | 21.6 | 9.42 ± 0.19 | 0.82 ± 0.04 | 29.39 ± 5.40 | 0.873 ± 0.07 | 0.372 ± 0.00 | 23.70 | 42.81 ± 1.32 | 71.28 ± 1.92 | 98.04 ± 1.47 |
| 141 | ICC-4593 | 26.6 | 2.24 ± 0.12 | 0.81 ± 0.02 | 32.97 ± 1.31 | 0.23 ± 1.13 | 0.67 ± 0.02 | 95.40 | 43.31 ± 4.33 | 70.43 ± 0.66 | 185.12 ± 1.60 |
| 142 | ICC-4639 | 17.1 | 10.58 ± 0.38 | 0.047 ± 0.0 | 44.85 ± 4.59 | 1.377 ± 0.07 | 0.267 ± 0.01 | 119.76 | 36.51 ± 2.44 | 69.01 ± 0.25 | 128.14 ± 1.24 |
| 143 | ICC-4657 | 16.4 | 8.42 ± 0.71 | 0.024 ± 0.0 | 121.56 ± 2.16 | 1.265 ± 0.05 | 0.255 ± 0.00 | 62.43 | 40.24 ± 1.68 | 70.78 ± 1.89 | 181.06 ± 2.90 |
| 144 | ICC-4814 | 13.4 | 6.75 ± 0.70 | 0.028 ± 0.0 | 66.79 ± 3.24 | 1.105 ± 0.13 | 0.243 ± 0.06 | 76.4 | 38.09 ± 1.51 | 69.24 ± 0.24 | 209.49 ± 1.63 |
| 145 | ICC-67 | 17.4 | 4.05 ± 0.10 | 0.42 ± 0.03 | 21.62 ± 0.70 | 0.37 ± 0.52 | 0.86 ± 0.02 | 19.21 | 41.03 ± 0.23 | 70.76 ± 0.40 | 140.25 ± 4.50 |
| 146 | ICC-95 | 16.6 | 4.75 ± 0.13 | 0.116 ± 0.01 | 20.67 ± 0.63 | 0.733 ± 0.03 | 0.073 ± 0.00 | 123.37 | 40.11 ± 1.28 | 71.32 ± 0.38 | 158.34 ± 1.49 |
| 147 | ICC-283 | 13.1 | 4.33 ± 0.31 | 0.104 ± 0.02 | 33.97 ± 0.54 | 1.303 ± 0.02 | 0.336 ± 0.06 | 78.16 | 33.41 ± 2.03 | 68.01 ± 0.20 | 191.57 ± 2.18 |
| 148 | ICC-440 | 16.7 | 4.46 ± 0.14 | 0.077 ± 0.01 | 49.36 ± 2.07 | 1.216 ± 0.05 | 0.224 ± 0.01 | 122.6 | 44.56 ± 1.04 | 71.82 ± 1.24 | 200.12 ± 1.70 |
| 149 | ICC-456 | 18.2 | 5.08 ± 0.31 | 0.123 ± 0.01 | 43.58 ± 1.53 | 1.023 ± 0.09 | 0.267 ± 0.00 | 112.52 | 39.11 ± 1.52 | 69.40 ± 0.26 | 127.06 ± 1.40 |
| 150 | ICC-506 | 25.1 | 1.81 ± 0.11 | 0.99 ± 0.12 | 30.16 ± 1.62 | 0.41 ± 1.10 | 0.12 ± 0.01 | 6.74 | 30.33 ± 0.66 | 72.73 ± 0.59 | 191.27 ± 4.23 |
| 151 | ICC-2990 | 13.1 | 2.63 ± 0.09 | 1.42 ± 0.09 | 29.85 ± 1.51 | 0.47 ± 1.12 | 0.21 ± 0.01 | 7.64 | 30.98 ± 1.40 | 71.46 ± 0.78 | 176.91 ± 3.07 |
| 152 | ICC-3218 | 15.7 | 4.92 ± 0.31 | 0.081 ± 0.01 | 93.51 ± 1.62 | 0.884 ± 0.04 | 0.201 ± 0.01 | 65.22 | 41.32 ± 1.21 | 71.11 ± 0.17 | 170.43 ± 0.78 |
| 153 | ICC-3230 | 18.2 | 3.92 ± 0.31 | 0.083 ± 0.0 | 162.02 ± 6.48 | 0.854 ± 0.01 | 0.540 ± 0.03 | 56.26 | 38.08 ± 1.27 | 69.26 ± 0.36 | 199.85 ± 2.59 |
| 154 | ICC-2884 | 14.0 | 5.58 ± 0.26 | 0.17 ± 0.0 | 89.63 ± 7.11 | 0.957 ± 0.03 | 0.448 ± 0.02 | 73.1 | 41.96 ± 0.45 | 72.25 ± 0.74 | 251.62 ± 1.80 |
| 155 | ICC-2919 | 14.1 | 4.25 ± 0.45 | 0.1120.08 | 143.51 ± 5.13 | 0.873 ± 0.08 | 0.269 ± 0.00 | 72.62 | 40.38 ± 1.20 | 68.04 ± 0.20 | 172.60 ± 1.30 |
| 156 | ICC-2969 | 17.6 | 5.17 ± 0.19 | 0.198 ± 0.01 | 126.84 ± 3.42 | 1.038 ± 0.09 | 0.316 ± 0.00 | 116.36 | 29.57 ± 1.22 | 71.12 ± 0.54 | 181.21 ± 2.61 |
| 157 | ICC-4841 | 22.3 | 4.50 ± 0.33 | 0.121 ± 0.01 | 42.24 ± 3.06 | 0.822 ± 0.03 | 0.266 ± 0.01 | 91.8 | 46.62 ± 0.19 | 69.31 ± 0.84 | 178.22 ± 2.15 |
| 158 | ICC-4872 | 31.4 | 5.58 ± 0.31 | 0.129 ± 0.0 | 141.92 ± 5.58 | 0.942 ± 0.03 | 0.327 ± 0.04 | 260.89 | 42.28 ± 1.51 | 68.02 ± 0.76 | 244.24 ± 1.29 |
| 159 | ICC-4918 | 21.1 | 1.48 ± 0.11 | 0.87 ± 0.04 | 37.78 ± 1.63 | 0.33 ± 5.11 | 0.17 ± 0.01 | 15.31 | 31.23 ± 1.20 | 73.11 ± 0.67 | 157.17 ± 1.17 |
| 160 | ICC-5135 | 28.7 | 2.42 ± 0.20 | 0.81 ± 0.03 | 32.33 ± 1.63 | 0.81 ± 2.73 | 0.3 ± 0.01 | 12.36 | 28.70 ± 0.52 | 68.26 ± 0.37 | 153.07 ± 1.53 |
| 161 | ICC-5337 | 15.5 | 2.41 ± 0.22 | 0.89 ± 0.02 | 35.22 ± 2.02 | 0.22 ± 0.90 | 0.5 ± 0.01 | 41.24 | 47.06 ± 0.50 | 71.25 ± 0.94 | 207.17 ± 1.93 |
| 162 | ICC-5383 | 21.6 | 4.38 ± 0.13 | 0.16 ± 0.0 | 23.85 ± 0.54 | 1.034 ± 0.06 | 0.355 ± 0.01 | 67.03 | 41.39 ± 1.86 | 69.54 ± 0.41 | 253.07 ± 2.44 |
| 163 | ICC-8350 | 12.8 | 4.29 ± 0.44 | 0.091 ± 0.0 | 50.76 ± 1.89 | 1.101 ± 0.03 | 0.201 ± 0.01 | 80 | 38.30 ± 0.92 | 70.02 ± 0.57 | 217.18 ± 1.37 |
| 164 | ICC-8384 | 17.9 | 2.36 ± 0.13 | 0.70 ± 0.06 | 31.01 ± 1.13 | 0.35 ± 1.00 | 0.35 ± 0.02 | 8.97 | 45.70 ± 0.51 | 68.44 ± 0.74 | 245.11 ± 6.93 |
| 165 | ICC-8522 | 21.4 | 5.08 ± 0.69 | 0.162 ± 0.01 | 26.91 ± 2.43 | 0.838 ± 0.03 | 0.118 ± 0.01 | 47.85 | 42.19 ± 1.62 | 68.12 ± 0.86 | 222.48 ± 2.11 |
| 166 | ICC-8607 | 12.9 | 5.67 ± 0.19 | 0.151 ± 0.01 | 84.29 ± 1.17 | 1.08 ± 0.14 | 0.360 ± 0.04 | 79.37 | 39.07 ± 0.69 | 70.44 ± 0.21 | 265.27 ± 4.34 |
| 167 | ICC-8621 | 6.7 | 1.82 ± 0.10 | 0.85 ± 0.05 | 28.42 ± 0.80 | 0.28 ± 1.03 | 0.29 ± 0.0 | 12.44 | 45.33 ± 0.57 | 70.83 ± 0.41 | 120.76 ± 0.76 |
| 168 | ICC-8740 | 14.4 | 2.30 ± 0.16 | 0.71 ± 0.04 | 40.84 ± 1.22 | 0.28 ± 1.02 | 0.2 ± 0.01 | 23.39 | 44.26 ± 0.05 | 70.86 ± 0.44 | 191.78 ± 3.55 |
| 169 | ICC-10341 | 16.3 | 6.83 ± 0.31 | 0.183 ± 0.01 | 24.05 ± 2.70 | 0.995 ± 0.03 | 0.255 ± 0.06 | 125.64 | 46.92 ± 2.24 | 71.32 ± 1.32 | 211.76 ± 1.88 |
| 170 | ICC-10393 | 19.5 | 9.13 ± 0.22 | 0.176 ± 0.02 | 26.02 ± 3.15 | 0.764 ± 0.17 | 0.154 ± 0.01 | 210.05 | 38.18 ± 1.73 | 70.04 ± 0.36 | 192.56 ± 1.40 |
| 171 | ICC-10399 | 19.2 | 2.42 ± 0.15 | 0.78 ± 0.03 | 20.50 ± 0.41 | 0.61 ± 1.72 | 0.45 ± 0.0 | 201.41 | 44.80 ± 0.86 | 71.12 ± 0.90 | 121.02 ± 2.47 |
| 172 | ICC-10755 | 22.3 | 9.46 ± 0.26 | 0.186 ± 0.01 | 33.40 ± 2.43 | 0.771 ± 0.18 | 0.225 ± 0.02 | 129.86 | 42.92 ± 2.44 | 71.28 ± 1.84 | 167.24 ± 1.45 |
| 173 | ICC-10885 | 13.4 | 11.17 ± 0.85 | 0.158 ± 0.02 | 25.06 ± 2.79 | 0.844 ± 0.1 | 0.212 ± 0.08 | 152.83 | 38.10 ± 0.93 | 70.22 ± 0.64 | 234.56 ± 1.39 |
| 174 | ICC-10945 | 15.8 | 7.25 ± 0.13 | 0.162 ± 0.01 | 60.37 ± 3.33 | 0.536 ± 0.1 | 0.212 ± 0.03 | 129.62 | 44.07 ± 0.41 | 70.61 ± 1.12 | 272.22 ± 2.30 |
| 175 | ICC-637 | 20.5 | 7.29 ± 0.89 | 0.184 ± 0.01 | 108.27 ± 3.42 | 0.881 ± 0.07 | 0.193 ± 0.01 | 199.80 | 41.92 ± 1.22 | 71.33 ± 0.33 | 193.09 ± 1.33 |
| 176 | ICC-708 | 22.9 | 9.13 ± 0.33 | 0.234 ± 0.03 | 25.76 ± 1.08 | 0.975 ± 0.04 | 0.279 ± 0.01 | 178.86 | 38.76 ± 1.92 | 69.48 ± 1.90 | 198.67 ± 1.77 |
| 177 | ICC-762 | 26.2 | 6.79 ± 0.36 | 0.088 ± 0.01 | 29.96 ± 6.48 | 0.871 ± 0.12 | 0.110 ± 0.03 | 156.33 | 44.22 ± 0.46 | 70.12 ± 1.55 | 214.19 ± 3.41 |
| 178 | ICC-791 | 24.1 | 4.38 ± 0.33 | 0.096 ± 0.0 | 34.10 ± 0.36 | 0.639 ± 0.07 | 0.157 ± 0.02 | 169.95 | 48.65 ± 1.05 | 71.36 ± 0.25 | 181.34 ± 1.78 |
| 179 | ICC-867 | 18.8 | 5.79 ± 0.71 | 0.276 ± 0.02 | 81.11 ± 2.43 | 0.858 ± 0.08 | 0.166 ± 0.05 | 217.87 | 45.28 ± 1.48 | 70.38 ± 1.66 | 159.49 ± 1.93 |
| 180 | ICC-1052 | 25.9 | 5.25 ± 0.25 | 0.226 ± 0.02 | 30.73 ± 4.86 | 0.979 ± 0.09 | 0.209 ± 0.05 | 158.14 | 39.81 ± 1.25 | 70.50 ± 0.84 | 162.61 ± 0.87 |
| 181 | ICC-1083 | 29.0 | 8.46 ± 0.52 | 0.233 ± 0.02 | 32.32 ± 1.26 | 0.926 ± 0.09 | 0.301 ± 0.05 | 141.24 | 43.07 ± 1.74 | 71.54 ± 1.34 | 187.26 ± 2.56 |
| 182 | ICC-1098 | 16.9 | 6.71 ± 0.38 | 0.301 ± 0.01 | 27.80 ± 3.15 | 0.759 ± 0.03 | 0.091 ± 0.01 | 121.18 | 41.46 ± 1.66 | 71.25 ± 1.55 | 129.28 ± 2.06 |
| 183 | ICC-1161 | 21.4 | 8.46 ± 0.29 | 0.276 ± 0.02 | 51.15 ± 3.51 | 0.771 ± 0.07 | 0.264 ± 0.01 | 95.70 | 32.49 ± 1.94 | 71.05 ± 0.88 | 272.10 ± 2.33 |
| 184 | ICC-1164 | 23.9 | 6.88 ± 0.38 | 0.226 ± 0.02 | 40.90 ± 2.52 | 1.098 ± 0.19 | 0.137 ± 0.01 | 60.58 | 37.19 ± 0.38 | 70.48 ± 0.62 | 121.02 ± 2.47 |
| 185 | ICC-1180 | 18.4 | 8.42 ± 0.26 | 0.233 ± 0.02 | 31.81 ± 1.71 | 0.606 ± 0.01 | 0.297 ± 0.06 | 55.65 | 48.76 ± 1.18 | 70.62 ± 0.28 | 212.32 ± 2.65 |
| 186 | ICC-1194 | 28.6 | 5.71 ± 0.52 | 0.301 ± 0.01 | 89.57 ± 1.71 | 0.543 ± 0.01 | 0.112 ± 0.02 | 286.43 | 41.12 ± 1.58 | 68.04 ± 1.34 | 196.08 ± 1.54 |
| 187 | ICC-1205 | 21.2 | 5.50 ± 0.45 | 0.301 ± 0.01 | 87.72 ± 3.06 | 1.188 ± 0.04 | 0.336 ± 0.06 | 193.20 | 39.67 ± 1.92 | 70.23 ± 1.33 | 218.70 ± 1.70 |
| 188 | ICC-1230 | 24.6 | 6.63 ± 0.38 | 0.301 ± 0.01 | 85.11 ± 5.94 | 0.878 ± 0.02 | 0.310 ± 0.00 | 83.25 | 42.62 ± 1.51 | 71.04 ± 0.25 | 234.96 ± 2.19 |
| 189 | ICC-1356 | 20.2 | 5.58 ± 0.19 | 0.291 ± 0.02 | 63.61 ± 3.33 | 1.093 ± 0.08 | 0.361 ± 0.02 | 101.38 | 47.05 ± 1.42 | 70.25 ± 0.26 | 199.47 ± 1.82 |
| 190 | ICC-1392 | 14.5 | 10.33 ± 0.63 | 0.311 ± 0.01 | 83.46 ± 5.49 | 0.764 ± 0.02 | 0.284 ± 0.05 | 141.24 | 46.81 ± 2.36 | 71.58 ± 0.29 | 167.58 ± 1.71 |
| 191 | ICC-1397 | 21.4 | 5.58 ± 0.56 | 0.307 ± 0.01 | 112.66 ± 1.80 | 0.702 ± 0.05 | 0.239 ± 0.02 | 95.70 | 34.10 ± 1.20 | 72.11 ± 0.16 | 192.61 ± 0.88 |
| 192 | ICC-1398 | 29.2 | 5.46 ± 0.14 | 0.213 ± 0.0 | 97.71 ± 1.35 | 1.188 ± 0.06 | 0.093 ± 0.03 | 70.13 | 41.75 ± 1.04 | 70.71 ± 0.28 | 188.16 ± 2.34 |
| 193 | ICC-1422 | 26.1 | 1.82 ± 0.11 | 1.18 ± 0.03 | 27.66 ± 0.53 | 0.64 ± 1.81 | 0.3 ± 0.01 | 197.25 | 32.36 ± 0.32 | 71.11 ± 0.12 | 131.27 ± 1.77 |
| 194 | ICC-1431 | 13.4 | 7.13 ± 0.25 | 0.2 ± 0.01 | 71.18 ± 3.51 | 0.775 ± 0.02 | 0.118 ± 0.01 | 216.11 | 40.11 ± 1.45 | 71.06 ± 0.10 | 236.48 ± 2.54 |
| 195 | ICC-1510 | 20.1 | 6.42 ± 0.40 | 0.206 ± 0.02 | 49.30 ± 3.69 | 0.845 ± 0.04 | 0.112 ± 0.01 | 203.78 | 32.37 ± 1.67 | 70.24 ± 0.61 | 187.82 ± 1.32 |
| 196 | ICC-1710 | 22.3 | 5.71 ± 0.31 | 0.181 ± 0.01 | 27.67 ± 5.67 | 1.024 ± 0.05 | 0.134 ± 0.04 | 183.67 | 41.12 ± 1.41 | 70.42 ± 0.46 | 208.10 ± 1.65 |
| 197 | ICC-1715 | 19.1 | 9.33 ± 0.31 | 0.222 ± 0.01 | 26.91 ± 1.62 | 0.583 ± 0.01 | 0.152 ± 0.01 | 214.45 | 45.05 ± 1.26 | 68.11 ± 0.21 | 264.16 ± 2.46 |
| 198 | ICC-1882 | 28.7 | 9.29 ± 0.44 | 0.241 ± 0.01 | 51.72 ± 1.35 | 0.819 ± 0.09 | 0.121 ± 0.01 | 285.43 | 49.11 ± 1.65 | 70.48 ± 0.96 | 268.90 ± 1.23 |
| 199 | ICC-2263 | 19.8 | 4.08 ± 0.52 | 0.257 ± 0.01 | 26.53 ± 1.08 | 0.719 ± 0.03 | 0.042 ± 0.00 | 103.43 | 36.08 ± 1.11 | 70.79 ± 0.29 | 210.44 ± 1.45 |
| 200 | ICC-2277 | 19.2 | 4.29 ± 0.07 | 0.211 ± 0.01 | 32.82 ± 2.43 | 0.731 ± 0.04 | 0.258 ± 0.04 | 53.33 | 40.70 ± 1.04 | 71.22 ± 0.32 | 190.41 ± 1.25 |
| 201 | ICC-2507 | 16.6 | 5.08 ± 0.56 | 0.326 ± 0.01 | 30.15 ± 1.89 | 1.162 ± 0.02 | 0.125 ± 0.04 | 174.45 | 39.61 ± 1.31 | 70.16 ± 0.71 | 177.21 ± 2.17 |
| 202 | ICC-2580 | 29.7 | 5.58 ± 0.31 | 0.67 ± 0.04 | 33.95 ± 1.20 | 0.27 ± 1.00 | 0.35 ± 0.02 | 21.47 | 29.72 ± 0.88 | 73.06 ± 0.80 | 136.14 ± 0.76 |
| 203 | ICC-2629 | 29.2 | 4.46 ± 0.40 | 0.45 ± 0.01 | 27.10 ± 2.16 | 1.012 ± 0.06 | 0.172 ± 0.06 | 35.06 | 45.15 ± 2.54 | 71.24 ± 0.61 | 144.72 ± 1.24 |
| 204 | ICC-2720 | 26.7 | 4.21 ± 0.31 | 0.198 ± 0.03 | 15.27 ± 2.16 | 0.884 ± 0.04 | 0.207 ± 0.01 | 76.70 | 35.83 ± 1.78 | 70.14 ± 0.46 | 298.39 ± 2.21 |
| 205 | ICC-3512 | 15.3 | 5.50 ± 0.45 | 0.311 ± 0.01 | 75.13 ± 0.90 | 0.992 ± 0.05 | 0.197 ± 0.00 | 66.92 | 42.04 ± 1.45 | 69.21 ± 0.19 | 179.26 ± 1.11 |
| 206 | ICC-3631 | 12.1 | 6.13 ± 0.57 | 0.14 ± 0.01 | 27.29 ± 3.78 | 1.575 ± 0.1 | 0.149 ± 0.03 | 119.66 | 36.10 ± 1.45 | 70.34 ± 0.28 | 234.67 ± 1.44 |
| 207 | ICC-3761 | 21.7 | 7.13 ± 0.25 | 0.129 ± 0.02 | 22.65 ± 2.07 | 1.027 ± 0.04 | 0.173 ± 0.02 | 47.18 | 28.22 ± 1.02 | 70.46 ± 0.09 | 274.27 ± 2.39 |
| 208 | ICC-3325 | 30.1 | 1.72 ± 0.11 | 1.09 ± 0.02 | 12.01 ± 0.51 | 0.45 ± 1.91 | 0.48 ± 0.03 | 122.93 | 40.26 ± 1.11 | 72.95 ± 0.92 | 167.42 ± 1.17 |
| 209 | ICC-3362 | 25.4 | 5.75 ± 0.33 | 0.128 ± 0.0 | 107.95 ± 5.22 | 1.167 ± 0.03 | 0.145 ± 0.04 | 80.62 | 42.12 ± 1.25 | 70.62 ± 0.47 | 192.61 ± 1.31 |
| 210 | ICC-3421 | 16.2 | 5.38 ± 0.25 | 0.174 ± 0.03 | 76.18 ± 1.40 | 0.903 ± 0.04 | 0.143 ± 0.01 | 89.38 | 33.60 ± 1.54 | 71.30 ± 0.10 | 218.66 ± 1.47 |
| 211 | ICC-4948 | 20.9 | 5.50 ± 0.33 | 0.204 ± 0.03 | 92.56 ± 0.54 | 1.174 ± 0.0 | 0.122 ± 0.01 | 93.94 | 34.29 ± 1.26 | 72.99 ± 0.46 | 246.08 ± 2.58 |
| 212 | ICC-4973 | 20.4 | 4.75 ± 0.13 | 0.129 ± 0.02 | 50.32 ± 1.80 | 0.836 ± 0.08 | 0.188 ± 0.04 | 70.98 | 41.61 ± 1.34 | 70.16 ± 0.24 | 281.56 ± 1.74 |
| 213 | ICC-12968 | 31.4 | 4.46 ± 0.71 | 0.128 ± 0.00 | 69.02 ± 2.79 | 0.939 ± 0.06 | 0.436 ± 0.01 | 32.61 | 40.77 ± 1.69 | 72.31 ± 0.12 | 248.19 ± 2.19 |
| 214 | ICC-15996 | 18.7 | 5.25 ± 0.22 | 1.10 ± 0.07 | 27.44 ± 1.01 | 1.098 ± 0.01 | 0.464 ± 0.04 | 8.88 | 29.93 ± 0.58 | 72.69 ± 0.78 | 240.52 ± 1.94 |
| 215 | ICC-16915 | 22.4 | 5.71 ± 0.26 | 0.204 ± 0.02 | 28.69 ± 3.33 | 1.036 ± 0.04 | 0.330 ± 0.01 | 22.85 | 27.10 ± 1.10 | 72.21 ± 0.44 | 277.34 ± 1.49 |
Total flavonoid content of these accessions resulted in a range of 0.04–1.57 mg/g and with average of 0.33 mg/g. The minimum flavonoid content was possessed by gene accession ICC-2263 while maximum content was observed in ICC-8058 followed by ICC-8318, ICC-8195, ICC-14098, ICC-5845, ICC-2072, ICC-67, ICC-5878, ICC-13863 and ICC-12654 gene accessions respectively compared to four chickpea checks. Phenolics group present in the flavonoids afford unique stability for executing biological activity. The intake of flavonoids includes reduction in osteoporosis, diabetes, obesity and cancer prevention (Martin et al. 2017). Aforesaid accessions can therefore be recommended for as a parent for developing elite cultivars.
DPPH radical scavenging activity
The antioxidant activity of mini-core accessions was experimented to highlight its radical scavenging performance. During the reaction, the hydroxyl group of the aromatic ring of phenol is substituted by donating the hydrogen atom (Zhang et al. 2011). DPPH free radical scavenging activity was observed in a range of 26.74–49.11%. The average content of free radical scavenging activity across all the accessions was observed to be 46.33%. Ten accessions was observed to be higher in all 211 accessions ICC-1882, ICC-1923, ICC-16903, ICC-1180, ICC-791, ICC-14778, ICC-1915, ICC-13219, ICC-12028 and ICC-12537. While, lowest in ICC-15618 compared to four Indian national checks.
The current results and those previously reported may attribute to differences in cultivated and core collected seed composition. The chickpea mini-core accessions represent both geographical and biological diversity leading to variations. Core and/or mini-core collections representing the reduced subsets of germplasm are effective in recognizing the accessions with desired agronomic traits (Upadhyaya and Ortiz 2001). Present results thus identify new sources of variation that can be used as a parent/functional food in agro-industry (Kaur et al. 2013). In summary, compared with other tested chickpea seeds ICC-14778 had higher antioxidant activity. Recently Quintero-Soto et al. (2018), investigated antioxidant activity in selected chickpea genotypes and observed that black seed desi chickpea contained highest scavenging activity.
Pulses with the high phenolic contents demonstrate highest antioxidant capacity. Antioxidant properties of food are widely believed to exhibit disease curing properties like cancer, diabetes, various respiratory diseases, eye diseases and schiozophrenia (Cai et al. 2004). The above said accessions may be employed as good sources of antioxidants for improvement of nutraceutical properties.
Hemagglutinating activity
Lectins are diverse group of carbohydrate binding proteins distributed ubiquitously in plant species and are the subject of intense investigations. Lectins preferably binds to a transition ion, usually manganese and calcium, thus exhibiting metal chelating activity. The specific activity of lectin depicted in a range of 0.07–330.32 HU/mg (Table 1) possessing an average of 75.1 HU/mg. The reprentative hemagglutinating activity is dispalyed in Fig. 2. Minimum specific activity was observed in ICC-6537 while highest specific activity was possessed by ICC-1923 (330.32 HU/mg) followed by ICC-14077, ICC-1194, ICC-1882, ICC-4872, ICC-867, ICC-1431, ICC-1715, ICC-10393 and ICC-1510 respectively as compared to the four national checks. Chickpea lectin demonstrate variation in agglutination activity to different human blood types (Roy et al. 2010). However, in the present study, only trypsin treated rabbit erythrocytes were able to agglutinate chickpea extract. This contradiction may be due to various features like cell surface interactions, lectin types and conditions of assay (Lis and Sharon 1986). Moreover, it has been reported that the seed type, harvesting time and area of cultivation can also bring change in the lectin concentration (Mekbungwan 2007). The amount of lectins vary significantly in legumes like kidney bean, soya bean and lima bean. In addition to nutritional properties, pulse lectin also exhibit potent clinical applications. For example, anti-HIV-1 reverse transcriptase activity was reported from kidney bean lectin (Zhang et al. 2009). To authors understanding, reports evaluating the lectin in chickpea core/mini-core collection was scanty. Compared to other legumes like pea and lentil, chickpea has lower lectin contents as revealed in the present study. Although scientific data has demonstrated that chickpea lectin has pharmacological properties vis-à-vis antifungal, antiproliferative (Gautam et al. 2018a; Kumar et al. 2014) and anti-HIV-1 reverse transcriptase activity (Gautam et al. 2018b).
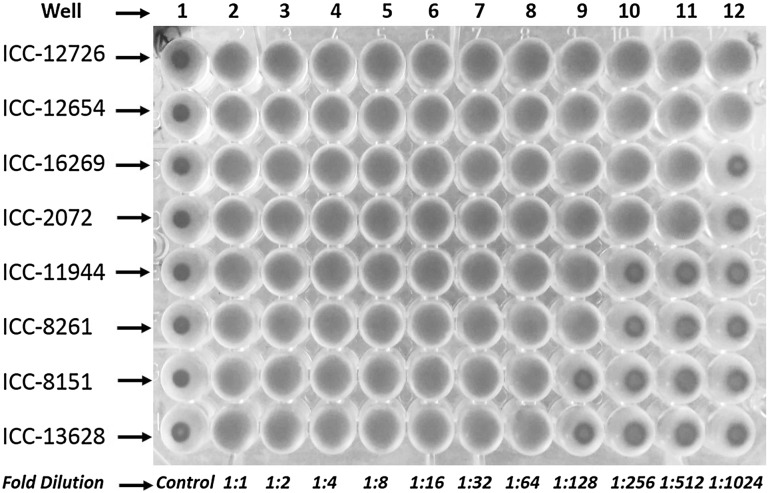
Fig. 2.
Hemagglutinating activity of representative chickpea accessions. Well 1: Control, PBS (50 µl) alone was added without protein. Hemagglutinating activity was checked by twofold serial dilution started from well 2 to 12. Minimum concentration of lectin required for hemagglutinating activity was found to be 7.1 µg shown in 7 well. Whereas maximum hemagglutinating activity was revealed at 8.3 µg up to 12 wells
Protein and starch digestibilities
In the present study, in vitro protein digestibility was observed in a range of 59.45–76.22%. The mean protein digestibility content in all the accessions was found to be 72.26%. The protein digestibility of ICC-14815, ICC-11764, ICC-11664, ICC-11879, ICC-4918, ICC-11879, ICC-2580, ICC-4533, ICC-4948 and ICC-3325 of these accessions were observed to be higher. While, lowest was observed in ICC-12028 in comparison to four Indian national checks.
In the present study, in vitro starch digestibility was observed in a range of 45.63–298.39%. The mean starch digestibility content among all the accessions was 252.81%. The starch digestibility of ten accessions viz. ICC-2720, ICC-8058, ICC-11944, ICC-9895, ICC-4973, ICC-169151, ICC-3761, ICC-11378, ICC-10945 and ICC-1161 were high. While, lowest was observed in ICC-13599 compared to four national checks ICC-4948, ICC-4973, ICC-12968 and ICC-15996.
The in vitro protein and starch digestibility constitutes an important indicator for physiological status of food samples. IVPD offers a hint for protein absorption whereas IVSD depicts the susceptibility of starch to digestive enzymes. Differences in seed coat and texture influence the digestibility. IVPD of desi chickpea is lower than kabuli due to high content of polyphenols that are present in the thick seed coats of desi chickpea (Singh and Jambunathan 1981). Also, IVPD of chickpea increases with fermentation period because of hydrolysis of seed storage proteins due to microbial proteases and possible break down of protease inhibitors (Xu et al. 2016). Another likely reason for increased IVPD might be due to reduced antinutritional factors where proteins are disrupted making the protein cross-linking more susceptible to proteolytic attack and thereby increasing their digestibility (Chitra et al. 1996). Chickpea protein has the highest digestibility amongst the dry edible legumes however, it can be enriched by cooking, roasting and autoclaving. The anti-nutritional factors like phytic acid and tannins inhibit the activity of α-amylase as a result decreasing the starch digestibility. Therefore, removal of anti-nutritional factors may contribute to enhancement of starch digestibility. It is well known that legume starch causes flatulence and discomfort to enzymatic digestion. It is further recommended that the consumption of the whole legume seed containing dietary fibers are helpful in decreasing the intestinal transit time. In case of diabetes reduced digestibility is beneficial as it lowers the amount of glucose into the blood stream (Muhammad et al. 2007).
Similar observations of increase in IVPD in chickpea flour were reported by earlier investigators (Doke and Guha 2015). This feature of chickpea is desirable as an ingredient for making protein rich food formulations from industrial perspective. Protein and starch digestibilities of core/mini-core collections of chickpea flour must be assessed in vivo to correlate with corresponding in vitro studies. Testing functional properties of chickpea flour (core/mini-core collections) is warranted for selection of donor parents.
PCA analysis and dendrogram
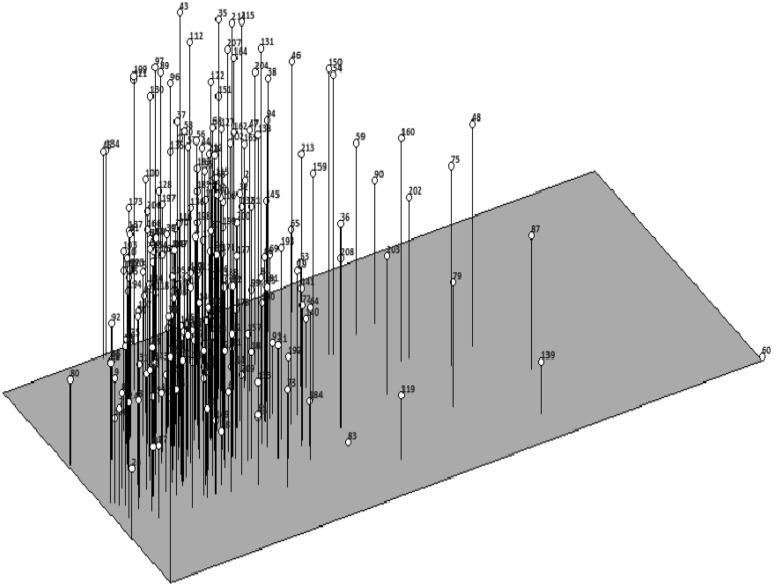
The principal component analysis of 215 mini-core chickpea accessions using the ten parameters viz., protein content, free amino acid, phytic acid, tannic acid, total phenols, total flavonoids, lectin specific activity, DPPH radical scavenging activity, IVPD and IVSD were studied. The PCA correlation depicted that the accessions viz., ICC-13599(60), ICC-9848(87), ICC-9942(90), ICC-14831(79), ICC-12947(75), ICC-7819(48), ICC-4533(139), ICC-4463(53), ICC-2580(202) and ICC-2629(203) possessed maximum protein content with lower levels of phenolics and flavonoid contents occupying specific position to the right side of the graph (Fig. 3). In contrast, the accessions viz., ICC-12851(2), ICC-7323(43), ICC-15264(80), ICC-9895(89), ICC-7272(100), ICC-15606(121), ICC-14778(130) and ICC-10885(173) occupied specific left position in the graph with maximum values for in vitro protein and starch digestability. It was further noticed that accessions existing towards left of the figure showed maximum DPPH radical scavenging activity while those towards the right demonstrated maximum free amino acid contents.
Fig. 3.
Multivariate principal component analysis of 215 mini-core chickpea accessions based on evaluated parameters
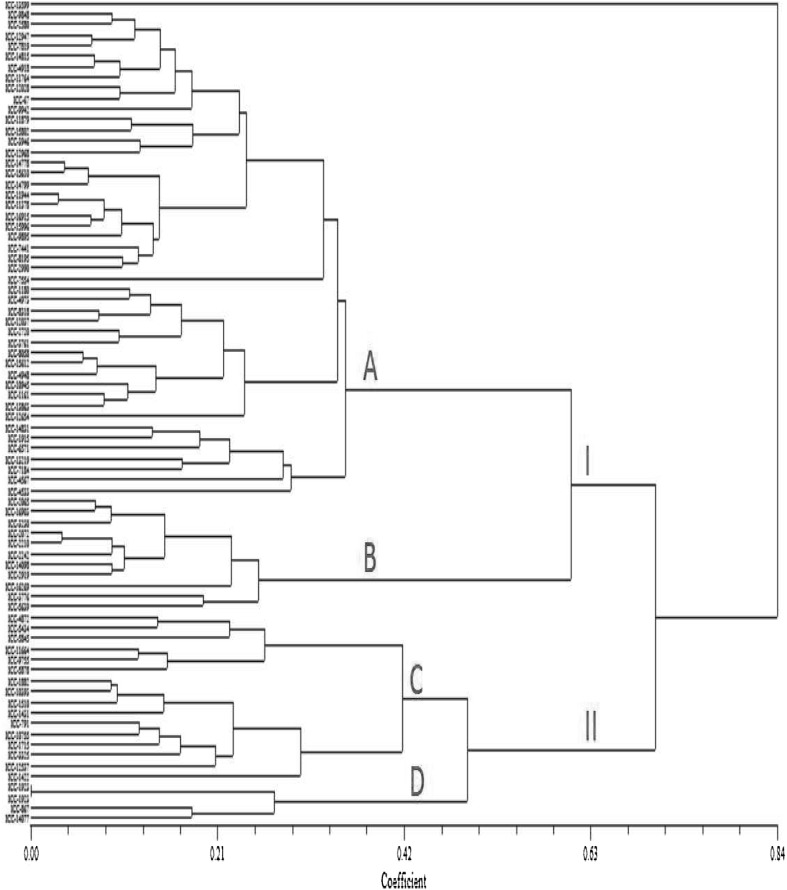
The dendrogram derived from the 100 accessions which is showing highest values for 10 experimeted parameters. Out of these, 12 accession showed equal values and therefore were not included in the dendrogram. The relationships among the chickpea accessions for above said parameters were graphed. The genetic similarity matrix was accessed by unweighted pair group method with arithmetic mean (UPGMA) (Fig. 4). The generated dendrogram distinguished into 2 clusters. The cluster-I subdivided into two clusters A and B, where first sub-cluster (A) was further subdivided into several small clusters that grouped chickpea accessions with maximum contents of protein (ICC-14831, ICC-13599, ICC-9843 etc.), free amino acids (ICC-7441, ICC-6571, ICC-3946 etc.), in vitro protein digestibility (ICC-11764, ICC-11879, ICC-4918 etc.), in vitro starch digestibility (ICC-2720, ICC-3761, ICC-8058 etc.). The sub cluster (B) consisted of accessions with high tannic acid content. Similarly the cluster-II was divided into 2 sub-clusters i.e. C and D. The sub cluster C possessed the chickpea accessions with high total phenolic content (ICC-5434, ICC-5845, ICC-1164) and DPPH radical scavenging activity (ICC-1882, ICC-791, ICC-3325, ICC-12537). While the other sub cluster D consisted of chickpea accessions possessing high lectin activity (ICC-867, ICC-14077). The accession ICC-13599 came as out group showing the maximum diversity. Thus, the dendrogram based information provides the existence of biochemical diversity among the mini-core lines of chickpea for these biochemical parameters.
Fig. 4.
Representative UPGMA dendrogram of 100 mini-core chickpea accessions of highest values for 10 experimeted parameters obtained using Pearson correlation coefficient. I and II are the major clusters; A and B sub-clusters of I; C and D sub-clusters of II
Currently, chickpea crop improvement programmes are being pursued vigorously worldwide. Germplasm with enhanced nutrients needs to be recognised. There has been no recorded attempts to biofortify chickpea via mini-core approach. Present investigation may offer a guideline to the breeders for designing biofortification strategies for chickpea. The elite trait specific accessions may be used for improving nutraceutical properties in chickpea, thus opening up new vista for further exploration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Ajay Kumar Gautam is grateful to University of Grants Commission (UGC), New Delhi for providing research fellowship under major research project (F.No. 40-154/2011 (SR). Authors are also grateful to ICRISAT, Hyderabad, (A.P.) India for providing the seed samples. Dr. Sushma Tiwari, Scientist, Rajmata Vijayaraje Scindia Krishi Vishwa Vidyalaya, Gwalior is thankfully acknowledged for providing statistical software support.
Compliance with ethical standards
Conflict of interest
No conflict of interest was reported by the authors.
References
- Alajaji AS, El-Adawy AT. Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. J Food Compos Anal. 2006;19:806–812. doi: 10.1016/j.jfca.2006.03.015. [DOI] [Google Scholar]
- Bersuder P, Hole M, Smith G. Antioxidants from a heated histidine glucose model system I: investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J Am Oil Chem Soc. 1998;75:181–187. doi: 10.1007/s11746-998-0030-y. [DOI] [Google Scholar]
- Bhagyawant SS, Gupta N, Shrivastava N. Biochemical analysis of chickpea accessions vis-a-vis; zinc, iron, total protein, proline and antioxidant activity. Am J Food Sci Technol. 2015;3(6):158–162. doi: 10.12691/ajfst-3-6-3. [DOI] [Google Scholar]
- Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitra U, Singh U, Rao PV. Phytic acid, in vitro protein digestibility, dietary fiber, and minerals of pulses as influenced by processing methods. Plant Foods Hum Nutr. 1996;49:307–316. doi: 10.1007/BF01091980. [DOI] [PubMed] [Google Scholar]
- Doke S, Guha M. Nutritional, physico-chemical and functional properties of ready-to-use chickpea and soybean flour. Int J Food Nutr Sci. 2015;4(5):2320–7876. [Google Scholar]
- Fan TY, Sosulski FW. Dispersibility and isolation of protein from legume flours. Can Inst Food Sci Technol J. 1974;7:256–259. doi: 10.1016/S0315-5463(74)73923-2. [DOI] [Google Scholar]
- Francis G, Makkar HPS, Becker K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture. 2001;199:197–227. doi: 10.1016/S0044-8486(01)00526-9. [DOI] [Google Scholar]
- Frankel OH. Genetic perspective of germplasm conservation. In: Arber W, Illmensee K, Peacock WJ, Starlinger P, editors. Genetic manipulation: impact on man and society. Cambridge: Cambridge University Press; 1984. pp. 161–170. [Google Scholar]
- Gautam AK, Gupta N, Narvekar DT, Bhadkariya R, Bhagyawant SS. Characterization of chickpea (Cicer arietinum L.) lectin for biological activity. Physiol Mol Biol Plants. 2018;24(3):389–397. doi: 10.1007/s12298-018-0508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam AK, Srivastava N, Nagar DP, Bhagyawant SS. Biochemical and functional properties of a lectin purified from the seeds of Cicer arietinum L. 3 Biotech. 2018;8:272. doi: 10.1007/s13205-018-1272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Shrivastava N, Bhagyawant SS. Multivariate analysis based on nutritional value, antinutritional profile and antioxidant capacity of forty chickpea genotypes grown in India. J Nutr Food Sci. 2017;7(3):1000600. doi: 10.4172/2155-9600.1000600. [DOI] [Google Scholar]
- Hsu HW, Vavak DL, Satterlee LD, Miller GA. A multienzyme technique for estimating protein digestibility. J Food Sci. 1977;42:1269–1273. doi: 10.1111/j.1365-2621.1977.tb14476.x. [DOI] [Google Scholar]
- Imura K, Okada A. Amino acid metabolism in pediatric patients. Nutrition. 1998;14(1):143–148. doi: 10.1016/S0899-9007(97)00230-X. [DOI] [PubMed] [Google Scholar]
- Kahraman A, Pandey A, Khan MK. Nutritional diversity assessment in chickpea—a prospect for nutrient deprived world. Harran J Agric Food Sci. 2017;21(3):357–363. [Google Scholar]
- Kashiwagi J, Krishnamurthy L, Upadhyaya HD, Krishna H, Chandra S, Vadez V, Serraj R. Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpea (Cicer arietinum L.) Euphytica. 2005;146:213–222. doi: 10.1007/s10681-005-9007-1. [DOI] [Google Scholar]
- Kaur K, Kaur N, Gupta AK, Singh I. Exploration of the antioxidative defense system to characterize chickpea genotypes showing differential response towards water deficit conditions. Plant Growth Regul. 2013;70:49–60. doi: 10.1007/s10725-012-9777-0. [DOI] [Google Scholar]
- Kaur N, Kumar A, Kaur K, Kaur S, Gupta AK, Singh I. Abiotic stress tolerance of chickpea genotypes depends upon antioxidative potential and nutritional quality of seeds. Proc Natl Acad Sci India Sect B Biol Sci. 2014;85(2):615–623. doi: 10.1007/s40011-014-0382-z. [DOI] [Google Scholar]
- Khoo HE, Azlan A, Ismail A, Abas F. Response surface methodology optimization for extraction of phenolics and antioxidant capacity in defatted dabai parts. Sains Malays. 2013;42(7):949–954. [Google Scholar]
- Kumar S, Kapoor V, Gill K, Singh K, Xess I, Das SN, Dey S. Antifungal and antiproliferative protein from Cicer arietinum: a bioactive compound against emerging pathogens. Biomed Res Int. 2014 doi: 10.1155/2014/387203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liener IE. Toxic factors in edible legumes and their elimination. Am J Clin Nutr. 1962;11:281–298. doi: 10.1093/ajcn/11.4.281. [DOI] [PubMed] [Google Scholar]
- Lis H, Sharon N. Lectin as molecules and as tools. Annu Rev Biochem. 1986;55:35–67. doi: 10.1146/annurev.bi.55.070186.000343. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luo XD, Basile MJ, Kennelly EJ. Polyphenolic antioxidants from the fruits of Chrysophyllum cainito L. (star apple) J Agric Food Chem. 2002;50:1379–1382. doi: 10.1021/jf011178n. [DOI] [PubMed] [Google Scholar]
- Martin LP, Rangel MB, Zamarreno MMD. Classification of lentils, chickpeas and beans based on their isoflavone content. Food Anal Methods. 2017;10:1191–1201. doi: 10.1007/s12161-016-0675-3. [DOI] [Google Scholar]
- Mekbungwan A. Application of tropical legumes for pig feed. Anim Sci J. 2007;78:343–350. doi: 10.1111/j.1740-0929.2007.00446.x. [DOI] [Google Scholar]
- Melki M, Gasouri A, Bouhadida M, Chograni H, Rezgui M. Phenotypic, genetic and biochemical characterization of five Tunisian varieties of chickpea. J Agric Stud. 2017;5(1):77–89. doi: 10.5296/jas.v5i1.10808. [DOI] [Google Scholar]
- Moore S, Stein WH. Analysis of amino acids. In: Colowick SP, Kaplan NO, editors. Methods in enzymology. New York: Academic Press; 1948. pp. 468–471. [Google Scholar]
- Muhammad ZH, Shahid I, Shakeel A, Muhammad I, Abdul N, Bhanger MI. Nutritional and compositional study of desi chickpea (Cicer arietinum L.) cultivars grown in Punjab, Pakistan. Food Chem. 2007;105:1357–1363. doi: 10.1016/j.foodchem.2007.05.004. [DOI] [Google Scholar]
- Pande S, Krishna Kishore G, Upadhyaya HD. Identification of sources of multiple disease resistance in mini-core collection of chickpea. Plant Disease. 2006;90(9):1214–1218. doi: 10.1094/PD-90-1214. [DOI] [PubMed] [Google Scholar]
- Parameshwarappa SG, Salimath PM, Upadhyaya HD, Patil SS, Kajjidoni ST. Genetic variability studies in minicore collection of chickpea (Cicer arietinum L.) under different environments. Karnataka J Agric Sci. 2012;25(3):305–308. [Google Scholar]
- Peng X, Zheng ZP, Cheng KW, Shan F, Ren GX, Chen F, Wang M. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advance glycation endproducts. Food Chem. 2008;106:475–481. doi: 10.1016/j.foodchem.2007.06.016. [DOI] [Google Scholar]
- Quintero-Soto MF, Saracho-Peña AG, Chavez-Ontiveros J, Garzon-Tiznado JA, Pineda-Hidalgo KV, Delgado-Vargas F, Lopez-Valenzuela JA. Phenolic profiles and their contribution to the antioxidant activity of selected chickpea genotypes from Mexico and ICRISAT collections. Plant Foods Hum Nutr. 2018 doi: 10.1007/s11130-018-0661-6. [DOI] [PubMed] [Google Scholar]
- Reddy NR, Sathe SK, Salunkhe DK. Phytate in legumes and cereals. Adv Food Res. 1982;28:1–92. doi: 10.1016/S0065-2628(08)60110-X. [DOI] [PubMed] [Google Scholar]
- Roorkiwal M, Jain A, Thudi M, Varshney RK. Advances in chickpea genomic resources for accelerating the crop improvement. In: Varshney R, Thudi M, Muehlbauer F, editors. The chickpea genome, compendium of plant genomes. Cham: Springer; 2017. pp. 53–67. [Google Scholar]
- Roy F, Boye JI, Simpson BK. Bioactive proteins and peptides in pulse crops: pea, chickpea and lentil. Food Res Int. 2010;43:432–442. doi: 10.1016/j.foodres.2009.09.002. [DOI] [Google Scholar]
- Saeed A, Darvishzadeh R. Genetic diversity in a minicore collection of Cicer accessions using amplified fragment length polymorphism (AFLP) Arch Agron Soil Sci. 2016;62(12):1711–1721. doi: 10.1080/03650340.2016.1159302. [DOI] [Google Scholar]
- Schandrel SH. Method in food analysis. New York: Academic Press; 1970. [Google Scholar]
- Serraj R, Krishnamurthy L, Upadhyaya HD. Screening chickpea minicore germplasm for tolerance to soil salinity. ICPN. 2004;11:29–32. [Google Scholar]
- Singh U, Jambunathan R. Studies on desi and kabull chickpea (Cicer arietinum L.) cultivars: levels of protease inhibitors, levels of polyphenolic compounds and in vitro protein digestibility. J Food Sci. 1981;46:1364–1367. doi: 10.1111/j.1365-2621.1981.tb04176.x. [DOI] [Google Scholar]
- Singh U, Kherdekar MS, Jambunathan R. Studies on desi and kabuli chickpea (Cicer arietinum L.) cultivars. The levels of amylase inhibitors, levels of oligosaccharides and in vitro starch digestibility. J Food Sci. 1982;47:510–512. doi: 10.1111/j.1365-2621.1982.tb10113.x. [DOI] [Google Scholar]
- Swain U, Hillis WE. The phenolic constituents of Prunus domestica. I. The quantitative analysis of phenolic constituents. J Agric Food Chem. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Tan GZH, Das Bhowmik SS, Hoang TML, Karbaschi MR, Johnson AAT, Williams B, Mundree SG. Finger on the pulse: pumping iron into chickpea. Front Plant Sci. 2017;8:1755. doi: 10.3389/fpls.2017.01755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thudi K, Roorkiwal M, Kudapa H, Chaturvedi SK, Singh NP, Varshey RK. An overview of chickpea research: from discovery to delivery. Pulse India. 2017;2(5):22–25. [Google Scholar]
- Upadhyaya HD, Ortiz R. A mini core subset for capturing diversity and promoting utilization of chickpea genetic resources in crop improvement. Theor Appl Genet. 2001;102:1292–1298. doi: 10.1007/s00122-001-0556-y. [DOI] [Google Scholar]
- Upadhyaya HD, Yadav D, Dronavalli N, Gowda CLL, Singh S. Mini core germplasm collections for infusing genetic diversity in plant breeding program. Electr J Plant Breed. 2010;1(4):1294–1309. [Google Scholar]
- Upadhyaya HD, Dronavalli N, Dwivedi SL, Kashiwagi J, Krishnamurthy L, Pande S, Sharma HC, Vadez V, Singh S, Varshney RK, Gowda CLL. Mini core collection as a resource to identify new sources of variation. Crop Sci. 2013;53(6):2506–2517. doi: 10.2135/cropsci2013.04.0259. [DOI] [Google Scholar]
- Vadivel V, Janardhanan K. Nutritional and antinutritional characteristics of seven Indian wild legumes. Plant Foods Hum Nutr. 2005;60:69–75. doi: 10.1007/s11130-005-5102-y. [DOI] [PubMed] [Google Scholar]
- Varshney RK, Ribaut JM, Buckler ES, Tuberosa R, Rafalski JA, et al. Can genomics boost productivity of orphan crops? Nat Biotechnol. 2012;30:1172–1176. doi: 10.1007/978-3-319-66117-9_6. [DOI] [PubMed] [Google Scholar]
- Vidal-Valverde C, Frias J, Estrella I, Gorospe MJ, Ruiz R, Bacon J. Effect of processing on some antinutritional factors of lentils. J Agric Food Chem. 1994;42:2291–2295. doi: 10.1021/jf00046a039. [DOI] [Google Scholar]
- Wilcox JK, Premchandra GS, Young KA, Raboy V. Isolation of high seed inorganic P, low-phytate soybean mutants. Crop Sci. 2000;40:1601–1605. doi: 10.2135/cropsci2000.4061601x. [DOI] [Google Scholar]
- Xu Y, Cartier A, Obielodan M, Jordan K, Hairston T, Shannon A, Sismour E. Nutritional and anti-nutritional composition, and in vitro protein digestibility of kabuli chickpea (Cicer arietinum L.) as affected by differential processing methods. J Food Meas Charact. 2016;10(3):625–633. doi: 10.1007/s11694-016-9346-8. [DOI] [Google Scholar]
- Yao Y, Sang W, Zhou MJ, Ren GX. Phenolic composition and antioxidant activities of 11 celery cultivars. J Food Sci. 2010;75:C9–C13. doi: 10.1111/j.1750-3841.2009.01392.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shi J, Illic S, Jun XS, Kakuda Y. Biological properties and characterization of lectin from red kidney bean (Phaseolus vulgaris) Food Rev Int. 2009;25:12–27. doi: 10.1080/87559120802458115. [DOI] [Google Scholar]
- Zhang D, Chu L, Liu YX, et al. Analysis of the antioxidant capacities of flavonoids under different spectrophotometric assays using cyclic voltammetry and density functional theory. J Agric Food Chem. 2011;59(18):10277–10285. doi: 10.1021/jf201773q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.