Abstract
Occurrence of salt stress with the soil borne fungus Fusarium oxysporum f. sp. cepa (FOC) are potential threat to the crop yield. This investigation reports effect of the concurrent stresses (salinity and FOC) on morpho-physiological and yield attributes in onion. In vitro growth tests revealed proliferation of FOC biomass at different levels of salinity (2–8 dS m−1). A greenhouse pot experiment with the proposed levels of salinity (2.5, 3.5 and 4.5 dS m−1) in combination with FOC inoculation showed more drastic effect of combined stress on disease severity, plant growth and bulb as compared to the individual stress. In general, osmotic potential, total chlorophyll content, membrane stability index and total protein content of the leaf were decreased, while total phenolics were increased due to the given stress/s. Total sugar content decreased due to effect of the individual stress of FOC, while it increased under the individual stress of salinity and in combination with FOC. FOC infection did not change activity of polyphenol oxidase (PPO), while it improved peroxidase (POX) and phenylalanine ammonia lyase (PAL) and decreased catalase (CAT) activity. Activities of POX and PPO increased, however PAL and CAT declined under individual as well as simultaneous stress of salinity and FOC. The research work concluded that FOC will be a more severe disease threat for onion cultivation in saline soils.
Keywords: Basal rot, Salt stress, Salt tolerant fungus, Physiology and biochemistry, Antioxidant enzyme
Introduction
Soil salinization imposes a major environmental threat to agriculture by limiting plant growth and reducing crop yield (Machado and Serralheiro 2017). In Pakistan, salinity is typical for irrigated agriculture where drainage is inadequate, and nearly 10 million ha area (12.9% of country land) is badly affected by salinity (Mahboob et al. 2016). Soil salinity interferes with plant nutrition and reduces the crop productivity, including that of most vegetable crops, which presents a high sensitivity to salt stress (Machado and Serralheiro 2017). Salts negatively alter water availability, respiration rate, mineral distribution, membrane stability, turgor pressure, growth rate and yield in the plants (Makhloufi et al. 2014). Reduction in the growth, biomass and yield has been reported in different vegetable crops under salt stress (Giuffrida et al. 2013). In addition to affecting the plant, salinity affected the survival and behavior of the phytopathogens in soil and increased susceptibility to the disease (Pandey et al. 2017). Therefore, salinity could be an important factor in the increasing disease incidence in the crops. Onion (Allium cepa L.) is among the world’s most important vegetable being cultivated in almost 170 countries with net production of 742.51 million tones. Pakistan occupied 8th position sharing 17.01 million tons production (FAO 2013). Fusarium basal rot caused by soil–borne fungus Fusarium oxysporum f. sp. cepa is one of the most harmful diseases of the onion (Saxena and Cramer 2009) which has been responsible for drastic losses in the productivity, both in the field and in the storage condition (Coşkuntuna and Özer 2008). Over 120 different formae speciales have been described for F. oxysporum and strains that cause basal rot disease in the onion as well as in other Allium species are classified as formae speciales cepa (FOC) (Sasaki et al. 2015). The fungus causes brown discoloration of the basal stem plate resulting in altering the physiological and growth responses. These changes due to the effects of FOC cause the death of plant through wilting, curling, yellowing eventually necrosis and decaying of the leaves from the tips to rotting of roots (Saxena and Cramer 2009).
Plant responses to the combined stress factors show several unique responses, along with other common responses (Pandey et al. 2017). Production of reactive oxygen species (ROS) is among the primary responses induced to combat the stress and resulting signal transduction triggers metabolic reprogramming towards defense (Bartoli et al. 2013) through response related factors (MAPKs, transcriptional factors, enzymes, antioxidants, heat-shock and PR protein) (Gechev et al. 2006). Increase in the activities of antioxidant enzymes like superoxide dismutase (SOD), peroxidase (POX) and catalase (CAT) are closely related to the salt endurance in many plants (Seckin et al. 2009). No specific and conclusive profiling of antioxidative system has been reported for the combined stress of disease and salinity. Antioxidative response can vary with the stress intensity, duration, the combined effects of other environmental factors and plant species/varieties (Gholizadeh and Kohnehrouz 2010). Understanding plant responses to simultaneous stresses is very important and will be helpful for agronomists and field pathologists in assessing the impact of the interactions between salinity and plant-pathogens on the onion crop performance. Therefore, the main objectives of the proposed study were to assess the interaction of FOC and salinity exclusively and synergistically on morphology, growth, biochemistry and yield of the onion.
Materials and methods
Laboratory assays to assess FOC behavior to saline conditions
Saline solutions consisted of 7 different EC ranging from 2 to 8 were prepared by adding measured amounts of four salts viz., NaCl, Na2SO4, CaCl2, MgSO4 in 100 mL of double-distilled water (Table 1). All salts were mixed well with constant shaking for 15 min with adjustment of final EC by EC meter (WTW series code720), and pH with a pH meter (BANTE instruments PHS-3 BW). To assess the effects of saline solution on the growth of F. oxysporum f. sp. cepa (FCBP 1114), experiments were conducted in 250 mL flask filled with a saline solution of different EC ranging from 2 to 8 dS m−1. Each of saline solution was mixed with 2 g ME 100 mL−1 while kept under constant shaking. After autoclaving, flasks were inoculated with 2 nm disc of FOC under aseptic conditions and incubated at 25 + 2 °C for 7 days. After the incubation period, the biomass of fungus was dried in the oven at 60 °C for 24 h and measured.
Table 1.
Concentration of different salts used to make saline solutions
| Sr. no | EC dS m−1 | NaCl, Na2SO4, CaCl2, MgSO4 (g 100 mL−1) |
|---|---|---|
| 1 | 2 | 0.024, 0.042, 0.020, 0.011 |
| 2 | 3 | 0.032, 0.067, 0.045, 0.024 |
| 3 | 4 | 0.036, 0.090, 0.072, 0.039 |
| 4 | 5 | 0.038, 0.011, 0.010, 0.057 |
| 5 | 6 | 0.039, 0.146, 0.154, 0.083 |
| 6 | 7 | 0.119, 0.221, 0.119, 0.064 |
| 7 | 8 | 0.134, 0.249, 0.136, 0.074 |
Pot experiment
Pre-trial soil physico-chemical analysis
Soil used in the experiment was loam (sand: 42.5%, silt: 32.3% and clay: 25.2%) (Bouyoucos 1962), with 36% saturation level, 7.6 pH (McLean 1982), 1.5 dS m−1 electrical conductivity (EC), 0.36% organic matter (Walkley 1947), 8 meq L−1 calcium (Ca++), 8 meq L−1 magnesium (Mg++), 3 sodium absorption ratios (SAR), 6 meq L−1 sodium (Na+), 3 meq L−1 chloride (Cl−), 5.5 meq L−1 sulfate (SO-24), 5 meq L−1 carbonates (CO3−), 4.5 meq L−1 and bicarbonates (HCO3−) (Richards 1954). Calcium + magnesium was assessed by EDTA titration, while sulfate was determined through barium sulfate precipitation. Sodium in the soil extract was measured on the flame photometer. Carbonate and bicarbonates were estimated by titrating soil extract with 0.01 N H2SO4.
Development of salinity in soil
Pot trials were performed in the plastic pots (15 cm diameter and 15 cm length). Soil was made saline by adding salts before transplanting plants. Sodium absorption ratio (SAR) and Quadratic Formula (Pang et al. 2010) were used to calculate the amount of salt required to add in soil. Double distilled water (10.6 L) containing calculated amount of different salts were added in 40 kg of soil for creating soil with EC 2.5 dS m−1 (NaCl: 1.27 g; Na2SO4: 3.26 g; CaCl2: 2.68 g and MgSO4, 1.44 g); 3.5 dS m−1 (NaCl: 1.52 g; Na2SO4: 6.136 g; CaCl2: 7.34 g and MgSO4, 3.64 g) and 4.5 dS m−1 (NaCl: 6.12 g; Na2SO4: 8.91 g; CaCl2: 11.42 g and MgSO4, 6.13 g). Four to five irrigations with water were given after every 20–25 days. After successive irrigations of 4 months, the salt accumulation in the soil was approached to equilibrium concentration through evaporation by leaving the salt behind in the soil.
Synergistic stress of salinity and pathogen (FOC)
Soil with each of three levels of EC was artificially infected by pouring 30 mL cultural suspensions of FOC. Twenty days old onion seedlings (Nasarpuri) were transplanted into the potted soil. There were three seedlings per pot and two were maintained after successful stand. There were eight treatments i.e. T1: Negative control; T2: Positive control (FOC); T3: EC 2.5 dS m−1; T4: EC 3.5 dS m−1; T5: EC 4.5 dS m−1; T6: EC 2.5 dS m−1 + FOC; T7: EC 3.5 dS m−1 + FOC and T8: EC 4.5 dS m−1 + FOC. The experiment was designed for a total 50 days and each treatment was triplicated. The pots were placed in a greenhouse (30 °C ± 3; 12 h photoperiod and 70% relative humidity). Plants were checked for basal rot disease symptoms on the basis of 1-5 disease rating scale (Coşkuntuna and Özer 2008). 1: no symptom of plant (0%); 2: curving, yellowing with tip die back (< 25%); 3: rot on the stem (25 > 50%); 4: brown watery appearance on the stem base (51 > 75%) and 5: rotten bulbs (76 > 100%). Disease incidence (%) and plant mortality (%) was calculated in inoculated treatments. The effects of the various treatments were checked on morphology, antioxidant enzyme activities growth and yield in the plants. Different physiological and biochemical tests of the onion leaf were conducted at 35th day after inoculation.
Physiological assays
Capell and Dörffling (1993) method was used to evaluate the osmotic potential of the cell sap from the leaf with vapor pressure osmometer (Wescor model VAPRO 5520). Total chlorophyll content and carotenoids of the leaf samples were measured according to Lichtenthaler and Wellburn (1983). Membrane stability index (MSI) of leaf samples was assessed according to Saneoka et al. (2004). Total sugar content was estimated by using the method of Nelson (1944) to take the absorbance of the sample at 620 nm. Lowry et al. (1951) method was used for the appraisal of protein content in the samples with the help of standard Bovine serum albumin (BSA).
Antioxidant enzymes assays
Leaves (0.5 g) were blended and homogenized with 10 mL of sodium phosphate buffer (pH 6.5). The suspension was centrifuged for 10 min at 10,000 rpm. Peroxidase (POX) activity was determined by purpurogallin formation from pyrogallol. The reaction mixture [1 mL enzyme extract, 2 mL phosphate buffer (pH 6.8; 0.1 mol L−1), 1 mL pyrogallol (0.01 mol L−1) and 1 mL H2O2 (0.05 mol L−1)] was incubated at 25 °C for 5 min. The reaction was stopped by adding 1 mL of 2.5 mol L−1 H2SO4 and absorbance was measured at 420 nm against blank consisting of all the chemicals used to prepare samples except pyrogallol (Kumar and Khan 1983). Catalase (CAT) activity was assessed in enzyme extract mixed with 1 mL of H2O2 (0.01 mol L−1) and 3 mL of phosphate buffer (pH 6.8; 0.1 mol L−1). After incubation at 20 °C, 10 mL of H2SO4 (1%) was added and the mixture was titrated against 0.005 pH 6.8; 0.1 mol L−1 KMNO4 (Maehly 1954). Phenylanaline ammonia lyase (PAL) activity was estimated in a 2 mL of reaction mixture [0.4 mL of enzyme extract made up to 1.5 mL by addition of 0.1 mol L−1sodium borate buffer (pH 8.8) and 0.5 mL of 12 mol L−1 phenylalanine]. After the incubation for an hour at 25 °C, the reaction was stopped by incubation at 47 °C for 10 min. Conversion of L-phenylalanine to transcinnamic acid was measured at 290 nm (Dickerson et al. 1984). Polyphenol oxidase (PPO) activity was assessed by the method of Mayer et al. (1966) in mixture consisting of enzyme extract (0.1 mL), sodium phosphate buffer (1.5 mL, 0.1 mol L−1) and catechol (0.2 mL of 0.01 mol L−1) at 495 nm (30 s interval for 3 min).
Growth assays
Plant (length and weight) and bulb (weight and diameter) attributes were recorded after 50 days of pathogen inoculation. The dry weight of the root and shoot was recorded by drying them in the oven at 80 °C for 24 h.
Statistical analysis
Kolmogorov–Smirnov’ test (normality test) and Levene’s test (variance test) were applied before any statistical analysis to check the normality. The differences among means were compared with Tukey’s test at level P ≤ 0.05 through latest version by using software Statistic 8.1.
Results
In vitro growth response of FOC to saline solution
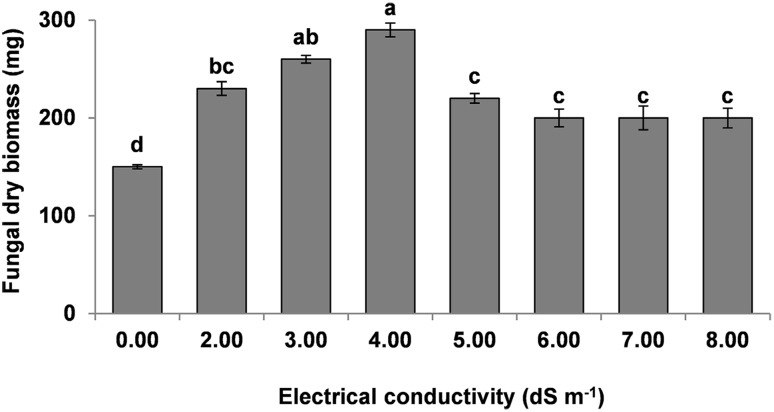
The fungus growth was significantly increased with increased EC levels (2–8 dS m−1) as compared to control. The biomass of FOC increased by 50–90 and 30–33% at EC levels of 2–4 and 5–8 dS m−1, respectively (Fig. 1).
Fig. 1.
Effect of different values of electrical conductivity on biomass of F. oxysporum f. sp. cepa (FOC) in 2% malt extract medium after 7 days of incubation. Values with different letters at their top show significant difference (P ≤ 0.05) as determined by Tukey’s HSD Test
In vivo pot assays
Disease and plant growth under stressed condition
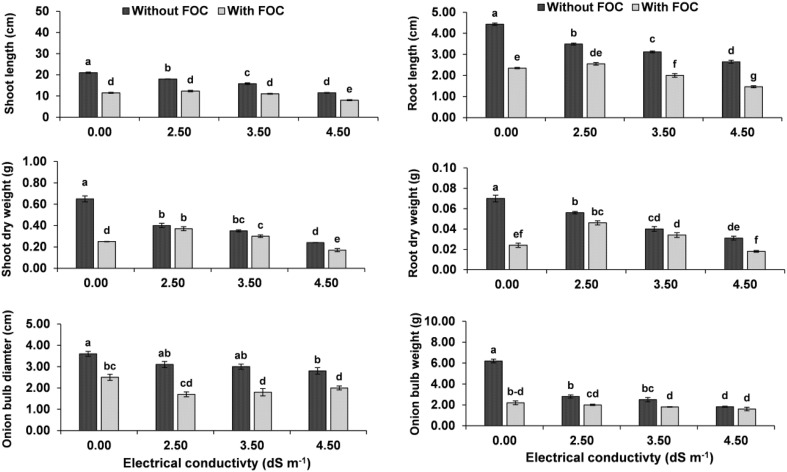
Inoculated plants in the positive control (T2) showed 48 and 26% disease incidence and mortality, respectively. The bulb was soft, irregular in the shape, discolored at the basal plate and showed significant (P ≤ 0.05) reduction of 50% in diameter and 60% in the weight as compared to the negative control (T1). Infected plants in T2 were categorized (DRS = 4) based on the disease rating scale. Length and biomass of the shoot were significantly (P ≤ 0.05) decreased by 42 and 54%, respectively and these attributes for root were significantly and more drastically decreased by 50 and 67%, respectively as compared to the negative control (Figs. 2, 3).
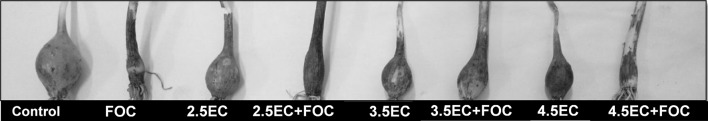
Fig. 2.
Effect of F. oxysporum f. sp. cepa (FOC) and different levels of salinity on bulb of A. cepa
Fig. 3.
a–f Effect of F. oxysporum f. sp. cepa (FOC) and different salinity levels on growth and biomass in A. cepa at 50th day of inoculation. Values with different letters show significant difference (P ≤ 0.05) as determined by Tukey’s HSD Test. Vertical bars show standard errors of means of three replicates
Increased soil salinity from 2.5 to 4.5 dS m−1 (T3–T5) significantly (P ≤ 0.05) decreased bulb diameter by 19–40% and weight by 50–70%. Affected bulbs were soft and dark colored. Shoot length and biomass decreased more significantly by 21–46 and 38–64% than the length and biomass of the root by 25–44 and 23–60% over the negative control (Figs. 2, 3).
Combined stress of FOC infection and salinity (T6–T8) significantly decreased plant biomass, increased the severity of disease and drastically deteriorated the bulb as compared to individual stress of FOC or salinity. The highest disease incidence (63%), plant mortality (50%) and disease severity (rotten bulbs 76 > 100%; rating scale: 5) were recorded due to simultaneous stress of FOC and salinity. The bulbs deformed and discolored at the basal region. The bulb diameter and weight were significantly (P ≤ 0.05) dropped to 50% and ~ 80%, respectively. Length and biomass of root were more drastically declined to 50–80% than those for the shoot 40–70% with increased salinity combined with FOC over the negative control (T1) (Figs. 2, 3).
Plant physiology and antioxidant enzymes under stressed condition
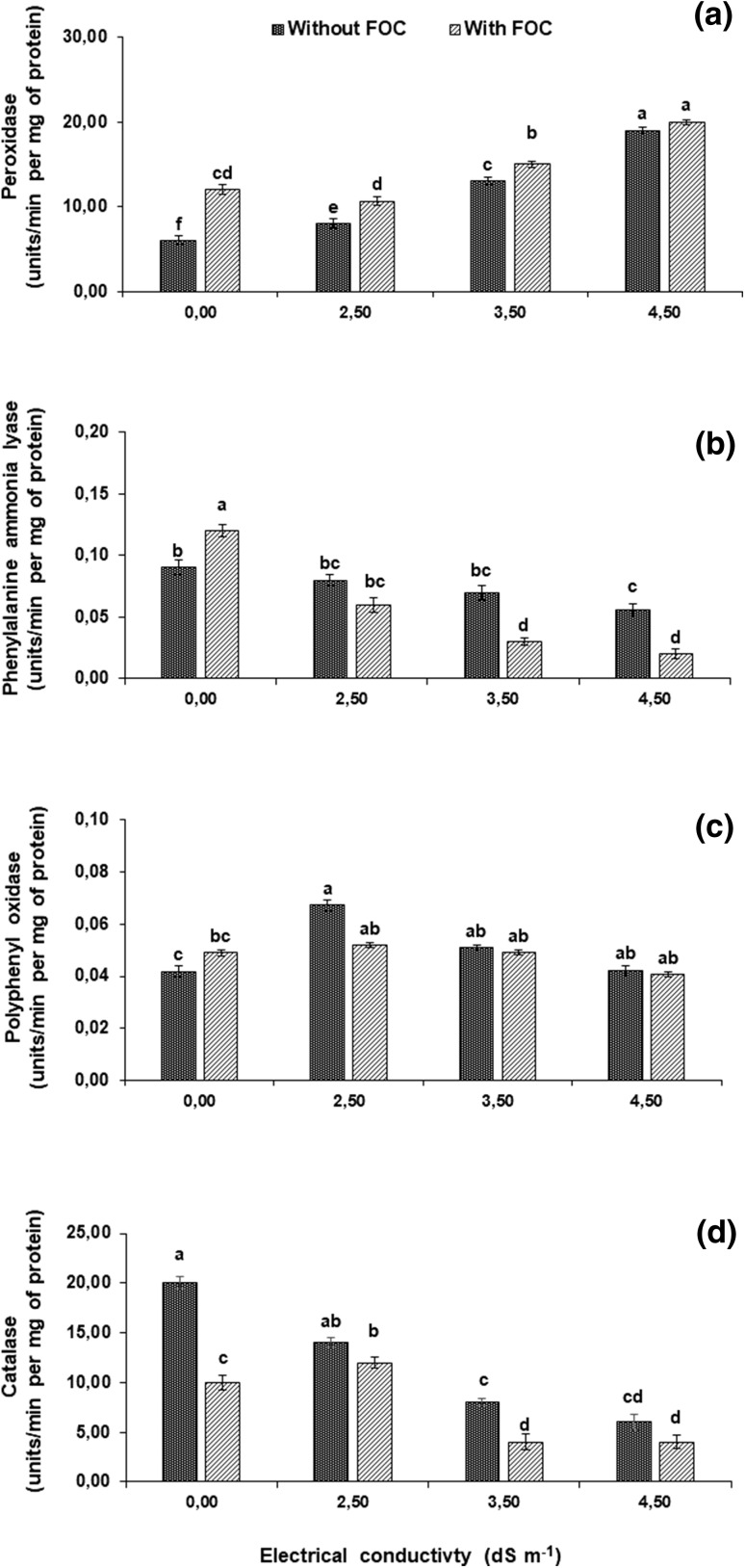
FOC (positive control) stress significantly decreased all of five physiological parameters (osmotic potential, total chlorophyll content, membrane stability index, total sugar content, and total protein content) of the leaf, while total phenolics increased considerably up to 40% over the negative control (Table 2). The activity of antioxidant enzymes like PPO was insignificantly affected, while that of POX and PAL were significantly increased by 100 and 70%, respectively, and CAT was decreased by 50% with respect to the negative control (Fig. 4).
Table 2.
Effect of F. oxysporum f. sp. cepa (FOC) and different salinity levels on the physiological attributes of onion at 35th day of inoculation
| # | Treatment | Osmotic potential (MPa) | Total chlorophyll content (mg/g) | Membrane stability index (%) | Total sugar content (mg/g) | Total phenolics (mg/g) | Total protein content (mg/g) |
|---|---|---|---|---|---|---|---|
| 1 | Negative control | − 0.97 a | 0.95 a | 87 a | 1.34 d | 3 d | 1.79 a |
| 2 | Positive control (FOC) | − 0.78 bc | 0.78 b | 71 b | 0.93 d | 4 c | 0.89 c |
| 3 | 2.5 dS m−1 | − 0.95 ab | 0.89 a | 61c | 1.43 d | 6.5 a | 1.47 b |
| 4 | 3.5 dS m−1 | − 0.79 bc | 0.84 b | 53 d | 2.27 c | 6 ab | 0.90 c |
| 5 | 4.5 dS m−1 | − 0.56 d | 0.82 b | 40 e | 2.40 c | 5.5 b | 0.63 d |
| 6 | FOC + 2.5 dS m−1 | − 1.05 a | 0.78 b | 52 d | 5.16 a | 6.4 a | 1.59 b |
| 7 | FOC + 3.5 dS m−1 | − 0.79 bc | 0.76 b | 47 d | 4.56 a | 5.1 b | 0.90 c |
| 8 | FOC + 4.5 dS m−1 | − 0.76 bc | 0.75 b | 46 de | 3.07 b | 4.5 c | 0.72 d |
Values with different letters show significant difference (P ≤ 0.05) as determined by Tukey’s HSD Test
Fig. 4.
a–d Effect of F. oxysporum f. sp. cepa (FOC) and different salinity levels on activities of antioxidant enzymes in A. cepa leaf at 35th day of inoculation. Values with different letters show significant difference (P ≤ 0.05) as determined by Tukey’s HSD Test. Vertical bars show standard errors of means of three replicates
Salinity stress (2.5–4.5 dS m−1) individually as well as in combination with FOC showed the similar trends of increase/decrease in various physiological and biochemical attributes. Therefore, osmotic potential, membrane stability index, total chlorophyll content and total protein content were significantly decreased by 20–40, 30–50, 13–20 and 10–60% in response to either salinity levels (2.5–4.5 dS m−1) or the combined stress factors. Salinity stress significantly improved the total sugar content and total phenolics up to twofolds and combined stress increased the said attributes up to two–threefolds. This increase became less pronounced with increasing soil salinity (2.5–4.5 dS m−1) (Table 2). The activities of POX and PPO were increased, while those for PAL and CAT declined as compared to individual as well as simultaneous stress. The activity of POX increased more intensively and progressively by 76–232% below the combined FOC stress and salinity level (2.5–4.5 dS m−1) and by 32–213% when salinity levels applied separately. Improvement in PPO activity gradually decreased from 61–37% and 38–24% with increased salinity levels and its combination with FOC, respectively. Reduction in PAL activity was significantly increased from 20–40% and 30–80% and in CAT activity from 30–70%, and 40–80% with increased in salinity levels and when given along with FOC stress, respectively (Fig. 4).
Discussion
Laboratory experiment
Laboratory trials conducted to assess the endurance of FOC at different levels of EC indicated that fungal biomass was increased by 50–90% at EC range of 2–4 dS m−1 and then by 33% with further increase in EC from 5 to 8 dS m−1 as compared to controls. Regragui and Lahlou (2005) and Boumaaza et al. (2015) also documented the stimulatory effects of NaCl up to 150 ppm on the mycelial growth, conidia production and conidia germination in Verticillium dahliae and Botrytis cinerea. They further reported inhibition in fungal growth attributes with further increase in salt concentration. On the contrary, Reid et al. (2001) reported potential use of NaCl to suppress F. oxysporum f. sp. asparagi and F. proliferatum responsible for Fusarium crown and root rot disease. Two primary mechanisms have been proposed for microbe’s endurance against high concentrations of soluble salts, (1) osmotic effect and (2) specific ion effects. Microorganisms adapt to salinity stress by accumulating organic (proline and glycine betaine) and inorganic (potassium cations) osmolytes (Sagot et al. 2010). However, synthesis of organic osmolytes requires high amounts of energy (Wichern et al. 2006) that can result in reduced growth and activity. Therefore, the increase in fungal biomass at EC range of 2–4 dS m−1 might show selective accumulation of solute (e.g., Na+, Cl−) necessary for metabolism (e.g. NH4+) by counteracting increase in osmotic pressure. While EC 5–8 dS m−1 probably resulted in increased concentration of solute with an increase in osmotic potential (more negative) of medium, which can draw water out of cells and can affect microbes through plasmolysis (Boumaaza et al. 2015).
Pot experiment
Effect of FOC stress
Fusarium basal rot is the second most important soil borne disease of the onion that has caused up to 60% economic losses to the onion farmers globally (Cramer 2000). In the present work, the FOC infection significantly decreased (30–70%) plant growth and biomass and caused 48% disease incidence and 26% mortality. Infected plants were chlorotic, stunted and wilted leave base showed the watery appearance, the stem plate discolored and the bulbs were rotted (Sasaki et al. 2015). Different physiological parameters (osmotic potential, total chlorophyll content, membrane stability index, total sugar content, and total protein content) of the infected host showed decline. Fusarium has been known to cause wilting in plants by systematic production of toxin mainly Fuasric acid that tends to decrease the stem hydraulic conductance and leaf water potential. Both changes not only induce membrane injury, but also cause water leakage from damaged cell (Van Alfen and Turner 1975). Wang et al. (2015) noticed that reduction in net photosynthesis lead to stomatal closure and disturbance in metabolic pathways of photosynthesis with a reduction in plant sugar accumulation and biomass. Kaushik and Roychoudhury (2014) stated over production of ROS in infected plant affected physiological and developmental aspects by increasing damage to membrane (lipid peroxidation), proteins, carbohydrate, nucleic acids, and pigments with decreased seed viability, root growth and increased leaf abscission. Disturbance in the plant physiology results in successful establishment of pathogen inside the host and weakening of the plant defense system. Therefore, although total phenolics were increased, but it seemed that its level was not enough to provide resistance against the invading pathogen. Antioxidant enzyme activity like CAT was significantly decreased, PPO was insignificantly increased, and that of POX and PAL were significantly increased. Reduced CAT activity may show over production and accumulation of H2O2 and enhanced proteolysis caused by peroxisomal endopeptidases induced by oxidative stress (Palma et al. 2002). Increased POX and PAL activities did not represent quick defense response against pathogens. These observations contribute to support that biochemical defense programming shown by the host against FOC was altered as a response to damage, but not as quick defense resulting in compatible host–pathogen interaction.
Effect of salinity stress
Increase in soil salinity (2.5, 3.5 and 4.5 dS m−1) caused 21–88% reduction in the plant length and biomass. The bulbs diameter and weight were drastically decreased by 19–40 and 50–70%, respectively and the affected bulbs became soft and turned dark colored. Inhibition in growth is the negative consequence of salinity on carbohydrate level and growth hormones (Mazher et al. 2007). Reduction in plant biomass under salinity stress might be associated with many events, including the absence of turgor maintenance, toxicity by sodium/chloride ions and disturbances in metabolic pathways (Shahid et al. 2012). Shoot length and biomass were however, more drastically affected than these attributes for the root which might be ascribed to reallocation of photosynthate into the root instead of the shoot under stress (Amirjani 2011). Disturbance in osmotic balance was further clear in the present study as increasing salinity significantly decreased the osmotic potential of the onion plant. This change was considered as one of the defensive means by which plants endure the harmful effects of salt accumulation inside the cell by increasing ability to absorb water (Cachorro et al. 1995). The reduction in osmotic potential was decreased with the increase in salinity stress, and the effects became insignificant at 4.5 dS m−1. It could be assumed that at low levels of salinity (2.5 dS m−1) the onion plant maintained its osmotic potential at levels below that of the osmotic potential of soil surrounding the plant (Zhu 2001). The presence of plasma membrane-compatible solutes (sugar and amino acids) could provide a link between capacity for osmotic adjustment and degree of membrane protection from the effects of dehydration. Hence, in the current findings, the least reduction in the membrane stability index was noticed at 2.5 dS m−1, while the membrane damage increased with increase in salinity levels (3.5 and 4.5 dS m−1) signifying inability of the onion plant to maintain membrane integrity under salt stress (Shahid et al. 2012; Roussos et al. 2013).
Cellular dehydration due to ionic imbalance and toxicity therefore negatively affected the total chlorophyll content of the onion plant possibly through inhibition in the synthesis of pigment (Youssef and Awad 2008). Many studies have shown decline in the content of photosynthetic pigments in rice, wheat and pea due to salinity stress (Shahid et al. 2012). Sugar, the final product of photosynthesis was significantly raised in the onion leaf in case of reduction in demand of carbon which may cause increase in glucose pool induced by salt stress in the leaves. Therefore, the reduction of photosynthesis and metabolic alteration by sugar accumulation could be attributed to salt sensitivity in the onion plants.
Salinity treatment caused a massive accumulation of total phenolics in the leaves at 2.5 dS m−1 as compared to two salinity levels. Total phenolics may act as an antioxidant agent that may help to protect protein integrity (Szabados and Savouré 2010) of the onion plants at low levels of salinity. Likewise, total protein content decreased at 3.5 and 4.5 dS m−1, indicative of stress conditions in the plants. Elimination of ROS is mainly achieved by POX, PPO, CAT and PAL, and in the present study, these enzymes undergo a differential modulation (increasing or decreasing) upon salt stress. The activities of PPO and POX increased, while that for CAT and PAL decreased drastically with increased soil salinity levels. Increase in POX might be attributed to increase in the activities cytosolic Cu/Zn-SOD and chloroplastic Cu/Zn-SOD as reported by Farag (2009). In addition, this increase might be linked with salinity endurance ability of the onion, where peroxidase increased constitutively (Mittova et al. 2004). CAT activity decreased might be the consequences of increasing H2O2 content, disturbance in redox balance with deactivation of catalase due to prevention of new enzyme synthesis at high salinity stress (Yamane et al., 2010). Increase in PPO activity with increase in salinity was not enough to oxidize and degrade the toxic substance such as phenolic compounds that were generally accumulated during salt stress (Weisany et al. 2012). PAL activity decreased that may result in high content of some phenolic components such as cinamic acid, which impedes flavonoid biosynthesis and phenylalanine ammonia lyase activity (Rice-Evans et al. 1996). These results suggested that the activation and the maintenance of POX, PAL, PPO and CAT activities under salinity are the most important factors against salt-induced oxidative stress.
Effect of combined stress of Fusarium infection and salinity
Combined stress of FOC + salinity increased disease severity with the highest disease incidence (63%), plant mortality (50%) and severely deteriorated bulb morphology (disease rating scale: 6) causing the highest reduction in its diameter and weight (50% and ~ 80%, respectively) as compared to individual stress of salinity or FOL. Most reduction in root growth attributes than the shoot could be attributed to increase in pathogen susceptibility through weakening of the plant defenses under abiotic stress of salinity (Mittler and Blumwald 2010). FOL sporulation and mycelial growth were motivated under salt stress conditions (Türkkan and Erper 2013) and it was also clear in our laboratory experiments. Likewise, salinity stress has been shown to increase the incidence and severity of Fusarium wilt of cotton, Fusarium crown and root rot in tomato and charcoal rot (Macrophomina phaseolina) of the bean (You et al. 2011). Under the combined stress of salinity and FOC infection, a general trend of increase/decrease in the investigated physiological and enzyme parameters were same as were observed with individual stress of FOC infection or salinity. Growth responses were correlated with accumulation of sugar and total phenolics, as these three attributes were decreased with increase in salinity, signifying the loss in a cellular osmotic adjustment under these stress conditions. Oxidative damage of cellular component could be attributed to enhance the production of POX and PPO, while decreasing production of CAT and PAL. These results suggested that the activation of antioxidant enzymes under salinity is quite variable in the onion plants and increase in POX could be a component of pivotal importance in this salt–induced onion antioxidative system.
Conclusions
Combined stress of FOC and salinity was more damaging for onion growth and yield than the individual stresses. The damage on the plants was increased with increase in soil salinity. Salinity stress imbalanced plant physiology through the weakening plants and encouraged pathogen virulence, resulting in more severe disease. This revealed the importance of salinity stress where FOC is a serious pathogen.
Acknowledgements
Authors are thankful to the University of the Punjab for providing funds to accomplish this research work.
References
- Amirjani MR. Effect of salinity stress on growth, sugar content, pigments and enzyme activity of rice. Int J Bot. 2011;7:73–81. doi: 10.3923/ijb.2011.73.81. [DOI] [Google Scholar]
- Bartoli CG, Casalongué CA, Simontacchi M, Marquez-Garcia B, Foyer CH. Interactions between hormone and redox signaling pathways in the control of growth and cross-tolerance to stress. Environ Exp Bot. 2013;94:73–88. doi: 10.1016/j.envexpbot.2012.05.003. [DOI] [Google Scholar]
- Boumaaza B, Benkhelifa M, Belkhoudja M. Effects of two salts compounds on mycelial growth, sporulation, and spore germination of six isolates of Botrytis cinerea in the Western North of Algeria. Int J microbiol. 2015;2015:1. doi: 10.1155/2015/572626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyoucos GJ. Hydrometer method improved for making particle size analysis of soil. Agron J. 1962;54:464–465. doi: 10.2134/agronj1962.00021962005400050028x. [DOI] [Google Scholar]
- Cachorro P, Martinez R, Ortiz A, Cerda A. Abscisic acid and osmotic relations in (Phaseolus vulgaris L.) under saline conditions. Plant Sci. 1995;95:29–32. [Google Scholar]
- Capell B, Dörffling K. Genotype-specific differences in chilling tolerance of maize in relation to chilling-induced changes in water status and abscisic acid accumulation. Physiol Plant. 1993;88:638–646. doi: 10.1111/j.1399-3054.1993.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Coşkuntuna A, Özer N. Biological control of onion basal rot disease using Trichoderma harzianum and induction of antifungal compounds in onion set following seed treatment. Crop Prot. 2008;27:330–336. doi: 10.1016/j.cropro.2007.06.002. [DOI] [Google Scholar]
- Cramer CS. Breeding and genetics of fusarium basal rot resistance in onion. Euphytica. 2000;115:159–166. doi: 10.1023/A:1004071907642. [DOI] [Google Scholar]
- Dickerson DP, Pascholati SF, Hagerman AE, Butler LG, Nicholson RL. Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol Plant Pathol. 1984;25:111–123. doi: 10.1016/0048-4059(84)90050-X. [DOI] [Google Scholar]
- FAO (2013) The Food and Agriculture Organization. FAO Global Aquaculture Production Volume and Value Statistics Database Updated to 2012
- Farag AA (2009) Increasing tolerance of Vigna sinensis L. to salt stress using an organic acid and a polyamine. M.Sc. thesis. Ain Shams University, Cairo, Eygpt
- Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays. 2006;28:1091–1101. doi: 10.1002/bies.20493. [DOI] [PubMed] [Google Scholar]
- Gholizadeh A, Kohnehrouz BB. Activation of phenylalanine ammonia lyase as a key component of the antioxidative system of salt-challenged maize leaves. Braz J Plant Physiol. 2010;22:217–223. doi: 10.1590/S1677-04202010000400001. [DOI] [Google Scholar]
- Giuffrida F, Scuderi D, Giurato R, Leonardi C. Physiological response of broccoli and cauliflower as affected by NaCl salinity. Acta Hortic. 2013;1005:435–441. doi: 10.17660/ActaHortic.2013.1005.52. [DOI] [Google Scholar]
- Kaushik D, Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci. 2014 doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- Kumar KB, Khan PA. Age-related changes in catalase and peroxidase activities in the excised leaves of Eleusine coracana Gaertn. cv PR 202 during senescence. Exp Gerontol. 1983;18:409–417. doi: 10.1016/0531-5565(83)90019-0. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H, Wellburn A. Determinations of total carotenoids and chlorophylls b of leaf extracts in different solvents. Biochem Soc Trans. 1983;11:591–592. doi: 10.1042/bst0110591. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Machado RM, Serralheiro RP. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae. 2017;3:30. doi: 10.3390/horticulturae3020030. [DOI] [Google Scholar]
- Maehly AC. The assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Mahboob W, Khan MA, Shirazi MU. Induction of salt tolerance in wheat (Triticum aestivum L.) seedlings through exogenous application of proline. Pak J Bot. 2016;48:861–8677. [Google Scholar]
- Makhloufi E, Yousfi FE, Marande W, Mila I, Hanana M, Bergs H, Mzid R, Bouzayen M. Isolation and molecular characterization of ERF1, an ethylene response factor gene from durum wheat (Triticum turgidum L. subsp. durum), potentially involved in salt-stress responses. J Exp Bot. 2014;65:6359–6371. doi: 10.1093/jxb/eru352. [DOI] [PubMed] [Google Scholar]
- Mayer AM, Harel E, Ben-Shaul R. Assay of catechol oxidase—a critical comparison of methods. Phytochemistry. 1966;5:783–789. doi: 10.1016/S0031-9422(00)83660-2. [DOI] [Google Scholar]
- Mazher AMA, El-Quesni EMF, Farahat MM. Responses of ornamental and woody trees to salinity. World J Agric Sci. 2007;3:386–395. [Google Scholar]
- McLean EO. Soil pH and lime requirement. In: Page AL, editor. Methods of soil analysis, part 2. Chemical and microbiological properties. Madison: American Society of Agronomy, Soil Science Society of America Journal; 1982. pp. 199–223. [Google Scholar]
- Mittler R, Blumwald E. Genetic engineering for modern agriculture: challenges and perspectives. Ann Rev Plant Biol. 2010;61:443–462. doi: 10.1146/annurev-arplant-042809-112116. [DOI] [PubMed] [Google Scholar]
- Mittova V, Guy M, Tal M, Volokita M. Salinity upregulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J Exp Bot. 2004;55:1105–1113. doi: 10.1093/jxb/erh113. [DOI] [PubMed] [Google Scholar]
- Nelson N. A photometric adaptation of Somogyi method for the determination of glucose. J Biol Chem. 1944;3:375–380. [Google Scholar]
- Palma JM, Sandalio LM, Javier CF, Romero-Puertas MC, McCarthy I, Del Río LA. Plant proteases, protein degradation, and oxidative stress: role of peroxisomes. Plant Physiol Biochem. 2002;40:521–530. doi: 10.1016/S0981-9428(02)01404-3. [DOI] [Google Scholar]
- Pandey P, Irulappan V, Bagavathiannan MV, Senthil-Kumar M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front Plant Sci. 2017;8:537. doi: 10.3389/fpls.2017.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang HC, Li YY, Yang JS, Liang YS. Effect of brackish water irrigation and straw mulching on soil salinity and crop yields under monsoonal climatic conditions. Agric Water Manag. 2010;97:1971–1977. doi: 10.1016/j.agwat.2009.08.020. [DOI] [Google Scholar]
- Regragui A, Lahlou H. Effect of salinity on in vitro Trichoderma harzianum antagonism against Verticillium dahliae. Pak J Biol Sci. 2005;8:872–876. doi: 10.3923/pjbs.2005.872.876. [DOI] [Google Scholar]
- Reid TC, Hausbeck MK, Kizilkaya K. Effects of sodium chloride on commercial asparagus and of alternative forms of chloride salt on Fusarium crown and root rot. Plant Dis. 2001;85:1271–1275. doi: 10.1094/PDIS.2001.85.12.1271. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Richards L (1954) Diagnosis and improvement of saline and alkali soils. Agriculture Handbook No 60 United States
- Roussos P, Gasparatos D, Kyriakou C, Tsichli K, Tsantili E, Haidouti C. Growth, nutrient status and biochemical changes in sour orange (Citrus aurantium L.) plants subjected to sodium chloride stress. Cοmmun Soil Sci Plant Anal. 2013;44:805–816. doi: 10.1080/00103624.2013.749438. [DOI] [Google Scholar]
- Sagot B, Gaysinski M, Mehiri M, Guigonis JM, Le Rudulier D, Alloing G. Osmotically induced synthesis of the dipeptide N-acetylglutaminylglutamine amide is mediated by a new pathway conserved among bacteria. Proc Natl Acad Sci. 2010;107:12652–12657. doi: 10.1073/pnas.1003063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneoka H, Moghaieb REA, Premachandra GS, Fujita K. Nitrogen nutrition and water stress effects on cell membrane stability and leaf water relations in Agrostis palustris Huds. Environ Exp Bot. 2004;52:131–138. doi: 10.1016/j.envexpbot.2004.01.011. [DOI] [Google Scholar]
- Sasaki K, Nakahara K, Tanaka S, Shigyo M, Ito S. Genetic and pathogenic variability of Fusarium oxysporum f. sp. cepae Isolated from onion and welsh onion in Japan. Phytopathology. 2015;105:525–532. doi: 10.1094/PHYTO-06-14-0164-R. [DOI] [PubMed] [Google Scholar]
- Saxena A, Cramer CS. Screening of onion seedlings for resistance against New Mexico isolates of Fusarium oxysporum f. sp. cepae. J Plant Pathol. 2009;91:199–202. [Google Scholar]
- Seckin B, Sekmen AH, Türkan I. An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. J Plant Growth Regul. 2009;28:12–20. doi: 10.1007/s00344-008-9068-1. [DOI] [Google Scholar]
- Shahid MA, Balal RM, Pervez MA, Abbas T, Ashfaq M, Ghazanfar U, Afzal M, Rashid A, Garcia-Sanchez F, Mattson NS. Differential response of pea (Pisum sativum L.) genotypes to salt stress in relation to the growth, physiological attributes antioxidant activity and organic solutes. Aust J Crop Sci. 2012;6:828–838. [Google Scholar]
- Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Türkkan M, Erper I. Evaluation of antifungal activity of sodium salts against. Plant Protect Sci. 2013;50:19–25. doi: 10.17221/9/2013-PPS. [DOI] [Google Scholar]
- Van Alfen NK, Turner NC. Influence of a Ceratocystis ulmi toxin on water relations of Elm (Ulmus americana) Plant Physiol. 1975;55:312–316. doi: 10.1104/pp.55.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley A. A critical examination of a rapid method for determining organic carbon in soils—effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947;63:251–264. doi: 10.1097/00010694-194704000-00001. [DOI] [Google Scholar]
- Wang M, Sun Y, Sun G, Liu X, Zhai L, Shen Q, Guo S. Water balance altered in cucumber plants infected with Fusarium oxysporum f. sp. cucumerinum. Sci Rep. 2015;5:1–7. doi: 10.1038/srep07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisany W, Sohrabi Y, Heidari G, Siosemardeh A, Ghassemi-Golezani K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.) Plant Omics J. 2012;5:60–67. [Google Scholar]
- Wichern J, Wichern F, Joergensen RG. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma. 2006;137:100–108. doi: 10.1016/j.geoderma.2006.08.001. [DOI] [Google Scholar]
- Yamane K, Mitsuya S, Taniguchi M, Miyake H. Transcription profiles of genes encoding catalase and ascorbate peroxidase in the rice leaf tissues under salinity. Plant Prod Sci. 2010;13:164–168. doi: 10.1626/pps.13.164. [DOI] [Google Scholar]
- You MP, Colmer TD, Barbetti MJ. Salinity drives host reaction in Phaseolus vulgaris (common bean) to Macrophomina phaseolina. Funct Plant Biol. 2011;38:984–992. doi: 10.1071/FP11137. [DOI] [PubMed] [Google Scholar]
- Youssef T, Awad MA. Mechanisms of enhancing photosynthetic gas exchange in date palm seedlings (Phoenix dactylifera L.) under salinity stress by a 5-aminolevulinic acid based fertilizer. J Plant Growth Regul. 2008;27:1–9. doi: 10.1007/s00344-007-9025-4. [DOI] [Google Scholar]
- Zhu JK. Plant salt tolerant. Trends plant Sci. 2001;6:66–71. doi: 10.1016/S1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]