Abstract
In this study, the effect of cadmium (Cd) uptake and concentration on some growth and biochemical responses were investigated in Malva parviflora under Cd treatments including 0, 10, 50 and 100 µM. The shoots and roots were able to accumulate Cd. However, increased Cd dose led to a considerable Cd content in the roots. Cd stress decreased growth, increased lipid peroxidation and also enhanced proline and ascorbic acid contents in both shoots and roots. Chlorophyll and carotenoid contents decreased in the plants with the increasing Cd concentration. While the activities of catalase (CAT) and superoxide dismutase (SOD) increased in the shoots under different Cd doses, these activities decreased in the roots as compared to the control. Both shoots and roots demonstrated a significant increase in guaiacol peroxidase activity in response to Cd stress. Contrary to the aboveground parts, the roots subjected to Cd doses showed a rise in protein content. Despite higher Cd content in the roots, it seems that CAT and SOD do not play a key role in detoxification of Cd-induced oxidative stress. These findings confirm that reduced biomass and growth under Cd stress can be due to an increase in oxidative stress and a decrease in photosynthetic pigment content. The present study clearly indicates that the shoots and roots exploit different tolerance behaviors to alleviate Cd-induced oxidative stress in M. parviflora.
Keywords: Ascorbate, Heavy metal, Photosynthetic pigments, Proline, Oxidative stress
Introduction
Cadmium (Cd) is a highly toxic metal due to its reactivity with S and N atoms of biomolecules. In addition, this metal is not known as an essential element for normal growth and metabolism in plants. Symptoms such as the reduction of biomass, chlorosis, and the inhibition of shoot and root elongation have been attributed to Cd phytotoxicity (Milone et al. 2003; Vecchia et al. 2005; Gill et al. 2012; Dong et al. 2016). The negative effect of Cd on the plant functions is exerted through its impact on respiration, photosynthesis, and water and nutrient uptake (Tran and Popova 2013). Cd, as one of the most important pollutants, is primarily released into various environments by metal smelting industries, cement factories, heating systems and the application of phosphate fertilizers. This metal is finally bioaccumulated in the food chains and can threaten the health of consumers (Sun et al. 2009). Although Cd is not an essential element, it can easily be absorbed by the roots and transferred to the shoots. According to the recommendation of the World Health Organization (2007), permissible limit of Cd content is 0.3 mg kg−1 dry weight (DW) of the plant for pharmaceutical and food applications. However, hyperaccumulators can accumulate Cd above 100 mg kg−1 DW of the shoots without any signs of phytotoxicity (Baker et al. 1994). Some plant species such as Thlaspi caerulescens (Baker et al. 1994), Arabis paniculata (Qiu et al. 2008) and Ricinus communis (Zhang et al. 2015) have been introduced as Cd hyperaccumulators. As potent biological tools, these plants can be considered for remediating soils contaminated by Cd.
Cd exposure induces reactive oxygen species (ROS) production and oxidative stress in plants. Oxidative stress has been found to disrupt the structure and function of lipids, proteins, nucleic acids, and pigments (Gill et al. 2012). Increased peroxidation of polyunsaturated fatty acids leads to an interruption in the function of cell membranes (Gill et al. 2012). Depending on the plant species and Cd concentration, plants employ a range of enzymatic antioxidants including Superoxide dismutase (SOD, EC1.15.1.1), Catalase (CAT, EC1.11.1.6), peroxidases (POXs, EC1.11.1.7) or non-enzymatic antioxidants such as ascorbic acid (AsA), carotenoids, glutathione (GSH), etc. In this way, plants can minimize the toxic effects of Cd on the growth and function of cells, and control the cellular redox status (Tran and Popova 2013). SOD is responsible for dismuting superoxide (O2˙−) anion to hydrogen peroxide (H2O2) and O2 under heavy metal stress. H2O2-reducing enzymes including CAT and POX have a significant role in eliminating H2O2. These enzymes can transfer electron to H2O2 and then produce H2O and O2. POXs exploit a variety of substrates, as electron donors, to reduce H2O2. For instance, POXs in the vacuoles and apoplastic spaces fundamentally use substrate guaiacol and are therefore called GPXs (EC1.11.1.7). It has been reported that Cd induces lipid peroxidation and leads to an increase in activities of antioxidant enzymes such as CAT, SOD and POX in some plants (Gill et al. 2012; Wu et al. 2015; Rui et al. 2016). Free proline content and antioxidant activity have been found to enhance under Cd stress in some plants (Yılmaz and Parlak 2011; Zouari et al. 2016). The studies suggest that proline accumulation under Cd stress leads to improvement in the function of antioxidant system and scavenge hydroxyl radicals by the enzymes. Furthermore, it is supposed that proline not only inhibits lipid peroxidation but it also maintains osmotic balance in cells exposed to heavy metals (Xu et al. 2009). Ascorbic acid, as an electron donor, is able to scavenge H2O2 by function of ascorbate peroxidase in the ascorbate–glutathione cycle; moreover, it can directly detoxify a range of ROS (Jouili et al. 2011). In some plants, Cd-induced oxidative stress has been found to increase ascorbic acid pool (Mohamed et al. 2012; Deng et al. 2017).
Ahvaz, as the center of Khouzestan province in the southwest of Iran, is an industrial city with a hot and arid climate and an average annual temperature of 25 °C. This city is located between 49°11′ east longitude and 31°50′ north latitude, typically in close proximity to regions with a tropical and subtropical climate. This area tends to have hot or extremely hot summers and warm to cool winters. Malva parviflora L. (Malvaceae) is an annual or perennial herb. This species widely grows in the area surrounding Khouzestan Steel Company in Ahvaz (Zoufan et al. 2015), where the industrial activities release metal particles into the environment. Moreover, this plant is important in traditional medicine and food habits of local people. A field survey showed that Cd accumulates in roots of M. parviflora grown in industrial area of Khouzestan Steel Company (Zoufan et al. 2017). This accumulation can threaten the health of people in this area. The main objective of the present study was to investigate the relationship between tissue Cd content and tolerance mechanism used by M. parviflora against Cd stress. Therefore, this study was conducted to evaluate the level of oxidative stress, Cd concentration of the tissue, and the performance and efficiency of some enzymatic and non-enzymatic antioxidants in Malva parviflora when the metabolism is affected by Cd stress. The results will probably be consequential to assess some tolerance strategies against Cd stress in M. parviflora grown in the area surrounding Khouzestan Steel Company.
Materials and methods
Plant materials, growth conditions and Cd treatments
The seeds of M. parviflora L. were collected from the industrial area of Khouzestan Steel Company, located in Ahvaz, Iran, surface-sterilized with 20% (v/v) sodium hypochlorite, and finally washed three times with distilled water. The seeds were germinated in the pots filled with commercial Peat Moss soil and maintained in plant growth room under 8/16 h light/dark photoperiod and 25/20 °C light/dark temperatures. The average light intensity was adjusted to 150 µmol photons m−2 s−1 using fluorescent lamps. The plants were irrigated three times a week with tap water. Five weeks after germination (5-leaf stage), the plants were transferred to nutrient solutions with 1/10 strength modified Johnson’s formulation (Siddiqi et al. 1990) for acclimation. The composition of essential elements in the liquid medium was as follows (in µM): H3BO3 2.5, MnSO4.H2O 0.2, ZnSO4.7H2O 0.2, CuSO4. 5H2O 0.05, Na2MoO4 0.05, Fe-EDTA 2.0, Ca (NO3)2.4H2O 1000, CaSO4.2H2O 400, KH2PO4 200, K2SO4 400. The pH of nutrient solution was adjusted to 6.0 ± 0.3 using 0.1 N HCl or KOH. Aquarium pumps were used to aerate the medium. The 42-day-old plants (7-leaf stage) were transferred to vessels containing 10 L of complete nutrient solution with 0 (control), 10, 50 and 100 µM Cd(NO3)2.4H2O. Three 10 L vessels, as 3 repetitions, were used for each experimental treatment. This means that a total of 12 vessels were prepared. Each vessel included 36 plants established on floating plastic plates. After being supplemented with different Cd concentrations, the plants were harvested on the tenth day (T10). Nutrient solutions were renewed every 3 days. Plant length and fresh weight (FW) were measured at harvest time. All 36 plants in each container were used to measure the length and fresh weight for Cd treatments. The plants were immediately separated into the roots and shoots. A part of the plants was transferred to − 80° C immediately to measure some biochemical parameters of the shoots and roots. The other part was oven dried to assay the amount of Cd.
Determination of Cd concentration
The roots were incubated in 0.1 M EDTA- Na2 solution for 10 min, and then thoroughly rinsed in distilled water to remove Cd adhered to external surfaces. Shoot and root samples were oven-dried at 72 °C for 48 h. The acid digestion method was chosen in consultation with the laboratories of chemistry and soil sciences. Heavy metal extraction was performed as previously explained by Zoufan et al. (2015) using the method of Kovács et al. (1996). A 1 g of dried and ground sample was extracted using 10 mL of 65% HNO3 and 1 mL of 30% H2O2 at 85–120 °C on an electric heating plate. The digests were filtered with Whatman paper (No. 42) and then the volume was adjusted to 50 mL with deionized water. Cd analysis of the plant samples was carried out using Flame Atomic Absorption Spectrometer (GBC, Avanta model, Australia) compared to Cd standard solutions. The efficiency of the acid digestion method was not determined. Therefore, Cd content in the roots and shoots was reported as an estimate.
Assay of pigment content
Fresh leaves (0.5 g) were extracted in 20 mL of 80% (v/v) acetone under dark and ice-cold conditions by the method of Lichtenthaler (1987). Subsequently, the samples were filtered by Whatman paper (No. 1). Chl a, Chl b, total chlorophyll, and carotenoid contents were assayed at 470, 663.2 and 646.8 nm using a UV–Visible spectrophotometer (Optision 2120UV PLUS, Korea).
Assay of thiobarbituric acid reactive substances (TBARS), proline and ascorbic acid contents
TBARS content, as a parameter of lipid peroxidation, was measured using thiobarbituric acid (TBA) reaction as described by Heath and Packer (1968). After homogenizing, 0.5 g of fresh root/leaf in 5 mL of 0.1% (w/v) trichloroacetic acid (TCA), the mixture was centrifuged at 10,000×g for 15 min. The supernatant (1 mL) was mixed with 4 mL of 20% (w/v) TCA containing 0.5% (w/v) thiobarbituric acid (TBA) and heated at 95 °C for 30 min. Then, the samples were immediately transferred on an ice bath. Extracted samples were centrifuged at 10,000×g for 10 min. The supernatant was used to read the absorbance at 532 and 600 nm. The content of TBARS was determined by an extinction coefficient (155 mM−1 cm−1).
To quantify free proline content, fresh root/leaf (0.5 g) was mixed and ground in 10 mL of 3% (w/v) sulfosalisylic acid on the ice bath as described by Bates (1973). The homogenate was centrifuged at 10,000×g for 10 min at 4 °C. A mixture of 2 mL of ninhydrin and 2 mL of glacial acetic acid were added to the supernatant. The samples were transferred to a water bath for 1 h at 100° C; then were rapidly incubated in the ice bath, and then combined with 4 mL of toluene. After shaking for 1 min, the mixture subsequently was kept constant to form two phases. The supernatant including toluene and proline was used to measure the absorbance at 520 nm using proline standards.
Ascorbic acid was assayed according to the method reported by Mukherjee and Choudhuri (1983). Fresh leaf/root (0.5 g) was homogenized in 10 mL of 5% (w/v) metaphosphoric acid, and then centrifuged at 10,000×g for 10 min. To assay total ascorbate (AsA + dehydroascorbate; DHA), the supernatant was mixed with 1 mL of deionized water. Moreover, 0.5 mL of 3 M 2, 6-dichloroindophenol sodium solution was added to 1 mL of the supernatant to measure DHA. All the samples were mixed with 1 mL of 1% (w/v) thiourea solution. After 20 min, 1 mL of 10 mM 2, 4-dinitrophenylhydrazine was added to the mixtures. The samples were heated at 50 °C for 1 h and then quickly cooled in an ice bath for 20 min. Under the ice bath, 2.5 mL of 85% (v/v) H2SO4 and 1 mL of 20% (v/v) H2SO4 were slowly added to the mixtures. Finally, the absorbance of the samples was measured spectrophotometrically at 520 nm. The contents of total AsA, DHA, and reduced AsA were determined using L-ascorbic acid standard curve.
Assay of enzyme activity
In order to extract soluble proteins in accordance with Qiu et al. (2008), 5 mL of the extraction buffer containing 100 mM potassium phosphate (PSB, pH 7.0), 0.1 mM EDTA, and 1% (w/v) polyvinyl pyrrolidone was added to fresh root/leaf (0.5 g) to homogenize using a pre-chilled mortar and pestle under ice-cold conditions. After centrifugation (15,000×g for 15 min) at 4 °C, the supernatant was used to assay soluble proteins and enzyme activities as mg g−1 FW and unit (U) mg−1 protein, respectively. The content of soluble proteins was analyzed according to Bradford (1976) using standard solutions of Bovine serum albumin.
CAT activity was determined as described by Aebi (1984) and analyzed by the consumption of H2O2 at 240 nm for 2 min. The reaction was started by adding 80 µl of protein extract to 920 µL of the reaction mixture, which contained 50 mM PSB (pH 7.8), deionized water, and 100 mM H2O2. SOD activity was measured following the procedure of Beauchamp and Fridovich (1971) and analyzed by its ability to inhibit photochemical reduction of nitroblue tetrazolium (NBT) at 560 nm. The reaction mixture (3 mL) was provided as 50 mM PSB (pH 7.8), 130 mM methionine, 750 µM NBT, 0.1 mM EDTA-Na2, deionized water, 20 µM riboflavin, and 100 µL of the enzyme extract. This mixture was illuminated for 15 min, and then assayed spectrophotometerically at 560 nm. One unit of SOD activity was considered as the amount of enzyme inhibiting 50% reduction of NBT under light conditions compared with a dark control. GPX activity was assayed using the method of Hemeda and Klein (1990) and determined with guaiacol oxidation by H2O2 at 470 nm within 2 min. The reaction mixture, with a total volume of 1.5 mL, contained 50 mM PSB (pH 6.0), 2% (v/v) H2O2, 50 mM guaiacol, and 70 µL of the protein extract.
Statistical analysis
All the assessments related to Cd treatments were replicated three times. The analysis of data sets was carried out using SPSS software (ver. 16). One-way analysis of variance (ANOVA) was followed by Duncan’s Multiple Range Test to determine significant differences at P < 0.05 confidence level.
Results
Cd concentration, growth response and pigment content
The content of Cd absorbed by the shoots and roots showed a significant increase (P < 0.05) with increasing Cd doses in the solution as compared to the control (Table 1). For 50 µM Cd treatment, shoot Cd concentration had not significant differences (P < 0.05) as compared to the plants exposed to 10 µM Cd, whereas the root Cd content was 84% (P < 0.05) higher than roots treated with 10 µM Cd concentration. For all the treatments, Cd concentration in the roots was always higher (P < 0.05) than that in the shoots. Compared to the aboveground parts, the root Cd concentrations were 41, 90 and 90.4% higher under the 10, 50 and 100 µM treatments, respectively. Growth parameters such as length and biomass of M. parviflora plants were considerably affected as the Cd concentration increased in the nutrient solution (Table 2). Compared to the control, Cd treatments led to a significant decline (P < 0.05) in the shoot and root length and fresh weight. The 100 µM Cd, with the highest inhibitory effect, considerably reduced the shoot and root length with the values of 58% and 58.6% as compared to the control group. As shown in Table 2, compared to the control, fresh weight in shoots and roots significantly (P < 0.05) decreased under 100 µM Cd by 59% and 63%, respectively. According to Table 3, under Cd stress, Chl a, Chl b, and the total chlorophyll content displayed a significant reduction (P < 0.05) compared with the control leaves. Compared with the control, Chl a and Chl b contents decreased by 34–35%, and 36–54% under Cd treatments, respectively. The assessment of Chl a amounts revealed no significant difference among the plants exposed to various Cd doses (10–100 µM). The Chl a/b ratio showed a significant increase (P < 0.05) in comparison to the control due to a sharp drop in Chl b content. Increased Cd concentration in the solution reduced total Chl content by 35–39% compared to the control. Cd treatments (10–100 μM) resulted in a significant reduction in the carotenoids contents with the values of 40, 37 and 30% as compared to the control, respectively (Table 3). The application of 100 µM Cd increased (P < 0.05) carotenoids contents nearly by 10–14% compared with the 10 and 50 µM Cd treatments.
Table 1.
Cd concentrations (mg g−1 DW) in shoots and roots of M. parviflora treated with 0 (control), 10, 50 and 100 µM Cd for 10 days
| Cd treatment (µM) | Cd concentration | |
|---|---|---|
| Shoot | Root | |
| 0 | 0.00c | 0.00d |
| 10 | 0.095 ± 0.006b | 0.16 ± 0.006c |
| 50 | 0.10 ± 0.014b | 1.01 ± 0.01b |
| 100 | 0.13 ± 0.004a | 1.35 ± 0.03a |
Data represent mean ± SD of three replicates; different letters indicate significant differences at P < 0.05
Table 2.
Length (cm) and fresh weight (g per plant) of shoots and roots in M. parviflora treated with 0 (control), 10, 50 and 100 µM Cd for 10 days
| Cd treatment (µM) | Length | Fresh weight | ||
|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |
| 0 | 11.1 ± 0.53a | 14.0 ± 0.96a | 1.13 ± 0.03a | 0.27 ± 0.01a |
| 10 | 6.40 ± 0.30b | 8.50 ± 0.66b | 0.63 ± 0.04b | 0.14 ± 0.00b |
| 50 | 5.60 ± 0.26c | 7.00 ± 0.92bc | 0.58 ± 0.02b | 0.12 ± 0.00c |
| 100 | 4.67 ± 0.35d | 5.80 ± 0.35c | 0.46 ± 0.01c | 0.10 ± 0.00d |
Data represent mean ± SD of three replicates and each replicate includes 36 plants; different letters indicate significant differences at P < 0.05
Table 3.
Leaf pigment contents (mg g−1 FW) in M. parviflora treated with 0 (control), 10, 50 and 100 µM Cd for 10 days
| Cd treatment (µM) | Chl a | Chl b | Chl a/b | Total Chl | Carotenoids |
|---|---|---|---|---|---|
| 0 | 1.05 ± 0.006a | 0.39 ± 0.02a | 2.68 ± 0.11c | 1.44 ± 0.02a | 0.30 ± 0.007a |
| 10 | 0.69 ± 0.03b | 0.25 ± 0.02b | 2.97 ± 0.17b | 0.94 ± 0.02b | 0.18 ± 0.005c |
| 50 | 0.69 ± 0.04b | 0.19 ± 0.005c | 3.45 ± 0.09a | 0.88 ± 0.03c | 0.19 ± 0.007c |
| 100 | 0.68 ± 0.03b | 0.18 ± 0.005c | 3.64 ± 0.2a | 0.88 ± 0.01c | 0.21 ± 0.003b |
Data represent mean ± SD of three replicates; different letters indicate significant differences at P < 0.05
TBARS, proline, and ascorbic acid contents
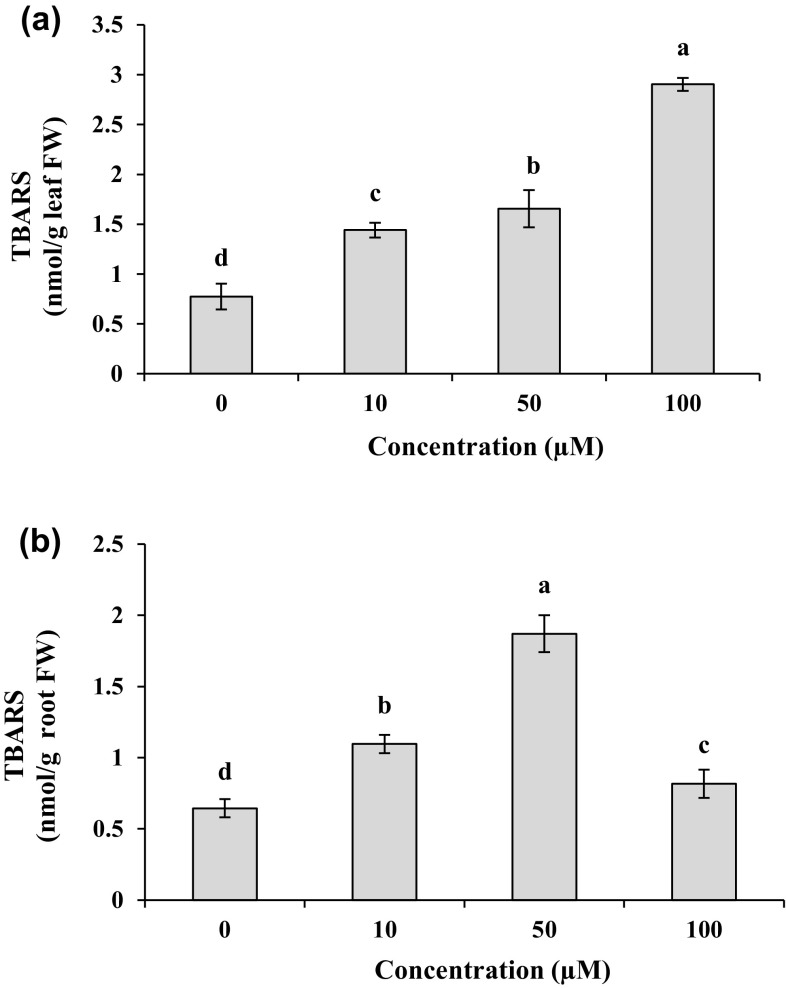
Increased Cd concentration significantly (P < 0.05) enhanced TBARS contents in both leaves and roots as compared to the control plants (Fig. 1a, b). For different Cd treatments, TBARS contents in the shoots were 1.8–3.7 folds higher than those in control plants. Under the treatments of Cd (≤ 50 µM), the root TBARS contents showed an increase by 1.7–3.0 folds compared to their control (Fig. 1b). Moreover, 100 µM Cd exhibited the lowest additive effect on the lipid peroxidation level in the roots with the value 1.3 folds higher than that in the control.
Fig. 1.
TBARS contents in leaves (a) and roots (b) of M. parviflora treated with 0 (control), 10, 50 and 100 µM Cd for 10 days. Values represent mean ± SD of three replicates. Different letters indicate significant differences at P < 0.05
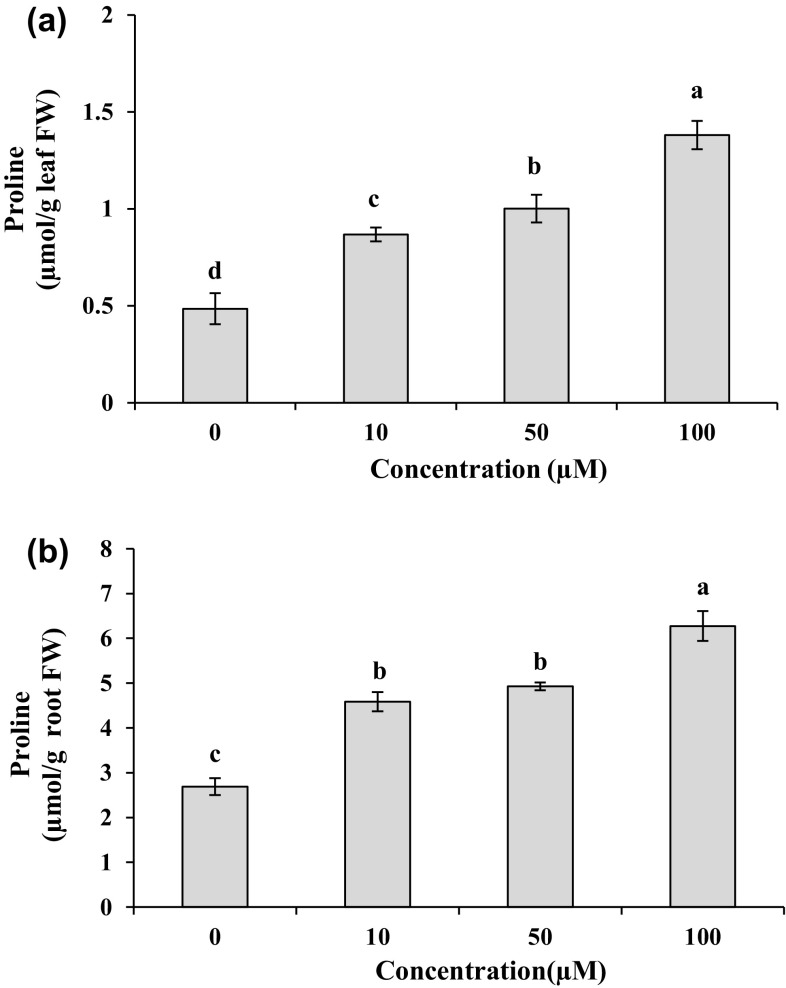
As shown in Fig. 2a, b, the leaf and root proline content was significantly (P < 0.05) enhanced with increasing Cd concentration in the medium. The plants treated with 100 µM Cd displayed the greatest proline content in the leaves and roots with the levels of 2.8 and 2.3 folds compared to the controls, respectively. For the 10 and 50 µM Cd treatments, the proline contents increased 1.8 and 2.0 folds in the leaves and 1.7 and 1.8 folds in the roots compared to the controls. Compared to the leaves, higher levels of proline were assayed in the roots with the values of 81, 80 and 78% in response to 10, 50 and 100 µM Cd, respectively. The content of ascorbic acid pool in the leaves and roots is presented in Fig. 3. Compared to the control, total AsA contents increased (P < 0.05) 1.2–1.6 folds in leaves and 1.3–1.7 folds in roots under different Cd treatments. These treatments enhanced significantly (P < 0.05) the DHA contents with the values of 1.3–1.8 folds in leaves and 1.2–1.6 folds in roots as compared to their control. The 50 µM Cd was found to accumulate the highest total AsA and DHA contents, with the values 1.6 and 1.8 folds in leaves and 1.7 and 1.6 folds in roots as compared to the control. Concentration of 100 µM Cd had a negligible stimulatory effect on reduced AsA in the leaves as compared to the control (Fig. 3c). Contrary to shoot, the treatment of root with 100 μM Cd led to a marked increase (2.2 folds) in reduced AsA contents compared to the control (Fig. 3f). The ascorbic acid pool contents in the leaves were higher than those in the roots in most cases (Fig. 3).
Fig. 2.
Proline contents in leaves (a) and roots (b) of M. parviflora treated with 0 (control), 10, 50 and 100 µM Cd for 10 days. Values represent mean ± SD of three replicates. Different letters indicate significant differences at P < 0.05
Fig. 3.
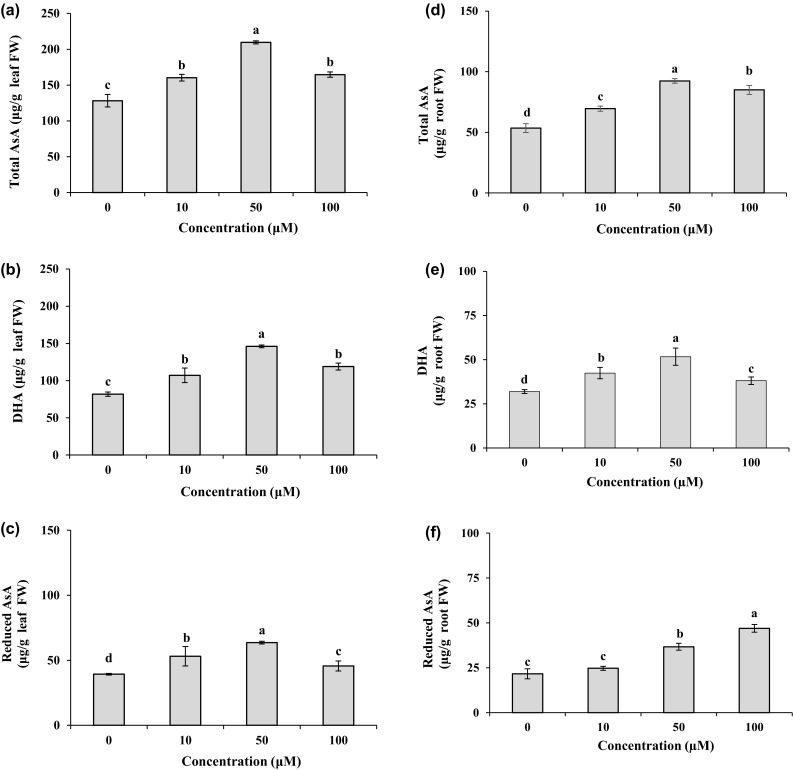
Total AsA, DHA, and reduced AsA contents in leaves (a, b, c) and roots (d, e, f) of M. parviflora treated with 0 (control), 10, 50 and 100 µM Cd for 10 days. Values represent mean ± SD of three replicates. Different letters indicate significant differences at P < 0.05
Antioxidant enzymes activity
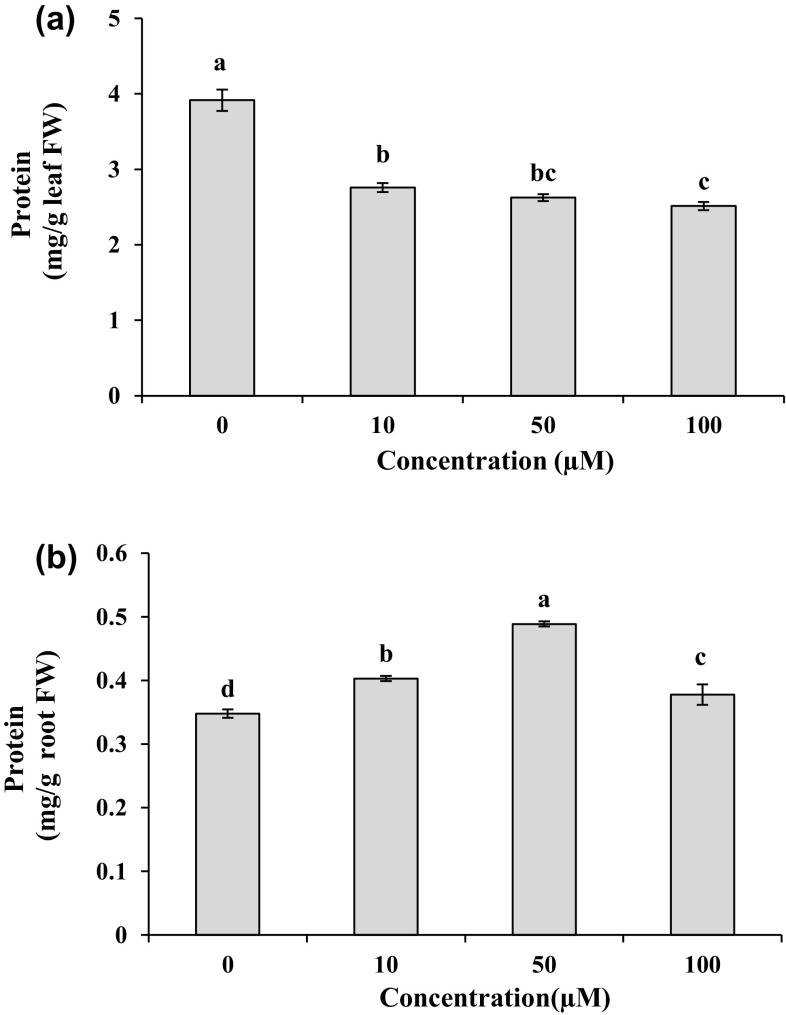
On the tenth day, the leaf protein content showed a tendency to decrease under different Cd treatments by 30–36% in comparison to the control (Fig. 4a). Under Cd treatments, protein contents exhibited a significant elevation (P < 0.05) in the roots with the levels of 1.1–1.4 folds higher than those in the control, and the maximum value was recorded at 50 µM Cd concentration (Fig. 4b). In the plants exposed to Cd, leaf CAT activity was significantly (P < 0.05) higher with the levels of 1.2–1.7 folds as compared to their respective controls (Fig. 5a). On the contrary, according to Fig. 5d, with increasing Cd dose in the medium, CAT activity in the roots significantly (P < 0.05) declined and was about 66–80% of the CAT activity in the control, while the minimum activity was found in the roots treated with 50 µM Cd for 10 days. For leaves, Cd treatments significantly (P < 0.05) increased SOD activity with the values of 13–17 folds higher than the control (Fig. 5b). There was a significant reduction (P < 0.05) in SOD activity by 11–33% in the roots exposed to different Cd concentrations as compared to the control group (Fig. 5e). The lowest SOD activity in the roots was detected at 50 µM Cd. The treatments of Cd markedly (P < 0.05) enhanced GPX activity as 6.0–10.3 folds in leaves and 7.1–16 folds in roots compared to untreated plants (Fig. 5c, f).
Fig. 4.
Protein contents in leaves (a) and roots (b) of M. parviflora treated with 0 (control), 10, 50 and 100 µM Cd for 10 days. Values represent mean ± SD of three replicates. Different letters indicate significant differences at P < 0.05
Fig. 5.
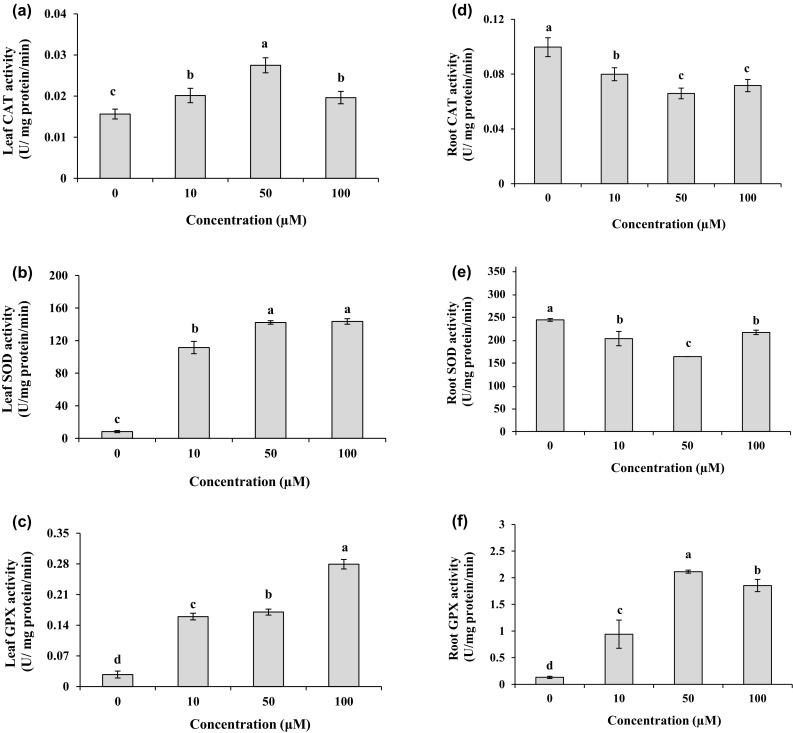
CAT, SOD, and GPX activities in leaves (a, b, c) and roots (d, e, f) of M. parviflora treated with 0 (control), 10, 50 and 100 µM Cd for 10 days. Values represent mean ± SD of three replicates. Different letters indicate significant differences at P < 0.05
Discussion
Malva parviflora was chosen due to its application in traditional medicine and food habits of the people in Khouzestan province, also due to its wide distribution in the area. It is well known that the therapeutic effects of Malva are mainly as the result of the presence of antioxidants, especially phenolic compounds (Gasparetto et al. 2011). Therefore, it was expected that the antioxidant mechanisms of M. parviflora would have a positive effect on the detoxification of Cd stress. In the roots, Cd content was approximately 1.5–10 folds higher than that in the shoots (Table 1). Thus, it seems that Cd absorbed by this plant is mainly maintained in the roots and a lower amount is translocated to the shoots. Plants are able to accumulate heavy metals according to physiological capacity. Contrary to our findings, in M. sinesis, as a Cd-accumulator, the aboveground parts showed a more ability to accumulate Cd compared to the roots (Zhang et al. 2010). Lower Cd root-to-shoot translocation is well known as a protective mechanism to tolerate Cd stress by non-hyperaccumulator plants and confirmed by many studies (for example, Sun et al. 2007; Zhang et al. 2015; Rui et al. 2016). To alleviate Cd toxicity, some plants restrict Cd root-to-shoot translocation and this tactic is supported by metal accumulation in the root apoplastic spaces, complex formation with organic compounds, and retention in the vacuoles of root cells (Hossain et al. 2012). Hence, the results of this experiment are partly indicative of the ability of M. parviflora to avoid Cd-caused stress through less metal translocation to the shoots.
The results of present study showed that increased Cd uptake led to a reduced growth in roots and shoots (Table 2), while TBARS content increased with increasing Cd doses from 10 to 100 µM (Fig. 1). The growth is used as one of the most important indicators to evaluate Cd toxicity in plants (Zhang et al. 2015). The inhibitory effect of Cd on growth has been reported by other researchers (for example, Sun et al. 2007; Gill et al. 2012; Dong et al. 2016; Marzban et al. 2017). Cd-induced growth inhibition is attributed to production of excess ROS, destruction of ultrastructure in chloroplasts (Chen et al. 2010; Wu et al. 2015) and reduction of net photosynthesis rate (Gill et al. 2012) as well as the deficiency of nutrient and the reduction of turgor pressure (Irfan et al. 2014). Cd has been reported to enhance membrane lipid peroxidation in many plants (for example, Sun et al. 2009; Mohamed et al. 2012; Tauqeer et al. 2016). Although Cd is not considered as a redox-active metal, it indirectly causes the generation of ROS by inhibiting electron transfer chains in chloroplast and mitochondria, and ultimately induces oxidative stress (Tran and Popova 2013). Extra ROS generation is considered as a key factor in inhibition of growth that can promote lipid peroxidation of biomembranes in response to Cd. (Chen et al. 2010; Wu et al. 2015). Increased electrolyte leakage in M. parviflora exposed to Cd (unpublished data) confirmed an increase in the lipid peroxidation of membrane that probably disturbs the absorption of essential elements and reduces the growth. The oxidative stress caused by Cd can be determined by TBARS content. Therefore, based on the present results, it is supposed that growth reduction caused by Cd in M. parviflora may be mainly due to an increase in lipid peroxidation and cell TBARS content (Fig. 1) as well as a decrease in photosynthetic pigments (Table 3).
In this study, the levels of chlorophylls and carotenoids decreased after treating with different Cd concentrations (Table 3). Increased Chl a/b ratio in M. parviflora treated with Cd could be due to a higher reduction in Chl b content than in Chl a. Under Cd stress, higher Chl a/b ratio has been attributed to changes in pigment composition as well as a decline in light harvesting chlorophyll proteins to acclimatize with Cd-induced oxidative stress (Gill et al. 2012). Chlorosis and reduced chlorophyll content are one of the primary symptoms in plants when they are exposed to Cd-contained mediums (Gill et al. 2012; Dong et al. 2016). In Capsicum annuum, it has been supposed that a decrease in chlorophyll content is due to reduced Mg uptake under Cd stress (Abdel Latef 2013). Increased enzymatic degradation, disturbance in electron transport of PSII and destruction of thylakoid membranes have been found as some reasons for the reduction of chlorophyll contents (Boonyapookana et al. 2002). Chloroplast membranes, as rich sources of polyunsaturated fatty acids, are one of the most important targets for peroxidation by Cd-induced ROS (Hattab et al. 2009). In agreement with our study, Cd treatments have been reported to reduce carotenoids contents (Hattab et al. 2009; Mohamed et al. 2012). Carotenoids, as non-enzymatic antioxidants, are known to protect photosynthetic apparatus and chlorophylls against the damage caused by photooxidation, and also prevent lipid peroxidation against stress conditions including heavy metal contamination (Ünyayar et al. 2005). Hence, an increase in TBARS content (Fig. 1) can be a main reason to reduce the contents of carotenoids and chlorophylls in M. parviflora treated with Cd doses. Additionally, according to the protective role of carotenoids, the results of this experiment suggest that a decrease in carotenoids contents under Cd stress may lead to ROS accumulation, biomembranes destruction such as chloroplast membrane, and finally oxidative degradation of chlorophylls.
As previously reported, heavy metals-induced oxidative stress is mainly due to ROS overproduction or reduced capacity of antioxidative responses (Hattab et al. 2009). To understand the effect of proline and ascorbate contents on modification of oxidative stress caused by Cd, these biochemical parameters were measured in M. parviflora. Our study showed an obvious increase in proline content of plants treated with different Cd concentrations (Fig. 2). Additionally, the roots exposed to Cd displayed a more significant rise in proline content in comparison to the leaves. In plants, de novo synthesis of proline has been mentioned as a common adaptive mechanism to alleviate the negative effects of abiotic stresses (Zouari et al. 2016). In agreement with our work, increased proline content was observed in response to Cd exposure in Arachis hypogaea (Dinakar et al. 2008), Groenlandia densa (Yılmaz and Parlak 2011). It has been suggested that proline functions as osmolyte for water balance status (Zouari et al. 2016), metal chelator and possibly ROS scavenger (Yılmaz and Parlak 2011) under Cd stress, hence protecting the plant cells against oxidative stress damage. Our results revealed that as a result of increasing Cd concentration in the roots and leaves, both proline content and lipid peroxidation level showed a coordinated increase. Thus, this study suggests that proline plays an effective role in reducing oxidative stress caused by Cd. In roots, proline probably reduces Cd stress by protecting proteins (Fig. 4b).
The role of ascorbate in the ascorbate–glutathione cycle for H2O2 reduction (Jouili et al. 2011) is indicative of its notable contribution in non-enzymatic antioxidant systems to modulate adverse conditions. Cd was found to increase ascorbate content in some plants such as Brassica juncea (Mohamed et al. 2012) and Brassica napus (Wu et al. 2015). In the present study, the plants supplemented with Cd treatments exhibited a rise in AsA pool (Fig. 3), simultaneously with increasing TBARS content indicating an important involvement of this metabolite in both ROS removal mechanism and cellular redox homeostasis in leaves and roots.
Based on our findings, with increasing Cd concentration in the solution, the level of leave soluble proteins showed a noticeable decrease, whereas this parameter increased in the roots (Fig. 4). Dinakar et al. (2008) postulated that a decrease in soluble proteins in response to Cd treatment is possibly due to degradation of proteins. In Alternanthera bettzickiana, increased protein content has been attributed to adaptation of this plant to Cd stress (Tauqeer et al. 2016). A well-known role of proline in the plants is the protection and stabilization of protein structure under adverse conditions (Zouari et al. 2016). In roots, these results imply that an increase in the protein content might be, at least in part, due to higher proline levels compared to the leaves. This strategy possibly is used as an acclimatization mechanism in the roots to overcome Cd-induced oxidative stress.
It is noteworthy that the efficiency of enzymatic antioxidants is evaluated to determine more precisely the strategies of Cd tolerance in plants. The present study indicates that the roots and leaves have different performances in enzymatic antioxidants to overcome oxidative stress when they are exposed to Cd treatments. Compared to the control, a considerable increase was observed in CAT, SOD, and GPX activities in the leaves as a result of increasing Cd doses (Fig. 5a–c). In the roots, a decrease in CAT and SOD activity was associated with increased GPX activity in response to Cd treatments (Fig. 5d–f). Under Cd stress, the most common form of ROS is H2O2. On the other hand, as already reported, the coordinated activities of CAT and SOD showed a crucial contribution in modifying oxidative stress in Cd-hyperaccumulators (Sun et al. 2007). Based on the results of the current study, it should be noted that inhibited CAT and SOD activities in the roots may be responsible for increasing ROS, especially H2O2. Therefore, an increase in the level of GPX activity may be effective in removing H2O2 when CAT and SOD are less active in the roots. Various plant species and varieties present different defensive responses in tolerating heavy metal stress. The difference in antioxidant capacity can be dependent on the plant species, type of tissue and organ, content of Cd, and the duration of exposure to Cd (He et al. 2011). One study on Lepidium sativum illustrated that CAT and SOD, as antioxidant enzymes, play an important role in reducing the oxidative stress caused by Cd in the leaves (Gill et al. 2012). In the leaves of A. bettzickiana, increased CAT, SOD, and POX activity has been proposed as a part of the defense mechanism to cope with Cd-induced oxidative stress (Tauqeer et al. 2016) that was in agreement with our work. Higher SOD activity in the shoots can probably be the result of a further increase in O2˙− generation (Nahar et al. 2016) and also the de novo synthesis of enzyme (Yılmaz and Parlak 2011). Based on present study, increased CAT, GPX, and SOD activities in the leaves of M. parviflora following Cd treatments indicate effectiveness of enzymatic antioxidant system to protect presumably photosynthetic parts against oxidative stress. In agreement with our results, some studies showed that increased Cd concentration in the medium led to a decline in CAT activity as well as an increase in POX activity in roots (Gopal and Nautiyal, 2011; Mohamed et al. 2012). The control of H2O2 level in plant tissues has been attributed to the activity of CAT and POX (Rui et al. 2016). According to Rui et al. (2016), in the roots, higher participation of GPX in H2O2 scavenging was associated with increased wall lignifications which might contribute to diminish Cd stress. Based on these results, it is suggested that reduced CAT activity in the roots may be compensated by substantial GPX activity, and also the reduced activity of CAT and SOD enzymes can be explained by the fact that Cd concentrations accumulated in the roots are probably higher than the threshold level for the function of these enzymes. In kenaf, low Cd concentration enhanced SOD activity, while under high Cd concentration, this activity was inhibited (Deng et al. 2017). It is well known that Cd is a potent enzyme inhibitor, and that Cd-induced oxidative stress reduces the activity of CAT possibly through disabling the enzyme-linked heme group (Willekens et al. 1997). Totally, due to the increased phenolic compounds in the roots of M. parviflora under Cd stress (unpublished data), it is supposed that non-enzymatic antioxidants such as phenolics, ascorbate as well as higher contents of proline and protein may have a more significant role in removing ROS when the roots are treated with Cd. Therefore, these results imply that apart from enzymatic strategies, the other mechanisms may also be exploited by the roots of M. parviflora to reduce oxidative load in response to Cd stress. In addition, it is suggested that the shoots and roots approximately have an equivalent ability to reduce Cd-induced oxidative stress, but with different mechanisms.
Conclusions
In summary, the results of this study revealed that increased Cd concentration in the solution led to a decrease in growth and pigment content as well as an increase in lipid peroxidation of M. parviflora. As the Cd concentration increased in the nutrient solution, Cd content showed an increase in the roots and shoots. Though, the concentration of Cd in the roots was considerably higher than that in the aboveground parts. It seems that Cd doses have obvious toxic effects on biomembranes and induce oxidative stress in this species. Therefore, to diminish Cd-induced damages, it is supposed that this plant uses some defense mechanisms such as the accumulation of proline and ascorbic acid, and also the modification of CAT, GPX and SOD activities as enzymatic antioxidants. Considering these results, we suggest that higher Cd concentration in the roots is possibly responsible for differences in the behaviors exhibited by the leaves and roots to cope to Cd stress in M. parviflora. In aboveground parts, activities of CAT, GPX, and SOD, together with free proline and ascorbic acid, appear to play a fundamental role to mitigate oxidative stress following the addition of Cd. In the roots, the data provides the evidence that CAT and SOD may be less involved in detoxification of Cd-induced oxidative stress due to the inhibitory effect of high Cd content on the activity of these enzymes. Consequently, to remove H2O2 in the roots, GPX might present a greater performance. Based on these results, increased proline, ascorbic acid, and soluble proteins as well as Cd retention by the roots can be mentioned as more effective mechanisms to alleviate Cd toxic effects in the roots in comparison with enzymatic antioxidants. Further research should be done to obtain more detailed information about metabolic pathways that lead to Cd tolerance in M. parviflora.
Acknowledgements
This work was supported by the Research Affairs of Shahid Chamran University of Ahvaz (Grant No. 94/3/02/31579).
Abbreviations
- AsA
Ascorbate
- CAT
Catalase
- DHA
Dehydroascorbate
- DW
Dry weight
- FW
Fresh weight
- GPX
Guaiacol peroxidase
- H2O2
Hydrogen peroxide
- TBARS
Thiobarbituric acid reactive substances
- POX
Peroxidase
- PSB
Potassium phosphate buffer
- ROS
Reactive oxygen species
- O2˙−
Superoxide anion
- SOD
Superoxide dismutase
Authors’ contribution
PZ designed and conducted the research, analyzed the data and wrote the paper; RJ performed all the assessments related to growth and biochemical parameters; EN measured cadmium concentration of plant tissues; PH and SR helped as consultant to carry out measurement protocols.
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest.
References
- Abdel Latef AA. Growth and some physiological activities of pepper (Capsicum annuum L.) in response to cadmium stress and mycorrhizal symbiosis. J Agric Sci Technol. 2013;15:1437–1448. [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Baker AJM, Reeves RD, Hajar ASM. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J & C Presl (Brassicaceae) New Phytol. 1994;127:61–68. doi: 10.1111/j.1469-8137.1994.tb04259.x. [DOI] [PubMed] [Google Scholar]
- Bates S. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Boonyapookana B, Upatham ES, Kruatrachue M, Pokethitiyook P, Singhakaew S. Phytoaccumulation and phytotoxicity of cadmium and chromium in duckweed Wolffia globosa. Int J Phytoremediation. 2002;4:87–100. doi: 10.1080/15226510208500075. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen F, Wang F, Sun H, Cai Y, Mao W, Zhang G, Vincze E, Wu F. Genotype-dependent effect of exogenous nitric oxide on Cd-induced changes in antioxidative metabolism, ultrastructure, and photosynthetic performance in barley seedlings (Hordeum vulgare) J Plant Growth Regul. 2010;29:394–408. doi: 10.1007/s00344-010-9151-2. [DOI] [Google Scholar]
- Deng Y, Li D, Huang Y, Huang S. Physiological response to cadmium stress in kenaf (Hibiscus cannabinus L.) seedlings. Ind Crops Prod. 2017;107:453–457. doi: 10.1016/j.indcrop.2017.06.008. [DOI] [Google Scholar]
- Dinakar N, Nagajyothi PC, Suresh S, Udaykiran Y, Damodharam T. Phytotoxicity of cadmium on protein, proline and antioxidant enzyme activities in growing Arachis hypogaea L. seedlings. J Environ Sci. 2008;20:199–206. doi: 10.1016/S1001-0742(08)60032-7. [DOI] [PubMed] [Google Scholar]
- Dong Y, Chen W, Xu L, Kong J, Liu S. Nitric oxide can induce tolerance to oxidative stress of peanut seedling under cadmium toxicity. Plant Growth Regul. 2016;79:19–28. doi: 10.1007/s10725-015-0105-3. [DOI] [Google Scholar]
- Gasparetto JC, Martins CAF, Hayashi SS, Otuky MF, Pontarolo R. Ethnobotanical and scientific aspects of Malva sylvestris L.: a millennial herbal medicine. J Pharm Pharmacol. 2011;64(2):172–189. doi: 10.1111/j.2042-7158.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- Gill SS, Khan NA, Tuteja N. Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.) Plant Sci. 2012;182:112–120. doi: 10.1016/j.plantsci.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Gopal R, Nautiyal N. Phytotoxic effects of cadmium exposure and metal accumulation in sunflower. J Plant Nutr. 2011;34:1616–1624. doi: 10.1080/01904167.2011.592559. [DOI] [Google Scholar]
- Hattab S, Boutheina D, Chouba L, Kheder MB, Bousetta H. Photosynthesis and growth responses of pea Pisum sativum L. under heavy metals stress. J Environ Sci. 2009;21:1552–1556. doi: 10.1016/S1001-0742(08)62454-7. [DOI] [PubMed] [Google Scholar]
- He JL, Qin JJ, Long LY, Ma YL, Li H, Li K, Jiang XN, Liu TX, Polle A, Liang ZS, Luo ZB. Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus × canescens. Physiol Plant. 2011;143:50–63. doi: 10.1111/j.1399-3054.2011.01487.x. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts 1. Kinetics and stoichiometry of fatty acid peroxidatoin. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hemeda HM, Klein BP. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci. 1990;55:184–185. doi: 10.1111/j.1365-2621.1990.tb06048.x. [DOI] [Google Scholar]
- Hossain MA, Piyatida P, de Silva JAT, Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot. 2012;2012:1–37. doi: 10.1155/2012/872875. [DOI] [Google Scholar]
- Irfan M, Ahmad A, Hayat S. Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J Biol Sci. 2014;21:125–131. doi: 10.1016/j.sjbs.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouili H, Bouazizi H, El Ferjani E. Plant peroxidases: biomarkers of metallicstress. Acta Physiol Plant. 2011;33:2075–2082. doi: 10.1007/s11738-011-0780-2. [DOI] [Google Scholar]
- Kovács B, Gŷori Z, Prokisch J, Loch J, Dániel P. A study of plant sample preparation and inductively coupled plasma emission spectrometry parameters. Commun Soil Sci Plant Anal. 1996;27:1177–1198. doi: 10.1080/00103629609369625. [DOI] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- Marzban L, Akhzari D, Ariapour A, Mohammadparast B, Pessarakli M. Effects of cadmium stress on seedlings of various rangeland plant species (Avena fatua L., Lathyrus sativus L. and Lolium temulentum L.): growth, physiological traits and cadmium accumulation. J Plant Nutr. 2017;40:2127–2137. doi: 10.1080/01904167.2016.1269347. [DOI] [Google Scholar]
- Milone MT, Sgherri C, Clijsters H, Navari-Izzo F. Antioxidative responses of wheat treated with realistic concentration of cadmium. Environ Exp Bot. 2003;50:265–276. doi: 10.1016/S0098-8472(03)00037-6. [DOI] [Google Scholar]
- Mohamed AA, Castagna A, Ranieri A, di Toppi LS. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol Biochem. 2012;57:15–22. doi: 10.1016/j.plaphy.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant. 1983;58:166–170. doi: 10.1111/j.1399-3054.1983.tb04162.x. [DOI] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MdM, Rahman A, Suzuki T, Fujita M. Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf. 2016;126:245–255. doi: 10.1016/j.ecoenv.2015.12.026. [DOI] [PubMed] [Google Scholar]
- Qiu RL, Zhao X, Tang XZ, Yu FM, Hu PJ. Antioxidative response to Cd in a newly discovered cadmium hyperaccumulator, Arabis paniculata F. Chemosphere. 2008;74:6–12. doi: 10.1016/j.chemosphere.2008.09.069. [DOI] [PubMed] [Google Scholar]
- Rui H, Chen C, Zhang X, Shen Z, Zhang F. Cd-induced oxidative stress and lignification in the roots of two Vicia sativa L. varieties with different Cd tolerances. J Hazard Mater. 2016;301:304–313. doi: 10.1016/j.jhazmat.2015.08.052. [DOI] [PubMed] [Google Scholar]
- Siddiqi MY, Glass ADM, Ruth TJ, Rufty T. Studies of the uptake of nitrate in barley: I. Kinetics of 13NO3 influx. Plant Physiol. 1990;93:1426–1432. doi: 10.1104/pp.93.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun RL, Zhou QX, Sun FH, Jin CX. Antioxidative defense and proline/phytochelatin accumulation in a newly discovered Cd-hyperaccumulator, Solanum nigrum L. Environ Exp Bot. 2007;60:468–476. doi: 10.1016/j.envexpbot.2007.01.004. [DOI] [Google Scholar]
- Sun YB, Zhou QX, Wang L, Liu W. Cadmium tolerance and accumulation characteristics of Bidens pilosa L. as a potential Cd-hyperaccumulator. J Hazard Mater. 2009;161:808–814. doi: 10.1016/j.jhazmat.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Tauqeer HM, Ali S, Rizwan M, Ali Q, Saeed R, Ahmad UR, Farid M, Abbasi GH. Phytoremediation of heavy metals by Alternanthera bettzickiana: growth and physiological response. Ecotoxicol Environ Saf. 2016;126:138–146. doi: 10.1016/j.ecoenv.2015.12.031. [DOI] [PubMed] [Google Scholar]
- Tran TA, Popova LP. Functions and toxicity of cadmium in plants: recent advances and future prospects. Turk J Bot. 2013;37:1–13. [Google Scholar]
- Ünyayar S, Keles Y, Çekiç FÖ. The antioxidative response of two tomato species with different drought tolerances as a result of drought and cadmium stress combinations. Plant Soil Environ. 2005;51:57–64. doi: 10.17221/3556-PSE. [DOI] [Google Scholar]
- Vecchia FD, La Rocca N, Moro I, De Faveri S, Andreoli C, Rascio N. Morphogenetic, ultrastructural and physiological damages suffered by submerged leaves of Elodea Canadensis exposed to cadmium. Plant Sci. 2005;168:329–338. doi: 10.1016/j.plantsci.2004.07.025. [DOI] [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraunder M, Langebartels C, Van-Montagu M, Inzé D, Van-Camp W. Catalase is a sink for H2O2 and is indispensable for stress defense in C3 plants. EMBO J. 1997;16:4806–4816. doi: 10.1093/emboj/16.16.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues. Geneva: WHO Publishers; 2007. [Google Scholar]
- Wu Z, Zhao X, Sun X, Tan Q, Tang Y, Nie Z, Qu C, Chen Z, Hu C. Antioxidant enzyme systems and the ascorbate–glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere. 2015;138:526–536. doi: 10.1016/j.chemosphere.2015.06.080. [DOI] [PubMed] [Google Scholar]
- Xu J, Yin HX, Li X. Protective effects of proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Rep. 2009;28:325–333. doi: 10.1007/s00299-008-0643-5. [DOI] [PubMed] [Google Scholar]
- Yılmaz DD, Parlak KU. Changes in proline accumulation and antioxidative enzyme activities in Groenlandia densa under cadmium stress. Ecol Indic. 2011;11:417–423. doi: 10.1016/j.ecolind.2010.06.012. [DOI] [Google Scholar]
- Zhang S, Chen M, Li T, Xu X, Deng L. A newly found cadmium accumulator-Malva sinensis Cavan. J Hazard Mater. 2010;173:705–709. doi: 10.1016/j.jhazmat.2009.08.142. [DOI] [PubMed] [Google Scholar]
- Zhang H, Guo Q, Yang J, Shen J, Chen T, Zhu G, Chen H, Shao C. Sub cellular cadmium distribution and antioxidant enzymatic activities in the leaves of two castor (Ricinus communis L.) cultivars exhibit differences in Cd accumulation. Ecotoxicol Environ Saf. 2015;120:184–192. doi: 10.1016/j.ecoenv.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Zouari M, Ahmed CB, Elloumi N, Bellassoued K, Delmail D, Labrousse P, Abdallah FB, Rouina BB. Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. cv Chemlali exposed to cadmium stress. Ecotoxicol Environ Saf. 2016;128:195–205. doi: 10.1016/j.ecoenv.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Zoufan P, Saadatkhah A, Rstegarzadeh S. Amount of Mn and Zn in herbaceous plants growing on industrial area of steel production companies in southeast of Ahvaz, Iran. Prog Biol Sci. 2015;5:181–193. [Google Scholar]
- Zoufan P, Jalali R, Karimiafshar A, Motamedi H. Assessment of heavy metal accumulation and antibacterial activity of some medicinal herbs grown in an industrial area of steel production, Ahvaz. Iran J Pharm Sci. 2017;13:73–86. [Google Scholar]