Abstract
An improved micropropagation protocol has been developed for a cosmetically important, dye yielding crop, henna (Lawsonia inermis). Quality of henna product is governed by naphthoquinone based pigment lawsone, thus in vitro multiplication of superior healthy plant to achieve enhanced productivity in terms of dye content and biomass deserve due attention. In the present study, nodal explants collected from an elite plant screened on the basis of superiority in lawsone content was cultured on MS medium supplemented with 0.5 μM benzyl adenine (BA) gave significantly (p < 0.05) high number of shoots (24.33). The explants placed on MS medium augmented with 0.5 μM BA and 2-isopentenyladenine (2-iP) resulted in the formation of maximum number of shoots (43.67) and was elongated (12.57 cm) within 4 weeks of culture period. Enhanced axillary bud proliferation and production of mass number of micro shoots was achieved by the continuous subculture in MS medium containing 0.5 μM BA and 2-iP. In vitro raised micro shoots were dipped in 0.44 mM NAA for 5 min followed by planting in polyethylene pots containing a soil: vermiculite (1:1 v/v) mixture produced rooted plantlets (100%). Different auxin types and its concentrations had significant role rooting of L. inermis. Rooting response of various size shoots of L. inermis treated with 0.44 mM NAA showed 100% rooting in 4.1–5 cm size class shoots. After two months of potting, survived (95%) plants were successfully transferred to medicinal plant garden of the Department. The lawsone content of one-year-old micropropagated plants (23.04 mg/g dw) growing in normal environmental conditions and elite mother plant (22.84 mg/g dw) was almost similar. Through the present study, efficient cloning of superior germplasm of L. inermis was established.
Keywords: Axillary bud, Explant, Ex vitro rooting, Lawsonia inermis L., Micro-shoots, MS medium
Introduction
Lawsonia inermis L. (family Lythraceae), also commonly known as ‘henna’ is one of the cosmetically important, dye yielding plant cultivated in different parts of the world. Leaf powder derived from this small tree is widely used to decorate skin, hair, fingernails, leather, silk and wool since time immemorial (Nadkarni 1982; Anand et al. 1992; Hema et al. 2010; Singh et al. 2014). The plant is widely used in cosmetic and medicinal industries due to its leaf which contains an active dye (red orange pigment), lawsone (2-hydroxy-1,4 naphthoquinone). Lawsone being an important secondary metabolite gets accumulated in aerial part of the plant with highest amount of 1.0–1.4% in young leaf petiole. Physical conditions influence the dye properties and percentage of lawsone in henna (Bakkali et al. 1997). Quality of henna dye is ranges from orange, auburn to burgundy. Lawsone occurs in the form of a glycosidic precursor that proficiently split the glycosidic bond when applied onto skin (Al-Tufail et al. 1999; Cartwright-Jones 2006; Gallo et al. 2008). Currently, numerous henna based formulations of natural dyes are available in the market. Hence, one of the most important factors affecting the economic value of the plant is its lawsone content. In addition to naphthoquinone dye-lawsone, flavonoids, tannins, phenolic compounds, alkaloids, terpenoids, quinones, coumarins and xanthones are present (Chaudhary et al. 2010) in henna. Henna leaves, flowers, seeds, stem bark and roots were used in traditional medicine to treat a variety of ailments such as rheumatoid arthritis, headache, ulcer, diarrhoea, leprosy, fever, diabetes, cardiac disease and liver disorders (Ali et al. 2001 Okpekon et al. 2004; Kamaraj et al. 2012).
The plant has been reported to have analgesic, hypoglycemic, hepato-protective, immune stimulant, anti-inflammatory, antibacterial, antifungal, antiviral, antiparasitic, anti-trypanosomal, anti-dermatophytic, protein glycation inhibition, anti-sickling, antioxidant, anti-fertility, tuberculostatic, wound healing, anticomplimentary and anticancer activities (Alia et al. 1995; Handa et al. 1997; Ali and Grever 1998; Mikhaeil et al. 2004; Nayak et al. 2007; Sultana et al. 2009; Hsouna et al. 2011; Chaudhary et al. 2012). Henna is now considered as a valuable source of unique natural products for the development of medicines against various diseases and also for the development of industrial products.
An elite mother plant proven superiority in lawsone content was used as explant source in the present study. Superior and healthy plants can be used as starting point for genetic resource management (Siva et al. 2009). When propagules derived from superior individuals were raised, improvement in terms of productivity, yield and specific metabolite such as dye content can be realized. Emergence of an efficient micropropagation method is therefore a prerequisite to evolve large scale production of superior planting materials to raise commercial plantations of L. inermis.
Previous reports on micropropagation of L. inermis through the culture of excised apical and axillary shoot buds (Rout et al. 2001; Phirke et al. 2010; Ram and Shekhawat 2011; Paiker and Kandir 2011) are available. Due to increasing commercial value and market demand, there is a great need to develop an efficient, easy and consistent in vitro regeneration protocol for this commercially important cosmetic plant. In the present study, we sought to develop an effective micropropagation protocol using low levels of BA singly or in combination with 2-iP without an intervention of auxin in the multiplication medium. Over the previous reports, in the forwarded method increase in shoot number without any callus formation, by avoiding auxin and organic additives in the medium was achieved and in vitro produced microshoots were successfully rooted through direct potting method.
Materials and methods
Explant selection and sterilization
A high yielding, 25 year-old L. inermis tree (CPT 16; Location: 10°09.820″N North latitude and 77°43.301″E East longitude, altitude of 325 m) was selected as ‘elite’ after an extensive field exploration survey and evaluation among 20 candidate plus trees (CPTs) based on various yield attributes such as total dry leaf biomass, lawsone content and lawsone yield of the plant (Table 1). The branch cuttings (15–20 cm, 15 nos.) were excised from elite plant (CPT 16) and rooted in the Department nursery beds. After 2 years of planting, these clonal plants were served as the source of explants. The fresh shoots of current season’s (May–June) growth were collected and brought to laboratory. The nodal segments (1–1.5 cm) of 4th or 5th position, counting from the tip portion were excised and were washed in running tap water for 30 min and soaked in 1% polysorbitol detergent (Labolene, Mfg. Fischer Scientific Chemicals, Mumbai, India) for 35 min and then treated in 0.1% (w/v) carbendazim fungicide (Bavistin, Mfg. BASF, Mumbai, India) for 40 min. Further processing was done in a sterile laminar air flow cabinet. Microcuttings of convenient size (1–5 cm) were prepared and surface-sterilized by serial passage through 80% ethanol for 20 s. After rinsing in distilled water, explants were surface sterilized with mercuric chloride solution (0.1%) for 5 min followed by 5–6 times washing in sterile distilled water was conducted. The surface sterilized explants were trimmed into 0.5–1.5 cm segments and were cultured on nutrient media.
Table 1.
A comparison on lawsone yield of candidate plus trees (CPTs) of L. inermis
| Collection code | Total leaf dry weight (g) | Lawsone content (mg/g dw) | Total lawsone yield (g) |
|---|---|---|---|
| CPT1 | 51.43gh | 21.2de | 1.09f |
| CPT2 | 29.56k | 21.4d | 0.63j |
| CPT3 | 57.35f | 21.2de | 1.22e |
| CPT4 | 75.09d | 18.4g | 1.38d |
| CPT5 | 38.56j | 17.1h | 0.66j |
| CPT6 | 92.80b | 15.4j | 1.43d |
| CPT7 | 43.10i | 22.8ab | 0.98g |
| CPT8 | 45.00i | 21.3de | 0.96g |
| CPT9 | 49.26h | 16.8i | 0.83h |
| CPT10 | 34.66j | 18.4g | 0.64j |
| CPT11 | 62.86e | 22.2bc | 1.39d |
| CPT12 | 43.42i | 20.4f | 0.89hh |
| CPT13 | 49.27h | 21.2de | 1.04fg |
| CPT14 | 52.00gh | 17.3h | 0.99g |
| CPT15 | 99.76a | 21.5cd | 2.14a |
| CEP16 | 86.66c | 23.1a | 2.01b |
| CPT17 | 53.18fg | 18.5g | 0.98g |
| CPT18 | 34.80j | 20.6ef | 0.72i |
| CPT19 | 87.57c | 20.7def | 1.81c |
| CPT20 | 46.09i | 16.2i | 0.75i |
| F value df = (n − 1) = 19 | 184.7*** | 90.8*** | 46.5*** |
***Significant at p < 0.001 level; Means within column followed by same letter are not significantly (p < 0.05) different as determined by Duncan’s Multiple Range test (DMRT)
Culture media and conditions
Basal MS medium (Murashige and Skoog 1962) containing 3% (w/v) sucrose and 0.75% agar (extra pure, Bactograde suitable for plant tissue culture, SISCO Research Laboratories, Mumbai, India) was used for the tissue culture experiments. Plant growth regulators viz, benzyl adenine (BA), kinetin (Kn) and 2-isopentanyl adenine (2-iP) (Sigma-Aldrich, St. Louis, US) at different concentrations were supplemented into the basal MS media. The pH of the medium was adjusted to 5.8 using 1 N KOH/1 N HCl and autoclaved at 121 °C and 108 kPa pressure for 15 min. At first, single explant was cultured in 25 × 150 mm culture tube containing 15 ml of sterilized MS medium for the development of cultures. After inoculation, cultures were maintained at 25 ± 2 °C in a culture room under 40 µmol m−2 s−1 irradiance provided by cool white fluorescent tubes (40 W; Philips, India) for 16 h photoperiod and relative humidity of 55 ± 5%.
Establishment of in vitro sterile culture, subculture and shoot propagation
Shoot tip or nodal segments were cultured on agar gelled MS medium fortified with diverse concentrations of plant growth regulator (PGR) (0.25, 0.5, 2.5, 5.0, and 10 µM) of BA, Kn and 2-iP. Nodal explants cultured on MS medium without plant growth regulators are taken as control. Nodal segments were inoculated in MS medium incorporated with 0.5 µM BA was used for the further experiments. After 30 days of culture, in vitro raised shoots were excised from the clump and further segmented (0.5–1 cm) and was subcultured on MS medium augmented with BA (0.5 µM). The shoots were multiplied further through the transfer of original explants at an interval of 2 weeks. After the third passage of frequent transfer, induced clumps of 2–4 shoots were transferred to MS medium containing BA (0.5 µM) in combination with 2-iP (0.5 µM) for shoot elongation and formation of new shoot buds from the axils of the transferred shoot clumps. After 3 weeks of culture, data on percentage response, total shoot number, and shoot length (cm) was scored.
Influence of position of node on shoot multiplications
In vitro response of nodal segments counting from tip portion (1st–8th node) of the young shoots were evaluated by culturing on MS medium incorporated with 0.5 µM BA and 0.5 µM 2-iP.
Rooting of microshoots
The microshoots after 3rd subculture onwards were used for rooting experiments. Microshoot length ranging from 3 to 5 cm was used for rooting. The basal portion of the individual shoots were taken from the clump with a scalpel and washed thoroughly in running tap water for the ex vitro rooting experiment. Excess water was separated by blotting and the cut ends were dipped in different concentrations of NAA (0.44, 0.88, 1.32 mM) or IBA solutions (0.49, 0.98, 1.47 mM) for 5 min. Micro shoots planted without plant growth regulator treatment served as control. Both growth regulator pulse treated micro shoots and untreated controls were then planted in polyethylene nursery pots with diameter 7 cm and height 8 cm containing a mixture of soil and sand (1:2). Initially, the potted plants were maintained in a hardening chamber (28 ± 2 °C, RH 90%; 40 µmol m−2 s−1 irradiance). The plants were enclosed with polyethylene bags which maintain high relative humidity and then, after 4 weeks, the polyethylene bags were removed. After 4 weeks of planting, rooting percentage, total root number and length of roots were scored. Rooted plants were then further maintained in a shade net nursery house and 2 month old plants were transferred to Department nursery.
Determination of lawsone content in both in vitro and acclimatized L. inermis plants compared with mother plant
For the quantification of lawsone, fresh leaves of mother plant, in vitro and 1 year old acclimatized plant were collected and shade dried. The dried leaves were grind into fine powder and used for the extraction of dye. Dried, powdered leaves (100 mg) of L. inermis were soaked in 10 ml methanol for 2 h and then, the mixture was centrifuged at 5000 rpm for 20 min and the clear supernatant was separated out into an empty clean dried boiling tube. The absorbance of supernatant was read out at 452 nm using UV–Vis spectrophotometer (Shimadzu, Japan, Model No. 1900). The quantification was made on the basis of pure standard of lawsone (Sigma Chem., St. Louis, USA). The lawsone content in dried leaves was calculated in mg/g dw.
Experimental design and statistical analysis
All experiments were done using a randomized complete block design (RCBD) method. All the treatment was made of three replication blocks and each block was represented by seven culture tubes. Subculture experiments were performed in culture bottles (300 ml capacity). Data recorded in percentages were subjected to arcsine transformation before the analysis, and then transformed back to percentages for inclusion in the tables (Snedecor and Cochran 1962). Data on diverse parameters were subjected to analysis of variance (both one way and two way ANOVA) to determine levels of significance and mean separation was done using Duncan’s Multiple Range Test (p < 0.05). Data on percentage of response, number of shoot and length of shoot (cm), were scored after 4 weeks of incubation.
Results and discussion
Lawsonia inermis is an important dye yielding, commercial crop used in traditional systems of medicinal as well as cosmetic industries. In view of its importance, the present work was undertaken to multiply superior healthy plant to achieve enhanced productivity in terms of dye content and biomass.
Establishment of in vitro culture and multiplication
Nodal segments cultured in vitro, primarily showed rapid phenolic exudation. Phenolic exudation is one of the prime crisis in culture establishment when explants were taken from tissues of woody plants (Rai et al. 2010). Media turned into slight yellowish colour and explants turned brown to blackish colour at the cut end within 24 h. Explants subjected to continuous three transfers every 2 week interval to fresh medium facilitated better establishment rate and survival of explants. Explants retained in the initial culture medium for 4 weeks without transferring into fresh medium had a reduced percentage of establishments and showed stunted growth response characterized by poorly elongated (1 cm) single shoots. Control sets cultured on medium devoid of plant growth regulator did not show any growth response.
The explant transfer every 2 week interval to fresh medium essentially alleviated problem of phenolics induced explant browning and facilitated emergence of multiple shoots within 4 weeks of culture. Repeated transfer of original explants for rapid production of shoots was previously reported in L. inermis (Ram and Shekhawat 2011). To achieve better culture establishment, several workers (Tiwari et al. 2002; Singh et al. 2008, 2012) have attempted regular transfer of explants to fresh medium. Frequency of axillary bud break and subsequent proliferation was low at the initial stage of cultures irrespective of hormones present in the medium. However, the capacity of bud break was increased after 2 weeks in case of optimal level of hormone added medium. ANOVA shown significant (p < 0.001) effect on types of cytokinin and different concentrations tested with regard to number of shoots and length of shoot.
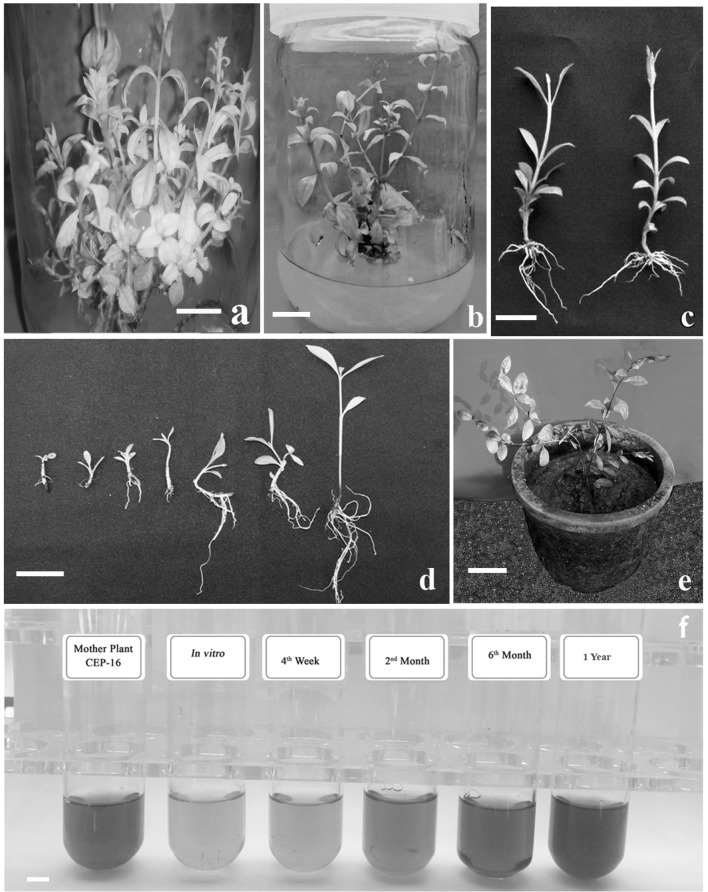
MS basal medium containing 0.5 µM BA was found to be effective for establishment of cultures (Fig. 1a). In this medium, maximum percentage response (79.67%), highest shoot number (24.33) and elongation of shoots (Table 2) were achieved after 6 weeks of initial culture (three transfer every 2 week interval). In the previous reports on in vitro multiplication of L. inermis (Ram and Shekhawat 2011), 11.8 shoots/culture was reported in MS medium supplemented with 0.5 mg l−1 BA. BA is one of the commercially important cytokinin used for shoot proliferation that causes reinvigoration of mature/old tissues (Zhang et al. 2010; Rathore et al. 2013). In the present study, as the concentration of BA increased, it was noticed that the number of multiple shoots decrease and became more dwarf shoots with basal callusing. Similar response that reduction in shoot number with increase in the concentration of cytokinin in media was reported in Ulmus species (Corchete et al. 1997). BA is one of the main commercial cytokinin used to reinvigoration of grown-up tissues and causes bud stimulation, a requirement for cloning of mature trees (Bonga and von Aderkas 1992; Bhatt and Dhar 2000; Rathore et al. 2010; Zhang et al. 2010). Kn was also used for axillary bud proliferation, where 0.5 µM Kn gave 4.33 shoots per explant. When the concentration of Kn was increased the quality of the shoots were deteriorated, with formation of dwarf (2.67 cm), pale green shoots. These shoots were failed to elongate further even 3 weeks after culture. However, in kinetin added medium also phenolic exudation was noticed. Elongation frequency of multiple shoot buds was less both in BA and Kn added medium. Lower concentration (0.5 µM) of 2-iP when tried, shoot elongation was achieved where new shoots grew 9.77 cm size with 91.33% response. Basal callusing did not occur due to both the Kn and 2-iP addition in the medium. There was also a remarkable differences were observed among the shoot formation with size differences. The same explants produced with the various size of shoots among the total number of shoots formed which was treated with 0.5 µM BA. Therefore to attain the standard length for the complete shoots formed, a new experiment was set with BA in combination with lower concentration (0.25, 0.5, 1, 2.5 and 0.5 µM) of other cytokinins such as Kn and 2-iP. The combinations of cytokinins were attempted to achieve better elongation of multiple shoots while sustaining BA induced rate of shoot multiplication. Nodal segments cultured on MS medium containing lower concentrations of BA (0.5 µM) and 2-iP (0.5 µM) showed proper shoot elongation (Table 3) and improved number of shoots (43.67). The effect of 2-iP on shoot elongation coupled with multiplication was reported earlier also (Chengalrayan and Gallo 2001). However, moderately high concentration of 2-iP (5 µM) resulted in the significant decrease in shoot length (6.40 cm). Guang-jie et al. (2008), found that 2-iP concentration influenced elongation of shoots and adversely affected by increased concentration of 2-iP. The optimized concentration of 2-iP could be used to improve rate of shoot multiplication. In present study, 2-iP showed significant influence on the shoot length. Cytokinin combinations were earlier shown to be critical for shoot elongation of many plant species (Rout et al. 2000; Rasmussen 2005). The greater effect of 2-iP over other cytokinins for shoot bud stimulation has been attributed to the capability of plant tissues to metabolize the natural plant growth regulator more readily than other artificial growth regulators (Khoshhal et al. 2012).
Fig. 1.
In vitro propagation using nodal explants and ex vitro rooting of L. inermis. a Multiple shoot induction of nodal segment explant cultured on MS medium supplemented with 0.5 μM each of BA and 2-iP (bar = 0.6 cm), b Axillary bud proliferation on MS medium supplemented with BA 0.5 μM with 2-iP 0.5 μΜ (bar = 0.5 cm), c Ex vitro rooted plantlets in different length class (< 0.5–1.0, 1.1–2.0, 2.1–3.0, 3.1–4.0, 4.1–5.0) treated with 0.44 mM of NAA (bar = 0.3 cm), d ex vitro rooted plantlets produced by treating treated with 0.44 mM of NAA (bar = 0.3 cm), e ex vitro rooted microshoots after 4 weeks of potting (bar = 0.26 cm) and f lawsone extracted from mother plant (CEP 16), different in vitro stages and micropropagagted plants of varying ages (4-week old, 2-month old, 6 month old and 1 year-old)
Table 2.
Effect of different cytokinins on shoot multiplication using nodal explants of L. inermis
| Cytokinin type | Concentration (µM) | Explant response (%) | No. of shoots/explant | Shoot length (cm) |
|---|---|---|---|---|
| BA | 0.25 | 46.67 ± 1.53ij | 8.33 ± 1.15e | 3.07 ± 0.47ghi |
| 0.5 | 79.67 ± 0.58bcd | 24.33 ± 0.58a | 5.53 ± 0.42d | |
| 1.0 | 68.33 ± 7.64e | 17.00 ± 1.00b | 4.60 ± 0.00e | |
| 2.5 | 61.67 ± 1.53f | 13.33 ± 0.58c | 4.17 ± 0.45e | |
| 5.0 | 53.33 ± 3.06gh | 11.33 ± 0.58d | 3.20 ± 0.20g | |
| 10 | 44.00 ± 6.08j | 8.00 ± 1.00ef | 2.37 ± 0.32hij | |
| 2-iP | 0.25 | 74.33 ± 1.15d | 5.00 ± 1.00g | 6.23 ± 0.21d |
| 0.5 | 91.33 ± 3.21a | 8.33 ± 1.53e | 9.77 ± 0.06a | |
| 1.0 | 84.67 ± 1.15b | 7.67 ± 0.58ef | 9.17 ± 0.15ab | |
| 2.5 | 77.67 ± 2.52cd | 7.00 ± 1.00ef | 8.83 ± 0.12b | |
| 5.0 | 80.33 ± 3.21bc | 6.67 ± 0.58f | 7.60 ± 0.17c | |
| 10 | 67.00 ± 2.00e | 4.67 ± 0.58gh | 4.27 ± 0.31e | |
| KIN | 0.25 | 28.67 ± 2.89kl | 3.00 ± 1.00ij | 2.30 ± 0.61jk |
| 0.5 | 60.67 ± 4.04f | 4.33 ± 0.58ghi | 4.31 ± 0.58e | |
| 1.0 | 57.00 ± 1.00fg | 3.33 ± 0.58hij | 3.40 ± 0.26f | |
| 2.5 | 50.33 ± 2.52hi | 2.67 ± 0.58j | 3.17 ± 1.04gh | |
| 5.0 | 33.67 ± 1.53k | 2.33 ± 0.58j | 2.60 ± 0.10ghij | |
| 10 | 27.00 ± 2.65l | 2.67 ± 1.15j | 2.00 ± 0.87k | |
| Control | 0.0 | 0.0m | 0.0k | 0.0i |
| Treatment df (n − 1) | 18 | 189.6*** | 133.14*** | 94.51*** |
| Cytokinin type (T) | 2 | 87.52*** | 18.8*** | 71.25*** |
| Cytokinin conc. (C) | 5 | 19.07*** | 2.34NS | 10.24*** |
| TxC | 10 | 6.64*** | 38.02*** | 7.88*** |
***Significant at p < 0.001 level; NS non significant; Means within column followed by same letter are not significantly (p < 0.05) different as determined by Duncan’s Multiple Range test (DMRT)
Table 3.
Effect of 0.5 µM BA and different concentrations of (KIN, 2-iP) on shoot multiplication and elongation using nodal explants of L. inermis
| Cytokinin conc. (µM) | Explant response (%) | No. of shoots/explant | Shoot length (cm) |
|---|---|---|---|
| BA 0.5 µM (control) | 71.67 ± 2.89b | 18.33 ± 0.58 cd | 4.90 ± .361 cd |
| KIN | |||
| 0.25 | 20.00 ± 1.00f | 5.00 ± 2.43f | 1.85 ± 0.24f |
| 0.5 | 60.67 ± 3.06c | 12.00 ± 1.00d | 5.00 ± 0.40d |
| 1 | 59.00 ± 2.29c | 8.00 ± 1.73e | 3.37 ± 0.32e |
| 2.5 | 41.67 ± 6.66d | 8.67 ± 1.53e | 3.20 ± 0.92e |
| 5 | 23.00 ± 1.00e | 6.00 ± 2.65e | 1.87 ± 0.32f |
| 2-iP | |||
| 0.25 | 72.00 ± 2.45b | 25.45 ± 1.34c | 5.54 ± 1.20c |
| 0.5 | 89.67 ± 0.58a | 43.67 ± 1.53a | 12.57 ± 0.74a |
| 1 | 86.00 ± 1.00a | 39.00 ± 1.00b | 11.97 ± 0.85a |
| 2.5 | 75.67 ± 5.5b | 38.33 ± 2.52b | 10.53 ± 0.81b |
| 5 | 73.00 ± 2.65b | 26.67 ± 1.53c | 6.40 ± 1.25c |
| Treatment df (n − 1) = 9 | 50.15*** | 239.78*** | 92.42*** |
| Cytokinin type (T) df (n − 1) = 1 | 45.09** | 121.61** | 64.34** |
| Cytokinin conc.(C) df (n − 1) = 4 | 5.89* | 3.46NS | 5.28NS |
| TxC df (n − 1) = 8 | 5.33* | 12.43*** | 7.85** |
***Significant at p < 0.001 level; **Significant at p < 0.001 level; *Significant at p < 0.05 level, NS non significant; Means within column followed by same letter are not significantly (p < 0.05) different as determined by Duncan’s Multiple Range test (DMRT)
Adventitious shoot proliferation was also observed in the same treatment after the continuous transfer of explants (Fig. 1b). Similar studies were reported earlier that the cytokinin combinations induce axillary shoots (Varisai et al. 1999). Multiple shoots developed with a combination of BA (0.5 µM) and 2-iP (0.5 µM) grew faster, while those initiated in the BA (0.5 µM) and Kn combinations showed slower growth. Of the various concentration tested, 0.5 μM BA combined with Kn (0.25 µM) found to be least effective for shoot induction and elongation. The inferior response of explants to both the combination of cytokinins BA and Kn was also reported in Rhododendron sp. (Fordham et al. 1982; Almeida et al. 2005) and Eclipta alba (Baskaran and Jayabalan 2005). However, explants response to the combinations of BA with 2-iP and Kn varied significantly. Combination of BA with 2-iP found to be more effective than BA–Kn combination. In the earlier reports on L. inermis (Table 4), the shoot number obtained was ranging from 1.18 to 7.8 per explant (Bakkali et al. 1997; Rout et al. 2001; Singh et al. 2012; Moharana et al. 2017) using MS medium supplemented with varying levels of different cytokinins such as BA. Ram and Shekhawat (2011) have reported MS medium provided with 0.25 mg l−1 BA, 0.25 mg l−1 l Kn, 0.1 mg l−1 IAA, 50 mg l−1 ascorbic acid, 25 mg l−1 ammonium sulphate resulted 28.5 shoots with basal callusing. High concentration of additives and auxin was supplemented with cytokinin in this medium. However, in the present study BA (0.5 µM) in combination with 2-iP (0.5 µM) was found to be the most suitable cytokinin combination for nodal explants to generate large number of shoots (43.67). In the previous report (Bakkali et al. 1997) both auxin (4 mg l−1, NAA), and cytokinin (2 mg l−1 BA) were used. In present study, use of auxin in shoot multiplication medium was avoided and this omission reduced chance of development of basal callus, thus prevents deterioration of cultures.
Table 4.
Summary of in vitro studies of L. inermis
| References | Explant and mode of regeneration | Treatment/medium | Result | Remarks |
|---|---|---|---|---|
| Bakkali et al. (1997) | Internode segments (1–2 cm length) from 6 to weeks old axenic seedlings | MS + 0.4 mg l−1 NAA + 2 mg l−1 BA + 0.25 µM of FeSO4 for shoots and hormone-free ½ MS solid medium for in vitro rooting | 3.8 shoots | Low frequency of multiplication. As the explant is seedling based quality of propagules cannot be ensured |
| Rout et al. (2001) | Apical shoot and node of actively growing young stems from green house growing plant | MS + 0.25 mg l−1 + BA, 0.25 mg l−1 + Kn + 0.5 mg l−1 ascorbic acid for shoot and 0.25 mg l−1 IBA with 2% (w/v) sucrose for in vitro rooting | 1.18 shoots | Low frequency of multiplication |
| Ram and Shekhawat (2011) | Apical shoot tip, below apical nodal segments and woody nodes and fresh hard nodal segments of mature plant (direct) | MS + 0.25 mg l−1 BA + 0.25 mg l−1 + Kn + 0.1 mg l−1 IAA + 50 mg l−1 ascorbic acid + 25 mg l−1 ammonium sulphate for shoot and 300 mg l−1 IBA and 100 mg l−1 NOA for rooting | 28.5 shoots with basal callus | High concentration of excess additives with cytokinins in combination with auxin were used for rising multiple shoots. Taken long (8–10) days for bud break even though medium was supplemented with hormones and addition of additives with higher concentration |
| Singh et al. (2012) | Apical shoot tip and meristems nodes of actively growing young stems from mature plant (indirect) | MS + 0.2 mg l−1 BAP + 0.2 mg l−1 Kn +0.2 mg l−1 CW for shooting and ½ MS + 200 mg l−1 AC + 0.5 mg l−1 NAA for rooting | 4.7 shoots | Low frequency of multiplication |
| Moharana et al. (2017) | Nodal segments belonging to different position from shoot apex from mature plant | MS + 1.0 mg l−1 BA for shoots and MS medium with free hormone for rooting | 7.8 shoots | Low frequency of multiplication |
| Present study | 4th and 5th nodes from elite plant (direct) | MS + 0.5 µM BA + 0.5 µM 2-iP for shoots and 0.44 mM NAA for rooting | 43.67 shoots | Used low concentration of cytokinin combination for multiplication, Continuous subculture attained high frequency (43.67) shoot multiplication. Use of auxin in multiplication medium, avoided-reduced callusing, chances for variation. Simple ex vitro rooting system was standardized. Evaluated the best size shoots for rooting. Time taken for rooting is less over previous method |
Influence of position of node on shoot multiplication
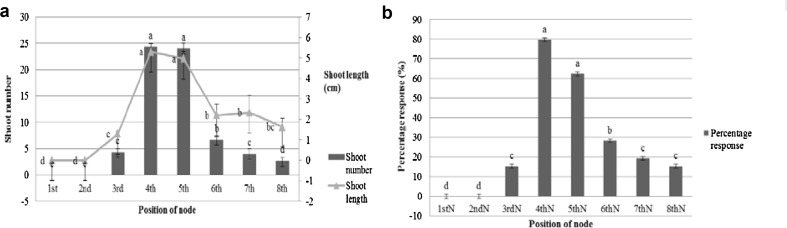
Browning at basal portion of the explant is a greater and common problem in tissue culture of woody plant genus (Rai et al. 2010). Therefore it is important to identify the optimally mature nodes for the successful culture of the species. Of the different nodal explants collected from the shoot cuttings (1–8th nodes), the 4th node from the tip produced maximum number of shoots (24.33) followed by the 5th node (24), while the maximum length (5.30 cm) was attained by shoots produced by the 4th node followed by that of the 5th node (Fig. 2A). The explant response was high in 4th and 5th node (79.67%; Fig. 2B). The shoot tip, first and second nodes failed to produce shoots. These cultures showed necrosis and dried after 6 days of the culture. This may be due to the nature of explants which cannot withstand the surface sterilization and lead to the death of the explants. The 3rd node produced fewer number of shoots compared to the bottom nodes. This may be due to the differential influence of the apical dominance on the axillary meristems located at varying distances from the tip. Where the auxin synthesized in the apical meristem moves basipetally along the length of the growing shoot. Only few numbers (6.67 and 2.67) of shoots were produced in 6th to 8th node of the explants. Hence 4th and 5th node is most suitable for large scale multiplication. Similar response of nodal segment explant was noticed previously (Moharana et al. 2017) in L. inermis. The position of the nodal explants significantly influenced the multiple shoot production in 0.5 µM BA added medium.
Fig. 2.
A Effect of nodal position on shoot multiplication of L. inermis by treatment of 0.5 µM BA on MS medium in terms of shoot number and shoot length. B Percentage response of explant
Ex vitro root formation and acclimatization
Ex vitro rooting have an important role in the development of efficient micropropagation protocol. Ex vitro rooting efforts results in the production plants with improved physiological functions consequently achieved high survival rate (Bonal and Monteuuis 1997; Hazarika 2003; Martin 2003; Feyissa et al. 2007). In the present study, an ex vitro rooting technique was performed as a substitute of conventional in vitro rooting methods in order to decrease time, cost, resources and labour of micropropagation (Krishnan and Siril 2016). Different types of auxin (IBA and NAA) and its concentrations had significant influence on ex vitro rooting of L. inermis. NAA or IBA (0.44 mM) treated (5 min) shoots were planted in polyethylene cups filled with soil: sand (1:2) mixture showed 100% rooting and maximum number of roots (Fig. 1c; Table 5). Microshoots planted without auxin treatment were failed to generate roots.
Table 5.
Effect of auxin pulse treatments (5 min) on ex vitro rooting of micropropagated shoots of L. inermis
| Auxin | Concentration (mM) | Rooting percentage (%) | Root number | Mean root length (cm) |
|---|---|---|---|---|
| NAA | 0.44 | 100.00 ± 0.00a | 19.33 ± 0.58a | 12.00 ± 0.87a |
| 0.88 | 96.50 ± 1.24ab | 16.67 ± 1.53bc | 10.10 ± 1.08a | |
| 1.32 | 95.00 ± 1.26b | 13.67 ± 0.58d | 6.43 ± 0.93b | |
| IBA | 0.49 | 100.00 ± 0.00a | 17.67 ± 0.58ab | 12.13 ± 0.81a |
| 0.98 | 98.60 ± 0.94ab | 15.67 ± 1.53c | 10.30 ± 2.14a | |
| 1.47 | 91.33 ± 2.05c | 12.00 ± 1.00d | 4.92 ± 1.38b | |
| Control | 0.0 | 0.0c | 0.0e | 0.0c |
| Treatment | Df (n−1) = 6 | 7.39*** | 19.29*** | 16.08*** |
| Auxin Type (T) | 1 | 0.33NS | 13.00* | 0.58NS |
| Auxin conc.(C) | 2 | 0.52NS | 0.01NS | 16.75* |
| TxC | 2 | 0.35NS | 0.79NS | 0.19NS |
***Significant at p < 0.001 level; *Significant at p < 0.05 level, NS non significant; Means within column followed by same letter are not significantly (p < 0.05) different as determined by Duncan’s Multiple Range test (DMRT)
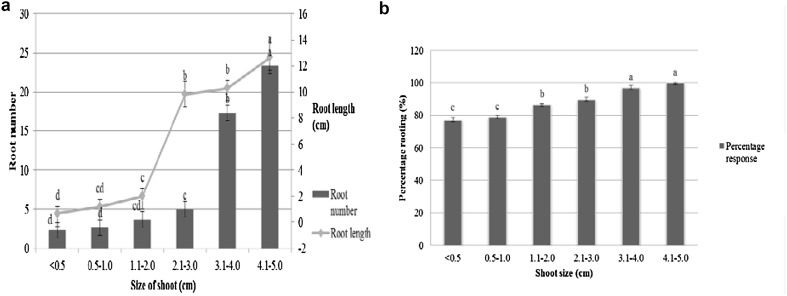
Rooting response of various size shoots of L. inermis treated with 0.44 mM NAA (Fig. 1d) was also evaluated and rooting response of different size shoots varied significantly (p < 0.001). Effect of different size of shoot on in vitro rooting response was reported in B. orellana (Joseph et al. 2011). Shoot length class 4.1–5 cm showed 99.33% rooting with 23.3 roots and were elongated maximum (Fig. 3A, B). Shoots with 3.1–4 cm also respond with 97.0% of rooting. Smaller shoots (< 0.5 cm and 2 cm) showed 77.0 and 89.67% rooting respectively. For the ex vitro treatments, soil: sand (1:2) was used which is more suitably aerated than an agar gelled medium, which shows positive effects on root initiation and growth. The soil: sand media also had superior water holding ability over agar gelled media and therefore it can avoid medium hypoxia is also an advantageous over in vitro rooting. There are previous reports that roots growing in agar gelled medium prove structural differences and abnormalities and frequently be short of root hairs which influence their establishment in the field soil under commercial-scale cultivation (Debergh and Maene 1981). Similar difficulty has also been showed in earlier report in case of in vitro developed shoots of blue honeysuckle (Karhu 1997). Ex vitro rooted plantlets showed superior growth with morphologically normal, robust and improved root system that sustains plantlets under field environment after the transplantation. Ex vitro rooting method also gives callus free rooting and better survival of the plantlets. These results show that the better aeration of soil media could assign for better root growth facilitated by superior diffusion of ethylene in the soil medium (Newell et al. 2005). Agar medium possibly caused reduced gas diffusion including ethylene and hence the accumulation of ethylene at the basal part of the stem. This accumulation can decrease root induction, elongation and growth (De Klerk 2002). As the shoot size 4.1–5 cm showed maximum rooting, this size class is recommended with 0.44 mM NAA pulse treatment (5 min) for the elicitation of adventitious roots of L. inermis microshoots. After 2 months, survived (95%) plants were transferred to Department nursery (Fig. 1e).
Fig. 3.
A Ex vitro rooting response of various size shoots of L. inermis treated with 0.44 mM NAA in terms of root number and root length. B Percentage rooting. Values followed by different letters on the bar are significantly different at p < 0.05 level as determined by Duncan’s test (DMRT)
Comparison of lawsone production
Lawsone concentration in mother plant was compared with different stages of in vitro and micropropagated, field transplanted plants of L. inermis. Spectrophotometric determination of lawsone content showed significant (p < 0.001) variation in different in vitro samples. Lawsone content was high in one-year-old micropropagated plant (23.04 mg/g dw) and is comparable to content of mother plant (22.84 mg/g dw; Fig. 1f). The plant material sampled from in vitro cultures raised on MS medium supplemented with 0.5 µM BA and 0.5 µM 2-iP, showed inferior level of lawsone content (8.8 mg/g dw) than both mother plant as well as acclimatized plant (Table 6). Similar observations were made in the production of anthraquinones in both the in vivo and in vitro samples of Oldenlandia umbellata (Siva et al. 2012) Lawsone is the major metabolite present in L. inermis. It is not only the colouring agent of the plant but also attributes numerous phyto pharmacological properties (Gevrenova 2010). In the present study, the quantification of lawsone from different stages of both in vitro and in vivo was carried out by spectrophotometric analysis. Through the present study, it is proved that plus tree selected on the basis of lawsone content can be cloned with superior lawsone yield.
Table 6.
Lawsone contents of mother plant, micropropagated plant and different in vitro culture sources of L. inermis
| Plant material | Lawsone content (mg/g dw) |
|---|---|
| Leaf from mother plant | 22.84 ± 0.04a |
| Leaf from in vitro culture | |
| On MS medium supplemented with 0.5 µMBA in combination with 0.5 µM 2-iP | 8.8 ± 0.28e |
| Leaf from acclimatized micropropagated plantlet | |
| I 4-week old | 11.56 ± 0.24d |
| II 2-Month old | 18.36 ± 0.48c |
| III 6-Month old | 22.36 ± 0.56b |
| IV 1-year old | 23.04 ± 0.24a |
| F value | 1648.18*** |
***Significant at p < 0.001 level; Means within column followed by same letter are not significantly (p < 0.05) different as determined by Duncan’s Multiple Range test (DMRT)
Conclusion
The protocol presented here is suitable for the large scale propagation of L. inermis and its efficiency in terms of number shoots (43.67) and number of rootable shoots per culture (100%), ex vitro rooting are superior over previous reports. The present procedure facilitates multiplication of L. inermis without using any organic additives thus ensures reproducibility. Present study also revealed the direct regeneration of plants from nodal segments and hence germplasm collection of elite plant can be propagated in large scale for the production of commercially important plant. The present study proved that micropropagated plants can provide better concentration of lawsone, thus ensure improvement of L. inermis germplasm and production of superior plants.
Acknowledgements
The authors thank Dr. Suhara Beevy S, Associate Professor and Head, Department of Botany, University of Kerala, for providing research facilities. PCS thank Kerala State Welfare Board, Government of Kerala for providing research Grant (B3-14931/06/11/2014).
Authors’ contributions
PCS conducted the experiments. PCS and EAS analyzed the data. PCS drafted the manuscript. EAS designed the experiments. EAS and PCS revised the manuscript. All authors read and approved the manuscript.
References
- Ali M, Grever MR. A cytotoxic naphtho-quinone from Lawsonia inermis. Fitoterapia. 1998;69:1810–1813. [Google Scholar]
- Ali NA, Jülich WD, Kusnick C, Lindequist U. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol. 2001;74:173–179. doi: 10.1016/S0378-8741(00)00364-0. [DOI] [PubMed] [Google Scholar]
- Alia BH, Bashi AK, Tanira MOM. Anti inflammatory, antipyretic and analgesic effects of Lawsonia inermis L. (henna) in rats. Pharmacology. 1995;51:356–363. doi: 10.1159/000139347. [DOI] [PubMed] [Google Scholar]
- Almeida R, Goncalves S, Romano A. In vitro micropropagation of endangered Rhododendron ponticum L. Subsp. Baeticum (Boissier & Reuter) Handel-Mazzetti. Biodivers Conserv. 2005;14:1059–1069. doi: 10.1007/s10531-004-8413-3. [DOI] [Google Scholar]
- Al-Tufail M, Krahn P, Hassan H, Mahier T, Al-Sedairy ST, Haq A. Rapid identification of phenylene diamine isomers in henna hair dye products by gas chromatography-mass spectrometry (GC–MS) Toxicol Environ Chem. 1999;71:241–246. doi: 10.1080/02772249909358795. [DOI] [Google Scholar]
- Anand KK, Singh B, Chand D, Chandan BK. An evaluation of Lawsonia alba extract as hepato protective agent. Planta Med. 1992;58:22–25. doi: 10.1055/s-2006-961382. [DOI] [PubMed] [Google Scholar]
- Bakkali AT, Jaziri M, Foriers A, Vander Heyden Y, Vanhaelen M, Homes J. Lawsone accumulation in normal and transformed cultures of henna, Lawsonia inermis. Plant Cell, Tissue Organ Cult. 1997;51:83–87. doi: 10.1023/A:1005926228301. [DOI] [Google Scholar]
- Baskaran P, Jayabalan N. An efficient micropropagation system for Eclipta alba—a valuable medicinal herb. In Vitro Cell Dev Biol Plant. 2005;41:532–539. doi: 10.1079/IVP2005667. [DOI] [Google Scholar]
- Bhatt ID, Dhar U. Combined effect of cytokinins on multiple shoot production from cotyledonary node explants of Bauhinia vahlii. Plant Cell, Tissue Organ Cult. 2000;62:79–83. doi: 10.1023/A:1006450516329. [DOI] [Google Scholar]
- Bonal D, Monteuuis O. Ex vitro survival, rooting and initial development of in vitro rooted vs. unrooted microshoots from juvenile and mature Tectona grandis genotypes. Silvae Genet. 1997;46:301–306. [Google Scholar]
- Bonga JM, von Aderkas P. In vitro cultures of trees. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- Cartwright-Jones C. Developing guidelines on henna: a geographical approach. Stow: TapDancing Lizard Publishing; 2006. [Google Scholar]
- Chaudhary G, Goyal S, Poonia P. Lawsonia inermis Linnaeus: a phytopharmacological review. Int J Pharm Sci Drug Res. 2010;2:91–98. [Google Scholar]
- Chaudhary GD, Poonia P, Kamboj P, Kalia AN. Hepatoprotective potential of Lawsonia inermis (seeds) Int J Phytopharmacol. 2012;3:66–73. [Google Scholar]
- Chengalrayan K, Gallo MM. Effect of various growth regulators on shoot regeneration of Sugarcane K. In Vitro Cell Dev Biol Plant. 2001;37:434–439. doi: 10.1007/s11627-001-0076-0. [DOI] [Google Scholar]
- Corchete MP, Fenning T, Gartcand JS, Valle T. Micropropagation of Ulmus species (elms) In: Bajaj YPS, editor. High-tech and micropropagation. Berlin: Springer; 1997. pp. 381–392. [Google Scholar]
- De Klerk GJ. Rooting of microcuttings: theory and practice. In Vitro Cell Dev Biol Plant. 2002;38:415–422. doi: 10.1079/IVP2002335. [DOI] [Google Scholar]
- Debergh PC, Maene LJA. A scheme for commercial propagation of ornamental plants by tissue culture. Sci Hortic. 1981;14:335–345. doi: 10.1016/0304-4238(81)90047-9. [DOI] [Google Scholar]
- Feyissa T, Welander M, Negash L. Genetic stability, ex vitro rooting and gene expression studies in Hagenia abyssinica. Biol Plant. 2007;51:15–21. doi: 10.1007/s10535-007-0004-1. [DOI] [Google Scholar]
- Fordham I, Stimart DP, Zimmerman RH. Axillary and adventitious shoot proliferation of Exbury azaleas in vitro. HortScience. 1982;17:738–739. [Google Scholar]
- Gallo FR, Multari G, Giambenedetti M, Federici E. Chemical fingerprinting of Lawsonia inermis L. using HPLC, HPTLC and densitometry. Phytochem Anal. 2008;19:550–559. doi: 10.1002/pca.1084. [DOI] [PubMed] [Google Scholar]
- Gevrenova R. Determination of natural colorants in plant extracts by high-performance liquid chromatography. J Serb Chem Soc. 2010;75:903–915. doi: 10.2298/JSC091027071G. [DOI] [Google Scholar]
- Guang-jie Z, Zhan-bin W, Dan W. In vitro propagation and ex vitro rooting of blueberry plantlets. Plant Cell, Tissue Organ Cult. 2008;18:187–195. [Google Scholar]
- Handa G, Kapil A, Sharma S, Jagdev S. Lawnermis acid a new anticomplementary tri-terpenoids from Lawsonia inermis seeds. Indian J Chem. 1997;36:252–256. [Google Scholar]
- Hazarika BN. Acclimatisation of tissue-cultured plants. Curr Sci. 2003;85:30–31. [Google Scholar]
- Hema R, Kumaravel S, Gomathi S, Sivasubramaniam C. Gas chromatography–mass spectroscopic analysis of Lawsonia inermis leaves. NY Sci J. 2010;3:142–143. [Google Scholar]
- Hsouna BA, Culioli MG, Blache Y, Jaoua S. Antioxidant constituents from Lawsonia inermis leaves: isolation, structure elucidation and antioxidative capacity. Food Chem. 2011;125:193–200. doi: 10.1016/j.foodchem.2010.08.060. [DOI] [Google Scholar]
- Joseph N, Siril EA, Nair GM. An efficient in vitro propagation methodology for Annatto (Bixa orellana L.) Physiol Mol Biol Plants. 2011;17:263–270. doi: 10.1007/s12298-011-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaraj C, Elango G, Zahir AA, Rajakumar G, Velayutham K. Lousicidal activity of synthesized silver nanoparticles using Lawsonia inermis leaf aqueous extract against Pediculus humanus capitis and Bovicola ovis. Parasitol Res. 2012;111:2023–2033. doi: 10.1007/s00436-011-2667-y. [DOI] [PubMed] [Google Scholar]
- Karhu ST. Rooting of blue honeysuckle microshoots. Plant Cell, Tissue Organ Cult. 1997;48:153–159. doi: 10.1023/A:1005768117246. [DOI] [Google Scholar]
- Khoshhal MS, Salehi H, Khosh MK. Micropropagation of Araucaria excelsa R. Br. var. glauca Carrier from orthotropic stem explants. Physiol Mol Biol Plants. 2012;18:265–271. doi: 10.1007/s12298-012-0115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan SSR, Siril EA. Enhanced in vitro shoot regeneration in Oldenlandia umbellata L. by using quercetin: a naturally occurring auxin-transport inhibitor. Proc Natl Acad Sci India Sect B Biol Sci. 2016;21:271–278. doi: 10.1007/s40011-015-0672-0. [DOI] [Google Scholar]
- Martin KP. Rapid in vitro multiplication and ex vitro rooting of Rotula aquatica Lour., a rare rhoeophytic woody medicinal plant. Plant Cell Rep. 2003;21:415–420. doi: 10.1007/s00299-002-0547-8. [DOI] [PubMed] [Google Scholar]
- Mikhaeil BR, Badria FA, Maatooq GT, Amer MM. Antioxidant and immuno-modulatory constituents of henna leaves. Z Naturforsch C. 2004;59:468–476. doi: 10.1515/znc-2004-7-803. [DOI] [PubMed] [Google Scholar]
- Moharana A, Das A, Subudhi E, Naik SK, Barik DP. Assessment of genetic fidelity using random amplified polymorphic DNA and inter simple sequence repeats markers of Lawsonia inermis L. plants regenerated by axillary shoot proliferation. J Crop Sci Biotech. 2017;20:405–416. doi: 10.1007/s12892-017-0002-0. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nadkarni KM. Indian materia medica 1. Bombay: Popular Book Depot; 1982. [Google Scholar]
- Nayak BS, Isitor G, Davis EM, Pillai GK. The evidence based wound healing activity of Lawsonia inermis L. Phytother Res. 2007;21:827–831. doi: 10.1002/ptr.2181. [DOI] [PubMed] [Google Scholar]
- Newell C, Growns DJ, McComb JA. A novel in vitro rooting method employing an aerobic medium. Aust J Bot. 2005;53:81–89. doi: 10.1071/BT04061. [DOI] [Google Scholar]
- Okpekon T, Yolou S, Gleye C, Roblot F, Loiseau P, Bories C, Grelllier P, Frappier F, Laurens A, Hocquemiller R. Antiparasitic activities of medicinal plants used in Ivory Coast. J Ethnopharmacol. 2004;90:91–97. doi: 10.1016/j.jep.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Paiker N, Kandir K. In vitro micropropagation and callus induction of Lawsonia inermis L. from shoot tips. The Bioscan. 2011;6:39–42. [Google Scholar]
- Phirke SS, Saha M, Chandra N. In vitro callus induction from leaf explants of Lawsonia inermis L. used as herbal dye. Asian J Exp Biol Sci. 2010;2010:118–120. [Google Scholar]
- Rai MK, Asthana P, Jaiswal VS, Jaiswal U. Biotechnological advances in guava (Psidium guajava L.): recent developments and prospects for further research. Trees. 2010;24:1–12. doi: 10.1007/s00468-009-0384-2. [DOI] [Google Scholar]
- Ram K, Shekhawat NS. Micropropagation of commercially cultivated Henna (Lawsonia inermis) using nodal explants. Physiol Mol Biol Plants. 2011;17:281–289. doi: 10.1007/s12298-011-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen HN. Terrestrial orchids: from seed to mycotrophic plant. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Rathore MS, Gehlot HS, Shekhawat NS. Biotechnological approaches for propagation and prospecting of important medicinal plants from Indian Thar Desert. Int J Plant Prod. 2010;4:67–72. [Google Scholar]
- Rathore MS, Rathore MS, Shekhawat NS. Ex vivo implication of phytohormones on various in vitro responses in Leptadenia reticulata (Retz.) Wight. & Arn.—an endangered plant. Environ Exp Bot. 2013;86:86–93. doi: 10.1016/j.envexpbot.2010.05.009. [DOI] [Google Scholar]
- Rout GR, Samantaray S, Das P. In vitro manipulation and propagation of medicinal plants. Biotechnol Adv. 2000;18:91–120. doi: 10.1016/S0734-9750(99)00026-9. [DOI] [PubMed] [Google Scholar]
- Rout GR, Das G, Samantaray S, Das P. In vitro micropropagation of Lawsonia inermis (Lythraceae) Rev Biol Trop. 2001;49:957–963. [PubMed] [Google Scholar]
- Singh A, Reddy MP, Patolia JS. An improved protocol for micropropagation of elite genotypes of Simmondsia chinensis (Link) Schneider. Biol Plant. 2008;52:538–542. doi: 10.1007/s10535-008-0105-5. [DOI] [Google Scholar]
- Singh P, Jain K, Jain B, Khare S. Micropropagation of Lawsonia inermis: an important medicinal plant. Int J Curr Res Rev. 2012;4:29–34. [Google Scholar]
- Singh KS, Luqman S, Mathur AJ. Lawsonia inermis L.—a commercially important primaeval dying and medicinal plants with diverse pharmacological activity: a review. Ind Crops Prod. 2014;65:269–286. doi: 10.1016/j.indcrop.2014.11.025. [DOI] [Google Scholar]
- Siva R, Rajasekaran C, Mudgal G. Induction of somatic embryogenesis and organogenesis in Oldenlandia umbellata L., a dye-yielding medicinal plant. Plant Cell, Tissue Organ Cult. 2009;98:205–211. doi: 10.1007/s11240-009-9553-7. [DOI] [Google Scholar]
- Siva R, Behera SK, Rajasekaran C. Anthraquinones dye production using root cultures of Oldenlandia umbellata L. Ind Crops Prod. 2012;37:415–419. doi: 10.1016/j.indcrop.2011.12.027. [DOI] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 4. Ames: The Iowa State University Press; 1962. [Google Scholar]
- Sultana N, Iqbal M, Choudhary AK. Protein glycation inhibitory activities of Lawsonia inermis and its active principles. J Enzyme Inhib Med Chem. 2009;24:257–261. doi: 10.1080/14756360802057500. [DOI] [PubMed] [Google Scholar]
- Tiwari SK, Tiwari KP, Siril EA. An improved micropropagation protocol for teak. Plant Cell, Tissue Organ Cult. 2002;71:1–6. doi: 10.1023/A:1016570000846. [DOI] [Google Scholar]
- Varisai SM, Jawahar M, Thiruvengadam M, Jeyakumar M, Jayabalan N. Effect of cytokinins on the proliferation of multiple shoots in Horsegram (Macrotyloma uniflorum (Lam) Verdc) J Plant Biotechnol. 1999;1:79–83. [Google Scholar]
- Zhang H, Horgan KJ, Reynolds PH, Jameson PE. 6-Benzyladenine metabolism during reinvigoration of mature Pinus radiata buds in vitro. Tree Physiol. 2010;30:514–526. doi: 10.1093/treephys/tpp130. [DOI] [PubMed] [Google Scholar]