Abstract
Hypoxia is linked to metastasis; however, how it affects metastatic progression is not clear due to limited consensus in the literature. We posit that this lack of consensus is due to hypoxia being studied using different approaches, such as in vitro, primary tumor, or metastasis assays in an isolated manner. Here, we review the pros and cons of in vitro hypoxia assays, highlight in vivo studies that inform on physiological hypoxia, and review the evidence that primary tumor hypoxia might influence the fate of disseminated tumor cells (DTCs) in secondary organs. Our analysis suggests that consensus can be reached by using in vivo methods of study, which also allow better modeling of how hypoxia affects DTC fate and metastasis.
Hypoxia in Cell Fate and Cancer
Evolution and organism development have revealed how natural hypoxic environments influence cell survival and reprogramming. During evolution, organisms capable of ef ciently handling oxidative stress and using oxygen for energy production exhibited survival and evolutionary advantages [1]. Normal mammalian development occurs in a moderate-to-severe hypoxic environment that is responsible for aspects of developmental morphogenesis. Oxygen concentrations in the uterine environment range from 1 to 5%, and the placenta and embryonic cardiovascular system are formed under low cellular oxygen conditions [2]. During development, the expression and activity of the hypoxia inducible factor (HIF) transcription factor, is tightly controlled in space and time by oxygen availability in different developmental processes including placental development, trophoblast differentiation, and heart development, among others [3]. In low oxygen tensions the HIF protein is stabilized and not targeted for degradation by the Von Hippel Landau (VHL) tumor suppressor. Stabilized HIF then translocates to the nucleus where it participates in the expression of genes that drive adaptation to low oxygen tension [3].
The above literature shows how oxygen is an essential morphogen that regulates cell fate. This is clearly recapitulated, albeit in an aberrant manner, in cancer where hypoxia is a strong regulator of tumor cell fate. In cancer, hypoxia is associated with tumor progression, resistance to therapy, and metastasis. Sustained hypoxia in a growing tumor was described as being associated with a clinically aggressive phenotype, increased invasive capacity, perifocal tumor cell spreading, regional and distant dissemination, and resistance to different therapies [4,5]. However, hypoxia can also induce growth arrest, cause cell death, decrease motility speed while increasing invasiveness and directionality [6], and induce a dormancy-like program [7].
In this review we highlight the role of hypoxia during the steps of metastasis occurring in the primary tumor (invasion and intravasation) and in secondary organs (extravasation, dormancy, and reactivation) (for details on the metastatic cascade see [8]). We address several unresolved differences in the literature, which we attribute to limitations of the standard assays utilized to evaluate hypoxia in cancer and metastasis. As these standard in vitro assays have been thoroughly reviewed elsewhere in the literature [5,9–12], we instead focus on new technological developments, which enable studies of hypoxia in more physiologically relevant contexts, and highlight some of the surprising new revelations that these assays have provided. We further dissect apparently opposing functions of hypoxia and discuss how the context-dependent effects of hypoxia shape solitary DTC biology and metastasis development.
Hypoxia, Motility, and Directionality in the Primary Tumor
Hypoxia is one microenvironmental parameter historically implicated in both metastasis initiation [13] and therapy resistance [14]. Early on, two studies performed in mouse models showed that tail vein injection of tumor cells previously exposed to hypoxic conditions (<0.1% O2), followed by reoxygenation, led to a dramatic increase in resulting metastases [13,15]. These experiments, along with the advent of a small polarographic needle sensor for measurement of tissue oxygen levels, enabled a series of clinical studies which showed that tumor oxygenation levels are prognostic for patient outcome in various cancers. For an excellent review of these studies, and the needle sensor that enabled them, see [16].
With the prognostic ability of tumor oxygen levels thus established, understanding how hypoxia influences the metastatic cascade is crucially important. Some researchers have taken a reductionist approach to analyzing hypoxia and relied on in vitro assays designed to break the metastatic process into its postulated steps and to test how hypoxia in uences these in isolation. However, as discussed below, traditional in vitro assays do not recapitulate in vivo biology from several aspects and are likely poor surrogates for migration and invasion during the metastatic cascade in vivo.
Traditional In Vitro Assays May Not Re ect In Vivo Biology
First, in vitro studies typically compare cells exposed to hypoxic conditions (typically <1%, but occasionally <0.1% O2) with cells exposed to the rather nonphysiological ‘normoxic’ conditions (atmospheric oxygen levels of 21%). This is problematic since physiological (median) oxygen levels of normal tissues range from 3 to 7.4%. The de nition of normoxia as 21% does not match the lower levels of oxygen universally found in normal tissues, a condition that has been termed physoxia [17]. In addition, the choice of oxygen level considered to represent hypoxia may vary as many cell types respond differently to different levels of hypoxia, limiting comparative studies [18]. Second, in vitro systems cannot accurately re ect the fact that tumor cells do not exist in isolation from vascular and lymphatic systems, growth factors, cytokines, stromal, and immune cells, all of which have a signi cant impact upon tumor cell phenotype [19]. Third, the majority of published studies have used invasion through Matrigel, an arti cial matrix composed of laminin, nidogen, and collagen, which has been measured to dramatically alter tumor cell migration mode (collective vs. single cell), velocity, and distance of migration depending upon its mechanical properties [20]. Even in a study that avoided Matrigel – which contains several extracellular matrix (ECM) molecules including laminin as a major component – and instead utilized only collagen I [21], the situation was not improved as differences in ECM stiffness can dramatically impact migration and invasion. For example, focal adhesion formation and cell motility were enhanced on cultures utilizing a stiff versus a soft substratum, and this effect of substrate stiffness was dependent upon HIF activity, confounding any ability to determine the role HIF activity plays in motility phenotypes [22]. Finally, the extensive use of 2D in vitro assays limits the interpretation of the results as qualitative and quantitative differences in cell movement between 2D and 3D environments (such as 3D in vitro cultures and real tissues) exist [23,24] (see Box 1). Thus, while these in vitro studies have provided information on hypoxia-driven migration and invasion, they should be reconsidered as more sophisticated in vivo-like tests are developed.
Box 1. Variability in the In Vitro Measurements of Tumor Cell Responses to Hypoxia.
While it is generally accepted that hypoxia increases migration and invasion, in vitro studies show a variable response to hypoxia across culture conditions [118] and cell lines [119]:
Hypoxia reduces tumor cell cycling, but tumor cell invasiveness is not affected [120]
Hypoxia increases ability of tumor cells to invade [121]
Overexpressing HIF-1a increases invasion [122]
Silencing HIF-1a with siRNA inhibits migration and invasion [123]
Acute hypoxia (≤6 h) enhances cell migration, invasion, and clonogenic survival [124]
Chronic (≥24 h) hypoxia results decreases cell proliferation and increases cell death [124]
Intermittent hypoxia inhibits cell proliferation and increases cell migration and motility compared to both chronic hypoxia and normoxia [118]
The crucial question is which of these phenotypes is observed in vivo and related to tumor progression in animals and in patients.
Accordingly, recent studies have begun to shift away from these standard techniques and develop assays that attempt to recapitulate the in vivo complexity. These efforts are summarized in Figure 1 and include: more accurate 3D culture assays, microfluidic and controlled gas exchange platforms, and increased tumor–host mimicry by incorporation of additional host cell types. 3D culture assays (Figure 1A) address the geometrical constraints placed on cells that are plated on a glass or plastic surface by culturing cells either as a single-cell suspension, or as small clusters (known as spheroids [25]) within a deep but loose (low concentration) ECM. The ability of the cells to grow into large spheroids and to spread and invade into the surrounding matrix under different culture conditions are taken as indicators of the aggressiveness and invasiveness of the tumor cells. The increased physical dimensionality thus allows for a more nuanced analysis of cell migration. For example, one study looked at the impact of hypoxia on different modes of migration and found that hypoxia induces a switch from collective to single cell migration [26] a switch that was revealed thanks to the 3D nature of the assay.
Figure 1. Moving away from 2D and toward More Sophisticated Assays.
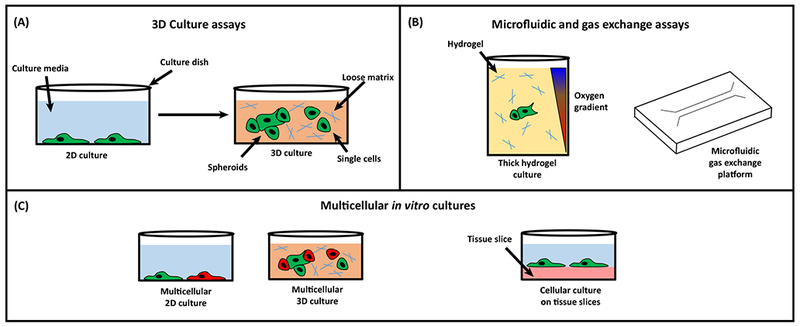
(A) Traditional 2D cultures consist of tumor cells plated on a glass or plastic surface. These geometrical constraints are alleviated by moving to 3D cultures where single tumor cells or tumor cell spheroids are grown in suspension in a deep but loose (low concentration) matrix. (B) Micro uidic and gas exchange assays provide precise control of oxygen levels for cell cultures allowing the utilization of physoixia, oxygen levels which are physiological for normal tissues and tumors. These assays have the added bene t of being able to create gradients of oxygen that can then be used to evaluate migration phenotypes. (C) Since tumors comprise multiple cell types, coculturing them together is another way to increase sophistication. This can be done either in standard 2D assays, 3D cultures, or even on top of tissue slices which can provide the most physiologically relevant extracellular matrix.
Closely related to 3D culture assays are microfluidic and controlled gas exchange platforms (Figure 1B) that both remove the dimensional constraint of 2D assays and also provide precise regulation and control of gas concentrations, either uniformly across the sample, or in gradients. These assays can also be combined with imaging systems to directly compare the motility of the cells under different oxygen conditions. These systems have revealed that tumor cells can respond to, and travel along, gradients of oxygen concentration leading to a net migration of cells towards higher oxygen levels [27].
Finally, since tumors comprise multiple cell types, coculturing them together is another way to increase sophistication and account for more complex cell–cell interactions (Figure 1C). Using Boyden chambers, one group determined that, in direct contrast to tumor cells, hypoxia dramatically inhibits monocyte and macrophage migration toward chemoattractants [28–30]. This led the group to speculate that macrophages and monocytes, which are observed to accumulate in hypoxic regions of tumors, do so by shutting down chemotaxis. This inhibition of migratory capacity in vitro was also seen in monocyte-derived dendritic cells [31]. Another group looked at the role of tumor-associated macrophages (TAMs) in gastric cancer using a 3D dynamic migration imaging system to directly visualize the motility of tumor cells under normal and hypoxic conditions, both alone and in coculture with macrophages. They found that tumor cells in 3D culture (i) exhibit reduced migration speeds when under hypoxia; (ii) show increased migration rates when cocultured with macrophages; and most surprisingly; (iii) show a variable response when cocultured with macrophages under hypoxia where the migration speed of some cell lines increase under hypoxic conditions, while others decrease [32]. These apparently conflicting data emphasize that in vitro models do not converge on a set of coherent conclusions, suggesting that the phenotypes observed in vitro may be more a property of the model than the functions these cells actually exhibit in vivo and ultimate validation of any conclusion obtained from these in vitro assays must come from the ability to observe cell phenotypes, in vivo.
Thus, while representing a step forward, these assays still show some limitations and have not significantly shifted understanding of tumor cell responses to hypoxia when compared to in vivo analyses. Since our goal is to highlight in vivo biology, we focus next on the assays that we propose will more accurately improve our understanding of hypoxia biology in metastasis.
Divergent Cell Motility Phenotypes in In Vitro versus In Vivo Hypoxia Assays
There is no doubt that advances in intravital imaging have revolutionized the analysis of biological systems [33,34]. Intravital imaging using multiphoton microscopy is a powerful technique capable of directly visualizing the phenotypes of individual cancer, immune, and other stromal cells, and their interactions in both intact solid tumors and in target organs. This technique demonstrated that tumor cells migrating in invasive carcinoma display two distinct motility patterns, slow and fast migratory phenotypes, which differ in migration speeds by a factor of ~10 [35,36]. The slow migratory phenotype in vivo, is characterized by cells that migrate with mean speeds of 8 mm/h, display increased numbers of invadopodia, increased matrix degradative ability, and increased intravasation over time [35]. Meanwhile, fast migratory cells (69 mm/h) comigrate in streams with macrophages along collagen fibers [37] (Figure 2); both of these phenotypes are required for metastatic dissemination [35,38].
Figure 2. Hypoxia-Regulated Migration and Metastasis Programs.
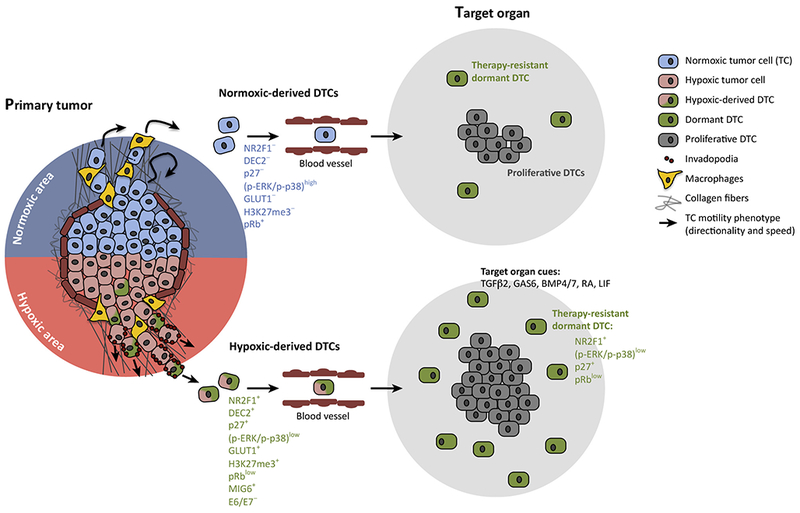
Tumor cells in solid tumors display two distinct motility patterns that are linked to hypoxic microenvironments: (Top Left) the fast migratory phenotype observed in normoxic cells, in which cells migrate rapidly, and in streams with macrophages (yellow cells), along collagen fibers; and (Bottom Left) the slow migratory phenotype observed in response to hypoxia, characterized by slower but more directional migration, where cells display increased numbers of invadopodia (red dots), increased matrix dissolving ability, and increased levels of intravasation over time. Tumor cells in hypoxic areas (red) are usually dormant or slow cycling, which is also true in secondary organs (green cells). DTCs originating from primary hypoxic microenvironments carry a hypoxia and dormancy signature (pink and green cells – DEC2+ p27+ (p-ERK/p-p38)low GLUT1+ H3K27me3+ pRblow MIG6+ E6/E7−), while cancer cells originating from normoxic regions (blue cells) do not carry a dormancy signature. Once in secondary organs, unlike normoxia-exposed cancer cells, cells emanating from hypoxic microenvironments can display a long-lived dormancy program (green cells – NR2F1+ (p-ERK/p-p38)low p27+ pRblow) reinforced by secondary organ signals (target organ cues). These hypoxic microenvironment-derived cells may have high ROS levels, silence RTK signaling or alter oncogene signaling evading chemo, targeted and radiation therapies. Adapted from [10].
Abbreviations: DTC, disseminated tumor cells; ROS, reactive oxygen species; RTK, receptor tyrosine kinase.
Importantly, comparison of the speeds reported in the few in vitro assays that measured this parameter [22,26,27,32,39,40] reveals that in vitro assays only recapitulate the slow migratory phenotype (Table 1). While treatment of the cells in culture to hypoxic conditions in some studies does result in an increase in migration rates, these elevated speeds still fall well within the slow migratory phenotype [35]. Thus, because these in vitro assays lack the appropriate microenvironment found in vivo, they lock tumor cells into the slow locomotion, degradative phenotype and completely suppress the fast migration phenotype.
Table 1.
Recapitulation of the In Vivo Slow and Fast Migratory Phenotypes with In Vitro Assays
| In vitro assay | Speed normoxia (μm/h) | Speed hypoxia (μm/h) | Refs |
|---|---|---|---|
| 3D collagen I microfluidic platform | 10 | 14 | [39] |
| 3D collagen I matrix | 6 | 6 | [26] |
| 3D collagen I matrix | 24 | 24–35 | [22] |
| 3D Matrigel matrix | 4–9 | 4–14 | [32] |
| 3D hydrogel platform | 0–8 | 4–18 | [27] |
| 1D fibronectin tracks | 60–90 | - | [41] |
| 1D fibronectin tracks | 60–90 | - | [40] |
The fast migratory phenotype in vitro can be recapitulated by reconstituting the chemotactic gradients and ECM support found in living tumors. One study built upon the known CSF1–EGF paracrine loop discovered to exist in vivo between fast comigrating macrophages and tumor cells [38] and added to the culture 2-mm diameter micropatterned fibers recreating the physiologically relevant ECM utilized by these streaming cells in vitro and forcing cell–cell interactions to occur only along this 1D surface [41] (Figure 2). Furthermore, another group [40] expanded upon this 1D coculture model by adding endothelial cells, thereby modeling the fast migration phenotype associated with blood vessel trophic streaming on collagen fibers in vivo ending on microanatomical sites within tumor high masses (composed of a Mena tumor cell, an endothelial cell, and a macrophage) [42]. These sites, called tumor microenvironments of metastasis (TMEM), act as the doorways for hematogenous dissemination [42]. Thus, this study determined that endothelial cells supply an additional HGF/c-Met axis (HGF is a powerful promigratory morphogen also known as scatter factor) to the TMEM that breaks the migration symmetry and allows sustained and persistent fast streaming migration toward blood vessels and intravasation [40,42,43].
Finally, the 3D assays in Figure 1 and Table 1 assume that invasion into the stromal matrix at tumor edges is key when assessing tumor cell metastatic capability. While it has long been speculated that the invasive front of tumors is the location of metastatic dissemination [44], this connection has been based upon associations and correlations between features observed either in in vitro models [45] or in fixed tissue histology and eventual metastatic outcome [46–49]. The intravasation of tumor cells at the invasive front of tumors has not been observed in live tissues nor functionally linked to metastasis. In contrast, studies quantifying intravasation sites across entire primary mouse mammary tumors (possessing histology like that seen in breast cancer patients) and human tumors themselves, show the location of intravasation sites to be throughout the entire tumor mass and not localized to the tumor edge [42,50,51].
Furthermore, numerous intravital imaging studies have directly observed, with single-cell resolution and in real time, the intravasation process within the core of the tumor [42,52–56]. The mechanisms underlying these intravasation events have been well elucidated [42,54,57–59] and can be directly inhibited in vitro and in vivo by blocking their underlying pathways [42,58–60].
Recent evidence using large-volume, high-resolution imaging in tumor models in chick and mouse tissues also supports the conclusion that intravasation occurs through the tumor mass [61], and a study of early-stage cancer showed that cancer cells can also disseminate very efficiently from sites associated with atypical ducts in the core of the mammary gland [56]. A recent extension of this work revealed that CD206high/Tie2high macrophages in TMEM assist early dissemination of tumor cells through vascular endothelial growth factor A signaling that causes a local loss of vascular junctions and induces transient vascular permeability and tumor cell intravasation [42], further supporting the importance of additional immune cell types in understanding and modeling dissemination [62]. Finally, the above studies correlate with clinical trials showing that TMEM sites for intravasation are found in the primary tumor interior and not the tumor stromal interface of breast cancer patients, and that TMEM numbers in the core are prognostic for distant metastatic recurrence [50,51,63,64].
In addition to advances in in vitro assays, a number of recently reviewed measurement techniques [65,66] have been developed to identify and measure hypoxic cells in vivo. We have summarized these techniques in Table 2 for easy comparison. The first of these techniques are those that label hypoxic areas of tissues in vivo and then analyze them ex vivo (i.e., in explanted tissues). This includes directly marking those tissue areas exposed to blood ow by utilizing an intravascular injection of a membrane permeable dye [67]. However, with this assay only areas perfused at the time of injection are marked. Tumor neovasculature, which is often only intermittently perfused, is therefore missed [68]. Techniques that can be applied directly to patients in the clinic include conjugated magnetic resonance imaging (MRI) [69] and positron emission tomography (PET) [70] probes and polarographic electrodes [71] or optical fibers coated with oxygen sensitive dyes [72,73]. However, these methods lack spatial resolution, do not provide information on cell type or viability, and cannot reveal oxygenation levels down to the single cell level.
Table 2.
Comparison of Different Technologies Used to Study Hypoxia In Vitro and In Vivo
| Assay | Pros | Cons | Refs |
|---|---|---|---|
| In vitro assays | |||
| Scratch assay | Easy, economical, establishes cell-cell contact before start of assay | 2D, nonphysiological, no chemical/oxygen gradients | [140,141] |
| Boyden chamber | Easy, economical, utilizes a chemical gradient | 2D, nonphysiological, removes cell-cell contacts prior to assay, no oxygen gradient | [142] |
| Invasion assay (Boyden chamber with matrix) | Easy, economical, utilizes a chemical gradient | 2D, nonphysiological, removes cell-cell contacts prior to assay, sensitive to matrix composition and density, no oxygen gradient | [143] |
| Culture dish hypoxia chambers | Easy, economical, creates gradients of oxygen | 2D, nonphysiological | [144] |
| 3D culture | Moderately easy, mimics 3D structure of tumors | Nonphysiological, sensitive to matrix composition and density | [145,146] |
| Invasion into tissue sections | Mimics 3D structure of tissue, realistic matrix | Lacks connection to vasculature | [123] |
| Thick hydrogel | Moderately easy, mimics 3D structure of tumors, creates gradient of oxygen | Complex, nonphysiological, limited to hydrogel | [27] |
| Microfluidic controlled gas exchange platforms | Mimics 3D structure of tumors, creates controllable oxygen gradients | Complex, nonphysiological | [39] |
| Ex vivo assays | |||
| Staining for endogenous hypoxia markers in sectioned tissue | Reflects true physiology at time of excision | Requires sectioning of tissue, different makers do not colocalize and reflect different timing post exposure to hypoxia | [76,147–149] |
| Nitroimidazole adducts for sectioned tissue | Reflects true physiology at time of excision, can be used in clinic, most sensitive at low oxygen levels | Requires sectioning of the tissue | [150–152] |
| Dye injections | Only stains perfused cells | Indirect marker only showing perfusion, requires sectioning of tissue | [67] |
| Soluble phosphorescent probes | Directly measures oxygen levels | Requires sectioning of tissue, does not indicate history of cells | [153,154] |
| In vivo assays | |||
| Polarographic electrodes | Gold standard, approved for use in clinic | Susceptible to oxygen consumption by probe, damaging to tissue, averages over many cells, limited to only a few locations | [155,156] |
| MRI, PET or CT probes | Noninvasive | Lacks single cell resolution | [69,157] |
| Fiber optic phosphorescent probes | Does notconsume oxygen, higher sensitivity at physiological oxygenation | Damaging to tissue, averages over many cells, variable results | [73,158] |
| Fluorescent protein biosensors | Genetically encoded, allows realtime, single-cell visualization | Cannot be used in clinic, may be delayed with respect to exposure to hypoxia | [6,80,86,159] |
Other techniques are designed around staining of excised tissues either for proteins within the hypoxia response pathway (e.g., HIF [74,75], glucose transporter (GLUT)1 [76], carbonic anhydrase (CA)IX [77]), or with molecules from the nitroimidazole class [78] (e.g., pimonidazole) that are reductively metabolized to form covalent adducts in hypoxic cells. These techniques provide the requisite spatial resolution to analyze hypoxia; however, as they are methods used on fixed tissue sections they cannot inform on cellular motility and invasion. In addition, these stains often do not correlate with each other owing to their dramatically different onset and decay lifetimes after hypoxic exposure. Comparison of HIF-1a, CAIX, and pimonidazole staining [79] showed that the onset of HIF-1a and pimonidazole only takes 1–2 h, while CAIX takes upwards of 16 h. Meanwhile, pimonidazole and CAIX last for days after exposure while HIF-1a rapidly decays (1–2 min).
Techniques that enable in vivo measurements of single-cell motility and invasion include the engineering of cells to express fluorescent or bioluminescent proteins that are expressed in response to hypoxic conditions. These hypoxia biosensors typically use multiple copies of the hypoxia response element (HRE) gene promoter to drive expression of the biosensor protein. Inclusion of the oxygen dependent degradation (ODD) domain of the HIF-1a protein further reduces background signal and increases hypoxia-speci city of the biosensor. Several reporter proteins were developed including GFP [80–82], YFP [83], and firefly luciferase [84,85]; albeit with some limitations. For example, GFP did not demonstrate expression at levels suf cient for unequivocal visualization in tumor sections or live tissues [86]. Additionally, the choice of GFP, YFP, and firefly luciferase complicated analyses as the formation of these chromophores depends on the presence of molecular oxygen [87,88]. Thus, the uorescence intensity is not necessarily proportional to the amount of synthesized reporter protein [89]. Recognizing these chromophore limitations, one group has developed a fluorescence resonance energy transfer (FRET)-based biosensor called FluBO using a combination of YFP and a avin-binding fluorescent protein [89]. Additionally, another study used mCherry, which is not dependent on oxygen for folding and fluorescence [6]. Although these reporters allow the tracking of the motility and invasive phenotypes of hypoxic cells with single cell resolution, only two of the studies utilizing these in vivo probes [6,85] analyzed migration and invasion of tumor cells.
Another study utilized a novel luciferase-based reporter [85] that could be induced to permanently mark tumor cells and their progeny at a speci c time; cells were traced by sacri cing mice at different time points post induction and compared to other staining markers such as HIF-1a and pimonidazole. While innovative, the interpretation of much of the data was dependent upon the assumption that HIF-1a and pimonidazole areas remain static in the tumor. However, in the rapidly growing tumor, this assumption does not hold.
The discrepancies raised above were resolved using intravital imaging [6] as this study was the first to directly visualize the motility of hypoxic tumor cells, with single-cell resolution, in living tissue. The authors found that hypoxic cells show dramatically reduced migration speeds (~24 mm/h) compared to normoxic cells (~66 mm/h). This places hypoxic cells into the slow migratory phenotype associated with invasion and dissemination as observed previously [35]. Concordant with this, hypoxic cells were also observed to have dramatically increased invadopodia with degradative activity and be more chemotactic toward flowing blood vessels with which hypoxic cells were found to associate in vivo [6] (Figure 1).
Using the in vivo invasion assay [6], 83% of collected tumor cells were observed to be hypoxic. In contrast, only ~33% of the tumor cells in the primary tumor were observed to be hypoxic. Thus, although hypoxic tumor cells show dramatically reduced migratory speeds, their increased degradative ability and chemotaxis toward blood vessel associated growth factors resulted in greater intravasation and dissemination.
The above studies highlight the importance of in vivo imaging of tumor cell phenotypes associated with invasion and intravasation. Importantly, the phenotype of hypoxic cells can be long lived, and the slow but efficient movement towards vessels that they exhibit is more persistent than anticipated from in vitro studies. These findings then raise questions as to how this long-lived migratory phenotype of hypoxic tumor cells is carried over to distant sites by disseminating cells. The next section will explore how the hypoxic or normoxic phenotypes affect DTC behavior and its ability to initiate a metastatic colony.
Routes by Which Hypoxia Affects DTC Fate
A large body of literature that has been expertly reviewed recently links in vitro hypoxia to the epithelial-to-mesenchymal transition or EMT, which allows epithelial cells to reduce their interaction with other epithelial cells and become motile [5,90,91]. Given that these mechanisms and papers were reviewed in the past we focus here primarily on in vivo studies. Also, because we attempt to understand how naturally occurring hypoxia in target organs affects DTC behavior, we do not focus on how therapies, either chemotherapy and/or antiangiogenic therapies affect hypoxia in tumors [5,92–96]. As mentioned earlier, most of the measurements and conclusions of the biology of hypoxia have been derived from primary tumor biology and in vitro studies. However, the impact of hypoxia on tumor phenotypes does not end in the primary site. How hypoxia affects intravasating cells also needs to be taken into consideration at the secondary organs where physiology and oxygen levels may vary. Additionally, acute, cycling, and chronic hypoxia can coexist in tumors and often lead to different biological consequences (See Box 2 and [5]), with both associated with poor clinical outcome. However, in clinical studies, the challenge is to determine where hypoxia speci cally in uences the endpoints of clinical outcome. We attempt to provide an analysis of this question, focusing mostly on in vivo experiments and mention the oxygen tension and exposure duration of the hypoxic conditions for context and comparisons.
Box 2. Acute versus Chronic Hypoxia.
Acute or perfusion hypoxia (0.05–2.3% O2) [5]:
is an abrupt and brief (of the order of minutes) exposure to hypoxia due to structural and functional abnormalities in the tumor microvessels [125];
Ieads to cell survival by activation of autophagic, apoptotic, and metabolic adaptation pathways and a decrease of oxidative metabolism [126,127].
Chronic or diffusion limited hypoxia (≤0.05% O2) [5]:
occurs due to hyperproliferation of cancer cells that surpasses the capabilities of the vascular supply leading to tumor cells being exposed to continuous hypoxia for long periods [125];
activates cell survival and progression programs through increased ROS production [128];
has been linked to a more aggressive tumor phenotype, usually measured by in vitro parameters of motility and invasion, but also through measurements of lung colonization and metastasis development [21,119,129–139].
Role of Hypoxia in Affecting Metastatic Capacity through Motility and Growth Programs
Limited oxygen supply has been historically proposed as a rate-limiting factor for primary tumor growth, where it is suggested that the tumor could not surpass 1 mm3 if hypoxic cells do not mount a neovascularization response [97]. However, in a mouse model of melanoma [98], inactivation of HIF1a or HIF-2a did not affect primary tumor formation but instead selectively abrogated metastasis. This result suggests that in some cancers tumor growth is actually independent of HIF signaling but HIF function is critical for metastasis development. This unexpected result supports the need to understand how hypoxia shapes niches in the metastatic setting. Along these lines another study described that the ECM protein, lysyl oxidase (LOX), is regulated by hypoxia and HIF [99]; LOX upregulation correlates with poor distant metastasis-free and overall survival in human breast and head and neck tumors. When LOX is secreted, it is responsible for the invasive properties of hypoxic cancer cells (1–2% O2 for 18 or 24 h) through focal adhesion kinase activity and cell-to-matrix adhesion [99,100]. Moreover, LOX blockade strikingly decreased metastasis, suggesting this enzyme to be a metastatic niche therapeutic target [99,100]. LOX secreted by hypoxic primary tumors was proposed to be critical for premetastatic niche formation as it results in crosslinked collagen IV and the recruitment of CD11b+ myeloid cells; all factors that are prometastatic. These data suggest that hypoxia in the primary site can affect the secondary organ [19]. It will be important to understand how these mechanisms of hypoxia-regulated premetastatic niche organization are sustained after primary tumor surgery, as patients may not develop metastasis for years after the source of these signals is removed.
Hypoxia and HIF1α also in uence the splicing of a specific variant of the scaffold protein A-kinase anchor protein 12 (AKAP12). AKAP12v2 is a direct HIF target and regulates protein kinase A (PKA) activity under hypoxia (2% O2 for 16 h), favoring tumor cell invasion and migration in vitro, but most importantly, metastasis in an in vivo orthotopic model of melanoma [101]. Additionally, metastatic samples have higher levels of AKAP12v2 than the primary tumor has [101–105]. It is possible that hypoxia-induced splicing of AKAP12v2 at the primary site provides an advantage for dissemination and/or colonization at the metastatic sites. The latter possibility would suggest that AKAP12v2 splicing is part of a posthypoxic program that is self-sustained in secondary organs.
Leukemia inhibitory factor (LIF), which is produced by osteoblast-lineage and bone marrow (BM) stromal cells, can induce disseminated breast cancer cell dormancy [106], a reversible growth arrest state. Accordingly LIF receptor (LIFR) functions as a metastasis inhibitor in different cancers [106,107] and, in breast cancer patients with bone metastases, low levels of LIFR are correlated with poor patient outcome. Intriguingly, hypoxia (0.5% O2 for 24 h) and HIF signaling reduce the LIFR–STAT3–SOCS3 signaling pathway in breast cancer cells in vitro leading to downregulation of dormancy related genes. These data suggest that hypoxia may awaken dormant cells by inhibition of LIFR–STAT3-mediated dormancy [106]. In vivo validation of these findings may support some of the speculation derived from the in vitro ndings [106] that are countered by the findings that dormant DTCs can persist in the BM for years despite this being a proposed hypoxic niche [108]. It would also be interesting to know if primary tumor hypoxia already renders DTCs insensitive to LIF signaling in secondary sites.
The above studies comprise a handful of recent papers with in vivo data that support the notion that hypoxia can activate traits that render cancer cells more migratory, invasive, and apt to escape dormancy. However, given the heterogeneous nature of the tumor cell population, their response to hypoxia may be also as heterogeneous.
Hypoxia as a Regulator of Dormancy and Stress-Tolerance Programs in DTCs
Numerous studies suggest that hypoxia can induce as an adaptive survival response growth arrest and stem-cell-related programs [4,109,110]. This allows cells to pause proliferation while adapting to hypoxia and then resume growth. Thus, this transient growth arrest in growing tumors contributes to the viability of cells that can resume growth. Hypoxia activates an unfolded protein response (UPR) that results in the attenuation of translation initiation and the induction of cell cycle arrest genes [4,109,110]. Intriguingly, the UPR was found in dormant cancer cells in experimental models [111] and transcripts for speci c UPR genes were also found to be upregulated in dormant DTCs isolated from BM of prostate cancer patients [112]. These genes could also be turned on in DTCs in the target organ by the fact that they carry a signature epigenetically imprinted in the primary tumors (i.e., the DTCs carry a remodeled chromatin in response to hypoxia in the primary site) [7,113]. Accordingly, hypoxic micro-environments in primary lesions contain a fraction of cells that concomitantly turn on hypoxia (GLUT1 and HIF1a) and long-term dormancy genes such as NR2F1, DEC2, and p27. Presumably, these would be the slow, but persistent migratory cells that ef ciently reach blood vessels for dissemination (see first section on motility) [6]. Additionally, both NR2F1 and HIF1a are required for p27 induction in dormant DTCs originating from hypoxic primary tumor microenvironments, but these DTCs do not display GLUT1high expression, supporting the concept that in secondary sites, a dormancy program is sustained even after the hypoxic response is resolved (Figure 2). The dormancy-prone hypoxic cells also display accumulation of transcriptionally repressive histone marks (H3K27me3), suggesting that an epigenetic change is taking place in the dormancy-inducing primary tumor hypoxic microenvironments. Interestingly, this histone H3 repressive mark is found in dormant cells [114], suggesting that it could be a regulator of a persistent dormancy program retained in DTCs originating from hypoxic primary tumor niches. Importantly, hypoxia-induced dormant-like DTCs survive chemotherapy [7]. These findings lead to the notion that these once hypoxic and now dormant DTCs may be the source of disease relapse and poor prognosis associated with hypoxia. Hypoxia-associated poor prognosis is derived from measurements in primary tumors, but survival is dictated by metastases. Thus, it is possible, that the dormancy-inducing capacity of hypoxia allows a subpopulation of DTCs to robustly survive treatment and then ultimately determine the fate of patients as they reactivate.
In an HPV+ head and neck squamous cell carcinoma model, tumors express the viral E6/E7 oncogenes, which inhibit p53 expression/function resulting in induced senescence or cell death [115]. What was found is that under hypoxic conditions where E6/E7 are repressed, but p53 is not induced; instead, these cells experience growth arrest upon reoxygenation in a reversible manner controlled via the nutrient sensing mTOR/REDD1/TSC2 signaling axis [115]. This suggests that HPV+ tumor cells low may enter a therapy resistant, p53low, dormant phenotype in response to hypoxia, serving as a source of recurrence [115]. Overall, these studies provide additional evidence that some cancer cell subpopulations respond to hypoxia by activating a growth arrest program that, like dormancy, can lead to increased therapeutic resistance.
Analysis of receptor tyrosine kinase (RTK) signaling in lung cancer cells also revealed that hypoxia induced dormancy of mutant epidermal growth factor receptor (EGFR) (ErbB1) primary lung cancer cells through upregulation of MIG6; MIG6 results in the disassembly of the ErbB receptor heterodimers, blocking their signaling for growth. Critically this phenotype renders mutant EGFR lung cancer cells resistant to specific EGFR inhibitors, while MIG6 downregulation restores EGFR signaling and sensitivity to tyrosine kinase inhibitors and radiation. Lung cancer patients carrying EGFR mutations and MIG6 up-regulation showed shorter disease free periods. Thus, coexistence of dormant hypoxic tumor cells and proliferative cells might allow lung tumors to adapt to therapeutic stress and eventually progress unrestricted [116].
It has been recently described that successful metastasizing melanomas underwent reversible metabolic changes that increased their oxidative stress resistance through NADPH-generating enzymes in the folate pathway [117]. Using cells with high primary tumor forming capacity, but low metastatic efficiency, the authors showed that tumor cells in the blood and visceral organs experienced oxidative stress not observed in the bulk primary tumors. Antioxidants promoted distant metastasis without significantly affecting primary tumor growth [117]. While the authors did not explore dormancy mechanisms, we propose that perhaps hypoxia induced oxidative stress may prime melanoma cells to enter dormancy, and as a consequence limit distant metastasis in vivo.
Concluding Remarks and Future Perspectives
Our analysis of recent literature reveals that understanding how hypoxia affects metastasis will require high resolution intravital imaging at single cell resolution and/or other methods that complement intravital imaging in vivo. These are key to help de ne the actual phenotypes of cells in primary tumors and secondary organs both in hypoxic and normoxic microenvironments. It is expected that in vivo studies will then inform more physiologic in vitro assays. Finally, the notion that hypoxia in primary sites can have long term effects on secondary organ DTC biology needs to be explored in more detail (see Outstanding Questions). The integration of efficient hypoxia biosensors with fate mapping tools that allow for longitudinal tracking of DTCs may be necessary to link primary tumor biology to secondary organ DTC fate. Overall, understanding the fate of DTCs in vivo will ultimately guide us to understand how to target these hypoxia-influenced events to prevent and treat metastasis more ef ciently.
Outstanding Questions.
How is hypoxia linked to the fate of DTCs?
How does hypoxia affect the manifestation of metastasis years after primary tumor surgery and what happens to premetastatic niches in uenced by hypoxia regulated mechanisms?
How could hypoxia lead to increased therapeutic resistance of DTCs, which may serve as reservoirs for tumor recurrence after reoxygenation?
What is the role of epigenetic mechanisms in driving hypoxia in uenced DTC fate and how do organ cues required to maintain organ homeostasis affect the fate of DTCs derived from hypoxic and normoxic microenvironments?
Highlights.
Hypoxia is associated with resistance to therapy and metastasis onset but mechanistically this link is still unclear.
In vitro assays to study hypoxia in metastasis have limitations that lead to limited consensus on how it drives metastasis.
New intravital imaging technologies enable studies of hypoxia and metastasis in physiological contexts, revealing unanticipated roles of hypoxia not observed in vitro.
In vivo studies revealed that hypoxia could decrease motility speed while increasing invasiveness and priming tumor cells for dormancy after dissemination.
In vivo analysis of hypoxia and DTC fate may reveal its link to metastasis and poor prognosis and how to prevent recurrence.
Acknowledgments
ARN and JAA-G were supported by the Samuel Waxman Cancer Research Foundation Tumor Dormancy Program, NIH/NCI CA163131 CA109182 CA218024 and CA196521. JC, YW, and DE acknowledge the support of Einstein’s Integrated Imaging Program, and the National Institutes of Health grants CA100324, and CA216248.
References
- 1.Fisher SA and Burggren WW (2007) Role of hypoxia in the evolution and development of the cardiovascular system. Antioxid. Redox Signal 9, 1339–1352 [DOI] [PubMed] [Google Scholar]
- 2.Okazaki K and Maltepe E (2006) Oxygen, epigenetics and stem cell fate. Regen. Med 1, 71–83 [DOI] [PubMed] [Google Scholar]
- 3.Dunwoodie SL (2009) The role of hypoxia in development of the mammalian embryo. Dev. Cell 17, 755–773 [DOI] [PubMed] [Google Scholar]
- 4.Hockel M and Vaupel P (2001) Tumor hypoxia: de nitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst 93, 266–276 [DOI] [PubMed] [Google Scholar]
- 5.Muz B et al. (2015) The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl) 3, 83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y et al. (2016) Direct visualization of the phenotype of hypoxic tumor cells at single cell resolution in vivo using a new hypoxia probe. Intravital 5, e1187803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fluegen G et al. (2017) Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic micro-environments. Nat. Cell Biol 19, 120–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert AW et al. (2017) Emerging biological principles of metastasis. Cell 168, 670–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan R and Graham CH (2007) Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev 26, 319–331 [DOI] [PubMed] [Google Scholar]
- 10.Vaupel P et al. (2004) Tumor hypoxia and malignant progression. Methods Enzymol 381, 335–354 [DOI] [PubMed] [Google Scholar]
- 11.Chang J and Erler J (2014) Hypoxia-mediated metastasis. Adv. Exp. Med. Biol 772, 55–81 [DOI] [PubMed] [Google Scholar]
- 12.Chan DA and Giaccia AJ (2007) Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev 26, 333–339 [DOI] [PubMed] [Google Scholar]
- 13.Young SD et al. (1988) Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc. Natl. Acad. Sci. U. S. A 85, 9533–9537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mottram J (1936) A factor of importance in the radio sensitivity of tumours. Br. J. Radiol 9, 606–614 [Google Scholar]
- 15.Young SD and Hill RP (1990) Effects of reoxygenation on cells from hypoxic regions of solid tumors: anticancer drug sensitivity and metastatic potential. J. Natl. Cancer Inst 82, 371–380 [DOI] [PubMed] [Google Scholar]
- 16.Vaupel P et al. (2007) Detection and characterization of tumor hypoxia using pO2 histography. Antioxid. Redox Signal 9, 1221–1235 [DOI] [PubMed] [Google Scholar]
- 17.McKeown SR (2014) De ning normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol 87, 20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krohn KA et al. (2008) Molecular imaging of hypoxia. J. Nucl. Med 49 (Suppl 2), 129S–148S [DOI] [PubMed] [Google Scholar]
- 19.Erler JT et al. (2009) Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premeta-static niche. Cancer Cell 15, 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes CS et al. (2010) Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886–1890 [DOI] [PubMed] [Google Scholar]
- 21.Romain CV et al. (2014) Targeting aurora kinase A inhibits hypoxia-mediated neuroblastoma cell tumorigenesis. Anticancer Res 34, 2269–2274 [PMC free article] [PubMed] [Google Scholar]
- 22.Gilkes DM et al. (2014) Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc. Natl. Acad. Sci. U. S. A 111, E384–E393 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Zaman MH et al. (2006) Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. U. S. A 103, 10889–10894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer AS et al. (2012) 2D protrusion but not motility predicts growth factor-induced cancer cell migration in 3D collagen. J. Cell Biol 197, 721–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franko AJ and Sutherland RM (1978) Rate of death of hypoxic cells in multicell spheroids. Radiat. Res 76, 561–572 [PubMed] [Google Scholar]
- 26.Lehmann S et al. (2017) Hypoxia induces a HIF-1-dependent transition from collective-to-amoeboid dissemination in epithelial cancer cells. Curr. Biol 27, 392–400 [DOI] [PubMed] [Google Scholar]
- 27.Lewis DM et al. (2016) Intratumoral oxygen gradients mediate sarcoma cell invasion. Proc. Natl. Acad. Sci. U. S. A 113, 9292–9297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negus RP et al. (1998) Hypoxia down-regulates MCP-1 expression: implications for macrophage distribution in tumors.J. Leukoc. Biol 63, 758–765 [DOI] [PubMed] [Google Scholar]
- 29.Turner L et al. (1999) Hypoxia inhibits macrophage migration. Eur. J. Immunol 29, 2280–2287 [DOI] [PubMed] [Google Scholar]
- 30.Grimshaw MJ and Balkwill FR (2001) Inhibition of monocyte and macrophage chemotaxis by hypoxia and in ammation – a potential mechanism. Eur. J. Immunol 31, 480–489 [DOI] [PubMed] [Google Scholar]
- 31.Qu X et al. (2005) Hypoxia inhibits the migratory capacity of human monocyte-derived dendritic cells. Immunol. Cell Biol 83, 668–673 [DOI] [PubMed] [Google Scholar]
- 32.Shen Z et al. (2013) Both macrophages and hypoxia play critical role in regulating invasion of gastric cancer in vitro. Acta Oncol 52, 852–860 [DOI] [PubMed] [Google Scholar]
- 33.Liu TL et al. (2018) Observing the cell in its native state: imaging subcellular dynamics in multicellular organisms. Science 360, eaaq1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Entenberg D et al. (2017) Time-lapsed, large-volume, high-resolution intravital imaging for tissue-wide analysis of single cell dynamics. Methods 128, 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gligorijevic B et al. (2014) Multiparametric classi cation links tumor microenvironments with tumor cell phenotype. PLoS Biol 12, e1001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W et al. (2002) Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular pro ling. Cancer Res 62, 6278–6288 [PubMed] [Google Scholar]
- 37.Sidani M et al. (2006) Probing the microenvironment of mam-mary tumors using multiphoton microscopy. J. Mammary Gland Biol. Neoplasia 11, 151–163 [DOI] [PubMed] [Google Scholar]
- 38.Wyckoff J et al. (2004) A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mam-mary tumors. Cancer Res 64, 7022–7029 [DOI] [PubMed] [Google Scholar]
- 39.Funamoto K et al. (2012) A novel micro uidic platform for high-resolution imaging of a three-dimensional cell culture under a controlled hypoxic environment. Lab Chip 12, 4855–4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung E et al. (2017) Blood vessel endothelium-directed tumor cell streaming in breast tumors requires the HGF/C-Met signaling pathway. Oncogene 36, 2680–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma VP et al. (2012) Reconstitution of in vivo macrophage-tumor cell pairing and streaming motility on one-dimensional micro-patterned substrates. Intravital 1, 77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harney AS et al. (2015) Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov 5, 932–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karagiannis GS et al. (2017) Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci. Transl. Med 9, eaan0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bryne M et al. (1989) New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. J. Oral Pathol. Med 18, 432–437 [DOI] [PubMed] [Google Scholar]
- 45.Cao C et al. (2012) alpha-Catulin marks the invasion front of squamous cell carcinoma and is important for tumor cell metastasis. Mol. Cancer Res 10, 892–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim SC et al. (2004) Predictive markers for late cervical metastasis in stage I and II invasive squamous cell carcinoma of the oral tongue. Clin. Cancer Res 10, 166–172 [DOI] [PubMed] [Google Scholar]
- 47.Sawair FA et al. (2003) Invasive front grading: reliability and usefulness in the management of oral squamous cell carcinoma. J. Oral Pathol. Med 32, 1–9 [DOI] [PubMed] [Google Scholar]
- 48.Eiro N et al. (2012) Impact of CD68/(CD3+CD20) ratio at the invasive front of primary tumors on distant metastasis development in breast cancer. PLoS One 7, e52796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalil AA et al. (2017) Collective invasion in ductal and lobular breast cancer associates with distant metastasis. Clin. Exp. Metastasis 34, 421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson BD et al. (2009) Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin. Cancer Res 15, 2433–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rohan TE et al. (2014) Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J. Natl. Cancer Inst 106, dju136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wyckoff JB et al. (2000) A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res 60, 2504–2511 [PubMed] [Google Scholar]
- 53.Entenberg D et al. (2013) Imaging tumor cell movement in vivo Curr. Protoc. Cell Biol Chapter 19, Unit 19.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyckoff JB et al. (2007) Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 67, 2649–2656 [DOI] [PubMed] [Google Scholar]
- 55.Kedrin D et al. (2008) Intravital imaging of metastatic behavior through a mammary imaging window. Nat. Methods 5, 1019–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harper KL et al. (2016) Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature 540, 589–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roussos ET et al. (2011) Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J. Cell Sci 124, 2120–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pignatelli J et al. (2016) Macrophage-dependent tumor cell transendothelial migration is mediated by Notch1/MenaINV-initiated invadopodium formation. Sci. Rep 6, 37874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pignatelli J et al. (2014) Invasive breast carcinoma cells from patients exhibit MenaINV- and macrophage-dependent transendothelial migration. Sci. Signal 7, ra112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harney AS et al. (2017) The selective Tie2 inhibitor rebastinib blocks recruitment and function of Tie2(Hi) macrophages in breast cancer and pancreatic neuroendocrine tumors. Mol. Cancer Ther 16, 2486–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deryugina EI and Kiosses WB (2017) Intratumoral cancer cell intravasation can occur independent of invasion into the adjacent stroma. Cell Rep 19, 601–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Linde N et al. (2018) Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sparano JA et al. (2017) A metastasis biomarker (MetaSite Breast Score) is associated with distant recurrence in hormone receptor-positive, HER2-negative early-stage breast cancer. NPJ Breast Cancer 3, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karagiannis GS et al. (2016) Signatures of breast cancer metastasis at a glance. J. Cell Sci 129, 1751–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Springett R and Swartz HM (2007) Measurements of oxygen in vivo: overview and perspectives on methods to measure oxygen within cells and tissues. Antioxid. Redox Signal 9, 1295–1301 [DOI] [PubMed] [Google Scholar]
- 66.Serganova I et al. (2008) Molecular imaging: reporter gene imaging. Handb. Exp. Pharmacol 185, 167–223 [DOI] [PubMed] [Google Scholar]
- 67.Chaplin DJ et al. (1985) Cell selection from a murine tumour using the fluorescent probe Hoechst 33342. Br. J. Cancer 51, 569–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Helmlinger G et al. (1997) Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med 3, 177–182 [DOI] [PubMed] [Google Scholar]
- 69.Rojas-Quijano FA et al. (2012) Synthesis and characterization of a hypoxia-sensitive MRI probe. Chemistry 18, 9669–9676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bollineni VR et al. (2013) PET imaging of tumor hypoxia using 18F-fluoroazomycin arabinoside in stage III-IV non-small cell lung cancer patients. J. Nucl. Med 54, 1175–1180 [DOI] [PubMed] [Google Scholar]
- 71.Vaupel P et al. (1991) Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res 51, 3316–3322 [PubMed] [Google Scholar]
- 72.Bussink J et al. (2000) Optical sensor-based oxygen tension measurements correspond with hypoxia marker binding in three human tumor xenograft lines. Radiat. Res 154, 547–555 [DOI] [PubMed] [Google Scholar]
- 73.Young WK et al. (1996) Measurement of oxygen tension in tumours by time-resolved fluorescence. Br. J. Cancer Suppl 27, S256–9 [PMC free article] [PubMed] [Google Scholar]
- 74.Wang GL and Semenza GL (1993) Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem 268, 21513–21518 [PubMed] [Google Scholar]
- 75.Talks KL et al. (2000) The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol 157, 411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cooper R et al. (2003) Glucose transporter-1 (GLUT-1): a potential marker of prognosis in rectal carcinoma? Br. J. Cancer 89, 870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giatromanolaki A et al. (2001) Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res 61, 7992–7998 [PubMed] [Google Scholar]
- 78.Varghese AJ et al. (1976) Hypoxia-dependent reduction of 1-(2-nitro-1-imidazolyl)-3-methoxy-2-propanol by Chinese hamster ovary cells and KHT tumor cells in vitro and in vivo. Cancer Res 36, 3761–3765 [PubMed] [Google Scholar]
- 79.Sobhanifar S et al. (2005) Reduced expression of hypoxiainducible factor-1alpha in perinecrotic regions of solid tumors. Cancer Res 65, 7259–7266 [DOI] [PubMed] [Google Scholar]
- 80.Vordermark D et al. (2001) Green fluorescent protein is a suitable reporter of tumor hypoxia despite an oxygen requirement for chromophore formation. Neoplasia 3, 527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wen B et al. (2004) A preclinical model for noninvasive imaging of hypoxia-induced gene expression; comparison with an exogenous marker of tumor hypoxia. Eur. J. Nucl. Med. Mol. Imaging 31, 1530–1538 [DOI] [PubMed] [Google Scholar]
- 82.Palmer GM et al. (2011) In vivo optical molecular imaging and analysis in mice using dorsal window chamber models applied to hypoxia, vasculature and fluorescent reporters. Nat. Protoc 6, 1355–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou W et al. (2011) Assessment of hypoxia inducible factor levels in cancer cell lines upon hypoxic induction using a novel reporter construct. PLoS One 6, e27460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Safran M et al. (2006) Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc. Natl. Acad. Sci. U. S. A 103, 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harada H et al. (2012) Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate towards tumour blood vessels. Nat. Commun 3, 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He F et al. (2008) Noninvasive molecular imaging of hypoxia in human xenografts: comparing hypoxia-induced gene expression with endogenous and exogenous hypoxia markers. Cancer Res 68, 8597–8606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reid BG and Flynn GC (1997) Chromophore formation in green fluorescent protein. Biochemistry 36, 6786–6791 [DOI] [PubMed] [Google Scholar]
- 88.Baldwin TO (1996) Fire y luciferase: the structure is known, but the mystery remains. Structure 4, 223–228 [DOI] [PubMed] [Google Scholar]
- 89.Potzkei J et al. (2012) Real-time determination of intracellular oxygen in bacteria using a genetically encoded FRET-based biosensor. BMC Biol 10, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nantajit D et al. (2015) The network of epithelial-mesenchymal transition: potential new targets for tumor resistance. J. Cancer Res. Clin. Oncol 141, 1697–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forte E et al. (2017) EMT/MET at the crossroad of stemness, regeneration and oncogenesis: the ying-yang equilibrium recapitulated in cell spheroids. Cancers (Basel) 9, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DeClerck K and Elble RC (2010) The role of hypoxia and acidosis in promoting metastasis and resistance to chemotherapy. Front. Biosci. (Landmark Ed) 15, 213–225 [DOI] [PubMed] [Google Scholar]
- 93.Rohwer N and Cramer T (2011) Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist. Updates 14, 191–201 [DOI] [PubMed] [Google Scholar]
- 94.Wilson WR and Hay MP (2011) Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 11, 393–410 [DOI] [PubMed] [Google Scholar]
- 95.Cooke VG et al. (2012) Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 21, 66–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hughes VS et al. (2018) Tumor oxygenation and cancer therapy – then and now. Br. J. Radiol. Published online March 14, 2018 10.1259/bjr.20170955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gimbrone MA Jr et al. (1972) Tumor dormancy in vivo by prevention of neovascularization. J. Exp. Med 136, 261–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hanna SC et al. (2013) HIF1alpha and HIF2alpha independently activate SRC to promote melanoma metastases. J. Clin. Invest 123, 2078–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Erler JT and Giaccia AJ (2006) Lysyl oxidase mediates hypoxic control of metastasis. Cancer Res 66, 10238–10241 [DOI] [PubMed] [Google Scholar]
- 100.Erler JT et al. (2006) Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440, 1222–1226 [DOI] [PubMed] [Google Scholar]
- 101.Finger EC et al. (2015) Hypoxic induction of AKAP12 variant 2 shifts PKA-mediated protein phosphorylation to enhance migration and metastasis of melanoma cells. Proc. Natl. Acad. Sci. U. S. A 112, 4441–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haqq C et al. (2005) The gene expression signatures of melanoma progression. Proc. Natl. Acad. Sci. U. S. A 102, 6092–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Talantov D et al. (2005) Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin. Cancer Res 11, 7234–7242 [DOI] [PubMed] [Google Scholar]
- 104.Bittner M et al. (2000) Molecular classi cation of cutaneous malignant melanoma by gene expression pro ling. Nature 406, 536–540 [DOI] [PubMed] [Google Scholar]
- 105.Xu L et al. (2008) Gene expression changes in an animal melanoma model correlate with aggressiveness of human melanoma metastases. Mol. Cancer Res 6, 760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnson RW et al. (2016) Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat. Cell Biol 18, 1078–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen D et al. (2012) LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat. Med 18, 1511–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spencer JA et al. (2014) Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rouschop KM et al. (2013) PERK/eIF2alpha signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc. Natl. Acad. Sci. U. S. A 110, 4622–4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rouschop KM et al. (2010) The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J. Clin. Invest 120, 127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ranganathan AC et al. (2006) Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res 66, 1702–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chery L et al. (2014) Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget 5, 9939–9951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bragado P et al. (2013) TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat. Cell Biol 15, 1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sosa MS et al. (2015) NR2F1 controls tumour cell dormancy via SOX9- and RARbeta-driven quiescence programmes. Nat. Commun 6, 6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoppe-Seyler K et al. (2017) Induction of dormancy in hypoxic human papillomavirus-positive cancer cells. Proc. Natl. Acad. Sci. U. S. A 114, E990–E998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Endo H et al. (2017) The induction of MIG6 under hypoxic conditions is critical for dormancy in primary cultured lung cancer cells with activating EGFR mutations. Oncogene 36, 2824–2834 [DOI] [PubMed] [Google Scholar]
- 117.Piskounova E et al. (2015) Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu L et al. (2017) Hypoxia-inducible factor 1 mediates intermittent hypoxia-induced migration of human breast cancer MDA-MB-231 cells. Oncol. Lett 14, 7715–7722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salnikov AV et al. (2012) Hypoxia induces EMT in low and highly aggressive pancreatic tumor cells but only cells with cancer stem cell characteristics acquire pronounced migratory potential. PLoS One 7, e46391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krtolica A and Ludlow JW (1996) Hypoxia arrests ovarian carcinoma cell cycle progression, but invasion is unaffected. Cancer Res 56, 1168–1173 [PubMed] [Google Scholar]
- 121.Cuvier C et al. (1997) Exposure to hypoxia, glucose starvation and acidosis: effect on invasive capacity of murine tumor cells and correlation with cathepsin (L + B) secretion. Clin. Exp. Metastasis 15, 19–25 [DOI] [PubMed] [Google Scholar]
- 122.Krishnamachary B et al. (2003) Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res 63, 1138–1143 [PubMed] [Google Scholar]
- 123.Fujiwara S et al. (2007) Silencing hypoxia-inducible factor-1alpha inhibits cell migration and invasion under hypoxic environment in malignant gliomas. Int. J. Oncol 30, 793–802 [PubMed] [Google Scholar]
- 124.Dai Y et al. (2011) Impact of hypoxia on the metastatic potential of human prostate cancer cells. Int. J. Radiat. Oncol. Biol. Phys 81, 521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bayer C and Vaupel P (2012) Acute versus chronic hypoxia in tumors: controversial data concerning time frames and biological consequences. Strahlenther. Onkol 188, 616–627 [DOI] [PubMed] [Google Scholar]
- 126.Mazure NM and Pouyssegur J (2010) Hypoxia-induced autophagy: cell death or cell survival? Curr. Opin. Cell Biol 22, 177–180 [DOI] [PubMed] [Google Scholar]
- 127.Rouschop KM et al. (2009) Autophagy is required during cycling hypoxia to lower production of reactive oxygen species. Radiother. Oncol 92, 411–416 [DOI] [PubMed] [Google Scholar]
- 128.Tafani M et al. (2016) The interplay of reactive oxygen species, hypoxia, inflammation, and sirtuins in cancer initiation and progression. Oxid. Med. Cell. Longev 2016, 3907147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ginis I and Faller DV (2000) Hypoxia affects tumor cell invasiveness in vitro: the role of hypoxia-activated ligand HAL1/13 (Ku86 autoantigen). Cancer Lett 154, 163–174 [DOI] [PubMed] [Google Scholar]
- 130.Zhang Q et al. (2006) Redox sensor CtBP mediates hypoxia-induced tumor cell migration. Proc. Natl. Acad. Sci. U. S. A 103, 9029–9033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cronin PA et al. (2010) Hypoxia increases the metastatic ability of breast cancer cells via upregulation of CXCR4. BMC Cancer 10, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Romain B et al. (2014) Hypoxia differentially regulated CXCR4 and CXCR7 signaling in colon cancer. Mol. Cancer 13, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guo M et al. (2014) Hypoxia promotes migration and induces CXCR4 expression via HIF-1alpha activation in human osteosarcoma. PLoS One 9, e90518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang MH and Wu KJ (2008) TWIST activation by hypoxia inducible factor-1 (HIF-1): implications in metastasis and development. Cell Cycle 7, 2090–2096 [DOI] [PubMed] [Google Scholar]
- 135.Svastova E et al. (2012) Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J. Biol. Chem 287, 3392–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eisinger-Mathason TS et al. (2013) Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov 3, 1190–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Graham CH et al. (1999) Hypoxia-mediated stimulation of carcinoma cell invasiveness via upregulation of urokinase receptor expression. Int. J. Cancer 80, 617–623 [DOI] [PubMed] [Google Scholar]
- 138.Gilkes DM et al. (2014) Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat. Rev. Cancer 14, 430–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rofstad EK et al. (2010) Tumors exposed to acute cyclic hypoxic stress show enhanced angiogenesis, perfusion and metastatic dissemination. Int. J. Cancer 127, 1535–1546 [DOI] [PubMed] [Google Scholar]
- 140.Todaro GJ et al. (1965) The initiation of cell division in a contact-inhibited mammalian cell line. J. Cell Physiol 66, 325–333 [DOI] [PubMed] [Google Scholar]
- 141.Liang CC et al. (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc 2, 329–333 [DOI] [PubMed] [Google Scholar]
- 142.Boyden S (1962) The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med 115, 453–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Repesh LA (1989) A new in vitro assay for quantitating tumor cell invasion. Invasion Metastasis 9, 192–208 [PubMed] [Google Scholar]
- 144.Oppegard SC et al. (2009) Modulating temporal and spatial oxygenation over adherent cellular cultures. PLoS One 4, e6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sutherland RM et al. (1971) Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J. Natl. Cancer Inst 46, 113–120 [PubMed] [Google Scholar]
- 146.Sutherland RM and Durand RE (1973) Hypoxic cells in an in vitro tumour model. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med 23, 235–246 [DOI] [PubMed] [Google Scholar]
- 147.Airley RE et al. (2003) GLUT-1 and CAIX as intrinsic markers of hypoxia in carcinoma of the cervix: relationship to pimonidazole binding. Int. J. Cancer 104, 85–91 [DOI] [PubMed] [Google Scholar]
- 148.Gruber G et al. (2004) Hypoxia-inducible factor 1 alpha in high-risk breast cancer: an independent prognostic parameter? Breast Cancer Res 6, R191–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bos R et al. (2001) Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J. Natl. Cancer Inst 93, 309–314 [DOI] [PubMed] [Google Scholar]
- 150.Chapman JD (1979) Hypoxic sensitizers – implications for radiation therapy. N. Engl. J. Med 301, 1429–1432 [DOI] [PubMed] [Google Scholar]
- 151.Chapman JD et al. (1981) A marker for hypoxic cells in tumours with potential clinical applicability. Br. J. Cancer 43, 546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lord EM et al. (1993) Detection of hypoxic cells by monoclonal antibody recognizing 2-nitroimidazole adducts. Cancer Res 53, 5721–5726 [PubMed] [Google Scholar]
- 153.Rumsey WL et al. (1988) Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science 241, 1649–1651 [DOI] [PubMed] [Google Scholar]
- 154.Torres Filho IP and Intaglietta M (1993) Microvessel PO2 measurements by phosphorescence decay method. Am. J. Physiol 265, H1434–8 [DOI] [PubMed] [Google Scholar]
- 155.Clark LC Jr et al. (1953) Continuous recording of blood oxygen tensions by polarography. J. Appl. Physiol 6, 189–193 [DOI] [PubMed] [Google Scholar]
- 156.Cater DB and Silver IA (1960) Quantitative measurements of oxygen tension in normal tissues and in the tumours of patients before and after radiotherapy. Acta Radiol 53, 233–256 [DOI] [PubMed] [Google Scholar]
- 157.Horsman MR et al. (2012) Imaging hypoxia to improve radio-therapy outcome. Nat. Rev. Clin. Oncol 9, 674–687 [DOI] [PubMed] [Google Scholar]
- 158.Griffiths JR and Robinson SP (1999) The OxyLite: a fibre-optic oxygen sensor. Br. J. Radiol 72, 627–630 [DOI] [PubMed] [Google Scholar]
- 159.Serganova I et al. (2004) Molecular imaging of temporal dynamics and spatial heterogeneity of hypoxia-inducible factor-1 signal transduction activity in tumors in living mice. Cancer Res 64, 6101–6108 [DOI] [PubMed] [Google Scholar]


