Abstract
Irradiation with red light or near-infrared (NIR) lasers can bio-modulate cellular processes or revitalize injured tissues and therefore, widely been used for therapeutic interventions. Mechanistically, this cellular or biological process, referred as Photobiomodulation (PBM), is achieved by the generation of oxide free radicals in cells and tissues. This explorative study using red light (636 nm) and Near Infra-Red (NIR, 825 nm) laser at various irradiation exposures reckons the level of oxidative stress induced by these free radicals in human primary fibroblasts. Freshly isolated dermal fibroblasts were irradiated with red light and NIR at power densities of 74 and 104 mV/cm2, respectively and, at varying fluences ranging from 5 to 25 J/cm2. Cellular oxidative stress, measured by Reactive Oxygen Species (ROS) upon quantifying fluorescently labelled oxide free radicals in cells, detected considerable variations between the irradiation exposures of red light and NIR laser. The NIR laser demonstrated high levels of ROS at all fluences, except 10 J/cm2 indicating its ability in generating of two types of oxide radicals in dermal fibroblasts, often illustrated as biphasic response. Further, the responses of these cells to variable fluences of red light and NIR laser were measured to evaluate the immediate effect of PBM on cellular activity. The production of cellular energy coincides with the amount of oxidative stress, which was two-fold higher in cells irradiated with the NIR laser, as compared with the red light. This outcome indicates that the ROS production within biological systems are more dependent on the wavelength of the laser rather than its fluences. Further studies are required to avoid ‘overdosing of PBM’ and to analyse ROS qualitatively for making the best use of the red light and NIR laser in clinics.
Keywords: Fibroblasts, Laser, Light, Photobiomodulation, Reactive Oxygen Species
1. Introduction
The mechanism of action of lasers in cells and tissues involves stimulation of the mitochondrial respiratory chain and release of molecules such as growth factors and inflammatory mediators [1,2]. The net effect of these biochemical processes is the changes in oxidative stress influencing cell signalling and metabolism, which orchestrate an altered cell functionality. Cytochrome c oxidase (CCO), within the mitochondrial respiratory chain, along with heme proteins and porphyrins are involved in the absorption of light [3,4]. The process of Photo-biomodulation (PBM) is displayed as the outburst of oxide radicals and calcium ions that can affect cellular signalling pathways through redox sensitive signalling molecules, mitogen-activated protein kinases (MAPKs), and transcription factors, like Nuclear factor kB (NFkB) [5–7]. Light affects by generating oxide radicals in mitochondrial respiratory complexes by the activity of CCO situated in the mitochondrial inner membranes [8,9]. Collectively, these ultra-short lived oxide radicals are referred as Reactive Oxygen Species (ROS) that leak into the cytoplasm causing a variety of cellular changes.
Red light at wavelengths 600 nm to 760 nm, as well as near-infrared at wavelengths 780 nm to 1000 nm, are capable of influencing a myriad of activities in living cells and tissues. Chemical effects of these wavelengths stems from the absorption of photons by chromophores in the mitochondria, and its reactions with respiratory oxygen and intracellular water [10,11]. As a result, singlet oxygen (O2) molecules build up creating oxidative stress and changing intracellular calcium levels, which are capable of altering cell signalling profile [12,13]. Biological effects of these reactions are manifested as changes in gene expression, cellular electrical capacitance, metabolic rearrangements and cytoskeleton reorganization [14,15]. Often, this is followed by a cascade of metabolic events resulting in various physiological changes such as cellular proliferation and differentiation [8,16]. Cell membrane permeability alters alongside these changes to biochemical processes and is responsible for alleviating symptoms of cell and tissue damages.
Regardless of the therapeutic benefits, there are instances where Low-Level Laser Therapy (LLLT) is unsuccessful, mostly due to inadequate dosimetry arising from the use of unsuitable wavelengths as well as excess or low irradiation. As a result, laser researchers and therapists often end up using lasers at wavelengths and fluences that are not suitable for optimal recovery. This study has been undertaken to provide a better understanding of the production of ROS in relation to the wavelengths and fluences of lasers that are most widely used in clinics. We quantified ROS in response to Low-Level Laser Irradiation (LILI) in freshly isolated human dermal fibroblasts in laboratory. We also looked at intracellular levels of lactate dehydrogenase and Adenosine Triphosphate (ATP) to reckon any possible metabolic shifts from cytoplasmic glycolysis to mitochondrial oxidative phosphorylation, often a prelude for proliferation and/or differentiation. The findings of this study could justify the use of lasers with different wavelengths and fluences generating variable amounts of ROS in vitro and subsequent effects. Ultimately, it could assist researchers and clinicians with choosing the correct parameters of their lasers for optimal therapeutic effect.
2. Material and Methods
2.1. Harvest of Cells from Human Tissue
Consent forms for adipose tissue donation were prepared in accordance with the University of Johannesburg’s’ institutional guidelines. Ethical approval (REC-01–186-2016) was obtained from the Research Ethics Committee of the Faculty of Health Sciences, University of Johannesburg in accordance with the Human Tissue Act 65, 1983). Tissue was obtained from a donor undergoing abdominoplasty in Linksfield hospital, Johannesburg, South Africa and the isolation was performed using the collagenase digestion method [17]. Briefly, donated tissue was subjected to enzymatic digestion with collagenase solution, followed by high-speed centrifugation and filtration of the infranatant through a 40 μm filter (BD Biosciences, 352,340) to obtain the stromal vascular fraction (SVF) containing high numbers of blood cells with traces of endothelial cells, fibroblasts, pericytes and stem cells. Cell pellet containing the SVF was re-suspended in Erythrocyte Lysis Buffer to remove blood cells and the solution was then spun at 0.5 ×g for 5 min. The residue was re-suspended in a complete culture medium consisting of Dulbecco’s Modified Eagle Medium (DMEM, Sigma, D5796) supplemented with 10% foetal bovine serum (FBS, Sigma, F6178), 0.1% v/v penicillin/streptomycin, and 0.5% v/v fungizone for incubation at 37 °C in a humidified chamber containing 5% CO2.
2.2. Characterization of Isolated Cells
Adherence and propagation of freshly isolated cells from human tissue on culture plates were monitored every 72 h over a period of 2–3 weeks. Cells were grown to semi-confluency in tissue culture flasks before proceeding to characterization by immunofluorescence staining of cell surface markers. Briefly, cells at the mid-log growth phase in 35 mm diameter culture plates (Corning, 430,165) were washed twice in ice-cold 1× PBS and blocked with 5% goat serum in 1× PBS for 30 min. Mouse anti-human CD44, CD90, CD133 and CD166 were added to each of these samples at a ratio of 1:100 and incubated for 30 min on ice. Further, cells were washed thrice in ice-cold 1× PBS and incubated with FITC-conjugated anti-mouse antibody at a ratio of 1:200 for another 30 min. This suspension was washed twice again and re-constituted in 1× PBS for analysing 10,000 events on Accuri C6 flow cytometer (BD Biosciences). Positive controls were set by incubating cells with BD CompBead Plus anti-mouse IgG, kappa chain (BD biosciences) with each of the above primary and flourochrome-conjugated secondary antibody. The kappa chain bound mouse beads with one drop of bovine serum albumin served as the negative control.
2.3. Staining for Oxidative Stress in Cells
Detection and quantification of oxidative stress was based on the cleavage of 5-(and-6)-carboxy-2′ 7′ -dichlorodihydroflourescein diacetate (carboxy-H2DCFDA) by cellular esterases. The non-florescent carboxy-H2DCFDA permeates live cells and deacetylates by non-specific intracellular esterases. In the presence of non-specific ROS the reduced fluorescein compound is oxidized and emits fluorescence at 488 nm. Primary dermal fibroblasts were grown up to semi-confluence and subcultured in 35 mm diameter culture plates for various treatments. The best detection of ROS in cultured fibroblasts has been achieved in 30 min and the protocol was standardized using 5, 10 and 20 μM carboxy-H2DCFDA. Prior to laser irradiations, culture media was discarded and cells were rinsed twice with 1× PBS and incubated at 37 °C in 10 μM carboxy-H2DCFDA for 30 min. Positive and negative controls were established by incubating the non-irradiated cells with tert-Butyl hydroperoxide (TBHP), an organic peroxide for cellular oxidation and N-acetyl Cysteine (NAC) for scavenging oxide free radicals, respectively. For optimization, fibroblasts were treated with 0.1 mM and 1 mM TBHP for 15 and 30 min each to identify the highest levels of ROS generated in each sample. Similarly, these cells were treated with 1 mM and 10 mM NAC for 15 and 30 min each followed by the detection of oxidative stress.
2.4. Laser Induction of Oxidative Stress in Cells
The diode lasers (LTIO00-PLT20) 636 nm and 825 nm (Oriel, USA) used in this study were supplied and set up by the National Laser Centre (NLC, South Africa). Semi-confluent monolayers of dermal fibroblasts were irradiated in the dark at room temperature at fluences 5, 10, 15, 20, 25 J/cm2 using these diode lasers. Laser irradiations were performed on the 35 mm diameter tissue culture plates via optical fibres with a spot size of 9.1 cm2 covering the entire surface of the culture dish uniformly. The power density of the laser was determined by dividing the initial power output (as determined by a laser power meter) by the spot size area and so the duration of irradiation could be calculate by dividing the fluence by this power density. On an average, power outputs of 75 and 104 mW were measured for each of the 636 and 825 nm lasers, which were used to irradiate cultured cells for specific durations to deliver fluences in the numerical order of five-fold. Details of LILI parameters viz. power, density, fluence and duration are given in the Table 1. Treated cells were detached from the 35 mm diameter tissue culture plates using TrypLE Select (Life Technologies, 12563–029) and re-suspended in 1× PBS. Detection of fluorescence was performed in 50,000 flow events (dye-stained cells) immediately following each treatments as above using Accuri™ C6 cytometer (BD Biosciences). Sample sizes were minimal (n = 3), since the assay had to be performed within a day for fluences 5, 10, 15, 20 and 25 J/cm2 for each of the groups in red and NIR lasers along with their controls. Laser untreated (non-irradiated or 0 J/cm2) cells were processed under the same conditions for background correction.
Table 1.
Laser parameters used for generating oxidative stress in human primary dermal fibroblasts.
| Wave length (nm) | 636 | 825 |
| Wave emission | Continuous wave | Continuous wave |
| Power output (mW) | 75 | 104 |
| Spot size (cm2) | 9.08 | 9.08 |
| Output density (mW/cm2) | 8.26 | 11.45 |
| Fluence (J/cm2)=duration of irradiation (seconds) | 5 J/cm2=605.28 | 5 J/cm2=436.50 |
| 10 J/cm2=1210.56 | 5 J/cm2=436.50 | |
| 15 J/cm2=1815.84 | 15 J/cm2=1309.50 | |
| 20 J/cm2=2421.12 | 20 J/cm2=1746.00 | |
| 25 J/cm2=3026.40 | 25 J/cm2=2182.50 |
2.5. Visualization of Oxidative Stress
Dermal fibroblasts were seeded on heat-sterilized coverslips placed at the bottom of 35 mm diameter culture plates for 72 h prior to staining carboxy-H2DCFDA (10 μM). Following incubation, these coverslips with adhered cells were washed twice in 1× PBS and irradiated with 5, 10, 15, 20, 25 J/cm2 of laser fluences at 636 and 825 nm wavelengths. Changes in cellular morphology and the level incorporation of ROS detection dye in both non-irradiated and irradiated cells were observed by inverted microscope (Olympus CKX41). Digital micrographs were captured immediately after the treatments using an Olympus CS30 digital camera coupled to this microscope. Laser treated cells were washed in 1× PBS to stain the nuclei with 300 nM DAPI (Life Technologies, D1306) followed by the mounting of glass coverslips on labelled glass slides containing a drop of aqeuous Flouromount™ aqueous medium (Sigma, F4680). Fluorescence was visualized and Differential Interference Contrast (DIC) micrographs were taken using the Axio Observer Z1 (Carl Zeiss) within a span of 15 min following each treatments. These digital micrographs were analysed using ImageJ (NIH, USA) by randomly picking ten spots for the measurement of fluorescence intensities followed by correction of background and normalization with the non-irradiated control.
2.6. Estimation of Cellular Viability
The proportion of viable cells in each group was estimated by trypan blue, 0.4% (w/v) (Sigma, H7901) following each treatment. The chromophore of this dye is negatively charged and react with the dermal fibroblasts only if there is a membrane damage. Therefore, viable fibroblasts that do not imbibe the dye can easily be identified from the nonviable cells that do so. Both viable and non-viable cells were counted using a haemocytometer with Neubauer rulings using a light microscope (Olympus CKX41). Percentage viability of laser treated samples were calculated by dividing the number of viable cells (translucent) by the total number of cells. Following elimination of background from non-irradiated cells, graphs were plotted using SigmaPlot v13.0 (Systat Software, Inc.). Primary fibroblasts treated with the optimized concentrations of TBHP and NAC were also subjected to viability staining and analysis as above.
2.7. Estimation of Metabolic Ability
Assessment of mitochondrial activity based on the quantitation of ATP will enable us to identify metabolic function of cells. CellTiter-Glo® luminescent cell viability assay (Promega, G7571) is a homogenous method for enumerating viable cells in culture by determining the presence of metabolically active cells. Following LILI, dermal fibroblasts were detached from culture plates, centrifuged and reconstituted as 10,000 cells in 100 μl of 1× PBS. Further, these cells were incubated with CellTiter-Glo® reagent at room temperature on a shaker (Labcon, 3081U) for two minutes, followed by incubation at room temperature for 10 min in the dark. Addition of CellTiter-Glo® reagent (1 ml buffer and 0.007 g substrate) to each group normalized to 10,000 cells results in lysis and generation of a luminescent signal that is proportional to the amount of ATP present. Luminescence was detected from each wells of treated samples as well as controls using Victor3 (Perkin Elmer) and the signal intensity was measured in Relative Light Units (RLU) after deduction of background from non-irradiated cells. Raw data was collected on MS Excel from the instrument software and statistical analysis was performed using SigmaPlot v13.0 (Systat Software, Inc.).
2.8. Estimation of Cellular Cytotoxicity
CytoTox 96® non-radioactive cytotoxicity assay measures lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released upon cell lysis. Following carboxy-H2DCFDA staining and laser treatments of fibroblasts samples, 50 ul of culture media was aspirated and used for the LDH assay. Equal volume of substrate was added to this culture medium per well, in a flat-bottomed 96-well clear plate. Mechanistically, LDH catalyse the conversion of lactate to pyruvate by reduction of NAD+ to NADH. An enzyme, diaphorase then uses NADH for reduction of tetrazolium salt (INT) to a red formazan product. Following incubation for 30 min in dark, 50 ul of the stop solution was added to each well and the absorbance was read at 490 nm using a microplate reader, Victor3 (Perkin Elmer). Absorbance of non-irradiated fibroblasts, which forms the background reading, was subtracted from the laser-treated samples and controls.
3. Results
3.1. Harvest and Culture of Adipose Tissue Cells for Differentiated Fibroblasts
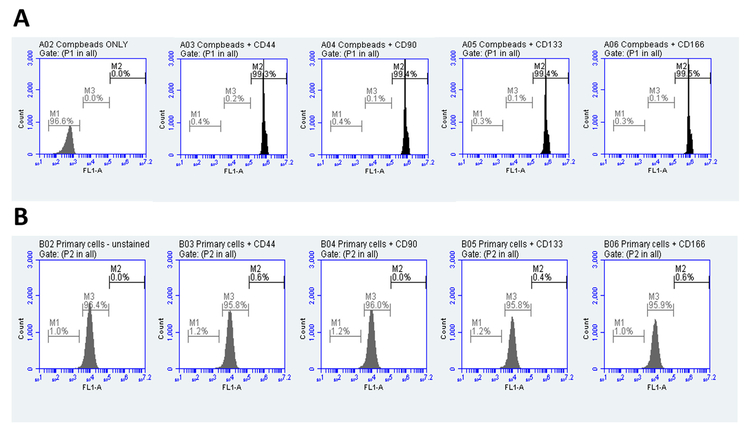
Adipose tissue from a human donor was subjected to enzymatic digestion followed by fractional separation to isolate cells. Harvested cells adhered to culture flasks when seeded and grown in DMEM supplemented with 10% FBS for a week. Grossly, these cells assumed various shapes and structures as they grew along and attached to the substrate. Dense cultures were spindle-shaped or elongated with bipolar or multipolar branching often overlapping with the neighbouring cells. Characterization of these cells based on the surface markers identified them as terminally differentiated fibroblasts with CD44-, CD90-, CD133- and CD166- much similar to the WS1, a laboratory adapted strain of human dermal fibroblasts supplied by ATCC. BD CompBead Plus anti-mouse IgG with kappa chain (BD biosciences), which is used to optimize fluorescence compensation for the multicolour flow cytometry settings served as control for the staining protocol. Positive staining was identified by a shift in the fluorescence peak intensity due to the binding of each of the above four antibodies to cell surface indicating the presence of cell markers (Fig. 1A and B).
Fig. 1.
Characterization of isolated human dermal fibroblasts: Polystyrene microparticles coupled to the kappa light chain of mouse immunoglobulin (BD CompBead plus, The Scientific Group) were incubated with these primary as well as the fluorochrome-conjugated secondary antibodies as primary cells to serve as the positive staining control (A). Primary cells in its mid-logarithmic phase were incubated with mouse anti-human polyclonal antibodies for CD44, CD90, CD133 and CD166 followed by FITC-conjugated rabbit anti-mouse antibody for 30 min each on ice (B). Unstained BD CompBead plus and primary cells were the controls.
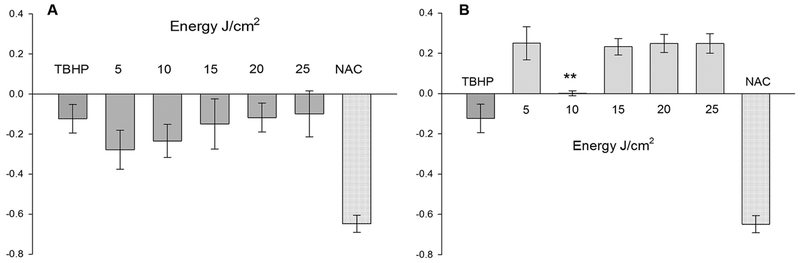
3.2. Laser Wavelength and Not its Fluence Defines the Level of Oxidative Stress
Laser irradiation using wavelengths 636 nm and 825 nm on human primary dermal fibroblasts resulted in patterns that were consistent as dose-response, but quite different in terms of yield. Strikingly, these two wavelength of lasers with matching fluences generates quite variable amount of ROS in primary cells (Fig. 2A and B). The amount of ROS generated using 636 nm laser was much lower than the non-irradiated cells (deducted as background fluorescence) in all these fluences. Further, there was a non-significant, but dose-dependent increase in ROS with fluences in the order of five starting from 5 J/cm2 to 25 J/cm2. However, with 825 nm laser, there was a quite significant increase in the level of ROS than the non-irradiated cells and controls. ROS was recorded high in 5 J/cm2 and in fluences ranging from 15 J/cm2 to 25 J/cm2; but, strikingly low with 10 J/cm2 (P ≤ 0.01). This suggests that the cellular redox status responds quite differently to certain fluences of 825 nm with some unknown mechanisms. ROS generated by 1 mM TBHP in 15 min matched with oxidative stress of primary fibroblasts irradiated with red light at higher fluences of 20 and 25 J/cm2. However, it was lower than the ROS generated by non-irradiated cells as well as 825 nm laser. Failure to measure higher levels of ROS generated by TBHP directly relates to its toxicity on primary cells. TBHP is a corrosive chemical that is highly reactive on organic surfaces and onset pathways of cell death. All these lifeless cells are eliminated while gating events in flow cytometry analysis ensuing lower yield of ROS with TBHP. Interestingly, primary fibroblasts tolerated higher concentrations of NAC (10 mM) for 15 min and was able to scavenge most of the oxide radicals from these cells.
Fig. 2.
Quantification of oxidative stress in dermal fibroblasts: Production of Reactive Oxygen Species (ROS) in dermal fibroblasts using lasers λ = 636 nm (A) and λ = 825 nm (B). Intracellular ROS is expressed in arbitrary units I = (It-Io)/Io on Y-axes, where It and Io are the mean fluorescence intensities of the treated group and untreated (0 J/cm2) groups, respectively (n = 3, mean ± SE). Tert-Butyl hydroperoxide (TBHP) and N-acetyl Cysteine (NAC) were used as positive and negative controls, respectively. **P ≤ 0.01 in 10 J/cm2 compared to the higher fluences of 825 nm laser.
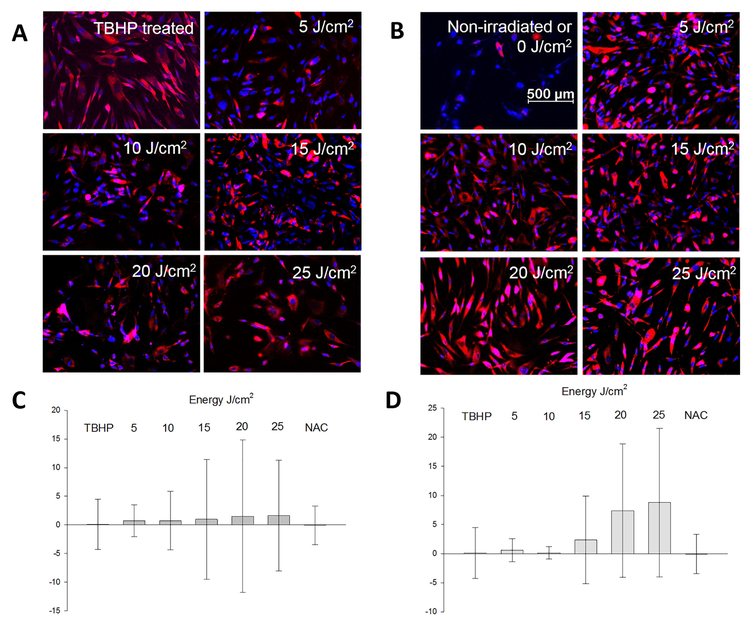
3.3. Visualization of Oxidative Stress Immediately Following Laser Irradiation
Primary fibroblasts stained with Carboxy-H2DCFDA and irradiated with lasers did not show any visible changes by gross morphology under an inverted light microscope. In brief, these cells tolerated 10 μM of Carboxy-H2DCFDA during dye incorporation followed by laser treatments at various irradiation exposures. TBHP (1 mM) treated cells started detaching and floating in the culture medium as early as 15 min when visualized under an inverted light microscope, signifying the upper limits of induction of oxidative stress (data not shown). However, there was an observable change in morphology and in the level of incorporation of ROS dye among these groups, when examined under Axio Observer Z1 fluorescence microscope. The intensity of ROS staining visualized on these cells followed a pattern comparable between the fluences of each laser (Fig. 3A and B). Based on the fluence, there was a gradual increment in the number of cells stained with ROS dye in 636 nm laser. Similar pattern of staining was observed with 825 nm laser, which is much intense than the non-irradiated fibroblasts. Overall, the intensity and the number of fibroblasts stained with ROS dye was higher for fluences above 15 J/cm2 in 825 nm laser. Primary cells treated with 1 mM TBHP for a duration of 15 min appeared larger in size and intensely staining, while those cells treated with 10 mM NAC for 15 min resembled closely to the non-irradiated cells. Fluorescence intensities in laser treated groups as well as controls were compared using ImageJ software (Fig. 3C and D). Comparatively fluorescence intensities were higher in NIR irradiated cells compared to red light.
Fig. 3.
Detection of oxidative stress in dermal fibroblasts: Primary cells were treated with 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate for detecting intracellular Reactive Oxygen Species (ROS) after exposure to lasers of wavelength, λ = 636 nm (A) or λ = 825 nm (B) and fluences ranging in the order of 5 J/cm2. ROS stained orange-red under kaede red and the nuclei stained blue with DAPI filters using Axiovision v4.8 (Zeiss microscopy). Fluorescence intensities were measured for laser wavelength, λ = 636 nm (C) or λ = 825 nm (D) from the digital micrographs using ImageJ (NIH, USA). Tert-Butyl hydroperoxide (TBHP) was the positive control and untreated (0 J/cm2) indicate background of ROS in cells. Scale bar is set as 500 μm across all images. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
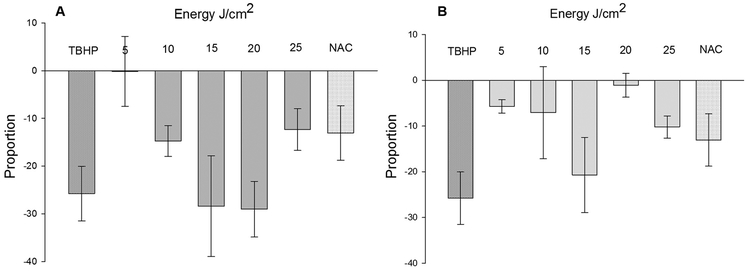
3.4. Cellular Viability Fluctuates with the Intensity of Laser Irradiation
Staining and analysis of the percent of live and dead cells identified by trypan blue showed interesting patterns. At large, the trypan blue assay showed that LILI reduced viability of human primary dermal fibroblasts for both wavelengths as compared to the non-irradiated cells (Fig. 4A and B). Although statistically insignificant, the percent of viable cells following exposure to 636 nm laser was lowest for both 15 J/cm2 and 20 J/cm2. However, with 825 nm laser irradiation, viability was better at 20 J/cm2 than fluences either 15 or 25 J/cm2 indicating the onset of cellular antioxidant mechanisms. Similarly, primary cells receiving 25 J/cm2 of irradiation with 636 nm laser had better viability indicating the onset of cell survival mechanisms. Clearly, the cellular mechanisms for maintaining redox status are fluctuating quite differently for both wavelength of lasers. Presumably, any fluctuation in the redox status have consequences in the viability of these cells. Lastly, the percent viability of TBHP treated cells were much lower than the laser irradiated and NAC treated cells indicating its exceptional toxicity on primary cells. Although, 1 mM TBHP for 15 min increased free radicals, it was drastically reducing viability of these primary cells resulting in a relatively low yield of ROS (refer Fig. 2). As mentioned elsewhere, TBHP treated primary cells detached from the culture plates at a faster rate and thus, recorded a lower yield of ROS upon analysis in flow cytometer. On the other side, 10 mM NAC for 15 min was less damaging to these cells and was quite successful as a negative control.
Fig. 4.
Viability of dermal fibroblasts following laser irradiations: Viability of dermal fibroblasts in response to laser λ = 636 nm (A) and λ = 825 nm (B) irradiations and fluences ranging in the order of 5 J/cm2. Y-axes represents the proportion of cells among the total cells (n = 3, mean ± SE) unstained with 0.4% (w/v) trypan blue after normalization with untreated (0 J/cm2) cells. Tert-Butyl hydroperoxide (TBHP) and N-acetyl Cysteine (NAC) were used as positive and negative controls, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
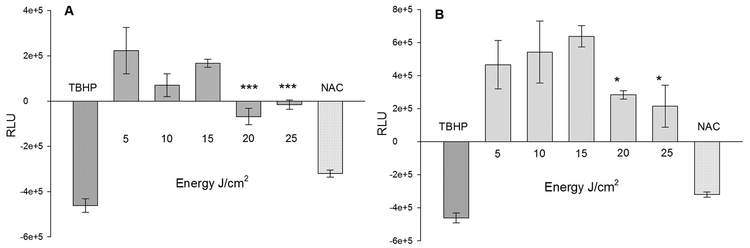
3.5. Metabolic Activity of Cells Correlates with the Level of Oxidative Stress
Primary fibroblasts had a comparable pattern of ATP production upon irradiation with two wavelengths of lasers. Although, ATP level was more with lower fluences, there was a profound decrease with fluences above 15 J/cm2 in both these lasers, indicating the vivid effects of oxidative stress on cellular metabolism (Fig. 5A and B). When irradiated with 636 nm laser, production of ATP in these cells was optimal until 15 J/cm2, but declined thereafter. ATP was significantly lower (P ≤ .001) at both 20 and 25 J/cm2, indicating that excessive oxidation is not congenial for cell survival. It seems a gradual increase in the oxidative stress until 15 J/cm2 results in an increased production of ATP with 636 nm laser. Further on, irradiation with this laser cause excessive oxidative stress resulting in a decreased ATP production and cellular viability (refer Fig. 4). On the contrary, the production of ATP was two-fold higher in fibroblasts irradiated with 825 nm laser than the 636 nm laser. Irradiation of dermal fibroblasts with 825 nm laser demonstrated a non-significant increase in the production of ATP at fluences up to 15 J/cm2 and a sharp decline (P ≤ 0.05) thereafter. It is clear that the ROS generated at higher fluences of 825 nm laser is damaging the cellular metabolic process. Further, ATP levels were recorded lower for both controls suggesting that an excessive oxidation by TBHP or elimination of ROS by NAC adversely affected cellular metabolism. Often, an optimal level of oxide free radicals indicates the uninterrupted functioning of cellular process of oxidative phosphorylation and ATP production.
Fig. 5.
Cellular responses of dermal fibroblasts to laser irradiations: Production of Adenosine Triphosphate (ATP) immediately following laser irradiations using λ = 636 nm (A) and λ = 825 nm (B) on 10,000 live cells (n = 3, mean ± SE). Y-axes indicates Relative Luminescence Units (RLU) in laser irradiated groups after normalization with untreated (0 J/cm2) group. Tert-Butyl hydroperoxide (TBHP) and N-acetyl Cysteine (NAC) were used as positive and negative controls respectively.***P ≤ 0.001 and *P ≤ 0.05 in 20 J/cm2 and 25 J/cm2 as compared to the 15 J/cm2 of both lasers.
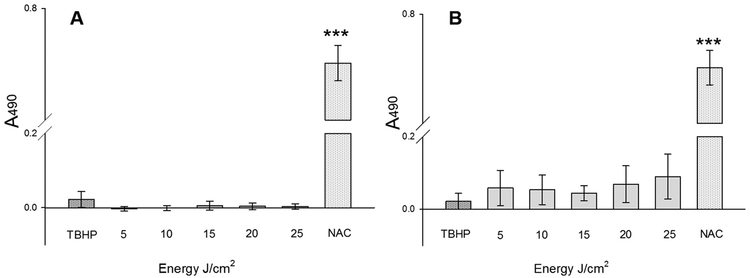
3.6. Cytotoxicity Reflects the Tolerance of Cells to Oxidative Stress
Analysis of cellular cytotoxicity by measure of LDH in the culture media using CytoTox 96® non-radioactive assay recorded extremely low values indicating that both these laser wavelengths and its fluences were not harmful to living cells. However, between the two lasers used here, 825 nm laser was more cytotoxic in accordance with an increase in fluence (Fig. 6A and B). This suggests that both the wavelength and fluences of these two lasers directly correlates to cellular damage and toxicity. Although, 1 mM TBHP was corrosive on these primary cells, LDH secreted into the medium was comparable to laser treated cells. However, a regimen of 1 mM TBHP for 15 min drastically reduced cellular viability and ATP production (refer Figs. 4 & 5). The cellular mechanism of TBHP involves lipoperoxidation of membrane phospholipids as well as depletion of glutathione by oxidation to its disulphide form. Perhaps, a lower amount of cytotoxicity detected in positive control is due to the inability of CytoTox 96® reagent to conjugate LDH in a high concentration of TBHP. A more suitable choice of inducers of oxidative stress would be rotenone or antimycin A that are inhibitors of mitochondrial complexes I and III, respectively. Additionally, Phorbol Myristate Acetate (PMA) or bacterial lipopolysaccharides (LPS) can activate cell surface receptors for the cytosolic production of free radicals such as superoxides. On the contrary, LDH activity was very high with 10 mM NAC treated primary cells suggesting that this high concentration for the elimination of free radicals was less suitable.
Fig. 6.
Effect of laser irradiations and treatments on dermal fibroblasts: Level of cytotoxicity as determined by the production of Lactate Dehydrogenase (LDH) in primary fibroblasts after irradiations using laser wavelengths, λ = 636 nm (A) and λ = 825 nm (B). Assay was performed using CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega 1781). Y-axes indicates absorbance at 490 nm (A490) in Victor3 spectrophotometer (Perkin Elmer) after normalization with the untreated (0 J/cm2). All groups are represented as mean ± SE (n = 3) and subtracted to the non-irradiated control. ***P ≤ 0.001 as compared to all other groups.
4. Discussion
The proliferative response and wound healing properties of 636 nm laser (red light) in fibroblasts is affected by modulating oxidative stress. Our understanding is red light at 5 J/cm2 is appropriate in stimulating proliferation, migration and wound healing of fibroblasts, while, fluences above 16 J/cm2 cause excessive oxidative stress [18,19]. Later studies using 660 nm laser has shown a significant decrease in the activity of mitochondrial complexes III and IV at 15 J/cm2 among normal, diabetic, and ischemic fibroblasts [20,21]. However, this research with a 636 nm laser and flow cytometry analysis shows that fluences up to 25 J/cm2 decreases ROS in the primary cells as compared to the nonirradiated control. Perhaps, fluences < 15 J/cm2 of red light could be decreasing oxidative stress for a desirable therapeutic effect and, the fluences above 15 J/cm2 would be fatiguing the mitochondrial electron transfer mechanisms. Increasing fluences above 100 J/cm2 may hinder mitochondrial functions and alter membrane potential leading to apoptosis and cell death [22]. Using NIR, it is shown that a higher level irradiation has phototoxic effects characterized by generation of ROS and rise in peripheral temperature by the upregulation of Activating Transcription Factor-4 (ATF-4) and Heat Shock Protein 70 (HSP70) [23]. However, the range of fluences used for the comparison of two wavelengths of lasers are widely used for PBM. Thus, our finding can reduce failures of LLLT by choosing the suitable lasers and precise fluences for desirable outcomes in clinics.
This study using 825 nm wavelength of laser clearly identifies that ROS is primarily responsible for the biphasic response of PBM. LILI at 5 J/cm2 using 825 nm laser generated ROS equivalent to LILI at 15, 20 and 25 J/cm2, while, there was a subdued expression at 10 J/cm2. Sharma et al. (2011) observed similar pattern of oxidative stress response by generation of ROS in the mouse primary cortical neurons [24]. Therefore, a failure of an inverse linear relationship between the laser wavelength and fluences used in LLLT indicates that there is threshold in every living cells, often influenced by the wavelength of light affecting bio-stimulation or bio-inhibition [25]. Such properties of light are manifested as biphasic response, a simulation of Arndt-Schulz law identified in living cells when administered with incremental doses of poison. In addition, much higher levels of ATP were recorded in primary fibroblasts irradiated with various fluences of 825 nm laser than its counterpart used in this study. Light-induced biphasic response has been shown in the production of ATP, maintenance of Mitochondrial Membrane Potential (MMP) in tissue culture models and animal experiments [26,27]. Mouse primary cortical neurons responded to 810 nm laser by a significant rise in ATP content at 3 J/cm2, although, fluences at 10 and 30 J/cm2 seemed harmful [24]. Similarly, ATP synthesis was supressed at fluences above 15 J/cm2 of 825 nm laser, a pattern much similar to the 636 nm laser indicating that oxidative stress does harm to mitochondrial functionalities. Therefore, this study confirmed that it is the laser wavelength and not fluence influencing the level of oxidative stress. Furthermore, there is an increase in ROS production using NIR laser irradiation compared to red light in human primary fibroblasts.
Oxidation and reduction processes within a growing or differentiating cell affects the function of heterotrimeric proteins Gi and Go and signal transduction mechanisms [28]. The relative outcome of this redox state balance is ROS species-specific as (i) the beneficial ROS resulting in cell signalling beneficial for survival and proliferation (ii) the undesirable ROS that can damage mitochondrial functions by decreasing MMP. In culture experiments using endothelial cells, 1 μM of H2O2 is proliferative, whereas, concentration above 10 μM decreases cell [29,30]. Similarly, cultured fibroblasts irradiated with a range of laser wavelengths showed that the red light was stimulatory while NIR was inhibitory at 10 J/cm2, suggesting a biphasic response [31]. Low levels of ROS could activate cellular gene expression profile towards increased production of antioxidants. This means that low to medium levels of ROS may be tolerated within cells (dependent of cell type and stage of growth) by the production of antioxidants such as glutathione. In general, cancer cells produce higher levels of ROS in response to hypoxic condition and upregulate antioxidants, especially cellular glutathione to favour survival and proliferative effects within the host environment [32]. Possibly, depletion of ROS is also altering cancer cell metabolism by production of lactates for increasing glycolysis rate towards its survival and rapid proliferation.
A biphasic response is also observed in vivo - when using NIR lasers with 810 nm on rheumatoid arthritis, a biphasic dose response with a highest level of MTT activity was observed at 8 J/cm2 compared to its lower and higher fluences [33]. Additional studies have shown that biphasic responses in animal models are not limited to any wavelength of light or tissue types. When using LED red light at 5 J/cm2, there was a faster healing to skin wounds than the 20 J/cm2 in a 3-week long trial on rats [34]. It was also noted in the healing of fresh excisional wounds in BALB/c mice with a 635 nm wavelength laser at fluences of 1, 2, 10 and 50 J/cm2. The result followed a biphasic curve with a maximal dose-dependent response at 1 and 2 J/cm2 indicated by faster wound closure possibly due to the beneficial ROS and a gradual decline at 10 and 50 J/cm2 marked by swelling and widening of the incisional site [35]. Even irradiations using 635 nm laser at 5 J/cm2 with a power density 4 mW/cm2 demonstrated a significant healing effect than with 15 mW/cm2 on rat models of open skin wounds [36,37]. Further, biphasic responses based on a range of fluences was illustrated with 635 nm laser over skin wounds on Swiss albino mice [38] giving a clear indication that this cellular response is achievable by irradiations with red light as well. This was also noted in mice models with pressure ulcers, where 670 nm light with a fluence of 5 J/cm2 and varying power densities of 0.7–40 mW/cm2 showed better response particularly at 8 mW/cm2 than the rest [39]. Thus, cross analyses of several clinical trials have stated that the failure of LLLT is a result of too much or too little laser fluences that is appropriate to deliver cure on affected body parts [40,41].
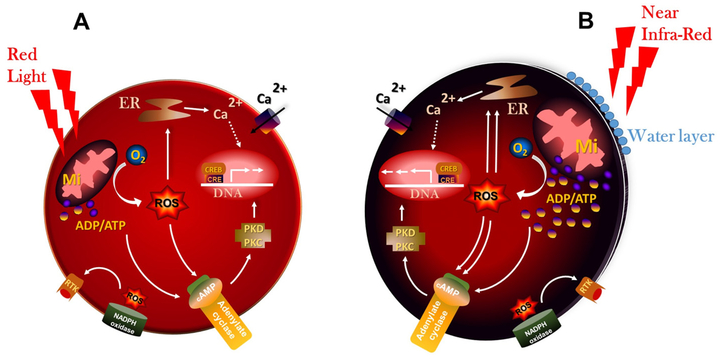
Unlike visible red light absorbed by electrons in atomic orbitals, NIR laser is absorbed by chemical bonds, which increases strain to molecules [42] (Fig. 7A and B). An overlap of the spectra of solar irradiance and water absorption is found in the region of 800–1300 nm giving the most therapeutic benefits of NIR light in the living systems [42,43]. In addition, the absorption spectra of CCO at its various oxidation states in a living cell is reciprocal to the biological effects of NIR [4,44]. Further, wavelength of 810 nm has better cellular absorption both by CCO and by aqueous medium while wavelengths above 980 nm (and within infrared range) is mostly absorbed by intracellular water [43]. Particularly, NIR activates two important pathways in cells (i) oxygen-dependent pathway responsible for the generation of high-energy molecules such as ATP and GTP by oxidative stress (ii) oxygen-independent pathways responsible for the non-linear oscillations due to the water-light interactions. The latter interactions create an exclusion zone (EZ) between non-polar water and its hydrophobic membranes of cells, often making it to function as a rechargeable bio-battery with electrolytic ions [45,46]. Interestingly, intracellular water is dynamic in nature and its electromagnetic absorption spectrum lies in the infrared region [47,48]. Cellular membranes have an ultra-thin layer of water between its hydrophobic surfaces and infrared light can deliver infinitively small amounts of vibrational energy to water molecules in this layer without raising temperature [11,49]. This study emphasizes the therapeutic benefits of NIR irradiation which may be a result of increased oxidative stress leading to increased cellular ATP levels. Thus, the beneficial effect of NIR on cellular oxidative stress levels and the cellular mechanisms affecting subsequent cell signalling pathways requires further investigation.
Fig. 7.
Generation of oxidative stress by red light and NIR laser: Red light (A) generates lesser amount of Reactive Oxygen Species (ROS) and Adenosine Triphosphate (ATP) as compared to the near infrared (B). Further, irradiations with near infrared is not only absorbed by CCO, but also by structured intracellular water at very low levels. Abbreviations: cAMP, cyclic Adenosine monophosphate; Ca2+, Calcium; ER, Endoplasmic Reticulum; Mi, Mitochondria; O2, Oxygen; PK C/D, Protein Kinases C/D; RTK, Tyrosine Kinase Receptors. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
5. Conclusions
Clinical trials have speculated that 630 to 660 nm may be the effective wavelength of light to bring desirable effects on cells and tissues. However, this analysis has identified that an 825 nm laser in the NIR region is more potent in generating ROS than the red light. Further, NIR laser was more potent in generating beneficial as well as detrimental ROS, as illustrated by a biphasic response in these cells. Biological effects of ROS are also dependent on the cell or tissue-types, which relates to its ability to produce enzymes, modulate signal transduction mechanisms and perform DNA repair. By comparing wavelengths 636 nm and 825 nm of PBM and using a range of fluences, we were able to identify ROS suitable for generating unique dose-responses in human primary fibroblasts. Current data confirms that fluences above 15 J/cm2 are damaging to the functioning of mitochondria, possibly from the excessive oxidative stress. Accordingly, clinicians and laser researchers should be able to identify wavelength and fluences of lasers that are well suited for their applications.
Declaration
This material has neither been published, nor is being considered elsewhere for publication. There is no conflict of interest among the authors. The data set and diagrams that accompany this manuscript are original products of the research conducted in the Laser Research Centre, University of Johannesburg, South Africa.
Acknowledgements
We acknowledge Dr. G Doucas, Linksfield Hospital, Johannesburg, South Africa for providing adipose tissue for the isolation of primary dermal fibroblasts from the consented healthy donors. This work is based on the research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant # 98337). We acknowledge the reward and infrastructure support by the University of Johannesburg, South Africa.
Footnotes
This manuscript is based on our original research and has neither been published, nor is being considered elsewhere for publication.
References
- [1].Hamblin MR, Mechanisms and applications of the anti-inflammatory effects of photobiomodulation, AIMS Biophys 4 (2017) 337–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gao X, Xing D, Molecular mechanisms of cell proliferation induced by low power laser irradiation, J. Biomed. Sci 16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Karu TI, Afanas NI, Cytochrome c oxidase as the primary photoacceptor upon laser exposure of cultured cells to visible and near IR-range light, Dokl. Akad. Nauk 342 (1995) 693–695. [PubMed] [Google Scholar]
- [4].Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI, Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation, J. Photochem. Photobiol. B 81 (2005) 98–106. [DOI] [PubMed] [Google Scholar]
- [5].Calles C, Schneider M, Macaluso F, Benesova T, Krutmann J, Schroeder P, Infrared A radiation influences the skin fibroblast transcriptome: mechanisms and consequences, J. Invest. Dermatol 130 (2010) 1524–1536. [DOI] [PubMed] [Google Scholar]
- [6].Groeger G, Quiney C, Cotter TG, Hydrogen peroxide as a cell-survival signaling molecule, Antioxid. Redox Signal 11 (2009) 2655–2671. [DOI] [PubMed] [Google Scholar]
- [7].Hamblin MR, Mechanisms and mitochondrial redox signaling in photo-biomodulation, Photochem. Photobiol 94 (2018) 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].de Freitas LF, Hamblin MR, Proposed Mechanisms of photobiomodulation or low-Level light therapy, IEEE J. Sel. Top. Quantum Electron 22 (2016), 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Farivar S, Malekshahabi T, Shiari R, Biological effects of low level laser therapy, J. Lasers Med. Sci 5 (2014) 58–62. [PMC free article] [PubMed] [Google Scholar]
- [10].Kim HP, Lightening up light therapy: activation of retrograde signaling pathway by photobiomodulation, Biomol. Ther. (Seoul) 22 (2014) 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sommer AP, Caron A, Fecht HJ, Tuning nanoscopic water layers on hydrophobic and hydrophilic surfaces with laser light, Langmuir 24 (2008) 635–636. [DOI] [PubMed] [Google Scholar]
- [12].Moreau D, Lefort C, Pas J, Bardet SM, Leveque P, O’Connor RP, Infrared neural stimulation induces intracellular Ca2+ release mediated by phospholipase C, J. Biophotonics 11 (2) (2018), 10.1002/jbio.201700020 (Epub 2017 Aug 9). [DOI] [PubMed] [Google Scholar]
- [13].Karu TI, Mitochondrial signaling in mammalian cells activated by red and near-IR radiation, Photochem. Photobiol 84 (2008) 1091–1099. [DOI] [PubMed] [Google Scholar]
- [14].Passarella S, Karu T, Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photo-acceptors results in photobiomodulation, J. Photochem. Photobiol. B 140 (2014) 344–358. [DOI] [PubMed] [Google Scholar]
- [15].Shapiro MG, Homma K, Villarreal S, Richter CP, Bezanilla F, Infrared light excites cells by changing their electrical capacitance, Nat. Commun 3 (2012) 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR, The nuts and bolts of low-level laser (light) therapy, Ann. Biomed. Eng 40 (2012) 516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mvula B, Mathope T, Moore T, Abrahamse H, The effect of low level laser irradiation on adult human adipose derived stem cells, Lasers Med. Sci 23 (2008) 277–282. [DOI] [PubMed] [Google Scholar]
- [18].Hawkins DH, Abrahamse H, The role of laser fluence in cell viability, proliferation, and membrane integrity of wounded human skin fibroblasts following helium-neon laser irradiation, Lasers Surg. Med 38 (2006) 74–83. [DOI] [PubMed] [Google Scholar]
- [19].Zungu IL, Hawkins Evans D, Abrahamse H, Mitochondrial responses of normal and injured human skin fibroblasts following low level laser irradiation–an in vitro study, Photochem. Photobiol 85 (2009) 987–996. [DOI] [PubMed] [Google Scholar]
- [20].Masha RT, Houreld NN, Abrahamse H, Low-intensity laser irradiation at 660 nm stimulates transcription of genes involved in the electron transport chain, Photomed. Laser Surg 31 (2013) 47–53. [DOI] [PubMed] [Google Scholar]
- [21].Houreld NN, Masha RT, Abrahamse H, Low-intensity laser irradiation at 660 nm stimulates cytochrome c oxidase in stressed fibroblast cells, Lasers Surg. Med 44 (2012) 429–434. [DOI] [PubMed] [Google Scholar]
- [22].Huang L, Wu S, Xing D, High fluence low-power laser irradiation induces apoptosis via inactivation of Akt/GSK3beta signaling pathway, J. Cell. Physiol 226 (2011) 588–601. [DOI] [PubMed] [Google Scholar]
- [23].Khan I, Tang E, Arany P, Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress, Sci. Rep 5 (2015) 10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sharma SK, Kharkwal GB, Sajo M, Huang YY, De Taboada L, McCarthy T, Hamblin MR, Dose response effects of 810 nm laser light on mouse primary cortical neurons, Lasers Surg. Med 43 (2011) 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huang YY, Chen AC, Carroll JD, Hamblin MR, Biphasic dose response in low level light therapy, Dose Response 7 (2009) 358–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang YY, Sharma SK, Carroll J, Hamblin MR, Biphasic dose response in low level light therapy - an update, Dose Response 9 (2011) 602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].El-Hussein A, Hamblin MR, ROS generation and DNA damage with photo-inactivation mediated by silver nanoparticles in lung cancer cell line, IET Nanobiotechnol 11 (2017) 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nishida M, Schey KL, Takagahara S, Kontani K, Katada T, Urano Y, Nagano T, Nagao T, Kurose H, Activation mechanism of Gi and Go by reactive oxygen species, J. Biol. Chem 277 (2002) 9036–9042. [DOI] [PubMed] [Google Scholar]
- [29].Day RM, Suzuki YJ, Fanburg BL, Regulation of glutathione by oxidative stress in bovine pulmonary artery endothelial cells, Antioxid. Redox Signal 5 (2003) 699–704. [DOI] [PubMed] [Google Scholar]
- [30].Davies KJ, The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress, IUBMB Life 48 (1999) 41–47. [DOI] [PubMed] [Google Scholar]
- [31].Moore P, Ridgway TD, Higbee RG, Howard EW, Lucroy MD, Effect of wavelength on low-intensity laser irradiation-stimulated cell proliferation in vitro, Lasers Surg. Med 36 (2005) 8–12. [DOI] [PubMed] [Google Scholar]
- [32].Day RM, Suzuki YJ, Cell proliferation, reactive oxygen and cellular glutathione, Dose Response (3) (2006) 425–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yamaura M, Yao M, Yaroslavsky I, Cohen R, Smotrich M, Kochevar IE, Low level light effects on inflammatory cytokine production by rheumatoid arthritis synoviocytes, Lasers Surg. Med 41 (2009) 282–290. [DOI] [PubMed] [Google Scholar]
- [34].Corazza AV, Jorge J, Kurachi C, Bagnato VS, Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources, Photomed. Laser Surg 25 (2007) 102–106. [DOI] [PubMed] [Google Scholar]
- [35].Demidova-Rice TN, Salomatina EV, Yaroslavsky AN, Herman IM, Hamblin MR, Low-level light stimulates excisional wound healing in mice, Lasers Surg. Med 39 (2007) 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kilik R, Lakyova L, Sabo J, Kruzliak P, Lacjakova K, Vasilenko T, Vidova M, Longauer F, Radonak J, Effect of equal daily doses achieved by different power densities of low-level laser therapy at 635 nm on open skin wound healing in normal and diabetic rats, Biomed. Res. Int 2014 (2014) 269253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gal P, Mokry M, Vidinsky B, Kilik R, Depta F, Harakalova M, Longauer F, Mozes S, Sabo J, Effect of equal daily doses achieved by different power densities of low-level laser therapy at 635 nm on open skin wound healing in normal and corticosteroid-treated rats, Lasers Med. Sci 24 (2009) 539–547. [DOI] [PubMed] [Google Scholar]
- [38].Prabhu V, Rao SB, Rao NB, Aithal KB, Kumar P, Mahato KK, Development and evaluation of fiber optic probe-based helium-neon low-level laser therapy system for tissue regeneration–an in vivo experimental study, Photochem. Photobiol 86 (2010) 1364–1372. [DOI] [PubMed] [Google Scholar]
- [39].Lanzafame RJ, Stadler I, Kurtz AF, Connelly R, Peter S, Brondon Ta P, Olson D, Reciprocity of exposure time and irradiance on energy density during photoradiation on wound healing in a murine pressure ulcer model, Lasers Surg. Med 39 (2007) 534–542. [DOI] [PubMed] [Google Scholar]
- [40].Bjordal JM, Couppe C, Chow RT, Tuner J, Ljunggren EA, A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders, Aust. J. Physiother 49 (2003) 107–116. [DOI] [PubMed] [Google Scholar]
- [41].Nogueira AC, Junior Mde J, The effects of laser treatment in tendinopathy: a systematic review, Acta Ortop. Bras 23 (2015) 47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tsai SR, Hamblin MR, Biological effects and medical applications of infrared radiation, J. Photochem. Photobiol. B 170 (2017) 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hale GM, Querry MR, Optical Constants of Water in the 200-nm to 200-microm Wavelength Region, Appl. Opt 12 (1973) 555–563. [DOI] [PubMed] [Google Scholar]
- [44].Karu TI, Kolyakov SF, Exact action spectra for cellular responses relevant to phototherapy, Photomed. Laser Surg 23 (2005) 355–361. [DOI] [PubMed] [Google Scholar]
- [45].Santana-Blank L, Rodriguez-Santana E, Santana-Rodriguez KE, Reyes H, “Quantum Leap” in photobiomodulation therapy ushers in a new generation of light-based treatments for cancer and other complex diseases: Perspective and mini-review, Photomed. Laser Surg 34 (2016) 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Santana-Blank L, Rodriguez-Santana E, Santana-Rodriguez KE, Photobiomodulation of aqueous interfaces as selective rechargeable bio-batteries in complex diseases: personal view, Photomed. Laser Surg 30 (2012) 242–249. [DOI] [PubMed] [Google Scholar]
- [47].Yao J, Liu B, Qin F, Rapid temperature jump by infrared diode laser irradiation for patch-clamp studies, Biophys. J 96 (2009) 3611–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chai B, Yoo H, Pollack GH, Effect of radiant energy on near-surface water, J. Phys. Chem. B 113 (2009) 13953–13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sommer AP, Zhu D, Mester AR, Forsterling HD, Pulsed laser light forces cancer cells to absorb anticancer drugs–the role of water in nanomedicine, Artif. Cells Blood Substit. Immobil. Biotechnol 39 (2011) 169–173. [DOI] [PubMed] [Google Scholar]