Abstract
Aims
Both sitagliptin (SIT) and mitiglinide (MIT) can lower postprandial hyperglycemia. The purpose of this study was to examine differences in insulin and glucagon secretion after SIT or MIT administration when similar levels of plasma glucose (PG) were achieved for both agents following an oral glucose load.
Patients and methods
We directly compared the effects of these two agents in 16 type-2 diabetic patients (M/F = 10/6, age 66 ± 3 years old, HbA1c 6.6 ± 0.5 %). Patients received SIT (50 mg qd for 1 week and 100 mg qd for an additional week) or MIT (10 mg tid for 2 weeks). After 2 weeks, patients crossed over to the other treatment. 75-g oral glucose tolerance tests were conducted before the study and after interventions.
Results
The area under the curve (AUC) up to 180 min for the PG response was similar for both agents. While basal insulin secretion rates (ISR) were similar, incremental AUC of ISR was significantly lower in the SIT treatment (522 ± 108 vs 702 ± 288 pmol/min min, p < 0.01), although the difference between the SIT and MIT treatments in the Matsuda index—which reflects insulin sensitivity—remained nonsignificant. Glucose-stimulated insulin secretion was similarly increased by the MIT and SIT treatments. Suppression of the AUC for glucagon was observed in the SIT treatment, while MIT treatment failed to suppress the glucagon concentration (−432 ± 2322 vs MIT 1116 ± 2520 pg/ml min, p < 0.05). The basal proinsulin/insulin ratio was lower in the SIT treatment (0.23 ± 0.04 vs MIT 0.26 ± 0.36, p < 0.05).
Conclusions
Although either SIT or MIT can be employed to reduce postprandial hyperglycemia, SIT induces changes in hormonal profiles that are more favorable to islet functions than MIT does.
Keywords: Sitagliptin, Mitiglinide, Proinsulin
Introduction
The importance of avoiding postchallenge hyperglycemia to improve the quality of glycemic control in type-2 diabetic subjects has been underscored [1]. Oral hyperglycemic agents that reduce postprandial hyperglycemia include alpha-glucosidase inhibitors, glinides, and dipeptidyl peptidase-4 (DPP-4) inhibitors. While a beneficial effect of alpha-glucosidase inhibitors has been demonstrated [2], their efficacy is limited due to adverse events associated with their use and their low potential to reduce glucose levels, given that they are not capable of inducing insulin secretion. On the other hand, both glinides and DPP-4 inhibitors facilitate insulin secretion in pancreatic beta cells. Lowering the postprandial plasma glucose concentration minimizes damage to vessel walls, thus reducing nateglinide-induced atherosclerosis [3]. It has been postulated that DPP-4 inhibitors work against cardiovascular events, and the administration of these inhibitors has been demonstrated to result in improvements in surrogate markers [4]. Prospective studies examining their effects on cardiovascular endpoints are in progress.
Both DPP-4 inhibitors and glinides reduce the plasma glucose (PG) concentration after an oral glucose load by facilitating insulin secretion in pancreatic beta cells, albeit through different mechanisms. Sitagliptin is a DPP-4 inhibitor that inhibits the degradation of incretins, i.e., glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) [5]. GLP-1 acts through its receptors on pancreatic beta cells by enhancing insulin secretion during the hyperglycemic state while also normalizing the glucagon response to glycemic states, while GIP works to enhance glucagon secretion [6]. However, glinides act through the sulfonylurea receptor (SUR)-1 of pancreatic beta cells, inducing insulin secretion [7]. Mitiglinide is a synthesized non-sulfonylurea hypoglycemic agent that exerts a rapid but short-lasting hypoglycemic effect. This compound has been reported to enhance glucagon secretion transiently following sustained suppression [8].
Therefore, the responses of insulin and glucagon to an oral glucose load following the administration of sitagliptin or mitiglinide are of great interest. However, there is no published report of a direct comparison of those two agents with regards to islet function, especially when similar levels of plasma glucose are achieved. In the work described in the present paper, we conducted a randomized crossover study to find differences in the effects of these two agents on hormonal levels following an oral glucose load when similar hypoglycemic potency was anticipated.
Patients and methods
Study design and participants
This randomized crossover multicenter study was conducted to examine the effects of sitagliptin (a DPP-4 inhibitor) and mitiglinide (a glinide compound) on insulin secretion and insulin sensitivity (possibly mediated by GLP-1 through its action on alpha cells as well as beta cells in the pancreas) during oral glucose tolerance tests (OGTTs). The main outcome was the area under the curve (AUC) for glucagon response during the OGTT following sitagliptin treatment or mitiglinide treatment.
Subjects had to be at least 20 years old and less than 75 years old. They also had to be type-2 diabetic patients with stable glycemic control for more than two months, and their HbA1c was required to be <9.4 %. Subjects taking pioglitazone or metformin were included. Subjects undergoing treatment with insulin, type-1 diabetic subjects, subjects taking alpha-glucosidase inhibitors or sulfonylureas, subjects with nephrotic syndrome, and subjects taking steroids, immune suppression medication, or azole antifungal medication were excluded. Subjects taking HIV protease inhibitors, subjects who had previously suffered serious adverse events while taking DPP-4 inhibitors and/or glinides, subjects who had a stroke or acute coronary syndrome within 6 months before enrollment, subjects with severe heart failure (NYHA class 3 or higher) or severe arrhythmia, subjects who had elevated liver enzymes, subjects with malignancy, subjects who were pregnant or were attempting to get pregnant, and subjects with other conditions that led the doctor in charge to decide that they were not eligible for this study were also excluded.
Informed consent was obtained before the study. The original protocol and consent form was approved by the ethics committee of Saitama Medical Center, Saitama Medical University, and the protocol and consent form for each institute was approved by local independent ethics committees or institutional review boards before trial initiation.
Procedures
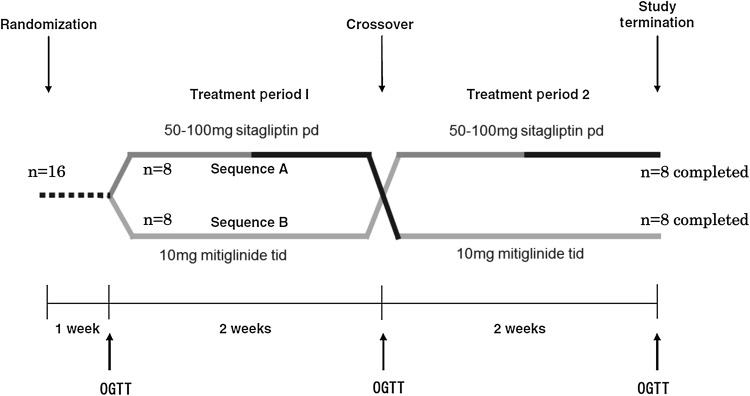
After obtaining written consent, the order in which sitagliptin and mitiglinide were administered to eligible patients was randomized using a central web response system. Patients received sitagliptin (50 mg qd for 1 week and 100 mg qd for an additional week) or mitiglinide (10 mg tid for 2 weeks) according to the randomization. These doses of both agents were designed to achieve similar glucose-lowering effects, based on the information in the Japan Pharmaceutical Reference (http://www.e-search.ne.jp/~jpr/). After two weeks, each patient crossed over to the other treatment. 75-g OGTTs were conducted before the study and after interventions. A flow diagram of the study is depicted in Fig. 1.
Fig. 1.
Flow diagram of the study. During week 1, 50 mg sitagliptin were administered orally in the morning after breakfast; in week 2, this was increased to 100 mg sitagliptin. Mitiglinide treatment consisted of 10 mg mitiglinide tid orally before each meal for two weeks. A 75-g OGTT was performed at the end of each treatment period
An OGTT was started after a 10–12 h overnight fasting period at 9 am after basal sampling of blood for glycoalbumin and HbA1c as well as substrates and hormones, as described below. After basal sampling, patients were asked to take mitiglinide 10 mg, when assigned, before the administration of an oral glucose load. A stopwatch was started when the thyroid cartilage moved down after the first swallow of Trelan®-G75 (Ajinomoto Pharmaceuticals Co. Ltd., Tokyo, Japan), which is equivalent to 75 g glucose as a metabolic product. Alternatively, patients were asked to take sitagliptin they had finished drinking the Trelan®-G75. Blood was sampled at 30, 60, 90, 120, and 180 min after the start of the test in order to measure glucose, triglyceride, insulin, C-peptide, proinsulin, glucagon, and GLP-1.
Plasma or serum samples were obtained according to the recommended procedure for assays. The samples were cooled at 4 °C before separation to obtain serum or plasma. They were then frozen at −80 °C before they were sent to the central laboratory of Mitsubishi Kagaku Bio-Clinical Laboratories, Inc. (Tokyo, Japan). Glucose and triglyceride were measured enzymatically, and insulin, C-peptide, proinsulin, glucagon, and GLP-1 were measured by ELISA using specific antibodies in the central laboratory. Samples obtained for the glucagon assay were placed into vials containing aprotinin. Samples were extracted for the GLP-1 assay and added to vials containing diprotin A (a DPP-4 inhibitor) and GLP-1 before measurements. Biologically active forms of GLP-1 [i.e., GLP-1(7-36)amide and GLP-1(7-37)] were measured by ELISA (Linco Research, St. Louis, MO, USA) according to the Guideline for Incretin Measurement (http://www.jds.or.jp/uploads/photos/786.pdf) issued by the Japan Diabetes Society.
Calculation and statistical analysis
The insulin secretion rate (ISR) during the OGTT was calculated by deconvolving the plasma C-peptide concentration using the ISEC software package developed by Hovorka et al. [9]. The ISR was related to the glucose stimulus by dividing the incremental area under the ISR curve by the incremental area under the plasma glucose curve. HOMA-IR and HOMA-beta were calculated as described previously [10]. The Matsuda index incorporates both hepatic and muscle components of insulin sensitivity, correlates well with the results of the euglycemic insulin clamp technique, and was calculated as reported previously [11]. This made it easier to understand values, since the simple raw value obtained from the calculation was too large and difficult to assess. The deposition index (insulin secretion/insulin resistance) was determined by dividing ΔISR/ΔPG by the severity of insulin resistance (IR) [ΔISR(AUC)/ΔG(AUC) divided by IR], where IR was measured as the inverse of the Matsuda index [12]. The incremental area under the concentration curve of hormone or substrate concentration was calculated according the trapezoid rule.
The Wilcoxon signed-rank test (Mann–Whitney U test) was used to compare the data obtained from the OGTTs. All statistical analyses were performed using SPSS version 21.0 (IBM SPSS Statistics, Chicago, IL, USA). Data are expressed as the mean ± SD. Data with p values of <0.05 were considered significant.
Results
Sixteen type-2 diabetic patients were randomized to start either medication in addition to the pills that they were already taking. We directly compared the effects of these two agents in the 16 type-2 diabetic patients, as shown in Table 1. Average age was 66.2 ± 3.4 years old, and average BMI was 24.4 ± 3.6 kg/m2. Average duration of diabetes was 11.5 ± 9.1 years, and average recent HbA1c was 6.6 ± 0.5 %. No advanced diabetic complications were reported. There were no differences between the features of the subjects who started mitiglinide first and those of the subjects who started sitagliptin first. Subjects taking pioglitazone (n = 2) and metformin (n = 9) were asked to continue taking them as prescribed during the whole study. The area under the curve (AUC) up to 180 min for PG response was similar regardless of the agent taken, and lower than before treatment (Table 2). The basal insulin secretion rates [0.86 ± 0.15 at baseline (BASE), 1.03 ± 0.38 after mitiglinide (MIT) treatment, and 1.11 ± 0.56 pmol/kg per min after sitagliptin (SIT) treatment] were similar before the study, and there were no differences after taking mitiglinide or sitagliptin.
Table 1.
Baseline characteristics of subjects
| Total | Mitiglinide treatment | Sitagliptin treatment | |
|---|---|---|---|
| n (M/F) | 16 (10/6) | 8 (4/4) | 8 (6/2) |
| Age (years old) | 66.2 ± 3.4 | 66.1 ± 3.9 | 66.3 ± 3.2 |
| BMI (kg/m2) | 24.4 ± 3.6 | 25.0 ± 4.4 | 23.7 ± 2.6 |
| Duration of diabetes (years) | 11.5 ± 9.1 | 14.5 ± 12.0 | 8.5 ± 3.7 |
| HbAlc (%) | 6.6 ± 0.5 | 6.5 ± 0.4 | 6.7 ± 0.7 |
| FPG (mg/dl) | 116 ± 27 | 113 ± 27 | 118 ± 28 |
| LDL-chol (mg/dl) | 94 ± 29 | 81 ± 16 | 107 ± 34 |
| HDL-chol (mg/dl) | 65 ± 19 | 64 ± 23 | 66 ± 17 |
| TG (mg/dl) | 110 ± 65 | 116 ± 86 | 103 ± 37 |
| Creatinine (mg/dl) | 0.82 ± 0.21 | 0.87 ± 0.19 | 0.78 ± 0.23 |
Data are expressed as the mean ± SD
Table 2.
Summary of parameters during OGTTs
| BASE | Mitiglinide | Sitagliptin | |
|---|---|---|---|
| Basal | |||
| GLP-1 (pmol/L) | 1.14 ± 0.32 | 1.37 ± 0.69 | 3.53 ± 0.96** |
| FPG (mg/dl) | 115.5 ± 26.5 | 118.9 ± 32.6 | 107.9 ± 4.9** |
| Glycoalbumin (%) | 18.0 ± 1.8 | 17.8 ± 1.9 | 17.7 ± 0.6 |
| Glucagon (pg/ml) | 73.9 ± 19.9 | 70.8 ± 18.1 | 76.6 ± 4.3 |
| Insulin (μU/ml) | 3.7 ± 2.1 | 4.2 ± 3.4 | 4.7 ± 1.2 |
| C-peptide (ng/ml) | 0.95 ± 0.20 | 1.16 ± 0.57 | 1.26 ± 0.20 |
| Proinsulin (pmol/L) | 5.7 ± 3.2 | 6.2 ± 2.3 | 5.3 ± 0.6* |
| TG (mg/dl) | 110 ± 65 | 92 ± 47 | 112 ± 14** |
| LDL-chol (mg/dl) | 93.8 ± 29.3 | 92.8 ± 23.4 | 88.6 ± 5.2 |
| HDL-chol (mg/dl) | 64.7 ± 19.4 | 65.3 ± 17.2 | 63.4 ± 4.8 |
| Proinsulin/insulin | 0.26 ± 0.15 | 0.26 ± 0.13 | 0.23 ± 0.04* |
| Proinsulin/C-peptide | 21.7 ± 11.7 | 20.7 ± 8.6 | 17.4 ± 8.4** |
| HOMA-IR | 1.1 ± 0.8 | 1.4 ± 1.5 | 1.4 ± 1.6 |
| HOMA-β | 30.0 ± 21.9 | 29.3 ± 15.9 | 40.7 ± 32.9 |
| Basal ISR (pmol/kg per min) | 0.86 ± 0.15 | 1.03 ± 0.38 | 1.11 ± 0.56 |
| OGTT | |||
| 2 h PG | 240 ± 88 | 187 ± 77 | 180 ± 75 |
| 3 h PG | 184 ± 92 | 134 ± 85 | 134 ± 66 |
| ΔAUC of GLP-1 | 756 ± 749 | 837 ± 934 | 2056 ± 2499** |
| ΔAUC of glucose | 19135 ± 7382 | 10771 ± 6809 | 11903 ± 7531 |
| ΔAUC of insulin | 2258 ± 1476 | 3244 ± 2069 | 2556 ± 2086* |
| ΔAUC of glucagon | −129 ± 2693 | 1110 ± 2513 | −428 ± 2330* |
| ΔAUC of C-peptide | 455 ± 204 | 628 ± 287 | 477 ± 228** |
| ΔAUC of proinsulin | 2077 ± 1165 | 2747 ± 1616 | 1803 ± 1182** |
| ΔAUC of TG | −2080 ± 4198 | −953 ± 1209 | −2379 ± 3065 |
| ΔAUC of ISR | 508 ± 214 | 693 ± 283 | 527 ± 101** |
| Matsuda index (MI) | 11.0 ± 6.5 | 9.9 ± 5.2 | 11.1 ± 6.6 |
| ΔAUC of ISR/AAUC of glucose × MI | 0.39 ± 0.44 | 1.16 ± 2.46 | 0.79 ± 0.81 |
OGTTs were conducted before (BASE) and 2 weeks after the start of intervention with mitiglinide and sitagliptin. Before calculating ratios, the units for insulin, C-peptide, and proinsulin were converted to pmol/l, nmol/l, and pmol/l, respectively. The AUC was calculated up to 180 min. Data are expressed as the mean ± SD of 16 subjects. Statistics were examined using the Wilcoxon rank-sum test
* p < 0.05, ** p < 0.01 vs mitiglinide
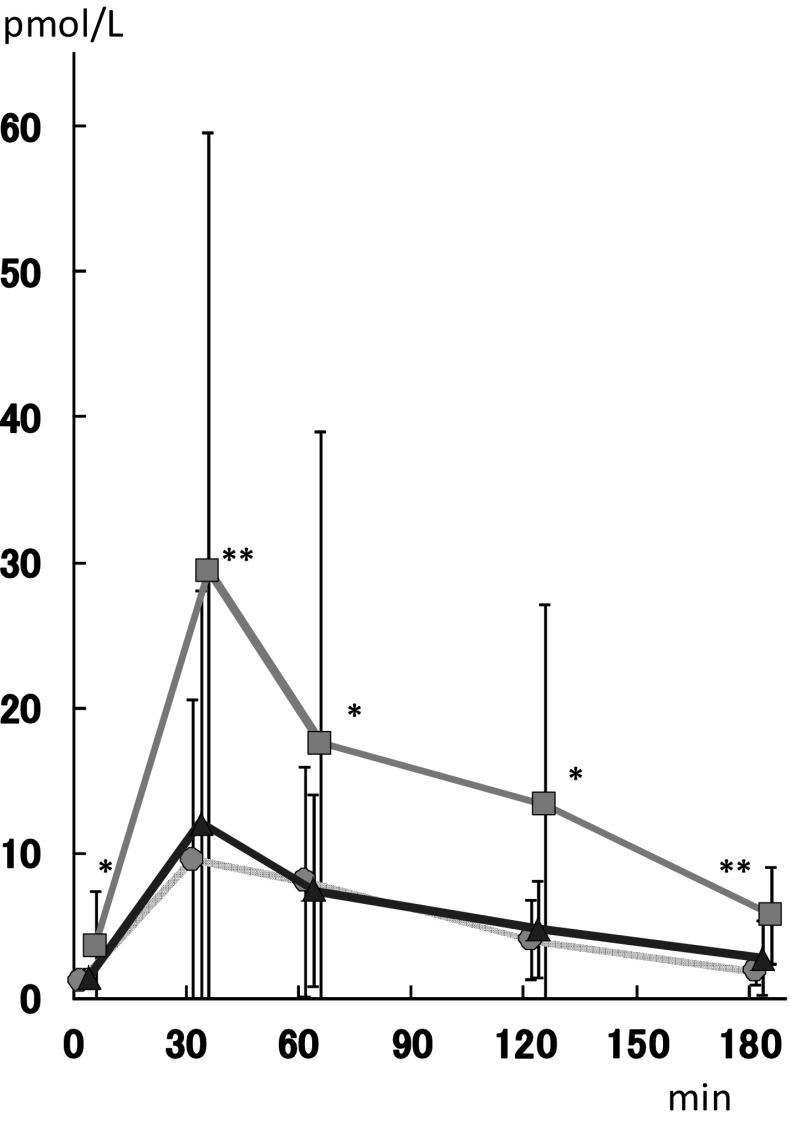
As expected, an increase in GLP-1 was only detected in the sitagliptin treatment, as shown in Fig. 2. The basal GLP-1 level was three times higher (3.53 ± 0.96 pmol/l) after sitagliptin treatment than those of BASE (1.14 ± 0.32) and MIT (1.37 ± 0.69).
Fig. 2.
Plasma GLP-1 concentrations after 75-g OGTTs performed before (gray circles) and two weeks after the start of mitiglinide treatment (blue triangles) or two weeks after the start of sitagliptin treatment (green squares). Each data value is expressed as the mean ± SD. Statistics were examined using the Wilcoxon rank-sum test. *p < 0.01, **p < 0.001 vs mitiglinide
Excursion curves for glucose, insulin, C-peptide, proinsulin, glucagon, and triglyceride during 75-g OGTTs in the subjects before and two weeks after the start of mitiglinide or sitagliptin intervention are depicted in Fig. 3. To facilitate a comparison of the substrate and hormone responses, we calculated the area under the curve in each case (AUC). The AUC for glucagon response during the OGTT after sitagliptin treatment was not significantly decreased compared to the baseline (76.9 ± 18.9 in MIT, 74.2 ± 22.4 in SIT, and 73.2 ± 19.9 ng/ml in BASE). Despite a slight, nonsignificant increase in basal glucagon levels in the SIT treatment (76.6 ± 4.3 vs 70.8 ± 18.1 in MIT, and 73.9 ± 19.9 pg/ml in BASE), a significant decrease in the AUC of glucagon was observed, whereas MIT treatment failed to suppress the glucagon concentration (−432 ± 2322 vs MIT 1116 ± 2520 pg/ml min, p < 0.05) (Fig. 4).
Fig. 3.
Excursion curves for a glucose, b insulin, c C-peptide, d proinsulin, e glucagon, and f triglyceride during 75-g OGTTs in subjects before (gray circles) and two weeks after the start of mitiglinide treatment (blue triangles) or two weeks after the start of sitagliptin treatment (green squares). Each data value is expressed as the mean ± SD. Statistics were examined using the Wilcoxon rank-sum test. *p < 0.05, **p < 0.01 vs mitiglinide
Fig. 4.
Excursion curves showing the AUC for glucagon during 75-g OGTTs in subjects before (gray circles) and two weeks after the start of mitiglinide treatment (blue triangles) or two weeks after the start of sitagliptin treatment (green squares). Each data value is expressed as the mean (bold horizontal bar) ± SD (vertical bar)
The SIT and MIT treatments did not yield significantly different values of HOMA-IR or HOMA-beta. There also were no differences between the treatments in the Matsuda index (11.1 ± 6.6 in SIT, 9.9 ± 5.2 in MIT).
While basal insulin secretion rates (ISRs) were similar for both treatments, the incremental AUC of ISR was significantly lower in the SIT treatment (522 ± 108 vs 702 ± 288 pmol/kg per min min, p < 0.01), and the ΔAUC of insulin (2556 ± 2088 vs 3240 ± 2070 μU/ml min in MIT, p < 0.05) and the ΔAUC of C-peptide (486 ± 234 vs 630 ± 288 ng/ml min in MIT, p < 0.01) were smaller in the SIT treatment. There was no difference between the treatments in the disposition index (Table 2). Figure 5a plots the ISR versus time. This excursion curve indicates that mitiglinide treatment increased the ISR as compared with the ISR afforded by sitagliptin treatment or the baseline rate. Plotting the ISR data against the concomitant plasma glucose concentrations measured during the OGTT enabled the glucose-stimulated insulin secretion (GSIS) to be assessed (Fig. 5b). The GSIS was not decreased by sitagliptin administration.
Fig. 5.
a The insulin secretion rate in subjects was calculated and plotted versus time during a 75-g OGTT performed before (gray circles) and two weeks after the start of mitiglinide treatment (blue triangles) or two weeks after the start of sitagliptin treatment (green squares). b Plotting the ISR data against the concomitant plasma glucose concentrations measured during the OGTT enabled the glucose-stimulated insulin secretion (GSIS) to be assessed in subjects before (gray circles) and two weeks after the start of mitiglinide treatment (blue triangles) or two weeks after the start of sitagliptin treatment (green squares). Each data value is expressed as the mean (bold horizontal bar) ± SD (vertical bar)
The AUC of proinsulin was decreased in the SIT treatment (2700 ± 684, vs MIT 3852 ± 1764, p < 0.01; vs CON 3096 ± 1548 pmol/l min, p < 0.05). Basal triglyceride levels were reduced by MIT treatment (92 ± 47 vs 112 ± 14 mg/dl in SIT, p < 0.01). Although this decrease was not significant, the ΔAUC of TG was lower (−2376 ± 3060 vs −954 ± 1206 mg/dl min in MIT).
There were no differences in the values of parameters for subjects who were treated with sitagliptin first and those treated with mitiglinide first in this study. There were no treatment-related abnormalities based on laboratory measurements such as liver function tests, creatinine, and urinalysis for ketones and proteins. Furthermore, there were no changes in vital signs such as heart rate and blood pressure with either treatment. There were no hypoglycemic episodes.
Discussion
Similar glycemic levels were achieved after an oral glucose load in this study after treatment with either sitagliptin or mitiglinide. Although the area under the curve (AUC) for glucagon response during an OGTT was slightly lower for the sitagliptin treatment, it was not significantly less than that seen with the mitiglinide treatment. However, a decrease in the AUC for glucagon was observed after treatment with sitagliptin, while treatment with mitiglinide failed to suppress glucagon concentration. There was no difference between the treatments in insulin resistance and insulin sensitivity as assessed by HOMA-IR and the Matsuda index. Although at first glance the ISR seemed to be greater for the mitiglinide treatment, the disposition indices were similar for both treatments. In addition, the GSIS was not lower in patients treated with sitagliptin. Since this study was designed to achieve similar hypoglycemic potencies with the two medications, there were no differences between the treatments in glycoalbumin and 2-h PG after OGTTs. However, the FPG was lower when sitagliptin was assigned, as the short duration of action of mitiglinide meant that it was not potent enough to counter glucose toxicity over consecutive days. On the other hand, it seems likely that sitagliptin continued to work until at least the next morning when the dose was 100 mg per day. Sitagliptin at 100 mg per day may significantly suppress DPP-4 overnight, as demonstrated by a dose–response increase in inhibition [5]. It is therefore no wonder that GLP-1 levels were increased during the fasting state, resulting in a reduced fasting PG. This may have caused the slight increase in the basal GSIS in the sitagliptin group compared with the baseline GSIS and that observed in the mitiglinide group (Fig. 4).
Although both sitagliptin and mitiglinide facilitated insulin secretion, reduced glucagon responses and reduced insulin responses were observed in the sitagliptin treatment compared to those seen in the mitiglinide treatment. In addition, levels of proinsulin/insulin and proinsulin/C-peptide (indicators of beta-cell working stress) were significantly lower with sitagliptin treatment.
The d-phenylalanine derivative nateglinide, a short-acting insulin secretagogue, has been reported to have DPP-4-inhibitor-like potency [13] or the ability to secrete GLP-1 through its amino-acid-like structure independent of a K-ATP channel [14]. However, mitiglinide has never been reported to act in this way, and we did not observe any additional increases in GLP-1 concentration after an oral glucose load during mitiglinide intervention (Fig. 2). Comparisons of the effects of a DPP-4 inhibitor and those of mitiglinide have been conducted in mice [15] and in humans [16]. Repaglinide also had no effect on GLP-1 in humans [17].
We also observed a significant acute decrease in the triglyceride concentration after glucose administration with sitagliptin treatment. In a previous study, when postprandial levels of both lipids and glucagon after three months of treatment with sitagliptin were compared with those after three months of treatment with nateglinide, triglycerides were found to be significantly suppressed in the sitagliptin group [7]. A significant decrease in the serum glucagon level was also observed at 1 h postprandially in the sitagliptin group in that study. Although the duration of treatment was different in our study, similar responses in terms of triglycerides and glucagon were observed. However, in our study, the fasting triglyceride and glucagon levels showed the opposite behavior after the two weeks’ intervention. Since the fasting PG was significantly lower after sitagliptin treatment due to increased fasting insulin concentrations and GLP-1 levels, glucagon secretion may be controlled and suppressed using this intervention, as previously proposed [18]. It is possible that glucagon levels were slightly elevated to avoid hypoglycemia, resulting in a slight increase in fasting triglycerides, which may help to avoid hypoglycemic attacks while using sitagliptin.
We believe that using the plasma C-peptide concentration as a measure of pre-hepatic insulin secretion provides a more direct measure of GSIS and beta-cell function, although another study [7, 19] suggested that hepatic insulin extraction does not affect insulin-derived calculations of beta-cell function. We conducted this study by administering an oral glucose load, since excluding the gastrointestinal tract by administering the glucose load intravenously may limit our physiologic understanding of beta-cell function in vivo.
This study was primarily designed to compare the acute glycemic responses of the patients to the two treatments, so a longer-term study was not considered necessary. Long-term use of these agents may yield different results, but they would be affected by any changes in body weight or other factors. However, for the first month of SIT treatment, the study reported in a previous study [7] gave similar results to those seen for the SIT treatment in our study, while the effect of nateglinide was limited in the same study. Although nateglinide exhibits DPP-4-inhibitor-like action [13, 14, 20], its beneficial effects were significantly exceeded by those of sitagliptin. In addition, nateglinide failed to show any beneficial effects on glucose metabolism and cardiovascular events in the NAVIGATOR study [21]. Possible reasons include increased body weight and the occurrence of hypoglycemia, which are not likely to be induced by sitagliptin.
One limitation of the present study was the lack of a medication washout period between the two treatment periods. However, since the half-lives of both sitagliptin (12 h) and mitiglinide (1.2 h) are short, and assessments were conducted two weeks after the patients had crossed over to the other treatment, any carryover effect is unlikely. A test for carryover using patients nested within sequence as the error term revealed no evidence of any carryover effect. Moreover, as the study was primarily designed to compare the acute glycemic responses of the patients to the two treatments, a longer study duration was not considered necessary; this approach was also adopted in a similar study [22]. In addition, terminating the administration of a hypoglycemic agent may cause glucose toxicity, which may affect the results. However, since the order of agent administration was randomized for the patients, the conclusions drawn based on the results of this study are scientifically robust.
There are other limitations to this study. The number of subjects included was not large. However, it should be possible to compare the responses of each patient to the two treatments in this study design, and all of the participants returned at the exact time scheduled for this study. Therefore, we believe that the results reflect basic pharmacological data for humans, and increasing the number of subjects may not be ethically appropriate. Also, we only administered an oral glucose load; we did not use mixed meals. However, we wanted to elucidate differences in the mechanisms of action of sitagliptin and mitiglinide, especially on insulin secretion and insulin sensitivity (possibly mediated by GLP-1 through its action on alpha cells as well as beta cells in the pancreas) during OGTTs. The data shown here should prove helpful when attempting to understand differences in the effects of DPP-4 inhibitors and glinides when they are used in clinical situations.
In conclusion, the glucagon and insulin responses were suppressed by sitagliptin treatment as compared to mitiglinide treatment after an oral glucose load. In addition, as demonstrated by reduced proinsulin levels, less beta-cell stress occurred in the sitagliptin treatment. Both the normalization of alpha-cell function and the protection of beta-cell function in the pancreatic islets by sitagliptin were successfully demonstrated in this study. It may be better to use DPP-4 inhibitors rather than mitiglinide when postprandial hyperglycemia is a concern in the treatment of type-2 diabetic patients.
Acknowledgments
This work was planned and conducted by the Saitama Incretin Study Group, and was funded via a grant from the Waksman Foundation of Japan, Inc. The authors are members of the Saitama Incretin Study Group, of which Shigehiro Katayama is the president. The number of this clinical trial in the UMIN clinical trial registration system is UMIN000005283. The UMIN Registry has been officially accepted by the ICMJE (International Committee of Medical Journal Editors). Part of the data obtained in this study was presented at the annual meeting of the American Diabetes Association in 2012, and the calculations of insulin secretion rates were presented as an abstract at the annual meeting of the Japan Diabetes Society in 2013.
Conflict of interest
Yoshimasa Aso received lecture fees from Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., and MDS K.K. Shigeharu Katayama received research grants from MSD K.K. and Daiichi Sankyo Pharma Ltd. Yoshitaka Akiyama, Tomoko Morita-Okubo, Natsuko Oshitani, Yuko Ohno, Toshihiko Inukai, Kakei Masafumi, Kawakami Masanobu, Takuya Awata, and Masafumi Matsuda declare that they have no conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its subsequent revision. Informed consent or a substitute for it was obtained from all patients before they were included in the study.
Footnotes
An erratum to this article is available at http://dx.doi.org/10.1007/s13340-017-0322-2.
References
- 1.DECODE Study Group, European Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354:617–621. [PubMed]
- 2.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 3.Mita T, Watada H, Shimizu T, Tamura Y, Sato F, Watanabe T, et al. Nateglinide reduces carotid intima-media thickening in type 2 diabetic patients under good glycemic control. Arterioscler Thromb Vasc Biol. 2007;27:2456–2462. doi: 10.1161/ATVBAHA.107.152835. [DOI] [PubMed] [Google Scholar]
- 4.Chrysant SG, Chrysant GS. Clinical implications of cardiovascular preventing pleiotropic effects of dipeptidyl peptidase-4 inhibitors. Am J Cardiol. 2012;109:1681–1685. doi: 10.1016/j.amjcard.2012.01.398. [DOI] [PubMed] [Google Scholar]
- 5.Herman GA, Bergman A, Stevens C, Kotey P, Yi B, Zhao P, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:4612–4619. doi: 10.1210/jc.2006-1009. [DOI] [PubMed] [Google Scholar]
- 6.Meier JJ, Gallwitz B, Siepmann N, Holst JJ, Deacon CF, Schmidt WE, et al. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia. 2003;46:798–801. doi: 10.1007/s00125-003-1103-y. [DOI] [PubMed] [Google Scholar]
- 7.Kojima Y, Kaga H, Hayashi S, Kitazawa T, Iimura Y, Ohno M, et al. Comparison between sitagliptin and nateglinide on postprandial lipid levels: the STANDARD study. World J Diabetes. 2013;4:8–13. doi: 10.4239/wjd.v4.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinukawa M, Ohnota H, Ajisawa Y. Effect of a non-sulphonylurea hypoglycaemic agent, KAD-1229 on hormone secretion in the isolated perfused pancreas of the rat. Br J Pharmacol. 1996;117:1702–1706. doi: 10.1111/j.1476-5381.1996.tb15342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovorka R, Soons PA, Young MA. ISEC: a program to calculate insulin secretion. Comput Methods Programs Biomed. 1996;50:253–264. doi: 10.1016/0169-2607(96)01755-5. [DOI] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 12.Abdul-Ghani MA, Matsuda M, Jani R, Jenkinson CP, Coletta DK, Kaku K, et al. The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2008;295:E401–E406. doi: 10.1152/ajpendo.00674.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffy NA, Green BD, Irwin N, Gault VA, McKillop AM, O’Harte FP, et al. Effects of antidiabetic drugs on dipeptidyl peptidase IV activity: nateglinide is an inhibitor of DPP IV and augments the antidiabetic activity of glucagon-like peptide-1. Eur J Pharmacol. 2007;568:278–286. doi: 10.1016/j.ejphar.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Kitahara Y, Miura K, Yasuda R, Kawanabe H, Ogawa S, Eto Y. Nateglinide stimulates glucagon-like peptide-1 release by human intestinal L cells via a K(ATP) channel-independent mechanism. Biol Pharm Bull. 2011;34:671–676. doi: 10.1248/bpb.34.671. [DOI] [PubMed] [Google Scholar]
- 15.Tahara A, Matsuyama-Yokono A, Shibasaki M. Effects of antidiabetic drugs in high-fat diet and streptozotocin-nicotinamide-induced type 2 diabetic mice. Eur J Pharmacol. 2011;655:108–116. doi: 10.1016/j.ejphar.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Jung JA, Kaku K, Kim JH, Kim JR, Ko JW, Lee SY, et al. Additive postprandial glucose-lowering effects of mitiglinide and sitagliptin in patients with type 2 diabetes mellitus. Adv Ther. 2013;30:1018–29. [DOI] [PubMed]
- 17.Stephens JW, Bodvarsdottir TB, Wareham K, Prior SL, Bracken RM, Lowe GD, et al. Effects of short-term therapy with glibenclamide and repaglinide on incretin hormones and oxidative damage associated with postprandial hyperglycaemia in people with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;94:199–206. doi: 10.1016/j.diabres.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239–1246. doi: 10.1210/jcem.87.3.8355. [DOI] [PubMed] [Google Scholar]
- 19.Malin SK, Solomon TP, Blaszczak A, Finnegan S, Filion J, Kirwan JP. Pancreatic beta-cell function increases in a linear dose-response manner following exercise training in adults with prediabetes. Am J Physiol Endocrinol Metab. 2013;305:E1248–E1254. doi: 10.1152/ajpendo.00260.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKillop AM, Duffy NA, Lindsay JR, Green BD, Patterson S, O’Harte FP, et al. Insulinotropic actions of nateglinide in type 2 diabetic patients and effects on dipeptidyl peptidase-IV activity and glucose-dependent insulinotropic polypeptide degradation. Eur J Endocrinol. 2009;161:877–885. doi: 10.1530/EJE-09-0547. [DOI] [PubMed] [Google Scholar]
- 21.Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, Hua TA, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1463–1476. doi: 10.1056/NEJMoa1001122. [DOI] [PubMed] [Google Scholar]
- 22.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943–2952. doi: 10.1185/03007990802418851. [DOI] [PubMed] [Google Scholar]