Abstract
Given GDF5 involvement in hip development, and osteoarthritis (OA) and developmental hip dysplasia (DDH) risk, here we sought to assess the role(s) of GDF5 and its regulatory sequence on the development of hip morphology linked to injury risk. The brachypodism (bp) mouse, which harbors a Gdf5 inactivating mutation, was used to survey how Gdf5 loss of function impacts the development of hip morphology. Two transgenic Gdf5 reporter BAC lines were used to assess the spatiotemporal expression of Gdf5 regulatory sequences. Each BAC line was also used to assess the functional roles of upstream and downstream sequence on hip morphology. bp/bp mice had shorter femora with smaller femoral heads and necks as well as larger alpha angles, smaller anterior offsets, and smaller acetabula, compared to bp/+ mice (p<0.04). Regulatory sequences downstream of Gdf5 drove strong prenatal (E17) expression and low postnatal (6 months) expression across regions of femoral head and acetabulum. Conversely, upstream regulatory sequences drove very low expression at E17 and no detectable expression at 6 months. Importantly, downstream, but not upstream Gdf5 regulatory sequences fully restored all the key morphologic features disrupted in bp/bp mice. Hip morphology is profoundly affected by Gdf5 absence, and downstream regulatory sequences mediate its effects by controlling Gdf5 expression during development. This downstream region contains numerous enhancers harboring risk variants related to hip OA, DDH, and dislocation. We posit that subtle alterations to morphology driven by changes in downstream regulatory sequence underlie this locus’ role in hip injury risk.
Introduction
Several hip conditions, including osteoarthritis (OA) and developmental dysplasia of the hip (DDH), are among the most common musculoskeletal injuries. The unique morphological features of the human proximal femur and pelvic acetabulum, such as the shape and size of the femoral head, and acetabular diameter and depth, are among major contributors to hip biomechanics and stability. Changes to these morphological features often result in altered joint loading and motion, which can lead to decreased joint stability and/or increased risk of cartilage degeneration over time. These findings are supported by reports correlating the deformities of the proximal femur (i.e. cam deformity) and acetabulum (i.e. acetabular dysplasia) to increased risk of hip dislocation and OA [1–3].
Hip morphology is initially determined during embryogenesis, when a series of flattened mesenchymal cells of the joint interzone form and delineate the boundary between the proximal femur and acetabulum. The interzone additionally provides progenitor cells that give rise to hip connective tissues (e.g. articular cartilage, ligaments, and labrum), while adjacent cells give rise to the bony elements. Over the course of late prenatal and early postnatal development, hip morphology matures and once it is mechanically loaded undergoes remodeling. Changes to this process can lead to hard and soft-tissue abnormalities, which can result in non-physiologic joint loading and ultimately increased risk of instability, injury, or degeneration.
The Growth and Differentiation Factor 5 (GDF5) gene encodes a bone morphogenic protein of the TGF- ß superfamily, found only in vertebrates [4]. It is one of the key components of the biological pathways involved in pre- and postnatal development of synovial joints (e.g. hip) [5]. In humans and mice, coding mutations in GDF5 can lead to a broad spectrum of skeletal abnormalities including short stature, misregistered and malformed joints, and missing digits [4, 6–11]. More prevalent, however, is the link established in several Genome-Wide Association Studies (GWAS) between common single nucleotide polymorphisms (SNPs) spanning a 130 kb interval containing GDF5 and OA [12–14]. This interval reflects an underlying haplotype found in hundreds of millions of people worldwide, the frequency of which has been attributable to a SNP residing within a growth plate enhancer (GROW1) that affects long bone size [7]. Interestingly, no variants affecting the GDF5 or downstream UQCC (ubiquitinol-cytochrome C reductase complex assembly factor 1) protein coding sequences can account for the specific population-level OA associations, leading researchers to focus on linked GDF5 5’UTR variants. While these variants reduce transcriptional activity of the core GDF5 promoter in vitro [12], and along with other variants in strong linkage disequilibrium, correlate with reduced GDF5 transcript levels in vivo [13], they do not reside in sequence capable of specifically impacting hip morphology [15].
Despite strong and reproducible evidence suggesting GDF5 associations with hip OA, DDH, and dislocation [12–14, 16–18] its mechanism of action is unknown. A recent investigation into the cis-regulatory architecture of the human GDF5 and mouse Gdf5 locus, using a Bacterial Artificial Chromosome (BAC) scan and fine-mapping approach, revealed a number of distinct GDF5 synovial joint regulatory enhancers [15]. Many of these enhancers reside downstream of GDF5, control hip expression, and harbor risk variants related to hip OA, DDH, and dislocation [12–14, 16–18]. Considering the importance of proximal femur and acetabulum morphology to hip biomechanics and stability, it is possible that the role of GDF5 in hip OA and dislocation is mediated by alterations in hip morphology. Thus, here we sought to assess the role(s) of GDF5 and its regulatory sequence on the development of key hip morphological features that are linked to hip injury risk.
Materials and methods
Ethics statement
All mouse procedures were specifically approved and performed in accordance with Harvard University Institutional Animal Care and Use Committee (IACUC) protocol and ethical standards (protocol # 13-04-161). Mice were euthanized following standard standardized CO2 inhalation followed by cervical dislocation.
Mouse strains
To study hip morphology and how Gdf5 loss-of-function impacts the development of the hip, we used the BALB/cJ bp3J strain (Jackson Laboratories), whose mutation has been described elsewhere [4, 15]. As previously described, mice with two copies of this non-functional allele (bp/bp) lack Gdf5 and exhibit brachypodism, while heterozygous mice (bp/+) have no overt developmental abnormalities [6]. In line with the findings of other authors [19–22], we also did not observe differences between +/+ and bp/+ mice, indicating that in the context of loss-of-function, dosage must be reduced below 50% to drive noticeable phenotypes. Due to the above, and because our breeding strategy yields only bp/bp and bp/+ animals, bp/+ mice were used as controls.
We also used two transgenic FVB-NJ strains (Upstream BAC and Downstream BAC; Friend Virus B—NIH Jackson) [6, 15], each expressing Gdf5 and lacZ from a BAC transgene containing mouse Gdf5 and ~110-kb of upstream or downstream flanking sequence, to study how broad regulatory regions impact Gdf5 function. Accordingly, each BAC, as previously described in (6, 15) contains an IRES-β-Geo cassette in the 3’UTR of Gdf5, permitting bicistronic transcription of Gdf5 and lacZ. For rescue experiments, Upstream BAC and Downstream BAC stable mouse lines were separately crossed to bp/bp mice. Upstream BAC;bp/+ and Downstream BAC;bp/+ were then crossed to bp/bp mice, and the resulting progeny were genotyped for the lacZ transgene and the bp allele in separate PCR reactions as described [15].
Skeletal preparations, staining, and section in situ hybridization
Skeletal preparations
Adult 8-week old mice were euthanized (see above) and then subjected to bone and cartilage staining protocols using Alizarin red and Alcian blue, respectively. In brief, after euthanasia, adult mice were skinned, eviscerated, and muscles were removed. The specimens were then dehydrated using an ethanol series, and then treated with acetone. Alcian blue/Alizarin red S was used to stain skeletal structures. Finally, specimens were cleared using 1% KOH/glycerol [6].
X-gal staining
Whole-mount staining and section staining on Tissue-Tek OCT-embedded histological sections for β-galactosidase activity proceeded as follows and as described in [15]: For whole mount, Upstream BAC and Downstream BAC embryos were placed in 4% paraformaldehyde (Sigma, 158127) at 4 °C based on stage-specific criteria. Embryos were then placed in wash buffer three times, and then stained for 24 hours with 1 mg/ml X-gal (Sigma, B4252) in staining buffer at room temperature in the dark. After, embryos were briefly rinsed in wash buffer and post-fixed in 4% paraformaldehyde for 6 hours. For histological section staining, 10 um sections were acquired from OCT-embedded snap-frozen Downstream BAC specimens using a cryostat. These sections were briefly fixed in 4% paraformaldehyde for 10 minutes. The slides were then passed through 3 rounds, 5 minutes each, of wash buffer, then stained for one hour, post-fixed, and then imaged at a later time.
In situ hybridization
E17 embryonic mouse hindlimbs (N = 2) were snap-frozen and embedded in Tissue-Tek OCT compound and sectioned for both lacZ staining (see above) and Gdf5 in situ hybridization as previously described [5].
Micro-computed tomography (microCT) imaging and morphology assessment
In order to quantify morphological changes (abnormalities) related to Gdf5 loss-of-function as well as to determine the influence of Gdf5 regulatory sequences on hip morphology, right femora and pelves of twenty 8-week old mice (N = 5 per genotype) were scanned using high-resolution MicroCT (μCT40, SCANCO Medical AG, Brüttisellen, Switzerland). Scanned images were then exported as Digital Imaging and Communications in Medicine (DICOM) images, used to measure multiple clinically relevant morphological features of the femur and pelvis. The measurements were done in Osirix MD v7.5 (Pixemo SARL, Bernex, Switzerland) using established protocols (S1 Fig) [23–26]. The measurer (AMK) was blinded to specimens’ genotype. Shapiro-Wilk’s test was used to assess normality in SPSS (IBM Corp., Armink, NY). Analysis of Variance (ANOVA) with a post hoc Tukey correction for multiple comparisons was used to compare normally distributed outcomes. Kruskal-Wallis test with a Benjamini Hochberg post hoc correction for multiple comparisons was used to compare not-normally distributed outcomes. (Prism, GraphPad Software Inc., La Jolla, CA). P-values are two-sided and the statistical significance was assessed at alpha = 0.05.
Results
Assessment of the role of Gdf5 in the development of hip morphology
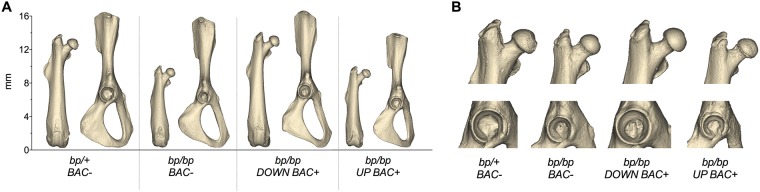
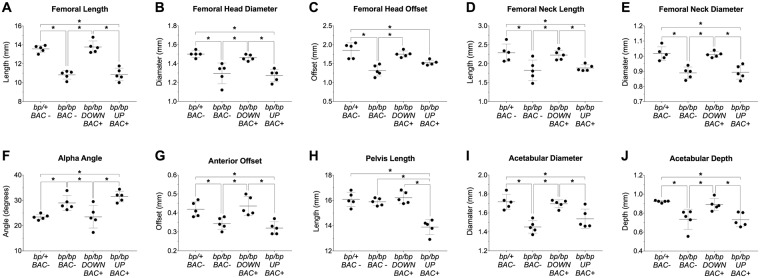
Examination of microCT imaged skeletal preparations of Gdf5 null (bp/bp) and control (bp/+) mice reveals that qualitatively, 8-week old bp/bp mice possess a normal looking hip joint, with all of the prominent morphological features of the proximal femur and acetabulum (Fig 1). However, detailed quantitative analyses reveal significant alterations to several hip features, especially those linked to risk of injury. Compared to bp/+ mice, bp/bp mice had shorter femora (p = 0.014), smaller femoral head diameters (p = 0.001), smaller femoral head offsets (p<0.001), shorter femoral neck lengths (p = 0.009), and smaller femoral neck diameters (p<0.001). Moreover, bp/bp mice had larger alpha angles (p = 0.038), smaller anterior offsets (p = 0.037), smaller acetabular diameters (p<0.001) and shallower acetabular depths (p = 0.004) (Fig 2). Conversely, there are no significant differences in valgus cut angles (p = 0.087), neck shaft angles (p = 0.220), femoral head tilt angles (p = 0.465), anteversion angles (p = 0.506) and pelvis lengths (p = 0.789) between genotypes. The mean (SD) of all quantified indices along with the P-values for all pairwise comparisons are presented in S1 Table.
Fig 1. Reconstructed three-dimensional models of hip bones from a representative specimen of each genotype.
(A) femur and pelvis and (B) close up views of proximal femur and acetabulum. BAC, Bacterial Artificial Chromosome; bp, brachypodism; DOWN BAC+, Downstream BAC; UP BAC+, Upstream BAC.
Fig 2. Quantitative morphological analysis of the femur and pelvis in the bp/bp and bp/bp+BAC rescue experiments.
Group differences in (A) femoral length (* p<0.02), (B) femoral head diameter (* p<0.01), (C) femoral head offset (* p<0.01), (D) femoral neck length (* p<0.03), (E) femoral neck diameter (* p<0.01), (F) alpha angle (* p<0.04), (G) anterior offset (* p<0.04), (H) pelvis length (* p<0.05), (I) acetabular diameter (* p<0.01), and (J) acetabular depth (* p<0.02). Mean±SD (n = 5/group). BAC, Bacterial Artificial Chromosome; bp, brachypodism; DOWN BAC+, Downstream BAC; UP BAC+, Upstream BAC.
Late prenatal and postnatal expression of Gdf5 regulatory domains
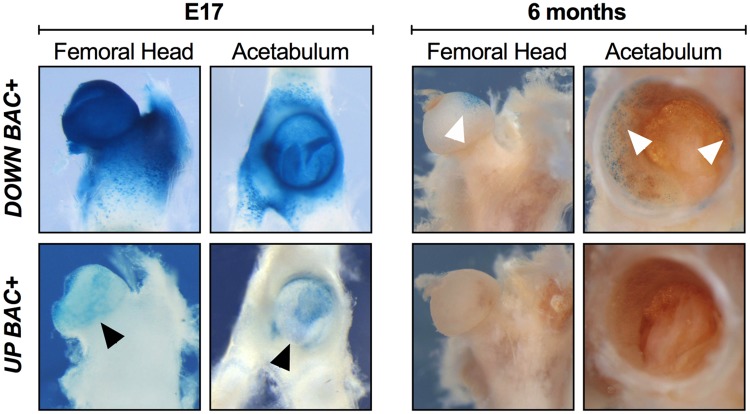
Given the conservation of the mouse and human GDF5 gene as well as its regulatory sequence function [7, 15], we next assessed in late fetal and postnatal mouse hips the expression patterns of two large Upstream BAC and Downstream BAC clones, each expressing Gdf5 and lacZ from a BAC transgene containing mouse Gdf5 and ~110-kb of upstream or downstream flanking sequence. During prenatal stages (at E17), the Downstream BAC drove expression ubiquitously throughout the hip joint with strong expression throughout the proximal femur and acetabulum (Fig 3 and S2 Fig); specifically, across the proximal femoral head articular cartilage, femoral neck, trochanters and intertrochanteric zones, and proximal chondrocyte growth zone. Within the acetabulum, the Downstream BAC drove expression across the labrum, the acetabular surface and rim, the lunate surface, ligaments, as well as the surrounding chondrocytes in the growth zone formed between the ilium, ischium, and pubis. In contrast, at E17, the Upstream BAC drove expression at a much lower level and only across the femoral head articular cartilage as well as partially in the labrum and acetabulum proper (Fig 3; black arrowheads). By 6 months postnatal, a much-reduced expression pattern was observed for the Downstream BAC construct: expression was observed only at the most proximal, anterior region of the femoral head articular cartilage as well as along the superior lip of the labrum and acetabulum rim (Fig 3; white arrowheads), locations of major joint loadings in mice. In contrast, at 6 months postnatal, no detectable lacZ expression was observed in the proximal femur and acetabulum using the Upstream BAC construct (Fig 3). Detailed expression patterns of each BAC are presented in S2 Table.
Fig 3. Comparison of Upstream and Downstream BAC expression in late prenatal hip development and in the postnatal hip.
X-gal-stained hips from Downstream BAC (DOWN BAC+) and Upstream BAC (UP BAC+) mice at E17 and 6 months are shown. At E17, the Downstream BAC drove a strong, ubiquitous expression throughout the hip joint covering the proximal femur and acetabulum, whereas the Upstream BAC drove expression at a much lower level and only across the femoral head articular cartilage as well as partially in the labrum and acetabulum proper (black arrowheads). At 6 months, the Downstream BAC construct drove a much-reduced expression pattern, with expression present only at the most proximal, anterior region of the femoral head articular cartilage as well as along the superior lip of the labrum and acetabulum rim (white arrowheads). No detectable expression was observed using the Upstream BAC construct at 6 months.
Assessment of Gdf5 regulatory sequence in shaping hip morphology
Given the dynamic expression patterns by both the Upstream and Downstream BAC constructs, and prior findings indicating their influence on long bone and knee morphology [6, 7, 15], we more deeply explored the regulatory control that each regulatory sequence block has on hip morphology. Taking advantage of our bicistronic Gdf5-lacZ construct design, we introduced a copy of each BAC transgene onto the bp/bp mouse background and gauged phenotypic rescue at 8 weeks using microCT and morphometric techniques. Transgenic expression of Gdf5 by the Downstream BAC restored the femoral length as well as all quantified morphological features of the proximal femur and acetabulum that were disrupted in the bp/bp mouse (Fig 2 and S1 Table; p>0.8 for all bp/+ vs. bp/bp Down BAC+ comparisons). In contrast, Gdf5 expression through the Upstream BAC failed to restore femur length and the dysmorphic morphology of the proximal femur and acetabulum of bp/bp mice (Fig 2 and S1 Table; p<0.03 for all bp/+ vs. bp/bp Up BAC+ comparisons).
Discussion
In this study, we investigated the influence that Gdf5 and its broad regulatory domains have on hip morphology, in the context of shape parameters relevant to OA, DDH, and dislocation. We revealed that: (1) Gdf5 loss results in substantial dysmorphologies to the proximal femur and acetabulum in line with known features involved in hip instability, developmental dislocation, injury, and adult onset OA; (2) Compared to upstream regulatory sequences, downstream regulatory sequences of Gdf5/GDF5 drive stronger, more ubiquitous prenatal hip expression and uniquely control postnatal hip expression; (3) This regulatory structure is functionally important as downstream sequences were capable of restoring Gdf5 loss-of-function phenotypes to normal, a situation not observed when using the upstream region. These latter two findings have important ramifications to how we understand GDF5 and its association with OA and hip dysplasia/dislocation.
Indeed, this is the first study to not only demonstrate a direct link between Gdf5 loss-of-function and hip dysmorphology in mice but to demonstrate that loss of Gdf5 influences proximal femur and acetabular morphology in a manner concordant with known hip joint features that are clinically relevant to hip ailments. Interestingly, unlike prior observations that the bp/bp knee joint is completely dysmorphic [6], gross morphology of its hip joint appeared relatively normal. However, our use of quantitative imaging approach revealed that fine-grained features of both the proximal femur and acetabulum were significantly affected. The anatomical alterations that we observed have been either directly linked to hip OA risk in humans (e.g. femoral head diameter, femoral neck length, and femoral neck diameter) or previously reported in patients with femoroacetabular impingement (FAI), DDH, and recurrent hip dislocation, which are proven risk factors for hip OA [1–3]. Abnormal development of these features can compromise hip stability (e.g. hip dislocation) and/or result in non-physiologic joint loading, leading to excessive cartilage wear and degeneration (e.g. OA) overtime.
Importantly, there is ample evidence revealing the importance of non-coding regulatory variants in the GDF5 locus as potentially underlying hip dysplasia’s and degenerative diseases [12–14, 16–18]. These types of regulatory mutations often have less pleiotropic effects on phenotypes. For example, recent reports have shown substantial variations in these hip morphologic features between subjects with progressive hip OA and matched controls [2, 27, 28], and candidate association and GWAS studies have repeatedly revealed the association of the GDF5 locus with OA, including that of the hip. In these studies, a large 130 kb haplotype underlies risk of OA at the locus [12–14]. Likewise, several studies further demonstrate that variants far downstream of GDF5, within the same risk haplotype are also associated with DDH [16–18]. In both cases, the underlying causal variants likely reside within developmental enhancers in relevant regulatory domains.
Accordingly, here we have revealed that a broad downstream regulatory domain, which completely spans the GDF5 hip OA and DDH risk interval, possesses the functional ability to significantly influence hip morphology. We specifically identified that the Downstream BAC allele restored all abnormal bp/bp measures of proximal femoral and acetabular morphology to normal control levels, whereas the regulatory sequences upstream of Gdf5 had little impact on phenotypic rescue. Within this downstream regulatory domain (contained with the Downstream BAC), at least four published (GROW1, R3, R4, and R5) and three unpublished GDF5 regulatory sequences exist (data not shown), several of which (GROW1, R4, and two others) drive hip expression, and harbor risk variants [7, 15]. Indeed, the ability of these enhancers to control specific phenotypes related to femoral morphology and length is exemplified by our previous findings on the GROW1 perichondrial growth plate enhancer that has a key role in mediating limb length and femoral neck length in mice and humans [7].
While these findings do shed light on the regulatory control of Gdf5 during hip formation, this study has some scientific limitations. First, given the nature of breeding the bp/bp line, all genotypic comparisons are made to heterozygous (bp/+) controls, which have been reported upon as having no overt limb abnormalities or evidence of OA or hip defects up through six months of age (see Methods). Interestingly, bp/bp mice also do not develop overt signs of OA in the hip or knee, but only do when challenged [19], indicating that OA development at this locus may have complex etiology. Likewise, we only concentrated on male mice given the opportunistic numbers acquired through breeding, though, at the GDF5 locus sex-specific effects in OA and DDH risk have not been reported.
In summary, this work helps to elucidate the role(s) that Gdf5 and its regulatory domains have in hip development and provides an important developmental context for subsequent studies that address the impact of human GDF5 genetic variants on a range of hip injuries including OA. In light of our findings and given that genetic variants in the GDF5 association interval, as well as hip morphology, are risk factors for OA and DDH, we propose a developmental-genetic model in which risk-associated variants in downstream regulatory sequences influence local GDF5 expression levels in developing hip structures, leading to subtle alterations in hip morphology that, in turn, predispose the joint to injury and subsequent degeneration. It is also possible that such variants act to decrease GDF5 levels in matured adult hips and that this may also influence OA risk by impairing homeostasis in healthy joints or by accelerating degeneration due to injury. The development of a conditional allele for disrupting Gdf5 expression at specific pre- and postnatal time-points and spatial domains, along with the targeted deletion of its hip joint enhancers will be essential for teasing apart potential roles of Gdf5 in hip development and/or homeostasis. Finally, an extensive functional interrogation of the downstream regulatory and its variants should prove fruitful for identifying causative variants underlying hip OA and DDH at this locus.
Supporting information
Measurements were conducted on microCT images acquired at 12 μm3 isotropic voxel size, 70 kVp peak x-ray tube intensity, 114 mA x-ray tube current, and 200 ms integration time. Femoral length (FL), femoral head diameter (FHD), femoral head offset (FHO), femoral neck length (FNL), femoral neck diameter (FND), valgus cut angle (VCA), neck shaft angle (NSA), femoral head tilt angle (FHTA), alpha angle (AA), anterior offset (AO), pelvis length (PL), acetabular diameter (ADI) and acetabular depth (ADE).
(TIFF)
Left image shows a histological section of a portion of the mouse hip revealing expression of lacZ driven by the Downstream BAC in chondrocytes of the proximal femoral head/acetabulum (red arrows) and inferior femoral neck perichondrium (black arrows), counter-stained with nuclear fast red. The image on the right is an adjacent histological section revealing endogenous Gdf5 expression in the same domains but as assessed using in situ hybridization.
(TIFF)
All outcome measures were defined as continuous variables (N = 5 per genotype). Normally distributed data were compared using Analysis of Variance (ANOVA) with a post hoc Tukey correction for multiple comparisons. Non-normally distributed data were compared between the groups using Kruskal-Wallis test with a Benjamini Hochberg post hoc correction for multiple comparisons. P values are two-sided and the statistical significance was assessed at alpha = 0.05 for all the comparisons.
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank David Kingsley at Stanford University, Hao Chen at Genentech, Michele Schoor at Miltenyi Biotec, Hari Reddi at the UC Davis Center for Tissue Regeneration and Repair, and Daniel Brooks and Mary Bouxsein from the Imaging and Biomechanical Testing Core of the Center for Skeletal Research (NIH P30 AR066261) at the Massachusetts General Hospital. This work was funded in part by the Milton Fund and Dean’s Competitive Fund of Harvard University, NIH (NIAMS) grants to TDC (R01AR070139) and AMK (R01AR065462), and the Department of Orthopaedic Surgery at Boston Children’s Hospital.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was funded in part by the Milton Fund (https://vpr.harvard.edu/milton-fund) and Dean’s Competitive Fund of Harvard University (https://research.fas.harvard.edu/deans-competitive-fund-promising-scholarship) to TDC, as well as NIH NIAMS grants (https://www.niams.nih.gov/) to TDC (R01AR070139) and AMK (R01AR065462), as well as the Department of Orthopaedic Surgery at Boston Children’s Hospital (AMK). The views expressed are those of the authors and not necessarily those of the funding agencies. None of the Sponsors/Funders had any involvement in the design, collection, analysis or interpretation of the data and no role in writing or submitting this manuscript for publication.
References
- 1.Ahedi HG, Aspden RM, Blizzard LC, Saunders FR, Cicuttini FM, Aitken DA, et al. Hip Shape as a Predictor of Osteoarthritis Progression in a Prospective Population Cohort. Arthritis Care Res (Hoboken). 2017;69(10):1566–73. Epub 2016/12/20. 10.1002/acr.23166 . [DOI] [PubMed] [Google Scholar]
- 2.Saberi Hosnijeh F, Zuiderwijk ME, Versteeg M, Smeele HT, Hofman A, Uitterlinden AG, et al. Cam Deformity and Acetabular Dysplasia as Risk Factors for Hip Osteoarthritis. Arthritis Rheumatol. 2017;69(1):86–93. Epub 2016/10/04. 10.1002/art.39929 . [DOI] [PubMed] [Google Scholar]
- 3.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012–8. Epub 2005/06/24. 10.1302/0301-620X.87B7.15203 . [DOI] [PubMed] [Google Scholar]
- 4.Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368(6472):639–43. Epub 1994/04/14. 10.1038/368639a0 . [DOI] [PubMed] [Google Scholar]
- 5.Shwartz Y, Viukov S, Krief S, Zelzer E. Joint Development Involves a Continuous Influx of Gdf5-Positive Cells. Cell Rep. 2016;15(12):2577–87. Epub 2016/06/14. 10.1016/j.celrep.2016.05.055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pregizer SK, Kiapour AM, Young M, Chen H, Schoor M, Liu Z, et al. Impact of broad regulatory regions on Gdf5 expression and function in knee development and susceptibility to osteoarthritis. Ann Rheum Dis. 2018;77(3):450 Epub 2018/01/10. 10.1136/annrheumdis-2017-212475 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capellini TD, Chen H, Cao J, Doxey AC, Kiapour AM, Schoor M, et al. Ancient selection for derived alleles at a GDF5 enhancer influencing human growth and osteoarthritis risk. Nat Genet. 2017;49(8):1202–10. Epub 2017/07/04. 10.1038/ng.3911 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas JT, Kilpatrick MW, Lin K, Erlacher L, Lembessis P, Costa T, et al. Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nat Genet. 1997;17(1):58–64. Epub 1997/09/01. 10.1038/ng0997-58 . [DOI] [PubMed] [Google Scholar]
- 9.Harada M, Takahara M, Zhe P, Otsuji M, Iuchi Y, Takagi M, et al. Developmental failure of the intra-articular ligaments in mice with absence of growth differentiation factor 5. Osteoarthritis Cartilage. 2007;15(4):468–74. Epub 2006/10/21. 10.1016/j.joca.2006.09.003 . [DOI] [PubMed] [Google Scholar]
- 10.Faiyaz-Ul-Haque M, Ahmad W, Zaidi SH, Haque S, Teebi AS, Ahmad M, et al. Mutation in the cartilage-derived morphogenetic protein-1 (CDMP1) gene in a kindred affected with fibular hypoplasia and complex brachydactyly (DuPan syndrome). Clin Genet. 2002;61(6):454–8. Epub 2002/07/18. . [DOI] [PubMed] [Google Scholar]
- 11.Langer LO Jr., Cervenka J, Camargo M. A severe autosomal recessive acromesomelic dysplasia, the Hunter-Thompson type, and comparison with the Grebe type. Hum Genet. 1989;81(4):323–8. Epub 1989/03/01. . [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto Y, Mabuchi A, Shi D, Kubo T, Takatori Y, Saito S, et al. A functional polymorphism in the 5’ UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat Genet. 2007;39(4):529–33. Epub 2007/03/27. 10.1038/2005 . [DOI] [PubMed] [Google Scholar]
- 13.Southam L, Rodriguez-Lopez J, Wilkins JM, Pombo-Suarez M, Snelling S, Gomez-Reino JJ, et al. An SNP in the 5’-UTR of GDF5 is associated with osteoarthritis susceptibility in Europeans and with in vivo differences in allelic expression in articular cartilage. Hum Mol Genet. 2007;16(18):2226–32. Epub 2007/07/10. 10.1093/hmg/ddm174 . [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Yao J, Xu P, Ji B, Luck JV, Chin B, et al. A comprehensive meta-analysis of association between genetic variants of GDF5 and osteoarthritis of the knee, hip and hand. Inflamm Res. 2015;64(6):405–14. Epub 2015/04/22. 10.1007/s00011-015-0818-9 . [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Capellini TD, Schoor M, Mortlock DP, Reddi AH, Kingsley DM. Heads, Shoulders, Elbows, Knees, and Toes: Modular Gdf5 Enhancers Control Different Joints in the Vertebrate Skeleton. PLoS Genet. 2016;12(11):e1006454 Epub 2016/12/03. 10.1371/journal.pgen.1006454 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai J, Shi D, Zhu P, Qin J, Ni H, Xu Y, et al. Association of a single nucleotide polymorphism in growth differentiate factor 5 with congenital dysplasia of the hip: a case-control study. Arthritis Res Ther. 2008;10(5):R126 Epub 2008/10/25. 10.1186/ar2540 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, Wang C, Hao Z, Dai J, Chen D, Xu Z, et al. A common variant of ubiquinol-cytochrome c reductase complex is associated with DDH. PLoS One. 2015;10(4):e0120212 Epub 2015/04/08. 10.1371/journal.pone.0120212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouault K, Scotet V, Autret S, Gaucher F, Dubrana F, Tanguy D, et al. Evidence of association between GDF5 polymorphisms and congenital dislocation of the hip in a Caucasian population. Osteoarthritis Cartilage. 2010;18(9):1144–9. Epub 2010/07/17. 10.1016/j.joca.2010.05.018 . [DOI] [PubMed] [Google Scholar]
- 19.Daans M, Luyten FP, Lories RJ. GDF5 deficiency in mice is associated with instability-driven joint damage, gait and subchondral bone changes. Ann Rheum Dis. 2011;70(1):208–13. Epub 2010/09/02. 10.1136/ard.2010.134619 . [DOI] [PubMed] [Google Scholar]
- 20.Settle SH Jr., Rountree RB, Sinha A, Thacker A, Higgins K, Kingsley DM. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol. 2003;254(1):116–30. Epub 2003/02/28. . [DOI] [PubMed] [Google Scholar]
- 21.Storm EE, Kingsley DM. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development. 1996;122(12):3969–79. Epub 1996/12/01. . [DOI] [PubMed] [Google Scholar]
- 22.Landauer W. Brachypodism: a recessive mutation of house-mice. Journal of Heredity. 1952;43(6):293–8. [Google Scholar]
- 23.Young EY, Gebhart J, Cooperman D, Ahn NU. Are the left and right proximal femurs symmetric? Clin Orthop Relat Res. 2013;471(5):1593–601. Epub 2012/11/28. 10.1007/s11999-012-2704-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unnanuntana A, Toogood P, Hart D, Cooperman D, Grant RE. Evaluation of proximal femoral geometry using digital photographs. J Orthop Res. 2010;28(11):1399–404. Epub 2010/09/28. 10.1002/jor.21119 . [DOI] [PubMed] [Google Scholar]
- 25.Toogood PA, Skalak A, Cooperman DR. Proximal femoral anatomy in the normal human population. Clin Orthop Relat Res. 2009;467(4):876–85. Epub 2008/09/02. 10.1007/s11999-008-0473-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawal B, Ribeiro R, Malhotra R, Bhatnagar N. Anthropometric measurements to design best-fit femoral stem for the Indian population. Indian J Orthop. 2012;46(1):46–53. Epub 2012/02/22. 10.4103/0019-5413.91634 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.An H, Marron JS, Schwartz TA, Renner JB, Liu F, Lynch JA, et al. Novel statistical methodology reveals that hip shape is associated with incident radiographic hip osteoarthritis among African American women. Osteoarthritis Cartilage. 2016;24(4):640–6. Epub 2015/12/02. 10.1016/j.joca.2015.11.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson AE, Stiller JL, Shi XA, Leyland KM, Renner JB, Schwartz TA, et al. Measures of hip morphology are related to development of worsening radiographic hip osteoarthritis over 6 to 13 year follow-up: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2016;24(3):443–50. Epub 2015/10/27. 10.1016/j.joca.2015.10.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Measurements were conducted on microCT images acquired at 12 μm3 isotropic voxel size, 70 kVp peak x-ray tube intensity, 114 mA x-ray tube current, and 200 ms integration time. Femoral length (FL), femoral head diameter (FHD), femoral head offset (FHO), femoral neck length (FNL), femoral neck diameter (FND), valgus cut angle (VCA), neck shaft angle (NSA), femoral head tilt angle (FHTA), alpha angle (AA), anterior offset (AO), pelvis length (PL), acetabular diameter (ADI) and acetabular depth (ADE).
(TIFF)
Left image shows a histological section of a portion of the mouse hip revealing expression of lacZ driven by the Downstream BAC in chondrocytes of the proximal femoral head/acetabulum (red arrows) and inferior femoral neck perichondrium (black arrows), counter-stained with nuclear fast red. The image on the right is an adjacent histological section revealing endogenous Gdf5 expression in the same domains but as assessed using in situ hybridization.
(TIFF)
All outcome measures were defined as continuous variables (N = 5 per genotype). Normally distributed data were compared using Analysis of Variance (ANOVA) with a post hoc Tukey correction for multiple comparisons. Non-normally distributed data were compared between the groups using Kruskal-Wallis test with a Benjamini Hochberg post hoc correction for multiple comparisons. P values are two-sided and the statistical significance was assessed at alpha = 0.05 for all the comparisons.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.