Abstract
Ischemia-reperfusion (IR) injury occurs when blood supply to an organ is disrupted and then restored, and underlies many disorders, notably myocardial infarction and stroke. While reperfusion of ischemic tissue is essential for survival, it also initiates cell death through generation of mitochondrial reactive oxygen species (ROS). Recent work has revealed a novel pathway underlying ROS production at reperfusion in vivo in which the accumulation of succinate during ischemia and its subsequent rapid oxidation at reperfusion drives ROS production at complex I by reverse electron transport (RET). Pharmacologically inhibiting ischemic succinate accumulation, or slowing succinate metabolism at reperfusion, have been shown to be cardioprotective against IR injury. Here, we determined whether ischemic preconditioning (IPC) contributes to cardioprotection by altering succinate kinetics during IR. Mice were subjected to a 30-minute occlusion of the left anterior descending coronary artery followed by reperfusion, with or without a protective IPC protocol prior to sustained ischemia. We found that IPC had no effect on ischemic succinate accumulation with both control and IPC mice having profound increases in succinate compared to normoxia. Furthermore, after only 1-minute reperfusion succinate was rapidly metabolised returning to near pre-ischemic levels in both groups. We conclude that IPC does not affect ischemic succinate accumulation, or its oxidation at reperfusion.
Keywords: ischemia-reperfusion injury, ischemic preconditioning, mitochondria, succinate
Introduction
Ischemia-reperfusion (IR) injury occurs when a tissue is rendered ischemic, for example by a thrombosis, and blood flow is subsequently restored when the obstruction is removed. IR injury is a major factor in a range of pathologies, notably heart attack and stroke, but also in many other clinically important situations such as transplantation. Consequently, there has been considerable interest in understanding the mechanisms of IR injury and in developing approaches to decrease it. We recently proposed a unifying mechanism for mitochondrial reactive oxygen species (ROS) production and associated myocardial damage, driven by the accumulation and subsequent oxidation of the tricarboxylic acid (TCA) intermediate, succinate [1]. Ischemic preconditioning (IPC) is one of the most reproducible and robust forms of cardioprotection. Since its initial description by Murry et al., [2, 3], there has been sustained interest in unravelling the underlying mechanisms of IPC with the hope of pharmacologically mimicking its beneficial effects. Despite extensive investigation, our understanding of the precise mechanisms of IPC remains incomplete. The modulation of ROS production has, however, been implicated as a key mechanism in preconditioning-mediated cardioprotection [4, 5]. We hypothesised that IPC may act by decreasing mitochondrial ROS production upon IR injury by either preventing the build-up of succinate during ischemia, or its oxidation upon reperfusion. However, while it has been reported that IPC has no effect on the accumulation of succinate during ischemia in ex vivo models [6, 7], no work has been done on the effect of IPC on succinate using in vivo models of IR injury. To address this outstanding question, we measured succinate levels in an in vivo model of IPC. We found that IPC-mediated cardioprotection of the mouse myocardium in vivo was independent of any changes in either succinate accumulation or oxidation.
Results & Discussion
Ischemic succinate is rapidly oxidised at reperfusion within minutes
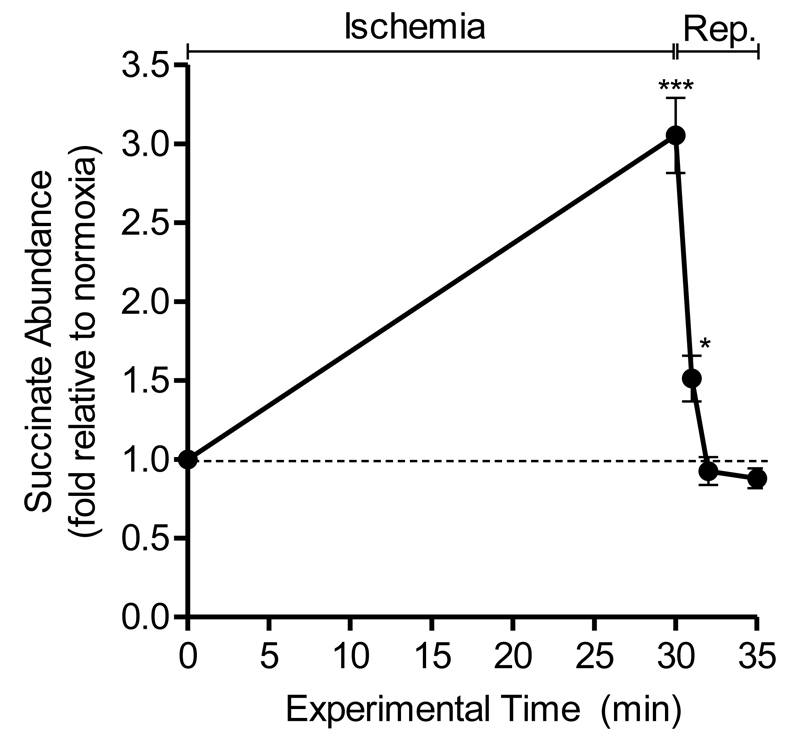
To assess the impact of IPC on myocardial succinate kinetics, we first established, a detailed time-course of the changes in succinate abundance during IR injury in vivo. In previous work by our lab demonstrated that succinate returns to near baseline levels 5 min after reperfusion in an in vivo mouse model of LAD occlusion [1]. We set out to increase the time resolution of the changes in succinate levels by isolating heart tissue from within the ischemic risk zone at earlier time-points following reperfusion. Metabolomic analysis revealed that the accumulated succinate decreased to pre-ischemic levels after 2 minutes of reperfusion. Interestingly, after 1 minute of reperfusion, ischemic succinate had fallen by 85%, to 1.5-fold, versus normoxic controls (Fig. 1). The fate of ischemic succinate following reperfusion is disputed with some arguing that post-ischemic succinate is released from the cell instead of being actively oxidised by mitochondrial complex II into fumarate [6]. Analysis of blood samples in patients with acute ST-elevated myocardial infarction indeed show that succinate is, in part, released from the ischemic myocardium into the blood following primary percutaneous coronary intervention [9]. Recent work, however, also supports succinate metabolism at reperfusion, with the rapid decline in succinate being slowed upon addition of the complex II inhibitor, malonate [8]. Thus upon reperfusion the accumulated succinate has two fates: oxidation by SDH and release from the cell.
Figure 1. Succinate rapidly declines in reperfused ischemic myocardium.
Mice were subjected to 30 min ischemia, 30 min ischemia plus 1, 2 or 5 min reperfusion or a normoxic time-matched procedure. Data is expressed as fold change relative to normoxic control (n=3-6). *p<0.05, ***p<0.001 vs normoxia (time-point zero) (one-way ANOVA with Tukey’s post-hoc analysis). Mean ± SEM (n=3-5).
Cardioprotection mediated by ischemic preconditioning is not due to changes in succinate accumulation or oxidation during IR injury
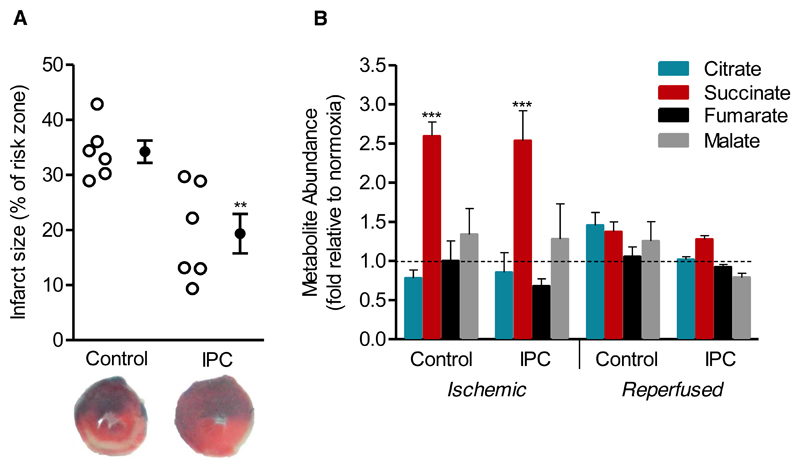
To assess the impact of ischemic preconditioning on succinate kinetics during IR injury we utilized the established protocol of three sets of 5-minute ischemia and 5-minute of reperfusion followed by left anterior descending artery (LAD) ligation [10]. Preconditioned hearts exhibited significantly smaller infarcts compared to controls after 2 hours of reperfusion in vivo, with infarct size reducing from 34.2 ± 2.0% to 19.4 ± 3.6% of the risk zone, respectively (Fig. 2A). We first hypothesised that the mechanism by which IPC may affect mitochondrial ROS production was through disruption of succinate accumulation during ischemia. To investigate this hypothesis, we assessed succinate kinetics during IR injury, and in conjunction with IPC. IPC had no effect on ischemic succinate accumulation with both control and IPC mice exhibiting increases in succinate of 2.6- and 2.5-fold compared to normoxia, respectively (Fig. 2B). However, despite succinate accumulating to the same extent during ischemia, IPC may act to slow the oxidation of succinate upon reperfusion thereby decreasing the proton motive force for reverse electron transport (RET) at complex I. IPC, however, had no effect on succinate metabolism at reperfusion, with succinate returning to near comparable levels in both IPC and control mice after 1 minute of reperfusion (Fig. 2B).
Figure 2. The effect of IPC on metabolite abundance during IR injury in the in vivo mouse heart.
A) Preconditioning reduced infarct size in vivo. Representative cross-sections from mouse hearts after myocardial infarction ± IPC are shown. Infarcted tissue is white, the area at risk is red, and non-risk tissue is dark blue (n=6). **p<0.01 (two-tailed Student’s t-test). B) Metabolite abundance following 30 min ischemia ± IPC, and following 1 min reperfusion ± IPC. Data is expressed as fold change relative to normoxic control (n=4-6). ***p<0.001 vs normoxia (one-way ANOVA with Tukey’s post-hoc analysis). Mean ± SEM.
IPC had no significant effect on other TCA cycle intermediates measured during ischemia or immediately upon reperfusion (Fig. 2B). This result suggests that succinate kinetics are unaffected by IPC. However, it should be noted that while no significant change in reperfusion-induced succinate levels was detected following IPC, we acknowledge the limitations of our tissue preservation and analytic methods, as they may be insufficiently sensitive to either detect minute changes in such a dynamic metabolite pool at the time-points investigated, or distinguish intracellular vs interstitial succinate accumulation.
While the formation and metabolism of ischemic succinate is central to the generation of ROS burst upon reperfusion, other mechanisms downstream of succinate could account for the ROS-attenuating effect of IPC. Indeed, any manipulation of the RET pathway could potentially influence the end outcome of ROS production, be that preventing the rapid re-activation of complex I, or reducing the driving hyperpolarisation of the mitochondrial membrane potential. Interestingly, IPC has also been demonstrated to induce S-nitrosation of complex I, a modification shown to inhibit complex I-mediated ROS production [13, 14]. During ischemia the ATP/ADP ratio progressively decreases, and accumulated AMP is further metabolised to hypoxanthine and xanthine. Upon reperfusion, ATP synthesis is compromised due to a delay in the repletion of adenine nucleotides [12]. Due to the influx of electrons from oxidised succinate the Coenzyme Q (CoQ) pool is maintained in a highly reduced state, which forces RET, resulting in a ROS burst. In one of the original descriptions of IPC, Murry et al. made the interesting observation that IPC slowed the rate of ATP decline during ischemia suggesting a reduction in ATP utilisation [3]. This effect was similarly observed by Kaplan et al. in which they demonstrated IPC preserved end-ischemic ATP in isolated perfused rat hearts [11]. The subsequent preservation of ADP during ischemia could enhance oxidative phosphorylation upon reperfusion, thereby decreasing the proton motive force and oxidizing the CoQ pool at reperfusion, ultimately reducing RET and subsequent ROS production.
Conclusion
We conclude that IPC has no effect on succinate kinetics during cardiac IR injury. The accumulation and metabolism of ischemic succinate seems to be a promising therapeutic target when targeting IR injury. It is, however, not the only target or mechanistic component by which IPC could act to disrupt RET-mediated ROS production upon reperfusion in vivo.
Methods
In vivo experiments
This research was conducted in accordance with the Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012 following ethical review by the University of Cambridge Animal Welfare and Ethical Review Body (AWERB) under Project Licence numbers 80/2374, and 70/8238.
An open-chest, in situ left anterior descending (LAD) coronary artery infarct model was used as previously described [15]. Briefly, C57BL/6 male mice (8-10 weeks of age; Charles River Laboratories, UK) were anesthetized with sodium pentobarbital (70 mg·kg-1 body weight intraperitoneally), intubated endotracheally and ventilated with 3 cm H2O positive-end expiratory pressure. Ventilation frequency was maintained at 110 breaths·min-1 with tidal volume between 125 and 150 μl. A thoracotomy was performed and the pericardium stripped to expose the heart. The LAD was surrounded by a 7-0 Prolene suture, which was then passed through a small plastic tube. Ischemia was induced by tightening the tubing against the heart surface to occlude blood flow. Mice in the IPC group were subjected to 3 x 5 min of ischemia/reperfusion episodes followed by an intervening 10 min period prior to the induction of prolonged ischemia. Infarct size was assessed following 30 min ischemia, 120 min reperfusion using 2% triphenyltetrazolium chloride staining, and is expressed as a percentage of the risk zone.
For metabolomic analyses myocardial tissue was removed from the ischemic risk zone at the end of ischemia, and following 1, 2 and 5 min reperfusion, rapidly snap-frozen in liquid nitrogen and stored at -80°C until analysis. Data was normalised to tissue isolated from normoxic time-matched animals.
Metabolomic analyses
Equal amounts of wet weight murine tissue were lysed in 250 ml extraction solution (30% acetonitrile, 50% methanol and 20% water) per 10 mg tissue in a Bullet Blender (Next Advance) following the manufacturer’s instructions. The suspension was immediately centrifuged (16,000g, 15 min at 4°C) and the supernatant analysed by liquid chromatography-mass spectrometry. Sample extracts were run twice on a liquid chromatography system fitted with a Sequant ZIC-HILIC column (5 μm, 4.6 x 150 mm) and afterwards with a Sequant ZIC-pHILIC column (5 μm, 2.1 x 150 mm), with the corresponding guard columns (both 2.1 x 20 mm, 5 μm) (all from Merck), and according to previously described gradient elution methods [1]. The mass spectrometer (Thermo Q Exactive) was operated in full scan mode with polarity switching. Samples were randomized to avoid bias due to machine drift and the operator was blinded to the sample key. Spectra were analysed using Xcalibur Quan Browser software (Thermo Fisher Scientific) by referencing to an internal library of compounds.
Statistics and experimental design
Data were expressed as mean ± SEM, and p values calculated using a two-tailed Student’s t-test for pairwise comparisons, and one-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons. Differences were considered significant if p<0.05. Histological analysis of infarct size was performed blinded by an independent researcher.
Highlights.
Succinate accumulates during cardiac ischemia and its oxidation drives ROS production upon reperfusion
IPC does not affect succinate accumulation or oxidation during cardiac IR injury
Changes in succinate metabolism do not contribute to IPC
Acknowledgements
The study was funded in part by a grant of the British Heart Foundation (PG/15/84/31670) to TK. VRP was funded by a BHF Studentship. MPM was supported by the Medical Research Council UK (MC_U105663142) and by a Wellcome Trust Investigator award (110159/Z/15/Z). CF and SC were supported by the Medical Research Council (MC_UU_12022/6).
Footnotes
Disclosures. None.
References
- [1].Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- [3].Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66(4):913–31. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- [4].Crestanello JA, Lingle DM, Kamelgard J, Millili J, Whitman GJ. Ischemic preconditioning decreases oxidative stress during reperfusion: a chemiluminescence study. J Surg Res. 1996;65(1):53–8. doi: 10.1006/jsre.1996.0342. [DOI] [PubMed] [Google Scholar]
- [5].Vanden Hoek T, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ Res. 2000;86(5):541–8. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- [6].Andrienko TN, Pasdois P, Pereira GC, Ovens MJ, Halestrap AP. The role of succinate and ROS in reperfusion injury - A critical appraisal. J Mol Cell Cardiol. 2017;110:1–14. doi: 10.1016/j.yjmcc.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jespersen NR, Yokota T, Stottrup NB, Bergdahl A, Paelestik KB, Povlsen JA, Dela F, Botker HE. Pre-ischaemic mitochondrial substrate constraint by inhibition of malate-aspartate shuttle preserves mitochondrial function after ischaemia-reperfusion. J Physiol. 2017;595(12):3765–3780. doi: 10.1113/JP273408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Valls-Lacalle L, Barba I, Miro-Casas E, Alburquerque-Bejar JJ, Ruiz-Meana M, Fuertes-Agudo M, Rodriguez-Sinovas A, Garcia-Dorado D. Succinate dehydrogenase inhibition with malonate during reperfusion reduces infarct size by preventing mitochondrial permeability transition. Cardiovasc Res. 2016;109(3):374–84. doi: 10.1093/cvr/cvv279. [DOI] [PubMed] [Google Scholar]
- [9].Kohlhauer M, Dawkins S, Costa ASH, Lee R, Young T, Pell VR, Choudhury RP, Banning AP, Kharbanda RK, S. Oxford Acute Myocardial Infarction. Saeb-Parsy K, et al. Metabolomic Profiling in Acute ST-Segment-Elevation Myocardial Infarction Identifies Succinate as an Early Marker of Human Ischemia-Reperfusion Injury. J Am Heart Assoc. 2018;7(8) doi: 10.1161/JAHA.117.007546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fisher SG, Marber MS. An in vivo model of ischaemia-reperfusion injury and ischaemic preconditioning in the mouse heart. J Pharmacol Toxicol Methods. 2002;48(3):161–9. doi: 10.1016/S1056-8719(03)00046-7. [DOI] [PubMed] [Google Scholar]
- [11].Kaplan LJ, Bellows CF, Blum H, Mitchell M, Whitman GJ. Ischemic preconditioning preserves end-ischemic ATP, enhancing functional recovery and coronary flow during reperfusion. J Surg Res. 1994;57(1):179–84. doi: 10.1006/jsre.1994.1128. [DOI] [PubMed] [Google Scholar]
- [12].Lindsay TF, Liauw S, Romaschin AD, Walker PM. The effect of ischemia/reperfusion on adenine nucleotide metabolism and xanthine oxidase production in skeletal muscle. J Vasc Surg. 1990;12(1):8–15. doi: 10.1067/mva.1990.19946. [DOI] [PubMed] [Google Scholar]
- [13].Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cocheme HM, Reinhold J, Lilley KS, Partridge L, et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med. 2013;19(6):753–9. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sun J, Nguyen T, Aponte AM, Menazza S, Kohr MJ, Roth DM, Patel HH, Murphy E, Steenbergen C. Ischaemic preconditioning preferentially increases protein S-nitrosylation in subsarcolemmal mitochondria. Cardiovasc Res. 2015;106(2):227–36. doi: 10.1093/cvr/cvv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Methner C, Lukowski R, Grube K, Loga F, Smith RA, Murphy MP, Hofmann F, Krieg T. Protection through postconditioning or a mitochondria-targeted S-nitrosothiol is unaffected by cardiomyocyte-selective ablation of protein kinase G. Basic Res Cardiol. 2013;108(2):337. doi: 10.1007/s00395-013-0337-1. [DOI] [PubMed] [Google Scholar]