Abstract
The aryl hydrocarbon receptor (AHR) is a ligand activated bHLH transcription factor that belongs to the Per-Arnt-Sim (PAS) superfamily of proteins involved in mediating responses to cellular environment regulating normal physiological and developmental pathways. The AHR binds a broad range of naturally derived and synthetic compounds, and plays a major role in mediating effects of certain environmental chemicals. Although our understanding of the physiological roles of the AHR in the immune system is evolving, there is little known about its role in hematopoiesis and hematopoietic diseases. Prior studies demonstrated that AHR null (AHR-KO) mice have impaired hematopoietic stem cell (HSC) function; they develop myeloproliferative changes in peripheral blood cells, and alterations in hematopoietic stem and progenitor cell populations in the bone marrow. We hypothesized mice lacking AHR expression only within hematopoietic cells (AHRVav1 mice) would develop similar changes. However, we did not observe a complete phenocopy of AHR-KO and AHRVav1 animals at 2 or 18 months of age. To illuminate the signaling mechanisms underlying the alterations in hematopoiesis observed in these mice, we sorted a population of cells highly enriched for HSC function (LSK cells: CD34-CD48-CD150+) and performed microarray analyses. Ingenuity Pathway and Gene Set Enrichment Analyses revealed that that loss of AHR within HSCs alters several gene and signaling networks important for HSC function. Differences in gene expression networks among HSCs from AHR-KO and AHRVav1 mice suggest that AHR in bone marrow stromal cells also contributes to HSC function. In addition, numerous studies have suggested a role for AHR in both regulation of hematopoietic cells, and in the development of blood diseases. More work is needed to define what these signals are, and how they act upon HSCs.
Introduction
All mature lineages of blood cells are generated from hematopoietic stem cells (HSCs), which reside primarily in bone marrow (BM) of adult mice and humans. One of the most important aspects of HSC biology is the precise regulation of their proliferation, differentiation, and self-renewal. This balance can be shifted due to genetic mutations, environmental exposures to toxicants, and age [1–5]. For example, exposure to environmental toxicants which activate the aryl hydrocarbon receptor (AHR) have been linked to blood diseases in humans.
The aryl hydrocarbon receptor (AHR) is an environment sensing transcriptional regulator that is expressed in hematopoietic and non-hematopoietic cells. While the normal, physiological role of AHR is not fully understood, it regulates aspects of HSC function, immune system development, and hematopoietic diseases [3, 6–11]. Several proposed physiological functions of AHR in non-hematopoietic tissues have been suggested from studies using AHR-null-allele (AHR-KO) mouse models [9, 12, 13]. We have summarized these previous data in Table 1. While these models have generated much information on possible roles of the receptor in a variety of tissues and cell types, few studies have sought to describe the role of AHR as an intrinsic regulator of BM stem cell functions. Hematopoietic cells, including HSCs, exist in the BM in close proximity to a variety of other cell types. Multiple studies that have described the role of these non-hematopoietic cells in the regulation of HSC function have led to the development of models that describe a hematopoietic “niche”, the cells of which can have significant regulatory effects on HSCs and greatly alter their function and output [14–19].
Table 1. Summary of phenotypes observed in global AHR-KO mice.
| Phenotypes Observed in Global AHR-KO mice |
| Increased numbers of peripheral white blood cells |
| Alterations in white blood cell subsets |
| Elevated HSC oxidative stress elevated |
| HSC DNA damage increased |
| HSC p16 expression decreased |
| Spleen weight increased |
| Decreased HSC self-renewal during serial transplants |
| 672 genes altered compared to WT using microarray |
In order to better understand the role of AHR signaling intrinsic to HSCs, we used a conditional knockout (AHRVav1) model that utilizes a Cre-loxP system to target loss of AHR expression to the hematopoietic system [20, 21]. Targeted deletion of AHR allows for better discrimination of genes that are regulated intrinsically, and avoids the potential confounding effects in global knockout because all cells, including BM stromal cells, lack AHR expression. Here we report the characterization of the morphology and hematopoietic phenotype of AHRVav1 mice, as well as the gene expression profiles of long-term HSC (LT-HSC) from these mice at 8 weeks or 18 months of age. LT-HSCs are the most primitive multipotent BM stem cell, and are responsible for long term multi-lineage cell generation. These data provide insight into the genes that AHR may intrinsically regulate within HSCs, and guide new approaches to elucidate how modulation or loss of AHR signaling can lead to diseases or dysfunction of the hematopoietic system.
Materials and methods
Mice
Breeding pairs of B6.Cg-Tg A2Kio/J (Vav1-Cre) and AHRtm3.1Bra/J (AHR-KO) mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Initial breeding stocks for Ahrfx/fxmice were provided by Dr. Christopher Bradfield (University of Wisconsin) [20]. All mice were maintained in micro-isolator housing according to approved protocols at the University of Rochester. Males expressing Vav1-Cre were crossed with females homozygous for the floxed Ahr allele. This breeding scheme produced males heterozygous for both transgenes, which were then crossed with female Ahrfx/fx mice, to produce female Vav1-cre+/-Ahrfx/fx (AHRVav1) mice. These female mice were used in the experiments presented. Female Ahrfx/fx (AHRFX) mice were used as experimental controls. The CD45.1+ (B6.SJL-Ptprc<a>/BoAiTac) mice used in the repopulation experiments were purchased from Taconic Farms (Rensselaer, New York).
Ethics statement
Animals were used at 2 or 18 months of age. All handling and experimental procedures were carried out in accordance with University of Rochester approved protocols. Our study was approved by both the University Committee on Animal Resources (UCAR) as well as the Institutional Biosafety Committee (IBC). Mice are housed in cages with air-handling systems designed to reduce ammonia build-up. Food and water are changed frequently by dedicated animal care technicians and were provided ab libitum for all studies described here. Mice receive nesting materials and red plastic houses to minimize distress and to encourage natural behaviors such as nesting and familial socialization. Prior to experiments, all mice were sacrificed using CO2 asphyxiation and cervical dislocation to ensure death and absence of pain due to tissue harvests. Mice are monitored daily by dedicated vivarium staff for health and well-being. These checks monitor appearance, behavior and food and water levels to ensure the health and comfort of all experimental animals.
Cell isolation and detection of Ahr excision by PCR
Cells were isolated from the bone marrow, spleen, lymph nodes, and lung as previously described [11, 22, 23]. After hypotonic lysis to remove erythrocytes, single cell suspensions were prepared. CD45negative (non-hematopoietic cells) were isolated from the lung. To do so, 1% low melting point agarose was inserted into the lung via the trachea, and the tissue was then digested with dispase. CD45negative cells were isolated from the resulting cell suspension using a magnetic enrichment beads conjugated to an anti-mouse CD45 antibody (Miltenyi Biotec, Auburn, CA). Genomic DNA was isolated from cell suspensions using a QIAamp DNA Mini Kit (QIAGEN), and PCR to detect the excised Ahr allele was performed as previously described [20, 23, 24].
Western Blotting for AHR protein in hematopoietic tissues
Cells from AHRVav1 tissues were washed twice with PBS, and lysed (Reporter Lysis Buffer, Promega, Madison, WI). Lysates were stored at −80°C. Lysate proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) (8% acrylamide resolving gel) and transferred to a polyvinylidene fluoride (PVDF) membrane, which was blocked using 5% nonfat milk in wash buffer (50mM Tris base, 150mM NaCl, and 0.2% Tween 20, pH 7.5). Antibodies used were anti-AHR (rabbit polyclonal, Enzo Life Sciences, Farmingdale NY) and anti-β-actin (rabbit polyclonal, Sigma, St Louis, MO). Horseradish peroxidase-conjugated secondary antibodies were purchased from Jackson Immunoresearch (West Grove, PA). Proteins were visualized by chemiluminescence using LumiGlo reagents (KPL, Gaithersburg, MD).
Tissue and cell collection and hematological profiles
Mice were euthanized with CO2 and peripheral blood was collected from the retroorbital plexus vein into BD microtainer tubes containing EDTA (Beckton Dickinson and Company). Hematological profiles were analyzed using a HESKA Hematology Analyzer (HESKA Corporation, Colorado). Organs were harvested to collect wet weights. BM cells from both femurs of each mouse were harvested as previously described [6]. The number of cells obtained was determined using a hemocytometer.
Bone marrow cell phenotyping
A single cell suspension of BM cells was prepared and stained with a cocktail of fluorochrome-conjugated antibodies against lineage markers defining more mature hematopoietic cells as previously described [6]. Lineage depleted cell suspensions from BM were stained with fluorochrome-conjugated antibodies to detect the following populations of cells: long-term repopulating HSCs (LT-HSCs) (Lin−CD34-Flt3-Sca-1highcKit+), short-term HSCs (ST-HSCs) (Lin-CD34+Flt3-Sca-1highcKit+), MPP (Lin-Sca-1+cKithighFlt3+), common lymphoid progenitors (CLP) (Lin-IL7Rα+Sca-1lowcKit+), common myeloid progenitors (CMP) (Lin−CD34+FcRlowIL7Rα−Sca-1−cKit+), granulocyte macrophage progenitors (GMP) (Lin−CD34+FcRhighIL7Rα−Sca-1−cKit+), and megakaryocyte erythroid progenitors (MEP) (Lin−CD34−FcRlowIL7Rα−Sca-1−cKit+). The antibody clones used to analyze BM cells were CD34 (RAM34), Sca-1 (E13-161.7), cKit (2B8), IL7R (A7R34), and FcR (2.4G2) (BD Pharmingen). Spleen cells were analyzed using fluorochrome-conjugated antibodies against CD4 (H129.19), CD8 (53–6.7), B220 (RA3-6B2), Gr-1(RB6-8C5), and Mac-1(M1/70) (BD Pharmingen). Bone marrow cells (Lin-) were also stained with CD48 (FITC clone Hm48-1; BD Pharmingen) and CD150 (APC Clone 459911; R&D Systems) to identify LT-HSCs for cell sorting. When indicated, cells were also stained with 10 μM 2’7’-dichlorofluorescein diacetate (DCFDA) (Abcam) to measure cellular oxidative status [7]. All flow cytometry was performed on a Becton Dickenson LSRII flow cytometer maintained by the University of Rochester Flow Cytometry Core. Flow data were analyzed using Flowjo software (Treestar, California).
Spleen colony forming unit assay
Recipient C57/Bl6 mice were sub-lethally irradiated (9.0 Gy) using a 137Cs gamma-ray source and after 4 h these mice were tail-vein injected with 50,000 total BM cells from donor AHRFX or AHRVav1 (prepared as described above) suspended in PBS. The recipient mice were euthanized after 8 or 12 days. Spleens were collected and fixed in Telleyesniczky’s solution. The nodular colonies formed on the spleens were counted macroscopically [2].
Serial Bone marrow transplant assay
Bone marrow cells from recipient (CD45.1+) and donor AHRVav1 or AHRFX (CD45.2+) mice were prepared as described earlier [7]. Cells isolated from AHRVav1 or AHRFX (1 x 106) and CD45.1+ (1 x 106) mice were injected together (in 100 μl PBS) into the tail vein of irradiated (550 + 550 rads, 4 h apart) CD45.1+ recipients. After 20 weeks, BM cells from recipient animals were harvested and injected (2 x 106 cells) into irradiated CD45.1+ secondary recipient mice. Similarly, BM cells isolated from secondary recipients and injected into tertiary recipients. Bone marrow cells were isolated from primary, secondary, and tertiary CD45.1+ recipients were analyzed for cells of donor (CD45.2+) and recipient (CD45.1+) origins. Bone marrow engraftment was analyzed at 20 weeks after each BM transplantation.
Sorting and microarray analysis of Lin-CD48-CD150+ (SLAM+) cells
BM cells from femurs and tibias were harvested from each animal (6 to 8 mice) separately and depleted for lineage positive cells as previously described [6]. The Lin- cells were stained with fluorochrome-conjugated antibodies against Sca-1 (V450 Clone D7; BD Pharmingen), cKit (PeCy7 Clone 2B8; BD Pharmingen), CD34 (AF700 Clone Ram34; eBiosciences), CD48 (FITC Clone Hm48-1; BD Pharmingen), and CD150 (APC Clone 459911; R&D Systems). HSC were obtained by fluorescence-activated cell sorting lineage depleted BM cells [7]. Cells were sorted into RLT+ buffer (Qiagen) and placed at -80°C. Total RNA was isolated from sorted LT-HSCs using an RNeasy Mini Kit (Qiagen). RNA was pre-amplified and cDNA was produced using a WT-Ovation PicoSL kit (Nugen). Each animal was ultimately prepared as a cDNA library for analysis using a distinct microarray chip. Microarray analysis was performed using Genechip Mouse Gene 2.0 ST Array (Affymetrix) by the UR Genomics Research Center. The IterPLIER algorithm was used to generate background-subtracted, quantile-normalized signals from the microarray data. Transcripts with a mean difference in expression of at least 1.5-fold and P<0.05 were examined for network/pathway associations by Ingenuity Pathway Analysis. Physiological functions were ascribed to the altered set of genes. Microarray data were also analyzed using Gene Set Enrichment Analysis (GSEA) as previously described [7]. The microarray data can be accessed through the Gene Expression Omnibus accession number GSE76276.
Reverse transcription (RT)-qPCR
LT-HSCs (SLAM+) were sorted as described above, and total RNA was extracted using an RNeasy Mini Kit (Qiagen). cDNA was prepared as described above and 10 ng of cDNA was used in qPCR reactions via a Bio-Rad CFX96 Real Time Thermal Cycler (Bio-Rad, Hercules, California), using primer/probe assays (Applied Biosystems). Expression of mRNA for each gene was normalized using the expression of Hprt and Gapdh. Gene expression was compared using the 2-ΔΔT method [25]. Differences were considered significant when relative mRNA expression was 1.5-fold higher or lower than indicated controls with a p-value of less than 0.05.
Statistics
For analyses of two groups, the Student’s t-test was used. For comparisons of two or more groups, a two-way ANOVA was used. For microarray data, The IterPLIER algorithm was used to generate background-subtracted, quantile-normalized signals from the microarray data and analyses were adjusted to account for multiple comparisons. Ingenuity Pathways Analysis (IPA) uses a proprietary platform knowledge base and Fischer’s Exact Test to determine if pathways and subsets of genes are altered.
Results
Validation of conditional deletion of Ahr gene in hematopoietic cells
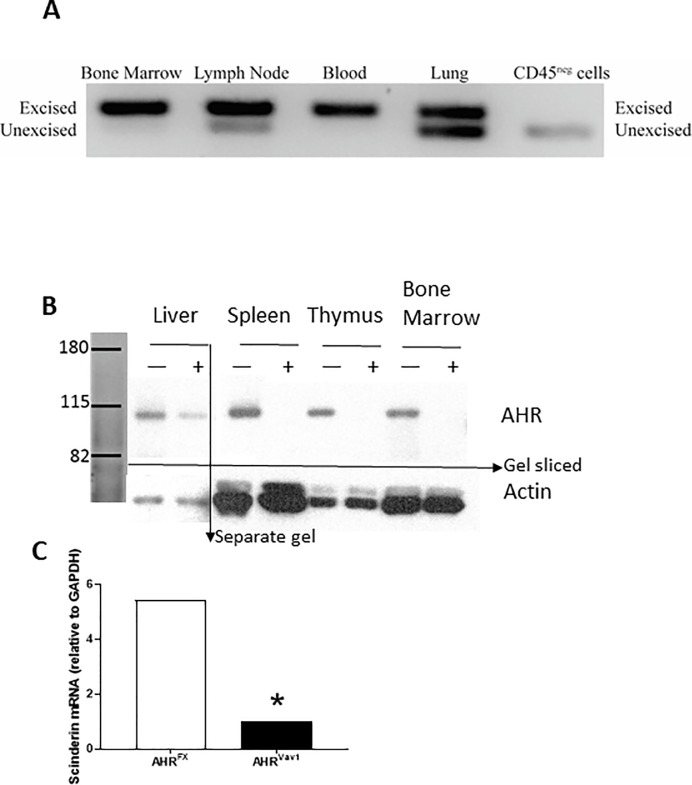
To verify that targeted excision is occurring, we isolated genomic DNA from spleen, liver, kidney, thymus and BM from AHRFX and AHRVav1 animals. PCR analyses revealed that hematopoietic tissues from AHRVav1 mice display excision of Ahr (Fig 1A). The tissues analyzed included the hematopoietic-derived cell enriched tissues BM, spleen, thymus. We determined that CD45 non-hematopoietic cells only display the unexcised allele. Western blotting confirmed that hematopoietic tissues from AHRVav1 mice have greatly reduced or undetectable AHR protein (Fig 1B). As an additional demonstration of functional loss of AHR, we treated isolated BM cells with the potent AHR agonist TCDD, and measured the expression level of the TCDD:AHR gene target Scinderin [3] by RT-qPCR. Relative to isolated Lin- BM cells from TCDD treated with control, Scinderin expression is 5-fold higher in Lin- BM cells from AHRFX mice exposed to TCDD ex vivo (Fig 1C). In contrast, Lin- BM cells from AHRVav1 mice failed to respond to TCDD, which further supports that functional deletion of AHR protein was achieved (Fig 1C). Some reports have suggested that Ahr excision is possible in non-hematopoietic tissues due to endothelial expression of Vav1-cre [26], but we did not observe this in our PCR screens. Combined with our confirmation that AHR protein levels are reduced in hematopoietic tissues in AHRVav1 mice, and that they do not respond to TCDD, AHRVav1 mice are a useful model for studying the hematopoietic role of AhR. Our data shows that we have deleted AhR within hematopoietic cells, and not other tissues.
Fig 1. Genotyping and gene induction in 8 week old AHRVav1 mice.
(A) Excised Ahr is detectable in hematopoietic tissues of AHRVav1 animals. Tissues from AHRVav1 and AHRFX mice were isolated as described and DNA was extracted for PCR analysis using a three primer reaction that amplifies either 180 bp fragments (excised allele) or 140 bp (unexcised allele) fragments. (B) AHRVav1 animals have reduced levels of AHR protein in hematopoietic cells. Various tissues (liver, spleen, thymus, and total bone marrow) were analyzed by western blot to detect AHR (~90kD). AHRFX mice (+) express the receptor in all tissues. AHRVav1 (-) mice display reductions or lack of AHR in the tissues examined. Hepatoma cells were used as a positive control as they express large amounts of AHR.(C) Lineage negative cells from AHRVav1 mice fail to upregulate Scinderin mRNA after 6h exposure to 10nM TCDD. Data expressed relative to DMSO vehicle, using HPRT as an endogenous control gene for normalization. N = 4 mice per treatment group. Relative fold change in expression was calculated using the 2ΔΔCt method.
Flanking Ahr with loxp sites and/or the expression of the Cre transgene could potentially have unintended off-target effects. AHRVav1 animals were healthy and did not display any gross alterations to non-hematopoietic organs (S1 Fig). No difference was observed in total body mass, indicating that the mice grow at the same rate as controls. Heart, lung, thymus, kidney, liver and spleen mass were unaltered in AHRVav1 mice relative to age-matched AHRFX controls. Together these data indicate that neither deletion of the Ahr gene in hematopoietic tissues nor expression of the Cre transgene is sufficient to induce observable gross changes in non-hematopoietic organs.
Alterations to peripheral blood cells can be reflective of defects in HSC function. AHR-KO mice display alterations to peripheral blood composition at 2- and 24-months [6, 7]. To determine if lack of AHR in hematopoietic cells is sufficient to produce similar alterations in peripheral blood, we examined complete blood counts and hematological parameters in 2- and 18-month old AHRVav1 mice compared to age-matched AHRFX mice. Young AHRVav1 mice displayed no alterations to the total white blood cell count or in the percentage of lymphocytes, monocyte or granulocyte in peripheral blood (Fig 2A and 2C). However, as these mice age, there was significant decrease in total white blood cell (WBC) number. In addition, there was significant increase in monocytes and granulocytes, but a decrease in lymphocytes in older AHRVav1 mice (Fig 2B and 2D).However, spleens from 8-week old AHRVav1 mice did not display alterations in cellularity compared to AHRFX controls, nor were there significant differences in the percentage or number of T-cells, B-cells, myeloid cells and erythroid cells (S2 Fig).
Fig 2. Aging AHRVav1 mice show changes in total and differential WBC counts.
Peripheral blood was collected from retro-orbital plexus and complete blood cell counts were done using HESKA blood cell counter: 8-week-old (A & C), 18-month-old (B & D). * = p<0.05 compared to AHRFX controls. N = 5 mice per group.
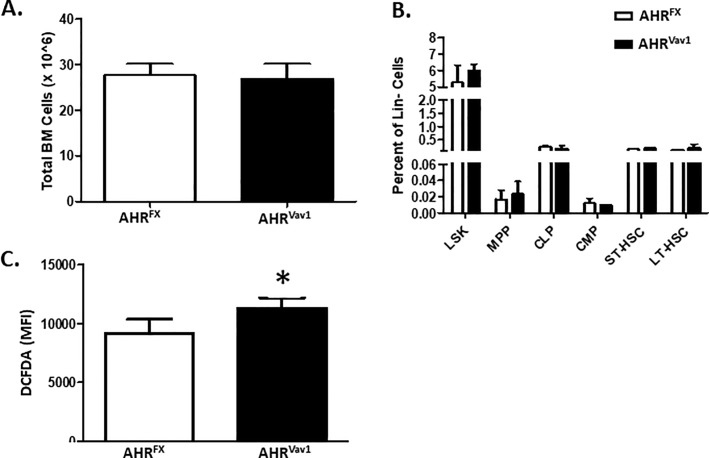
Given that it was previously reported that global AHR-KO mice display alterations in BM subpopulations [6], we examined the frequencies of hematopoietic subpopulations in the BM of AHRVav1 mice. In contrast to global AHR-KO mice, young AHRVav1 mice display no significant alterations in the percentage of LSK, MPP, CLP, CMP, ST-HSC or LT-HSC in BM cells (Fig 3A and 3B). Given that AhR-KO mice also displayed decreased expression of ROS detoxifying enzymes such as Stra13 [7], we examined ROS levels in AHRVav1 mice. LSK cells from AHRVav1 mice had a small, but statistically significant, enhanced oxidative capacity as indicated by DCFDA staining (Fig 3C).
Fig 3. 8-week old AHRVav1 animals do not display alterations in total bone marrow cell counts or subpopulations and display heightened levels of ROS in hematopoietic LSK cells.
(A). Total BM counts were determined for both AHRVav1 and AHRFX animals. (B) Flow cytometry was used to determine percentage of various hematopoietic subpopulations. N = at least 5 mice per group. (C) Flow cyometry was used to identify LSK cells, which had been co-stained with DCFDA to determine levels of oxidative species. Data presented as mean ± S.D. n = 5 per group. * Values significantly different from control (p<0.05).
In order to determine if there are functional alterations in the hematopoietic system of young AHRVav1 mice, we utilized the spleen colony forming assay [27] (S3 Fig). We observed no significant alterations in colony forming units at either 8 or 12 days following irradiation and injection of AHRVav1 or AHRFX cells. These data are consistent with the above data indicating no alterations in phenotype of BM from AHRVav1 mice.
To determine whether conditional ablation of AHR from only the hematopoietic lineage affects LT-HSC function, we measured their ability to serially engraft and repopulate irradiated recipient mice. Serial transplantation forces LT-HSCs to exit quiescence and undergo regulated proliferation and differentiation, in order to repopulate blood lineages in the irradiated recipients. Thus, if conditionally ablating the AHR from only hematopoietic cells affects LT-HSC function, we would detect differences in cellular populations after serial transplantation. We observed a slight decrease in the total repopulation ability of cells from AHRVav1 animals as indicated by the decreased number of CD45.2+ cells in primary recipients of AHRVav1 cells (Fig 4A). However, AHRVav1 cells appear to have enhanced proliferative potential at secondary transplant, indicated by the increase in the percentage of CD45.2+ cells in secondary recipients. This proliferative advantage did not persist to tertiary engraftment, as AHRVav1 cells appear to exhaust prematurely when compared with AHRFX cells at the same time point.
Fig 4. Cells from AHRVav1 mice display a slight defect in serial transplantation.
(A) Serial repopulation experiments were performed, and BM counts and (B) % CD45.2+ cell engraftment was determined at each successive stage. The extent of CD45.2+ cell engraftment was determined by flow cytometry. Data presented as mean ± S.D. n = 5 per group. * Values significantly different compared to AHRFX donors as a control (p<0.05).
AHRVav1 mice have altered gene expression and signaling networks related to normal HSC function
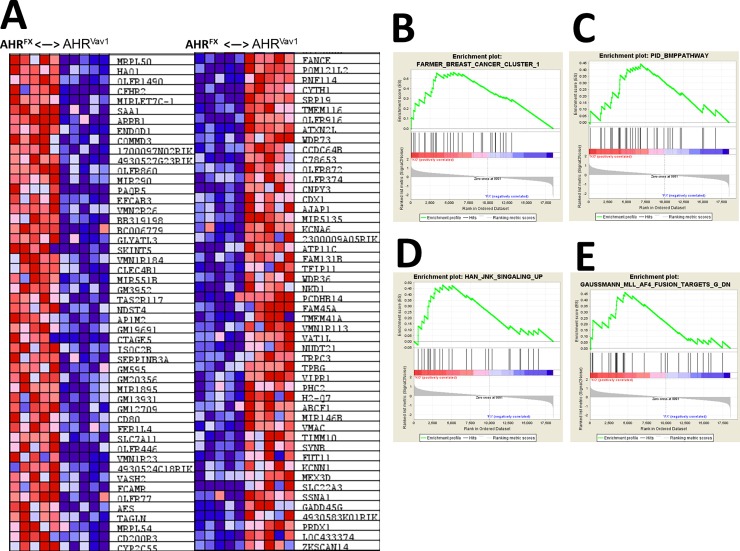
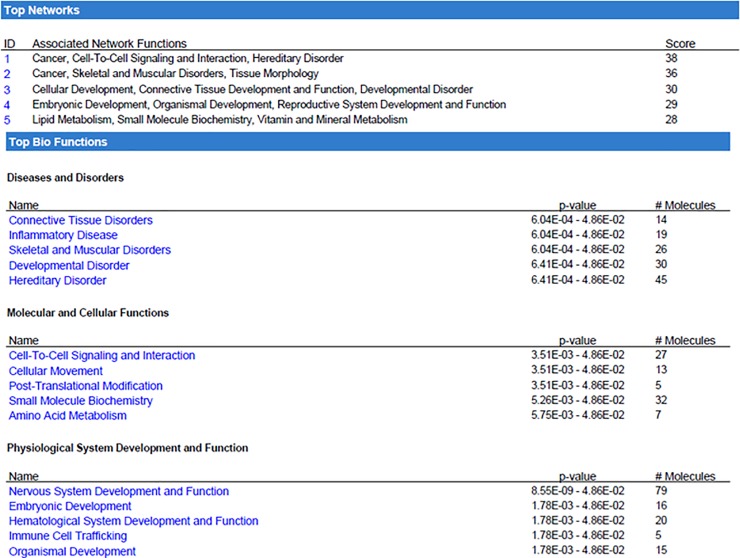
To determine the possible differential expression of genes due to lack of AHR, we analyzed sorted LT-HSCs from 2-month old female mice. As hypothesized, lack of AHR expression in hematopoietic cells is sufficient to drive differences in gene expression relative to AHRFX controls (Fig 5A). Array data were also analyzed by Gene Set Enrichment Analysis (GSEA). Enrichment was determined in a variety of genes sets related to hematological disease or proliferative capacity (Fig 5B, 5C, 5D and 5E). To examine the potential biological impact of the genes determined to be altered by microarray, we next utilized Ingenuity Pathway Analysis (IPA). IPA indicated that there are likely alterations in AHRVav1 LT-HSCs in pathways such as Cell-to-Cell Signaling and Interaction, Cellular Development, and Cancer (Fig 6). Consistent with the hypothesized role of AHR as a regulator of the hematopoietic system, the genes changed in LT-HSCs of 2-month old AHRVav1 participate in hematological system development and function. Together, these proposed physiological functions generated by IPA are consistent with the hypothesis that lack of AHR is sufficient to induce alterations in gene expression in LT-HSCs, and that the induced alterations may play a role in the subsequent development of hematological disease or dysfunction. IPA was used to filter genes which change in LT-HSCs from both 8 week old AHRVav1 and AHR-KO mice (AHR-KO microarray data from Gene Expression Omnibus database accession number: GSE 46976) and which also have putative AHR binding sites on their promoter (Supported by SABiosciences' proprietary database known as DECODE (DECipherment Of DNA Elements). These genes are presented in Table 2.
Fig 5. 8 week old AHRVav1 mice display altered gene expression in LT-HSCs: LT-HSCs from AHRFx and AHRVav1 were sorted and analyzed by microarray.
These analyses revealed differences in gene expression profiles between AHRFx and AHRVav1 mice (A). These changes also indicate changes in specific pathways as revealed by Gene Set Enrichment Analyses (B—E).
Fig 6. 8-week old AHRVav1 mice display alterations in signaling networks.
Array data was analyzed using Ingenuity Pathway Analysis to determine which subcellular pathways and signaling networks might be altered with LT-HSCs due to lack of AHR expression within the hematopoietic compartment.
Table 2. Genes differentially regulated in HSCs from 2-month-old AHR-KO and AHRVav1 mice, and which also contain AHR binding sites in their promoter region.
| CKO vs FX | Fold Change | P | KO vs WT | Fold Change | P |
|---|---|---|---|---|---|
| Stra13 | -4.52 | 0.02982 | Stra13 | -3.54 | 0.0014 |
| Fam164c | 1.83 | 0.01883 | Fam164c | 2.42 | 0.0434 |
| Vmn1r218 | 2.58 | 0.04922 | Vmn1r218 | 4.23 | 0.049 |
| Fut11 | -5.72 | 0.0216 | Fut11 | 4.22 | 0.0193 |
| Krtap7-1 | 2.84 | 0.01732 | Krtap7-1 | -3.07 | 0.0179 |
| Cnpy3 | -7.13 | 0.01154 | Cnpy3 | 2.89 | 0.0154 |
| Ankrd29 | 2.32 | 0.04337 | Ankrd29 | 1.65 | 0.0497 |
| Olfr1057 | 2.12 | 0.01515 | Olfr1057 | -2.81 | 0.0025 |
| Olfr1058 | -3.66 | 0.01532 | Olfr1058 | 2.54 | 0.0147 |
| Slc7a11 | 2.91 | 0.00689 | Slc7a11 | -42.12 | 0.0452 |
| Cited4 | 1.51 | 0.03988 | Cited4 | -1.51 | 0.0244 |
| Gm13040 | 1.54 | 0.04411 | Gm13040 | -3.23 | 0.0452 |
| Pdp1 | 8.32 | 0.04899 | Pdp1 | 5.42 | 0.0393 |
| Arl9 | 2.55 | 0.02796 | Arl9 | -2.21 | 0.0233 |
| Coro1c | 2.52 | 0.03759 | Coro1c | -2.96 | 0.0375 |
| Gm19265 | 2.18 | 0.0448 | Gm19265 | -2.13 | 0.0003 |
| C030030A07Rik | 1.51 | 0.02305 | C030030A07Rik | -1.96 | 0.0049 |
| Wdr88 | 1.52 | 0.03527 | Wdr88 | -1.58 | 0.006 |
| Vat1l | -1.92 | 0.00617 | Vat1l | -2.14 | 0.0027 |
| Olfr836 | 3.98 | 0.00839 | Olfr836 | 3.95 | 0.0482 |
| Cmc1 | -2.41 | 0.01346 | Cmc1 | 1.75 | 0.0141 |
To examine the potential functional impact of lack of AHR in the hematopoietic system throughout aging, we repeated the array and GSEA/pathway analyses in 18-month old AHRVav1 mice. These analyses indicated that LT-HSCs from old AHRVav1 mice have differential gene expression compared to floxed controls (Fig 7A). Array data were also analyzed by GSEA. Enrichment was determined in a variety of genes sets related to hematological disease or proliferative capacity and HSCs aging (Fig 7B–7G). IPA analysis indicated that there are alterations in AHRVav1 LT-HSCs in genes functionally significant for hematopoietic stem cell function or activity (Fig 8). A different subset of altered genes was examined which possess putative AHR binding sites in aged AHRVav1 and AHR-KO (AHR-KO microarray data from Gene Expression Omnibus database accession number: GSE 67378) are presented in Table 3. Ingenuity Pathway Analysiswas used to filter this list, and which also have putative AHR binding sites confirmed using SA Biosciences website. These data are presented Table 3.
Fig 7. 18-month old AHRVav1 LT-HSCs display altered gene expression compared to AHRFX controls.
(A) Heatmap of array data from aging (Old) and young (Yng) AHRFX and AHRVav1 showing differential expression of genes. (B—G) Gene Set Enrichment Analysis was performed on array data from LT-HSCs from 18-month old AHRFX and AHRVav1 mice. Gene profiles are altered in AHRVav1 mice.
Fig 8. Ingenuity Pathway Analysis of differentially expressed genes in LT-HSCs from 18-month-old AHRVav1 mice.
Array data was uploaded to IPA, which was set to include to include only observations that have been experimentally observed, using direct and indirect relationships.
Table 3. Overlapping genes differentially regulated in HSCs from both 18-month-old AHR-KO and AHRVav1 mice.
| Up-regulated genes in 18-month-old AHR-KO and AHRVav1 | Down-regulated genes up-regulated genes in 18-month-old AHR-KO and AHRVav1 |
|---|---|
| 1700030F18Rik | 1700003H04Rik |
| A630023A22Rik | 1700100L14Rik |
| AGBL4 | 2610305D13Rik (includes others) |
| ANAPC1/ANAPC1P1 | 4930459I23Rik |
| BIRC6 | AP4S1 |
| EBF3 | ARAP1 |
| FOXB1 | C16orf93 |
| GINS1 | CYP2D6 |
| Gm4836 | Gm19404 (includes others) |
| KRTAP2-3 (includes others) | IL1RAP |
| MBIP | Lce1a2 |
| MRPS31 | MAGEL2 |
| Mup1 (includes others) | Mir3087 |
| Olfr887 | PERP |
| PSMG3 | RUNX1T1 |
| RPS15A | SEMA3C |
| SF3B4 | Speer4a (includes others) |
| Trhr2 | Ssty1 (includes others) |
| Zfp932 (includes others) | TMEM159 |
| Vmn1r188 (includes others) | |
| Vmn2r66 (includes others) | |
| Zscan4b (includes others) |
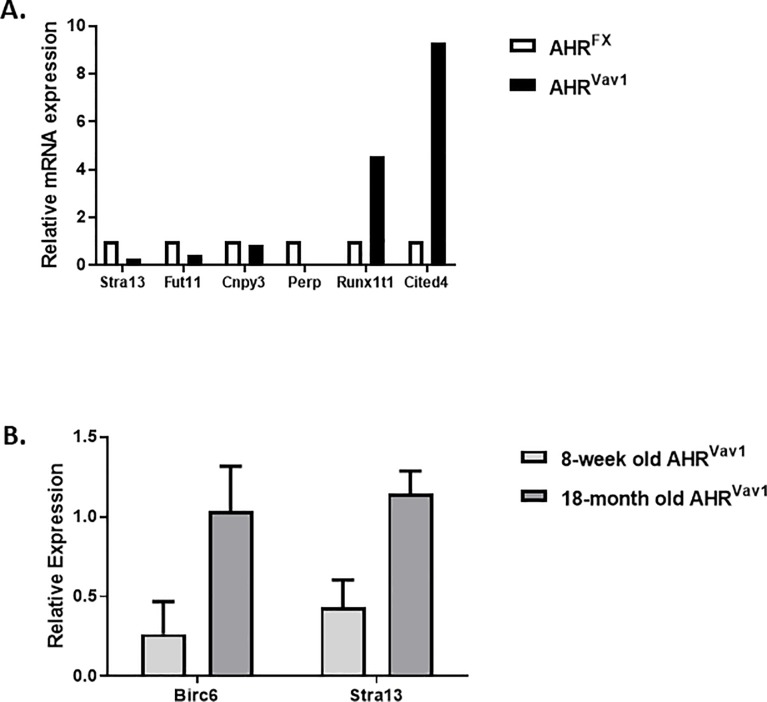
To confirm that certain genes identified utilizing microarray and bioinformatics approaches are truly differentially expressed in AHRVav1 mice, we chose to examine genes linked to hematopoietic development. We used qPCR to examine the expression of genes related to hematopoiesis at both ages, which included Birc6 and Stra13 (Fig 9A and 9B). Consistent with the hypothesis that AHR may regulate these genes, and hematopoietic aging in a broad sense, we observed altered relative expression of both Stra13 and Birc6 in LT-HSCs for both ages of AHR Vav1 animals.
Fig 9. LT-HSCs from AHRVav1 mice have altered expression of genes directly involved in hematopoiesis (A) LT-HSC samples were sorted from BM cells and analyzed for selected genes proposed to have a role in HSC.
Samples are from 6-7-week old animals. (B) Samples are from 6-8-week old female animals for the young comparison, 18-month old female mice for the old comparison. Each gene is normalized relative to GAPDH expression in the AHRFX animals at the indicated age. Data shown as mean +/- SD for at least three replicates. * = P<0.05.
Discussion
The data presented here are consistent with the hypothesized role of AHR as a potential intrinsic regulator of genes in HSCs. In consideration of data previously reported for AHR-KO mice, where we observed altered functional capacity and abnormal proliferation [6], they also suggest that AHR signaling in non-hematopoietic “niche” tissue assists in the regulation of HSCs, and the absence of this signaling in AHR-null allele mice is partially responsible for producing the phenotypes observed.
Interestingly, AHRVav1 mice do appear to phenocopy enhanced oxidative capacity of LSK cells, and this finding is consistent with a prior report in AHR-KO mice [7] and with the described crosstalk between AHR and the anti-oxidative transcription factor Nrf2. [28]. Functional assays of progenitor and HSC function indicate that AHRVav1 mice are not altered at the level of progenitors as indicated by spleen colony forming units. This finding is consistent with the lack of alterations observed in BM progenitors assessed by flow cytometry. However, AHRVav1 mice do display alterations in serial repopulation assays that resemble expansion and early exhaustion at the secondary and tertiary transplant analyses, respectively. While surprising that a defect only became apparent at the secondary stage of engraftment, these data are is consistent with AHR regulating at least some of the aspects of engraftment in BM, and with a previous report that activation of AHR in HSCs alters gene expression related to migration and trafficking [3].
The lack of phenocopy between AHR-KO mice and AHRVav1 mice can potentially be due to the presence of AHR in non-hematopoietic cells in the AHRVav1 mice. In addition, the young age of the mice examined could potentially play a role in the absence of the AHR-KO phenotype. Aging is a significant risk factor for hematological diseases [29], and dysfunction of hematopoietic system [30–32]. It is possible that the AHRVav1 mice could develop a phenotype with aging that is more consistent with observed phenotypes in AHR-KO mice such as splenomegaly, white cell expansion, or LSK expansion [6, 7]. Future studies will address this issue. However, notably there were significant deficits observed following the serial transplant of HSCs from young animals. Serial transplantation mimics some aspects of HSC aging. The observation that a defect becomes apparent in the secondary stage of engraftment with AHRVav1 mice as donors is consistent with the hypothesis that AHR promotes long term fitness of hematopoietic stem cells. Serial repopulation mimics proliferative stress and aging, and it is intriguing that a defect became apparent at later iterations of engraftment. This is suggestive that expression of AHR is required for long term HSC maintenance and function.
In order to determine what gene expression changes could potentially contribute to HSC dysfunction, we examined gene expression profiles in LT-HSCs from AHRVav1 mice. Gene expression profiles in LT-HSCs from young AHRVav1 mice are substantially altered. Hierarchical clustering of the genes reveals alterations in genes such as FANCE, which is a critical component of DNA repair machinery in HSCs [33], and CFHR2 which may have regulatory roles for immune cells and complement activation [34]. Gene Set Enrichment Analysis indicates enrichment for genes in hematopoietic and cancer related gene sets from the Molecular Signatures Database. These genes include Jun and Fos, which are important regulators of HSC function [35, 36]. The genes altered examined fall into gene sets and pathways that are likely to have functional impact on hematopoietic stem cell function or output.
Ingenuity Pathway Analysis indicated that the genes differentially expressed in AHRVav1 mice are related to cell networks such as Cancer, Cell-to-Cell Signaling and Interaction, and Cellular Development. This finding is consistent with known and hypothesized roles for AHR in a variety of cell types [37–39]. The top biological functions predicted by IPA include Cellular Movement, Amino Acid Metabolism, Immune Cell Trafficking, Small Molecule Biochemistry and Hematological System Development and Function. These proposed networks are again consistent with known and hypothesized roles for AHR [3], and with the proposed activation of AHR by a variety of endogenous ligands [40–43]. qPCR confirmation of a subset of genes significant to the hematopoietic system revealed that at both young and old ages, AHRVav1 mice have differential gene expression. Stra13 is involved in the detoxification of reactive oxygen species. We have previously identified Stra13 as differentially regulated in HSCs lacking AHR using a global deletion mouse (AHR-KO) model [7]. This finding suggests that AHR is an important regulator of genes which control ROS detoxification, as Stra13 has been decreased in both AhR-KO and AhR Vav1 mice. Birc6 is a regulator of apoptosis and has been described as being deregulated in acute myeloid leukemia [44]. Interestingly, knockdown of Birc6 in cell lines used to model promyelytic leukemia impairs neutrophil differentiation, and AhR regulation of Birc6 is suggested by our data. This finding is consistent with the hypothesis that AHR is a critical regulator of gene expression in LT-HSCs. Many of the genes we observed to be changed are differentially altered (directionality) between the two types of mouse model used to study AhR (globally deficient, vs HSC deficient). This lack of phenocopy is consistent with our hypothesis that non-hematopoietic effects are driving some of the changes in global AHR-KO animals, and further highlights the utility of using AHRVav1 mice to identify genes directly regulated by AHR in hematopoietic cells.
Our lab has recently published work which suggests that AHR-KO mice have changes in the cellular composition of BM stromal cells [45]. We also observed higher BM cell counts in chimeric mice having WT hematopoietic cells transplanted into AHR-KO host. This study suggests that lack of AHR within BM stromal cells has a role in determining HSC phenotype and function in AHR-KO mice. HSCs from AHR-KO mice have reduced expression of Angiopoietin 1 (Angpt1). It has been reported that changes in Angpt1 gene expression in HSCs may induce secondary changes in BM niche [46]. Angpt1 is also expressed by niche osteoblast cells, which promotes quiescent HSCs in BM niche [47]. The observed phenotypic differences between AHR-KO and AHRVav1 HSCs may be not only due to AHR cell-intrinsic regulation of HSCs, but an extrinsic effect of an AHR-regulated BM niche environment that may play a role in HSC homeostasis and function.
Although we did not observe the same phenotype, function and gene profiles of AHRVav1 HSCs as in AHR-KO mice, common changes in several genes were observed. The expression of pdp1 (up-regulated) and stra13 (down-regulated) were altered in both global and conditional AhR KO models. These genes are associated with aging and longevity, as well as oxidative stress [48,49]. When these mice age they have several common genes up-regulated (agbl4, birc6, anapc1) and down-regulated (runx1t1, perp); these play a role in megakaryopoiesis, apoptosis, cell cycle and aging related changes, apoptosis and leukemia [45, 50, 51].
Notably, Vav-1, and, in this model, Cre-recombinase, are expressed in hematopoietic cells at about mouse gestational day 11 [21, 26]. As such, it is possible that despite the multiple gene changes in HSCs from AHRVav1 mice, some compensatory gene and functional changes occurring (in HSCs and/or BM niche cells) during the lifetime of the AHRVav1 animals could contribute to the lack of complete phenocopy compared to AHR-KO mice. It is also possible that some AHR-dependent pre-programmed event taking place prior to gestational day 11 could “lock in” some aspects of HSC regulation. Other studies, such as the examination of an inducible KO model, may address some of these issues.
In conclusion, the data presented here suggest that lack of AHR in HSCs is sufficient to induce alterations in HSC gene expression, but these gene changes do not appear to induce significant alterations in HSC output/function at 2-months of age, other than in the context of serial transplantation, that were observed in AHR-KO mice. These findings indicate the need to examine the role of AHR signaling in non-hematopoietic stromal cells of the BM (or more distant tissues), and how such signaling can ultimately influence the function or activity of HSCs. The enhanced levels of oxidative species in both AHRVav1 and AHR-KO mice are supportive of a role for AHR:Nrf2 crosstalk in maintaining HSC oxidative levels and may represent an important protective mechanism in HSC biology. These data also suggest the need to examine aging of AHRVav1 mice to determine the impact that deficiency in AhR may have on the development of hematological disease.
Supporting information
Organs were collected and wet mass was determined. No significant differences were detected.
(TIF)
(A) Cell counts for a single spleen of the indicated genotypes. (B) Flow cytometric analysis of splenic cell subpopulations.
(TIF)
Spleen colony forming assays were performed as described, and colonies counted at 8 and 12 days. Slightly higher but non-significant differences were observed.
(TIF)
Acknowledgments
The authors wish to thank Dr. Ellen Henry for help in western blotting for AHR protein and critical review of the manuscript, Dr. Zeenath Unnisa for helping with the qPCR, and Jason Walrath and Catherine Donegan for maintaining the mouse colonies used in this publication.
Data Availability
The microarray data can be accessed through the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) accession number GSE76276.
Funding Statement
This work was supported by NIH Grants P30 ES01247, T32ES07026, ES023068 and ES04862. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Akada H, Akada S, Hutchison RE, Sakamoto K, Wagner KU, Mohi G. Critical role of Jak2 in the maintenance and function of adult hematopoietic stem cells. Stem cells (Dayton, Ohio). 2014;32(7):1878–89. 10.1002/stem.1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh KP, Wyman A, Casado FL, Garrett RW, Gasiewicz TA. Treatment of mice with the Ah receptor agonist and human carcinogen dioxin results in altered numbers and function of hematopoietic stem cells. Carcinogenesis. 2009;30(1):11–9. 10.1093/carcin/bgn224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casado FL, Singh KP, Gasiewicz TA. Aryl hydrocarbon receptor activation in hematopoietic stem/progenitor cells alters cell function and pathway-specific gene modulation reflecting changes in cellular trafficking and migration. Molecular pharmacology. 2011;80(4):673–82. 10.1124/mol.111.071381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garrett RW, Gasiewicz TA. The aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin alters the circadian rhythms, quiescence, and expression of clock genes in murine hematopoietic stem and progenitor cells. Molecular pharmacology. 2006;69(6):2076–83. 10.1124/mol.105.021006 [DOI] [PubMed] [Google Scholar]
- 5.Sun D, Luo M, Jeong M, Rodriguez B, Xia Z, Hannah R, et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell stem cell. 2014;14(5):673–88. 10.1016/j.stem.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh KP, Garrett RW, Casado FL, Gasiewicz TA. Aryl hydrocarbon receptor-null allele mice have hematopoietic stem/progenitor cells with abnormal characteristics and functions. Stem cells and development. 2011;20(5):769–84. 10.1089/scd.2010.0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh KP, Bennett JA, Casado FL, Walrath JL, Welle SL, Gasiewicz TA. Loss of aryl hydrocarbon receptor promotes gene changes associated with premature hematopoietic stem cell exhaustion and development of a myeloproliferative disorder in aging mice. Stem cells and development. 2014;23(2):95–106. 10.1089/scd.2013.0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes MD, Ovcinnikovs V, Smith AG, Kimber I, Dearman RJ. The aryl hydrocarbon receptor: differential contribution to T helper 17 and T cytotoxic 17 cell development. PloS one. 2014;9(9):e106955 10.1371/journal.pone.0106955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esser C, Rannug A. The Aryl Hydrocarbon Receptor in Barrier Organ Physiology, Immunology, and Toxicology. Pharmacological reviews. 2015;67(2):259–79. 10.1124/pr.114.009001 [DOI] [PubMed] [Google Scholar]
- 10.Ahrenhoerster LS, Leuthner TC, Tate ER, Lakatos PA, Laiosa MD. Developmental exposure to 2,3,7,8 tetrachlorodibenzo-p-dioxin attenuates later-life Notch1-mediated T cell development and leukemogenesis. Toxicology and applied pharmacology. 2015;283(2):99–108. 10.1016/j.taap.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winans B, Nagari A, Chae M, Post CM, Ko CI, Puga A, et al. Linking the Aryl Hydrocarbon Receptor with Altered DNA Methylation Patterns and Developmentally Induced Aberrant Antiviral CD8+ T Cell Responses. Journal of immunology (Baltimore, Md: 1950). 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawajiri K, Fujii-Kuriyama Y. The aryl hydrocarbon receptor: a multifunctional chemical sensor for host defense and homeostatic maintenance. Experimental animals. 2017;66(2):75–89. 10.1538/expanim.16-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esser C. The immune phenotype of AhR null mouse mutants: not a simple mirror of xenobiotic receptor over-activation. Biochemical pharmacology. 2009;77(4):597–607. 10.1016/j.bcp.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 14.Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd AL, Salci KR, Shapovalova Z, McIntyre BA, Bhatia M. Nonhematopoietic cells represent a more rational target of in vivo hedgehog signaling affecting normal or acute myeloid leukemia progenitors. Experimental hematology. 2013;41(10):858–69 e4. 10.1016/j.exphem.2013.05.287 [DOI] [PubMed] [Google Scholar]
- 16.Charbord P, Pouget C, Binder H, Dumont F, Stik G, Levy P, et al. A systems biology approach for defining the molecular framework of the hematopoietic stem cell niche. Cell stem cell. 2014;15(3):376–91. 10.1016/j.stem.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 17.Hooker RA, Chitteti BR, Egan PH, Cheng YH, Himes ER, Meijome T, et al. Activated leukocyte cell adhesion molecule (ALCAM or CD166) modulates bone phenotype and hematopoiesis. Journal of musculoskeletal & neuronal interactions. 2015;15(1):83–94. [PMC free article] [PubMed] [Google Scholar]
- 18.Gori JL, Butler JM, Chan YY, Chandrasekaran D, Poulos MG, Ginsberg M, et al. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. The Journal of clinical investigation. 2015;125(3):1243–54. 10.1172/JCI79328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer S, Brooks R, Gumbleton M, Kerr WG. SHIP1-Expressing Mesenchymal Stem Cells Regulate Hematopoietic Stem Cell Homeostasis and Lineage Commitment During Aging Stem cells and development; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walisser JA, Glover E, Pande K, Liss AL, Bradfield CA. Aryl hydrocarbon receptor-dependent liver development and hepatotoxicity are mediated by different cell types. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(49):17858–63. 10.1073/pnas.0504757102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. European journal of immunology. 2003;33(2):314–25. 10.1002/immu.200310005 [DOI] [PubMed] [Google Scholar]
- 22.Wheeler JL, Martin KC, Lawrence BP. Novel cellular targets of AhR underlie alterations in neutrophilic inflammation and inducible nitric oxide synthase expression during influenza virus infection. Journal of immunology (Baltimore, Md: 1950). 2013;190(2):659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boule LA, Burke CG, Jin GB, Lawrence BP. Aryl hydrocarbon receptor signaling modulates antiviral immune responses: ligand metabolism rather than chemical source is the stronger predictor of outcome. Scientific reports. 2018;8(1):1826 10.1038/s41598-018-20197-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin GB, Winans B, Martin KC, Lawrence BP. New insights into the role of the aryl hydrocarbon receptor in the function of CD11c(+) cells during respiratory viral infection. European journal of immunology. 2014;44(6):1685–98. 10.1002/eji.201343980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif). 2001;25(4):402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 26.Georgiades P, Ogilvy S, Duval H, Licence DR, Charnock-Jones DS, Smith SK, et al. VavCre transgenic mice: a tool for mutagenesis in hematopoietic and endothelial lineages. Genesis (New York, NY: 2000). 2002;34(4):251–6. 10.1002/gene.10161 [DOI] [PubMed] [Google Scholar]
- 27.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. 1961. Radiat Res. 2012;178(2):AV3–7. [DOI] [PubMed] [Google Scholar]
- 28.Lu H, Cui W, Klaassen CD. Nrf2 protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced oxidative injury and steatohepatitis. Toxicology and applied pharmacology. 2011;256(2):122–35. 10.1016/j.taap.2011.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell DR, Van Zant G. Stem cells, aging, and cancer: inevitabilities and outcomes. Oncogene. 2004;23(43):7290–6. 10.1038/sj.onc.1207949 [DOI] [PubMed] [Google Scholar]
- 30.Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(12):5465–70. 10.1073/pnas.1000834107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5(8):e201 10.1371/journal.pbio.0050201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–9. 10.1038/nature05862 [DOI] [PubMed] [Google Scholar]
- 33.Gordon SM, Alon N, Buchwald M. FANCC, FANCE, and FANCD2 form a ternary complex essential to the integrity of the Fanconi anemia DNA damage response pathway. J Biol Chem. 2005;280(43):36118–25. 10.1074/jbc.M507758200 [DOI] [PubMed] [Google Scholar]
- 34.Zipfel PF, Skerka C. Complement factor H and related proteins: an expanding family of complement-regulatory proteins? Immunol Today. 1994;15(3):121–6. 10.1016/0167-5699(94)90155-4 [DOI] [PubMed] [Google Scholar]
- 35.Liebermann DA, Gregory B, Hoffman B. AP-1 (Fos/Jun) transcription factors in hematopoietic differentiation and apoptosis. Int J Oncol. 1998;12(3):685–700. [DOI] [PubMed] [Google Scholar]
- 36.Lee SY, Yoon J, Lee MH, Jung SK, Kim DJ, Bode AM, et al. The role of heterodimeric AP-1 protein comprised of JunD and c-Fos proteins in hematopoiesis. J Biol Chem. 2012;287(37):31342–8. 10.1074/jbc.M112.387266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Contador-Troca M, Alvarez-Barrientos A, Barrasa E, Rico-Leo EM, Catalina-Fernandez I, Menacho-Marquez M, et al. The dioxin receptor has tumor suppressor activity in melanoma growth and metastasis. Carcinogenesis. 2013;34(12):2683–93. 10.1093/carcin/bgt248 [DOI] [PubMed] [Google Scholar]
- 38.Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13(2):144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vezina CM, Lin TM, Peterson RE. AHR signaling in prostate growth, morphogenesis, and disease. Biochemical pharmacology. 2009;77(4):566–76. 10.1016/j.bcp.2008.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiaro CR, Morales JL, Prabhu KS, Perdew GH. Leukotriene A4 metabolites are endogenous ligands for the Ah receptor. Biochemistry. 2008;47(32):8445–55. 10.1021/bi800712f [DOI] [PubMed] [Google Scholar]
- 41.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–34. 10.1146/annurev.pharmtox.43.100901.135828 [DOI] [PubMed] [Google Scholar]
- 42.Kawasaki H, Chang HW, Tseng HC, Hsu SC, Yang SJ, Hung CH, et al. A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy. 2014;69(4):445–52. 10.1111/all.12346 [DOI] [PubMed] [Google Scholar]
- 43.Nguyen LP, Hsu EL, Chowdhury G, Dostalek M, Guengerich FP, Bradfield CA. D-amino acid oxidase generates agonists of the aryl hydrocarbon receptor from D-tryptophan. Chem Res Toxicol. 2009;22(12):1897–904. 10.1021/tx900043s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlafli AM, Torbett BE, Fey MF, Tschan MP. BIRC6 (APOLLON) is down-regulated in acute myeloid leukemia and its knockdown attenuates neutrophil differentiation. Experimental hematology & oncology. 2012;1(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unnisa Z, Singh KP, Henry EC, Donegan CL, Bennett JA, Gasiewicz TA. Aryl Hydrocarbon Receptor Deficiency in an Exon 3 Deletion Mouse Model Promotes Hematopoietic Stem Cell Proliferation and Impacts Endosteal Niche Cells. Stem cells international. 2016;2016:4536187 10.1155/2016/4536187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–61. 10.1016/j.cell.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 47.Schepers K, Hsiao EC, Garg T, Scott MJ, Passegue E. Activated Gs signaling in osteoblastic cells alters the hematopoietic stem cell niche in mice. Blood. 2012;120(17):3425–35. 10.1182/blood-2011-11-395418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narasimhan SD, Yen K, Bansal A, Kwon ES, Padmanabhan S, Tissenbaum HA. PDP-1 links the TGF-beta and IIS pathways to regulate longevity, development, and metabolism. PLoS genetics. 2011;7(4):e1001377 10.1371/journal.pgen.1001377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vercherat C, Chung TK, Yalcin S, Gulbagci N, Gopinadhan S, Ghaffari S, et al. Stra13 regulates oxidative stress mediated skeletal muscle degeneration. Human molecular genetics. 2009;18(22):4304–16. 10.1093/hmg/ddp383 [DOI] [PubMed] [Google Scholar]
- 50.Lam K, Zhang DE. RUNX1 and RUNX1-ETO: roles in hematopoiesis and leukemogenesis. Frontiers in bioscience (Landmark edition). 2012;17:1120–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kong CS, Cao H, Kwok S, Nguyen CM, Jordan RC, Beaudry VG, et al. Loss of the p53/p63 target PERP is an early event in oral carcinogenesis and correlates with higher rate of local relapse. Oral surgery, oral medicine, oral pathology and oral radiology. 2013;115(1):95–103. 10.1016/j.oooo.2012.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Organs were collected and wet mass was determined. No significant differences were detected.
(TIF)
(A) Cell counts for a single spleen of the indicated genotypes. (B) Flow cytometric analysis of splenic cell subpopulations.
(TIF)
Spleen colony forming assays were performed as described, and colonies counted at 8 and 12 days. Slightly higher but non-significant differences were observed.
(TIF)
Data Availability Statement
The microarray data can be accessed through the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) accession number GSE76276.