Abstract
We investigated the short-term bronchodilator effects of RPL554 (an inhaled dual phosphodiesterase 3 and 4 inhibitor) combined with other bronchodilators in chronic obstructive pulmonary disease patients with reversibility (>150 mL to short-acting bronchodilators).
Study 1 was a six-way, placebo-controlled crossover study (n=36) with single doses of RPL554 (6 mg), salbutamol (200 µg), ipratropium (40 µg), RPL554 (6 mg)+salbutamol (200 µg), RPL554 (6 mg)+ipratropium (40 µg) or placebo. Study 2 was a three-way crossover study (n=30) of tiotropium (18 µg) combined with RPL554 (1.5 or 6 mg) or placebo for 3 days. Forced expiratory volume in 1 s (FEV1), lung volumes and specific airway conductance (sGaw) were measured.
In study 1, peak FEV1 change compared with placebo was similar with RPL554, ipratropium and salbutamol (mean 223, 199 and 187 mL, respectively). The peak FEV1 was higher for RPL554+ipratropium versus ipratropium (mean difference 94 mL; p<0.0001) and RPL554+salbutamol versus salbutamol (mean difference 108 mL; p<0.0001). In study 2 (day 3), both RPL554 doses caused greater peak FEV1 effects than placebo. The average FEV1(0–12 h) increase was greater with RPL554 6 mg only versus placebo (mean difference 65 mL; p=0.0009). In both studies, lung volumes and sGaw showed greater RPL554 combination treatment effects versus monotherapy.
RPL554 combined with standard bronchodilators caused additional bronchodilation and hyperinflation reduction.
Short abstract
The dual PDE3 and PDE4 inhibitor RPL554 causes additional bronchodilation when combined with commonly used short- or long-acting bronchodilators http://ow.ly/CUYi30lDcYW
Introduction
RPL554 is a first-in-class, dual inhibitor of both phosphodiesterase (PDE) 3 and 4 isoforms [1, 2]. PDE3 inhibitors principally target smooth muscle cells to cause bronchodilation [3–5], whereas PDE4 inhibitors exert anti-inflammatory effects across a range of immune cell types [6, 7]. RPL554 therefore represents a novel drug class combining bronchodilator and anti-inflammatory effects in a single molecule. Initial clinical trials showed that inhaled RPL554 caused bronchodilation in patients with asthma and chronic obstructive pulmonary disease (COPD), likely due to PDE3 inhibition, and demonstrated significant anti-inflammatory effects in the healthy volunteer lipopolysaccharide (LPS) inhalation model of neutrophilic lung disease, likely due to PDE4 inhibition [2]. However, cell and animal models have shown that combined PDE3 and PDE4 inhibition causes additive or synergistic anti-inflammatory and bronchodilator effects [8]. Inhaled RPL554 delivery minimises systemic exposure, thereby reducing the potential for PDE3- or PDE4-mediated side-effects, and has been well tolerated in early-phase clinical trials to date [2].
While pre-clinical data demonstrate that combining RPL554 with other bronchodilators produces additional bronchodilation [9, 10], this concept has not been investigated in COPD clinical trials. The future use of RPL554 in clinical practice is likely to be in conjunction with other bronchodilators. We report two phase II clinical trials in COPD patients investigating the bronchodilator effects of RPL554 combined with other bronchodilators. In one study, RPL554 was combined with short-acting bronchodilators; in another study, RPL554 was combined with the long-acting muscarinic antagonist (LAMA) tiotropium.
Methods
Subjects
Both studies were performed at the Medicines Evaluation Unit, Manchester, UK (www.clinicaltrials.gov identifiers NCT02542254 and NCT03028142). Inclusion and exclusion criteria are listed in full in the supplementary material. For both studies, patients with a diagnosis of COPD and a post-bronchodilator forced expiratory volume in 1 s (FEV1) 40–80% predicted were recruited, and COPD patients with significant cardiovascular disease including angina or recent myocardial infarction were excluded. For study 1, FEV1 reversibility >150 mL after inhalation of salbutamol (200 µg) and ipratropium (40 µg) together was required. For study 2, FEV1 reversibility >150 mL after inhalation of salbutamol (400 µg) was required. One patient participated in both studies. Ethical approval was obtained and participants provided written informed consent before screening.
Study design
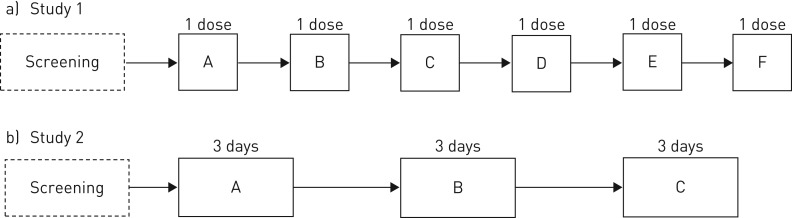
Study 1 was a randomised, double-blind, placebo-controlled, double-dummy, complete-block six-way crossover study to investigate combination treatment with nebulised RPL554 (6 mg) and salbutamol (200 µg) or ipratropium (40 µg) compared with salbutamol or ipratropium alone (figure 1a). The salbutamol and ipratropium doses are those approved for COPD patients. Long-acting bronchodilator treatment was withdrawn at screening. There were six treatment visits separated by washout periods of 3–14 days. The pre-dose FEV1 at treatment visits was required to be within ±15% of the value at the first treatment visit. On each treatment visit, patients received a single dose (two puffs) from a blinded pressurised metered dose inhaler (pMDI) of salbutamol (200 µg) or matched placebo followed, within 1 min, by a single dose (two puffs) from a second blinded pMDI of ipratropium (40 µg) or matched placebo. This was followed immediately (within 2 min) by a single double-blind dose of either RPL554 (6 mg) or placebo. Spirometry was performed pre-dose and at various times up to 12 h post-dose. Whole-body plethysmography was performed pre-dose and up to 4 h post-dose to obtain measurements of functional residual capacity (FRC), residual volume (RV), total lung capacity and specific airway conductance (sGaw).
FIGURE 1.
Overview of study designs. a) Study 1 randomised treatments (A–F): RPL554 (6 mg), salbutamol (200 µg), ipratropium (40 µg), RPL554 (6 mg)+salbutamol (200 µg), RPL554 (6 mg)+ipratropium (40 µg) or placebo. b) Study 2 randomised treatments (A–C): tiotropium (18 µg) once daily and RPL554 (6 mg) twice daily, tiotropium (18 µg) once daily and RPL554 (1.5 mg) twice daily or tiotropium (18 µg) once daily and placebo twice daily.
Study 2 was a randomised, double-blind, placebo-controlled, complete-block three-way crossover study to investigate combination treatment with nebulised RPL554 (1.5 or 6 mg) and tiotropium (18 µg using Handihaler; Boehringer Ingelheim Pharmaceuticals, Ridgefield, CT, USA) compared with tiotropium alone (figure 1b). Long-acting bronchodilator treatment was withdrawn at screening. There were three treatment visits separated by washout periods of 7–21 days. On each treatment visit, patients received one of the following treatments for 2 days and on the morning of day 3: 1) tiotropium (18 µg) once daily and RPL554 (6 mg) twice daily (tiotropium+RPL554 6 mg), 2) tiotropium (18 µg) once daily and RPL554 (1.5 mg) twice daily (tiotropium+RPL554 1.5 mg) or 3) tiotropium (18 µg) once daily and placebo twice daily (tiotropium+placebo).
Tiotropium was administered open label, while RPL554 or placebo was administered double blind. Spirometry was performed on days 1 and 3 at pre-dose and at various times up to 12 h post-dose, and on day 2 at pre-dose and up to 4 h post-dose. Whole-body plethysmography was performed at pre-dose on day 1 and at pre-dose and 1.25 h post-dose on day 2.
For both studies, patients using inhaled corticosteroids were allowed to continue this treatment during the study. Patients were allowed to use short-acting bronchodilators throughout, but these were withheld for at least 8 h prior to spirometry. RPL554 manufacture and administration using a PARI LC Sprint (PARI, West Byfleet, UK) jet nebuliser is described in the supplementary material. The RPL554 doses were selected based on the results of a preliminary study [11]. Spirometry assessments were performed in accordance with guidelines with three technically acceptable measurements recorded [12]; predicted values were calculated using European Community for Coal and Steel reference equations [13].
Statistical analysis
Sample size calculations are given in the supplementary material. The study 1 primary end-points were change from baseline in peak and average FEV1 over 8 h comparing RPL554+salbutamol versus salbutamol and RPL554+ipratropium versus ipratropium. The study 2 primary end-points were change from baseline (pre-dose day 1) in peak and average FEV1 over 12 h on day 3 comparing RPL554 versus placebo. For both studies, the secondary end-points included onset of action (defined as time to reach 10% increase in FEV1 from baseline), forced vital capacity (FVC), body plethysmography measurements (FRC, RV and sGaw) and safety. ANCOVA models were used as described in the supplementary material.
Results
For both studies, flow diagrams showing the number of patients screened, reasons for screen failure and withdrawals are given in the supplementary material.
Study 1
Table 1 lists the characteristics of the 36 enrolled COPD patients; the mean post-bronchodilator FEV1 was 58.3% predicted with 17.4% reversibility. Six patients did not complete the study: two patients were withdrawn due to chest infections, while four patients failed to meet the criteria for pre-dose FEV1 variability and were withdrawn.
TABLE 1.
Demographics and baseline characteristics
| Study 1 | Study 2 | |
| Subjects | 36 | 30 |
| Sex | ||
| Male | 19 | 17 |
| Female | 17 | 13 |
| Age years | 61.3±5.2 | 62.0±7.0 |
| BMI kg·m−2 | 25.7±3.1 | 26.4±3.6 |
| FEV1 % pred | 50.4±12.2 | 50.8±10.4 |
| FEV1 L | 1.44±0.5 | 1.52±0.7 |
| Bronchodilator reversibility % | 17.4±11.1# | 19.5±8.6¶ |
| Smoking status | ||
| Current smoker | 24 | 10 |
| Ex-smoker | 12 | 20 |
| Cigarette pack-years | 47.1±19.3 | 38.6±17.2 |
Data are presented as n or mean±sd. BMI: body mass index; FEV1: forced expiratory volume in 1 s. Pre-bronchodilator values shown. #: 30 min post-salbutamol and ipratropium; ¶: 30 min post-salbutamol.
FEV1
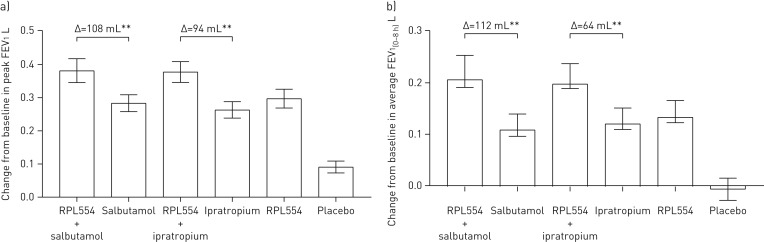
The change from baseline in peak FEV1 compared with placebo was similar with RPL554 (mean 223 mL), ipratropium bromide (mean 199 mL) and salbutamol (mean 187 mL) (figure 2a), with no differences between treatments (p>0.05) (table 2). The combination of RPL554 with ipratropium bromide caused a greater increase in peak FEV1 compared with placebo (mean 292 mL; p<0.0001) and compared with ipratropium alone (mean 94 mL; p<0.0001). RPL554 combined with salbutamol caused a greater peak FEV1 compared with placebo (mean 295 mL; p<0.0001) and compared with salbutamol alone (mean 108 mL; p<0.0001).
FIGURE 2.
Study 1: forced expiratory volume in 1 s (FEV1) changes caused by RPL554 (6 mg), salbutamol (200 µg) and ipratropium (40 µg) alone and in combination (single doses). a) Peak FEV1 and b) average FEV1(0–8 h). Data are presented as mean±sem. **: p<0.01.
TABLE 2.
Statistical analysis of treatment effects in study 1
| Treatment difference: peak FEV1 L | Treatment difference: FEV1 average effect 0–8 h L | |||
| Difference (95% CI) | p-value | Difference (95% CI) | p-value | |
| Salbutamol versus placebo | 0.187 (0.142–0.232) | <0.001 | 0.123 (0.087–0.159) | <0.001 |
| Ipratropium versus placebo | 0.199 (0.153–0.244) | <0.001 | 0.165 (0.128–0.201) | <0.001 |
| RPL554 versus placebo | 0.223 (0.178–0.269) | <0.001 | 0.165 (0.133–0.206) | <0.001 |
| Salbutamol+RPL554 versus placebo | 0.292 (0.250–0.340) | <0.001 | 0.235 (0.199–0.271) | <0.001 |
| Ipratropium+RPL554 versus placebo | 0.292 (0.247–0.337) | <0.001 | 0.229 (0.192–0.265) | <0.001 |
| RPL554 versus salbutamol | 0.039 (−0.009–0.081) | 0.120 | 0.046 (0.010–0.082) | 0.0136 |
| RPL554 versus ipratropium | 0.024 (−0.021–0.070) | 0.294 | 0.004 (−0.032–0.041) | 0.8148 |
| Salbutamol versus ipratropium | −0.012 (−0.057–0.034) | 0.614 | −0.042 (−0.078–0.005) | 0.0245 |
| Salbutamol+RPL554 versus salbutamol | 0.108 (0.063–0.153) | <0.001 | 0.112 (0.076–0.148) | <0.001 |
| Ipratropium+RPL554 versus ipratropium | 0.094 (0.049–0.139) | <0.001 | 0.064 (0.028–0.100) | 0.0006 |
FEV1: forced expiratory volume in 1 s.
The average FEV1(0–8 h) increase compared with placebo was similar for RPL554 (mean 169 mL) and ipratropium (mean 165 mL), while RPL554 was statistically superior to salbutamol (mean 123 mL; p=0.014) (figure 2b and statistical analysis shown in table 2). RPL554 combined with ipratropium caused a mean increase of 229 mL compared with placebo (p<0.0001) and 64 mL compared with ipratropium alone (p=0.0006). RPL554 combined with salbutamol caused a mean increase of 235 mL compared with placebo (p<0.0001) and 112 mL compared with salbutamol alone (p<0.0001).
The median time of onset of action (≥10% increase from baseline) for RPL554 was 14.6 min, while for ipratropium bromide and salbutamol this was 18.4 and 6.0 min, respectively. RPL554 administered in combination with either salbutamol or ipratropium bromide reduced the median time of onset to 3.6 min (p=0.009 versus salbutamol alone) and 4.8 min (p<0.0001 versus ipratropium bromide alone), respectively.
Analysis of peak FVC and average FVC(0–8 h) showed greater effects of RPL554 combined with another bronchodilator compared with a bronchodilator alone (analysis shown in supplementary tables S1 and S2).
Body plethysmography
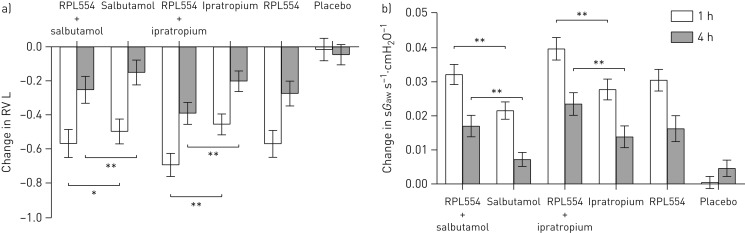
Each treatment alone reduced RV to a similar extent (figure 3a). The combination of RPL554 with ipratropium bromide caused a 695 mL reduction in RV at 1 h, which was significantly greater than ipratropium alone (treatment ratio 0.91, 95% CI 0.88–0.95; p<0.001). Likewise, RPL554 with salbutamol caused a significantly greater reduction in RV than salbutamol alone at both 1 and 4 h. For sGaw, the effects of combination treatment involving RPL554 were significantly greater than ipratropium or salbutamol alone (treatment ratios at 1 h: 1.18, 95% CI 1.08–1.28; p<0.001 and 1.20, 95% CI 1.11–1.31; p<0.001, respectively) (figure 3b). The FRC results are shown in supplementary table S3; there was a greater effect of combination treatment involving RPL554 than ipratropium alone or salbutamol alone (e.g. treatment ratios at 4 h: 0.96, 95% CI 0.94–0.99; p=0.006 and 0.97, 95% CI 0.94–0.99; p=0.01, respectively).
FIGURE 3.
Study 1: a) Residual volume (RV) and b) specific airway conductance (sGaw) changes caused by RPL554 (6 mg), salbutamol (200 µg) and ipratropium (40 µg) alone and in combination (single doses). Data are presented as mean±sem. *: p<0.05; **: p<0.01.
Safety
RPL554 was well tolerated alone or in combination with salbutamol or ipratropium with a similar rate of adverse events compared with placebo (table 3).
TABLE 3.
Adverse events: study 1
| Placebo | RPL554 (6 mg) | Salbutamol (200 µg) |
Salbutamol (200 µg) + RPL554 (6 mg) |
Ipratropium (40 µg) |
Ipratropium (40 µg) + RPL554 (6 mg) |
|
| Subjects | 31 | 31 | 32 | 31 | 32 | 33 |
| Cough | 4 (12.9) | 4 (12.9) | 7 (21.9) | 5 (16.1) | 2 (6.3) | 8 (24.2) |
| Dyspnoea | 0 | 2 (6.5) | 2 (6.3) | 0 | 1 (3.1) | 1 (3.0) |
| Catheter site bruise | 0 | 0 | 1 (3.1) | 1 (3.2) | 1 (3.1) | 1 (3.0) |
| Dizziness | 1 (3.2) | 0 | 2 (6.3) | 0 | 1 (3.1) | 1 (3.0) |
| Headache | 1 (3.2) | 1 (3.2) | 2 (6.3) | 0 | 0 | 0 |
| Oropharyngeal pain | 3 (9.7) | 0 | 0 | 1 (3.2) | 0 | 0 |
| Rash | 0 | 1 (3.2) | 2 (6.3) | 0 | 0 | 2 (6.1) |
| Confusion | 0 | 1 (3.2) | 1 (3.1) | 0 | 1 (3.1) | 1 (3.0) |
| Back pain | 0 | 0 | 2 (6.3) | 0 | 1 (3.1) | 0 |
| COPD | 0 | 1 (3.2) | 0 | 0 | 2 (6.3) | 0 |
| Diarrhoea | 1 (3.2) | 1 (3.2) | 1 (3.1) | 0 | 0 | 0 |
| Chest discomfort | 1 (3.2) | 0 | 0 | 0 | 0 | 1 (3.0) |
| Haematoma | 1 (3.2) | 0 | 0 | 1 (3.2) | 0 | 0 |
| Migraine | 1 (3.2) | 0 | 0 | 1 (3.2) | 0 | 0 |
| Nasopharyngitis | 0 | 0 | 0 | 2 (6.5) | 0 | 0 |
Data are presented as n or n (%). COPD: chronic obstructive pulmonary disease. Treatment emergent adverse events reported by more than one patient are presented. Patients may have experienced the same adverse event following different study treatments.
Study 2
Table 1 lists the characteristics of the 30 enrolled COPD patients; the mean post-bronchodilator FEV1 was 60.1% predicted with 19.5% reversibility. Four patients did not complete the study: two patients were withdrawn because of worsening COPD symptoms, one patient withdrew with pneumonia and one patient withdrew consent.
FEV1
The FEV1 change from baseline (pre-dose on day 1) over 12 h on day 3 is shown in figure 4a. The peak FEV1 changes from baseline for tiotropium combined with placebo or RPL554 1.5 or 6 mg were 373, 477 and 500 mL, respectively (figure 4b). The effects of tiotropium+RPL554 1.5 and 6 mg were significantly greater than tiotropium+placebo (p=0.002 and p<0.0001, respectively). The average FEV1(0–12 h) increase on day 3 was greater with tiotropium+RPL554 6 mg (331 mL) compared with tiotropium+placebo (266 mL; p=0.0009) (figure 4c). There was no difference for tiotropium+RPL554 1.5 mg (317 mL) versus tiotropium+placebo (p=0.09).
FIGURE 4.
Study 2: lung function (forced expiratory volume in 1 s (FEV1)) on day 3. a) FEV1 change from baseline (pre-dose on day 1). b) Peak FEV1 change from baseline (pre-dose on day 1) caused by tiotropium (18 µg), tiotropium (18 µg)+RPL554 (1.5 mg) and tiotropium (18 µg)+RPL554 (6 mg). c) Average FEV1(0–12 h) change from baseline (pre-dose on day 1) caused by tiotropium (18 µg), tiotropium (18 µg)+RPL554 (1.5 mg) and tiotropium (18 µg)+RPL554 (6 mg). Data are presented as mean±sem. **: p<0.01.
The mean change in morning trough FEV1 on day 3 compared with baseline (day 1 pre-dose) was greater with tiotropium+RPL554 6 mg (230 mL) compared with tiotropium+placebo (114 mL; p=0.0003), while there was no difference for tiotropium+RPL554 1.5 mg (168 mL) versus tiotropium+placebo (p=0.35).
On day 1, there were significantly greater FEV1 improvements with tiotropium+RPL554 6 mg compared with tiotropium+placebo, while tiotropium+RPL554 1.5 mg was not significantly different to tiotropium+placebo; these results are shown in the supplementary material. Notably, for peak FEV1 the mean difference between tiotropium+RPL554 6 mg and tiotropium+placebo was 95 mL (p=0.0039). The median time of onset of action on day 1 for tiotropium+RPL554 1.5 and 6 mg was 4.2 and 4.6 min, respectively, compared with 37.6 min for tiotropium+placebo (p<0.0001 for comparisons of RPL554 versus placebo).
Analysis of peak FVC and average FVC(0–12 h) showed a greater effect of RPL554 6 mg compared with placebo (shown in the supplementary material).
Body plethysmography
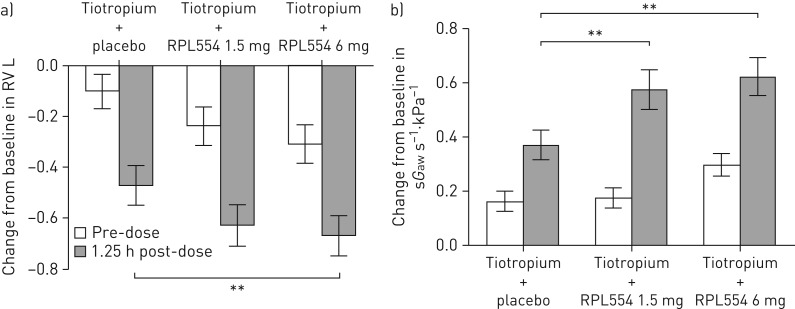
On day 2, there was a reduction in RV with all treatments at 1.25 h post-dose (figure 5a). Tiotropium+RPL554 6 mg caused a significantly greater effect than tiotropium+placebo (p=0.0048), while the effect of tiotropium+RPL554 1.5 mg was not significant (p=0.071). The improvements in FRC were significantly greater with tiotropium+RPL554 1.5 and 6 mg compared with tiotropium+placebo (treatment ratios: 0.96, 95% CI 0.94–0.99; p=0.027 and 0.97, 95% CI 0.95–1.00; p=0.047, respectively). Similarly, sGaw improvements were significantly greater with tiotropium+RPL554 1.5 and 6 mg compared with tiotropium+placebo (treatment ratios: 1.24, 95% CI 1.14–1.35 and 1.31, 95% CI 1.20–1.43, respectively; p<0.0001 for both comparisons) (figure 5b).
FIGURE 5.
Study 2: a) Residual volume (RV) and b) specific airway conductance (sGaw) changes on day 3 caused by tiotropium (18 µg), tiotropium (18 µg)+RPL554 (1.5 mg) and tiotropium (18 µg)+RPL554 (6 mg). The change from baseline (pre-dose on day 1) is shown. Data are presented as mean±sem. **: p<0.01.
Safety
Tiotropium+RPL554 was well tolerated with a similar rate of adverse events compared with tiotropium+placebo (table 4). Withdrawals due to COPD worsening occurred during washout periods and were not attributed to RPL554.
TABLE 4.
Adverse events: study 2
| System organ class preferred term# |
Tiotropium (18 µg) + RPL554 (1.5 mg) |
Tiotropium (18 µg) + RPL554 (6 mg) |
Tiotropium (18 µg) + placebo |
|||
| Events | Patients | Events | Patients | Events | Patients | |
| Subjects | 29 | 27 | 28 | |||
| Total TEAEs | 16 | 12 (41.4) | 20 | 12 (44.4) | 17 | 12 (42.9) |
| General disorders and administration site conditions | 5 | 4 (13.8) | 4 | 4 (14.8) | 7 | 6 (21.4) |
| Medical device site reaction | 4 | 4 (13.8) | 1 | 1 (3.7) | 4 | 4 (14.3) |
| Chest discomfort | 1 | 1 (3.4) | 1 | 1 (3.7) | 2 | 2 (7.1) |
| Nervous system disorders | 2 | 2 (6.9) | 8 | 5 (18.5) | 3 | 3 (10.7) |
| Headache | 2 | 2 (6.9) | 8 | 5 (18.5) | 3 | 3 (10.7) |
| Respiratory, thoracic and mediastinal disorders | 3 | 3 (10.3) | 3 | 3 (11.1) | 3 | 2 (7.1) |
| Dyspnoea | 1 | 1 (3.4) | 1 | 1 (3.7) | 3 | 2 (7.1) |
| Infections and infestations | 1 | 1 (3.4) | 1 | 1 (3.7) | 3 | 2 (7.1) |
| Musculoskeletal and connective tissue disorders | 2 | 2 (6.9) | 2 | 2 (7.4) | 0 | 0 |
Data are presented as n or n (%). TEAE: treatment emergent adverse event. TEAEs reported by more than one patient are presented. Patients may have experienced the same adverse event following different study treatments. #: MedDRA terms (www.meddra.org).
Discussion
We show that RPL554 combined with short-acting bronchodilators or tiotropium caused additional improvements in FEV1 and hyperinflation in reversible COPD patients. In study 1, a single dose of RPL554 6 mg in addition to salbutamol or ipratropium caused significantly greater peak FEV1 improvements compared with either short-acting bronchodilator alone. In study 2, additional improvements in peak, trough and average (0–12 h) FEV1 were observed when RPL554 6 mg was administered with tiotropium for 3 days.
Study 1 was designed to test the mechanistic hypothesis that RPL554 could provide additional bronchodilation in combination with either a β2-agonist or a muscarinic antagonist. This can be regarded as a “proof-of-pharmacology” study to investigate effects caused by different mechanisms of action alone or combined. This study was focused on peak FEV1, and showed similar effects of RPL554, salbutamol and ipratropium administered alone, suggesting that RPL554 causes clinically relevant bronchodilation. The additional effects of RPL554 when combined with short-acting bronchodilators provided mechanistic insights that PDE3/4 inhibition, which increases cAMP levels [7], can cause additional effects when combined with a β2-agonist, which also acts through regulation of cAMP levels [10], or a muscarinic antagonist. Study 1 therefore provided mechanistic support to investigate RPL554 further in combination with long-acting bronchodilator treatment.
Tiotropium was chosen for study 2 as it is a commonly used long-acting bronchodilator. On day 3, the magnitude of additional bronchodilation caused by RPL554 was 116 mL at trough (pre-dose), suggesting that twice-daily RPL554 dosing provides sustained additional bronchodilation persisting for 12 h post-dose. RPL554 6 mg had a greater effect on FEV1 parameters than the 1.5 mg dose and appears to be a suitable twice-daily dose for further investigation. Although study 2 demonstrated an additional effect of RPL554 when administered with a LAMA, it would also be relevant to investigate the effects of RPL554 administered with a long-acting β2-adrenergic agonist (LABA) or a LAMA/LABA combination.
Hyperinflation and gas trapping are major causes of the sensation of dyspnoea, and improvements in lung volumes can improve dyspnoea and exercise performance [14]. RV, a measurement of gas trapping, was reduced by RPL554 in both studies, suggesting a possible effect of RPL554 on small airway function. The onset of bronchodilator action was also faster in both studies when RPL554 was used in combination. A faster onset of bronchodilation may be important to some patients in terms of providing symptom relief.
Pre-clinical studies using human isolated bronchial smooth muscle preparations have demonstrated that RPL554 added to other bronchodilators caused additional bronchodilation, with some evidence of synergistic effects when combined with a muscarinic antagonist [9, 10, 15]. In our clinical trials, additional bronchodilation was observed when using RPL554 in combination with other bronchodilators. While the RPL554 bronchodilator effects are likely to be mainly attributable to PDE3 inhibition, pre-clinical studies have suggested that PDE4 inhibition relaxes inherent tone in isolated human airway tissue [16, 17].
Roflumilast is an orally administered PDE4 inhibitor that reduces exacerbation rates, but the frequency of side-effects, including nausea, weight loss and gastrointestinal disturbance, limits its use in clinical practice [18, 19]. RPL554 was well tolerated in these short-term studies. Clinical trials with a longer duration and larger sample size are needed for proper safety evaluation. RPL554 has the potential for fewer side-effects compared with orally administered PDE4 inhibitors due to reduced systemic exposure, although there may also be intrinsic differences in the pharmacological potential of these different molecules to cause side-effects.
The limitations of the studies reported here include: 1) longer studies are needed to evaluate effects on key clinical end-points, including symptoms and exacerbations, and to properly evaluate safety; 2) the COPD patients included had evidence of reversibility (>150 mL) at screening and the effects of RPL554 in broader population groups need to be studied; 3) the effects of RPL554 in addition to combination treatments that are commonly used in clinical practice remain to be studied, such as LAMA/LABA or triple (inhaled corticosteroid+LABA+LAMA) combinations; and 4) while the anti-inflammatory effects of RPL554 have previously been demonstrated in the LPS challenge model in healthy volunteers [2], further studies of anti-inflammatory effects in COPD patients would be informative.
In conclusion, RPL554 provided additional bronchodilation, reduced gas trapping, improved airway conductance and showed a more rapid onset of action when administered in combination with either a β2-agonist or muscarinic antagonist. These short-term bronchodilator studies provide support to further study RPL554 in longer-term COPD studies focused on other end-points, including symptoms and exacerbations.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01074-2018_supp (297KB, pdf)
Supplementary figures ERJ-01074-2018_supp_figs (186.4KB, pdf)
Footnotes
This article has supplementary material available from erj.ersjournals.com
These studies are registered at www.clinicaltrials.gov with identifier numbers NCT02542254 and NCT03028142. This article contains considerable details of the group-level data. The protocol, statistical analysis plan and patient-level data can be accessed by contacting Verona Pharma plc.
Conflict of interest: D. Singh reports receiving personal fees from Verona during the conduct of the study; and personal fees from Apellis, Cipla, Genentech, Peptinnovate and Skyepharma, and grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Glenmark, Menarini, Merck, Mundipharma, Novartis, Pfizer, Pulmatrix, Teva, Therevance and Verona, outside the submitted work.
Conflict of interest: K. Abbott-Banner has nothing to disclose.
Conflict of interest: T. Bengtsson reports receiving personal fees for statistical consultancy from Verona Pharma plc during the conduct of the study and outside the submitted work.
Conflict of interest: K. Newman was previously an employee of Verona Pharma.
Support statement: This study was supported by Verona Pharma plc.
References
- 1.Boswell-Smith V, Spina D, Oxford AW, et al. The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]isoquinolin-4-one]. J Pharmacol Exp Ther 2006; 318: 840–848. [DOI] [PubMed] [Google Scholar]
- 2.Franciosi LG, Diamant Z, Banner KH, et al. Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: findings from four clinical trials. Lancet Respir Med 2013; 1: 714–727. [DOI] [PubMed] [Google Scholar]
- 3.de Boer J, Philpott AJ, van Amsterdam RG, et al. Human bronchial cyclic nucleotide phosphodiesterase isoenzymes: biochemical and pharmacological analysis using selective inhibitors. Br J Pharmacol 1992; 106: 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardin PG, Dorward MA, Lampe FC, et al. Effect of selective phosphodiesterase 3 inhibition on the early and late asthmatic responses to inhaled allergen. Br J Clin Pharmacol 1998; 45: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myou S, Fujimura M, Kamio Y, et al. Bronchodilator effect of inhaled olprinone, a phosphodiesterase 3 inhibitor, in asthmatic patients. Am J Respir Crit Care Med 1999; 160: 817–820. [DOI] [PubMed] [Google Scholar]
- 6.Page CP, Spina D. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handb Exp Pharmacol 2011: 391–414. [DOI] [PubMed] [Google Scholar]
- 7.Banner KH, Press NJ. Dual PDE3/4 inhibitors as therapeutic agents for chronic obstructive pulmonary disease. Br J Pharmacol 2009; 157: 892–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbott-Banner KH, Page CP. Dual PDE3/4 and PDE4 inhibitors: novel treatments for COPD and other inflammatory airway diseases. Basic Clin Pharmacol Toxicol 2014; 114: 365–376. [DOI] [PubMed] [Google Scholar]
- 9.Calzetta L, Cazzola M, Page CP, et al. Pharmacological characterization of the interaction between the dual phosphodiesterase (PDE) 3/4 inhibitor RPL554 and glycopyrronium on human isolated bronchi and small airways. Pulm Pharmacol Ther 2015; 32: 15–23. [DOI] [PubMed] [Google Scholar]
- 10.Calzetta L, Page CP, Spina D, et al. Effect of the mixed phosphodiesterase 3/4 inhibitor RPL554 on human isolated bronchial smooth muscle tone. J Pharmacol Exp Ther 2013; 346: 414–423. [DOI] [PubMed] [Google Scholar]
- 11.Singh D, Banner K, Newman K. RPL554, an inhaled PDE3/4 inhibitor, causes profound and sustained bronchodilation in healthy volunteers and COPD patients. Eur Respir J 2016; 48: PA4052. [Google Scholar]
- 12.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 13.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Eur Respir J 1993; 6: Suppl. 16, 5–40. [DOI] [PubMed] [Google Scholar]
- 14.Langer D, Ciavaglia CE, Neder JA, et al. Lung hyperinflation in chronic obstructive pulmonary disease: mechanisms, clinical implications and treatment. Expert Rev Respir Med 2014; 8: 731–749. [DOI] [PubMed] [Google Scholar]
- 15.Venkatasamy R, Spina D. Novel relaxant effects of RPL554 on guinea pig tracheal smooth muscle contractility. Br J Pharmacol 2016; 173: 2335–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naline E, Qian Y, Advenier C, et al. Effects of RP 73401, a novel, potent and selective phosphodiesterase type 4 inhibitor, on contractility of human, isolated bronchial muscle. Br J Pharmacol 1996; 118: 1939–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt DT, Watson N, Dent G, et al. The effect of selective and non-selective phosphodiesterase inhibitors on allergen- and leukotriene C4-induced contractions in passively sensitized human airways. Br J Pharmacol 2000; 131: 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009; 374: 695–703. [DOI] [PubMed] [Google Scholar]
- 19.Rennard SI, Calverley PM, Goehring UM, et al. Reduction of exacerbations by the PDE4 inhibitor roflumilast – the importance of defining different subsets of patients with COPD. Respir Res 2011; 12: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01074-2018_supp (297KB, pdf)
Supplementary figures ERJ-01074-2018_supp_figs (186.4KB, pdf)