Abstract
This systematic review aimed to evaluate the efficacy of neurofeedback (NF) compared to stimulant medication in treating children and adolescents with attention-deficit/hyperactivity disorder (ADHD). Included in this review are eight randomized controlled trials that compared an NF condition, either alone or combined with medication, to a medication condition, which was mainly methylphenidate. Outcome measures included behavioral assessments by parents and teachers, self-reports, neurocognitive measures, electroencephalogram power spectra and event-related potentials. When only trials are considered that include probably blinded ratings or those that are sham-NF or semi-active controlled or those that employed optimally titration procedures, the findings do not support theta/beta NF as a standalone treatment for children or adolescents with ADHD. Nevertheless, an additive treatment effect of NF was observed on top of stimulants and theta/beta NF was able to decrease medication dosages, and both results were maintained at 6-month follow-up. This review concludes that the present role of NF in treating children diagnosed with ADHD should be considered as complementary in a multimodal treatment approach, individualized to the needs of the child, and may be considered a viable alternative to stimulants for a specific group of patients. Particularly patients with the following characteristics may benefit from NF treatment: low responders to medication, intolerable side effects due to medication, higher baseline theta power spectra and possibly having no comorbid psychiatric disorders. Future research should prioritize the identification of markers that differentiate responders from nonresponders to NF treatment, the potential of NF to decrease stimulant dosage, the standardization of NF treatment protocols and the identification of the most favorable neurophysiological treatment targets.
Keywords: ADHD, EEG, biofeedback, theta/beta training, methylphenidate, randomized trials
Introduction
The prevalence of attention-deficit/hyperactivity disorder (ADHD) in children and adolescents is estimated to be 5%, making it one of the most common diagnoses in children.1 In fact, prevalence of core symptoms, which comprise attention deficiency, hyperactivity and impulsivity, is estimated to be 20%, as evaluated by reports of teachers and parents.2 However, concerns have been raised regarding the overdiagnosis and overmedication of ADHD and its adverse effects on children.3,4 On the other hand, other evidence suggests underdiagnosis of ADHD is another cause for concern.5 Two systematic reviews show that untreated patients with ADHD have poor long-term outcomes, including addictive behavior and problems in academic performance, interpersonal relationships and overall functioning.6,7
Currently, the first line of treatment for children and adolescents diagnosed with ADHD from age 6 years onward consists of psychostimulant medication, which is primarily methylphenidate (MPH).8 There is substantial evidence for MPH improving functioning on symptom domains of attention, impulsivity and social behavior, with high effect sizes ranging from 0.63 to 0.85.9 Some limitations of psychostimulants, however, are the short duration of treatment effects, achieving no or only partial response in some patients, the lack of achieving long-term remission and short-term adverse effects such as fatigue, nausea and loss of appetite.1,10 Further issues include the significant portion of parents who have nondrug treatment preferences and the compliance of stimulants.11 Long-term adverse effects are yet to be identified, but there is increased awareness about their potential for adverse cardiovascular effects and suppression of growth in children.12,13
In response to these shortcomings, researchers have increasingly focused on different forms of treatment for children and adolescents with ADHD. Neurofeedback (NF), or electroencephalogram (EEG) biofeedback, is a relatively new, noninvasive approach for treating multiple brain-related conditions. Epilepsy has been one of the first therapeutic applications of NF that has been subject to extensive NF research. More conditions in which NF is being used include ADHD, learning disabilities, strokes, head injury, insomnia, depression, obsessive–compulsive disorder and drug addiction.14 NF attempts to normalize the disrupted brain waves that are associated with these conditions by means of repeated training based largely on operant conditioning.15 Although the overall working mechanisms of NF are partially explained by operant conditioning principles, the implications of how such training may influence biological processes at the hormonal or cellular level remains not fully understood. The assumption is that brain waves reflect neural functions, and that training in brain waves may improve neural functions, subsequently leading to improvements in ADHD symptom domains and behavior. NF is thus a method that assists subjects to control their brain waves consciously. The most frequently used type of NF used to treat ADHD is frequency/power NF, which is used to change the amplitude or speed of specific brain waves in particular brain locations, such as the frontal or parietal lobes. Another type of NF that is sometimes used to treat ADHD is slow cortical potential (SCP)-NF, which improves the direction of SCPs. Other types of NF include hemoencephalographic NF, functional MRI NF, low-resolution electromagnetic tomography and near-infrared spectroscopy NF, which are used for the treatment of various disorders.
One of the most consistent findings reported in the qualitative EEG literature on ADHD are those of increased anterior absolute power theta activity.16–18 The literature is less consistent about reduced absolute beta in ADHD, although reduced relative beta has been reported more often.16–18 Despite these consistent findings, previous studies have shown a low diagnostic value of excessive theta or theta/beta ratio, with accuracies below 65%.16,19,20 Although detailed discussion is beyond the scope of this paper, these EEG differences have contributed to the development of different NF treatment protocols in ADHD. The goal of theta/beta NF is to reduce brain activity in the theta band and to increase its activity in the beta band (or to decrease the theta/beta ratio), which aims to improve inattention.15 SCP-NF targets the decreased contingent negative variation amplitudes. Sensorimotor rhythm (SMR) is a form of beta protocol that is also used in ADHD, targeting frequencies in the range of 12–15 Hz to address hyperkinetic behavior. Other protocols such as alpha, delta and gamma have found less robust results in the context of ADHD and are used more frequently in the treatment of other disorders.
A substantial amount of research has been conducted on the efficacy of NF compared to placebo, semi-active controls or sham-NF in the treatment of ADHD. The meta-analysis of Arns et al21 used studies with different designs (controlled/noncontrolled and randomized/nonrandomized), showing large effect sizes for NF on impulsivity and inattention, but medium to low effect sizes on hyperactivity, and concluded that NF can be considered “Efficacious and Specific”. Three randomized controlled trials (RCTs) have shown NF to be superior to a semi-active control condition, such as electromyographic biofeedback, which can be regarded as a credible sham control.21 Moriyama et al22 reported medium to large effect sizes when assessing only nonrandomized trials, but medium to low effect sizes when only randomized trials were considered. Three randomized sham-NF controlled trials found no treatment differences between NF and sham-NF in children with ADHD. However, some authors argued that the negative results from these sham-NF controlled trials are in part due to effective blinding, using suboptimal treatment protocols and not employing adequate conditioning procedures.22 In short, based on the few randomized, semi-active controlled trials, NF seems moderately efficacious in treating children with ADHD, but the exact role of NF remains unclear in part due to the contrasting results from controlled trials.
A particularly relevant question for clinical practice is how NF compares to stimulant medication. In recent years, several head-to-head RCTs have been published. However, the systematic reviews and meta-analyses that have been published on NF in relation to ADHD have focused primarily on studies comparing NF to nonpharmacological controls (placebo, semi-active controls or sham-NF). To date, one meta-analysis21 and two reviews22,23 have incorporated a section focused on trials comparing NF to stimulants. However, due to the lack of trials that compared NF to stimulants at the time, the authors noted that for hyperactivity and inattention, there was not enough data available for a valid comparison between MPH and NF.21 With the data of five studies that compared NF to MPH, the meta-analysis did not find any differences for impulsivity ratings, although those studies were not randomized or blinded.21 The review of Moriyama et al22 reported three trials that compared NF to stimulants, none of which found NF to be inferior to stimulants, but these trials were nonrandomized and two of them only focused on neurophysiological outcomes. The systematic review of Holtmann et al,23 available only in German, showed encouraging results for NF and included three controlled studies that compared NF to MPH, but emphasized the need for further controlled studies with sufficient sample sizes, appropriate measures and follow-up. The more recent review of Holtmann et al24 included three head-to-head RCTs (NF vs stimulants). Out of these three RCTs, two found no difference in efficacy between NF and medication,25,26 whereas one found NF to be inferior to medication.27 Since 2013, eight more studies have been published that compare NF to MPH in a randomized controlled design, including several follow-up publications, that are not included in the currently available reviews. In view of the contrasting results from the first comparison RCTs and the emergence of more recent RCTs, a more up-to-date review is warranted. To date, this is the only systematic review that is specifically aimed at RCTs comparing NF to stimulants in children or adolescents with ADHD.
Materials and methods
The literature was searched for studies that compared frequency/power NF to MPH (or other stimulants) in children with ADHD. A search in the electronic PubMed/MEDLINE database was performed with the following inclusionary terms: (“ADHD” or “ADD” or “attention defi-cit hyperactivity disorder” or “attention deficit disorder”) and (“neurofeedback” or “NFB” or “EEG biofeedback” or “slow cortical potentials” or “SCP” or “neurotherapy” or “brainwave training” or “theta beta” or “sensorimotor rhythm” or “SMR”) and (“methylphenidate” or “stimulant medication” or “psychostimulants” or “dexamphetamine” or “atomoxetine”). Studies were only included if: 1) the study design was an RCT; 2) the article was peer reviewed and 3) the language of publication was English. There were no exclusion criteria based on comorbidity and comedication.
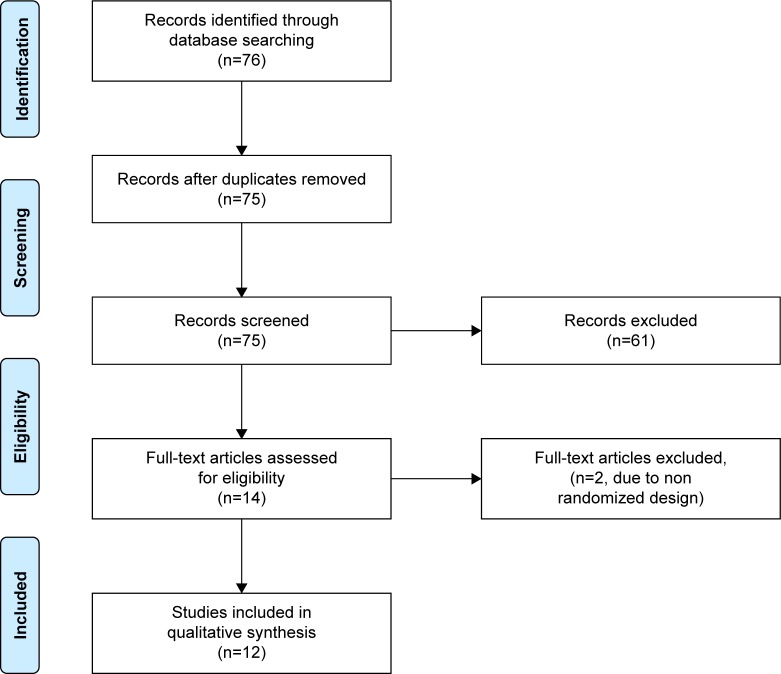
As shown in Figure 1, this search yielded 76 abstracts, which were all screened. An abstract was selected when it contained both NF and stimulant medication as interventions for at least two separate groups of children or adolescents diagnosed with ADHD and compared their efficacy on treating ADHD, measured either through subjective or objective outcome measures. This selection resulted in 15 abstracts. After this process, the full-text articles of the remaining abstracts were screened and assessed for inclusion. Two studies were excluded due to the study design not including randomization. One article was excluded since this was an identical reprint of another. The remaining 12 articles were included in this review. While screening the full-text articles, cross-references were checked and a forward search was done to identify any missing articles. Of the remaining 12 articles, several were related by sharing the same sample and group of authors, but differed in outcome measures or were follow-up assessments. These were clustered together, leading to eight distinct RCTs (Table 1).
Figure 1.
PRISMA flow diagram of study selection.
Table 1.
Overview of 12 RCT publications on the comparison of NF to MPH in treating children and adolescents with ADHD
| Studies | n | Age range and means in years | Treatment and control conditions (n) | Number of sessions, duration of treatment, duration per session | Psychiatric comorbidity | Outcome measures | Results |
|---|---|---|---|---|---|---|---|
| Duric et al (2012, 2014, 2017)25,32,33 | 130 | 6–18 Mean: 11.2±2.8 |
Treatments: theta/beta (42), theta/beta+MPH (44) Control: MPH (44) |
30 sessions, 2.5 months, 40 minutes | Not excluded | Behavioral assessments by parents and teachers, self-reports, with 6-month follow-up | All groups improved significantly on inattention and hyperactivity, with no treatment differences both posttreatment and at 6-month follow-up, although the most marked improvements were observed in the combination group |
| Li et al (2013)36 | 64 | 7–16 Mean: 10.6±2.8 |
Treatment: theta/SMR+MPH (32) Control: MPH+non- feedback attention training (32) |
40 sessions, 5 months, 25–35 minutes | Not excluded | Behavioral assessments by parents and teachers, self-reports, EEG, with 6-month follow-up | Compared to control, treatment showed significant reduction of ADHD symptoms and improvement on brain and behavioral functions, maintained at 6-month follow-up; 6-month follow-up also showed significant increase in MPH dosage in the control group |
| Ogrim and Hestad (2013)27 | 32 | 7–16 Means: 10.6±2.0, 11.2±2.7 |
Treatment: theta/beta (16) Control: medication (16) |
30 sessions, 4 months, 45 minutes | Not excluded | Behavioral assessments by parents and teachers, EEG, ERPs | Compared to NF, MPH showed significant improvement on behavioral assessments; both groups did not decrease in theta activity |
| Bink et al (2014)29 | 90 | 12–24 Mean: 16.1±3.3 |
Treatment: theta/SMR+TAU (45) Control: TAU (26) |
40 sessions, 25 weeks, 30 minutes | Not excluded | Neurocognitive measures | Neurocognitive outcomes improved in both groups with no significant difference between groups |
| Meisel et al (2014)26 | 27 | 7–14 Means: 9.53±1.8, 8.9±1.53 |
Treatment: theta/beta (12) Control: MPH (11) |
40 sessions, 5 months, 35 minutes | Excluded | Behavioral assessments by parents and teachers, with 6-month follow-up | Both groups improved significantly on ADHD symptoms with no significant differences between groups; 8 of 12 children in the NF group were medicated at 6-month follow-up |
| Geladé et al (2016, 2017)30,31 | 112 | 7–13 Mean: 9.63±1.76 |
Treatment: theta/beta (39) Controls: MPH (36), physical activity (37) |
29 sessions, 10–12 weeks, 45 minutes | Not excluded | Behavioral assessments by parents and teachers, neurocognitive measures | Compared to NF and physical activity, MPH showed significant improvement on behavioral and neurocognitive assessments, with no difference between NF and physical activity |
| Janssen et al (2016, 2016)34,35 | 112 | 7–13 Mean: 9.63±1.76 |
Treatment: theta/beta (39) Controls: MPH (36), physical activity (37) |
29 sessions, 10–12 weeks, 45 minutes | Not excluded | EEG power spectra, ERPs | Compared to physical activity, both NF and MPH showed significant reductions in theta power in the “eyes open” condition, but only MPH showed reductions in theta power in the active task condition. Only MPH showed increases in P3 no go ERP amplitudes |
| Lee and Jung (2017)28 | 36 | 6–12 Mean: 8.75±2.12 |
Treatment: theta/beta+medication (18) Control: medication (18) |
20 sessions, 2.5 months, 50 minutes | Excluded | Behavioral assessments by parents, intelligence measures, EEG | Compared to control, treatment showed significant reduction in ADHD symptoms; both groups did not improve on intelligence measures; treatment showed reductions in theta power |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; EEG, electroencephalography; ERP, event-related potential; MPH, methylphenidate; NF, neurofeedback; RCT, randomized controlled trial; SMR, sensorimotor rhythm; TAU, treatment as usual.
Results
Table 1 gives an overview of the eight RCTs (12 publications) that are included in the current review. All studies included children and/or adolescents diagnosed with ADHD. The age range of participants was 6–18 years in eleven studies and 12–24 years in one study. The number of sessions of NF treatment ranged from 20 to 40, and the duration per session ranged from 25 to 50 minutes across the studies. The sample sizes ranged from n=32 to n=130. Six studies used a theta/beta protocol, whereas two studies used a theta/SMR protocol. Stimulant medication usage during NF treatment was allowed in four studies; hence, these studies compared a combination condition (NF and medication) to a medication only condition, and one of these also included an NF only condition. Four studies compared NF as a standalone intervention to a medication condition. The most common outcome measures were behavioral assessments by parents and teachers, employed by seven studies. Other outcome measures were self-reports, neurocognitive tests, EEG parameters and event-related potentials (ERPs). Three studies included a 6-month follow-up.
With regard to psychiatric comorbidity, two studies excluded psychiatric comorbidity26,28 while all other studies allowed psychiatric comorbidity. Of the studies that allowed comorbidity, only four studies reported the data of different comorbid disorders in the sample.27,29–31 The most common comorbid disorders were oppositional defiant disorder, conduct disorder, mood disorders, anxiety disorders and autism spectrum disorders. All studies excluded patients with IQ scores below 70 or 80.
Contrasting results have been found. Of the studies that compared NF alone to stimulant medication, two studies showed improvements in both treatment groups with no significant differences between NF and stimulants on the outcome measures,25,26,32,33 whereas three studies found stimulant medication to be superior to NF.27,30,31,34,35 Of the studies that compared combination treatment (NF and stimulant medication) to stimulant medication, two studies found combination treatment to be superior to stimulant treatment only,33,36 whereas one study showed no difference in efficacy between treatments.29 Notably, in that study, the combination treatment comprised NF and treatment as usual, which includes both medication and behavioral interventions.29 All three studies that included 6-month follow-up found NF to be equally effective to stimulants at 6-month follow-up.26,33,36
The following results regard subjective outcome measures (ie, behavioral assessments, self-reports). Three out of six studies that used behavioral assessments by parents and teachers found NF to reduce ADHD symptoms (inattention, hyperactivity) similar to stimulant medication, with effects maintained at 6-month follow-up.25,26,33 One study found the combination treatment to be superior to medication in reducing ADHD symptoms.36 On the other hand, two studies found medication to be superior to NF in reducing ADHD symptoms based on behavioral assessments by parents and teachers.27,31 The two studies that used self-reports found favorable results for NF treatment, with effects maintained at 6-month follow-up.32,33,36
The following results regard objective outcome measures (ie, neurocognitive tests, EEG parameters and ERPs). Of the two studies that used neurocognitive outcome measures, Bink et al29 found equal improvements on neurocognitive tests between combination treatment and medication only, whereas Geladé et al30 found medication to improve significantly compared to NF. Four studies used brain functions (EEG spectra, ERPs) as outcome measures. In the study of Ogrim and Hestad,27 posttreatment theta activity did not differ between NF and stimulants. In the study of Janssen et al,34 both the NF group and the medication group showed significant reduction in theta power in the “eyes open” condition, but only the medication group showed reduction in theta power in the “active task” condition. In another publication, the same authors showed that the P3 no go ERP amplitudes increased only in the medication group.35 The study of Lee and Jung28 found reduction in theta power in the combination treatment. The study of Li et al36 found that the average dominant probability of alpha waves decreased significantly in the combination treatment and was maintained at 6-month follow-up. An additional outcome was change in medication dosage after NF treatment, reported by two studies:26,36 the study of Li et al36 showed significant reduction of MPH dosage at 6-month follow-up and the other study did not find statistical differences.26
Discussion
The present review examined eight RCT studies that compared NF treatment (six theta/beta, two theta/SMR), either alone or combined with stimulant medication, to stimulant medication in the treatment of children or adolescents with ADHD. Noteworthy is the change in medication dosage after NF treatment reported by two studies. Medication dosage was reduced when NF was given in conjunction with MPH, implying that NF may be particularly useful for low responders to single-drug treatment or children who experience side effects with MPH.25,36 In the study of Li et al36 (n=64), the combination treatment showed significant reduction of MPH dosage at 6-month follow-up. In the study of Meisel et al,26 33% of NF patients (4/12) maintained improvements after 6-month follow-up and did not require further medication, while 67% (8/12) was medicated after NF treatment. The relatively small sample size of this study (n=23) may have contributed to a lack of statistical significance. Other studies in this review did not report data of medication use or dosage after NF treatment. Future studies should further explore the possibility of NF decreasing medication dosage.
Similar to the reviews of Holtmann et al24 and Moriyama et al,22 the current review has found contrasting results. The review of Moriyama et al, which focused on studies that assessed the efficacy of NF, found that most non-RCTs reported medium to large effect sizes, whereas the effects were less robust when only RCTs were considered.22 The meta-analysis of Cortese et al37 reported significant effects for proximal ratings on ADHD symptoms, but failed to find significant effects when probably blinded ratings were the outcome or the trials included active/sham controls, which persisted when only frequency band training trials were analyzed. Some factors that may account for the contrasting results include different NF protocols, titration procedures, washout procedures, patient selection, sample sizes, number of treatment sessions and exclusion of comorbidity. Another limitation is that studies were not powered to detect differences. NF treatment protocols are currently not standardized in ADHD, which likely plays a role in the contrasting study results; also, the frequency band protocols may not be the most suitable targets for NF in ADHD. One frequently recurring point of debate concerns whether behavioral improvements are the result of specific NF treatment effects or nonspecific treatment effects, that is, placebo effects, such as therapist engagement or personal motivation. These may have contributed to the contrasting results found in the current RCT studies, despite the use of randomization. A better motivation to change ADHD may be particularly relevant due to the fact that NF is an intense treatment that may be facilitated through supportive parents and the child’s own motivation. Clinically, pretreatment motivation of patient or parents may be useful as a potential predictor for NF treatment response. Future research should identify the exact role of motivation by parents and children in NF treatment outcome.
Some authors have suggested that these nonspecific effects should be addressed by employing a double-blind sham-NF controlled study design. The only RCT in the present review that used a double-blind design found the combination of NF and medication to be superior to medication with nonfeedback training, that is, sham-NF, supporting the additive treatment effect of NF to medication.36 Although a double-blinded study design seems promising, employing such a design has practical limitations in the case of NF. For example, in addition to controlling neurophysiological parameters, psychosocial factors must also be controlled, such as perceptibility, controllability, motivation and learnability.38 Another potential route to overcome nonspecific effects can be found in the studies using longer follow-up durations, since it is less likely for nonspecific effects to last over longer periods. Two RCTs included in the current review found that treatment effects were maintained at 6-month follow-up, while the double-blind sham-NF controlled trial found that medication dosage was significantly increased in the sham condition.36 It would be relevant for future studies to investigate how the specific effects of NF compare to medication treatment effects by using a double-blind study design with four treatment arms: NF+medication, NF+placebo, sham-NF+medication and sham-NF+placebo.
An alternative method for overcoming the issues regarding the blinding procedures in NF studies is by placing more weight on the teacher ratings when comparing the results of teacher and parent ratings, as teacher ratings are considered to be probably blind.37,39,40 Out of the five RCTs that reported both teacher and parent ratings, three studies did not find any differences in parent or teacher ratings.27,33,36 The other two studies did find differences in treatment effects in favor of parental ratings. One of these found larger improvements on inattention according to parents compared to teachers after theta/beta training.26 The other study reported that, in contrast to parents, teachers reported no improvements in all ADHD symptoms in both the theta/beta group and semi-active controls, while both parents and teachers reported improvements in the medication group.30,31 These findings suggest that parental expectations and investment may contribute to the parent-reported improvements on ADHD symptoms, which has also been suggested by the meta-analysis of Cortese et al based on the results of three trials that included probably blinded ratings.37
Another particularly important factor of influence is the specific titration procedure of stimulants that is used. Through suboptimal titration methods in trials, the treatment effects of stimulants may be underestimated compared to the clinical use of stimulants, leading to overestimating NF treatment effects in these trials. The RCT studies of Ogrim and Hestad27 and Geladé et al30 used optimally titrated stimulant medication through a double-blind, placebo-controlled procedure and found medication to be superior to theta/beta in both behavioral and neurocognitive outcome measures. Other studies in the current review reported using standard dosages for MPH (1 mg/kg bodyweight) ranging across 10–60 mg daily. The findings of the two RCTs with double-blind optimal titration procedures do not support theta/beta NF as a standalone treatment for children or adolescents with ADHD.
As mentioned, the majority of studies in the present review did not exclude children based on psychiatric comorbidity, except for two studies.26,28 Not excluding patients based on psychiatric comorbidity enhances the generalizability of these studies, since comorbidity is a characteristic part of the clinical ADHD youth population.1 The two RCTs that excluded patients with comorbid disorders in the selection process found favorable results for theta/beta NF treatment,26,28 whereas the studies that did not exclude comorbidity found contrasting results. Even though two studies do not suffice to substantiate conclusions, it could suggest that children without comorbid disorders may be more receptive to theta/beta NF treatment, whereas children with ADHD and comorbid disorders (ie, psychiatrically more complex cases) may be in higher need of medication treatment. Another interpretation is that due to the high heterogeneity in ADHD, especially in those cases with comorbid disorders, different electrophysiological treatment targets should be considered depending on the exact presentation type of ADHD and the comorbid disorders, which might be another potential area of interest in future NF research. The current results indicate the need to reevaluate the rationale for the theta/beta treatment protocol in ADHD. Emerging technological developments in the technique of NF seem promising and may aid in this regard, for example, through connectivity-based NF, real-time-functional MRI-based NF or other imaging methods with higher specificity and spatial resolution, allowing for more accurate targeting of compromised brain structures in ADHD, such as the right inferior prefrontal cortex.41
For NF to be clinically applicable, it is important to identify markers that differentiate between responders and nonresponders. Potential markers could range across neurophysiological parameters, environmental influences or psychological factors. One finding in the study of Ogrim and Hestad was that posttreatment theta activity did not differ between groups.27 The researchers suggested that theta may not be a good indicator of symptom change, but rather a marker for ADHD at a more basic level.27 However, the study of Janssen et al34 showed that children with increased baseline theta activity showed the greatest improvements, suggesting its potential utility in the identification of responders to theta/beta NF. Ogrim and Hestad suggested the P3 no go ERP as a potential marker for treatment response, which was increased in 8 out of 12 MPH responders.27 The P3 no go ERP is a specific ERP seen in EEG measures when the subject successfully reacts to a no go cue during cognitive tasks. The presence of the P3 no go ERP in EEG measures is assumed to reflect inhibitory control.42 In another publication, Janssen et al35 further investigated the P3 no go ERP and found that amplitudes only increased in the stimulant group and not in the theta/beta group, suggesting its potential utility as a predictor of medication response, but less utility as a predictor of successful theta/beta treatment.35
To conclude, it appears that when only trials are considered that include probably blinded ratings or those that are sham-NF or semi-active controlled or those that employed optimally titration procedures, the findings do not support theta/beta NF as a standalone treatment for children or adolescents with ADHD. However, when used in combination with medication, theta/beta NF may decrease medication dosage in children who already use medication to treat their ADHD symptoms and has additive treatment effects on top of stimulant medication. There seems to be an unidentified group of patients who may benefit from NF treatment, also after 6-month follow-up, possibly those without comorbid disorders, those with higher baseline theta, those with low response to stimulant medication and those who are more prone to experience side effects due to stimulants. Thus, based on the current state of the art, the current review concludes that the present role of theta/beta NF in treating children diagnosed with ADHD should be considered as complementary in a multimodal treatment approach, individualized to the needs of the child, and may be considered a viable alternative to stimulants for a specific group of patients. NF is a relatively expensive treatment, and it is essential to expand our knowledge about patients who may benefit from this treatment. Future research should prioritize the following: the identification of markers that differentiate responders from nonresponders to NF treatment, the potential of NF to decrease stimulant dosage, the standardization of NF treatment protocols and the identification of the most favorable neurophysiological treatment targets.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Gadow KD, Sprafkin J, Nolan EE. DSM-IV symptoms in community and clinic preschool children. J Am Acad Child Adolesc Psychiatry. 2001;40(12):1383–1392. doi: 10.1097/00004583-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013–1023. doi: 10.1542/peds.2014-1778. [DOI] [PubMed] [Google Scholar]
- 4.Partridge B, Lucke J, Hall W. Over-diagnosed and over-treated: a survey of Australian public attitudes towards the acceptability of drug treatment for depression and ADHD. BMC Psychiatry. 2014;14(1):74–85. doi: 10.1186/1471-244X-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen PS. Current concepts and controversies in the diagnosis and treatment of attention deficit hyperactivity disorder. Curr Psychiatry Rep. 2000;2(2):102–109. doi: 10.1007/s11920-000-0053-z. [DOI] [PubMed] [Google Scholar]
- 6.Arnold LE, Hodgkins P, Kahle J, Madhoo M, Kewley G. Long-term outcomes of ADHD: academic achievement and performance. J Atten Disord. 2015 Jan 12; doi: 10.1177/1087054714566076. Epub. [DOI] [PubMed] [Google Scholar]
- 7.Shaw M, Hodgkins P, Caci H, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012;10(1):99–114. doi: 10.1186/1741-7015-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management. Wolraich M, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–1022. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown RT, Amler RW, Freeman WS, et al. Treatment of attention-deficit/hyperactivity disorder: overview of the evidence. Pediatrics. 2005;115(6):e749–e757. doi: 10.1542/peds.2004-2560. [DOI] [PubMed] [Google Scholar]
- 10.Clavenna A, Bonati M. Safety of medicines used for ADHD in children: a review of published prospective clinical trials. Arch Dis Child. 2014;99(9):866–872. doi: 10.1136/archdischild-2013-304170. [DOI] [PubMed] [Google Scholar]
- 11.Kropotov JD, Grin-Yatsenko VA, Ponomarev VA, Chutko LS, Yakovenko EA, Nikishena IS. ERPs correlates of EEG relative beta training in ADHD children. Int J Psychophysiol. 2005;55(1):23–34. doi: 10.1016/j.ijpsycho.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Reddy DS. Current pharmacotherapy of attention deficit hyperactivity disorder. Drugs Today. 2013;49(10):647–665. doi: 10.1358/dot.2013.49.10.2008996. [DOI] [PubMed] [Google Scholar]
- 13.Swanson JM, Arnold LE, Molina BSG, et al. Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: symptom persistence, source discrepancy, and height suppression. J Child Psychol Psychiatry. 2017;58(6):663–678. doi: 10.1111/jcpp.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond DC. What is neurofeedback? J Neurother. 2007;10(4):25–36. [Google Scholar]
- 15.Heinrich H, Gevensleben H, Strehl U. Annotation: neurofeedback – train your brain to train behaviour. J Child Psychol Psychiatry. 2007;48(1):3–16. doi: 10.1111/j.1469-7610.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- 16.Arns M, Conners CK, Kraemer HC. A decade of EEG theta/beta ratio research in ADHD: a meta-analysis. J Atten Disord. 2013;17(5):374–383. doi: 10.1177/1087054712460087. [DOI] [PubMed] [Google Scholar]
- 17.Clarke AR, Barry RJ, Mccarthy R, Selikowitz M. EEG-defined subtypes of children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2001;112(11):2098–2105. doi: 10.1016/s1388-2457(01)00668-x. [DOI] [PubMed] [Google Scholar]
- 18.Lazzaro I, Gordon E, Whitmont S, et al. Quantified EEG activity in adolescent attention deficit hyperactivity disorder. Clin Electroencephalogr. 1998;29(1):37–42. doi: 10.1177/155005949802900111. [DOI] [PubMed] [Google Scholar]
- 19.Liechti MD, Valko L, Müller UC, et al. Diagnostic value of resting electroencephalogram in attention-deficit/hyperactivity disorder across the lifespan. Brain Topogr. 2013;26(1):135–151. doi: 10.1007/s10548-012-0258-6. [DOI] [PubMed] [Google Scholar]
- 20.Ogrim G, Kropotov J, Hestad K. The quantitative EEG theta/beta ratio in attention deficit/hyperactivity disorder and normal controls: sensitivity, specificity, and behavioral correlates. Psychiatry Res. 2012;198(3):482–488. doi: 10.1016/j.psychres.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 21.Arns M, de Ridder S, Strehl U, Breteler M, Coenen A. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clin EEG Neurosci. 2009;40(3):180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- 22.Moriyama TS, Polanczyk G, Caye A, Banaschewski T, Brandeis D, Rohde LA. Evidence-based information on the clinical use of neurofeedback for ADHD. Neurotherapeutics. 2012;9(3):588–598. doi: 10.1007/s13311-012-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holtmann M, Stadler C, Leins U, Strehl U, Birbaumer N, Poustka F. Neurofeedback for the treatment of attention-deficit/hyperactivity disorder (ADHD) in childhood and adolescence. Z Kinder Jugendpsychiatr Psychother. 2004;32(3):187–200. doi: 10.1024/1422-4917.32.3.187. [DOI] [PubMed] [Google Scholar]
- 24.Holtmann M, Sonuga-Barke E, Cortese S, Brandeis D. Neurofeedback for ADHD: a review of current evidence. Child Adolesc Psychiatr Clin N Am. 2014;23(4):789–806. doi: 10.1016/j.chc.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Duric NS, Assmus J, Gundersen D, Elgen IB. Neurofeedback for the treatment of children and adolescents with ADHD: a randomized and controlled clinical trial using parental reports. BMC Psychiatry. 2012;12(1):107. doi: 10.1186/1471-244X-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meisel V, Servera M, Garcia-Banda G, Cardo E, Moreno I. Reprint of “Neurofeedback and standard pharmacological intervention in ADHD: a randomized controlled trial with six-month follow-up”. Biol Psychol. 2014;95(1):116–125. doi: 10.1016/j.biopsycho.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Ogrim G, Hestad KA. Effects of neurofeedback versus stimulant medication in attention-deficit/hyperactivity disorder: a randomized pilot study. J Child Adolesc Psychopharmacol. 2013;23(7):448–457. doi: 10.1089/cap.2012.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee EJ, Jung CH. Additive effects of neurofeedback on the treatment of ADHD: a randomized controlled study. Asian J Psychiatr. 2017;25:16–21. doi: 10.1016/j.ajp.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Bink M, van Nieuwenhuizen C, Popma A, Bongers IL, van Boxtel GJ. Neurocognitive effects of neurofeedback in adolescents with ADHD: a randomized controlled trial. J Clin Psychiatry. 2014;75(5):535–542. doi: 10.4088/JCP.13m08590. [DOI] [PubMed] [Google Scholar]
- 30.Geladé K, Bink M, Janssen TW, van Mourik R, Maras A, Oosterlaan J. An RCT into the effects of neurofeedback on neurocognitive functioning compared to stimulant medication and physical activity in children with ADHD. Eur Child Adolesc Psychiatry. 2017;26(4):457–468. doi: 10.1007/s00787-016-0902-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geladé K, Janssen TW, Bink M, van Mourik R, Maras A, Oosterlaan J. Behavioral effects of neurofeedback compared to stimulants and physical activity in attention-deficit/hyperactivity disorder: a randomized controlled trial. J Clin Psychiatry. 2016;77(10):e1270–e1277. doi: 10.4088/JCP.15m10149. [DOI] [PubMed] [Google Scholar]
- 32.Duric NS, Aßmus J, Elgen IB. Self-reported efficacy of neurofeedback treatment in a clinical randomized controlled study of ADHD children and adolescents. Neuropsychiatr Dis Treat. 2014;10:1645–1654. doi: 10.2147/NDT.S66466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duric NS, Assmus J, Gundersen D, Duric Golos A, Elgen IB. Multimodal treatment in children and adolescents with attention-deficit/hyperactivity disorder: a 6-month follow-up. Nord J Psychiatry. 2017;71(5):386–394. doi: 10.1080/08039488.2017.1305446. [DOI] [PubMed] [Google Scholar]
- 34.Janssen TW, Bink M, Geladé K, van Mourik R, Maras A, Oosterlaan J. A randomized controlled trial into the effects of neurofeedback, methylphenidate, and physical activity on EEG power spectra in children with ADHD. J Child Psychol Psychiatry. 2016;57(5):633–644. doi: 10.1111/jcpp.12517. [DOI] [PubMed] [Google Scholar]
- 35.Janssen TW, Bink M, Geladé K, van Mourik R, Maras A, Oosterlaan J. A randomized controlled trial investigating the effects of neurofeedback, methylphenidate, and physical activity on event-related potentials in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2016;26(4):344–353. doi: 10.1089/cap.2015.0144. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Yang L, Zhuo CJ, Wang YF. A randomised controlled trial of combined EEG feedback and methylphenidate therapy for the treatment of ADHD. Swiss Med Wkly. 2013;143:2–5. doi: 10.4414/smw.2013.13838. [DOI] [PubMed] [Google Scholar]
- 37.Cortese S, Ferrin M, Brandeis D, et al. Neurofeedback for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2016;55(6):444–455. doi: 10.1016/j.jaac.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Gaume A, Vialatte A, Mora-Sánchez A, Ramdani C, Vialatte FB. A psychoengineering paradigm for the neurocognitive mechanisms of biofeedback and neurofeedback. Neurosci Biobehav Rev. 2016;68:891–910. doi: 10.1016/j.neubiorev.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Zuberer A, Minder F, Brandeis D, Drechsler R. Mixed-effects modeling of neurofeedback self-regulation performance: moderators for learning in children with ADHD. Neural Plast. 2018;2018:15. doi: 10.1155/2018/2464310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonuga-Barke EJ, Brandeis D, Cortese S, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170(3):275–289. doi: 10.1176/appi.ajp.2012.12070991. [DOI] [PubMed] [Google Scholar]
- 41.Alegria AA, Wulff M, Brinson H, et al. Real-time fMRI neurofeedback in adolescents with attention deficit hyperactivity disorder. Hum Brain Mapp. 2017;38(6):3190–3209. doi: 10.1002/hbm.23584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kropotov JD, Grin-Yatsenko VA, Ponomarev VA, Chutko LS, Yakovenko EA, Nikishena IS. ERPs correlates of EEG relative beta training in ADHD children. Int J Psychophysiol. 2005;55(1):23–34. doi: 10.1016/j.ijpsycho.2004.05.011. [DOI] [PubMed] [Google Scholar]