Abstract
Background/Aims
The SYNERGY (Saizen® for Your New Life and Brighter Tomorrow without Growth Deficiency) study is the first randomised multi-centre, open-label study to assess the short-term efficacy and safety of this recombinant human growth hormone (r-hGH) preparation for prepubertal children with idiopathic short stature in South Korea.
Methods
The SYNERGY study (ClinicalTrials.gov NCT01746862) was conducted at 9 centres throughout South Korea between December 2012 and March 2015. The primary endpoint was difference in height velocity from baseline to 6 months in the treatment and control arms.
Results
97 children were screened; 90 were randomly assigned: 60 children to 0.067 mg/kg/day r-hGH for 12 months (treatment) and 30 children to 6 months of no treatment followed by 0.067 mg/kg/day r-hGH for 6 months (control). The 6-month mean height velocity in the treatment group increased from 5.63 cm/year (SD 1.62) to 10.08 cm/year (SD 1.92) (p < 0.0001) and from 4.94 cm/year (SD 1.91) to 5.92 cm/year (SD 2.01) (p = 0.0938) in the control group (between-group difference 3.47 cm/year, 95% CI 2.17–4.78; p < 0.0001). Adherence was > 90% throughout the study. The safety profile was consistent with that already known for r-hGH.
Conclusion
Treatment with r-hGH in the SYNERGY study demonstrated a statistically significant increase in height velocity at 6 months.
Keywords: Idiopathic short stature, Recombinant human growth hormone, Height velocity, Efficacy, Safety
Introduction
Short stature [1] and growth failure [2, 3] are some of the most common causes for referral to paediatric endocrinology clinics, general paediatricians, and primary health care physicians [3].
Although many of the causes of short stature and growth failure have been identified, children who fulfil the criteria for these disorders but who have no evidence of systemic, endocrine, nutritional, or chromosomal abnormalities, and who have normal simulated growth hormone (GH) concentrations, are commonly diagnosed under the umbrella term of idiopathic short stature (ISS). The causes of ISS are likely to be heterogeneous and related to subtle disorders of GH secretion or sensitivity or a combination of genetic factors that influence growth plate biology, and are unlikely to be picked up during conventional diagnostic workup [4, 5]. Even with the advent of comprehensive molecular studies, the diagnosis of ISS remains based on auxological assessment (height below −2 standard deviation scores [SDS]) and the exclusion of other causes [6].
Children with ISS are of normal size at birth but grow slowly during early childhood, meaning their height generally falls within the range for ISS by the time they start school [3]. In the long term, untreated children with ISS will usually be shorter than their predicted adult heights and significantly below their genetic target height, potentially resulting in detrimental effects on quality of life, necessitating early identification and treatment [7, 8, 9, 10, 11].
Treatment of ISS with somatropin (recombinant human GH [r-hGH]) was approved by the United States Food and Drug Administration (US FDA) in 2003, and several formulations of r-hGH have shown short-term efficacy (improvements in height velocity) and long-term efficacy (attainment of near-adult height) in children with ISS [4, 6, 12, 13]. In South Korea, the r-hGH preparation Saizen® (Merck KGaA, Darmstadt, Germany) is approved for the treatment of children with growth failure associated with GH deficiency, chronic renal failure, Turner syndrome and for children born small for gestational age. Saizen® is not currently approved for ISS in South Korea.
The SYNERGY (Saizen® for Your New Life and Brighter Tomorrow without Growth Deficiency) study is the first randomised multi-centre, open-label study to assess the short-term efficacy and safety of r-hGH for children with ISS in South Korea. The aim of the SYNERGY study is to expand the indication of this preparation of r-hGH as an additional option for the treatment of children with ISS in Korea.
Methods
Study Participants
The SYNERGY study (ClinicalTrials.gov NCT01746862) was a 12-month, open-label, randomised, two-arm, parallel group, delayed-treatment group-controlled (6 months of no treatment followed by 6 months of treatment with r-hGH 0.067 mg/kg/day, 6 days per week) phase III study. SYNERGY was conducted at 9 centres throughout South Korea between December 2012 and March 2015. The aim of SYNERGY was to assess the efficacy and safety of treatment with r-hGH in prepubertal children with ISS.
Children were eligible for inclusion if they had official height records available and were ≥5 years of age; were prepubertal; had height < 3rd percentile for the Korean National Growth Charts for the same age and sex; had peak serum GH > 10 µg/L in at least 2 GH stimulation tests; were naïve to r-hGH therapy; had a gestational age > 34 weeks and an appropriate birth weight, normal thyroid function and karyotype (girls); and had bone age ≤9 years in girls and ≤10 years in boys with no more than 3 years’ difference from chronological age. Children were excluded if they were diagnosed with predesignated pathological processes. Children were also excluded if they entered puberty (testicular volume ≥4 mL in boys or breast Tanner stage ≥2 in girls) during their participation in the study.
The SYNERGY study was conducted in compliance with the clinical study protocol, Good Clinical Practice, and the applicable Korean regulatory requirements. The institutional review board at each study site provided favourable opinion/approval, and the principal investigators were responsible for the conduct of the study. Written assent of the child and written informed consent of the parent/guardian to participate in the study were obtained before any study-related activities were carried out.
Study Procedures
Following informed consent, screening-related procedures were carried out during a period of up to 28 days. Eligible children were then randomly assigned in a 2: 1 ratio, using a permuted block randomisation method based on the order of recruitment at each participating centre, to receive 0.067 mg/kg/day r-hGH for 12 months (treatment group) or no treatment for 6 months followed by 6 months of treatment with r-hGH 0.067 mg/kg/day (control group). Randomisation codes were generated centrally by a contract research organisation. No masking to treatment allocation was carried out.
The primary endpoint of SYNERGY was the change in height velocity from baseline to 6 months between the treatment and the control groups. Secondary endpoints were the between-group change in height velocity at 12 months; height and height SDS; IGF-1 and IGFBP-3 concentrations at 6 and 12 months; treatment adherence; and overall safety profile.
At each centre, children were assessed at weeks 13, 26, 39, and 52 for auxological parameters (e.g., height and weight measurements), potential adverse events (AEs), dispensing of the study drug and diary entries (to evaluate adherence and concomitant medications), and review of the techniques for reconstitution using click easy® and injection using easypod®. An additional visit was conducted at 56 weeks to assess any AEs that may have developed or persisted since the last dose of r-hGH. Radiological evaluation of bone age [14] was undertaken at screening and at weeks 26 and 52 by an independent investigator blinded to treatment group and verified by a second investigator at a different centre. Routine serological and haematological tests were done at screening and at week 26 and week 52. IGF-1, IGFBP-3, HbA1c, and thyroid function tests were done at the screening visit and at weeks 13 (treatment group only), 26, 39, and 52. Serum IGF-1 and IGFBP-3 concentrations were assessed at an independent central laboratory (Seoul Medical Science Institute, Seoul, South Korea) using commercial assays (Immulite, Siemens Healthcare Korea, Seoul, South Korea). Sex-adjusted and age-adjusted IGF-1 SDS and IGFBP-3 SDS were calculated using commercial software (SDSeasy, Medidiagnost, Reutlingen, Germany). Cross-calibration of the assays showed that data from the Immulite IGF-1 assay could be used directly to calculate the IGF-1 SDS, whereas data from the Immulite IGFBP-3 assay required conversion using the following formula: IGFBP-3 (Mediagnost) = IGFBP-3 (Immulite) × 0.3644 + 1.392.
Statistical Analyses
72 children with ISS were estimated to provide > 90% power to detect a statistically significant difference in height velocity, expecting a difference of 2 cm/year between groups after 6 months. To achieve this number, an estimated 87 children with ISS were required for randomisation. Only the primary endpoint was used to determine statistical significance of the treatment effect. All other p values are presented for information only.
The primary endpoint was analysed in the intention-to-treat (ITT) population (all children who were randomly assigned to a treatment group and had at least 1 post-baseline height measurement) using two-sided analysis of covariance (ANCOVA), adjusted for baseline age and height as covariates and with last observation carried forward (LOCF). Between-group differences are least-squares mean differences. Sensitivity analyses of the primary endpoint were done in the ITT population without LOCF and in the children who completed the study (per-protocol population).
Analyses of the secondary endpoints were exploratory and reported in children in the ITT population who completed the study. Only available data were used in the analyses of the secondary endpoints. Safety outcomes were reported in children who received at least 1 dose of r-hGH and had post-baseline safety data. The occurrence of AEs and serious AEs, according to entries in the treatment diaries, was compared using Fisher's test. Adherence was calculated from the proportion of prescribed doses administered according to drug diaries. Nonadherence was defined as receipt of < 75% of expected injections.
Results
Children
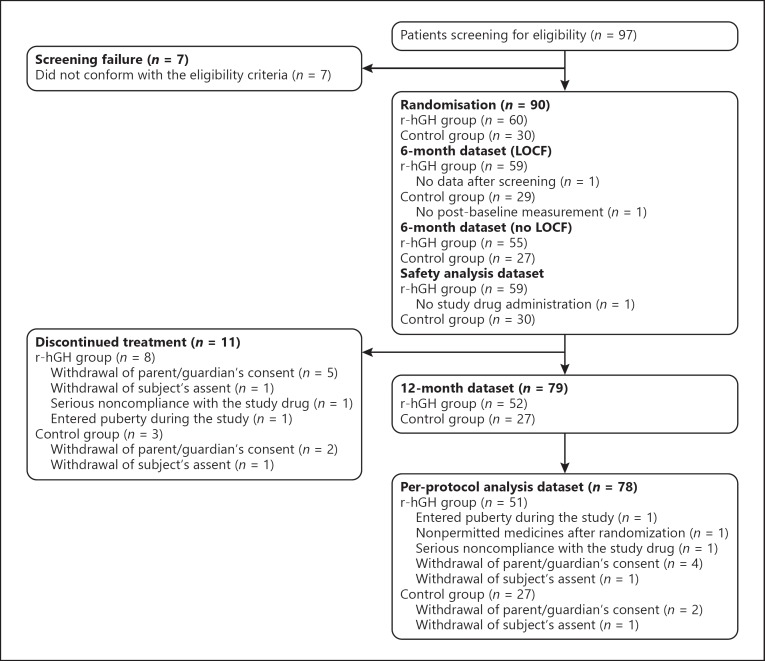
97 children with ISS were enrolled and screened across the 9 study sites in South Korea. Of these, 90 children met the eligibility criteria and were randomised (60 to the treatment group and 30 to the control group; ITT population [Fig. 1]).
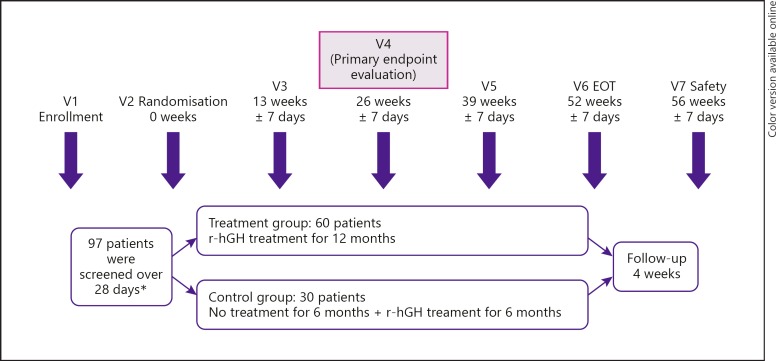
Fig. 1.
SYNERGY study design. * Seven children did not meet the screening criteria; therefore, 90 patients were enrolled and randomised in the intention-to-treat population. EOT, end of treatment; r-hGH, recombinant human growth hormone.
Demographics were similar between the two groups (Table 1). The flow of children through the study is shown in Figure 2. 89 children in the ITT population received ≥1 dose of r-hGH (safety population). Analysis of the primary endpoint was done in the ITT LOCF population (59 children in the treatment group [1 child had no data after screening] and 29 children in the control group [1 child had no post-baseline measurements]; Fig. 2). Six of these children (4 in the treatment group and 2 in the control group) were missing measurements at 6 months; the 6-month values for these children were reported as LOCF. One subject who was born at a gestational age of 30 weeks was enrolled in the study before the protocol was amended to only include subjects > 34 weeks of gestation; however, this subject was judged to have a normal weight (1.5 kg) based on gestational age and was included in the analyses. 79 children completed the study to 12 months, 78 without any protocol deviations (per-protocol population).
Table 1.
Demographics at baseline
| Treatment (r-hGH) group (n = 59) | Control group (n = 30) | ||
|---|---|---|---|
| Male/female | 30 (51%)/29 (49%) | 17 (57%)/13 (43%) | |
| Chronological age, years | 6.79 (1.54) | 6.83 (1.61) | |
| Height1, cm | 108.21 (7.72) | 108.9 (8.34) | |
| Height SDS | –2.26 (0.37) | –2.33 (0.34) | |
| Weight, kg | 18.09 (3.43) | 18.24 (3.11) | |
| Weight SDS | –1.95 (0.96) | –1.85 (1.05) | |
| BMI SDS | –0.55 (0.97) | –0.37 (1.12) | |
| Birth weight, kg | 2.95 (0.38) | 3.04 (0.30) | |
| Height velocity, cm/year | 5.65 (1.65) | 4.98 (1.95) | |
| Bone age2, years | 5.21 (1.56) | 5.04 (1.56) | |
| Ratio of bone age to chronological age | 0.76 (0.13) | 0.73 (0.11) | |
| Target height3, cm | 162.93 (7.20) | 162.80 (6.66) | |
| Target height4 SDS | –0.59 (0.48) | –0.70 (0.45) | |
| Peak GH, µg/L | 20.01 (14.24) | 22.25 (12.51) | |
| Serum IGF-1, µg/L | 113.56 (42.81) | 125.01 (65.68) | |
| Serum IGF-1 SDS | –0.60 (0.99) | –0.52 (1.33) | |
| Serum IGFBP-3, µg/L | 3,838.98 (814.25) | 3,935.67 (866.42) | |
| Serum IGFBP-3 SDS | 0.43 (0.57) | 0.52 (0.57) |
Data are n (%) or mean (SD). Data are reported in the safety population (treatment n = 59 [1 child did not receive the study drug and was excluded]). BMI, body mass index; GH, growth hormone; IGF, insulin-like growth factor; IGFBP-3, insulin-like growth factor binding protein 3; r-hGH: recombinant human growth hormone; SD, standard deviation; SDS, standard deviation score.
Mean of 2–3 measurements per child.
Bone age calculated from radiographs of the wrist assessed by 2 investigators according to the standards of Greulich and Pyle [14].
Target height was estimated from sex-adjusted midparental height (father's height – mother's height)/2; gender adjustment was achieved by adding 6.5 cm for boys and subtracting 6.5 cm for girls [30].
Target height SDS was calculated as 0.72 multiplied by (father's height SDS – mother's height SDS)/2 [31].
Fig. 2.
Flow of children through the study. LOCF, last observation carried forward; r-hGH, recombinant human growth hormone.
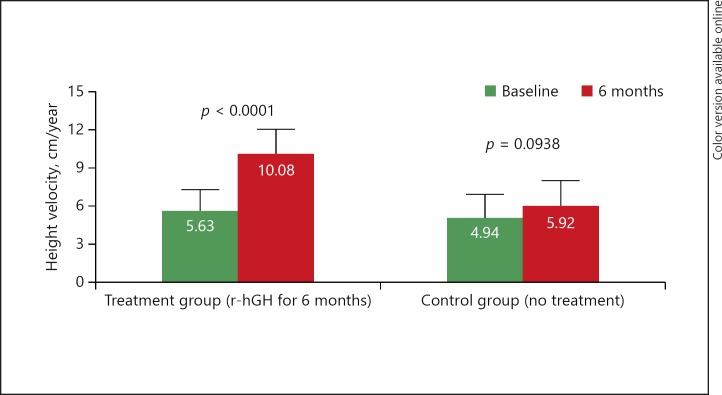
Primary Endpoint
The mean increase (±SD) in height velocity from baseline to 6 months was significant in the treatment group (from 5.63 cm/year [1.62] to 10.08 cm/year [1.92]; p < 0.0001) but not in the control group (from 4.94 cm/year [1.91] to 5.92 cm/year [2.01]; p = 0.0938). The between-group difference was 3.47 cm/year (95% confidence in terval [CI] 2.17–4.78; p < 0.0001 by ANCOVA); this between-group increase was clinically relevant (Fig. 3). Sensitivity analyses gave similar between-group differences to the LOCF analysis in both the ITT population without LOCF (3.75 cm/year, 95% CI 2.51–5.00; p < 0.0001 by ANCOVA) and in the per-protocol population (3.85 cm/year, 95% CI 2.58–5.12; p < 0.0001 by ANCOVA).
Fig. 3.
Change in height velocity at 6 months. Data are mean (SD); only positive SD are shown. p values are for within- group comparisons. Intention-to-treat with LOCF. Treatment group: n = 59 (1 patient was excluded from the baseline and change from baseline analyses owing to no height value recorded for 6 months prior to screening; this patient withdrew and there were no data after visit 1). Control group: n = 29 (6-month height measurements were missing for 3 children; these were replaced by measurements at withdrawal for 2 children; 1 child had no post-baseline measurement and, therefore, was not included in the analysis). LOCF, last observation carried forward; r-hGH, recombinant human growth hormone; SD, standard deviation.
Secondary Endpoints
Adherence was > 90% throughout the study (treatment group: 94.38% at 6 months and 93.27% at 12 months; control group: 95.69% at 12 months).
The mean increase (±SD) in height velocity from baseline at 12 months was 3.77 cm/year (2.21) in the children who had received r-hGH for 12 months (treatment group) and 2.86 cm/year (1.95) in the children who had received r-hGH for only the last 6 months of the study (control group). The between-group difference at 12 months was 0.96 cm/year (95% CI −0.04 to 1.97, p = 0.0598 by ANCOVA).
Children in the treatment group had a statistically and clinically greater increase in mean height compared with those in the control group at 6 months (difference 2.27 cm, 95% CI 1.83–2.71; p < 0.0001 by ANCOVA) and 12 months (difference 1.50 cm, 95% CI 0.87–2.13; p < 0.0001 by ANCOVA) (Fig. 4). The mean change in height SDS was also statistically and clinically greater in the treatment group than in the control group at 6 months (difference 0.51, 95% CI 0.42–0.60; p < 0.0001 by ANCOVA) and at 12 months (difference 0.33, 95% CI 0.21–0.45; p < 0.0001 by ANCOVA) (Fig. 4).
Fig. 4.
Change in height (a) and height SDS (b) at 6 and 12 months. Data are mean (SD); only positive SD are shown. Children completing the study at 12 months: treatment, n = 52; control, n = 27. Reasons for discontinuation in the treatment group: 5 parents/guardians withdrew consent; 1 child withdrew consent; 1 child had serious noncompliance with the study drug; 1 girl entered puberty (breast Tanner stage ≥2). Reasons for discontinuation in the control group: 2 parents/guardians withdrew consent; 1 child withdrew consent. SD, standard deviation; SDS, standard deviation score.
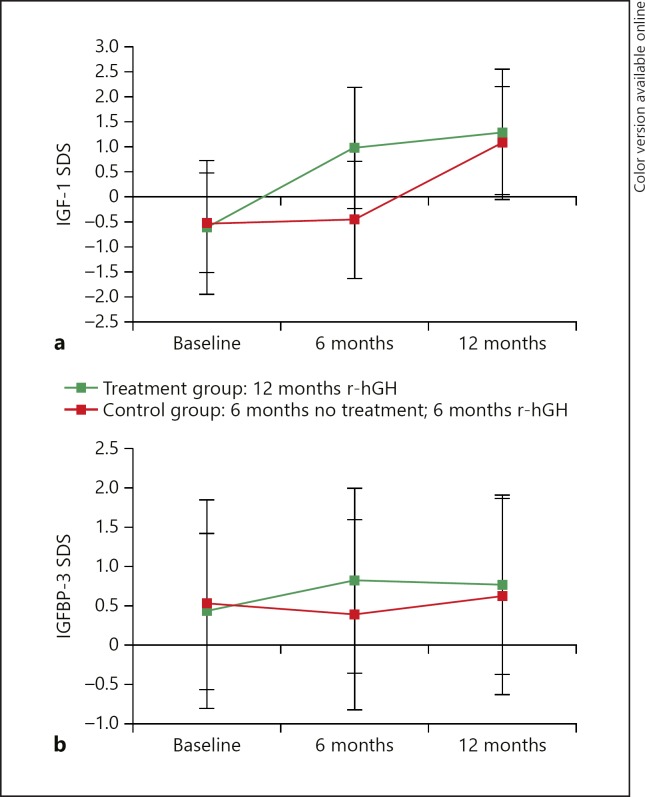
At baseline, there were no differences between groups in age-adjusted and sex-adjusted serum IGF-1 SDS (–0.6 ± 1.0 vs. −0.5 ± 1.3) and IGFBP-3 SDS (0.4 ± 0.6 vs. 0.5 ± 0.6) (Table 1). In the control group, mean IGF-1 SDS and mean IGFBP-3 SDS did not change during the first 6 months but increased after treatment with r-hGH started (IGF-1 SDS: from −0.5 ± 1.2 to 1.1 ± 1.3; IGFBP-3 SDS: from 0.4 ± 0.5 to 0.6 ± 0.5). In the treatment group, IGF-1 SDS increased to 1.0 ± 1.2 after 6 months and 1.3 ± 1.1 after 12 months, and IGFBP-3 SDS increased to 0.8 ± 0.5 after 6 months and 0.8 ± 0.5 after 12 months (Fig. 5). The mean between-group increase at 6 months, with baseline SDS (IGF-1 or IGFBP-3, as appropriate) and age as covariates, was significant for both biomarkers (p < 0.0001). Throughout r-hGH treatment, the mean IGF-1 SDS and IGFBP-3 SDS values were within the upper normal range. At 12 months, IGF-1 SDS was > 2 in 19 of 79 (24%) patients. At 12 months, IGF-1 SDS was > 3 in 4 of 79 (5%) patients, all of whom had IGFBP-3 SDS > 1.2.
Fig. 5.
Change in IGF-1 SDS (a) and IGFBP-3 SDS (b) from baseline at 6 months and 12 months. Data are mean (SD). Children completing the study at 12 months: treatment, n = 52; control, n = 27. Reasons for discontinuation in the treatment group: 5 parents/guardians withdrew consent, 1 child withdrew consent, 1 child had serious noncompliance with the study drug, and 1 girl entered puberty (breast Tanner stage ≥2). Reasons for discontinuation in the control group: 2 parents/guardians withdrew consent and 1 child withdrew consent. SD, standard deviation; SDS, standard deviation score.
In a post hoc analysis, no differences in BMI SDS were seen between the treatment and control groups at 6 months (least-squares mean difference between groups −0.15, 95% CI −0.42 to 0.12; p = 0.8513 by ANCOVA) or at 12 months (–0.15, 95% CI −0.39 to 0.09; p = 0.4854 by ANCOVA).
AEs were reported in 42 (71%) children (179 events) in the treatment group and 18 (60%) children (83 events) in the control group throughout the full 12-month period (Table 2). The most common AEs reported in both groups were nasopharyngitis (24 children [41%] in the treatment group and 9 children [30%] in the control group), upper respiratory tract infection (14 children [24%] in the treatment group and 9 children [30%] in the control group) and pyrexia (4 children [7%] in the treatment group and 3 children [10%] in the control group). Five serious AEs were reported for 3 children in the treatment group (1 chronic tonsillitis, 2 adenoidal hypertrophy, 1 tonsillar hypertrophy, and 1 croup infectious) and 3 serious AEs (1 chronic sinusitis, 1 pyrexia, and 1 T-cell lymphoma) were reported for 1 child in the control group. This child experienced T-cell lymphoma during the nontreatment period and had not been exposed to r-hGH during the whole study period. None of the serious AEs were considered by the study investigators to be treatment related.
Table 2.
Safety outcomes: treatment-emergent adverse events
| r-hGH 12 months (n = 59) |
Control (n = 30) |
|||
|---|---|---|---|---|
| with event, n (%) | events, n | with event, n (%) | events, n | |
| 12 months of treatment (baseline to last visit) | ||||
| Any TEAE | 42 (71.2) | 179 | 18 (60.0) | 83 |
| Any serious TEAE | 3 (5.1) | 5 | 1 (3.3) | 3 |
| Any treatment-related TEAE | 0 (0) | 0 | 1 (3.3) | 2 |
| Any treatment-related serious TEAE | 0 (0) | 0 | 0 (0) | 0 |
| Any TEAE leading to study treatment discontinuation | 0 (0) | 0 | 0 (0) | 0 |
| Any TEAE leading to death | 0 (0) | 0 | 0 (0) | 0 |
| 6 months of treatment (baseline to 6-month visit) | ||||
| Any TEAE | 38 (64.4) | 93 | 15 (50.0) | 42 |
| Any serious TEAE | 3 (5.1) | 5 | 1 (3.3) | 3 |
| Any treatment-related TEAE | 0 (0) | 0 | 0 (0) | 0 |
| Any treatment-related serious TEAE | 0 (0) | 0 | 0 (0) | 0 |
| Any TEAE leading to study treatment discontinuation | n/a | n/a | n/a | n/a |
| Any TEAE leading to death | 0 (0) | 0 | 0 (0) | 0 |
r-hGH, recombinant human growth hormone; TEAE, treatment-emergent adverse event; n/a, not applicable.
Two AEs (hypothyroidism and headache) in 1 child in the control group during the treatment period in the second 6 months of the study were possibly related to the study treatment. No clinically significant changes in laboratory values, physical examinations, or vital signs were observed and no deaths or AEs leading to treatment withdrawal were reported.
Discussion
The results reported in this publication are from the first randomised clinical study (SYNERGY) on the short-term height outcomes of r-hGH in prepubertal Korean children with ISS. Several studies have previously been conducted on r-hGH treatment in mixed populations of children with ISS across other regions [12]. However, only a few of these were randomised nontreatment controlled trials in prepubertal children that reported on short-term (up to 12 months) improvements in height outcomes, as are reported in this paper [15, 16, 17, 18]. Furthermore, the parallel group design and the large number of well-defined children reported here make this study one of only a few that provide an objective assessment of r-hGH treatment in a population that is representative of children with ISS. The population reported here will most probably comprise the target population for this indication.
The results of the SYNERGY study show that Korean children treated with r-hGH had clinically relevant and statistically significant increases in height velocity over 6 months, and continuous treatment led to clinically relevant growth enhancement for up to 12 months. The mean change in height SDS was statistically significant and clinically relevant at 6 months but not at 12 months, which is consistent with other clinical studies of r-hGH in children with ISS. The safety profile of r-hGH treatment in this new indication was comparable to the already approved indications. Although our data cannot be extrapolated to long-term height gains, the short-term responses to r-hGH in the first 6–12 months of treatment have correlated with long-term efficacy. This finding has been reported in prediction models based on large cohorts treated in standard clinical practice whose progress is followed in observational databases [13, 19, 20]. Furthermore, in one published randomised controlled trial of somatropin treatment versus nontreatment in a small cohort of short girls who were prepubertal at the start of treatment, favourable gains in near-final height were achieved over a mean period of 6.2 years of treatment, with no untoward effect on pubertal progression [21]. In accordance with these data, there is good reason to expect that the children treated with r-hGH in the SYNERGY study are likely to have a good chance of reaching an improved near-final height.
Despite the paucity of available diagnostic tests for ISS, height deficits will already be evident in children by the time they start school. This means that investigations and the commencement of treatment can usually be achieved at an early age, giving a child the best chance of achieving the most favourable height outcomes as an adult [4, 12, 22, 23, 24]. Older children, including those who have started pubertal development, may still also benefit from treatment with r-hGH. To remove the confounding effect of the pubertal growth spurt on the short-term growth outcomes, we included only younger children who had not entered puberty in the SYNERGY study. There are several published trials that report favourable final-height outcomes following treatment in pubertal or peripubertal children [21, 23, 25, 26]. Furthermore, although the children in the control group were still prepubescent during the second phase of the study, these children achieved a height velocity that was similar to that achieved in the treatment group after 6 months of treatment with r-hGH. This finding further supports the clinical relevance of the treatment effect and possibly provides evidence of catch-up growth response to r-hGH in older children.
Based on previously published data reporting on dose-dependent effects on first-year height velocity and subsequent gain in adult height [23, 24], a dose of 0.067 mg/kg for 6 days per week (0.4 mg/kg/week) was chosen as the dose most likely to give an optimum response over the initial 6 months. This dose is within the generally accepted range of doses used for the treatment of children who do not have GH deficiency [6].
After the start of high-dose r-hGH therapy, supra-normal IGF-1 concentrations (> 2 SDS) were observed in 24% of patients; this is comparable to the proportion of patients with supra-normal IGF-1 concentrations in other studies of high-dose r-hGH treatment [27]. Increased IGF-1 concentrations may be cause for concern, on the basis of epidemiological studies in healthy adults that showed a slight association between high IGF-1 and the development of some forms of cancer [28]. However, the relevance of such studies for paediatric patients treated with r-hGH has not been established, according to the consensus statement of paediatric societies [29].
There are some limitations that must be taken into account when interpreting the results of this study. In studies of children with ISS, the cohorts are commonly heterogeneous regarding the causes of short stature. Furthermore, treatment responses with r-hGH may fluctuate in clinical practice, even though the population reported here was considered to be representative of children with ISS who would receive treatment with r-hGH. Although this study was adequately powered, the population was still small. To present the full picture of the effect of treatment length and timing on the final height that is achieved in this population, a greater number of children would need to be treated with r-hGH and followed up for longer. Finally, although the trends reported here are in line with those reported for final height, the effect on final height after a longer duration of treatment has been reported for several studies [13, 21, 23, 25].
In conclusion, continuous treatment with r-hGH in the SYNERGY study demonstrated clinically relevant and statistically significant growth enhancement after 6 months, which was maintained over 12 months of continuous treatment. Despite a delayed start to treatment, children in the control group showed catch-up growth in response to 6 months of treatment that was comparable to that seen in the treatment group after the first 6 months of treatment. The safety profile for the SYNERGY study was consistent with the known profile of r-hGH and no new safety concerns were reported for the children with ISS treated with r-hGH.
Disclosure Statement
W.Y.C. has served as a consultant to LG. H.-W.Y. has served on advisory boards for Merck KGaA and Sanofi-Genzyme and has received speaker honoraria from Sanofi-Genzyme. J.S.H. has no disclosures to declare. C.W.K. has served as a consultant to LG, Dong-A, and Takeda and has received speaker honoraria from Novo Nordisk. H.-S.K. has served as a consultant to LG. D.-K.J. has no disclosures to declare. K.-H.L. has served as a consultant to LG and Novo Nordisk. H.-S.H. has served as a consultant to LG and Dong-A. B.-K.S. has served as a consultant to LG. At the time of the study, P.P. was an employee of Merck Ltd, Korea, an affiliate of Merck KGaA, Darmstadt, Germany.
Author Contributions
J.S.H. and B.-K.S. contributed to the development of the study concept, the design of the study, the interpretation of the data, and revision of the manuscript. W.Y.C., H.-W.Y., C.W.K., H.-S.K., D.-K.J., K.-H.L., H.-S.H., and P.P. contributed to the writing and review of the manuscript. All authors have read and approved the final version of the manuscript.
Acknowledgements
The authors would like to thank all the children and their parents and guardians who took part in the study. They would also like to thank all the investigators who contributed to the SYNERGY study. The authors would particularly like to thank Charmain Quigley (Sydney Children's Hospital, Sydney, Australia), Ekaterina Koledova (Merck KGaA, Darmstadt, Germany), and Werner Blum (Children's Hospital, University of Giessen, Giessen, Germany) for their contributions to the SYNERGY study.
The study was sponsored by Merck Ltd, Korea, an affiliate of Merck KGaA, Darmstadt, Germany. Medical writing support was provided by Steven Goodrick, inScience Communications, UK, funded by Merck KGaA, Darmstadt, Germany.
References
- 1.American Academy of Pediatrics Committee on Drugs and Committee on Bioethics Considerations related to the use of recombinant human growth hormone in children. Pediatrics. 1997 Jan;99((1)):122–9. doi: 10.1542/peds.99.1.122. [DOI] [PubMed] [Google Scholar]
- 2.Gharib H, Cook DM, Saenger PH, Bengtsson BA, Feld S, Nippoldt TB, et al. American Association of Clinical Endocrinologists Growth Hormone Task Force American Association of Clinical Endocrinologists medical guidelines for clinical practice for growth hormone use in adults and children—2003 update. Endocr Pract. 2003 Jan-Feb;9((1)):64–76. doi: 10.4158/EP.9.1.64. [DOI] [PubMed] [Google Scholar]
- 3.Growth Hormone Research Society; GH Research Society Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab. 2000 Nov;85((11)):3990–3. doi: 10.1210/jcem.85.11.6984. [DOI] [PubMed] [Google Scholar]
- 4.Wit JM, Clayton PE, Rogol AD, Savage MO, Saenger PH, Cohen P. Idiopathic short stature: definition, epidemiology, and diagnostic evaluation. Growth Horm IGF Res. 2008 Apr;18((2)):89–110. doi: 10.1016/j.ghir.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Savage MO, Burren CP, Rosenfeld RG. The continuum of growth hormone-IGF-I axis defects causing short stature: diagnostic and therapeutic challenges. Clin Endocrinol (Oxf) 2010 Jun;72((6)):721–8. doi: 10.1111/j.1365-2265.2009.03775.x. [DOI] [PubMed] [Google Scholar]
- 6.Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al. 2007 ISS Consensus Workshop participants Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008 Nov;93((11)):4210–7. doi: 10.1210/jc.2008-0509. [DOI] [PubMed] [Google Scholar]
- 7.Wit JM, Kamp GA, Rikken B. Spontaneous growth and response to growth hormone treatment in children with growth hormone deficiency and idiopathic short stature. Pediatr Res. 1996 Feb;39((2)):295–302. doi: 10.1203/00006450-199602000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Buchlis JG, Irizarry L, Crotzer BC, Shine BJ, Allen L, MacGillivray MH. Comparison of final heights of growth hormone-treated vs. untreated children with idiopathic growth failure. J Clin Endocrinol Metab. 1998 Apr;83((4)):1075–9. doi: 10.1210/jcem.83.4.4703. [DOI] [PubMed] [Google Scholar]
- 9.Sheiner E, Levy A, Katz M, Mazor M. Short stature—an independent risk factor for Cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2005 Jun;120((2)):175–8. doi: 10.1016/j.ejogrb.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Price DA. Spontaneous adult height in patients with idiopathic short stature. Horm Res. 1996;45(Suppl 2):59–63. doi: 10.1159/000184850. [DOI] [PubMed] [Google Scholar]
- 11.Magnusson PK, Gunnell D, Tynelius P, Davey Smith G, Rasmussen F. Strong inverse association between height and suicide in a large cohort of Swedish men: evidence of early life origins of suicidal behavior? Am J Psychiatry. 2005 Jul;162((7)):1373–5. doi: 10.1176/appi.ajp.162.7.1373. [DOI] [PubMed] [Google Scholar]
- 12.Quigley CA. Growth hormone treatment of non-growth hormone-deficient growth disorders. Endocrinol Metab Clin North Am. 2007 Mar;36((1)):131–86. doi: 10.1016/j.ecl.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Ranke MB, Lindberg A, Price DA, Darendeliler F, Albertsson-Wikland K, Wilton P, et al. KIGS International Board Age at growth hormone therapy start and first-year responsiveness to growth hormone are major determinants of height outcome in idiopathic short stature. Horm Res. 2007;68((2)):53–62. doi: 10.1159/000098707. [DOI] [PubMed] [Google Scholar]
- 14.Greulich WW, Pyle SI. ed 2nd Edition. Stanford CA: Stanford University Press; 1959. Radiographic Atlas of Skeletal Development of the Hand and Wrist. [Google Scholar]
- 15.Genentech Collaborative Study Group Idiopathic short stature: results of a one-year controlled study of human growth hormone treatment. J Pediatr. 1989 Nov;115((5 Pt 1)):713–9. doi: 10.1016/s0022-3476(89)80647-x. [DOI] [PubMed] [Google Scholar]
- 16.Cowell CT, Australasian Paediatric Endocrine Group Effects of growth hormone in short, slowly growing children without growth hormone deficiency. Acta Paediatr Scand Suppl. 1990;366(s366):29–30. doi: 10.1111/j.1651-2227.1990.tb11593.x. [DOI] [PubMed] [Google Scholar]
- 17.McCaughey ES, Mulligan J, Voss LD, Betts PR. Growth and metabolic consequences of growth hormone treatment in prepubertal short normal children. Arch Dis Child. 1994 Sep;71((3)):201–6. doi: 10.1136/adc.71.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soliman AT, abdul Khadir MM. Growth parameters and predictors of growth in short children with and without growth hormone (GH) deficiency treated with human GH: a randomized controlled study. J Trop Pediatr. 1996 Oct;42((5)):281–6. doi: 10.1093/tropej/42.5.281. [DOI] [PubMed] [Google Scholar]
- 19.Zucchini S. Growth hormone use in the treatment of idiopathic short stature. Curr Opin Investig Drugs. 2008 Apr;9((4)):396–401. [PubMed] [Google Scholar]
- 20.Bakker B, Frane J, Anhalt H, Lippe B, Rosenfeld RG. Height velocity targets from the national cooperative growth study for first-year growth hormone responses in short children. J Clin Endocrinol Metab. 2008 Feb;93((2)):352–7. doi: 10.1210/jc.2007-1581. [DOI] [PubMed] [Google Scholar]
- 21.McCaughey ES, Mulligan J, Voss LD, Betts PR. Randomised trial of growth hormone in short normal girls. Lancet. 1998 Mar;351((9107)):940–4. doi: 10.1016/S0140-6736(05)60604-6. [DOI] [PubMed] [Google Scholar]
- 22.Bryant J, Baxter L, Cave CB, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev. 2007 Jul;((3)):CD004440. doi: 10.1002/14651858.CD004440.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Albertsson-Wikland K, Aronson AS, Gustafsson J, Hagenäs L, Ivarsson SA, Jonsson B, et al. Dose-dependent effect of growth hormone on final height in children with short stature without growth hormone deficiency. J Clin Endocrinol Metab. 2008 Nov;93((11)):4342–50. doi: 10.1210/jc.2008-0707. [DOI] [PubMed] [Google Scholar]
- 24.Wit JM, Rekers-Mombarg LT, Cutler GB, Jr, Crowe B, Beck TJ, Roberts K, et al. Growth hormone (GH) treatment to final height in children with idiopathic short stature: evidence for a dose effect. J Pediatr. 2005 Jan;146((1)):45–53. doi: 10.1016/j.jpeds.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 25.Leschek EW, Rose SR, Yanovski JA, Troendle JF, Quigley CA, Chipman JJ, et al. National Institute of Child Health and Human Development-Eli Lilly & Co. Growth Hormone Collaborative Group Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004 Jul;89((7)):3140–8. doi: 10.1210/jc.2003-031457. [DOI] [PubMed] [Google Scholar]
- 26.Volta C, Bernasconi S, Tondi P, Salvioli V, Ghizzoni L, Baldini A, et al. Combined treatment with growth hormone and luteinizing hormone releasing hormone-analogue (LHRHa) of pubertal children with familial short stature. J Endocrinol Invest. 1993 Nov;16((10)):763–7. doi: 10.1007/BF03348921. [DOI] [PubMed] [Google Scholar]
- 27.Juul A, The role of insulin-like growth factors in growth hormone deficiency . In: Growth Hormone Therapy in Pediatrics: 20 Years of KIGS. Mb R, Da P, Eo R, editors. Basel: Karger Medical and Scientific; 2007. pp. pp. 70–82. [Google Scholar]
- 28.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004 Apr;363((9418)):1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 29.Allen DB, Backeljauw P, Bidlingmaier M, Biller BM, Boguszewski M, Burman P, et al. GH safety workshop position paper: a critical appraisal of recombinant human GH therapy in children and adults. Eur J Endocrinol. 2016 Feb;174((2)):1–9. doi: 10.1530/EJE-15-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chae HW, Suh I, Kwon AR, Kim YJ, Kim YH, Kang DR, et al. Longitudinal standards for height and height velocity in Korean children and adolescents: the Kangwha study. [corrected] J Korean Med Sci. 2013 Oct;28((10)):1512–7. doi: 10.3346/jkms.2013.28.10.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermanussen M, Cole J. The calculation of target height reconsidered. Horm Res. 2003;59((4)):180–3. doi: 10.1159/000069321. [DOI] [PubMed] [Google Scholar]