Abstract
Background/Aims
Microelectrode recording (MER)-guided deep brain stimulation (DBS) aims to place the DBS lead in the optimal electrophysiological target. When single-track MER or test stimulation yields suboptimal results, trajectory adjustments are made. The accuracy of these trajectory adjustments is unknown. Intraoperative computed tomography can visualize the microelectrode (ME) and verify ME adjustments. We aimed to determine the accuracy of ME movements in patients undergoing MER-guided DBS.
Methods
Coordinates following three methods of adjustment were compared: (1) those within the default “+” configuration of the ME holder; (2) those involving rotation of the default “+” to the “x” configuration; and (3) those involving head stage adjustments. Radial error and absolute differences between coordinates were determined.
Results
87 ME movements in 59 patients were analyzed. Median (IQR) radial error was 0.59 (0.64) mm. Median (IQR) absolute x and y coordinate errors were 0.29 (0.52) and 0.38 (0.44) mm, respectively. Errors were largest after rotating the multielectrode holder to its “x”-shaped setup.
Conclusion
ME trajectory adjustments can be made accurately. In a considerable number of cases, errors exceeding 1 mm were found. Adjustments from the “+” setup to the “x” setup are most prone to inaccuracies.
Keywords: Deep brain stimulation, Microelectrode recording, Intraoperative computed tomography, Microelectrode movement
Introduction
Deep brain stimulation (DBS) is an established and effective treatment for a variety of movement disorders and neuropsychiatric conditions [1, 2, 3, 4, 5]. Most centers perform the procedure in awake patients using a combination of preoperative imaging, a frame-based approach, microelectrode recording (MER) and/or test stimulation [6, 7]. Centers performing MER-guided DBS commonly use a BenGun multielectrode holder which allows up to 5 microelectrodes (ME) to be lowered to the target simultaneously [6, 8]. Some groups, such as ours, use the multielectrode holder but load only one channel at a time to minimize the number of parenchymal penetrations. When unsatisfactory MER signals or side effects at low intensity test stimulation warrant an additional MER track, trajectory adjustments are made in 1 of 3 ways: (1) adjustments with the multielectrode holder in its default “+” position, allowing for 2-mm adjustments in the x or y plane (anterior, posterior, medial or lateral adjustments); (2) rotating the holder around its central channel to the “x”-shaped configuration, allowing for 2-mm movements in the combined x-y plane (anteromedial, anterolateral, posteromedial or posterolateral adjustments) (Fig. 1) – both these methods allow the central guide tube to remain in place, which in effect “anchors” the brain; (3) repositioning the head stage; this requires removal of the guide tube which may be a source of targeting error [9]. ME trajectory adjustments are performed under the assumption that the ME travels in a straight trajectory and reaches the adjusted target as planned. However, this has been difficult to confirm, as intraoperative imaging techniques were lacking until recently [9, 10]. The objective of our current study is to determine whether ME trajectory adjustments result in the intended repositioning, using intraoperative CT (iCT). Moreover, this study compares the accuracy of three commonly performed methods of intraoperative ME trajectory adjustments.
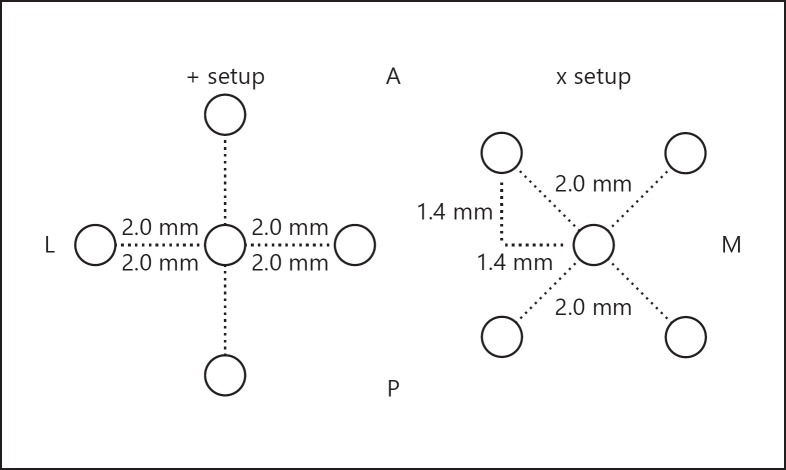
Fig. 1.
Multielectrode configurations. The figure illustrates the two BenGun configurations. The “+” setup allows for a 2-mm track adjustment in the x or y plane (anterior, posterior, medial or lateral). The “x” setup allows for a 1.4 mm adjustment in the combined x-y plane (anteromedial, anterolateral, posteromedial or posterolateral). The dotted lines represent the distance in millimeters from the central channel; this remains the same when rotating the holder. A, anterior; M, medial; P, posterior; L, lateral.
Methods
Patients
Data of patients who underwent MER-guided DBS for movement disorders between January 2014 and December 2016 were collected and reviewed. Included were consecutive patients who required more than one MER track and underwent iCT scans after consecutive tracks. This study was approved by the Rush University Medical Center institutional review board and in accordance with the 1964 Helsinki declaration and its later amendments. For this type of study formal consent is not required.
Surgical Procedure
MER-guided DBS was performed using a frame-based approach (Leksell, Elekta, Stockholm, Sweden) under local anesthesia. Preoperative MR images were fused with a stereotactic volumetric frame-based CT on the day of surgery using a Stealthstation S7 (Medtronic, Minneapolis, MN, USA) equipped with Framelink planning software (version 5.1, Medtronic, Minneapolis, MN, USA) and used for direct anatomical targeting. Surgery was started on the left side in bilateral cases, with patients in inclined position with the head of the bed at 30°. Following burr hole placement, fibrin glue was applied to prevent excessive cerebrospinal fluid loss. MER was performed by lowering a single ME (Alpha Omega, Nazareth, Israel) through the central channel of a multielectrode holder (BenGun, Alpha Omega, Nazareth, Israel) with 4 additional channels (anterior, posterior, medial and lateral) spaced 2 mm parallel to the central channel, in the “+” configuration. In case of a suboptimal MER track, a new trajectory was planned based on a combination of MER data, stimulation side effect profile and iCT images. Track corrections were made by choosing 1 of those 4 channels or by rotating the holder to the “x”-shaped setup, offering 4 additional options (anteromedial, anterolateral, posteromedial and posterolateral), 2 mm away from the central channel in the combined x-y plane. When rotating the holder from the “+” to the “x”-shaped configuration, the central guide tube was left in place, and a second guide tube was inserted in the desired (anteromedial, anterolateral, posteromedial or posterolateral) position – in effect “tethering” the brain in place. For any adjustment not achieved by using a different channel in the default position or by rotating the holder, the central guide tube was raised and a stage move performed after which the next ME track was made through the central channel. The guide tube is fixed to the head stage by tightening a screw, locking it into its desired configuration. iCT (O-arm technology, Medtronic, Minneapolis, MN, USA) was systematically performed at target depth after the initial MER track to visualize the ME. iCT was repeated if additional tracks required intraoperative verification, for instance when the electrophysiological data did not match results following test stimulation. After the optimal track had been determined based on neurophysiological data and test stimulation, the DBS electrode (Medtronic model 3389, Minneapolis, MN, USA) was implanted.
Intraoperative CT Parameters
The O-arm gantry was positioned concentrically with the patients’ head. Two preset positions were used during surgery. The ring was lowered and tilted towards the feet of the patient when a scan was performed. During the surgical procedure itself, the ring was positioned more vertically to allow unrestricted access for the surgical team. Each scan allowed for visualization of the cranial structures from the skull base to the vertex. Visualization of these cranial structures is important for accurate merging with preoperative CT and MR images. Fusion of the iCT with the preoperative images was visually inspected for accuracy. In case of an inadequate merge, iCT was repeated. Scan parameters were as follows: field of view 20 cm; scan range 15 cm; total slices, 192; slice thickness 0.78 mm; acquisition time 13 s.
ME Tip on iCT
iCT images were fused with preoperative MR images and with the frame-based volumetric CT scan using the planning software. The ME trajectory was delineated by setting an entry and target point along the ME artifact. The ME appears as a clear hyperdense artifact on CT images, surrounded by brain parenchyma and intracranial structures which are not clearly distinguishable on iCT [10]. The most caudal hyperintense aspect of the artifact on axial, coronal and sagittal images was localized, and the center of the artifact was selected to represent the tip of the ME. The center of the artifact was chosen because the tip of electrodes is conically shaped, smaller but not unlike DBS electrodes [11]. The ME tip was set as the target of the trajectory. The same method was used to select an entry point more cranial along the ME trajectory. The “trajectory view” setting was used after selecting an entry and target point to refine the selected trajectory and was particularly useful in refining the depth of the tip artifact.
Comparison between Intended and Visualized ME Position
Differences between the intended and visualized ME position were calculated using the radial error. The radial error defines the distance between the two trajectories along the same axial plane, parallel to the anterior commissure – posterior commissure plane. This is different from the absolute difference between two points in 3-dimensional space, the Euclidean distance, which also takes depth into account. Radial error was calculated using the following formula:
Coordinates of the intended tip location were calculated by adding/subtracting the chosen millimetric adjustment to the x and y coordinates of the previously visualized ME tip. Adjustments were grouped in those using the “+”-shaped setup, those using the rotated holder (or “x”-shaped setup) and those employing stage moves. A stage move is defined as any adjustment that cannot be achieved by using one of the predefined channels in the “+” or “x” setup and thus requires adjusting the head stage. Intended coordinates following adjustments were compared to the coordinates of the subsequently visualized ME tip. Tip coordinates were obtained using the planning software.
Outcome and Analysis
Radial error and absolute x and y coordinate errors between intended ME tip and visualized ME tip were calculated and compared. Subgroup analysis was performed to explore any target-specific differences. Kruskal-Wallis tests were performed to compare error differences among 3 methods of adjustment. Values are given as median (interquartile range, IQR). Statistical analysis was performed with SAS 9.1 (SAS Institute Inc., NC, USA). Statistical significance was defined as p < 0.05.
Results
Patients
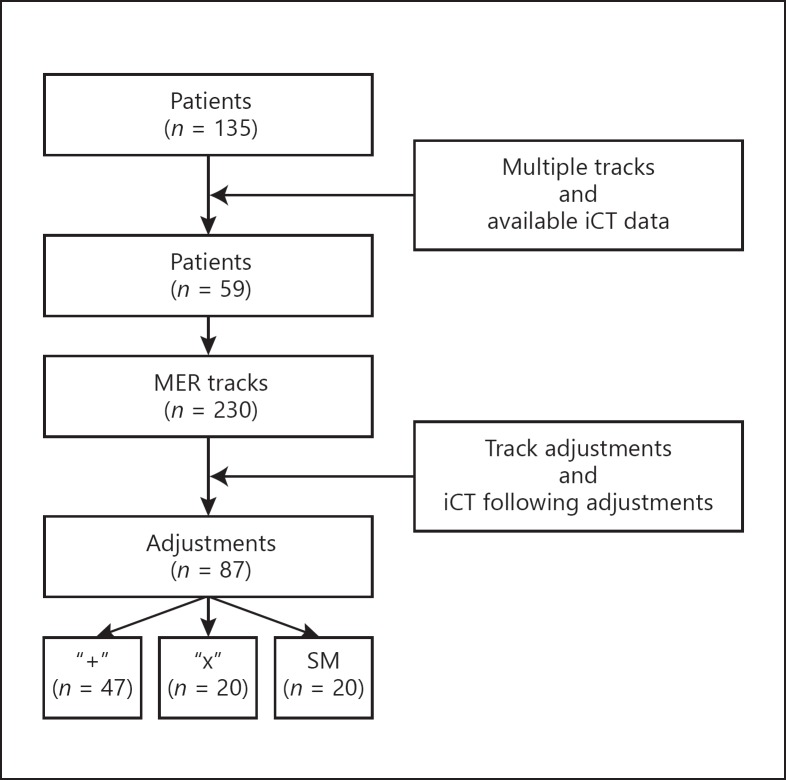
A total of 238 DBS electrodes were implanted in 135 patients. Of those patients, 59 required multiple MER tracks and had a sufficient amount of consecutive iCT images for subsequent analysis. A total of 230 MER tracks were performed. Not all procedures required MER track adjustments, and not all adjustments were monitored by iCT, leaving 87 track adjustments available for analysis. Of these 87 adjustments, 47 were performed by using the “+” setup, 20 by rotating to the “x” setup and 20 by moving the head stage. This is summarized in Figure 2. A total of 104 electrodes were implanted in these 59 patients. Fourteen patients underwent unilateral implantations. There were 38 male and 21 female patients. The subthalamic nucleus (STN) was targeted in 41 cases, the globus pallidus interna (GPi) in 6, the ventral intermediate thalamic nucleus (Vim) in 11 and the zona incerta (ZI) in 1. The ME tip artifact could be clearly delineated in all cases. Table 1 lists patient demographics.
Fig. 2.
Flowchart summarizing reported adjustments, illustrating how the number of analyzed adjustments was derived. Patients were included based on the availability of iCT data and if they had more than 1 track. The adjustments could only be analyzed if track adjustments were required, noted in patient charts, and if subsequent adjustments were monitored by iCT. SM, stage moves.
Table 1.
Patient demographics
| n (%) | |
|---|---|
| Patients | 59 |
| Male | 38 |
| Female | 21 |
| Age (range), years | 62 (27–77) |
| Disease | |
| PD | 45 (76) |
| Dys | 4 (7) |
| ET | 9 (15) |
| OST | 1 (2) |
| Target | |
| STN | 41 (69) |
| Gpi | 6 (10) |
| Vim | 11 (19) |
| ZI | 1 (2) |
| MER tracks | 230 |
| Left | 125 |
| Right | 105 |
| DBS leads | 104 |
| Left | 53 |
| Right | 51 |
PD, Parkinson's disease; Dys, dystonia; ET, essential tremor; OST, orthostatic tremor; STN; subthalamic nucleus; GPi, globus pallidus interna; Vim, ventral intermediate thalamic nucleus; ZI, zona incerta; MER, microelectrode recording; DBS, deep brain stimulation.
Comparing All Track Adjustments
The median (IQR) radial error for all 87 track adjustments was 0.59 (0.58) mm. Median (IQR) absolute x and y coordinate differences between intended and visualized ME tip coordinates were 0.29 (0.47) and 0.39 (0.43) mm, respectively. Twenty-one adjustments showed a radial error exceeding 1 mm, with a median (IQR) radial error of 1.42 (0.52) mm. Of these adjustments, 10 were associated with moves using the “+” configuration, 6 using the “x” setup and 5 following stage moves. Of tracks with a radial error exceeding 1 mm, median (IQR) errors of 0.96 (0.58) and 0.87 (0.79) were found between x and y coordinates, respectively. Figure 3 illustrates the radial difference between intended and actual ME trajectory adjustment in a selected case.
Fig. 3.
Difference between intended and actual ME movement. The difference is shown between the intended (indicated by the arrows) and the actual ME tip location (the more lateral and anterior trajectory in the figure) following a 2-mm anteromedial move after the first MER track, achieved by rotating from the “+” to the “x” setup. Both trajectories are projected on a 3-tesla susceptibility-weighted image. Target in this case was the STN. Intended track coordinates were −10.1 (x), −1.34 (y) and −4.16 (z). ME tip coordinates were −11 (x), 0.10 (y) and −4.16 (z). Both tracks were corrected for the intended surgical target depth, which was 4.16 mm below the anterior-posterior commissure plane (z coordinate). The radial error between intended and actual ME movement was 1.69 mm. The ME ended up 0.91 mm more lateral and 1.42 mm more anterior than intended.
Comparison between Methods of Adjustment
No statistically significant differences were found between errors comparing errors following the three methods of adjustment. The numerically largest errors were found after adjustments made using the “x”-shaped setup. Median (IQR) x coordinate, y coordinate and radial errors of 0.38 (0.55) mm, 0.51 (0.39) mm and 0.66 (0.72) mm were found following moves using the “x”-shaped setup. Table 2 illustrates radial and absolute coordinate differences for all methods of trajectory adjustment.
Table 2.
Errors compared following trajectory adjustments
| “+” setup | “x” setup | Stage move | P | |
|---|---|---|---|---|
| (n = 47) | (n = 20) | (n = 20) | value | |
| x coordinate | 0.29 (0.35) | 0.38 (0.55) | 0.16 (0.33) | 0.36 |
| y coordinate | 0.39 (0.42) | 0.51 (0.39) | 0.34 (0.51) | 0.69 |
| Radial error | 0.51 (0.52) | 0.66 (0.72) | 0.51 (0.73) | 0.61 |
This table illustrates the median (IQR) error following each method of adjustment, x and y coordinate errors are absolute errors. No statistically significant differences were found comparing x and y coordinate errors or radial errors. The largest numerical errors were found following adjustments made using the “x” setup.
Comparison between Targets
Of 87 total adjustments, 63 were made targeting the STN, 9 in the GPi, 13 in the Vim and 2 in the ZI. No statistically significant differences were found between targets when comparing radial error, x coordinate and y coordinate errors. The absolute x coordinate error was numerically largest in the STN (n = 63), with a median (IQR) error of 0.30 (0.55). The largest absolute y coordinate error and radial error were seen targeting the Vim, median (IQR) errors of 0.55 (0.24) mm and 0.69 (0.48) mm were recorded, respectively. Results are summarized in Table 3.
Table 3.
Errors compared in different targets
| GPi (n = 9) | STN (n = 63) | Vim (n = 13) | ZI (n = 2) | p value | |
|---|---|---|---|---|---|
| x coordinate | 0.29 (0.16) | 0.3 (0.55) | 0.19 (0.28) | 0.26 (0.4) | 0.92 |
| y coordinate | 0.21 (o.17) | 0.4 (0.5) | 0.55 (0.24) | 0.22 (0.24) | 0.22 |
| Radial error | 0.38 (0.15) | 0.60 (0.67) | 0.69 (0.48) | 0.34 (0.46) | 0.45 |
This table illustrates the median (IQR) error for each target. GPi, globus pallidus interna; STN, subthalamic nucleus; Vim, ventral intermediate thalamic nucleus; ZI, zona incerta. n refers to the number of adjustments made per target. x and y coordinate errors are absolute errors. No statistically significant differences were found comparing x and y coordinate errors or radial errors.
Comparing adjustments in STN cases (n = 63), we found no statistically significant differences between using the “+” setup (n = 31), the “x” setup (n = 15) and stage moves (n = 17). We did not have a sufficient number of GPi or Vim cases to perform a similar analysis. Results are summarized in Table 4.
Table 4.
Subgroup analysis of adjustments made targeting the STN
| “+” setup | “x” setup | Stage move | P | |
|---|---|---|---|---|
| (n = 31) | (n = 15) | (n = 17) | value | |
| x coordinate | 0.29 (0.42) | 0.51 (0.59) | 0.16 (0.35) | 0.52 |
| y coordinate | 0.42 (0.49) | 0.50 (0.45) | 0.35 (0.47) | 0.48 |
| Radial error | 0.51 (0.69) | 0.69 (0.67) | 0.52 (0.42) | 0.53 |
This table illustrates the median (IQR) errors found when targeting the subthalamic nucleus (STN) following adjustments using the “+” setup, after rotating the holder to its “x”-shaped setup or moving the head stage. n refers to the number of adjustments made per target. x and y coordinate errors are absolute errors. The largest numerical errors were found following moves performed by rotating the holder.
Discussion
This study reports on the accuracy of ME movements during MER-guided DBS for movement disorders. No statistically significant differences were found when comparing errors following all three methods of correction. In the majority of ME movements, 76% (66/87), errors were less than 1 mm, which suggests that ME movements can generally be made with a high degree of accuracy.
When comparing methods of adjustment, the largest median errors were found after adjustments from the “+” setup to the “x” setup. ME movements may have fallen into a previous trajectory in some cases, as they showed similar electrophysiological recordings to the previous track. A reason for this could be that the distance of the intended correction is simply too small relative to the diameter of the ME itself, making it more prone to fall into a previous trajectory [12].
Twenty-one out of 87 adjustments (24%) showed a radial error exceeding 1 mm. Overrepresentation of adjustments using the “+” setup in this group (n = 10) may be explained by the larger sample of these moves in our study. Limiting errors is important, as suboptimal adjustments potentially result in suboptimal recordings which would require additional passes. MER passes are not without risk, and multiple MER passes have been associated with an increased risk of intracranial hemorrhage [12, 13].
Literature Review
A previous study by Holloway and Docef [14] describes errors after movements using the “+”-shaped setup, with a mean Euclidean distance of 1.12 ± 0.74 mm. They also note a significant tendency for tracks to be more medial and superior than intended, and a nonsignificant trend for tracks to be more posterior than intended. We found a radial error after adjustments using the “+” configuration (n = 47) of 0.51 (0.52) mm. We also found a tendency for the ME to be more medial and posterior than intended, though the differences in the mediolateral (median 0.06, IQR 0.76 mm) and anteroposterior (0.17, IQR 0.57 mm) plane were so small they can hardly be considered clinically relevant. Differences between both our studies are to be expected, as different methods to quantify error were used. Holloway and Docef present their results in Euclidean distances, whereas we corrected tracks for planned depth allowing for assessment of the radial distance between the coordinates. This explains the higher reported error in their study as their group takes depth differences into account as well. We consider the radial error to be more relevant in our case, as we were specifically interested in the accuracy of ME adjustments made in the x and y planes. Holloway and Docef did not review movements following rotation of the holder or stage moves, as this was not their primary objective. A review of the literature did not find any reports on accuracy of adjustments made by rotating the holder from the “+” to the “x” configuration or following stage moves.
Strengths and Limitations
Accurate comparison between the intended ME and the actual ME position following adjustment is largely dependent on accuracy of (intraoperative) imaging and fusion using the planning software. Merging of intraoperative CT images with the preoperative stereotactic CT and MR images is not without error, which is inherent to the use of the coregistration software. A previous report by Mirzadeh et al. [15] validates CT-MRI fusion. Their group compared leads visualized on fused images to postoperative MRI and concluded that measurement errors could be attributed to differences in lead measurement on postoperative MRI, which they attributed to the larger, less well-defined MRI artifact. Barnaure et al. [16], comparing fused images to final leads on postoperative MR images, reported mean differences of 0.17–0.97 mm. Both groups did not focus on ME visualization but showed that iCT and CT-MRI fusion is an accurate method of lead (artifact) visualization. To limit potential fusion error, image fusion was visually inspected for each scan consistently. However, it remains difficult to determine the impact of these (inherent) errors on our results. Another source of error is the O-arm itself, which has a reported measurement error of 0.70 ± 0.38 mm, an error that was found after comparing lead visualization on the O-arm with visualization on postoperative CT [14]. It should be noted that the O-arm, a flat panel cone beam scanner, is not a “true” intraoperative CT scanner as it uses fluoroscopy and 3-dimensionally reconstructs the image. The O-arm, however, is perfectly suited to visualize lead artifacts, as previously reported by Holloway and Docef [14], Smith and Bakay [9] and Shahlaie et al. [17]. Our selective use of iCT introduces bias, as we only monitored ME tracks when there were discrepancies between recorded electrophysiology and side effect profile. Performing iCT after every MER track would have limited this. However, as those scans would not have had any clinical consequences and would have introduced additional and unnecessary radiation exposure, this would raise considerable ethical concerns. Brain shift during surgery is also a factor to consider and has been shown to alter intracranial anatomy and influence DBS placement accuracy as it tends to move the brain caudally and posteriorly [18, 19, 20]. Standard surgical precautions, described in detail in the Methods section, were taken following burr hole placement, but a certain degree of brain shift is unavoidable. As we measured radial error and corrected for depth (z coordinate), errors introduced by potential shift in the z plane are limited.
Strengths of this study include the largest sample size to date and the direct comparison of three different and widely used methods of intraoperative ME trajectory adjustment. To the best of our knowledge, this is the first direct comparison of these three methods. Our results are applicable to other indications for DBS implantations using the same multielectrode holder and confirm that in most cases the intended ME adjustment is achieved. Confirming accuracy of these trajectory adjustment methods may prove to be of continued value when “asleep” DBS procedures become more common practice, as intraoperative DBS lead corrections may be necessary due to unexpected targeting errors. Without electrophysiological recording and subsequent review of side effect profile thresholds, the accuracy of intraoperative DBS lead adjustments is crucial. While this study provides a framework for the accuracy of the most commonly used correction methods, our results are not directly applicable to DBS lead corrections as dimensions between ME and DBS leads differ.
Conclusion
ME trajectory adjustments can be made accurately, with median errors less than 1 mm in most cases following all three reviewed methods. However, in a considerable amount of cases errors exceeding 1 mm were found. When considering ME movements, inaccuracies may result in an unintended mismatch between assumed location, obtained recordings and side effect thresholds. Adjustments from the “+” setup to the “x” setup seem most prone to these inaccuracies.
Disclosure Statement
L.V.M. has received fees for consulting activities, advisory boards and educational activities from Medtronic Inc., Boston Scientific, St. Jude Medical, AbbVie, Britannia Pharmaceuticals Ltd. and US WorldMeds LLC. Rush is supported by the Parkinson Foundation. S.S. has received fees for consulting activities and educational activities from Medtronic Inc. and Globus Medical Inc. S.B. received financial support from the Vreedefonds Foundation, Parkinson's Foundation of the Netherlands and the Amsterdam Foundation for Promoting Neurosurgical Development.
Funding Sources
No funding was received for this research.
References
- 1.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 2.Volkmann J, Mueller J, Deuschl G, Kühn AA, Krauss JK, Poewe W, et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol. 2014;13:875–884. doi: 10.1016/S1474-4422(14)70143-7. [DOI] [PubMed] [Google Scholar]
- 3.Schuurman PR, Bosch DA, Merkus MP, Speelman JD. Long-term follow-up of thalamic stimulation versus thalamotomy for tremor suppression. Mov Disord. 2008;23:1146–1153. doi: 10.1002/mds.22059. [DOI] [PubMed] [Google Scholar]
- 4.Goodman WK, Alterman RL. Deep brain stimulation for intractable psychiatric disorders. Annu Rev Med. 2012;63:511–524. doi: 10.1146/annurev-med-052209-100401. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168:502–510. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 6.Gross RE, Krack P, Rodriguez-Oroz MC, Rezai AR, Benabid A-L. Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson's disease and tremor. Mov Disord. 2006;21((suppl 14)):S259–S283. doi: 10.1002/mds.20960. [DOI] [PubMed] [Google Scholar]
- 7.Machado A, Rezai AR, Kopell BH, Gross RE, Sharan AD, Benabid A-L. Deep brain stimulation for Parkinson's disease: surgical technique and perioperative management. Mov Disord. 2006;21((suppl 14)):S247–S258. doi: 10.1002/mds.20959. [DOI] [PubMed] [Google Scholar]
- 8.Benabid AL, Koudsie A, Benazzouz A, Vercueil L, Fraix V, Chabardes S, et al. Deep brain stimulation of the corpus luysi (subthalamic nucleus) and other targets in Parkinson's disease. Extension to new indications such as dystonia and epilepsy. J Neurol. 2001;248((suppl)):III37–III47. doi: 10.1007/pl00007825. [DOI] [PubMed] [Google Scholar]
- 9.Smith AP, Bakay RAE. Frameless deep brain stimulation using intraoperative O-arm technology. Clinical article. J Neurosurg. 2011;115:301–309. doi: 10.3171/2011.3.JNS101642. [DOI] [PubMed] [Google Scholar]
- 10.Gupta R, Cheung AC, Bartling SH, Lisauskas J, Grasruck M, Leidecker C, et al. Flat-panel volume CT: fundamental principles, technology, and applications. Radiographics. 2008;28:2009–2022. doi: 10.1148/rg.287085004. [DOI] [PubMed] [Google Scholar]
- 11.Pinto S, Le Bas JF, Castana L, Krack P, Pollak P, Benabid AL. Comparison of two techniques to postoperatively localize the electrode contacts used for subthalamic nucleus stimulation. Neurosurgery. 2007;60((suppl 2)):285–294. doi: 10.1227/01.NEU.0000255353.64077.A8. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Haim S, Asaad WF, Gale JT, Eskandar EN. Risk factors for hemorrhage during microelectrode-guided deep brain stimulation and the introduction of an improved microelectrode design. Neurosurgery. 2009;64:754–762. doi: 10.1227/01.NEU.0000339173.77240.34. [DOI] [PubMed] [Google Scholar]
- 13.Xiaowu H, Xiufeng J, Xiaoping Z, Bin H, Laixing W, Yiqun C, et al. Risks of intracranial hemorrhage in patients with Parkinson's disease receiving deep brain stimulation and ablation. Parkinsonism Relat Disord. 2010;16:96–100. doi: 10.1016/j.parkreldis.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Holloway K, Docef A. A quantitative assessment of the accuracy and reliability of O-arm images for deep brain stimulation surgery. Neurosurgery. 2013;72:47–57. doi: 10.1227/NEU.0b013e318273a090. [DOI] [PubMed] [Google Scholar]
- 15.Mirzadeh Z, Chapple K, Lambert M, Dhall R, Ponce FA. Validation of CT-MRI fusion for intraoperative assessment of stereotactic accuracy in DBS surgery. Mov Disord. 2014;29:1788–1795. doi: 10.1002/mds.26056. [DOI] [PubMed] [Google Scholar]
- 16.Barnaure I, Pollak P, Momjian S, Horvath J, Lovblad KO, Boëx C, et al. Evaluation of electrode position in deep brain stimulation by image fusion (MRI and CT) Neuroradiology. 2015;57:903–908. doi: 10.1007/s00234-015-1547-z. [DOI] [PubMed] [Google Scholar]
- 17.Shahlaie K, Larson PS, Starr PA. Intraoperative computed tomography for deep brain stimulation surgery: technique and accuracy assessment. Neurosurgery. 2011;68((suppl 1)):114–124. doi: 10.1227/NEU.0b013e31820781bc. [DOI] [PubMed] [Google Scholar]
- 18.Khan MF, Mewes K, Gross RE, Škrinjar O. Assessment of brain shift related to deep brain stimulation surgery. Stereotact Funct Neurosurg. 2007;86:44–53. doi: 10.1159/000108588. [DOI] [PubMed] [Google Scholar]
- 19.Miyagi Y, Shima F, Sasaki T. Brain shift: an error factor during implantation of deep brain stimulation electrodes. J Neurosurg. 2007;107:989–997. doi: 10.3171/JNS-07/11/0989. [DOI] [PubMed] [Google Scholar]
- 20.Van Den Munckhof P, Contarino MF, Bour LJ, Speelman JD, De Bie RMA, Schuurman PR. Postoperative curving and upward displacement of deep brain stimulation electrodes caused by brain shift. Neurosurgery. 2010;67:49–53. doi: 10.1227/01.NEU.0000370597.44524.6D. [DOI] [PubMed] [Google Scholar]