Abstract
Objective:
ADAMTS13 cleaves von Willebrand factor (VWF). This process is essential for hemostasis. Severe deficiency of plasma ADAMTS13 activity, most commonly resulting from autoantibodies against ADAMTS13, causes thrombotic thrombocytopenic purpura (TTP). Therapeutic plasma exchange is the standard of care to date, which removes autoantibodies and replenishes ADAMTS13. However, such a therapy is often ineffective to raise plasma ADAMTS13 activity and in-hospital mortality rate remains as high as 20%.
Approach and results:
To overcome the inhibition by autoantibodies, we developed a novel approach by delivering recombinant ADAMTS13 (rADAMTS13) using platelets as vehicles. We show that both human and murine platelets can uptake rADAMTS13 ex vivo. The endocytosed rADAMTS13 within platelets remains intact, active, and is stored in α-granules. Under arterial shear (100 dyne/cm2), the rADAMTS13 in platelets is released and effectively inhibits platelet adhesion and aggregation on a collagen-coated surface in a concentration-dependent manner. Transfusion of rADAMTS13-loaded platelets into Adamts13−/− mice dramatically reduces the rate of thrombus formation in the mesenteric arterioles after FeCl3 injury. An ex vivo transfusion of rADAMTS13-loaded platelets to a reconstituted “whole” blood containing plasma from a patient with immune-mediated TTP and the cellular components (e.g. erythrocytes and leukocytes) from a healthy individual, as well as a fresh whole blood obtained from a patient with congenital or immune-mediated TTP also dramatically reduces the rate of thrombus formation under arterial flow.
Conclusion:
Our results demonstrate that transfusion of rADAMTS13-loaded platelets may be a novel and potentially effective therapeutic approach for arterial thrombosis, including congenital and immune-mediated TTP.
Keywords: Platelet transfusion, recombinant ADAMTS13, endocytosis, von Willebrand factor, thrombosis, thrombotic thrombocytopenic purpura
Graphical Abstract
Recombinant ADAMTS13 in platelets is protected from being targeted by circulating IgG autoantibodies against ADAMTS13 in patients with immune-mediated thrombotic thrombocytopenic purpura (iTTP). At the site of vascular injury, quiescent platelets can be activated by arterial shear, inflammatory cytokines, and thrombin. The activated platelets release their granular contents, including stored recombinant ADAMTS13, which then binds and cleaves ultra large VWF multimers and/or VWF/platelet aggregates under flow. This results in the dissociation of VWF/platelet aggregates and inhibition of thrombus formation in microvasculature, the pathognomonic process of iTTP.
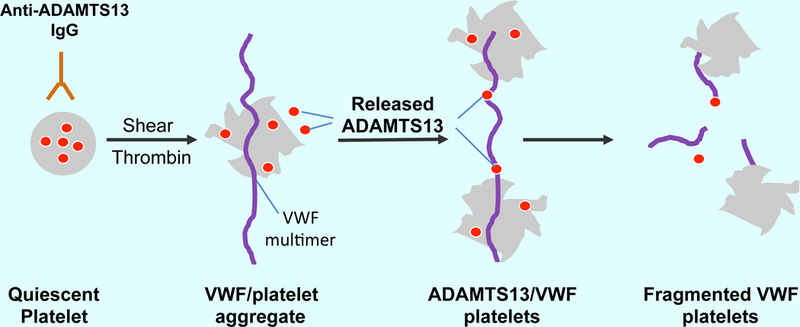
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare but potentially life-threatening clotting disorder 1. It is characterized by severe thrombocytopenia and microangiopathic hemolytic anemia with varying degrees of damage to the brain, heart, pancreas, kidneys, and adrenal glands, etc. 2, 3. There are two main types of TTP: hereditary or congenital TTP (cTTP) and acquired or immune-mediated TTP (iTTP). cTTP is caused by mutations in ADAMTS13 gene 4, 5, while iTTP is primarily caused by acquired autoantibodies targeting plasma ADAMTS13 protein 6, 7. The mechanisms of cTTP 1, 4 and iTTP 8, 9 have been extensively studied in the past decade.
In most cases of cTTP, mutations in ADAMTS13 result in secretion defect of ADAMTS13 protein 10, thus little to no ADAMTS13 protein and proteolytic activity can be detected in patients’ plasma. However, in iTTP an immunoglobulin G (IgG) type autoantibody binds ADAMTS13 protein 8, 11, particularly to the spacer domain, a region critical for recognition and proteolysis of von Willebrand factor (VWF) 12, 13. Modifications to the ADAMTS13 spacer domain are shown to weaken or eliminate the autoantibody-binding sites while preserving ADAMTS13 activity 14. Such antibody-resistant ADAMTS13 variants might be useful for therapy of iTTP. However, only 80–85% of patients may be benefited from such a strategy, since 15–20% patients harbor autoantibodies that still recognize the antibody-resistant ADAMTS13 variants 14. Other autoantibody-bypassing strategies, such as anti-VWF aptamer 15, 16, anti-VWF nanobody caplacizumab 17–19, and anti-glycoprotein 1b snake venom anfibatide 20–22, and N-acetylcysteine 23, 24, have been tested for treatments for TTP with some success. However, none of these strategies would have addressed the underlying mechanism of lacking ADAMTS13.
In our previous study, we developed a transgenic mouse line in which a human recombinant ADAMTS13 (rADAMTS13) was expressed exclusively in platelets in the Adamts13−/− background 25. Platelet-expressed rADAMTS13 was efficacious in blocking arterial thrombosis and preventing a murine model of TTP induced by shigatoxin-2 and recombinant VWF25. To translate these proof-of-concept findings into patient care, we sought to determine whether platelets could be loaded with rADAMTS13 ex vivo after collection and whether transfusion of rADAMTS13-loaded platelets would be as efficacious as the transgenic platelets expressing high levels of rADAMTS13 for inhibiting arterial thrombosis under flow in human whole blood under flow and in vivo in a mouse model.
Here, we show that human platelets are able to endocytose rADAMTS13 in a time-, concentration- and temperature-dependent manner; the endocytosed rADAMTS13 in platelets remains intact, proteolytically active, and releasable under arterial shear. Addition of rADAMTS13-loaded platelets to normal, TTP patient or reconstituted TTP blood inhibits thrombus formation under arterial flow. Finally, transfusion of rADAMTS13-loaded murine platelets into Adamts13−/− mice also dramatically inhibits thrombus formation in the mesenteric arterioles after oxidative injury. Our findings demonstrate that transfusion of rADAMTS13-loaded platelets may be developed as a novel therapeutic strategy for arterial thrombosis, including both cTTP and iTTP.
Data available on request from the corresponding authors
Materials and Methods
Isolation of human platelets for uptake of rADAMTS13
The Institutional Review Board (IRB) and the Institutional Animal Care and Use Committee (IACUC) of the University of Alabama at Birmingham have approved the studies involving human and animal, respectively. Venous blood was collected from healthy donors, cTTP, and iTTP patients for the study after informed consent. Criteria for diagnosing cTTP and iTTP include: severe thrombocytopenia, microangiopathic hemolytic anemia and various signs and symptoms of organ damage as previously described26, 27. No evidence of cancer, hematopoietic progenitor transplantation, sepsis and disseminated intravascular coagulation is present. Plasma ADAMTS13 activity <10 U/dL without (cTTP) or with (iTTP) inhibitors or anti-ADAMTS13 IgG.
10 mL whole blood was collected from each individual and anticoagulated with 10 μM of thrombin inhibitor D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK) (Sigma-Aldrich). The anticoagulated blood was then centrifuged at 150 ×g for 15 minutes to separate large blood cells (erythrocytes and leukocytes) from small cells (platelets). A Tyrode’s buffer (10 mM HEPES, pH 7.4 containing 134 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, 12 mM NaHCO3 and 0.34 mM Na2HPO4) was added to platelet-rich plasma (PRP) and centrifuged for additional 10 minutes at 900 ×g. The platelet pellet was re-suspended in the Tyrode’s buffer before addition of rADAMTS13 at various concentrations. The rADAMTS13 was expressed using HEK293 cells and was purified to homogeneity as previously described 28, 29.
Isolation of murine platelets for uptake of rADAMTS13
Similarly, mouse blood (0.7–1.0 mL) was obtained through cardiac puncture after anesthesia with ketamine/xylazine cocktail and anti-coagulated with 100 μM of PPACK. One μM of prostaglandin E1 (Sigma-Aldrich) and 0.1 U/mL of apyrase (Sigma-Aldrich) were added to prevent platelet activation. The anticoagulated blood was then centrifuged at 100 ×g for 10 min to obtain PRP, then centrifuged again at 400 ×g for 10 minutes to obtain platelet pellets. The platelet pellets were re-suspended in Tyrode’s buffer containing glucose (0.1%, wt/vol), bovine serum albumin (0.35%, wt/vol), and magnesium (1 mM). The platelets were incubated with human rADAMTS13 at various concentrations at 25°C for 120 minutes.
Following incubation of rADAMTS13, both human and mouse platelets were washed twice with 1 mL of Tyrode’s buffer. Platelets were then used for the microfluidic assays or lysed with 20 mM HEPES and 150 mM NaCl, pH 7.4, containing 1% Triton X-100 to quantify levels of rADAMTS13 protein and activity in platelets using a fully automated Western blotting system (WES) (ProteinSimple) and in-house fluorescent resonance energy transfer (FRET)-rVWF73 assay 30, 31, respectively.
Western blotting using the WES system
Platelet lysates, prepared as stated in the section above, were used immediately for Western blotting using the automated WES system. This size-based capillary electrophoresis system allowed for clean and quantitative results of protein signals. Two antibodies, a polyclonal antibody against human ADAMTS13, raised against human (80%) and murine (20%) recombinant ADAMTS13 (F481) 32 and a secondary anti-rabbit IgG peroxidase conjugated (ProteinSimple) were used.
Assay for platelet ADAMTS13 activity
The proteolytic activity of rADAMTS13 in platelet lysate was determined by FRET-rVWF73 assay as described previously 30, 31. Normal human plasma (CryoCheck) was used for calibration, defined as having 1 U/mL of ADAMTS13 activity.
Immunofluorescent staining and confocal microscopy
Platelets were spread on a fibrinogen-coated surface and then fixed with ice cold 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 minutes. The platelets were permeabilized with 0.1% Triton X-100 and incubated with a mouse anti-ADAMTS13 IgG (clone360) (1:50) (Green Mountain Antibodies) conjugated with Alexa488, a rabbit anti-VWF IgG (1:100) conjugated with Alexa594 (Life Technologies). The fluorescent images were obtained using a Nikon A1 confocal fluorescence microscope.
Platelet adhesion and aggregation on a collagen surface under flow
BioFlux microfluidic channels (Fluxion Bioscience) were coated with a fibrillar collagen (100 μg/mL) (Chrono-Log) in 0.01 N of hydrochloric acid under flow (shear rate of 500s−1) for 10 minutes. The surface was blocked with 0.5% bovine serum albumin in PBS. The platelets were labeled with fluorescein isothiocyanate (FITC) conjugated anti-human (1:10) or anti-murine (1:100) CD41 antibody for 15 minutes, then added into whole blood samples from a healthy donor, TTP patients, and Adamts13−/− mice. The native or reconstituted whole blood samples were then perfused (100 dyne/cm2) over the collagen-coated surfaces for 3 minutes. The time-lapse digital images were collected every 3 seconds for a total of 3 minutes. The relative fluorescent intensity or platelet coverage was determined offline using Montage software and data were analyzed using GraphPad Prism7 software 33.
For high resolution imaging, unbound platelets, leukocytes, and red blood cells were washed off from the channels with PBS for 10 min. The platelets adhered to the collagen surface were then fixed with an ice-cold paraformaldehyde (4%) in PBS for 10 min. After being extensively washed and blocked with 2% BSA in PBS, the platelets were incubated with a FITC-conjugated monoclonal anti-CD41 or glycoprotein IIb (GP-IIb) IgG (ThermoFisher Scientific) and an Alexa594-conjugated rabbit anti-VWF IgG (in-house conjugated with reagents from ThermoFisher Scientific). Images were obtained with Nikon A1 confocal fluorescence microscope (Nikon).
Intravital microscopic imaging of mesenteric arterial thrombosis in mice
Mice (~4 weeks) were anesthetized with intraperitoneal injection of ketamine/xylazine cocktail. 100 μL of rhodamine-6G (200 μg/mL) in PBS was infused via retro orbital sinus plexus. Mesenteric vessels were exposed to 10% (wt/vol) FeCl3 for 3 minutes. A Nikon fluorescent microscope equipped with a high-resolution digital camera determined the accumulation of fluorescent platelets and leukocytes in injured vessels. The time lapse was set for a total of 30 minutes, with acquisition of an image every 10 seconds. The relative fluorescent intensity was determined off-line, using the NIS-Elements software (Melville)25.
Statistical analysis:
Mann-Whitney test were used for unpaired non-parametric data when two groups were compared. Kruskal-Wallis test were used for comparing data of three or more groups with non-parametric data. All graphs and statistical analysis were performed using Prism 7 software (GraphPad).
Results
Human and mouse platelets uptake rADAMTS13 ex vivo in a time, temperature, and concentration-dependent manner
Normal human platelets are known to contain trace amounts of ADAMTS13, which may be synthesized in megakaryocytes or endocytosed from plasma 34. To determine whether platelets were able to uptake rADAMTS13 ex vivo, human platelets were isolated, washed, and incubated with varying concentrations of rADAMTS13 (0, 5, 20, 40, and 80 μg/mL) at three different temperatures (4, 25, and 37°C) for 0–120 minutes. After unbound rADAMTS13 was removed by gentle washing, remaining platelets (less than 50% of initial number of platelets) were either lysed or fixed for Western blotting or proteolytic activity or fluorescent confocal microscopy. Fig. 1 showed that at low temperature (4°C) there was no rADATMS13 detected inside and/or on human platelets even after 2 hours of incubation at the highest concentration of 80 μg/mL; at 25°C and 37°C, a concentration-dependent increase of rADAMTS13 protein signal and activity in platelet lysates was detected. Interestingly, there was no statistically significant difference in the amount of rADAMTS13 protein in platelets after being incubated with rADAMTS13 at 25°C compared to incubation at 37°C. Surprisingly, even at much lower concentrations (5–10 μg/mL), platelets could endocytose detectable amount of rADAMTS13 (Fig. S1). Together, these results suggest that rADAMTS13 can be sufficiently loaded into platelets ex vivo at temperatures where platelets are normally stored prior to transfusion.
Fig. 1. Platelets uptake rADAMTS13 ex vivo.
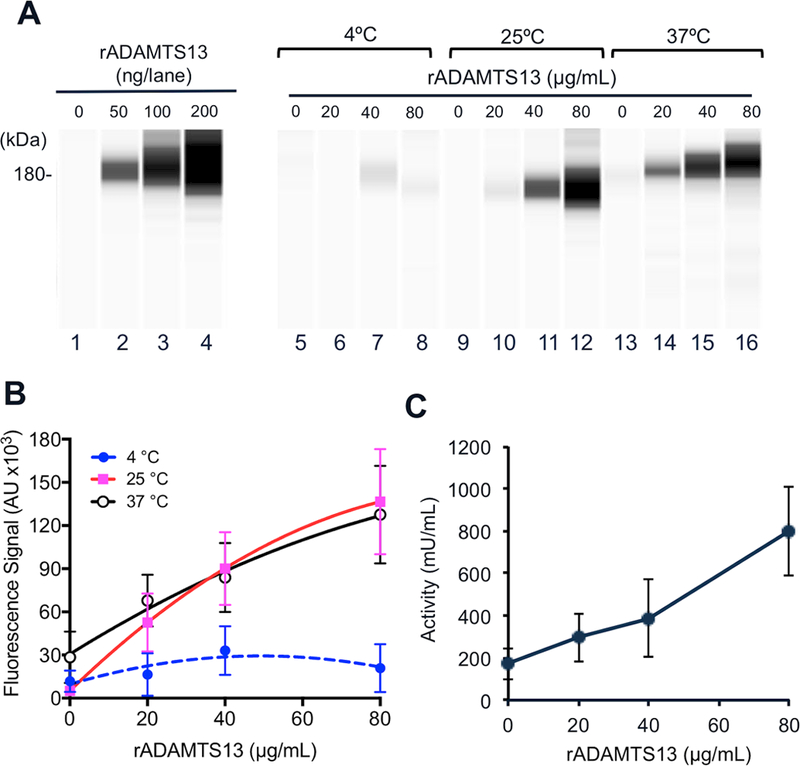
A. Representative Western blotting images demonstrate a full-length rADAMTS13 protein in platelet lysate after isolated human platelets were incubated with increasing concentrations of rADAMTS13 (0, 20, 40, and 80 μg/mL) at 4°C, 25°C, and 37°C for 2 hours. A calibration standard with increasing amounts of rADAMTS13 (0, 50, 100, and 200 ng) per lane is also shown on the left panel. A rabbit anti-human ADAMTS13 IgG, followed by a peroxidase-conjugated anti-rabbit IgG, and chemiluminescent reagents, detected the rADAMTS13 signals using the fully automated capillary WES system. B. Quantitation of rADAMTS13 signals (arbitrary unit) in platelet lysates as a function of increasing concentrations of rADAMTS13 added during the incubation at various temperatures. C. Proteolytic activity determine by FRETS-VWF73 assay in platelet lysates after platelets were incubated with an increasing concentration of rADAMTS13 at 37°C. The data in panels B and C were the means and standard errors of the means (SEM) from three independent experiments.
Localization of rADAMTS13 in human platelets
To assess where rADAMTS13 endocytosed was stored in platelets, we performed immunofluorescent analysis after fixation. A positive signal of rADAMTS13 was only detected in human platelets that were incubated with rADAMTS13 (5–20 μg/mL) for 2 hours (Fig. 2A and G), but not in those incubated with buffer alone (Fig. 2B). As expected, all platelets were stained positive for VWF (Fig. 2C, D, and H), which served as an internal control. The merged images of multiple platelets at the low magnification (20×) (Fig. 2E & F) or a single platelet at the high magnification (100×) (Fig. 2G, H, and I) revealed punctuated staining patterns of both rADAMTS13 and VWF. The blue staining is F-actin. These results indicate that rADAMTS13 and VWF are at least partially co-localized to the platelet α-granules.
Fig. 2. Immunofluorescent microscopy demonstrates the co-localization of rADAMTS13 with VWF in human platelets.
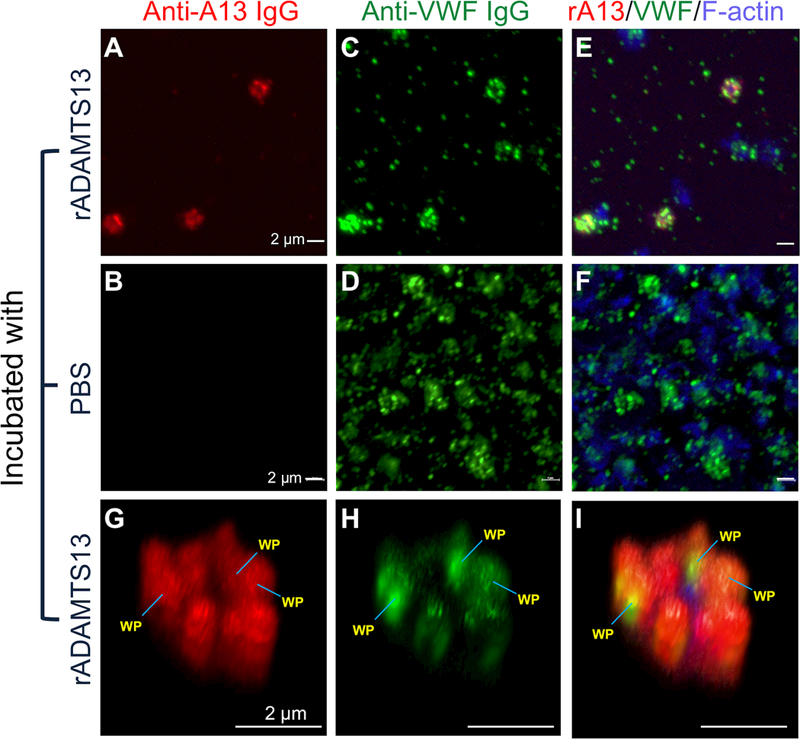
Isolated and washed human platelets were incubated with rADAMTS13 (20 μg/mL) at 37°C for 2 hours and fixed with 4% paraformaldehyde in PBS for 10 min. Alexa594-conjugated (red) monoclonal anti-ADAMTS13 IgG (A, B, and G) and Alexa488-conjugated (green) rabbit anti-VWF IgG (C, D, and H) were used to determine the presence of rADAMTS13 and VWF, respectively. Combined images including rADAMTS13, VWF, and F-actin (stained with Alexa350-conjugated actin binding protein phalloidin) are shown in E, F, and I. The images in A-F were obtained by confocal microscope under a magnification of 20×, while 3D images in panels (G, H, and I) were obtained using the same confocal microscope under a magnification of 100×. WP indicates the Weibel-Palade bodies. The bars represent 2 μm in length.
rADAMTS13 in platelets is releasable, which inhibits platelet/VWF aggregates in normal “whole” blood under flow
To determine whether the rADAMTS13 endocytosed in platelets is able to be released and cleave VWF/platelet aggregates under flow, a microfluidic assay was performed. Platelets were incubated with an increasing concentration of rADAMTS13 (0, 5, and 10 μg/mL). A reconstituted “whole” blood sample was created by mixing the rADAMTS13-loaded platelets after washing with a Tyrode’s buffer and washed red/white blood cells from healthy individuals. Due to the removal of plasma ADAMTS13 and VWF during the washing step, purified VWF (10 μg/mL) was added to restore normal VWF function in the reconstituted “whole” blood. Platelets incubated with a Tyrode’s buffer alone were used as a control. The reconstituted “whole” blood, including control platelets and rADAMTS13-loaded platelets, was then flowed under shear (100 dyne/cm2) through the microfluidic channels coated with a fibrillar collagen.
As shown, the rate of accumulation (Fig. 3A & 3B) and the final surface coverage (Fig. 3C & 3D) of fluorescent platelets on the collagen-coated surface were dramatically reduced in the channel where rADAMTS13-loaded platelets were included compared with the control (with same number of platelets containing no rADAMTS13). Such an anti-thrombotic effect of rADAMTS13-loaded platelets was dependent on the concentration rADAMTS13 used during platelet uptake: the more rADAMTS13 was used for uptake, the better anti-thrombotic efficacy of these platelets was (Fig. 3). These results indicate that rADAMTS13 endocytosed by platelets is functional and able to be released to cleave VWF and VWF-platelet aggregates under arterial shear.
Fig. 3. The rADAMTS13-loaded platelets exhibit an anti-thrombotic activity under flow in a whole blood from a healthy donor.

Isolated and washed platelets were incubated with rADAMTS13 at 0, 5, and 10 μg/mL at 37°C for 2 hours. The residual rADAMTS13 was removed by centrifugation and three washes. The rADAMTS13-loaded platelets were then added back to the washed cellular components including RBC and WBC with supplementation of VWF (10 μg/mL). The washes removed all plasma ADAMTS13. The reconstituted whole blood with 150×103/μL of platelets containing rADAMTS13 (0, 5, and 10 μg/mL) was perfused over a fibrillar collagen-coated surface under arterial shear (100 dyne/cm2) for 3 min. The rate of accumulation (A and B) and the final coverage (C and D) of fluorescently labeled platelets on a collagen-coated surface were determined using the BioFlux microfluidic system. The data shown are the means and standard errors of the means (SEM) (n=3).
rADAMTS13 in platelets is efficacious in inhibiting thrombus formation under flow in TTP patients’ blood
To assess whether transfusion of rADAMTS13-loaded platelets would be efficacious in treating TTP, we preloaded platelets with rADAMTS13 (0, 5, and 10 μg/mL) on the day of admission of a TTP patient as described in the Method. Whole blood was collected from a patient with cTTP or iTTP (Table S1) prior to the initiation of plasma therapy and anti-coagulated with the thrombin inhibitor PPACK (10 μg/mL). As shown, when added to the whole blood from a patient with cTTP (Fig. 4A-D) or two iTTP patients (Fig. 4E-H), washed rADAMTS13-loaded platelets (5 and 10 μg/mL) exhibited anti-thrombotic effect under arterial flow. In both cases, the rate of accumulation (Fig. 4A & 4B) and the final coverage (Fig. 4C & 4D) of fluorescently-labelled platelets on the collagen-coated surface was significantly inhibited compared with those with the control platelets.
Fig. 4. The rADAMTS13-loaded platelets can dramatically inhibit thrombus formation in TTP patients’ whole blood under flow.
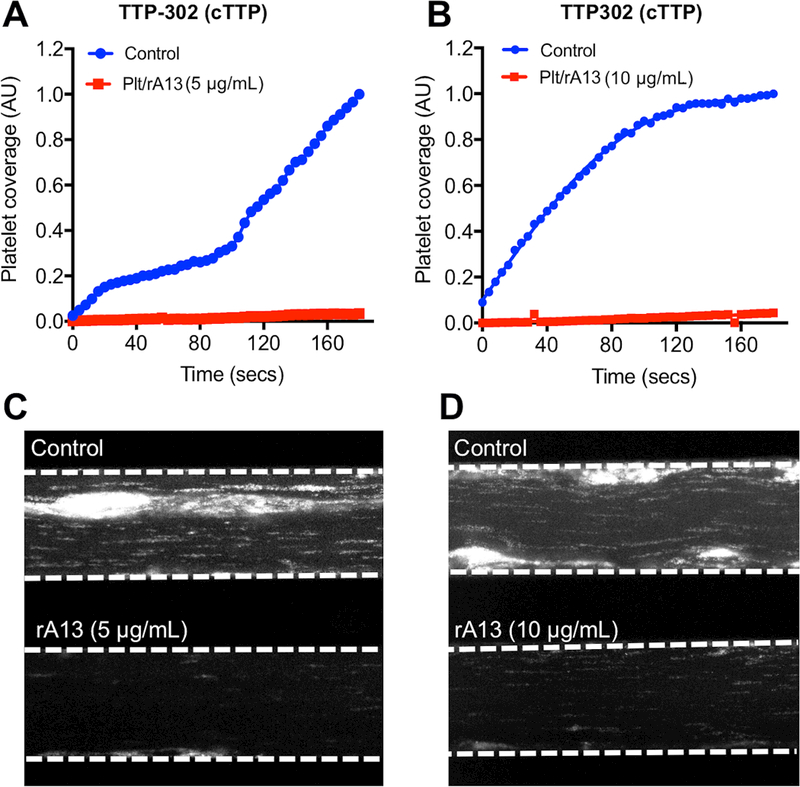
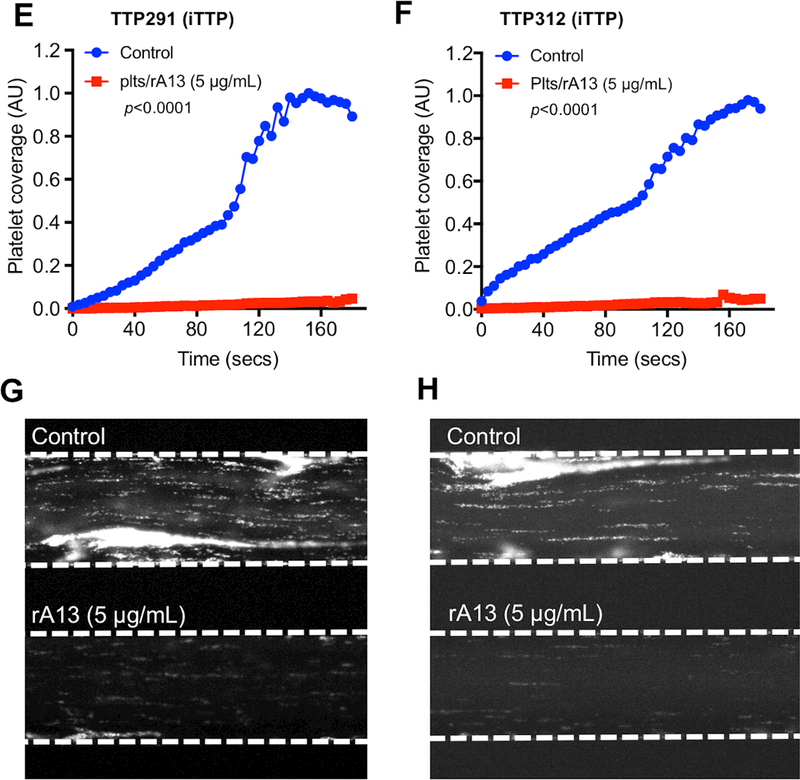
The rADAMTS13-loaded or the control platelets were added to a PPACK-anticoagulated fresh whole blood sample obtained from a patient (#302) with congenital TTP (plasma ADAMTS13 activity was <5% and no inhibitor or anti-ADAMTS13 IgG was present) (A-D) and two separate patients (#291 and #312) with immune-mediated TTP (E-H). The reconstituted whole blood was flowed over a fibrillar collagen-coated surface under arterial shear (100 dyne/cm2) for three minutes. The rate of accumulation (A & B, E & F) and the final surface coverage (C & D, G & H) of fluorescently-labeled platelets were determined under an inverted microscope. The rates of platelet accumulation as a function of time in panels A, B, E and F were plotted using Prism 7 software.
Also, the elongated VWF/platelet strings and large aggregates were detected on the collagen-coated surface in the controls, but not in those supplemented with rADAMTS13-loaded platelets (estimated ~0.05 ng of rADAMTS13/106 platelets) (Fig. 4C, D, G, and H). However, there was no significant dose-dependency regarding the rADAMTS13 used for platelet preparation (Fig. 4A and 4B), suggesting that only a small amount of rADAMTS13 within platelets is required for such an anti-thrombotic activity under flow. Together, our results demonstrate that rADAMTS13-loaded platelets are efficacious for inhibiting arterial thrombosis under flow in patients with either cTTP or iTTP.
rADAMTS13 in platelets is efficacious in inhibiting thrombus formation under flow in a reconstituted “whole” blood containing various titers of autoantibodies
To demonstrate the variability and efficacies of rADAMTS13-loaded platelets for inhibiting thrombus formation in iTTP patients, we took advantage of the Alabama TTP Patient Cohort, a large collection of iTTP patient plasma samples with various titers of inhibitors (Table S1). Normal human platelets pre-loaded with rADAMTS13 (0, 5, and 10 μg/mL) were added to a reconstituted “whole” blood containing plasma from an iTTP patient and washed cellular components (primarily erythrocytes and leukocytes) from a healthy donor in the presence of 10 μg/mL of purified VWF. The control contained normal human platelets that were not loaded with rADAMTS13. The “whole” blood samples were then flown over the fibrillar collagen-coated surface under arterial shear (100 dyne/cm2). The rates of accumulation and the final surface coverage of fluorescently labeled platelets were determined using the microfluidic system. As shown, the addition of rADAMTS13-loaded platelets (5 and 10 μg/mL) dramatically reduced the rates and final surface coverage when compared to the controls (Fig. 5A-D). Each experiment was repeated four times using four different patient plasma samples and four different healthy donor blood cells for reconstitution; the means and standard errors of the means were determined (Fig. 5A & 5C). These results suggest that the anti-thrombotic effect of rADAMTS13-loaded platelets under arterial shear is highly reproducible.
Fig. 5. The rADAMTS13-loaded platelets inhibit arterial thrombosis under flow in reconstituted “whole” blood consisting of iTTP patient plasma and normal RBC and WBC.
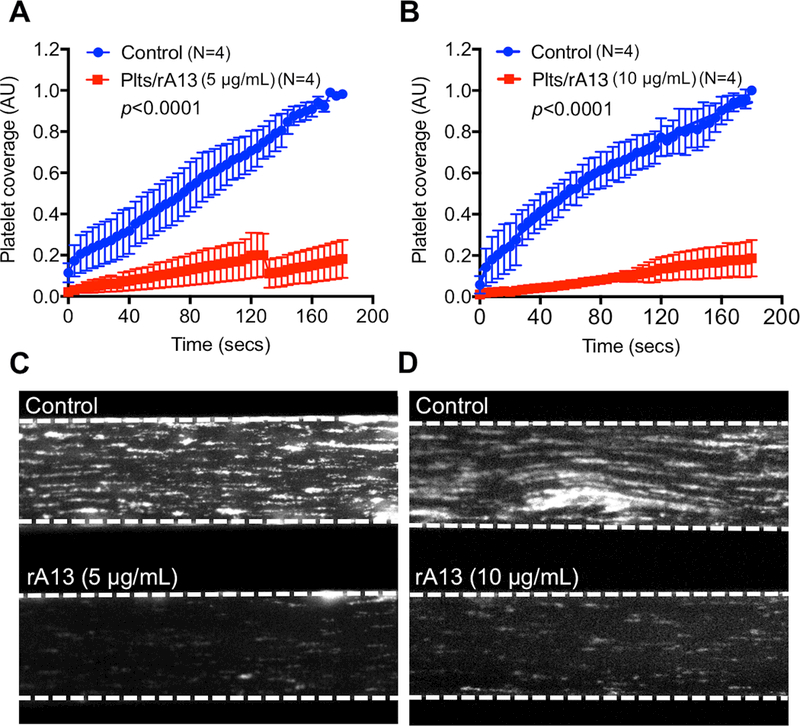
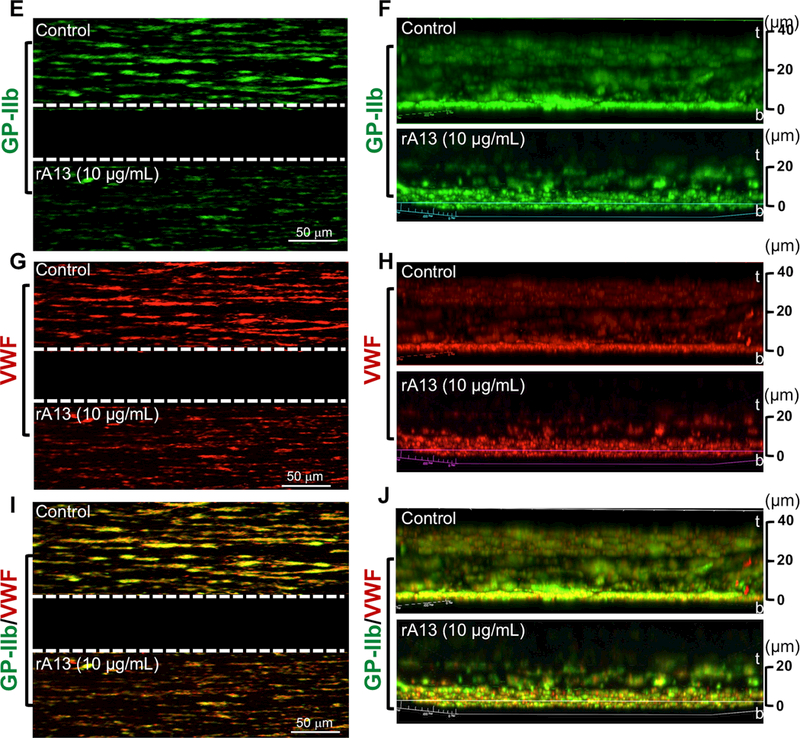
rADAMTS13-loaded platelets (5 and 10 μg/mL) were added to a reconstituted “whole” blood consisting of iTTP patient plasma with various titers of inhibitor and washed cellular components (RBC and WBC) from a healthy donor with an addition of purified VWF (10 μg/mL). The reconstituted “whole” blood samples with 150×103/μL of platelets (control platelets vs. rADAMTS13-loaded platelets) were perfused over a fibrillar collagen-coated surface under arterial shear (100 dyne/cm2) for 3 minutes. The rates of accumulation (A & B) and the final coverage (C & D) of fluorescently labeled platelets on the collagen-coated surfaces were determined using the microfluidic system. The data shown are the means ± SEM of 4 independent experiments. Immunofluorescent staining with FITC-conjugated monoclonal anti-CD41 IgG (GP-IIb) (green) (E & F) and alexa594-conjugated rabbit anti-VWF IgG (red) (G & H) was performed after fixation of the control and experimental channels with 4% paraformaldehyde in PBS. Merged confocal images of platelet GP1b and VWF staining are shown in I and J. Z-stacked images show the thickness of platelet accumulation stained by anti-CD41 (green) (F), VWF deposition (H), and co-localization of platelets and VWF (J) in the control (top) and experimental (bottom) channels. Here, t and b indicate top and bottom of the channel, respectively.
Immunohistochemistry and confocal analyses demonstrated the reduction of platelet adhesion and aggregation (Fig. 5E) corresponded to the reduction of VWF accumulation (Fig. 5F) in the channels containing rADAMTS13-loaded platelets when compared with those in the controls. Z-stack confocal image analysis revealed that the mean thicknesses of platelet aggregates (Fig. 5H) and VWF accumulation (Fig. 5I) in the channel treated with control platelets (~45 μm) was significantly greater than those in the channel treated with rADAMTS13-platelets (~24 μm). One could better appreciate more for the differences between the control and the experimental group in the merged confocal images of platelet/VWF staining (Fig. 5G & 5J). These results further support the hypothesis that rADAMTS13-loaded platelets are able to release rADAMTS13 at the site of injury, which inhibits the thrombus growth under flow despite the presence of circulating antibodies against ADAMTS13.
rADAMTS13 in murine platelets inhibits thrombosis in a mesenteric arteriole of Adamts13−/− mice after FeCl3 injury
To determine if platelets loaded with rADAMTS13 was able to inhibit arterial thrombosis in vivo, murine platelets were isolated from Adamts13−/− mice and incubated at 37°C for 1 hour with rADAMTS13 (0, 5, and 10 μg/mL) in Tyrode’s buffer. We also isolated platelets from transgenic mice expressing rADAMTS13 exclusively in platelets as a positive control and platelets from Adamts13−/− mice as a negative control. We then transfused platelets that lack ADAMTS13 (KO-Plts), platelets pre-loaded with rADAMTS13 (5 μg/mL) (rA13-Plts) or transgenic platelets containing rADAMTS13 (TG-Plts) into Adamts13−/− mice via the retro orbital plexus. Injury was induced using 10% FeCl3 to the mesenteric arterioles 2 min after platelet transfusion and real time images were obtained under an inverted fluorescent microscope.
As shown, transfusion of rA13-Plts or TG-Plts at the concentration of 10% of total platelets (~60×103/μL, final), resulted in a dramatic reduction in the rates of platelet adhesion and aggregation (i.e. thrombus formation) at the site of injury (Fig. 6A-C). As a control, transfusion of KO-Plts, which contained no rADAMTS13, had no effect (Fig. 6B & C). Within 12 minutes, the rate of thrombus formation in this group reached a plateau (Fig. 6B & C), similar to what we observed in Adamts13−/− mice without being transfused (not shown). The rate of thrombus formation in the mesenteric arterioles of mice that received rA13-Plts or TG-Plts was parallel to that observed in wild type (WT) mice that contained plasma ADAMTS13 (~1 μg/mL) (Fig. 6B and 6C). The estimated concentrations of platelet-delivered rADAMTS13 in murine whole blood and plasma were ~50 ng/mL and ~100 ng/mL, respectively, which is at least 20-folds less than the concentrations of soluble ADAMTS13 plasma of normal mice. However, a direct comparison of the anti-thrombotic efficacy between platelet-delivered rADAMTS13 and soluble rADAMTS13 in cTTP and iTTP patients’ blood should be performed in our future studies. Nevertheless, these results demonstrate that transfusion of rADAMTS13-loaded platelets may be efficacious for inhibiting arterial thrombosis under (patho) physiological conditions.
Fig. 6. The rADAMTS13-loaded murine platelets inhibit the rate of thrombus formation in mesenteric arterioles of Adamts13−/− mice after FeCl3 injury.
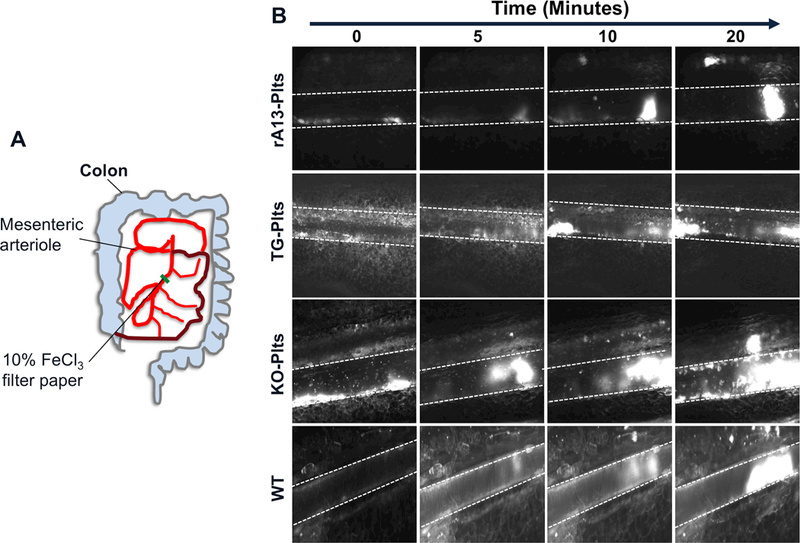
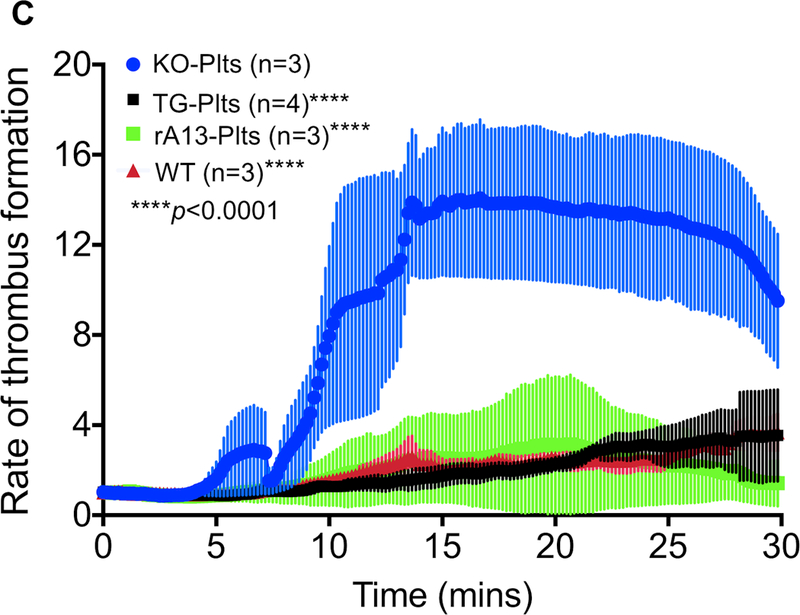
A. Schematic representation of mesenteric arteriole network and the position where a piece of filter paper with 10% FeCl3 (wt./vol.) was placed. B. The means and standard errors of the means (SEM) of the rates of thrombus formation as a function of time in each group were quantified off-line using the NIS Elements software and plotted using the Prism 7 software. C. Time lapses demonstrate the rates of thrombus formation induced by 10% FeCl3 injury to the mesenteric arterioles in Adamts13−/− mice after being transfused with 10% of rADAMTS13-loaded platelets (rA13-Plts), transgenic platelets (TG-Plts) expressing rADAMTS13, and Adamts13−/− platelets (KO-Plts) or in wild type mice (WT). All platelets (both infused and endogenous) were labeled with a rhodamine-6G. Representative digital images at 0, 5, 10, and 20 minutes in four different experimental groups are shown.
Discussion
In the present study, we demonstrate that human and murine platelets are able to endocytose rADAMTS13 in vitro at room temperature. This uptaking condition is compatible with that used for storing platelet products prior to transfusion in the blood bank. The endocytosed rADAMTS13 protease within platelets remains intact, proteolytically active, and is stored in α-granules. The rADAMTS13-loaded platelets show an anti-thrombotic activity ex vivo under arterial shear and in vivo in the absence or presence of autoantibodies against ADAMTS13. These findings support a working hypothesis that transfusion of rADAMTS13-loaded platelets may be therapeutically efficacious for inhibiting arterial thrombosis, such as in patients with TTP and other types of pathological thrombosis associated with an absolute or relative deficiency of ADAMTS13 activity.
Platelets are the first cells to arrive at the site of injury, and are known to participate in many physiological and pathophysiological processes, including inflammation, wound healing, atherosclerosis, antimicrobial host defense, angiogenesis, and protection against malignancy in addition to hemostasis and thrombosis 35. Each platelet contains 50–80 α-granules filled with a variety of proteins, including those synthesized in megakaryocytes (e.g., VWF, P-selectin, integrin αIIbβ3, and growth factors) and those endocytosed through cell surface receptors from plasma environments (e.g., fibrinogen, fibronectin, and factor V) 35, 36. Therefore, platelets are considered to be an excellent vehicle to store and deliver a therapeutic agent (or protein) for treatments of various disorders including bleeding diathesis, thrombotic disorders, and malignancy.
Previous studies have evaluated the therapeutic efficacy of platelet-delivered hemostatic agents by transgenic expression of such proteins under a platelet-specific promoter in megakaryocytes, which directs the storage of a therapeutic protein to the α-granules 37–39. For instance, an ectopic expression of factor VIII (FVIII) or factor IX (FIX) in platelets results in improvement of clinical phenotype of murine model of hemophilia A 40, 41 or hemophilia B42. Transplantation of hematopoietic progenitor cells transduced with a lentiviral vector carrying a therapeutic gene such as FVIII or FIX under platelet-specific promoter was also effective. Platelet-delivered FVIII is more effective than an equal amount of infused FVIII in the presence of inhibitors 40, 41. However, such a similar protective role for platelet-delivered FIX was only observed without an inhibitor, but not in mice with anti-FIX inhibitors 42.
In our study, we demonstrate a dramatic anti-thrombotic effect of platelet-delivered rADAMTS13 ex vivo using a microfluidic system, which mimics the formation of shear-dependent and platelet/VWF-rich thrombi inside small arterioles; additionally, we show that platelet-delivered rADAMTS13 inhibits thrombus formation in Adamts13−/− mice. Such an effect is sustained in a “whole” blood reconstituted and from a real TTP patient with or without anti-ADAMTS13 inhibitors. While only small amount of rADAMTS13 protease may be released from pre-loaded platelets, this amount appears to affect the formation of ultra-long VWF strings or to inhibit the formation of large and thick VWF-platelet aggregates on the collagen coated surface under flow. These results are consistent with those we have previously reported in transgenic mice, in which the ectopically expressed rADAMTS13 in platelets is highly efficacious for inhibiting the rate of thrombus formation in the mesenteric arterioles of mice after FeCl3 injury, and protecting mice from developing a “TTP-like” syndrome after shigatoxin-2 or recombinant VWF challenge 25.
Our current study has not only confirmed and extended our previous proof-of-concept findings in transgenic mice, but also demonstrated that transfusion of a small number of rADAMTS13-loaded platelets (~10% of total number of platelets) into a recipient that lacks circulating ADAMTS13, such as in Adamts13−/− mice or in patients with cTTP or iTTP, may be also efficacious for inhibiting microvascular thrombosis. Such an anti-thrombotic effect is reproducible under the conditions used, and even observed after the rADATMS13-loaded platelets are stored at 4°C for more than 24 hours (data not shown).
Our findings offer the possibility of translating this novel approach into patient care once rADAMTS13 receives the approval from Food and Drug Administration of the United States of America for therapy of cTTP 43. A clinical trial will be designed to test the efficacy of transfusion of rADAMTS13-loaded platelets for the treatment of both cTTP and iTTP. This can be accomplished by adding rADAMTS13 into the single donor platelets at the concentration of 5–10 μg/mL using an aseptic technique. After several hours of incubation, the rADAMTS13-loaded platelets will be ready for transfusion into patients.
In addition to the data presented, we are able to show that detectable amounts of rADAMTS13 can be endocytosed in the presence of normal plasma proteins in vivo in Adamts13−/− mice (Fig. S2). We anticipate that platelet-delivered rADAMTS13 may be more efficacious than plasma rADAMTS13 for both cTTP and iTTP because rADAMTS13 can be delivered to the site of thrombus formation at a more concentrated fashion and is likely shielded from inhibition by anti-ADAMTS13 antibodies. Additionally, the half-life of rADAMTS13 inside platelets may be much longer than that in solution, which is typically 2–3 days in the absence of inhibitors 43, 44, but can be dramatically reduced due to the rapid clearance of immune complexes 45, 46. This may avoid the need for daily plasma exchange as the standard of care for now for iTTP.
Platelet transfusions are currently avoided in patients with TTP due to a perceived risk for exacerbating thrombosis according to anecdotal reports 47, 48. However, a large cohort study has demonstrated that TTP patients who have received platelet transfusion do not exhibit an increased morbidity or mortality, nor has it increased thrombotic events when compared with those who do not 49, 50. Our in vitro experiments with reconstituted blood or real-time iTTP patient whole blood samples, as well as in vivo thrombus formation experiments have demonstrated the anti-thrombotic effects of rADAMTS13-loaded platelets when added or transfused into whole blood both ex vivo and in vivo.
A potential pitfall in our present study is that only is the prophylactic anti-thrombotic effect of rADAMTS13-loaded platelets tested. The efficacy of rADAMTS13-loaded platelets in the presence of established large and small thrombi is yet to be determined in our future studies. Nevertheless, we believe that transfusion of rADAMTS13-loaded platelets may be developed as a potential novel therapeutic for various arterial thromboses, including those seen in congenital and immune-mediated TTP.
Supplementary Material
Highlights.
Human and murine platelets are able to endocytose rADAMTS13 ex vivo and store it in the α-granules, co-localized with von Willebrand factor.
The endocytosed rADAMTS13 in platelets is intact, active, and able to be released upon activation by fluidic shear stress
The rADAMTS13-loaded platelets dramatically inhibit arterial thrombosis under flow in normal and TTP patient whole blood, as well as in Adamts13−/− mice.
Our findings demonstrate that transfusion of rADAMTS13-loaded platelets may be a potential therapeutic for arterial thrombosis including congenital and acquired immune-mediated TTP.
Acknowledgements
This study was supported in part by grants from National Heart, Lung, and Blood Institute (R01HL126724 and R01HL115187) to X.L.Z. and the institutional funds from the University of Alabama at Birmingham, AL.
Abbreviations
- cTTP and iTTP
congenital and immune-mediated thrombotic thrombocytopenic purpura
- TPE
therapeutic plasma exchanges
- ADAMTS13
A Disintegrin And Metalloprotease with ThromboSpondin type 1 repeats-13
- IgG
immunoglobulin
- PPACK
D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone, a thrombin inhibitor
- VWF
von Willebrand factor
- VWF73
a 73-amino acid peptide derived from the central A2 domain of VWF
- FITC
fluorescein isothiocyanate
Footnotes
Authorship statement
M.S.A, W.C, L.Z., N.K.K., and X.L.Z. designed the research, analyzed the data, and wrote manuscript. L.A.W. and X.L.Z. have helped recruit TTP patients and finalized the manuscript.
Conflict-of-interest statements
X.L.Z. serves in the speaker’s bureau of Alexion and is a consultant for Ablynx. All other authors declare no relevant conflict of interest associated with this work.
References
- 1.Zheng XL, Sadler JE. Pathogenesis of thrombotic microangiopathies. Annu Rev Pathol. 2008:3:249–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, Spasoff RA. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian apheresis study group. N Engl J Med. 1991:325:393–397 [DOI] [PubMed] [Google Scholar]
- 3.Hosler GA, Cusumano AM, Hutchins GM. Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome are distinct pathologic entities. A review of 56 autopsy cases. Arch Pathol Lab Med. 2003:127:834–839 [DOI] [PubMed] [Google Scholar]
- 4.Levy GG, et al. Mutations in a member of the adamts gene family cause thrombotic thrombocytopenic purpura. Nature. 2001:413:488–494 [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto M, et al. Molecular characterization of adamts13 gene mutations in japanese patients with upshaw-schulman syndrome. Blood. 2004:103:1305–1310 [DOI] [PubMed] [Google Scholar]
- 6.Tsai HM, Lian EC. Antibodies to von willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Eng J Med. 1998:339:1585–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng XL, Wu HM, Shang D, Falls E, Skipwith CG, Cataland SR, Bennett CL, Kwaan HC. Multiple domains of adamts13 are targeted by autoantibodies against adamts13 in patients with acquired idiopathic thrombotic thrombocytopenic purpura. Haematologica. 2010:95:1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casina V, Hu WB, Mao JH, Lu RN, Hanby H, Pickens B, Kan ZY, Lim WK, Mayne L, Ostertag E, Kacir S, Sigel D, Walter SW, Zheng XL. High resolution epitope mapping by hx ms reveals the pathogenic mechanism and a possible therapy for autoimmune ttp syndrome. PNAS. 2015:112:9620–9625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pos W, Sorvillo N, Fijnheer R, Feys HB, Kaijen PH, Vidarsson G, Voorberg J. Residues arg568 and phe592 contribute to an antigenic surface for anti-adamts13 antibodies in the spacer domain. Haematologica. 2011:96:1670–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, Tamai H, Konno M, Kamide K, Kawano Y, Miyata T, Fujimura Y. Mutations and common polymorphisms in adamts13 gene responsible for von willebrand factor-cleaving protease activity. Proc Natl Acad Sci U S A. 2002:99:11902–11907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luken BM, Turenhout EA, Hulstein JJ, Van Mourik JA, Fijnheer R, Voorberg J. The spacer domain of adamts13 contains a major binding site for antibodies in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2005:93:267–274 [DOI] [PubMed] [Google Scholar]
- 12.Ai J, Smith P, Wang S, Zhang P, Zheng XL. The proximal carboxyl-terminal domains of adamts13 determine substrate specificity and are all required for cleavage of von willebrand factor. J Biol Chem. 2005:280:29428–29434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng X, Nishio K, Majerus EM, Sadler JE. Cleavage of von willebrand factor requires the spacer domain of the metalloprotease adamts13. J Biol Chem. 2003:278:30136–30141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jian C, Xiao J, Gong L, Skipwith CG, Jin SY, Kwaan HC, Zheng XL. Gain-of-function adamts13 variants that are resistant to autoantibodies against adamts13 in patients with acquired thrombotic thrombocytopenic purpura. Blood. 2012:119:3836–3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cataland SR, Peyvandi F, Mannucci PM, Lammle B, Kremer Hovinga JA, Machin SJ, Scully M, Rock G, Gilbert JC, Yang S, Wu H, Jilma B, Knoebl P. Initial experience from a double-blind, placebo-controlled, clinical outcome study of arc1779 in patients with thrombotic thrombocytopenic purpura. Am J Hematol. 2012:87:430–432 [DOI] [PubMed] [Google Scholar]
- 16.Knobl P, Jilma B, Gilbert JC, Hutabarat RM, Wagner PG, Jilma-Stohlawetz P. Anti-von willebrand factor aptamer arc1779 for refractory thrombotic thrombocytopenic purpura. Transfusion. 2009:49:2181–2185 [DOI] [PubMed] [Google Scholar]
- 17.Callewaert F, Roodt J, Ulrichts H, Stohr T, van Rensburg WJ, Lamprecht S, Rossenu S, Priem S, Willems W, Holz JB. Evaluation of efficacy and safety of the anti-vwf nanobody alx-0681 in a preclinical baboon model of acquired thrombotic thrombocytopenic purpura. Blood. 2012:120:3603–3610 [DOI] [PubMed] [Google Scholar]
- 18.Holz JB. The titan trial--assessing the efficacy and safety of an anti-von willebrand factor nanobody in patients with acquired thrombotic thrombocytopenic purpura. Transfus Apher Sci. 2012:46:343–346 [DOI] [PubMed] [Google Scholar]
- 19.Peyvandi F, et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016:374:511–522 [DOI] [PubMed] [Google Scholar]
- 20.Lei X, Reheman A, Hou Y, Zhou H, Wang Y, Marshall AH, Liang C, Dai X, Li BX, Vanhoorelbeke K, Ni H. Anfibatide, a novel gpib complex antagonist, inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis. Thromb Haemost. 2014:111:279–289 [DOI] [PubMed] [Google Scholar]
- 21.Li TT, Fan ML, Hou SX, Li XY, Barry DM, Jin H, Luo SY, Kong F, Lau LF, Dai XR, Zhang GH, Zhou LL. A novel snake venom-derived gpib antagonist, anfibatide, protects mice from acute experimental ischaemic stroke and reperfusion injury. Br J Pharmacol. 2015:172:3904–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng L, Mao Y, Abdelgawwad MS, Li M, Dai X, Li B, Zheng XL. Therapeutic efficacy of anfibatide in a murine model of thrombotic thrombocytopenic purpura (ttp). Blood Adv. 2016: 1:75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tersteeg C, Roodt J, Van Rensburg WJ, Dekimpe C, Vandeputte N, Pareyn I, Vandenbulcke A, Plaimauer B, Lamprecht S, Deckmyn H, Lopez JA, De Meyer SF, Vanhoorelbeke K. N-acetylcysteine in preclinical mouse and baboon models of thrombotic thrombocytopenic purpura. Blood. 2017:129:1030–1038 [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Reheman A, Gushiken FC, Nolasco L, Fu X, Moake JL, Ni H, Lopez JA. N-acetylcysteine reduces the size and activity of von willebrand factor in human plasma and mice. J Clin Invest. 2011:121:593–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickens B, Mao Y, Li D, Siegel DL, Poncz M, Cines DB, Zheng XL. Platelet-delivered adamts13 inhibits arterial thrombosis and prevents thrombotic thrombocytopenic purpura in murine models. Blood. 2015:125:3326–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saha M, McDaniel JK, Zheng XL. Thrombotic thrombocytopenic purpura: Pathogenesis, diagnosis and potential novel therapeutics. J Thromb Haemost. 2017:15:1889–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scully M, Cataland S, Coppo P, de la Rubia J, Friedman KD, Kremer Hovinga J, Lammle B, Matsumoto M, Pavenski K, Sadler E, Sarode R, Wu H, International Working Group for Thrombotic Thrombocytopenic P. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017:15:312–322 [DOI] [PubMed] [Google Scholar]
- 28.Zhang P, Pan W, Rux AH, Sachais BS, Zheng XL. The cooperative activity between the carboxyl-terminal tsp1 repeats and the cub domains of adamts13 is crucial for recognition of von willebrand factor under flow. Blood. 2007:110:1887–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plaimauer B, Kremer Hovinga JA, Juno C, Wolfsegger MJ, Skalicky S, Schmidt M, Grillberger L, Hasslacher M, Knobl P, Ehrlich H, Scheiflinger F. Recombinant adamts13 normalizes von willebrand factor-cleaving activity in plasma of acquired ttp patients by overriding inhibitory antibodies. J Thromb Haemost. 2011:9:936–944 [DOI] [PubMed] [Google Scholar]
- 30.Raife TJ, Cao W, Atkinson BS, Bedell B, Montgomery RR, Lentz SR, Johnson GF, Zheng XL. Leukocyte proteases cleave von willebrand factor at or near the adamts13 cleavage site. Blood. 2009:114:1666–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Lawson HL, Harish VC, Huff JD, Knovich MA, Owen J. Creation of a recombinant peptide substrate for fluorescence resonance energy transfer-based protease assays. Anal Biochem. 2006:358:298–300 [DOI] [PubMed] [Google Scholar]
- 32.Li D, Xiao J, Paessler M, Zheng XL. Novel recombinant glycosylphosphatidylinositol (gpi)-anchored adamts13 and variants for assessment of anti-adamts13 autoantibodies in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2011:106:947–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao J, Xiao J, Mao Y, Zheng XL. Carboxyl terminus of adamts13 directly inhibits platelet aggregation and ultra large von willebrand factor string formation under flow in a free-thiol-dependent manner. Arterioscler Thromb Vasc Biol. 2014:34:397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L, Choi H, Bernardo A, Bergeron AL, Nolasco L, Ruan C, Moake JL, Dong JF. Platelet-derived vwf-cleaving metalloprotease adamts-13. J Thromb Haemost. 2005:3:2536–2544 [DOI] [PubMed] [Google Scholar]
- 35.Blair P, Flaumenhaft R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009:23:177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Q, Montgomery RR. Platelets as delivery systems for disease treatments. Adv Drug Deliv Rev. 2010:62:1196–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanaji S, Kuether EL, Fahs SA, Schroeder JA, Ware J, Montgomery RR, Shi Q. Correction of murine bernard-soulier syndrome by lentivirus-mediated gene therapy. Mol Ther. 2012:20:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarovoi HV, Kufrin D, Eslin DE, Thornton MA, Haberichter SL, Shi Q, Zhu H, Camire R, Fakharzadeh SS, Kowalska MA, Wilcox DA, Sachais BS, Montgomery RR, Poncz M. Factor viii ectopically expressed in platelets: Efficacy in hemophilia a treatment. Blood. 2003:102:4006–4013 [DOI] [PubMed] [Google Scholar]
- 39.Damon AL, Scudder LE, Gnatenko DV, Sitaraman V, Hearing P, Jesty J, Bahou WF. Altered bioavailability of platelet-derived factor viii during thrombocytosis reverses phenotypic efficacy in haemophilic mice. Thromb Haemost. 2008:100:1111–1122 [DOI] [PubMed] [Google Scholar]
- 40.Shi Q, Wilcox DA, Fahs SA, Weiler H, Wells CW, Cooley BC, Desai D, Morateck PA, Gorski J, Montgomery RR. Factor viii ectopically targeted to platelets is therapeutic in hemophilia a with high-titer inhibitory antibodies. J Clin Invest. 2006:116:1974–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Q, Fahs SA, Wilcox DA, Kuether EL, Morateck PA, Mareno N, Weiler H, Montgomery RR. Syngeneic transplantation of hematopoietic stem cells that are genetically modified to express factor viii in platelets restores hemostasis to hemophilia a mice with preexisting fviii immunity. Blood. 2008:112:2713–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang G, Shi Q, Fahs SA, Kuether EL, Walsh CE, Montgomery RR. Factor ix ectopically expressed in platelets can be stored in alpha-granules and corrects the phenotype of hemophilia b mice. Blood. 2010:116:1235–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scully M, Knobl P, Kentouche K, Rice L, Windyga J, Schneppenheim R, Kremer Hovinga JA, Kajiwara M, Fujimura Y, Maggiore C, Doralt J, Hibbard C, Martell L, Ewenstein B. Recombinant adamts-13: First-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood. 2017:130:2055–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furlan M, Robles R, Morselli B, Sandoz P, Lammle B. Recovery and half-life of von willebrand factor-cleaving protease after plasma therapy in patients with thrombotic thrombocytopenic purpura. Thrombosis and haemostasis. 1999:81:8–13 [PubMed] [Google Scholar]
- 45.Ferrari S, Palavra K, Gruber B, Kremer Hovinga JA, Knobl P, Caron C, Cromwell C, Aledort L, Plaimauer B, Turecek PL, Rottensteiner H, Scheiflinger F. Persistence of circulating adamts13-specific immune complexes in patients with acquired thrombotic thrombocytopenic purpura. Haematologica. 2014:99:779–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas MR, de Groot R, Scully MA, Crawley JT. Pathogenicity of anti-adamts13 autoantibodies in acquired thrombotic thrombocytopenic purpura. EBioMedicine. 2015:2:942–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duffy SM, Coyle TE. Platelet transfusions and bleeding complications associated with plasma exchange catheter placement in patients with presumed thrombotic thrombocytopenic purpura. J Clin Apher. 2013:28:356–358 [DOI] [PubMed] [Google Scholar]
- 48.Harkness DR, Byrnes JJ, Lian EC, Williams WD, Hensley GT. Hazard of platelet transfusion in thrombotic thrombocytopenic purpura. JAMA. 1981:246:1931–1933 [PubMed] [Google Scholar]
- 49.Otrock ZK, Liu C, Grossman BJ. Platelet transfusion in thrombotic thrombocytopenic purpura. Vox Sang. 2015:109:168–172 [DOI] [PubMed] [Google Scholar]
- 50.Zhou A, Mehta RS, Smith RE. Outcomes of platelet transfusion in patients with thrombotic thrombocytopenic purpura: A retrospective case series study. Ann Hematol. 2015:94:467–472 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


