Abstract
A review on cis-1-aminoindan-2-ol derived asymmetric syntheses is described.
Keywords: asymmetric synthesis, 1-aminoindan-2-ol, protease, inhibitor
1. Introduction
Stereochemically defined synthesis of organic molecules in enantiomerically pure form is one of the most important areas in organic synthesis.1 With the unprecedented advances in molecular biology and modern spectroscopic techniques, the pathogenesis of many complex human diseases is now well understood at the molecular level. In addition, a growing number of new complex natural products with important biological functions have been isolated and characterized. Concurrent to these remarkable achievements came new challenges and opportunities in asymmetric synthesis. The design and synthesis of enzyme inhibitors and receptor agonists or antagonists, very often targets a molecular probe that contains multiple stereocenters. For meaningful biological studies, the preparation of such molecules in enantiomerically pure form is highly desirable if not mandatory. Therefore, the development of methodologies for efficient asymmetric synthesis is of great interest in the field of medicinal chemistry and related disciplines.
The sophistication of asymmetric synthesis has now reached the point that many complex organic molecules can be synthesized with near complete enantioselectivity. Indeed, several asymmetric transformations can be carried out with enantio- or diastereoselectivities rivaling enzymatic transformations. The development of asymmetric catalysts or ‘abiological catalysts’ for asymmetric hydrogenation with chiral bis(phosphine)rhodium complexes,2 asymmetric dihydroxylations,3 asymmetric epoxidation of allylic alcohols,4 asymmetric epoxidation of unfunctionalized olefins,5 and asymmetric reductions with chiral oxazaborolidines6 are examples of the sophistication of catalytic asymmetric synthesis. Also, the development of chiral auxiliaries for asymmetric syn-aldol reactions,7 asymmetric alkylations,8 asymmetric Diels-Alder reactions,9 and asymmetric conjugate additions10 has reached levels of diastereoselectivity of >99:1. One of the key advantages of abiological systems over enzymatic transformations is that either enantiomer of the target molecule can be conveniently synthesized by the proper choice of catalyst or auxiliary.
Further development of asymmetric chiral catalysts and auxiliaries in the areas of novel asymmetric reactions, substantive enhancement of stereocontrol, efficiency and ready accessibility of the chiral templates will be of enormous benefit to synthetic communities. The α-amino acid derived amino alcohols have been utilized in numerous efficient auxiliary-directed and catalytic asymmetric transformations.11 However, development of catalysts or auxiliaries that are not derived from natural amino acids often renders advantage in terms of manipulation of structural properties, conformational rigidities and availability of either enantiomer for asymmetric synthesis. In the context of design and synthesis of potent, selective and orally active HIV-protease inhibitors for the treatment of AIDS, the researchers at Merck Research Laboratories first demonstrated the utility of (1S, 2R)- 1-aminoindan-2-ol as an effective ligand for protease inhibitors in 1990.12 Subsequently, the clinically effective protease inhibitor indinavir was discovered which incorporates this important amino alcohol into its structure.13 In combination with reverse transcriptase inhibitors, indinavir was approved by the U.S. Food and Drug Administration in 1996 for treatment of AIDS under the trade name of Crixivan®.14 Numerous effective syntheses and resolutions of cis-1-amino- indan-2-ol have been developed in this context and either enantiomer is readily available. Since its discovery as a ligand for HIV-protease inhibitors, cis-1-aminoindan-2-ol has become a vital source of enantio- and diastereoselec-tivity in asymmetric synthesis. cis-Aminoindanol is structurally related to phenylglycinol, and indeed is often used as a phenylglycinol surrogate. The advantage of amino- indanol over phenylglycinol is that aminoindanol is conformationally constrained by the methylene link between the aromatic ring and the alcohol moiety. This rigid skeleton is the key to the high selectivities often obtained with aminoindanol-based catalysts and auxiliaries. The rigid skeleton often limits transition state geometries far more effectively than corresponding phenylglycinol-based catalysts and auxiliaries. As either enantiomer of aminoin- danol is now commercially available, syntheses using aminoindanol-based catalysts or auxiliaries can be tailored to produce either enantiomer of the target mole- cule.15 Already, many impressive asymmetric synthetic methodologies have been recorded utilizing cis-1-amino- indan-2-ol derived catalysts and auxiliaries. The present review is intended to focus on the cis-1-aminoindan-2-ol derived asymmetric syntheses and their applications in organic synthesis.
2. Aminoindanol-Based Chiral Catalysts and Auxiliaries
Enantiomerically pure cis-1-aminoindan-2-ol (1) and its derivatives have been utilized in numerous asymmetric syntheses since 1991. These auxiliaries and catalysts are shown in Figure 1. One of the early uses of cis-amino- indanol 1 in asymmetric synthesis was reported by Didier et al. as a ligand for chiral oxazaborolidine based reductions of ketones.16 The Sepracor group later developed ligands 3–10 for oxazaborolidine reductions of a-chloro- acetophenone.17 cis-1-Aminoindan-2-ol derived tetrahy- dro-4H-oxazinone 2 was investigated for synthesis of α- amino acids.18 Ghosh et al. developed auxiliaries 11–16 for asymmetric reductions of α-keto esters,19 asymmetric Diels-Alder reactions,20 and for asymmetric syn-aldol reactions.21 Ghosh et al. also demonstrated the utility of chiral sulfonamides 11 and 15 in ester derived titanium- enolate based stereoselective asymmetric syn- and anti- aldol reactions.22 Ghosh et al. and Davies et al. independently developed bis(oxazoline) ligands 1723 and 1824 for catalytic asymmetric Diels-Alder reactions. The bis(oxazoline) ligand 17 was utilized by Ghosh et al. in asymmetric hetero Diels-Alder reactions.25 Davies et al. also developed py-box ligand 19 for asymmetric cyclopropa- nations.26 Armstrong has developed auxiliary 20 for asymmetric homoaldol reactions.27
Figure 1.
Auxiliaries and Catalysts Used in Asymmetric Synthesis
Aminoindanol has also been used extensively in the asymmetric syntheses of several HIV-protease inhibitors. (1S, 2R)-cis-aminoindanol was first introduced as a ligand for protease inhibitors in 1990.12 Intensive research efforts resulted in Crixivan®.14 Four of the five stereocenters in Crixivan® are set by aminoindanol: two in aminoindanol itself and two by chirality transfer processes in the synthesis of Crixivan®.
3. Syntheses of Aminoindanol 1
The synthesis of racemic cis-1-aminoindan-2-ol (1) was reported in 1951 by Lutz and Wayland.28 As shown in Scheme 1, trans-2-bromoindan-1-ol (21) was treated with excess ammonia to form trans- 1-aminoindan-2-ol (22). This reaction presumably proceeds via an indene oxide intermediate, which is then opened by ammonia. Treatment with 4-nitrobenzoyl chloride afforded the corresponding amide 23. Reaction of 23 with thionyl chloride gave the corresponding cis-oxazoline 24. Acidic hydrolysis of 24 furnished racemic cis-1-aminoindan-2-ol (1).
Scheme 1.
Heathcock and co-workers have synthesized racemic cis- aminoindanol 1 from indene via iodine isocyanate addition to indene as depicted in Scheme 2.29 Treatment of indene with iodine isocyanate prepared in situ by reaction of iodine and silver cyanate in ethanol afforded trans-1- (ethoxycarbonylamino)-2-iodoindan (26). Pyrrolysis of 26 in refluxing diglyme afforded the corresponding cis- oxazolidinone 27. Basic hydrolysis of 27 yielded racemic cis-aminoindanol 1.
Scheme 2.
Ghosh et al. have synthesized racemic aminoindanol by stereoselective reduction of α-hydroxy oxime-ether 30 which was obtained from indan-1-one (28).30 As shown in Scheme 3, treatment of 28 with potassium hydroxide and iodobenzenediacetate in methanol according to the procedure of Moriarty et al. yielded the corresponding 2-hydroxy-1-dimethyl ketal 29.31 Acidic hydrolysis followed by reaction of the resulting α-hydroxy ketone with benzyl- oxyamine hydrochloride in pyridine afforded the mixture of oximes 30. Reduction of 30 with borane-THF complex gave an 88:12 mixture of cis- and trans-aminoindanols. A related asymmetric reduction of keto oxime ethers catalyzed by oxazaborolidine-borane complex in the tetralone series has been reported by Tillyer and co-workers.32
Scheme 3.
Laksman and Zajc prepared cis-aminoindanol 1 from cis- indandiol 31 as outlined in Scheme 4.33 Reaction of 31 with α-acetoxyisobutyryl chloride afforded the trans-chloroacetate 32. The stereo and regiochemical outcome of this reaction is dictated by initial formation of an ace- toxonium species which is attacked by the chloride at the most stable carbocationic site, giving the trans-chloro-acetate 32.34 Treatment of 32 with lithium azide in DMF yielded the cis-azido acetate 33. Removal of acetate group by hydrolysis with sodium methoxide, followed by hydrogenation of the resulting cis-azido alcohol afforded the racemic cis-aminoindanol 1.
Scheme 4.
An early synthesis from the Merck group35 used a modification of the method of Lutz and Wayland.28 As shown in Scheme 5, racemic 2-bromoindan-1-ol (21) was first reacted with concentrated ammonium hydroxide. The resulting trans-aminoindanol 22 was then treated with benzoyl chloride followed by thionyl chloride to provide the oxazoline 34. Hydrolysis of oxazoline 34 with 6 N sulfuric acid at reflux afforded the racemic cis-aminoindanol. Resolution of the racemic amino alcohol was carried out by reaction of Boc-phenylalanine followed by removal of the Boc-group with trifluoroacetic acid and chromatographic separation of the corresponding phenylalanine amide 35.36 Hydrolysis of 35 with sodium methoxide gave optically pure (1S, 2R)-aminoindanol (1).
Scheme 5.
An enantioselective synthesis of either isomer of cis-amino- indanol was reported by Didier et al. using baker’s yeast reduction.16 As depicted in Scheme 6, baker’s yeast reduction of 1-(methoxycarbonyl)-indan-2-one 36 resulted in formation of optically active hydroxy ester 37 with 99.5% ee and >99% de. The ester was then hydrolyzed using pig liver esterase (PLE), and the resulting acid 38 subjected to a Curtius rearrangement with diphenylphos- phoryl azide to give oxazolidinone 40.37 Basic hydrolysis resulted in optically pure (1R, 2S)-aminoindanol 1. Hydrolysis of hydroxy ester 37 with aqueous sodium hydroxide gave a 2:1 mixture of cis- and trans- hydroxy acids, of which only the trans- isomer 39 was isolated in pure form. This isomer was then subjected to a Curtius rearrangement with diphenylphosphoryl azide in ethanol to provide the ethyl carbamate 41.37 Treatment with thionyl chloride afforded the oxazolidinone, which was hydrolyzed with potassium hydroxide to provide (1S, 2R)-aminoindanol 1.
Scheme 6.
Boyd has oxidized 2-bromoindan with whole cell cultures of Pseudomonas putida UV4 to enantiomerically pure (1S, 2R)-cis-2-bromoindan-1-ol (43).38 This cis-bromo- indanol 43 was then converted to both enantiomers of cis- aminoindanol 1 and ent-1. As illustrated in Scheme 7, direct application of a Ritter reaction with sulfuric acid in acetonitrile followed by basic hydrolysis with potassium hydroxide as described by Senanayake et al. afforded (1S, 2R)-aminoindanol 1.39 Inversion of the C-1 center by me- sylation followed by treatment with potassium hydroxide to effect inversion and epoxidation afforded (1R, 2S)-in- dene oxide 44. A Ritter reaction followed by hydrolysis of amine followed by displacement of the resulting mesylate with cesium acetate in toluene in the presence of 18- crown-6 to provide acetate derivative 48 in 64% yield. Alternatively, Mitsunobu inversion of the alcohol 46 with p-nitrobenzoic acid, diethyl azodicarboxylate, and triphe- nylphosphine afforded the azido ester 49 in 75% yield. Ester hydrolysis of 49 with sodium methoxide afforded azido alcohol 50 which upon hydrogenation over palladium on carbon afforded (1S, 2R)-aminoindanol 1. The azido acetate 47 was hydrolyzed with potassium carbonate in methanol to give azido alcohol ent-46. (1R, 2S)-1-Amino- indan-2-ol (ent-1) was synthesized from ent- 46 via a similar reaction sequence as described above (Scheme 8).the resulting oxazoline with potassium hydroxide yielded (1R, 2S)-1-aminoindan-2-ol (ent-1).
Scheme 7.
Scheme 8.
Ogasawara and Takahashi 40 and Ghosh et al. 41 have independently resolved trans-1 -azidoindan-2-ol with Pseudomonas sp. Amano lipase. As shown in Scheme 8, treatment of indene with NBS in THF/water afforded trans-bromohydrin 21. Reaction of racemic 21 with sodium hydroxide yielded the corresponding epoxide, which was opened with sodium azide to give racemic azido alcohol 45. Treatment of 45 with Lipase PS and vinyl acetate in tert-butyl methyl ether gave 48% of (1S, 2S)-azido in- danol 46 in >99% ee and 49% of (1R, 2R)-azido acetate 47 in 98% ee.40 Inversion of C-2 center of alcohol 46 was carried out by reaction with mesyl chloride and triethyl-triethylamine followed by displacement of the resulting mesylate with cesium acetate in toluene in the presence of 18- crown-6 to provide acetate derivative 48 in 64% yield. Alternatively, Mitsunobu inversion of the alcohol 46 with p-nitrobenzoic acid, diethyl azodicarboxylate, and triphenylphosphine afforded the azido ester 49 in 75% yield. Ester hydrolysis of 49 with sodium methoxide afforded azido alcohol 50 which upon hydrogenation over palladium on carbon afforded (1S, 2R)-aminoindanol 1. The azido acetate 47 was hydrolyzed with potassium carbonate in methanol to give azido alcohol ent-46. (1R, 2S)-1-Aminoindan- 2-ol (ent-1) was synthesized from ent-46 via a similar reaction sequence as described above (Scheme 8).
Ghosh’s synthesis of enantiopure aminoindanol proceeds via direct epoxidation of indene with MCPBA, followed by opening of the racemic epoxide 44 with sodium azide to give racemic trans-azidoindanol 45 (Scheme 9).41 Resolution of 45 was carried out with immobilized Lipase PS on Celite,42 and isopropenyl acetate in dimethoxyethane, yielding 46% of (1S, 2S)-azidoindanol 46 and 44% of (1R, 2R)-azido acetate 47, both in >96% ee. The resulting azi- doindanols 46 and ent-46 were each hydrogenated in the presence of diethylpyrocarbonate. The respective carbamates 51 and ent-51 were treated with thionyl chloride to afford the corresponding oxazolidinones. Basic hydrolysis of the resulting oxazoldinone with potassium hydroxide afforded the respective optically pure aminoindanols 1 and ent-1.
Scheme 9.
Senanayake et al. have developed one of the most practical syntheses of either enantiomer of cis-aminoindanol.39, 43 The key step involves Jacobsen epoxidation of indene to optically active indene oxide.44 As shown in Scheme 10, the epoxidation is carried out with 0.7 mol% (S,S)- (salen)Mn(III)Cl, 3 mol% 4-(3-phenylpropyl)pyridine N- oxide (P3NO), and 1.5M aqueous NaOCl in chlorobenzene. Indene oxide ent-44 is produced in 89% yield and 88% ee under these conditions. Detailed mechanistic studies have revealed that P3NO serves several purposes in this epoxidation: (i) P3NO is the axial ligand on manganese and stabilizes the catalyst while increasing the rate of the reaction, and (ii) P3NO assists in drawing the active oxidant, HOCl, into the organic layer. The same study showed that oxidation of the manganese catalyst is the rate-limiting step.45 Furthermore, hydroxide concentration in the aqueous layer has been shown to be of critical importance; commercial 2 M NaOCl typically is 0.030.18 M in hydroxide. Raising this value to 0.3 M helped to stabilize the hypochlorite, and also helped prevent the side reaction of oxidation of P3NO to isonicotinic acid and benzoic acid.43
Scheme 10.
In the Merck process, chiral indene oxide ent- 44 is then treated with oleum in acetonitrile in a Ritter reaction, producing the corresponding methyl oxazoline 53. Studies have shown that this reaction proceeds through a cyclic sulfate intermediate 52, then on to the favored cis-5,5 ring system.39 The oxazoline 53 is hydrolyzed with water, then fractionally crystallized with l-tartaric acid to yield (1S,2R)-aminoindanol in 50% overall yield from indene, and in >99% ee. This sequence also proceeds through optically pure indene oxide.
4. Applications of Aminoindanol as Chiral Auxiliaries
Amino alcohols derived from α-amino acids have been utilized in numerous efficient asymmetric syntheses.11 The development of new chiral auxiliaries that are not derived from natural amino acids however, offers opportunities in terms of manipulation of structural properties and conformational rigidities necessary for a particular asymmetric process.46 Conformationally constrained cyclic amino alcohol derived ligands and chiral auxiliaries are of particular interest since the transition state leading to asymmetric induction may be somewhat more predictable. The conformational rigidity of cis-1-aminoindan-2- ols (1) has been already exploited in a number of efficient asymmetric processes both as covalently bound chiral auxiliaries as well as ligands in asymmetric catalysis as described in the following sections.
4.1. Aldol and Homoaldol Reactions
Ghosh et al. have first demonstrated that the potential of (1S, 2R)-cis-aminoindanol derived chiral oxazolidinone 16 in asymmetric syn-aldol reactions.21 As shown in Scheme 11, propionyl imide 56 provided a similar level of diastereoselectivities and isolated yields as the valinol or phenylalaninol derived auxiliaries developed by Evans.7 An advantage of the aminoindanol derived auxiliary is that both enantiomers are readily available.15 The synthesis of the auxiliary is carried out by treatment of aminoindanol with disuccinimidyl carbonate in the presence of triethylamine. Aldol condensation and hydrolytic removal of the chiral auxiliary are then carried out under standard conditions.7, 21 A list of representative aldol reactions is shown in Scheme 11. Aldol condensation with various aldehydes proceeded with complete diastereofacial selectivity (>99% de).
Scheme 11.
This aminoindanol derived auxiliary has been used in several syntheses. The C11-C15 segment of tylosin has been synthesized by use of the corresponding crotonyl oxazolidinone, as shown in Scheme 12.21 Treatment of crotonyl oxazolidinone 59 with dibutylboron triflate and triethyl- amine, followed by propionaldehyde afforded aldol product 60. The auxiliary was removed by LAH reduction to the corresponding diol 61. A series of standard synthetic steps gave the C11-C15 tylosin intermediate synthesized by Nicolaou and co-workers.47
Scheme 12.
Aminoindanol based chiral oxazolidinone has also been utilized in the enantioselective total synthesis of hapalosin 65.48 Hapalosin is a novel cyclodepsipeptide isolated from the blue-green alga Hapalosiphon welwitschii. This molecule has shown important multidrug-resistance reversing activity.49 As shown in Scheme 13, aldol condensation of N-propionyl oxazolidinone 56 and octanaldehyde, fol-lowed by hydrolysis of the aldol condensation product 63 with lithium hydroperoxide provided the key synthetic intermediate 64 for the enantioselective total synthesis of hapalosin.
Scheme 13.
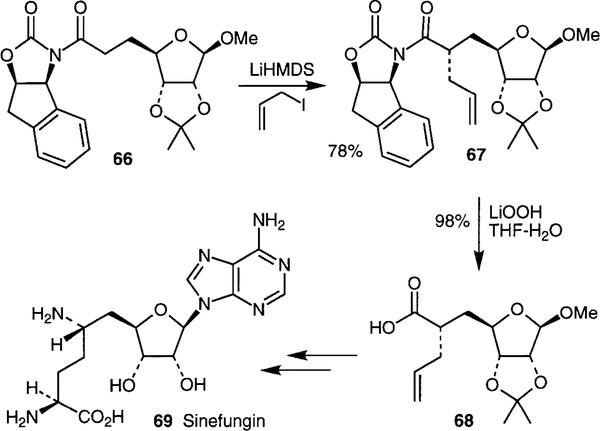
The C-6 amine stereochemistry of nucleoside antibiotic sinefungin 69 was set by a highly diastereoselective allyl- ation (>99% de) of a (1S, 2R)-1-aminoindan-2-ol derived oxazolidinone 66 followed by a Curtius rearrangement of the resulting acid 68 (Scheme 14).50 The C-9 amino acid stereochemistry of sinefungin (69) was established by a rhodium chiral bis(phosphine)-catalyzed asymmetric hydrogenation of an α-acylaminoacrylate derivative.51
Scheme 14.
The (1S, 2R)-1-aminoindan-2-ol derived oxazolidinone has also recently been utilized in the synthesis of the core unit of the HIV protease inhibitor Saquinavir®.52 As depicted in Scheme 15, N-acylation was carried out with the pivalic anhydride of hydrocinnamic acid and N-lithio- oxazolidinone. Aldol reaction with benzyloxyacetalde- hyde followed by lithium hydroperoxide hydrolysis afforded the key acid intermediate 73. This intermediate was converted by several standard synthetic steps to hydroxyethylamine dipeptide isostere 74.53
Scheme 15.
Ghosh and Onishi have developed N-tosyl-aminoindanol as a chiral auxiliary in an interesting ester derived titanium enolate based anti selective aldol reaction.22 While stereoselective generation of syn-aldol products54 has become very sophisticated, the development of corresponding enantioselective anti-aldol methodologies55 has received much less attention. As shown in Scheme 16, the chiral sulfonamide ent- 11 is readily prepared by reaction of cis-aminoindanol with tosyl chloride in the presence of triethylamine and 4-(dimethylamino)pyridine. The sulfonamide derivative ent- 11 is O-acylated by propionyl chloride, or with the corresponding acid in the presence of dicyclohexylcarbodiimide and 4-(dimethylamino)pyri- dine.22 The titanium enolate is formed by titanium tetrachloride and diisopropylethylamine. This enolate is then added to a solution of aldehyde precomplexed to titanium tetrachloride to yield the anti-aldol product, as shown in Scheme 16.22a
Scheme 16.
The stereochemical outcome of these reactions has been rationalized in terms of a Zimmerman-Traxler type tran-sition state model A, as shown in Figure 2.22, 56 The model is derived based on the following assumptions; that the geometry of the titanium enolate is Z, the titanium enolate is a seven-membered metallocycle with a chair-like conformation and a second titanium metal is involved in the transition state where it is chelated to the indanyloxy group as well as to the aldehyde carbonyl in a six mem- bered chair-like transition state. The involvement of two titanium metal atoms is supported by the fact that the titanium enolate derived from 75a does not react with aldehydes without precomplexation with TiCl4. Based on this possible transition state assembly, we subsequently hypothesized that the incorporation of a chelating substituent on the aldehyde side chain would adopt a transition state model B and thereby alter the stereochemical out-come from an anti-aldol to a syn-aldol product. It should be noted that the present syn-stereoselectivity can also be explained by an acyclic transition state similar to that proposed by Gennari et al.57
Figure 2.
Zimmerman-Traxler Type Transition States
As shown in Scheme 17, the reactions of the titanium enol- ates derived from esters ent-75 a-c with three representative bidentate oxyaldehydes such as benzyloxyacetalde- hyde, benzyloxypropionaldehyde and benzyloxybutyral- dehyde proceeded with excellent syn-diastereoselectivity (up to 99% de) with good to excellent isolated yields (Scheme 17).22b Thus, with proper choice of chiral template and aldehyde one can prepare either syn- or anti- aldol product in a stereopredictable fashion.
Scheme 17.
These reactions have been used in the asymmetric synthesis of a hydroxyethyl(sulfonamide) dipeptide isostere, and also a hydroxyethylene dipeptide isostere as depicted in Scheme 18.58 Aldol reaction of hydrocinnamate ester ent- 75b with benzyloxyacetaldehyde produced the syn-aldol product 78d in 97% yield as a single diastereomer. Removal of the chiral auxiliary with lithium hydroperoxide followed by a series of standard synthetic steps resulted in the hydroxy ethyl(sulfonamide) isostere 83.59 Similarly, aldol reactions with E-cinnamaldehyde provided a single diastereomer in 48% yield. Precomplexing the aldehyde with dibutylboron triflate instead of titanium tetrachloride increased the yield to 68%. However, the anti:syn diaste- reoselectivity was 6.1:1.60 Hydrolytic removal of the chiral auxiliary, followed by several standard synthetic steps provided hydroxyethylene isostere 82. The γ-lactone 81 and the sulfonamide isostere 83 are the intermediates of numerous potent and selective inhibitors of HIV protease and other aspartyl proteases.58, 61 The main advantage of the present synthesis is that the side chain substi- tutents of either isostere are not limited to amino acid derived side chains.62
Scheme 18.
Armstrong et al. have shown that isopropylidene aminoin- danol is an excellent chiral auxiliary for stereocontrolled homoaldol reactions, as depicted in Scheme 19.27 The titanium homoenolate species was formed by sonication of the corresponding iodide with Zn/Cu couple to form the zinc homoenolate, followed by transmetallation with dichlorotitanium diisopropoxide.27a Reaction with Boc- phenylalaninal provided product 87a as a single isomer in 55% yield. An improved method of forming the ho- moenolate involved treating the hydrocinnamate amide with BuLi, then with di(iodomethyl)zinc in the presence of benzyloxylithium.27b The mechanism of formation of the zincate is thought to proceed via stereoselective 1,2- migration of a zincate dianion, along with loss of lithium iodide to give the zincate anion. The stereoselective migration sets the absolute configuration of the α-center. Transmetallation with trichlorotitanium isopropoxide, then addition of aldehyde, affords the homoaldol products 87. Representative examples are shown in Scheme 19.
Scheme 19.
4.2. Diels-Alder Reactions
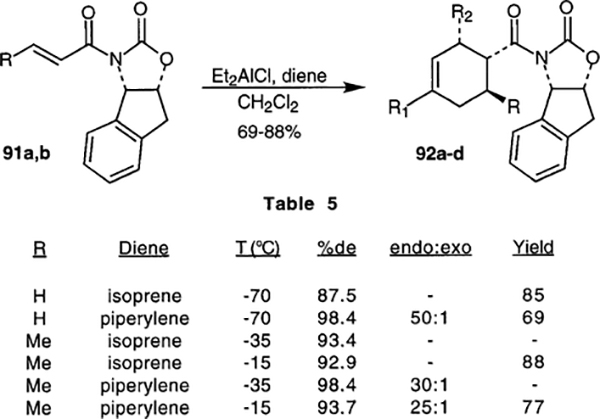
Merck researchers have used the previously described21 oxazolidinone derived from aminoindanol as a chiral auxiliary for Diels-Alder reactions.63 The auxiliary was prepared from aminoindanol and triphosgene, then acylated with acryloyl anhydride or crotonyl anhydride. Diels-Al-der reactions with isoprene and piperylene were studied under various conditions. The results are shown in Scheme 20. N-Acyl oxazolidinones were also prepared for the corresponding 6- and 7- membered ring homologs, and for phenylglycinol. In each case, low levels of stereocontrol were observed (30–35% de), demonstrating the importance of the rigidity of the aminoindanol platform.
Scheme 20.
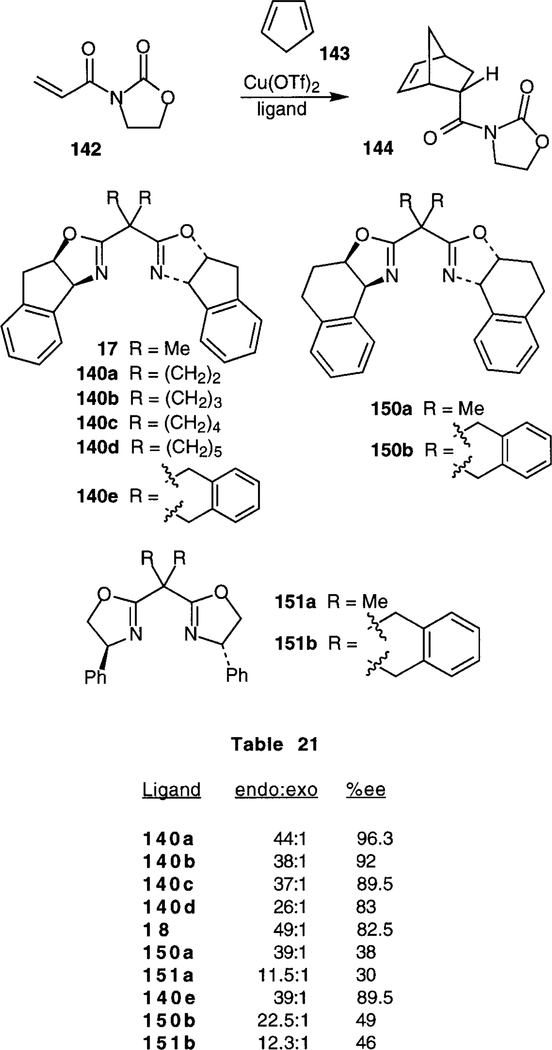
Ghosh has shown that 1-(arylsulfonamido)indan-2-ols are excellent chiral auxiliaries for Diels-Alder reactions.20 The aryl sulfonamides were prepared as previously de- scribed.22 Ο-Acylation is carried out with acryloyl chloride and triethylamine. Reaction with cyclopentadiene and a variety of Lewis acids demonstrated the synthetic utility of this auxiliary. endo/exo-Ratios of >99:1, and endo diastereoselectivities from 86:14 to 96:4 were observed, along with good to excellent yields, as shown in Scheme 21.
Scheme 21.
4.3. Reduction of a-Keto Esters
The use of 1-arylsulfonamido-2-aminoindanols as chiral auxiliaries for the stereoselective reduction of α-keto esters was demonstrated by Ghosh and Chen.19 As shown in Scheme 22, several hydride reagents and reaction conditions were examined. Although the selectivity using sodium borohydride was poor (2:1), the use of bulky alkyl hydride reagents afforded good to excellent selectivities, ranging from 4:1 to as high as >99:1. Indeed, reduction using L-selectride and zinc chloride afforded products 99a-b in 96% yield and >99:1 diastereoselectivity. Reduction with L-selectride and zinc chloride afforded 99c in 90% yield and 49:1 diastereoselectivity. Results of the reductions using various hydride reagents and aryl sulfonamides are shown in Scheme 22.
Scheme 22.
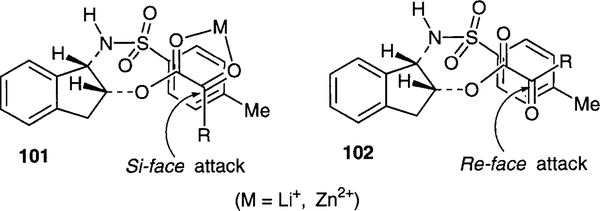
The high degree of stereoselection associated with this asymmetric reduction can be attributed to the chelation of carbonyl oxygens with a metal ion. As shown in Figure 3, in the α-keto esters 97a, reduction by L-Selectride most likely proceeds through the s-cis conformation 101 because of the metal chelation. The presence of the vicinal toluenesulfonamide blocks the approach of the hydride from the re-face and therefore, si-face hydride attack leads to the preferential formation of 99 a-c. Consistent with this rationale, a more sterically demanding 1-naph- thalenesulfonamide bearing chiral auxiliary 97b, has exhibited an even higher degree of stereoselection. The stereoselectivity has been reversed by using an additive to chelate the metal ion, such as K-selectride with 18-crown-6. This leads to predominately re-face attack of the hydride which proceeds through the s-trans conformation 102.
Figure 3.
Conformations 101 and 102 During L-Selectride Reduction
4.4. Miscellaneous Reactions
An asymmetric [2,3]-sigmatropic rearrangement of amide enolates of 103 containing isopropylidene aminoindanol as chiral auxiliary has been described by Kress et al64 As shown in Scheme 23, the diastereomeric ratio of 104:105 of the reaction has been shown to be a function of the counter ion. The syn-stereoselectivity increased in the order K < Na < Li < Zr. Using HMPA along with LiHMDS has given the optimal combination of selectivity and yield. Although the selectivity was greater with the zirconium enolate than the lithium enolate, the yield was much lower. Increased amounts of the HMPA additive resulted in slightly increased syn-selectivity. The rearrangement proceeds with good to excellent diastereoselectivity, as well as in good yield.
Scheme 23.
The scope and utility were further examined with amide- acetonides 106a-e as depicted in Scheme 24. The rearrangement was carried out under similar conditions as described above. In general, trans-disubstituted olefin exhibited excellent syn-diastereoselectivity. Unsubstituted allyl ether 106d furnished greater than 98% 2R selectivity.
Scheme 24.
Asymmetric synthesis of α-amino acids via electrophilic amination has been demonstrated by Zheng and Armstrong and coworkers.65 The electrophile used was lithium tert-bu- tyl-N-tosyloxycarbamate. The reaction proceeds via a lithium enolate, which was transmetallated with copper(I) cyanide. Zinc and lithium enolates were also tested for this reaction, but no product was observed. The amide cuprate thus generated adds to the electrophile in good yield, and with excellent diastereoselectivity, as shown in Scheme 25. The chiral auxiliary was then removed with 6 N HCl.
Scheme 25.
5. Applications of Aminoindanol as Ligands in Asymmetric Catalysis
5.1. Catalytic Asymmetric Reductions
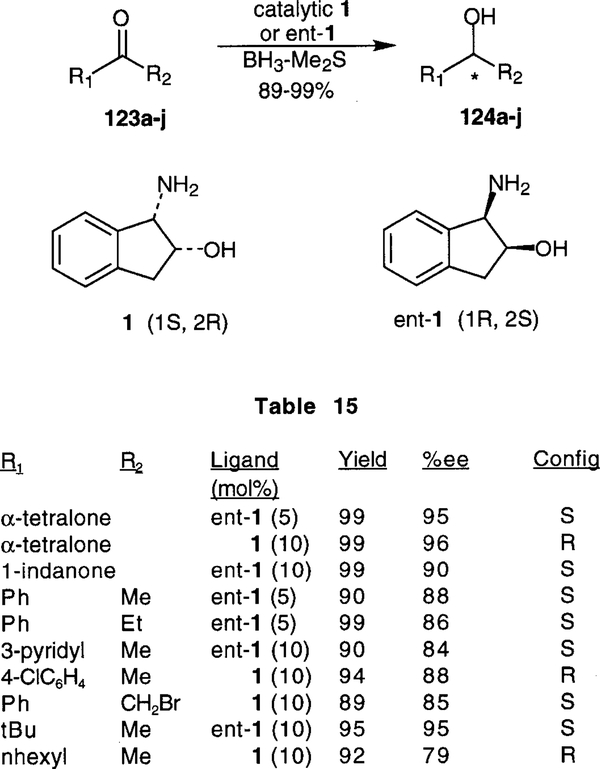
Enantioselective reduction of prochiral ketones by using oxazaborolidines as the chiral catalyst has been one of the most spectacular advances in organic synthesis.6,66 In this context, catalytic efficiency of chiral oxazaborolidines derived from numerous natural and unnatural amino alcohols have been examined. 67 cis -Aminoindanol has been used as a ligand for chiral oxazaborolidine reduction of several ketones. The use of cis-aminoindanol used as a ligand for chiral oxazaborolidine reductions of ketones was first explored by Didier et al.16 As shown in Scheme 26, several chiral cyclic and acyclic amino alcohols were tested for the asymmetric reduction of acetophenone in stoichiometric amounts. In selected instances, substoichiometric amounts of ligands were examined. In all cases, reactions proceeded with good yields and aminoindanol 1 derived catalyst afforded the highest enantioselectivity (87%).
Scheme 26.
Asymmetric reduction of anti-acetophenone oxime methyl ether was also investigated by Didier et al.16 As outlined in Scheme 27, good to excellent yields were reported in all cases, when a stoichiometric amount of ligand was utilized. The highest enantioselectivities were observed for 1-(N-methylamino)cyclopentan-2-ol ligand 114a (95%), and for aminoindanol 1 (94.5%). However, use of a substoichiometric amount of ligands 1, 114a, and 115a all resulted in decreased enantioselectivities, as shown in Scheme 27.
Scheme 27.
Sepracor researchers have developed practical catalyst systems derived from optically active cis-aminoindanol.17 As shown in Scheme 28, several oxazaborolidine catalysts based on aminoindanol were investigated. The B-methyl oxazaborolidines 4, 6–10 were prepared from aminoindanol and the corresponding A-alkylated aminoindanols (for catalysts 6–10) were prepared by reductive amination of the corresponding aldehydes. The ß-methyl oxaza- borolidene ligands 4, 6–10 were prepared by reaction of the corresponding N-alkyl aminoindanol with trimethylboroxine. The B-hydrogen oxazaborolidene ligand 3 was prepared in situ. These ligands were studied in the reduction of a-chloroacetophenone. The B-methyl oxazaborolidine catalysts 4 and ent-4 were found to be the most selective, as shown in Scheme 28. The incorporation of an additional coordinating heteroatom to the N-alkyl group greatly decreased the enantioselectivity (ligands 9–10).
Scheme 28.
As shown in Scheme 29, several other aromatic ketones were then examined using catalyst 4.17 All reactions were carried out using 5–10 mol% of catalyst. The α-halogen- ated ketones were reactive enough to be completely consumed at −20°C. Less reactive methyl and ethyl ketones required a temperature of 0°C to be completely reduced. All reductions proceeded with >95% isolated yields, and enantioselectivities ranging from 80–96% ee. The more reactive α-halogenated ketones generally proceeded with higher enantioselectivities than the ketones which required higher temperatures to react to completion.
Scheme 29.
Reduction of several cyclic and acyclic ketones with amino- indanol and borane-methyl sulfide complex was studied by Umani-Ronchi et al.68 The results are shown in Scheme 30. Catalytic amounts (5–10 mol%) of amino- indanol are treated with borane-methyl sulfide complex to provide the oxazaborolidene in situ. Isolated yields were at least 89%, and enantioselectivities were all >85%. The highest enantioselectivities were observed with cyclic and hindered ketones, as shown in Scheme 30.
Scheme 30.
The proposed mechanism of reduction involves initial addition of borane to aminoindanol, with the loss of two moles of hydrogen to form the oxazaborolidine. A second molecule of borane coordinates to the nitrogen with a trans-relationship to the indanyl substituent. The ketone carbonyl coordinates to the boron center providing a possible boat-like transition state 125 or a chair-like transition state as shown in Figure 4. In either case, intramolecular hydride attack by the re-face of the ketone is most probable. The boat-like transition state 125 is thought to be more accessible than the chair-like transition state 126.68
Figure 4.
Transition States 125 and 126
Asymmetric addition of diethyl zinc to aromatic aldehydes with 6 mol% N-alkyl aminoindanol was also examined by Umani-Ronchi et al68 As illustrated in Scheme 31, the N-dibutyl- or N-diallylaminoindanol (129a or ent-129a and 129b or ent- 129b, respectively) were prepared by alkylation with the corresponding iodide in the presence of sodium carbonate. Addition of 2 equivalents of diethylzinc with 0.6 mol% ligand to a solution of aldehyde gave the corresponding chiral alcohol in good yield. However, enantioselectivities were only fair, ranging from 4050% ee as shown in Scheme 31.
Scheme 31.
The researchers in Sepracor laboratories have recently utilized oxazaborolidine catalyst ent-4 in the asymmetric synthesis of R,R-formoterol, a β2-agonist for the treatment of asthma and bronchitis.69 As shown in Scheme 32, asymmetric reduction of bromo ketone 130 with (1R,2S)- B-methyl oxazaborolidine ent-4 (20 mol%) and borane/ tetrahydrofuran complex resulted in bromohydrin 131 in 98% yield and 96% ee. A series of standard synthetic transformations yields R,R-formoterol (133).
Scheme 32.
A detailed study was conducted to determine optimal conditions for the reduction of ketone 130.70 As depicted in Scheme 33, both B-methyl and B-hydrogen oxazaboro- lidines 4 and 3 were studied, using various temperatures and borane reagents. Although several reactions yielded enantioselectivities of >90%, the highest enantiomeric excess was observed using B-methyl oxazaborolidine 4 at −10°C, using borane/methyl sulfide as the active reductant. These conditions afforded an enantioselectivity of 96%. The optimal temperature using borane-tetrahydrofu- ran complex with ligand 4 was also −10°C, with an enantioselectivity of 95% ee. The optimal conditions using ligand 3 were the use of borane/THF complex at 0°C. With either 5 or 10 mol% catalyst, the enantioselectivity was 93%. However, using borane-m ethyl sulfide complex, the optimal temperature for ligand 3 was 25 °C, with an enantioselectivity of 90% ee.
Scheme 33.
cis-Aminoindanol has been shown to be an excellent ligand for transfer hydrogenation of several aromatic ketones.71 As outlined in Scheme 34, reduction of the ketone in isopropanol in the presence of 1 mol% ligand, 2.5 mol% potassium hydroxide, and 0.25 mol% [RuCl2- (arene)]2 afforded the corresponding alcohols in good yield and generally high enantioselectivity when cis-ami- noindanols were employed. In comparison, using phe- nylglycinol 136 or N-methyl aminoindanol 137 as ligand provided much lower enantioselectivities (23 and 27% ee).
Scheme 34.
5.2. Chiral Bis(oxazoline) Ligands (Inda-Box)
The utilities of C2-symmetric chiral bis(oxazoline) ligands in numerous catalytic asymmetric processes have been well documented since 1989.72 The C2-symmetric bis(oxazoline) ligands were designed based upon the semicorrin platform pioneered by Pfaltz et al.,73 Masam- une et al. first demonstrated the utility of bis(oxazoline)- Cu(II) complexes in enantioselective cyclopropanation reaction.74 In 1991, Corey et al. first demonstrated the catalytic potential of bis(oxazoline)-metal complexes in enan- tioselective Diels-Alder reactions.75 Evans et al. have also documented the utility of bis(oxazoline)-Cu complexes in numerous intermolecular and intramolecular Diels-Alder reactions.76 In 1996, Ghosh et al., 23 and the Merck group24 independently reported on the use of aminoindanol derived bis(oxazoline)-metal complexes as catalysts for asymmetric Diels-Alder reactions. The synthesis of bis(oxazoline) ligand 17 is shown in Scheme 35. Treatment of malononi- trile with anhydrous HCl in ethanol afforded the amide enol ether dihydrochloride 138.77 Condensation of the imidate salt with optically active amino alcohol furnished the bis(oxazoline) ligands 17 and ent-17 (inda-box) in multigram quantities in 60–65% yield.23
Scheme 35.
Constrained bis(oxazoline) ligand 18 was prepared by the Merck group by using a Ritter type reaction.24 As shown in Scheme 36, reaction of optically active 1S, 2R-indan- diol and dinitriles in the presence of trifluoromethane- sulfonic acid afforded various bis(oxazoline) derivatives. The use of malonitrile with indandiol afforded the bis(ox- azoline) 17 in 60% yield and the corresponding monoox- azoline in 10% yield. Alternatively, reaction of dimethyl malonitrile resulted in bis(oxazoline) 18 in only 30% yield and the corresponding mono(oxazoline) was obtained as the major product. Dimethyl bis(oxazoline) 18 was prepared by dialkylation of the parent bis(oxazoline) 17 with LDA and methyl iodide. Spiro bis(oxazoline) ligands 140a-d were also prepared by alkylation of 17 with the corresponding terminal diiodides.78
Scheme 36.
Py-box ligand 19 has also been made from aminoindanol and 2,6-pyridine dicarbonyl dichloride 141 as shown in Scheme 37.26 Reaction of 141 with (1S, 2R)-aminoin-danol in the presence of potassium bicarbonate in isopropyl acetate provided the corresponding bis-hydroxy- amide. Cyclization of bis-hydroxyamide by treatment with BF3∙OEt2 at 120°C furnished the py-box ligand 19. This protocol is convenient for the synthesis of other constrained py-box ligands derived from cyclic cis-amino alcohol with retention of configuration.
Scheme 37.
5.2.1. Diels-Alder Reactions
The Merck group disclosed the use of several bis(oxazo- line) ligands with copper(II) triflate in Diels-Alder reactions of cyclopentadiene with acryloyl-N-oxazolidinone 142.79 The results are summarized in Scheme 38. Ligand 18, which forms a six-membered copper chelate, has been shown to be the most selective of the ligands studied. Ligands 145a-d, which form 7 or 8 membered metal chelates, were far less selective. These ligands are postulated to have increased flexibility over the ligand 18, which lowers the enantioselectivity.
Scheme 38.
Ghosh et al. have investigated ligands 17 and ent-17 with copper(II) and magnesium as Lewis acid in the reaction of cyclopentadiene with several N-acyl oxazolidinones.23 These results are summarized in Scheme 39. A reversal of enantioselectivity was shown between copper and magne-sium complexes. Excellent enantioselectivities were observed using copper(II) as Lewis acid. Moderate enantio- selectivities were observed using magnesium as Lewis acid. Diels-Alder reaction of acryloyl-A-oxazolidinone 142 and cyclopentadiene in the presence of 10 mol% cationic aqua complex80 derived from bis(oxazoline) ent-17 and Cu(ClO4)2∙6H2O afforded cycloadduct 147a in 98% ee.81
Scheme 39.
These results have been rationalized in terms of a square planar conformation of copper, and s-cis conformation of the dieneophile.23, 76a In this model, endo-si-face attack by the diene is preferred. The reversal caused by use of magnesium complexes has been rationalized in terms of a Corey-Ishihara transition state model,75b which assumes tetrahedral magnesium geometry. This leaves the endo- re-face less hindered for attack by the diene (Figure 5).
Figure 5.
Transition Models 148 and 149
Merck researchers have examined the effect of bite angle of the bis(oxazoline) catalyst on the enantioselectivity of the Diels-Alder reaction between cyclopentadiene and acryloyl-A-oxazolidinone.78 Again, copper(II) triflate is the Lewis acid, and ligands 18 and 140a-d were examined (Scheme 40). The bite angle was calculated using the molecular mechanics program OPTIMOL.82 As shown in Scheme 40, the larger the bite angle, the better the enantio- selectivity. For example, cyclopropyl bis(oxazoline) ligand 140a has afforded the best enantioselectivity studied (96% ee), and also has the largest bite angle. The role of the conformation of the aromatic ring has also been studied.83 Ligands 150a,b were prepared from 1-amino- tetrahydronaphthalen-2-ol, and ligands 151a,b were prepared from phenylglycinol. Diels-Alder reaction of cyclopentadiene and acryloyl-N-oxazolidinone, with copper (II) triflate as Lewis acid, was examined. All the conformationally constrained aminoindanol-based ligands 18 and 140a-e have demonstrated far better enantioselectiv- ities than either tetrahydronaphthalene ligands 150a,b or phenylglycinol ligands 151a,b. Indeed, the highest enantioselectivity observed with any of these four ligands was 49%. The greater enantioselectivities have been attributed to the conformational rigidity of aminoindanol over tet- rahydronaphthalene or the phenyl group.
Scheme 40.
5.2.2. Hetero Diels-Alder Reactions
Ghosh et al. have investigated asymmetric hetero Diels- Alder reactions of Danishefsky’s diene and glyoxylate esters.25a As shown in Scheme 41, ligands 152, 153, and ent- 17 were studied using copper(II) triflate as Lewis acid. Aminoindanol derived ligand ent-17 afforded the highest yields and enantioselectivities. A modest reversal of enantioselectivity was again observed with magnesium complexes. This reaction proceeds in a stepwise manner via Mukaiyama aldol reaction, followed by ring closing with trifluoroacetic acid. Indeed, the Mukaiyama aldol product and the dihydropyrone were both observed before treatment with trifluoroacetic acid.84 Previous rigorous investigations by Danishefsky et al. have described the immense synthetic utility of this reaction.85
Scheme 41.
Hetero Diels-Alder reactions of Danishefsky’s diene and either benzyloxyacetaldehyde or 1,3-dithiane carboxaldehyde also provided the corresponding cycloadduct in high enantiomeric excess. As depicted in Scheme 42, conformationally constrained inda-box ligands 17 and ent-17 exhibited better enantioselectivity than the phe-box ligand 152 and the bu-box ligand 153.25b The C3-C14 segment of the antitumor macrolide Laulimalide,86 was synthesized by utilizing dihydropyran 157. As shown, reaction of Danishefsky’s diene with benzyloxyacetaldehyde, using 5 mol% copper(II) triflate and 6 mol% ent-17 provided dihydropyrone 157 in 76% yield and 85% ee. A series of standard synthetic steps gave the C3-Cj4 segment of Laulimalide, protected as the MOM ether.25b
Scheme 42.
5.2.3. Cyclopropanation Reactions
Bis(oxazoline)-metal complex catalyzed enantioselective cyclopropanation reactions of various alkyl diazoacetate and a variety of olefins were previously studied by a number of research groups.87 Davies et al. have examined Ru(II)-pybox complexes 19 and 162–164 for the cyclo- propanation of styrene (Scheme 43).83 Reaction of ethyl diazoacetate with styrene in the presence of 0.2 mol% [RuCl2(p-cymene)]2 and 0.8 mol% pybox ligand afforded cyclopropane 161 in moderate yield and fair to good enan- tioselectivity. Interestingly, py-box ligand 162 gave the best yields, diastereoselectivities, and trans-enantioselec- tivities compared to conformationally constrained py- inda-box ligand 19.
Scheme 43.
5.2.4. Free Radical Conjugate Additions
Chiral bis(oxazoline)-metal complex catalyzed enantio- selective free radical carbon-carbon bond forming reactions have been described by Porter et al. and Sibi et al.88 Recently, Sibi et al. have investigated various bis(oxazo- line) ligands for free radical conjugate additions to cin- namates using oxazolidinone template 146b.89 Stoichiometric amounts of metal-ligand complexes derived from a number of bis(oxazoline) ligands and a variety of Lewis acids including MgBr2, MgI2, Mg(OTf)2, Mg(ClO4)2 and Zn(OTf)2 were examined. The ligand-metal complexes of MgI2 afforded good to excellent enantioselectivities and isolated yields as shown in Scheme 44 and Table 25.89a The cyclopropyl bis(oxazoline) ligand 140a was most effective in providing maximum enantioselectivity. The use of 20–50 mol% ligand at −78°C resulted in at least 88% chemical yield and greater than 96% product enantiose- lectivites. The use of 30 mol% catalyst at temperatures ranging from −20 to 25°C also afforded excellent yields (at least 87%) and enantioselectivities (at least 93% ee). In comparison, conjugate additions to the pyrazole template using stoichiometric amounts of zinc(II) triflate and ligand afforded moderate enantioselectivities at best.89b
Scheme 44.
6. Aminoindanol in HIV-Protease Inhibitor Synthesis
Much of the explosion of interest in the chemistry of aminoindanol is a direct result of its use as an amino acid surrogate for HIV-protease inhibitors. Numerous potent and selective HIV protease inhibitors incorporating (1S, 2R)- aminoindanol were designed and synthesized by the researchers at Merck Research Laboratories.35, 90 These efforts have culminated the discovery of a clinically effective protease inhibitor indinavir which has been approved by the U.S. Food and Drug Administration for the treatment of AIDS in a combination therapy with reverse transcriptase inhibitors under the trade name Crixivan®.14 In the clinical setting, Crixivan® has shown remarkable evidence of effectiveness.91 As many as 90% of the clinical-trial participants who received Crixivan® have shown reduced viral load and increased CD4+ lymphocyte counts. There are five stereogenic centers in indinavir and cost effective synthesis of such inhibitor is one of the biggest challenges in asymmetric synthesis. In current commercial synthesis, of the five chiral centers in indinavir, two are in aminoindanol, and two more chiral centers are set by the chirality in aminoindanol. The design of aminoindanol as a ligand in HIV-protease inhibitor led to the discovery of potent inhibitor L-685,434 (167) with an enzyme inhibitory potency of (IC50) value of 0.35 nM and antiviral potency (CIC95) of 400 nM.12 However, L-685,434 suffered from aqueous insolubility and inadequate antiviral potency. Attempts were made to improve solubility and antiviral potency by adding hydrophilic substituents to each of the two phenyl rings in L-685,434. These investigations resulted in the discovery of two potent inhibitors, L-689,502 (168, IC50 = 0.45 nM; CIC95= 6–50 nM)35 and L-693,549 (169, IC50 = 0.1 nM; MIC100 = 25–50 nM).92 As shown in Figure 6, each inhibitor contains a polar, hydrophilic substituent on the 4-position of the P1’ phenyl ring.
Figure 6.
Structures of Inhibitors 167–169
For a concise and practical synthesis of this class of protease inhibitors, diastereoselective alkylations of chiral amide enolate derived from (1S, 2R)-aminoindanol were investigated by Askin et al.93 As depicted in Scheme 45, alkylation of N-hydrocinnamoyl aminoindanol acetonide 84a with methyl iodide provided alkylation product 84h in 76% yield and 97:3 diastereomeric ratio. This diastere- oselective alkylation protocol was conveniently employed in the synthesis of (1S, 2R)-aminoindanol containing pseudo-C2 symmetric inhibitor 171. The alkylation of the amide enolate of 84a was carried out with 0.5 equivalents of 3-iodo-2-(iodomethyl)prop-1-ene, to afford the alkylation product 170 in 74% yield. Ozonolysis of 170 followed by reductive workup with borohydride and subsequent deprotection of the isopropylidene group afforded to pseudo-C2 symmetric inhibitor 171.94
Scheme 45.
The synthesis of protease inhibitors 167-169 containing hydroxyethylene isostere were efficiently carried out as shown in Scheme 46. Reaction of 2 equivalents of BuLi in a mixture of 1 equivalent of known95 epoxide 172 and 1 equiv of amide at −78 to −25°C for 2 h furnished the epoxide opening product 173 in >90% yield and >99:1 dia-stereoselectivity. Presumably, lithium carbamate salt of 172 activated the epoxide toward the electrophilic epoxide opening. The removal of the isopropylidene group of 173 by treatment with camphorsulfonic acid in methanol provided the protease inhibitor 167 in excellent yield. Protease inhibitors 168 and 169 were also prepared conveniently with appropriate substitution at the 4-position of the amide 84a.93
Scheme 46.
As shown in Figure 7, (1S, 2R)-aminoindanol was also incorporated in a number of different dipeptide isosteres. A tyrosine-proline hydroxyethylene isostere containing aminoindanol as a ligand was synthesized however, this compound 174 was not very potent (IC50=110 nM).96 The researchers at Upjohn also incorporated aminoindanol into a dihydroxyethylene isostere providing protease inhibitor 175 (K = 200 nM).97 Compared to inhibitors 167–169, both enzyme inhibitory and antiviral potencies of inhibitor 175 were poor.97 (1S,2R)-Aminoindanol containing inhibitor 176 with greatly reduced peptide character has also been synthesized.98 However, enzyme inhibitory or antiviral potencies of this inhibitor has not been reported. Incorporation of 3(S)-tetrahydrofuranyl urethane in place of Boc-functionality in 167 resulted inhibitor 177 (IC50 < 0.03 nM; CIC95= 3 nM) with remarkable enhancement of both enzyme inhibitory and antiviral potencies.99
Figure 7.
Structures of Different Dipeptide Isosteres
In 1994, Merck researchers disclosed the synthesis and pharmacological properties of a novel (1S,2A)-aminoin- danol containing protease inhibitor, L-735,524 (180) which eventually became the current therapeutic agent Crixivan®.14, 90 Inhibitor L-735,524 evolved from a structure-based designed strategy in which features of the inhibitor 167 and Ro 31–8959 (178, Saquinavir®)100 were combined as shown in Figure 8. The lead inhibitor 179 incorporating N-teri-butylcarbonyl-(4a(S)-8a(S)-decahy- droisoquinoline was systematically modified using various cyclic amines to produce L-735,524 with a 2(S)-(N- tert-butylcarbonyl)piperazine moiety. Numerous N-sub- stituents to this piperazine were investigated prior to the discovery of Crixivan®.90
Figure 8.
Structural Features of Indinavir (180)
For industrial production of Crixivan, Merck researchers developed a novel alkylation-epoxidation sequence which utilizes the existing chirality of (1S,2A)-aminoindanol to set two of the three remaining chiral centers.101 As shown in Scheme 47, diastereoselective allylation of the lithium enolate of 84a with allyl bromide afforded the allylation product 181 in 94% yield and 96:4 diastereoselectivity.102 Treatment of 181 with N-chlorosuccinimide/sodium iodide in a two-phase isopropyl acetate/sodium hydrogen carbonate mixture afforded iodohydrin 182 in 92% yield.Reaction of iodohydrin 182 with sodium methoxide in THF afforded the corresponding oxirane 183 in 99% yield and impressive diastereoselectivity of 97:3.
Scheme 47.
As shown in Scheme 48, the reaction is proposed to proceed via cyclic imidate intermediate 184.102b A13 strain between the benzyl group and amine substituents forces the benzyl group to a pseudoaxial position. This results in a high bias for the 2,4-trans product. Hydrolysis of this cyclic imidate proceeds through intermediate 185 in two different pathways, depending upon the reaction conditions. Use of acidic media or unbuffered conditions results in formation of the iodolactone 186 (path A). Mildly basic hydrolysis with sodium bicarbonate results in the formation of iodohydrin amide 182 in excellent yields (path B). Rossen et al. at Merck recently reported an operationally simple electrochemical epoxidation of 181 which provided the epoxide 183 in excellent yield (86%) and high di- astereofacial selectivity (94:6).103
Scheme 48.
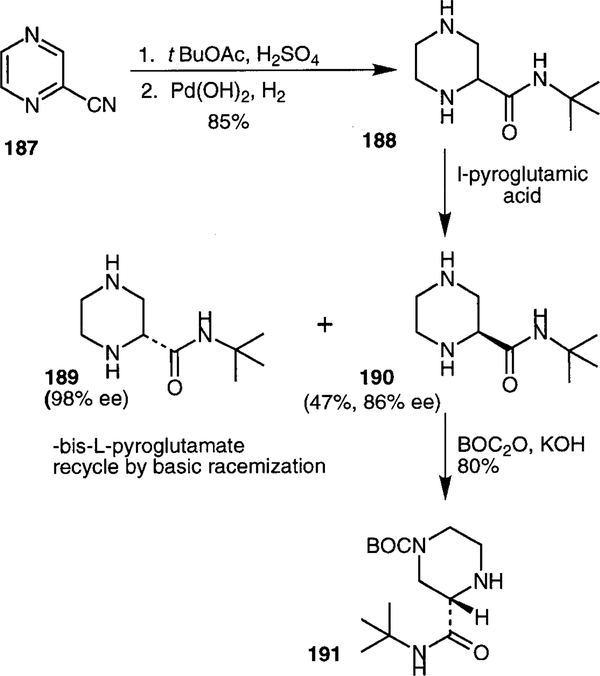
The 2(S)-piperazine carboxamide fragment of Crixivan® is synthesized from cyanopyrazine 187. As shown in Scheme 49, hydrolysis of nitrile 187 with tert-butyl acetate and sulfuric acid, followed by catalytic hydrogenation of the aromatic ring affords racemic piperazine carboxamide 188. Selective crystallization as the bis(l-pyro- glutamic) acid salt gives (S)-piperazine carboxamide 190 in 47% yield and 86% ee. The A-isomer 189 is recycled by base catalyzed racemization. The S-isomer is treated with di-tert-butyl dicarbonate and potassium hydroxide to afford the Boc-protected piperazine 191.104 Merck researchers have also developed an enantioselective route to 191 by asymmetric hydrogenation of the corresponding tetrahydropyrazine derivative with 2 mol% [(R)-BI- NAP(COD)Rh]OTf catalyst.105
Scheme 49.
To complete the synthesis of Crixivan®, epoxide 183 was reacted with with Boc-piperazine 191 in refluxing metha-nol and the resulting epoxide opening was subjected to treatment of HCl gas to effect the deprotection of the Boc and acetonide protecting groups (Scheme 50). Treatment of piperazine derivative 192 with 3-picolyl chloride in sulfuric acid provided indinavir sulfate 180 in 75% yield from the epoxide. The overall yield for the process from aminoindanol is 56%.104
Scheme 50.
Researchers at Merck laboratories have continued to search for more effective protease inhibitors incorporating aminoindanol as the P2’-ligand.106, 107 As shown in Figure 9, replacement of both P1’ and P3 substituents of indinavir with pyridylmethyl and [3,2-b]thienothiophene moieties resulted in inhibitor 193 (l-748,496, IC50=0.12 nM; IC95 = 6–12 nM).106 This inhibitor is reported to be more potent than indinavir in cell culture assay and also the compound has exhibited excellent pharmacokinetic properties in laboratory animals.106 Merck researchers have also attempted to replace the picolyl piperazine frag-ment with several aromatic substituents.107 The lowest IC50 observed with this strategy was 1.7 nM for inhibitor 194 (antiviral potency, CIC95 = 400 nM). Lehr et al. at Sandoz have incorporated aminoindanol into a 2-hetero- substituted-4-amino-3-hydroxy-5-phenylpentanoic acid (AHPPA) isostere.108 The most potent of these inhibitors, 195, only showed an IC50 of 47 nM.
Figure 9.
Structures of 193–195
7. Conclusion
This review reports on recent developments on the use of cis-1-aminoindan-2-ol (1) in asymmetric synthesis. Since the discovery of cis-1-aminoindan-2-ol (1) as a ligand for HIV-protease inhibitors in 1990, its use in asymmetric synthesis has blossomed. cis-1-Aminoindan-2-ol based chiral auxiliaries have proven to be effective for asymmetric reductions, aldol reactions, homoaldol reactions, alkylations, and Diels-Alder reactions. Furthermore, cis-1- aminoindan-2-ol derived chiral catalysts have been shown to be effective for asymmetric reductions, Diels-Alder and hetero Diels-Alder reactions, and conjugate radical additions. It should also be noted that related cis-2-ami- noindan-1-ol derivatives have been found to be efficient chiral auxiliaries for asymmetric syn-aldol, Diels-Alder reactions as well as chiral ligands for asymmetric reductions.109 The use of cis-1-aminoindan-2-ol (1) as a key inducer of asymmetry in the synthesis of the HIV-protease inhibitor Crixivan® has demonstrated its utility on an industrial scale. However, many potential applications of cis-aminoindanols and related cyclic amino alcohols in synthesis have yet to be explored. Hopefully, this review will help to stimulate the creativity and ingenuity of synthetic organic chemists to find novel applications of cis-1- aminoindan-2-ol (1) in organic synthesis.
Acknowledgments
Financial support of our work by the National Institute of Health (GM 55600) is gratefully acknowledged. We thank Merck Research Laboratories, Sepracor Inc. and BASF, Germany for providing us optically active aminoindan-2-ol used for our studies. The authors also thank Professor George Gould for helpful discussions.
Biographical Sketches
 Arun K. Ghosh was born in India in 1958. He received his Ph.D. from the University of Pittsburgh under the supervision of Professor Alan P. Kozikowski (1985). Following his postdoctoral studies at Harvard University with Professor E. J. Corey (1985–1988), he joined the department of Medicinal Chemistry at Merck Research Laboratories as a Senior Research Chemist in 1988. He moved to University of Illinois at Chicago in 1994 as an Assistant Professor. He is currently Associate Professor of Organic and Bioorganic Chemistry.
Arun K. Ghosh was born in India in 1958. He received his Ph.D. from the University of Pittsburgh under the supervision of Professor Alan P. Kozikowski (1985). Following his postdoctoral studies at Harvard University with Professor E. J. Corey (1985–1988), he joined the department of Medicinal Chemistry at Merck Research Laboratories as a Senior Research Chemist in 1988. He moved to University of Illinois at Chicago in 1994 as an Assistant Professor. He is currently Associate Professor of Organic and Bioorganic Chemistry.
 Steve Fidanze was born in Chicago, Illinois in 1969. He studied chemistry at the University of Illinois at Urbana-Champaign where he received his B.S. in chemistry in 1992. In 1996, he joined Professor Ghosh’s group and is currently working for his Ph.D. on the development of asymmetric syntheses and their applications in bioactive natural products. He is the recipient of 1997 University Graduate Fellowship from the University of Illinois at Chicago.
Steve Fidanze was born in Chicago, Illinois in 1969. He studied chemistry at the University of Illinois at Urbana-Champaign where he received his B.S. in chemistry in 1992. In 1996, he joined Professor Ghosh’s group and is currently working for his Ph.D. on the development of asymmetric syntheses and their applications in bioactive natural products. He is the recipient of 1997 University Graduate Fellowship from the University of Illinois at Chicago.
 Chris H. Senanayake was born in Sri Lanka. He obtained his Ph.D. from Wayne State University under the guidance of Professor James H. Rigby in 1987. He remained at Wayne State as a postdoctoral fellow with Professor Carl R. Johnson (1987–1989). In 1989, he moved to Dow Chemical Co. as a Senior Research Chemist and in 1990, he joined the Merck Process Research Group as a Senior Research Chemist. In 1996, he moved to Sepracor Inc. as Director of Chemical Process Research.
Chris H. Senanayake was born in Sri Lanka. He obtained his Ph.D. from Wayne State University under the guidance of Professor James H. Rigby in 1987. He remained at Wayne State as a postdoctoral fellow with Professor Carl R. Johnson (1987–1989). In 1989, he moved to Dow Chemical Co. as a Senior Research Chemist and in 1990, he joined the Merck Process Research Group as a Senior Research Chemist. In 1996, he moved to Sepracor Inc. as Director of Chemical Process Research.
Footnotes
Dedicated to the memory of Professor Wolfgang Oppolzer for his invaluable contributions in the field of asymmetric synthesis
References
- (1).For recent reviews, see: Chiral Auxiliaries and Ligands in Asymmetric Synthesis; Seyden-Penne J, Ed.; Wiley Interscience: New York, 1995.Stereoselective Synthesis; Nogradi M, Ed.; VCH Publishers: New York, 1995.Asymmteric Catalysis in Organic Synthesis, Noyori R; Wiley Interscience: New York, 1994.Catalytic Asymmetric Synthesis; Ojima I, Ed.; VCH Publishers: New York, 1993, and references cited therein.
- (2).Takaya H; Ohta T; Noyori R Asymmetric hydrogenation In Catalytic Asymmetric Synthesis; Ojima I, Ed.; VCH Publishers: New York, 1993; pp 1–39. [Google Scholar]
- (3).(a) Kolb HC; VanNieuwenhze MS; Sharpless KB Chem. Rev 1994, 94, 2483. [Google Scholar]; (b) Johnson RA; Sharpless KB In Catalytic Asymmetric Synthesis; Ojima I, Ed.; VCH Publishers: New York, 1993; pp 227–272. [Google Scholar]; (c) Lohray BB Tetrahedron: Asymmetry 1992, 3, 1317, and references cited therein. [Google Scholar]
- (4).(a) Sharpless KB Tetrahedron 1994, 50, 4235. [Google Scholar]; (b) Johnson RA; Sharpless KB In Catalytic Asymmetric Synthesis; Ojima I, Ed.; VCH Publishers: New York, 1993; pp 103–158. [Google Scholar]
- (5).Jacobsen EN In Catalytic Asymmetric Synthesis; Ojima I, Ed.; VCH Publishers: New York, 1993; pp 159–202. [Google Scholar]
- (6).(a) Corey EJ; Bakshi RK; Shibata S J. Am. Chem. Soc 1987,109, 5551. [Google Scholar]; (b) Corey EJ; Bakshi RK; Shibata S; Chen C-P; Singh VK J. Am. Chem. Soc 1987, 109, 7927. [Google Scholar]; (c) Wallbaum S; Martens J Tetrahedron: Asymmetry 1992, 3, 1475. [Google Scholar]; (d) Singh VK Synthesis 1992, 605, and references cited therein. [Google Scholar]
- (7).(a) Evans DA; Nelson JV; Taber TR Top. Stereochem 1983, 13, 1. [Google Scholar]; (b) Evans DA Aldrichimia Acta. 1982, 15, 23. [Google Scholar]; (c) Evans DA; Bartroli J; Shih TL J. Am. Chem. Soc 1981,103, 2127. [Google Scholar]
- (8).Evans DA; Ennis MD; Mathre DJ J. Am. Chem. Soc 1982,104, 1737. [Google Scholar]
- (9).(a) Oppolzer W Angew. Chem 1984, 96, 840; Angew. Chem. Int. Ed. Engl 1984, 23, 876. [Google Scholar]; (b) Evans DA; Chapman KT; Bisaha JJ Am. Chem. Soc 1988,110, 1238. [Google Scholar]
- (10).(a) Oare DA; Heathcock CH Top. Stereochem 1989, 19, 227. [Google Scholar]; (b) Oppolzer W; Kingma AJ; Poli G Tetrahedron 1989, 45, 479. [Google Scholar]
- (11).Ager DJ; Prakash I; Schaad DR Chem. Rev 1996, 96, 835, and references cited therein. [DOI] [PubMed] [Google Scholar]
- (12).Lyle TA; Wiscount CM; Guare JP; Thompson WJ; Anderson PS; Darke PL; Zugay JA; Emini EA; Schlief WA; Quintero JC; Dixon RAF; Sigal IS; Huff JR J. Med. Chem 1991, 34, 1228. [DOI] [PubMed] [Google Scholar]
- (13).Vacca JP; Dorsey BD; Guare JP; Holloway MK; Hungate RW; EP 5 411 68 A1, Merck & Co Inc. (1993); Chem. Abstr 1994, 120, 54 552. [DOI] [PubMed] [Google Scholar]
- (14).Vacca JP; Dorsey BD; Schleif WA; Levin RB; McDaniel SL; Darke PL; Zugay J; Quintero JC; Blahy OM; Roth E; Sardana VV; Schlabach AJ; Graham PI; Con- dra JH; Gotlib L; Holloway MK; Lin J; Chen I-W; Vastag K; Ostovic D; Anderson PS; Emini EA; Huff JR Proc. Natl. Acad. Sci. USA 1994, 91, 4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Enantiomerically pure cis-1-aminoindan-2-ol is available from Aldrich Chemical Co., Milwaukee, WI. For a highlight of cis-1- aminoindan-2-ol in synthesis, see, Senanayake CH Aldrichi- mica Acta, 1998, P-3.
- (16).Didier E; Loubinoux B; Ramos Tombo GM; Rihs G Tetrahedron 1991, 47, 4941. [Google Scholar]
- (17).Hong Y; Gao Y; Nie X; Zepp CM Tetrahedron Lett. 1994, 35, 6631. [Google Scholar]
- (18).Oxazinone 2 was prepared according to the procedure of Williams RM; Zhai W Tetrahedron 1988, 44, 5425 Synthesis of a-amino acids utilizing oxazinone 2 is currently in progress; Ghosh AK; Cho H, unpublished results. [Google Scholar]
- (19).Ghosh AK; Chen Y Tetrahedron Lett. 1995, 36, 6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ghosh AK; Mathivanan P Tetrahedron: Asymmetry 1996, 7, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ghosh AK; Duong TT; McKee SP Chem. Comm 1992, 1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).(a) Ghosh AK; Onishi M J. Am. Chem. Soc 1996, 118, 2527. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh AK; Fidanze S; Onishi M; Hussain KA Tetrahedron Lett. 1997, 38, 7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ghosh AK; Mathivanan P; Cappiello J Tetrahedron Lett. 1996, 37, 3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Davies IW; Senanayake CH; Larsen RD; Verhoeven TR; Reider PJ Tetrahedron Lett. 1996, 37, 813. [Google Scholar]
- (25).(a) Ghosh AK; Mathivanan P; Cappiello J; Krishnan K Tetrahedron: Asymmetry 1996, 7, 2165. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh AK; Mathivanan P; Cappiello J Tetrahedron Lett. 1997, 38, 2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Davies IW; Gerena L; Lu N; Larsen RD; Reider PJ J. Org. Chem 1996, 61, 9629. [Google Scholar]
- (27).(a) Armstrong JD III; Hartner FW Jr.; DeCamp AE; Volante RP; Shinkai I Tetrahedron Lett. 1992, 33, 6599. [Google Scholar]; (b) McWilliams JC; Armstrong JD III; Zheng N; Bhupa- thy M; Volante RP; Reider PJ J. Am. Chem. Soc 1996, 118, 11970. [Google Scholar]
- (28).Lutz RE; Wayland RL Jr. J. Am. Chem. Soc 1951, 73, 1639. [Google Scholar]
- (29).Hassner A; Lorber ME; Heathcock C J. Org. Chem 1967, 32, 540. [DOI] [PubMed] [Google Scholar]
- (30).Ghosh AK; McKee SP; Sanders WM Tetrahedron Lett. 1991, 32, 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Moriarty RM; Hu H; Gupta SC Tetrahedron Lett. 1981, 22, 1283. [Google Scholar]; (b) Moriarty RM; Prakash O; Thachet CT Syn. Comm 1984, 14, 1373. [Google Scholar]
- (32).Tillyer RD; Boudreau C; Tschaen D; Dolling U-H; Reider PJ Tetrahedron Lett. 1995, 36, 4337. [Google Scholar]
- (33).Laksman MK; Zajc B Tetrahedron Lett. 1996, 37, 2529. [Google Scholar]
- (34).Greenberg S; Moffatt JG J. Am. Chem. Soc 1973, 95, 4016. [DOI] [PubMed] [Google Scholar]
- (35).Thompson WJ; Fitzgerald PMD; Holloway MK; Emini EA; Darke PL; McKeever BM; Schleif WA; Quintero JC; Zugay JA; Tucker TJ; Schwering JE; Homnick CF; Nunberg J; Springer JP; Huff JR J. Med. Chem 1992, 35, 1685. [DOI] [PubMed] [Google Scholar]
- (36).Rittle KE; Evans BE; Bock MG; Dipardo RM; Whit- ter WL; Homnick CF; Veber DF; Freidinger RM Tetrahedron Lett. 1987, 28, 521. [Google Scholar]
- (37).(a) Ninomiya K; Shiori T; Yamada S Tetrahedron 1974, 30, 2151. [Google Scholar]; (b) Grünewald GL; Ye QJ Org. Chem 1988, 53, 4021. [Google Scholar]; (c) Ghosh AK; McKee SP; Thompson WJ; Darke PL; Zugay JC J. Org. Chem 1993, 58, 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Boyd DR; Sharma ND; Bowers NI; Goodrich PA; Groocock MR; Blacker AJ; Clarke DA; Howard T; Dalton H Tetrahedron: Asymmetry 1996, 7, 1559. [Google Scholar]
- (39).Senanayake CH; Roberts FE; DiMichele LM; Ryan KM; Liu J; Fredenburgh LE; Foster BS; Douglas AW; Larsen RD; Verhoeven TR; Reider PJ Tetrahedron Lett. 1995, 36, 3993. [Google Scholar]
- (40).Ogasawara K; Takahashi M Synthesis 1996, 954. [Google Scholar]
- (41).Ghosh AK; Kincaid JF; Haske MG Synthesis 1997, 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).(a) Ghosh AK; Chen Y Tetrahedron Lett. 1995, 36, 505. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bianchi D; Cesti P; Battistel EJ Org. Chem 1988, 53, 5531. [Google Scholar]
- (43).Senanayake CH; Smith GB; Ryan KM; Fredenburgh LE; Liu J; Roberts FE; Hughes DL; Larsen RD; Verhoeven TR; Reider PJ Tetrahedron Lett. 1996, 37, 3271. [Google Scholar]
- (44).(a) Jacobsen EN; Zhang W; Muci AR; Ecker JR; Deng L J. Am. Chem. Soc 1991, 113, 7063. [Google Scholar]; (b) Jacobsen EN; Larrow FJ J. Am. Chem. Soc 1994, 116, 12129, and references cited therein. [Google Scholar]
- (45).Hughes DL; Smith GB; Liu J; Dezeny GC; Senanayake CH; Larsen RD; Verhoeven TR; Reider PJ J. Org. Chem 1997, 62, 2222. [DOI] [PubMed] [Google Scholar]
- (46).(a) Curran DP; Jeong K-S; Heffner TA; Rebek J Jr. J. Am. Chem. Soc 1989, 111, 9238. [Google Scholar]; (b) Boeckman RK Jr.; Liu Y J. Org. Chem 1996, 61, 7984; [DOI] [PubMed] [Google Scholar]; (c) Palomo C; Oiarbide M; Gonzalez A; Garcia JM; Berree F Tetrahedron Lett. 1996, 37, 4565, and also see Ref. 1. [Google Scholar]
- (47).(a) Nicolaou KC; Pavia MR; Seitz SP J. Am. Chem. Soc 1982, 104, 2027. [Google Scholar]; (b) Nicolaou KC; Pavia MR; Seitz SP ibici. 1982, 104, 2030. [Google Scholar]
- (48).Ghosh AK; Liu W; Xu Y; Chen Z Angew. Chem 1996, 108, 73; Angew. Chem. Int. Ed. Engl. 1996, 35, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Stratmann K; Burgoyne DL; Moore RE; Patterson GML; Smith CD J. Org. Chem 1994, 59, 7219. [Google Scholar]
- (50).Ghosh AK; Liu WJ Org. Chem 1996, 61, 6175. [DOI] [PubMed] [Google Scholar]
- (51).(a) Knowles WS; Sabacky MJ; Vineyard BD; Wein- kauff DJ J. Am. Chem. Soc 1975, 97, 2567. [Google Scholar]; (b) Vineyard BD; Knowles WS; Sabacky MJ; Bachman GL; Weinkauff DJ J. Am. Chem. Soc 1977, 99, 5946, and also see Ref. 2. [Google Scholar]
- (52).Ghosh AK; Hussain KA; Fidanze SJ Org. Chem 1997, 62, 6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).(a) Ghosh AK; Kincaid JF; Walters DE; Chen Y; Chaudhuri NC; Thompson WJ; Culberson C; Fitzgerald PMD; Lee HY; McKee SP; Munson PM; Duong TT; Darke PL; Zugay JA; Schleif WA; Axel MG; Lin J; Huff JR J. Med. Chem 1996, 39, 3278. [DOI] [PubMed] [Google Scholar]; (b) Rich DH; Sun C-Q; Vara Prasad JVN; Pathiasseril A; Toth MV; Marshall GR; Clare M; Mueller RA; Houseman KJ Med. Chem 1991, 34, 1222, and references cited therein. [DOI] [PubMed] [Google Scholar]
- (54).For references for syn-aldol methodologies, see: Kim B-M; Williams SF; Masamune S In Comprehensive Organic Synthesis; Trost BM; Fleming I; Eds.; Pergamon Press; Oxford, 1991; Vol 2 (Heathcock CH, Ed.), Chapter 1.7, 239.Franklin AS; Paterson I; Contemp. Org. Synth 1994, 317 and references cited therein.
- (55).For references for anti-aldol methodologies, see: Fleming I In Comprehensive Organic Synthesis, Vol 2; Heathcock CH, Ed., Pergamon Press; Oxford, 1991; pp 563.Abiko A; Liu J-F; Masamune SJ Am. Chem. Soc 1997, 119, 2586, and references cited therein.
- (56).Zimmerman HE; Traxler MD J. Am. Chem. Soc 1957, 79, 1920. [Google Scholar]
- (57).Gennari C; Cozzi GJ Org. Chem 1988, 53, 4015, and references cited therein. [Google Scholar]
- (58).Ghosh AK; Fidanze S, submitted to J. Org. Chem [Google Scholar]
- (59).Vazquez ML; Bryant ML; Clare M; DeCrescenzo GA; Doherty EM; Freskos JN; Getman DP; Houseman KA; Julien JA; Kocan GP; Mueller RA; Shieh H-S; Stallings WC; Stegeman RA; Talley JJ J. Med. Chem 1995, 38, 581. [DOI] [PubMed] [Google Scholar]
- (60).Mechanistic details of this reaction is the subject of ongoing investigation in our laboratory, Ghosh AK; Fidanze S, unpublished results.
- (61).Greenlee WJ; J. Med. Res. Rev/ 1990, 10, 173, and references cited therein. [DOI] [PubMed] [Google Scholar]
- (62).For previous nonamino acid derived synthesis, see: Ghosh AK; McKee SP; Thompson WJ J. Org. Chem 1991, 56, 6500.Ghosh AK; McKee SP; Lee HY; Thompson WJJ Chem. Soc., Chem. Commun 1992, 273, and Ref. 52.
- (63).Davies IW; Senanayake CH; Castonguay L; Larsen RD; Verhoeven TR; Reider PJ Tetrahedron Lett. 1995, 36, 7619. [Google Scholar]
- (64).Kress MH; Yang C; Yasuda N; Grabowski EJ J. Tetrahedron Lett 1997, 38, 2633. [Google Scholar]
- (65).Zheng N; Armstrong JD III; McWilliams C; Volante RP Tetrahedron Lett. 1997, 38, 2817. [Google Scholar]
- (66).Itsuno S; Sakurai Y; Ito A; Hirao A; Nakahama S Bull. Chem. Soc. Jpn 1987, 60, 395, and references cited therein. [Google Scholar]
- (67).(a) Wallbaum S; Martens J Tetrahedron:Asymmetry 1992, 3, 1475. [Google Scholar]; (b) Deloux L; Srebnlk M Chem. Rev 1993, 93, 763, and references cited therein. [Google Scholar]
- (68).DiSimone B; Savoia D; Tagliavini E; Umani-Ronchi A Tetrahedron: Asymmetry 1995, 6, 301. [Google Scholar]
- (69).Hett R; Fang QK; Gao Y; Hong Y; Butler HT; Nie X; Wald SA Tetrahedron Lett. 1997, 38, 1125. [Google Scholar]
- (70).Hett R; Senanayake CH; Wald SA Submitted to Tetrahedron. [Google Scholar]
- (71).Palmer M; Walsgrove T; Wills MJ Org. Chem 1997, 62, 5226. [Google Scholar]
- (72).Ghosh AK; Mathivanan P; Cappiello J Tetrahedron: Asymmetry 1998, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).(a) Pfaltz A Acta Chem. Scan 1996, 50, 189. [Google Scholar]; (b) Pfaltz A Acc. Chem. Res 1993, 26, 339. [Google Scholar]; (c) Pfaltz A Chimia 1990, 44, 202. [PubMed] [Google Scholar]
- (74).(a) Lowenthal RE; Abiko A; Masamune S Tetrahedron Lett. 1990, 31, 6005. [Google Scholar]; (b) Lowenthal RE; Masamune S Tetrahedron Lett. 1991, 32, 7373. [Google Scholar]
- (75).(a) Corey EJ; Imai N; Zhang H J. Am. Chem. Soc. 1991, 113, 728. [Google Scholar]; (b) Corey EJ; Ishihara K Tetrahedron Lett. 1992, 33, 6807. [Google Scholar]
- (76).(a) Evans DA; Miller SJ; Lectka T J. Am. Chem. Soc 1993, 115, 6460. [Google Scholar]; (b) Evans DA; Kozlowski MC; Tedrow JS Tetrahedron Lett. 1996, 37, 7481. [Google Scholar]; (c) Evans DA; Johnson JS J. Org. Chem 1997, 62, 786, and references cited therein. [Google Scholar]
- (77).Hall J; Lehn JM; DeCian A; Fischer J Helv. Chim. Acta 1991, 74, 1. [Google Scholar]
- (78).Davies IW; Gerena L; Castonguay L; Senanayake CH; Larsen RD; Verhoeven TR; Reider PJ J. Chem Soc. Chem. Commun 1996, 1753. [Google Scholar]
- (79).Davies IW; Senanayake CH; Larsen RD; Verhoeven TR; Reider PJ Tetrahedron Lett. 1996, 37, 1725. [Google Scholar]
- (80).Kanemasa S; Oderaotoshi Y; Yamamoto H; Tanaka J; Wa- da E; Curran DP J. Org. Chem 1997, 62, 6454. [Google Scholar]
- (81).Ghosh AK; Cappiello J; Cho H Unpublished work.
- (82).Holloway MK; Wai JM; Halgren TA; Fitzgerald PMD; Vacca JP; Dorsey BD; Levin RB; Thompson WJ; Chen LJ; deSolms SJ; Gaffin N; Ghosh AK; Giuliani EA; Graham SL; Guare JP; Hungate RW; Lyle TA; Sanders WM; Tucker TJ; Wiggins M; Wiscount CM; Woltersdorf OW; Young SD; Darke PL; Zugay JA J. Med. Chem 1995, 38, 305. [DOI] [PubMed] [Google Scholar]
- (83).Davies IW; Gerena L; Cai D; Larsen RD; Verhoeven TR; Reider PJ Tetrahedron Lett. 1997, 38, 1145. [Google Scholar]
- (84).(a) Corey EJ; Cywin CL; Roper TD Tetrahedron Lett.1992, 33, 6907. [Google Scholar]; (b) Mikami K; Motoyama Y; Terada MJ Am. Chem. Soc 1994, 116, 2811. [Google Scholar]; (c) Motoyama Y; Mikami KJ Chem. Soc. Chem. Commun 1994,1563. [Google Scholar]; (d) Keck GE; Li X-Y; Krishnamurthy D J. Org. Chem 1995, 60, 5998, and references cited therein. [Google Scholar]
- (85).For synthetic application of wide variety of dihydropyranones, see: Danishefsky SJ Aldrichim. Acta 1986, 19, 59.Bednarski MD; Lyssikatos JP Compr. Org. Synth 1991,2, 661.
- (86).(a) Quinoa E; Kakou Y; Crews P J. Org. Chem 1988, 53, 3642. [Google Scholar]; (b) Corley DG; Herb R; Moore RE; Scheuer PJ; Paul VJ J. Org. Chem 1988, 53, 3644. [Google Scholar]
- (87).(a) Evans DA; Woerpel KA; Scott MJ Angew. Chem.1992, 104, 439; Angew. Chem. Intl. Ed. Engl. 1992, 31, 430. [Google Scholar]; (b) Gant TG; Noe MC; Corey EJ Tetrahedron Lett. 1995, 36, 8745. [Google Scholar]; (c) Pfaltz A Acta Chem. Scand. 1996, 50, 189, and references cited therein. [Google Scholar]
- (88).(a) Wu JH; Radinov R; Porter NA J. Am. Chem. Soc 1995, 117, 11029. [Google Scholar]; (b) Sibi MP; Ji J; Wu J-H; Gurtler S; Porter NA J. Am. Chem. Soc 1996, 118, 9200. [Google Scholar]
- (89).(a) Sibi MP; Ji J J. Org. Chem 1997, 62, 3800. [Google Scholar]; (b) Sibi MP; Shay JJ; Ji J Tetrahedron Lett. 1997, 38, 5955. [Google Scholar]
- (90).Dorsey BD; Levin RB; McDaniel SL; Vacca JP; Guare JP; Darke PL; Zugay JA; Emini EA; Schleif WA; Quintero JC; Lin JH; Chen I-W; Holloway MK; Fitzgerald PMD; Axel MG; Ostovic D; Anderson PS; Huff JR J. Med. Chem 1994, 37, 3443, and references cited therein. [DOI] [PubMed] [Google Scholar]
- (91).(a) First HIV Protease Inhibitors Approved by FDA. Antiviral Agents Bull. 1995, 8 (12) 353. [Google Scholar]; (b) Cohen J Science, 1996, 272, 1882. [DOI] [PubMed] [Google Scholar]
- (92).Young SD; Payne LS; Thompson WJ; Gaffin N; Lyle TA; Britcher SF; Graham SL; Schultz TH; Deana AA; Darke PL; Zugay J; Schleif WA; Quintero JC; Emini EA; Anderson PS; Huff JR J. Med. Chem 1992, 35, 1702. [DOI] [PubMed] [Google Scholar]
- (93).Askin D; Wallace MA; Vacca JP; Reamer RA; Volante RP; Shinkai IJ Org. Chem 1992, 57, 2771. [Google Scholar]
- (94).Bone R; Vacca JP; Anderson PS; Holloway MK J. Am. Chem. Soc 1991, 113, 9382. [Google Scholar]
- (95).Evans BE; Rittle KE; Homnick CF; Springer JP; Hirshfield J; Veber DF; J. Org. Chem 1985, 50, 4615, and also see Ref. 58. [Google Scholar]
- (96).Thompson WJ; Ball RG; Darke PL; Zugay JA; Thies JE Tetrahedron Lett. 1992, 33, 2957. [Google Scholar]
- (97).Thaisrivongs S; Turner SR; Strohbach JW; TenBrink RE; Tarpley WG; McQuade TJ; Heinrickson RL; To- masselli AG; Hui JO; Howe WJ J. Med. Chem 1993, 36, 941. [DOI] [PubMed] [Google Scholar]
- (98).Dorsey BD; Plzak KJ; Ball RG Tetrahedron Lett. 1993, 34, 1851. [Google Scholar]
- (99).Ghosh AK; Thompson WJ; McKee SP; Duong TT; Lyle TA; Chen JC; Darke PL; Zugay JA; Emini EA; Schleif WA; Huff JR; Anderson PS J. Med. Chem 1993, 36, 292. [DOI] [PubMed] [Google Scholar]
- (100).Roberts NA; Martin JA; Kinchington D; Broadhurst AV; Craig JC; Duncan IB; Galpin SA; Handa BK; Kay J; Krohn A; Lambert RW; Merrett JH; Mills JS; Parkes KEB; Redshaw S; Ritchie AJ; Taylor DL; Thomas GJ; Machin PJ Science 1990, 248, 358. [DOI] [PubMed] [Google Scholar]
- (101).Askin D; Eng KK; Rossen K; Purick RM; Wells KM; Volante RP; Reider PJ Tetrahedron Lett. 1994, 35, 673. [Google Scholar]
- (102).(a) Maligres PE; Upadhyay V; Rossen K; Cianciosi SJ; Purick RM; Eng KK; Reamer RA; Askin D; Volante RP; Reider PJ Tetrahedron Lett. 1995, 36, 2195. [Google Scholar]; (b) Maligres PE; Weissman SA; Upadhyay V; Cianciosi SJ; Reamer RA; Purick RM; Sager J; Rossen K; Eng KK; Askin D; Volante RP; Reider PJ Tetrahedron 1996,52, 3327. [Google Scholar]
- (103).Rossen K; Volante RP; Reider PJ; Tetrahedron Lett.1997,38, 777. [Google Scholar]
- (104).(a) Reider PJ Chimia 1997, 51, 306. [Google Scholar]; (b) Davies IW; Reider PJ Chem. & Ind. (London) 1996, 412. [Google Scholar]
- (105).Rossen K; Weissman SA; Sager J; Reamer RA; Askin D; Volante RP; Reider PJ Tetrahedron Lett. 1995,36, 6419. [Google Scholar]
- (106).Dorsey BD; McDaniel SL; Levin RB; Vacca JP; Darke PL; Zugay JA; Emini EA; Schleif WA; Lin JH; Chen I-W; Holloway MK; Anderson PS; Huff JR Bioorg. & Med. Chem. Lett 1994, 4, 2769. [DOI] [PubMed] [Google Scholar]
- (107).Coburn CA; Young MB; Hungate RW; Isaacs RCA; Vacca JP; Huff JR Bioorg. & Med. Chem. Lett 1996, 6, 1937. [Google Scholar]
- (108).Lehr P; Billich A; Charpiot B; Ettmayer P; Scholz D; Rosenwirth B; Gstach HJ Med. Chem 1996, 39, 2060. [DOI] [PubMed] [Google Scholar]
- (109).(a) Hashimoto Y; Kai A; Saigo K Tetrahedron Lett. 1995, 36, 8821. [Google Scholar]; (b) Sudo A; Saigo K Tetrahedron: Asymmetry 1996, 7, 2939. [Google Scholar]; (c) Sudo A; Saigo K Chem. Lett 1997, 97, and references cited therein. [Google Scholar]