Abstract
A review on the syntheses of bioactive compounds published since 1995 using tartaric acid and its derivatives as synthons is presented.
Keywords: tartaric acid, tartrate, synthesis, natural products, bio-active
1. Introduction
Asymmetric syntheses of organic molecules containing multiple stereocenters are of considerable importance in synthetic organic and medicinal chemistry. Efficient and cost-effective syntheses of such molecules are often significant synthetic challenges. For practical and economic reasons, it is ideal if such syntheses can be achieved by selective manipulation of readily available and inexpensive starting materials. Also, enantioselective syntheses of natural products are increasingly important since isolation from natural sources often can only be accomplished in minute quantities. Thus, stereochemically defined synthons are critical for meaningful biological studies. The use of tartaric acid and tartrate esters offers several important advantages in this field. This inexpensive chiron provides convenient access to an enantiomerically pure material for use in synthesis. It allows two stereocenters to be unambiguously set in a target molecule. This provides for structure elucidation including absolute stereochemical assignment of complex natural products, as well as the synthesis of structural analogs for structure-function studies.
Over the years there has been an impressive number of papers dealing with the use of tartaric acid and its derivatives in synthesis.1 An overview of tartaric acid as a resolving agent, chiral auxiliary and the majority of tartrate-derived synthons can be found in the 1998 publication by Gawronski and Gawronska.2 There are a number of other publications covering the application of tartaric acid and tartrates in 1,3-dipolar cycloadditions,3 as a “tether control group” in the Diels–Alder reaction,4 in combinatorial Diels–Alder reactions5 and their uses in asymmetric synthesis in general.6 In this review we intend to cover the use of tartaric acid and tartrate esters in the synthesis of biologically active products since 1995. The compounds covered will be distributed into six categories including: acyclic 1,2-diols, carbocycles, furans and pyrans, lactones and lactams, nitrogen heterocycles and miscellaneous compounds.
2. Acyclic 1,2-Diols
A very common subunit in the structure of biologically active molecules is the 1,2-diol. Control over the stereo-chemical formation of this subunit in the synthesis of these products is of great importance to synthetic chem ists. A convenient means of controlling this moiety is by its introduction through the incorporation of a tartaric acid-derived synthon. This allows the synthetic chemist explicit control over two stereocenters in a target molecule. Several bioactive compounds have been synthesized in this manner.
2.1. PQ-8
Several cytotoxic C14 and C17 polyacetylene compounds with potent antitumor activity have been isolated from the oriental medicinal plants Panax quinguefolium and Panax ginseng. Tartaric acid was found to be a convenient chiral precursor for the synthesis and elucidation of absolute stereochemistry of the compounds in this class. PQ-8 is a C14 representative of this class of cytotoxic polyacetylenes. The first synthesis of this compound and elucidation of its absolute configuration at the C6 and C7 centers were reported by Satoh and co-workers.7 The epoxide functionality was constructed using the 1,2-diol of (+)-diethyl-L-tartrate, as shown in Scheme 1. Both possible enantiomers were prepared starting with the same chiral template. The tartrate ester was transformed in seven steps into an epoxy alcohol 2,8 which was opened with a bisacetylene moiety. Tosylation of the resulting diol provided a mixture of the 6-tosyloxy and 7-tosyloxy alcohols. Each was converted into one enantiomer of the epoxy derivative upon treatment with potassium carbonate in methanol. Based on the optical rotation of the final products, the absolute stereo-chemistry of naturally occurring PQ-8 was assigned to be (6R,7S).
Scheme 1.
2.2. Panaxydol
The first enantioselective total synthesis of another cytotoxic polyacetylene, panaxydol, was recently reported by Lu and co-workers.9 The absolute configuration at the C9 and C10 centers was established using (+)-diethyl-L-tartrate as a chiral template. The key steps were preparation of protected epoxy alcohol 8, epoxide opening using an acetylenic moiety and coupling with another acetylene containing fragment (Scheme 2). As a result, panaxydol was obtained in 14 steps and 10% overall yield.
Scheme 2.
2.3. Panaxytriol
In order to elucidate the absolute configuration at the C3 center of panaxytriol, a potent antitumor agent, Lu and coworkers performed the synthesis of each of the two possible diastereomers [(3R,9R,10R) and (3R,9S,10S)] starting from optically active precursors.11 Retrosynthetic analysis of the molecule resulted in two fragments: bromoacety lene 15, which contains both the C9 and C10 stereo-centers, and terminal acetylene 14 (Figure 1).
Figure 1.
Retrosynthesis of panaxytriol
The bromoacetylene precursor for the (3R,9S,10S) diastereomer was enantioselectively prepared starting with L-tartaric acid as a chiral template (Scheme 3). Synthesis of the bromoacetylene fragment of the (3R,9R,10R) diastereomer was accomplished starting from D-arabinose. The coupling of each diastereomer of the bromoacetylene fragment with the terminal acetylene fragment followed by deprotection yielded each diastereomer of panaxytriol. As a result, the absolute stereochemistry of the natural product was assigned as (3R,9R,10R).
Scheme 3.
2.4. (+)-Polyoxin J
(+)-Polyoxin J is a natural product containing a polyhydroxylated amino acid moiety. This molecule has exhibited potent antifungal properties as well as high inhibitory potencies against isolated chitin synthetase from the human pathogen Candida albicans. Its synthesis, reported by Ghosh and Wang, was achieved in a convergent manner.12 The retrosynthesis shown in Figure 2 reveals the two major fragments of the synthesis, structures 19 and 20.
Figure 2.
Retrosynthesis of (+)-polyoxin J
The synthesis of the western, polyoxamic acid containing fragment 19 began with dimethyl-L-tartrate, and through a multi-step sequence, it was converted into allylic alcohol 22 (Scheme 4).13 This allylic alcohol was subjected to Sharpless asymmetric epoxidation followed by regioselective epoxide opening using diisopropoxytitanium diazide and hydrogenation to yield the corresponding amino diol as a mixture of two regioisomers, 23a and 23b. Multiple steps allowed the transformation of amino diol 23a into western fragment 19.
Scheme 4.
The eastern fragment 20 was achieved starting from the known methyl glycoside 24 (Scheme 5).14 It was converted via known procedures to produce epoxy alcohol 25.15 This epoxide was opened regioselectively as above, using diisopropoxytitanium diazide, to yield the corresponding diol, again as a mixture of regioisomers. The eastern fragment was completed by multiple transformations including coupling with Thymine-bis-TMS and hydrogenation of the azide to the corresponding amine.16 Thus, the two fragments, 19 and 20, were coupled using benzotriazol-1-yloxytris(dimethylamino)phosphomium hexafluorophosphate (BOP) followed by deprotection to yield (+)-polyoxin J.
Scheme 5.
2.5. Sphingofungin F
Sphingofungin F, another 1,2-diol-containing natural product, belongs to a group of sphingosine-like natural products containing an α-substituted alanine unit. Recently, Lin and co-workers reported a total synthesis of sphingofungin F based on a Lewis acid-catalyzed intramolecular epoxide opening reaction using a nitrogen nucleophile as a key step.17 Tartaric acid was used as a precursor in the synthesis of the polyhydroxylated starting material 6 (Scheme 6).10b Wittig olefination and Sharpless asymmetric epoxidation (resulting in a 3:1 mixture of isomers) were the key steps for the preparation of epoxide 29 (major isomer). The side chain was attached to the core structure in several steps including Wittig olefination, resulting in epoxide 30. The nitrogen functionality was introduced regioselectively via Hatakeyama’s method.18 Treatment of the epoxide moiety with trichloroacetonitrile and DBU followed by intramolecular epoxide opening catalyzed by BF3·(OEt)2 resulted in oxazoline 31. The oxazoline was subsequently converted into sphingofungin F over several steps.
Scheme 6.
2.6. (+)-Bengamide E
The heavily oxygenated polyhydroxylated molecule (+)-bengamide is a natural product that contains a 1,2-diol moiety. This molecule was synthesized via a Lewis acid-promoted aldol reaction starting from diisopropyl-D-tartrate, as shown in Scheme 7.19 Aldol reaction between silyl enol ether 37 and tartrate-derived aldehyde 36 proceeded in a highly stereoselective manner, resulting in only one diastereomer of cobalt-complexed thioester 38. Precomplexation of the acetylenic moiety with cobalt hexacarbonyl improved the stereoselectivity of this reaction. The total synthesis was completed after formation of the amide with (S)-3-aminoazepan-2-one and reduction of the alkyne moiety to the trans olefin.
Scheme 7.
3. Carbocycles
3.1. (–)-Rishitin
(–)-Rishitin is a defensive agent (phytoalexin) against Phytophora infestanus, a microorganism, which infests potatoes. It was synthesized by Chen and Marx21 from the aldol product of 42, derived from D-tartaric acid following the sequence of Iida and co-workers,13 and enol ether 43, derived from R-(–)-carvone.22 Radical cyclization of the corresponding iodide 45 followed by acidic hydrolysis led to (–)-rishitin (47, Scheme 8).
Scheme 8.
3.2. (–)-Methyl Jasmonate and (+)-Methyl Epijasmonate
Roth and co-workers developed a three-step method for the synthesis of optically active cyclopentanes from tartaric acid employing phosphorus ylide chemistry. Several cyclopentanoid natural products were obtained using these chiral building blocks. Recently, the authors reported the synthesis of two carbocyclic natural products, the structurally related fragrance constituents of jasmine oil (–)-methyl jasmonate (48) and (+)-methyl epijasmonate (49, Figure 3), employing the reported method.23
Figure 3.
Structures of (–)-methyl jasmonate and (+)-methyl epijasmonate
Tartaric acid-derived cyclopentenone 51 was used as a precursor in the synthesis of both jasmonates, as shown in Scheme 9.24 The Diels–Alder reaction of 51 with 2-methoxybutadienone in LiClO4 and Et2O resulted in the cis bicyclic ketone 53, which was converted in four steps into (–)-methyl jasmonate. As a result, (–)-methyl jasmonate was prepared in 6% overall yield from cyclopentenone 51. The same bicyclic ketone 53 was used as a precursor in the synthesis of (+)-methyl epijasmonate. A modification to the synthesis was made as the carbonyl functionality had to be protected to prevent enolization and preserve a cis configuration of the side chains. (+)-Methyl epijas-monate was prepeared from intermediate 51 in 14% overall yield.
Scheme 9.
3.3. (+)- and (–)-Isoterrein
Mikolajczyk and co-workers developed an efficient method for the production of substituted cyclopentenones and used it in their syntheses of both enantiomers of isoter-rein.25 These molecules are structural isomers of terrein, a metabolite of the mold Aspergillus terreus, which has the corresponding trans diol moiety. Both of the target molecules were synthesized beginning with the condensation between meso-tartaric acid and (+)-camphor (Scheme 10). The resulting spiro cycle 60 was treated with dimethyl lithiomethanephosphonate and yielded the two diastereomers of protected 3-phosphonomethylcyclopentenone 61a and 61b. The two isomers were separable by chromatography and were independently carried through to the final product by Horner–Emmons–Wittig reaction followed by acidic hydrolysis of the camphor moiety to afford (+)- and (–)-isoterrein.
Scheme 10.
3.4. Erythrina alkaloids
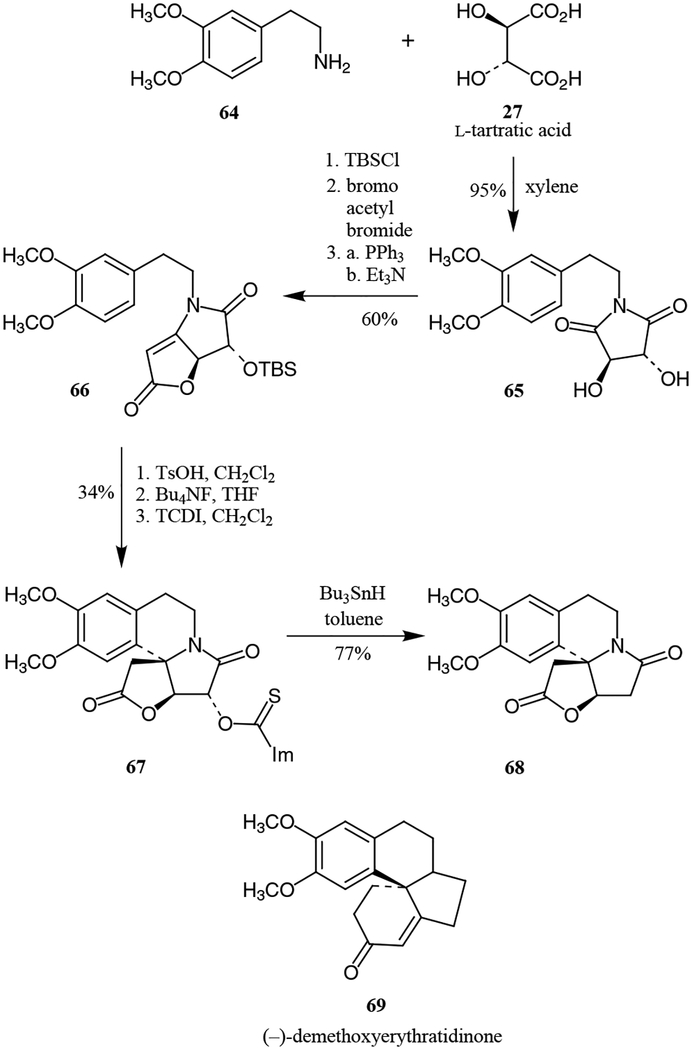
The tetracyclic heterocycle furo[3’,2’:2,3]pyrrolo[2,1-a]isoquinoline 68 is an important intermediate towards synthesis of the Erythrina alkaloids such as (–)-demethoxyerythratidinone 69. The synthesis by Lee and co-workers began with the coupling of L-tartaric acid with 2-(3,4-dimethoxyphenyl)ethylamine to produce the hydroxy imide 65, as shown in Scheme 11.26 After production of the unsaturated lactone moiety of 66, N-acyliminium ion cyclization with p-TsOH yielded the tetracyclic core which, upon deprotection and deoxygenation, afforded the tetracyclic target molecule 68.
Scheme 11.
3.5. (+)-Conduritol-E
Many compounds of a series, known as conduritols, have been shown to inhibit the action of glycosidases. These compounds are also useful as building blocks for more complex molecules. (+)-Conduritol-E is one in this class of compounds and the synthesis of its dimethoxy analog from diethyl-D-tartrate was reported by Lee and Chang in 1999.27
As shown in Figure 4, the synthesis of this conduritol derivative began with diethyl-L-tartrate and continued using several protection steps and a stereoselective Grignard addition to yield intermediate 71. This compound was exposed to ring-closing olefin metathesis conditions utilizing Grubbs’ catalyst followed by deprotection of the acetate groups to produce the target molecule.28
Figure 4.
Retrosynthesis of (+)-conduritol-E
4. Furans and Pyrans
4.1. (–)-Koninginin A
The members of the koninginin family of natural products contain a polyhydroxylated pyran ring system and have shown substantial antibiotic activity. Synthesis from tartaric acid has been used to determine the absolute configuration of several natural products in this family. (–)-Koninginin A was first synthesized by Mori,29 although the absolute stereochemistry was not correctly determined until the enantioselective synthesis of several possible isomers reported by Xu and co-workers.30 In Xu’s route, natural L-(+)-tartaric acid was used to introduce the C9 and C10 stereocenters. As shown in Figure 5, tartaric acid was converted into alkene 74 followed by hydroboration, Suzuki coupling and the addition of singlet oxygen to form intermediate 73. Acid-catalyzed deprotection of the isopropylidene moiety spontaneously led to cyclization, and after hydrogenation over Pd/C, (–)-koninginin A was isolated. The authors also prepared a diastereomer starting from the enantiomeric tartaric acid, and after extensive NMR studies of the two diastereomeric products, the absolute configuration of (–)-koninginin A was assigned as shown below (1S,4R,5S,6S,9S,10S).
Figure 5.
Retrosynthesis of (–)-koninginin A
4.2. (+)-Koninginin D
A structural analog of (–)-koninginin A called (+)-koninginin D was synthesized as shown in Scheme 12, by Liu and Wang.31 It was assumed that the absolute configuration at the C9 and C10 centers in (+)-koninginin D was the same as in (–)-koninginin A. Therefore, L-tartaric acid again could be used as a chiral building block in order to establish the stereochemistry of the two vicinal hydroxyl groups. Tartrate-derived aldehyde 77 was coupled with 1,3-cyclohexadione according to Paquette’s procedure.32 Treatment of thionide 78 with dilute hydrochloric acid resulted in deprotection and cyclization to afford 79, which was converted into (+)-koninginin D in several steps. As a result, the relative and absolute stereochemistry of (+)-koninginin D was established and this natural product was stereoselectively synthesized starting from L-tartaric acid with 4.1% overall yield.
Scheme 12.
4.3. Thromboxane B2
Another pyran-containing natural product molecule, thromboxane B2, is an important substance for the study of a wide variety of biological processes as well as an important synthetic intermediate for the platelet aggregating agent thromboxane A2. The synthetic route to thromboxane B2, devised by Masaki and co-workers in 1996, begins from diethyl-L-tartrate.34 Conversion of this tartaric ester into bicyclic compound 81 is accomplished in a known six step sequence.35 This bicyclic compound was carried through several transformations including a stereoselective epoxidation and a Claisen rearrangement to set the necessary chirality about the pyran ring, before completion of the synthesis (Scheme 13).
Scheme 13.
4.4. (–)-Kumausallene
(–)-Kumausallene is a natural product isolated from the red algae Laurencia nipponica. It contains a 2,6-dioxabicyclo[3.3.0]octane ring system in its core. Lee and coworkers developed a way to build this structure using two concomitant radical cyclizations of β-alkoxyacrylates.36 Diethyl-D-tartrate was converted into the corresponding bis(phenylselenide) via preparation of ditosylate 88 (Scheme 14). Treatment of bis(selenide) 89 with methyl propionate resulted in the corresponding bis(β-alkoxyacrylate) which was subjected to radical cyclization conditions to yield the corresponding bicyclic compound as a mixture of diastereomers (10:1). The resulting product was transformed in several steps into primary alcohol 91c (found in Overman’s synthesis of (–)-kumausallene),37 thus completing it’s formal total synthesis.
Scheme 14.
4.5. Zaragozic Acid C
Tartaric acid was found to be an excellent precursor for the synthesis of the heavily oxygenated furan core of zaragozic acids, also known as squalestatins. These natural products have become attractive synthetic targets due to their tremendous biological activity as squalene synthase inhibitors and challenging structures. The acyclic precursor of the bicyclic ketal of zaragozic acid C was prepared via several routes.
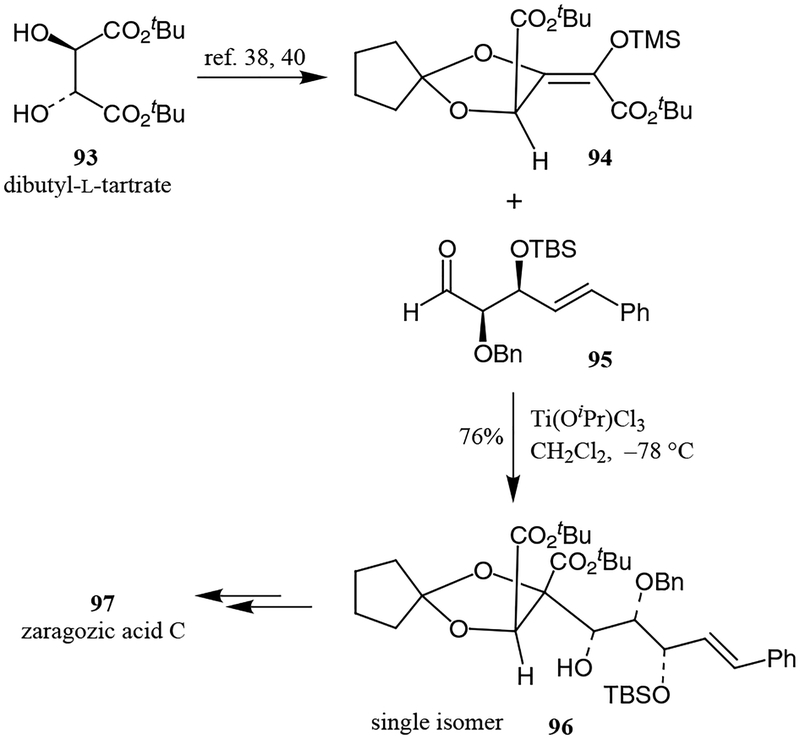
In 1981, Seebach demonstrated that dimethyl tartrate acetonide forms a stable enolate that can be trapped with various electrophiles with high stereoselectivity.38 Evans and co-workers first approached the synthesis of the acyclic precursor 96 using this method (Scheme 15).39 Thus, the synthesis of precursor 96 via a Lewis acid [Ti(OiPr)Cl3]-catalyzed aldol addition of aldehyde 95 to tartaric acid-derived silylketene acetal 94 resulted in adduct 96 as a single isomer.40 The aldol product was transformed to zaragozic acid C over several steps.
Scheme 15.
Sato and co-workers, in their highly convergent synthesis, also used a Lewis acid [Sn(OTf)2]-promoted aldol coupling reaction as a key step leading to formation of the C4–C5 bond with simultaneous creation of the contiguous quaternary carbon centers.41 In this approach however, both of the coupling components 98 and 99 were formed from the L- and D-isomers of tartaric acid, respectively, as shown in Scheme 16.42 The key aldol reaction resulted in a 1.6:1 mixture of 100 and 5-epi-100.
Scheme 16.
5. Lactones and Lactams
Lactones and lactams are very important and common structural units in biologically active molecules. Their stereoselective construction has been the focus of many research groups. Below is a list of natural products, containing this moiety, that have been synthesized through the use of tartaric acid and its derivatives.
5.1. (–)-Cinatrin C1 and C3
Cinatrin C1 and C3 are recently isolated members of a family of phospholipase inhibitors. Both γ-lactones can be derived from dioxolane 101, which resembles the acyclic precursor for zaragozic acid (Scheme 16). Therefore, the same Lewis acid-promoted aldol reaction that was used by Evans and co-workers as a key step in the synthesis of zaragozic acid C can be used in the synthesis of these two compounds.43 As shown in Scheme 17, tartrate-derived silylketene acetate 102 underwent a stereoselective Lewis acid-catalyzed aldol condensation resulting in aldol 104 as a single diastereomer. This compound was converted into both (–)-cinatrin C1 (106) and (–)-cinatrin C3 (105).
Scheme 17.
5.2. (S,S)-Sapinofuranone B
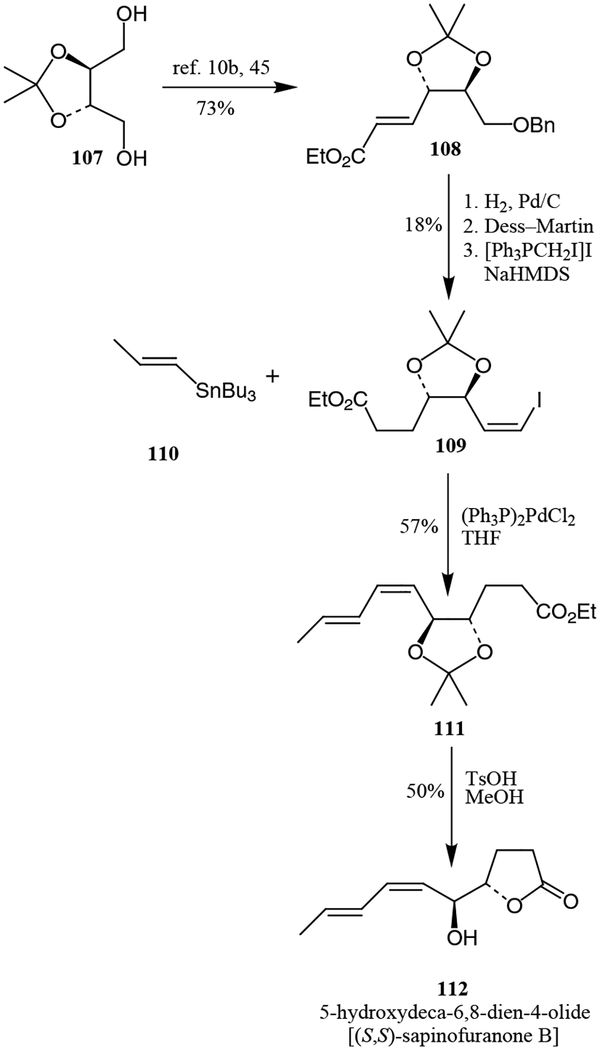
(4S,5S,6Z,8E)-5-Hydroxydeca-6,8-dien-4-olide, also known as (S,S)-sapinofuranone B, is a recently isolated γ-lactone metabolite of Sphaeropsis sapinae. Cluogh and co-workers reported the structure and stereochemical assignment of this unsaturated analog of L-factor.44 The stereoselective total synthesis, shown in Scheme 18, provided definitive proof for the structure and stereo-chemistry of (4S,5S,6Z,8E)-5-hydroxydeca-6,8-dien-4-olide [(S,S)-sapinofuranone B]. Diol 107, derived from L-tartrate, was converted into unsaturated ester 108 in several steps.10b,45 Conversion of unsaturated ester 108 to iodide 109 followed by Stille coupling led to diene 111. Finally, deprotection and lactonization provided (S,S)-sapinofuranone B in 50% yield.
Scheme 18.
5.3. (+)-Muricatacin
(+)-Muricatacin is an important biologically active natural product that has shown cytotoxicity against various lines of tumor cells. The synthetic route for the construction of this γ-lactone-containing natural product was devised by Somfai and begins with diethyl-L-tartrate.46 This tartrate ester was converted into derivative 113 through known procedures.47 Appending of the C11 side chain was followed by conversion of the protected diol moiety into the corresponding epoxide 115, as shown in Scheme 19. Nucleophilic opening of the epoxide with an alkynyl anion was followed by lactonization and deprotection to yield (+)-muricatacin.
Scheme 19.
5.4. Syringolide, Secosyrin and Syributin
Syringolides 1 and 2 (117) are compounds produced by P. syringae pv. tomato and function as specific molecular signals which cause hypersensitive responses. The co-products of the syringolides, namely the secosyrins and syributins, are also isolated from P. syringae pv. tomato but are not active elicitors themselves. However, these compounds are of interest for the understanding of their biosynthetic pathways. One of the common structures of this family of compounds is their γ-lactone moiety (Figure 6).
Figure 6.
Structures of syringolide, secosyrin and syributin
The syntheses of syringolides 1 and 2 by Kuwahara and co-workers are shown in Scheme 20.48 These syntheses begin with a D-tartrate ester and transform it into the protected threitol derivative 120.49 Conversion to β-keto ester 121 (R = 3-oxodecanoic acid leads to formation of syringolide 2 while R = 3-oxooctanoic acid leads to formation of syringolide 1) was followed by Knoevenagel condensation catalyzed by silica gel in a hexane–ethyl acetate mixture to yield 122. Exposure of the unsaturated lactone to an acidic resin allowed for spontaneous cyclization to form syringolide 2.
Scheme 20.
Mukai and co-workers reported the first total synthesis of secosyrin 1.50 As shown in Scheme 21, this synthesis started from diisopropyl-D-tartrate and proceeded by converting it into the protected threitol derivative 124.51 This compound was subjected to several transformations to yield alkyne 125. Deoxygenation to form furan 126 followed by lactonization, deprotection and condensation with hexanoic anhydride provided secosyrin 1. The appropriate choice of anhydride can be used to generate secosyrin 2. In addition, treatment of secosyrin 1 with lithium hexamethyldisilazide afforded syributin 1 (119), thus completing the synthesis of two natural products via the same synthetic route.
Scheme 21.
5.5. (+)-Boronolide
(+)-Boronolide is a δ-lactone-containing natural product reported to be effective against malaria. Its synthesis, beginning from diethyl-D-tartrate was completed by Ghosh and Bilcer in 2000.51 Conversion of the tartrate ester to threitol derivative 75 was accomplished through known procedures.10b This threitol derivative was then transformed to the corresponding Weinreb amide and further reacted with butyl Grignard. The resulting ketone was selectively reduced to afford alcohol 128 (Scheme 22). The benzyloxy side chain was converted into diene 129 over several steps and further subjected to ring-closing olefin metathesis conditions.52 Completion of the synthesis was accomplished through a deprotection/reprotection sequence.
Scheme 22.
5.6. Pinolidoxin
Pinolidoxin (Figure 7) is the main phytotoxin produced by Aschochyta pinodes, a species responsible for crop diseases. This macrolactone, as well as two of its structural analogs, were recently isolated and characterized by de Napoli and co-workers.53 In the course of the reported investigation of structure-activity relationships, the authors determined the absolute stereochemistry at the C7, C8 and C9 centers. The C6–C18 fragment was stereoselectively synthesized and its spectral data were compared with those of the corresponding analog obtained via degradation of natural pinolidoxin.
Figure 7.
Structure of pinolidoxin
Four stereoisomers corresponding to the C6–C18 fragment with syn relative stereochemistry at the C7 and C8 centers were synthesized as fully benzoylated heptanetetrols. All four isomers were easily accessible starting with meso-tartaric acid and were obtained as two racemic mixtures as shown in Figure 8. Spectral data of one of the resulting benzoates correlated well with the degradation sample. The syn, syn diastereomer of TBS-protected alcohol 134a and pinolidoxin itself were subjected to Mosher ester analysis in order to determine the absolute configuration of the natural product. As a result, the configuration at the C7, C8 and C9 centers was assigned as shown in Figure 7 above.
Figure 8.
Retrosynthesis of pinolidoxin
5.7. L-Biopterin
L-Biopterin is an important molecule in many biological processes including: the conversion of Phe to Tyr and Tyr to DOPA, melanine synthesis and Trp hydroxylation. Because of its numerous activities, tetrahydrobiopterin has been proposed for treatment of diseases such as Alzheimer’s, Parkinson’s, phenylketonuria and depression. The synthesis envisioned by Duhamel and Fernandez involves the condensation of 2,5,6-triamino-4-pyrimidinol with 5-deoxy-L-arabinose phenylhydrazone prepared directly from 5-deoxy-L-arabinose.54 L-Tartaric acid was used as the synthon for the production of the deoxy sugar as shown in Scheme 23. Therefore, L-tartaric acid was converted into pivaloyl aldehyde 136 following literature procedures.55 Nucleophilic addition to the alde-hyde followed by hemiacetal formation led to 5-deoxy-L-arabinose (137). Conversion of the deoxy sugar to the phenylhydrazone, followed by coupling with 2,5,6-tri-amino-4-pyrimidinol and deprotection led to the target molecule, L-biopterin (139).
Scheme 23.
The authors have also shown in later work that a change in Grignard reagent can lead to the formation of other biologically active natural products.56 For example, when an n-butyl Grignard is used, this synthetic route can be modified to produce quercus lactone (Figure 9).56b The use of an n-octyl Grignard has been shown to lead to the formation of dodecanolactone,56b avenaciolide56c and tetrahydrocerulenin.56d
Figure 9.
Construction of other natural products through modification of L-biopterin
6. Nitrogen Heterocycles
6.1. (–)-Anisomycin and (+)-Polyoxamic Acid
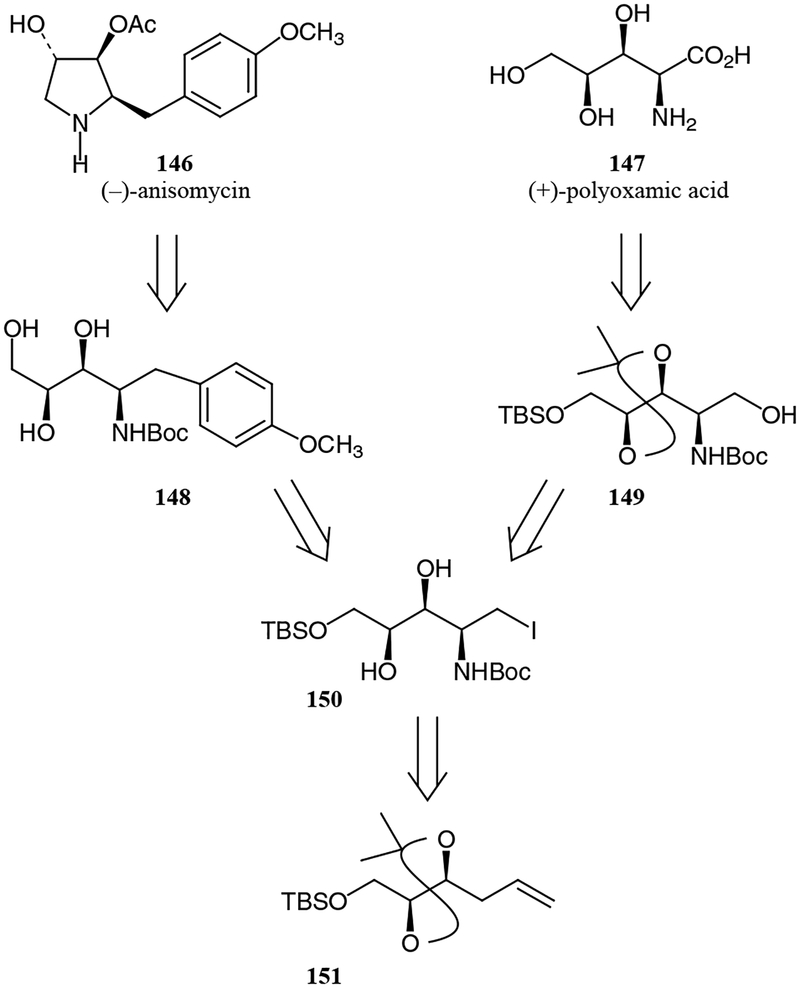
(–)-Anisomycin (146) is a potent antibiotic, antitumor and antifungal agent. Kang and Choi utilized a tartaric acid-derived amino polyol as a precursor in the synthesis of this compound, as shown in Figure 10.57 The key step in this synthetic route was an asymmetric amidation of alkene 151 eventually leading to protected aminotriol 150 that was further converted in nine steps into (–)-anisomycin (Scheme 24).
Figure 10.
Retrosynthesis of (–)-Anisomycin and (+)-Polyoxamic Acid
Scheme 24.
Tartrate-derived tris(trichloroacetimidate) 152 was reacted with iodine in the presence of sodium bicarbonate to afford a 4–5:1 mixture of dihydro-1,3-oxazine 153 and oxazoline 154 (reaction of 152 with IBr in the presence of potassium carbonate results in 6-membered ring formation only). Their major isomers were determined to be cis-153 and trans-154, respectively. Hydrolysis of the mixture followed by protection afforded a 12:1 mixture of carbamates 155b and 155a, respectively, in 84% yield. The same synthetic scheme can be used for the production of (+)-polyoxamic acid (147),57 a compound that was widely used as an agricultural fungicide as well as a general antifungal agent.
6.2. (–)-Deacetylanisomycin
(–)-Deacetylanisomycin, a deacetylated derivative of anisomycin, also has strong antifungal activity. The synthesis of this compound was attempted earlier starting from various chiral building blocks, including tartaric acid; however, these routes were either non-stereoselective or inefficient. Recently, deacetylanisomycin was synthesized from the same aminotriol used in the synthesis of anisomycin (148, Figure 10 above). Deacetylanisomycin was prepared stereoselectively via a shorter route, namely, reductive alkylation of the nitrile derived from tartaric acid, as shown in Scheme 25.58 The use of a Grignard re-agent in the reductive alkylation resulted in a mixture of diastereomers in a ratio of 81:19 (syn:anti). The synthetic route continued with several deprotections, mesylation and an intramolecular cyclization to afford deacetylanisomycin in 64% yield from 158a. This stereoselective approach offers an efficient access to a variety of anisomycin derivatives as well as polyhydroxylated pyrrolidines in general.
Scheme 25.
6.3. Nectrisine
Many polyhydroxylated pyrrolidines and piperidines possess remarkable biological activity as inhibitors of glycosidases and mannosidases. Figure 11 shows several examples of this class of molecules that were synthesized using a tartaric acid or tartrate derivative.
Figure 11.
Structures of several polyhydroxylated pyrrolidines
Nectrisine is a fungal metabolite isolated from Nectria lucida. Several routes for the synthesis of this compound have been reported starting from various sugars as chiral precursors. Kim and co-workers reported a new synthetic route for the preparation of nectrisine and 4-epinectrisine60 starting from (–)-diethyl-D-tartrate via lactam intermediates. As shown in Scheme 26, diethyl tartrate was converted into the corresponding aminonitrile 165, followed by oxidative lactam formation.62 The key step in this synthetic route is the reduction of lactam 167 to amino alcohol 168 using lithium triethylborohyride. This reduction was successful only with BOC protection of the nitrogen. A similar route was used to synthesize the C4 epimer, 4-epi-nectrisine.
Scheme 26.
6.4. DAB-1, LAB-1
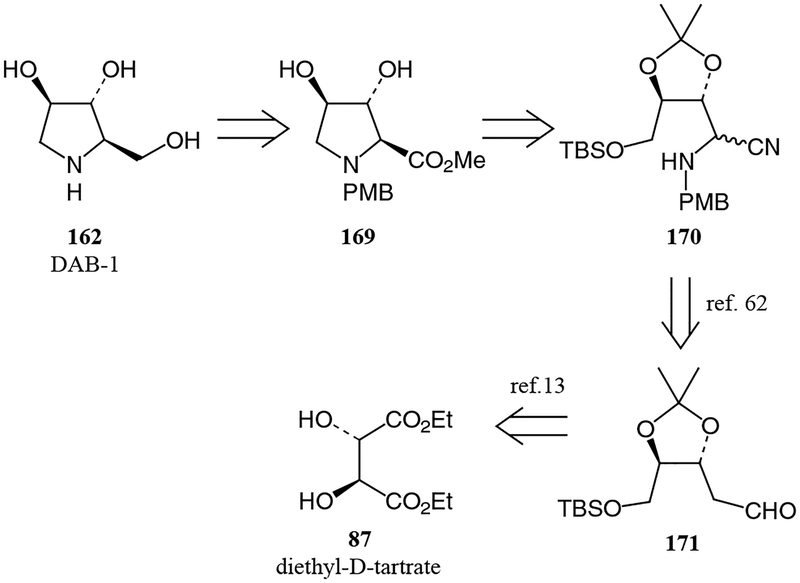
DAB-1 and LAB-1 (Figure 11), naturally occurring α-glycosidase inhibitors, were recently synthesized using an analogous approach to that of nectrisine.63 The pyrrolidine precursor to DAB-1 was derived from the corresponding open-chain aminonitrile by cyclization and methanolysis of the nitrile (Figure 12). The diastereomeric mixture of aminonitriles was prepared according to the Strecker procedure62 through aldehyde 171,13 derived from (–)-diethyl-D-tartrate for preparation of DAB-1 or (+)-diethyl-L-tartrate for preparation of LAB-1. With this new approach to the nectrisine, DAB-1 and LAB-1 structures could produce various potentially biologically active synthetic intermediates.
Figure 12.
Retrosynthesis of DAB-1
6.5. Lentiginosine
Polyhydroxylated indolizines such as castanospermine, swainsonine and lentiginosine (Figure 13) are also known as specific glycosidase inhibitors, and some have shown anti-HIV properties. Several compounds of this class have been synthesized from tartaric acid or its derivatives.
Figure 13.
Structures of various indolizines
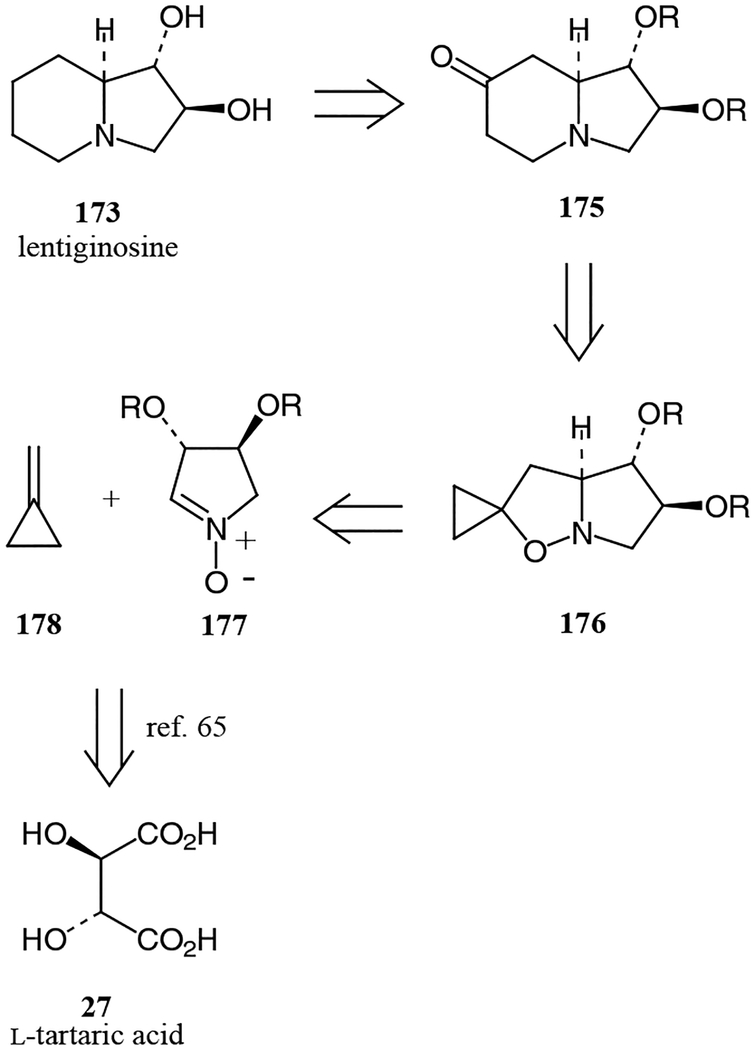
Brandi and co-workers developed a practical and versatile method for the synthesis of polyhydroxylated indolizines via the thermal rearrangement of 5-spirocyclopropanes. This method was recently applied to the synthesis of lentiginosine.64 Retrosynthetically, the target molecule was produced from 175 by deoxygenation (Figure 14). Thermal rearrangement of spirocyclopropane derviavtive 176 led to 175. This spirocycle was the product of a nitrone cycloaddition between 177 and methylenecyclopropane. Nitrone 177 was derived from L-tartaric acid following the procedure of Nagel and co-workers.65
Figure 14.
Retrosynthetic analysis of lentiginosine
In 1996, Goti and co-workers synthesized a structural analog of lentiginosine, hydroxy lentiginosine 179, following a similar route.66 Thus, 175 was synthesized via a nitrone cycloaddition followed by a thermal rearrangement, as above. It was converted into the target molecule 179, (1S,2S,7R,8aS)-trihydroxyoctahydroindolizine, via a stereoselective reduction followed by deprotection of the hydroxyl moieties (Figure 15).
Figure 15.
Retrosynthesis of hydroxy lentiginosine
Another synthesis of lentiginosine was reported by Giovannini and co-workers in 1995.67 As shown in Scheme 27, this synthesis also started from L-tartaric acid and proceeded through nitrone 180.68 This nitrone was then reacted with 4-benzyloxybutylmagnesium bromide to form 181 which was converted in four steps into lentiginosine.
Scheme 27.
Recently, McCaig and co-workers reported a new approach to the synthesis of various trihydroxylated pyrrolidines and indolizidines related to swainsonine, as well as the synthesis of (+)- and (–)-lentiginosine.69 This method is based on the use of 1,3-dipolar cycloadditions of suitably functionalized cyclic nitrones. The dihydroxyl moiety in all of McCaig’s swainsonine analogs comes from D- or L-arabinose derivatives. On the other hand, L-tartrate derivatives can be used as precursors for compounds with a 1,2-trans-dihydroxylation moiety, such as in lentiginosine (Scheme 28).
Scheme 28.
6.6. Trapoxin B
Trapoxin B, a cyclotetrapeptide, isolated from the fungus Helicoma ambient, profoundly affects mammalian cell growth and morphology. Schreiber and co-workers reported the total synthesis of this natural product71 derived from tartaric acid,72 a radioisotope labeled analog and a trapoxin-based affinity reagent which allowed the first molecular characterization of histone deacetylase, trapoxin’s target. The key steps, as shown in Scheme 29, include preparation of the Grignard reagent from bromide 187, coupling with Cbz-protected serine β-lactone and coupling of the resulting product 189 with amine 190 in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide and 1-hydroxybenzotriazole (EDC/HOBT). The final cyclization yielded the desired alcohol 191 in 51%. Tosylation followed by deprotection, epoxide formation and oxidation resulted in formation of trapoxin B.
Scheme 29.
6.7. (+)-FR900482
(+)-FR900482 is a complex natural product isolated from the culture broth of Streptomyces sandaenis No.6897. It displays potent antitumor activity against a variety of mammalian solid tumors. The retrosynthetic analysis applied by Yoshino and co-workers reveals two key intermediates (Figure 16).73 One of the intermediates, 195, is generated over 15 steps from 5-hydroxyisophthalic acid while the other, 196, is generated from diethyl-L-tartrate.
Figure 16.
Retrosynthetic analysis of (+)-FR900482
The synthesis of fragment 196 begins with the transformation of diethyl-L-tartrate into the protected threitol derivative 198 using known procedures.74 Transformation of this derivative into diol 199 followed by epoxidation and nucleophilic opening using sodium azide led to a mixture of regioisomers. Reduction and several protection steps yielded the target molecule 196 (Scheme 30).
Scheme 30.
Coupling of the two fragments 195 and 196 as shown in Scheme 31, was accomplished using sodium hydride followed by conversion into dialdehyde 202. Macrocyclization was achieved via treatment of 202 with lithium bis(trimethylsilyl)amide to afford 194 which was converted over several steps into (+)-FR900482.
Scheme 31.
6.8. Curasin A
Curasin A is a potent antimitotic agent isolated from the Caribbean cyanobacterium Lyngbya majuscula. The retrosynthetic analysis applied by Ononda and co-workers reveals that the molecule can initially be broken into two fragments as shown in Figure 17.75 Further disconnections of 205 lead to four synthons: L-cysteine, compound 206, geraniol and 1,4-butanediol. The cyclopropane fragment 204 is obtained from diethyl-L-tartrate (Scheme 32).
Figure 17.
Retrosynthetic analysis of curacin A
Scheme 32.
The cyclopropane-containing fragment 204 was synthesized starting from diethyl-L-tartrate. It was transformed into 210 in several steps.76 The ester moieties were removed and the resulting diene was subjected to a double asymmetric Simmons–Smith cyclopropanation. Completion of this fragment was achieved via periodic cleavage of the diol followed by oxidation of the resulting aldehyde to yield the target fragment 204.
7. Miscellaneous Compounds
Many biologically active products that do not fit into the categories defined above can also be prepared through tartrates and tartaric acids. For example, a publication by King and co-workers discusses the synthesis and potency of several dicaffeoyltartaric acids (DCTAs, 213) against HIV-1 (Figure 18).77 The series of compounds discussed are synthesized through the acylation of various bis(diphenylmethyl) tartrates (D-, L- and meso) with protected caffeoyl chloride. The syntheses of other anti-HIV agents were also started from tartrates. Schreiner and coworkers78 as well as Kaltenbach and co-workers79 each synthesized a series of cyclic ureas 214 from tartrate. Yet another example is the synthesis by Neuß and co-workers of the β-lactamase inhibitor shown below (215).80 While this is a small sample of the use of tatrates in the synthesis of bioactive molecules, they have been and will continue to be an important building block for synthetic chemists in years to come.
Figure 18.
Miscellaneous biologically active compounds from tart-rates
8. Conclusion
Tartaric acid-derived synthons have broad application in organic synthesis. As we have shown in the previous pages, tartaric acid and its derivatives are very useful in the synthesis of biologically active products. From their use as chiral resolving agents and chiral templates in asymmetric synthesis to the synthesis of bioactive organic molecules, optically active tartaric acid and its derivatives are employed in all areas of organic chemistry. Without doubt, these inexpensive starting materials will have more new applications in synthesis in forthcoming years. Hopefully, this review will further stimulate their use as an important chiron in organic synthesis.
Acknowledgement
Financial support for this work was provided by the National Institutes of Health (GM 55600).
Biographies

Arun K. Ghosh was born in India in 1958. He received his Ph.D. from the University of Pittsburgh with Professor Alan P. Kozikowski (1985). Following his postdoctoral studies at Harvard University with Professor E.J. Corey (1985–1988), he joined the department of Medicinal Chemistry at Merck Research Laboratories as a Senior Research Chemist in 1988. He moved to the University of Illinois at Chicago in 1994 as an Assistant Professor and is currently Professor of Chemistry. His current research interests include: development of asymmetric synthetic methodologies; asymmetric catalysis; total synthesis of biologically important natural products; design and synthesis of molecular probes for biological systems; peptidomimetic design and molecular modelling.

Elena S. Koltun was born in Russia in 1973. She received her M.S. from High Chemical College, Russian Academy of Science, Moscow, in 1994 and her Ph.D. from the University of Minnesota in 1999 under the supervision of Professor Steven R. Kass. In 2000 she joined Professor Ghosh’s group as a postdoctoral fellow and is currently working on the development of asymmetric aldol reactions.

Geoffrey Bilcer was born in Chicago, Illinois in 1973. He studied chemistry at Northwestern University where he received his B.A. in chemistry in 1995. In 1997, he joined Professor Ghosh’s group and is currently working towards his Ph.D. on the synthesis of bioactive natural products. He was awarded a National Science Foundation fellowship for the 2000–2001 academic year.
Footnotes
Dedicated to Professor Seebach for his invaluable contributions to the field of tartaric acid chemistry
References
- (1).Seebach D; Hungerbühler E In Modern Synthetic Methods, Vol. 2; Scheffold R, Ed.; Otto Salle-Sauerländer: Frankfurt-Aarau, Germany, 1980, 91–171. [Google Scholar]
- (2).Gawronski J; Gawronska K Tartaric and Malic Acids in Synthesis: A Source Book of Building Blocks, Ligands, Auxiliaries, and Resolving Agents; Wiley: New York, 1999. [Google Scholar]
- (3).Brandi A Molecules 1999, 4, 1. [Google Scholar]
- (4).Fallis A Pure Appl. Chem 1997, 69, 495. [Google Scholar]
- (5).Vogel P Curr. Org. Chem 1998, 2, 255. [Google Scholar]
- (6).(a) Couty F Amino Acids 1999, 16, 297. [DOI] [PubMed] [Google Scholar]; (b) Ukaji Y Kagaku to Kogyo 1997, 50, 167. [Google Scholar]; (c) Ukaji Y Yuki Gosei Kagaku Kyokai Shi 1999, 57, 581. [Google Scholar]; (d) Inomata K; Ukaji Y Yuki Gosei Kagaku Kyokai Shi 1998, 56, 11. [Google Scholar]
- (7).Satoh M; Takeuchi N; Fujimoto Y Heterocycles 1997, 45, 177. [Google Scholar]
- (8).Fujimoto Y; Satoh M; Takeuchi N; Kirisawa M Chem. Pharm. Bull 1990, 38, 1447. [DOI] [PubMed] [Google Scholar]
- (9).Lu W; Zheng G; Haji A; Aisa A; Cai J Tetrahedron Lett. 1998, 39, 9521. [Google Scholar]
- (10).(a) Feit PW J. Med. Chem 1964, 7, 14. [DOI] [PubMed] [Google Scholar]; (b) Hungerbühler E; Seebach D Helv. Chim. Acta 1981, 64, 687. [Google Scholar]
- (11).(a) Lu W; Zheng G; Gao D; Cai J Tetrahedron 1999, 55, 7157. [Google Scholar]; (b) Lu W; Zheng G; Gao D; Cai J Synlett 1998, 737. [Google Scholar]
- (12).Ghosh A; Wang YJ Org. Chem 1999, 64, 2789. [DOI] [PubMed] [Google Scholar]
- (13).Iida H; Yamazaki N; Kibayashi CJ Org. Chem 1987, 52, 3337 and references cited therein. [Google Scholar]
- (14).(a) Ghosh A; McKee S; Sanders W; Darke P; Zugay J; Emini E; Schleif W; Quintero J; Huff J; Anderson P Drug Des. Discovery 1993, 10, 77. [PubMed] [Google Scholar]; (b) Levene P; Stiller EJ Biol. Chem 1934, 299. [Google Scholar]
- (15).(a) Isono K J. Antibiot 1988, 41, 1711. [DOI] [PubMed] [Google Scholar]; (b) Isono K; Negatsu J; Kobinata K; Sesaki K; Suzuki S Agric. Biol. Chem 1967, 31, 190. [Google Scholar]; (c) Isono K; Negatsu J; Kawashima Y; Suzuki S Agric. Biol. Chem 1965, 29, 848. [Google Scholar]
- (16).Vorbrüggen H; Krolikiewicz K; Bennua B Chem. Ber 1981, 114, 1234. [DOI] [PubMed] [Google Scholar]
- (17).Liu D-G; Wang B; Lin G-QJ Org. Chem 2000, 65, 9114. [DOI] [PubMed] [Google Scholar]
- (18).Hatakeyama S; Matsumoto H; Fukuyama H; Mukugi Y; Irie HJ Org. Chem 1997, 62, 2275. [DOI] [PubMed] [Google Scholar]
- (19).(a) Mukai C; Moharram SM; Kataoka O; Hanaoka M J. Chem. Soc., Perkin Trans 1 1995, 2849. [Google Scholar]; (b) Mukai C; Kataoka O; Hanaoka M Tetrahedron Lett. 1994, 35, 6899. [Google Scholar]
- (20).Corey EJ; Fuchs P Tetrahedron Lett. 1972, 13, 3769. [Google Scholar]
- (21).Chen J; Marx J Tetrahedron Lett. 1997, 38, 1889. [Google Scholar]
- (22).(a) Mori K; Fukamatsu K Liebigs Ann. Chem 1992, 489. [Google Scholar]; (b) Rubottom G; Kim C-WJ Org. Chem 1983, 48, 1550. [Google Scholar]; (c) Vig O; Sharma S; Raj I Indian J. Chem 1966, 4, 129. [Google Scholar]
- (23).Roth GJ; Kirschbaum S; Bestmann HJ Synlett 1997, 618. [Google Scholar]
- (24).(a) Betsmann H; Roth D Synlett 1990, 751. [Google Scholar]; (b) Bestmann H; Roth D Angew. Chem 1990, 102, 95. [Google Scholar]; (c) Bestmann H; Roth D Angew. Chem., Int. Ed. Engl 1990, 20, 99. [Google Scholar]
- (25).Mikolajczyk M; Mikina M; Wieczorek W; Blaszczyk J Angew. Chem., Int. Ed. Engl 1996, 35, 1560. [Google Scholar]
- (26).Lee J; Lee Y; Chung B; Park H Tetrahedron 1997, 53, 2449. [Google Scholar]
- (27).Lee W-W; Chang S Tetrahedron: Asymmetry 1999, 10, 4473. [Google Scholar]
- (28).(a) Grubbs R; Chang S Tetrahedron 1998, 54, 4413. [Google Scholar]; (b) Miller S; Blackwell H; Grubbs RJ Am. Chem. Soc 1996, 118, 9606. [Google Scholar]; (c) Holder S; Blechert S Synlett 1996, 505. [Google Scholar]; (d) Hammer K; Undheim K Tetrahedron 1997, 53, 5925. [Google Scholar]
- (29).Mori K; Abe K Polish J. Chem 1994, 68, 2255. [Google Scholar]
- (30).Xu X-X; Zhu Y-H Tetrahedron Lett. 1995, 36, 9173. [Google Scholar]
- (31).(a) Liu G; Wang Z Chem. Commun 1999, 1129. [Google Scholar]; (b) Liu G; Wang Z Synthesis 2001, 119. [Google Scholar]
- (32).Fuchs K; Paquette LA J. Org. Chem 1994, 59, 528. [Google Scholar]
- (33).Kang SH; Lee SB Chem. Commun 1998, 761. [Google Scholar]
- (34).Masaki Y; Yoshizawa K; Itoh A Tetrahedron Lett. 1996, 37, 9321. [Google Scholar]
- (35).(a) Masaki Y; Iwata I; Imaeda T; Oda H; Nagashima H Chem. Pharm. Bull 1988, 36, 1241. [DOI] [PubMed] [Google Scholar]; (b) Masaki Y; Nagata K; Serizawa Y; Kaji K Tetrahedron Lett. 1984, 25, 95. [Google Scholar]
- (36).Lee E; Yoo S-K; Choo H; Song HY Tetrahedron Lett. 1998, 39, 317. [Google Scholar]
- (37).Grese TA; Hutchinson KD; Overman LE J. Org. Chem 1993, 58, 2468. [Google Scholar]
- (38).Naef R; Seebach D Angew. Chem., Int. Ed. Engl 1981, 20, 1030. [Google Scholar]
- (39).Evans DA; Barrow JC; Leighton JL; Robichud AJ; Sefkow MJ Am. Chem. Soc 1994, 116, 12111. [Google Scholar]
- (40).Corey EJ; Gross AW Tetrahedron Lett. 1984, 25, 495. [Google Scholar]
- (41).Sato H; Nakamura S-I; Watanabe N; Hashimoto S-I Synlett 1997, 451. [Google Scholar]
- (42).(a) Angelastro MR; Peet NP; Bey P J. Org. Chem 1989, 54, 3913. [Google Scholar]; (b) Mukaiyama T; Suzuki K; Yamada T Chem. Lett 1982, 929. [Google Scholar]
- (43).Evans DA; Trotter BW; Barrow JC Tetrahedron 1997, 53, 8779. [Google Scholar]
- (44).Cluogh S; Raggatt ME; Simpson TJ; Willis CL; Whiting A; Wrigley SKJ Chem. Soc., Perkin Trans 1 2000, 2475. [Google Scholar]
- (45).(a) Seyforth D; Vaughan LG J. Organomet. Chem 1963, 1, 138. [Google Scholar]; (b) Barrett AG; Hamprecht D; Ohkubu MJ Org. Chem 1997, 62, 9376. [Google Scholar]
- (46).Somfai PJ Chem. Soc., Perkin Trans 1 1995, 817. [Google Scholar]
- (47).Somfai P; Olsson R Tetrahedron 1993, 49, 6645. [Google Scholar]
- (48).(a) Kuwahara S; Moriguchi M; Miyagawa K; Konno M; Kodama O Tetrahedron Lett. 1995, 36, 3201. [Google Scholar]; (b) Kuwahara S; Moriguchi M; Miyagawa K; Konno M; Kodama O Tetrahedron 1995, 51, 8809. [Google Scholar]
- (49).Iida H; Yamazaki N; Kibayashi CJ Org. Chem 1986, 51, 1069. [Google Scholar]
- (50).Mukai C; Moharram S; Hanaoka M Tetrahedron Lett. 1997, 38, 2511. [Google Scholar]
- (51).Ghosh A; Bilcer G Tetrahedron Lett. 2000, 41, 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).For a review see: Grubbs R; Chang S Tetrahedron 1998, 54, 4413. [Google Scholar]
- (53).deNapoli L; Messere A; Palomba D; Piccialli VJ Org. Chem 2000, 65, 3432. [DOI] [PubMed] [Google Scholar]
- (54).Fernandez A-M; Duhamel LJ Org. Chem 1996, 61, 8698. [Google Scholar]
- (55).(a) Duhamel L; Plaquevent J J. Org. Prep. Proced. Int 1982, 14, 347. [Google Scholar]; (b) Duhamel L; Herman T; Angibaud P Synth. Commun 1992, 22, 735. [Google Scholar]
- (56).(a) Fernandez A-M; Plaquevent J-C; Duhamel L J. Org. Chem 1997, 62, 4007. [Google Scholar]; (b) Bloch R; Gilberty LJ Org. Chem 1987, 52, 4603. [Google Scholar]; (c) Tsuboi S; Sakamoto J; Sakai T; Utaka M Chem. Lett 1989, 1427. [Google Scholar]; (d) Vigneron J; Blanchard J Tetrahedron Lett. 1980, 21, 1739. [Google Scholar]
- (57).Kang SH; Choi H-W Chem. Commun 1996, 1521. [Google Scholar]
- (58).Hutin P; Haddad M; Larcheveque M Tetrahedron: Asymmetry 2000, 11, 2547. [Google Scholar]
- (59).(a) Chelucci G; Cabras MA; Botteghi C; Basoli C; Marchetti M Tetrahedron: Asymmetry 1996, 7, 885. [Google Scholar]; (b) Musich JA; Rapoport HJ Am. Chem. Soc 1978, 100, 4865. [Google Scholar]; (c) Batsanov AS; Begley MJ; Fletcher RJ; Murphy JA; Sherburn MSJ Chem. Soc., Perkin Trans 1 1995, 1281. [Google Scholar]
- (60).Kim YJ; Kitahara T Tetrahedron Lett. 1997, 38, 3423. [Google Scholar]
- (61).Kuwahara S; Moriguchi M; Miyagawa K; Konno M; Kodama O Tetrahedron 1995, 51, 8809. [Google Scholar]
- (62).Harusawa S; Hamada Y; Shioiri T Tetrahedron Lett. 1979, 48, 4663. [Google Scholar]
- (63).Kim YJ; Kido M; Bando M; Kitahara T Tetrahedron 1997, 53, 7501. [Google Scholar]
- (64).Cordero F; Cicchi S; Goti A; Brandi A Tetrahedron Lett. 1994, 35, 949. [Google Scholar]
- (65).Nagel U; Kinzel E; Andrade J; Prescher G Chem. Ber 1986, 119, 3326. [Google Scholar]
- (66).(a) Goti A; Cardona F; Brandi A Synlett 1996, 761. [Google Scholar]; (b) Goti A; Cardona F; Brandi A Tetrahedron: Asymmetry 1996, 7, 1659. [Google Scholar]
- (67).Giovannini R; Marcantoni E; Petrini MJ Org. Chem 1995, 60, 5706. [Google Scholar]
- (68).Ballini R; Marcantoni E; Petrini MJ Org. Chem 1992, 57, 1316. [Google Scholar]
- (69).McCaig AE; Meldrum KP; Wightman RH Tetrahedron 1998, 54, 9429. [Google Scholar]
- (70).Dulphy H; Gras J-L; Lejoy T Tetrahedron 1996, 52, 8517. [Google Scholar]
- (71).Taunton J; Collins JL; Schreiber SL J. Am. Chem. Soc 1996, 118, 10412. [Google Scholar]
- (72).McDougal PG; Rico JG; Oh YI; Condon BD J. Org. Chem 1986, 51, 3388. [Google Scholar]
- (73).(a) Katoh T; Itoh E; Yoshino T; Terashima S Tetrahedron Lett. 1996, 37, 3471. [Google Scholar]; (b) Yoshino T; Nagata Y; Itoh E; Hashimoto M; Katoh T; Terashima S Tetrahedron 1997, 53, 10229; and references cited therein. [Google Scholar]; (c) Yoshino T; Nagata Y; Itoh E; Hashimoto M; Katoh T; Terashima S Tetrahedron 1997, 53, 10239; and references cited therein. [Google Scholar]
- (74).(a) Ohno M; Fujita K; Nakai H; Kobayashi S; Inoue K; Nojima S Chem. Pharm. Bull 1985, 33, 572. [DOI] [PubMed] [Google Scholar]; (b) Al-Hakim A; Haines A; Morley C Synthesis 1985, 207. [Google Scholar]; (c) Carreaux F; Duréault A; Depezay J Synlett 1992, 527. [Google Scholar]
- (75).(a) Onoda T; Shirai R; Kawai N; Iwasaki S Tetrahedron 1996, 52, 13327. [Google Scholar]; (b) Taunton J; Collins JL; Shreiber SL J. Am. Chem. Soc 1996, 118, 10412. [Google Scholar]
- (76).(a) Krief A; Dumont W; Pasau P; Lecomte P Tetrahedron 1989, 45, 3039. [Google Scholar]; (b) Carmack M; Kelley CJ Org. Chem 1968, 33, 2171. [Google Scholar]
- (77).King P; Ma G; Miao W; Jia Q; McDougall B; Reinecke M; Cornell C; Kuan J; Kim T; Robinson WJ Med. Chem 1999, 42, 497. [DOI] [PubMed] [Google Scholar]
- (78).Screiner E; Pruckner AJ Org. Chem 1997, 62, 5380. [Google Scholar]
- (79).Kaltenbach IIIR; Nugiel D; Lam P; Klabe R; Seitz SJ Med. Chem 1998, 41, 5113. [DOI] [PubMed] [Google Scholar]
- (80).Neuß O; Furman B; Kaluza Z; Chmielewski M Heterocycles 1997, 45, 265. [Google Scholar]