Abstract
The concept of the neonatal window of opportunity assigns the early postnatal period a critical role for lifelong host-microbial and immune homeostasis. It is supported by epidemiological evidence that links postnatal environmental exposure with disease susceptibility and mechanisms in the neonate host that facilitate the postnatal transposition, establish a stable microbiome, and promote immune maturation. During the conference on “The neonatal window of opportunity – early priming for life,” postnatal micro-biome and immune maturation, epidemiological evidence, and fundamental mechanisms were discussed to identify new targets for future preventive and interventional measures.
From December 5 to 7, 2016, the Herrenhausen Conference “The neonatal window of opportunity – early priming for life” took place at Hannover, Germany, sponsored by the Volkswagen Foundation. The concept of the “neonatal window of opportunity,” that is, a critical nonredundant time frame in a newborn’s life during which environmental factors drive immune and tissue maturation and influence the susceptibility to immune-mediated and other diseases in adult life, was discussed.
NONCOMMUNICABLE DISEASES AS THE GRAND CHALLENGE FOR PREVENTION
Our body is exposed continuously to many danger signals. An effective immune system is essential to protect and repair. Unbalanced immune reactivity seems to play a key role in noncommunicable diseases such as chronic obstructive pulmonary disease, allergies, asthma, diabetes, cancer, and cardiovascular diseases as well as obesity. Prevention is the grand challenge, because causal therapies for noncommunicable diseases are still not available and prevalences and incidences are currently on the rise.
MATERNAL/FETAL/NEONATAL INTERACTION
The first “window of opportunity” in life is defined as the prenatal or perinatal period. Particularly in this period, microbial factors have a strong impact on the development of immune responses. This includes not only the birthplace and mode of delivery, breast-feeding, medication (eg, antibiotic use), and introduction of solid foods but also the exposure to siblings and pets. Challenging the immune tolerance of neonatal mice, germ-free mice exhibited an exaggerated allergic response when exposed to allergens, as compared with conventionally raised mice. Once their airway microbiome had formed after 2 post-natal weeks, the mice were protected against this overshooting allergic inflammation.1 However, oral antigen administration in early life, even in the presence of protective breast milk–derived factors, does not guarantee the induction of oral tolerance and the prevention of allergic disease. This implies the necessity to identify limiting factors for oral tolerance induction in early life.2
Healthy nutrition during pregnancy and early life represents a key factor for proper immune development. Human milk still remains the criterion standard.
DEVELOPMENT OF IMMUNE TOLERANCE IN THE NEONATAL PERIOD
This goes hand in hand with the ontogeny of the enteric mucosal tissue and its influence on the establishment of the enteric microbiota and long-term host-microbial homeostasis as well as infection susceptibility. Age-dependently expressed features of the innate and adaptive immune system contribute to the postnatal acquisition of mucosal homeostasis and the particular susceptibility of the neonate host to infection.3
EARLY EVENTS AND NEW INSIGHTS INTO MECHANISMS
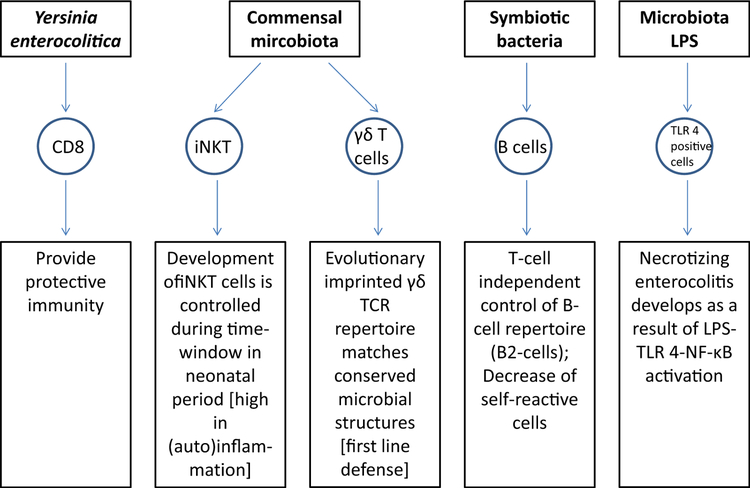
Streptococcus pneumonia is a leading cause of bacterial infection of the upper and lower respiratory tract in children. This pathogen spreads to its host following acquisition of very few organisms that quickly proliferate and dominate the niche in the upper respiratory tract. This work established a new paradigm for understanding pathogen transmission and the initial establishment of the colonizing microbiota in newborns. This is also influenced by the exposure to maternal microbiota metabolites in utero and in early life during lactation that prepares the newborn for colonization with its own microbiota and sets the baseline for a regulated immune system.4 Important novel mechanisms are summarized in Fig 1. Neonates are often highly susceptible to intestinal pathogens. An exception is seen after infection of murine neonates with Yersinia enterocolitica. Here, multiple innate and adaptive components including (surprisingly) CD8+ T lymphocytes cells participate in protective immunity. Natural killer T cells function as a kind of checkpoint regulating the crosstalk between the microbiota and the immune system. Invariant natural killer T cells are resident cells of the gut mucosa that establish their niche during early life when they home to and expand during a restricted period of neonatal life. Studies performed in germ-free mice suggest a critical neonatal time period for the development and proliferation of these immune cells.5 γδ cells in mice represent a first line of defense with respect to both early in life during T-cell development and ontogeny as well as in terms of mucosal tissue localization. The human neonate γδ TCR repertoire contains considerable frequencies of innate “public” TCR-δ sequences matching conserved microbial structure. Symbiotic bacteria control the selection processes that determine the antibody repertoire of naive B cells in neonatal mice. Interestingly, this training event appears to be particularly influential during a narrow window of opportunity after birth as commensal bacteria exposure of adult germ-free mice showed no effect on the positive selection events. Tissue-specific memory T cells are established as noncirculating resident memory T cells at various tissue sites in response to microbial stimulation.6 Necrotizing enterocolitis is the leading cause of death from gastrointestinal disease in premature infants and develops after an exuberant proinflammatory response to the colonizing microbiota and this requires activation of the LPS receptor, Toll-like receptor 4.7
FIG 1.
New mechanisms of microbe-immune interaction. For details please see text. iNKT, Invariant natural killer T cells; TLR 4, Toll-like receptor 4.
ROLE OF ENVIRONMENTAL MICROBES
Epidemiological studies show a strong correlation between early microbial exposure to allergens and a reduced risk of hypersensitivity leading to asthma or hay fever later in life.8 The diversity and duration of microbial exposure early in life are important for the development of tolerogenic immune functions. In this regard, mild proinflammatory signals including IL-6 play a critical role to orchestrate early activation signals.9
NEW METHODS
Stem cell–derived organoids represent a new 3-dimensional model system of the gut epithelium that allows the study of organ development, immune homeostasis, and tissue regeneration. Moreover, epithelial stem cell organoids now also open the possibilities to study host-microbe-interaction.
System biology approaches applied to the global transcriptome generated from the RNA isolated from a few drops of blood from preterm babies have helped to reveal how regulatory mechanisms determine the set-point for immune homeostasis in early life by integrating specific immune and metabolic pathways so that interpatient differences can be overcome and uncovered a novel immune-metabolic axis that accurately predicts sepsis in neonates and infants. These studies lay the foundation for future translation of host pathways in advancing diagnostic, prognostic, and therapeutic strategies for neonatal sepsis.10
CONCLUSIONS
We are only starting to appreciate the critical impact of the postnatal period for lifelong health and disease susceptibility. Recent discoveries provide the proof of principle, characterize the critical time window(s), and identify underlying mechanisms. However, a number of important questions remain: The cause-effect relationship between microbial exposure and health needs to be established and the mechanisms operating during this “window of opportunity” need to be elucidated. This must consequently lead to effective primary prevention strategies. Future research will further deepen our understanding and extend from animal models to human studies to develop interventional strategies to foster immune homeostasis and prevent the development of diseases.
Acknowledgments
We thank Susanne Zapf for her excellent editorial assistance.
The conference and the preparation of this manuscript were supported by an unrestricted grant of the Volkswagen Foundation, Hannover, Germany. J.G. receives grants from Nutricia Research Foundation, Dutch government funding (NWO, CCC, STW, RAAK/PRO, TKI), the European Union, and EDB. Research in M.W.H.’s laboratory is supported by the German Research Foundation (SPP1656, SPP1580, IRTG1273, and Ho-2237/12–1) and the Niedersachsen Research Network on Neuroinfectiology (N-RENNT). K.D.M. is supported by a grant from the SNSF (SNSF310030_134902) and the European Research Council (FP/2007–2013) agreement number 281785. J.P. is supported by a VIDI grant from the Netherlands Organisation for Scientific Research and a Joint Programme Initiatives grant (Intestinal Microbiomics GI-MDH) within the Healthy Diet for Healthy Living programme. I.P. is supported by the grant of the SFB900 (project B8). H.R. is supported by the German Lung Center, Disease Area of Allergy and Asthma. E.M. receives research grants from the German Research Foundation (DFG) and the German Federal Ministry of Education and Research (BMBF).
Disclosure of potential conflict of interest: J. Garssen received Dutch government grants (STW, NWO, RAAK/PRO, CCC) and a Nutricia Research Foundation grant for this work. P. Ghazal has a patent for Molecular Predictors of Sepsis. D. J. Hackam has a patent pending, Agents for treating necrotizing enterocolitis. K. D. McCoy received a Swiss National Science Foundation grant and a European Research Council grant for this work. I. Prinz received SFB900, project SFB900, and project B8 from Deutsche Forschungsgemeinschaft for this work. E. von Mutius received consultancy fees from PharmaVentures, OM Pharma, Decision Resources, and Novartis Pharma SAS; assessment fees from the Chinese University of Hongkong and the University of Copenhagen; speaker’s fees from HAL Allergie GmbH, Ökosoziales Forum Oberösterreich, Mundipharma, the American Thoracic Society, and AbbVie Deutschland GmbH & Co KG; expert witness fees from the University of Tampere and the European Commission; and honorarium from the Massachusetts Medical Society and the American Academy of Allergy, Asthma & Immunology. The rest of the authors declare that they have no relevant conflicts of interest.
Footnotes
Please note: An extended version of this article can be found in this article’s Online Repository at www.jacionline.org.
REFERENCES
- 1.Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med 2014;20:642–7. [DOI] [PubMed] [Google Scholar]
- 2.Turfkruyer M, Rekima A, Macchiaverni P, Le Bourhis L, Muncan V, van den Brink GR, et al. Oral tolerance is inefficient in neonatal mice due to a physiological vitamin A deficiency. Mucosal Immunol 2016;9479–91. [DOI] [PubMed] [Google Scholar]
- 3.Torow N, Hornef MW. The neonatal window of opportunity: setting the stage for life-long host-microbial interaction and immune homeostasis. J Immunol 2017; 198:557–63. [DOI] [PubMed] [Google Scholar]
- 4.Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, et al. The maternal microbiota drives early postnatal innate immune development. Science 2016;351:1296–302. [DOI] [PubMed] [Google Scholar]
- 5.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science 2016;352:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zens KD, Chen JK, Guyer RS, Wu FL, Cvetkovski F, Miron M, et al. Reduced generation of lung tissue-resident memory T cells during infancy. J Exp Med 2017; 214:2915–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest 2016;126:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Mutius E Environmental factors influencing the development and progression of pediatric asthma. J Allergy Clin Immunol 2002;109:S525–32. [DOI] [PubMed] [Google Scholar]
- 9.Conrad ML, Ferstl R, Teich R, Brand S, Bl€umer N, Yildirim AO, et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med 2009;206:2869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith CL, Dickinson P, Forster T, Craigon M, Ross A, Khondoker MR, et al. Identification of a human neonatal immune-metabolic network associated with bacterial infection. Nat Commun 2014;5:4649. [DOI] [PMC free article] [PubMed] [Google Scholar]