Abstract
Precancerous or cancerous lesions of the gastrointestinal tract often require surgical resection via endomucosal resection. Although excision of the colonic mucosa is an effective cancer treatment, removal of large lesions is associated with high morbidity and complications including bleeding, perforation, fistula formation, and/or stricture, contributing to high clinical and economic costs and negatively impacting patient quality of life. The present study investigates the use of a biologic scaffold derived from extracellular matrix (ECM) to promote restoration of the colonic mucosa following short segment mucosal resection. Six healthy dogs were assigned to ECM-treated (tubular ECM scaffold) and mucosectomy only control groups following transanal full circumferential mucosal resection (4 cm in length). The temporal remodeling response was monitored using colonoscopy and biopsy collection. Animals were sacrificed at 6 and 10 wk, and explants were stained with hematoxylin and eosin (H&E), Alcian blue, and proliferating cell nuclear antigen (PCNA) to determine the temporal remodeling response. Both control animals developed stricture and bowel obstruction with no signs of neomucosal coverage after resection. ECM-treated animals showed an early mononuclear cell infiltrate (2 weeks post-surgery) which progressed to columnar epithelium and complex crypt structures nearly indistinguishable from normal colonic architecture by 6 weeks after surgery. ECM scaffold treatment restored colonic mucosa with appropriately located PCNA+ cells and goblet cells. The study shows that ECM scaffolds may represent a viable clinical option to prevent complications associated with endomucosal resection of cancerous lesions in the colon.
Keywords: Extracellular matrix, Colonic mucosal resection, Bioscaffold, Constructive tissue remodeling
Introduction
Disorders of the lower gastrointestinal tract affect more than six million patients worldwide.1–3 Diseases such as inflammatory bowel disease and colorectal cancer are treated by medical and/or surgical methods. Endomucosal resection (EMR) is often required for severe colonic pathologies and particularly, cancer resection. Recent work has shown that neoplasia confined to the epithelium (T1) can be treated with transanal excision of the colonic mucosa,4 but removal of large lesions can be associated with complications including bleeding, perforation, fistula formation, and/or stricture.5–7 The incidence of such complications is correlated well with the size of mucosal resections. Therefore, larger lesions are often too risky for local excision and require colectomy, which though an effective solution, is associated with increased morbidity and a significant impact on quality of life.8–10
A biomaterial-based strategy involving endoscopic deployment of a biologic scaffold composed of extracellular matrix (ECM) has shown promising results following excision of large areas of neoplasia confined to the mucosa of the esophagus.11,12 To date, 13 patients with esophageal adenocarcinoma (T1a) confined to the mucosa or Barrett’s disease with high-grade dysplasia have been treated with ECM bio-scaffolds following long-segment mucosal resection.12–14 All patients showed rapid mucosal restoration, and none of the patients experienced recalcitrant stricture formation or cancer recurrence. These promising outcomes suggest that ECM bioscaffolds may enable aggressive endomucosal resection (EMR) in the lower gastrointestinal tract by promoting mucosal healing and mitigating stricture formation. The objective of the present study was to determine the ability of small intestinal submucosa (SIS) ECM to promote mucosal healing and prevent stricture following short segment colonic mucosal resection in a canine pilot study.
Materials and methods
Overview of study design
All animal procedures were conducted in accordance with the University of Pittsburgh’s Institutional Animal Care and Use Committee. The ability of SIS-ECM to promote mucosal remodeling was evaluated in a dog model of rectal mucosectomy. Six mongrel dogs were divided into two experimental groups. Four animals were treated with multilaminate SISECM sheets, and two animals served as mucosectomy only controls. Biopsies were taken biweekly to evaluate histologic changes following surgery. Colonoscopies were conducted biweekly postoperatively to examine gross changes in the colon. Animals were euthanized at 6 or 10 wk after surgery.
Production of bioscaffold materials
SIS-ECM was produced as previously described.15 Briefly, the small intestine was isolated from market weight pigs (240–260 lbs, Tissue Source, Lafayette, IN). The intestine was mechanically abraded to remove the tunica muscularis externa and the majority of the tunica mucosa. The remaining tunica submucosa and basilar portion of the tunica mucosa was then disinfected and decellularized in a 0.1% peracetic acid solution followed by two rinses in phosphate-buffered saline solution and deionized water. A tubular scaffold device was created by vacuum pressing eight, sequentially wrapped sheets of SIS-ECM around a mandrel (outer diameter = 30 mm). Dry, multilaminate tubular scaffolds were terminally sterilized by exposure to ethylene oxide.
Surgical procedure
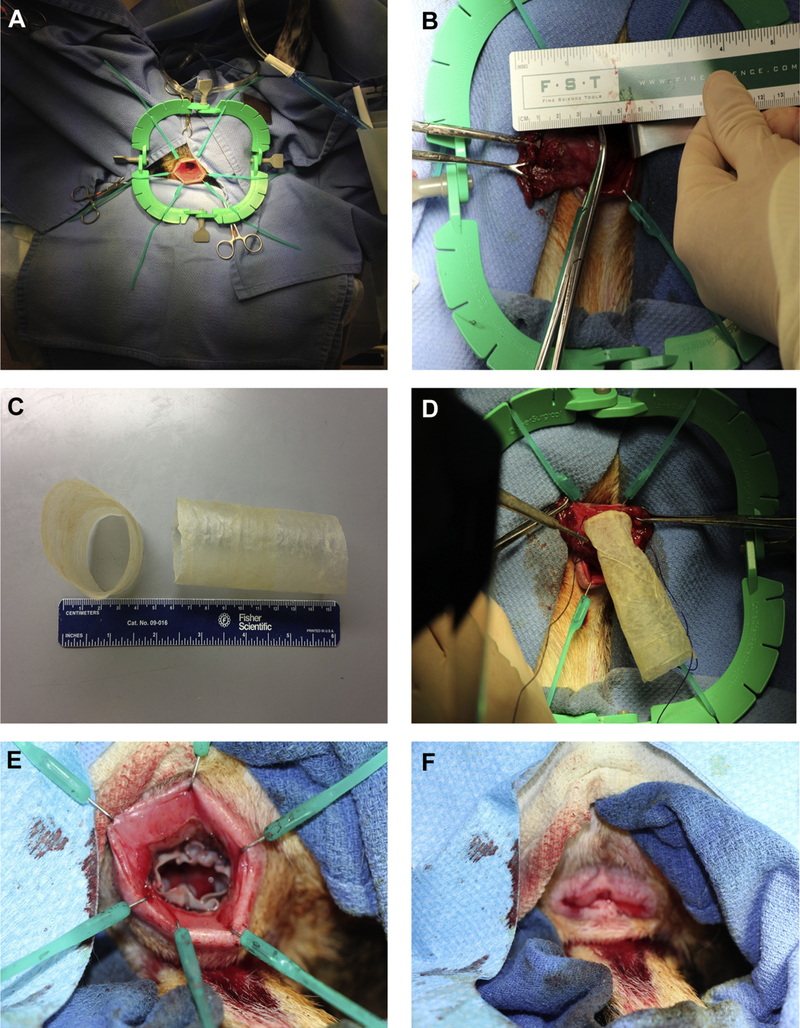
All procedures were performed in accordance with the Institutional Animal Care and Use Committee of the University of Pittsburgh guidelines. Six mongrel dogs (18–25 kg) were subjected to short segment (4 cm) rectal mucosectomy using a transanal approach to remove the mucosa of the rectum and distal sigmoid colon. After bowel preparation, each dog was placed in the supine position under general anesthesia and a Lone Star Retractor was used for transanal access (Fig. 1A). A mucosectomy was performed by circumferential sharp incision at the dentate line for a distance of 4 cm (Fig. 1B), and electrocautery was used to achieve hemostasis. Control animals (n = 2) were subjected to mucosectomy without the implantation of a scaffold. In the experimental group (n = 4), size-matched tubular SIS-ECM bioscaffolds were implanted into the mucosal defect using vicryl sutures, with four sutures placed at wound margins at 12-, 3-, 6-, and 9-o’clock positions (Fig. 1C–F). The dogs were closely monitored daily for weight loss, bloody stools, and rectal bleeding. Colonoscopy was performed biweekly postoperatively and included imaging and biopsy evaluation of the mucosa from the distal anus to the most proximal suture line of the defect. Blood was collected for a complete blood count preoperatively and at 24 h, 72 h, and 1 wk postoperatively (Table 1). Animals were sacrificed at 6 or 10 wk postoperatively, and the distal colon was resected en bloc using nonabsorbable proximal and distal marker sutures to identify the defect site. The tissue was imaged grossly, fixed in neutral buffered formalin, and then serially sectioned for histologic evaluation.
Fig. 1 –
Surgical procedure. (A, B) A 4-cm colonic mucosal resection was performed in all animals. (C, D) In the ECM-treated group, a multilaminate, tubular SIS-ECM bioscaffold was sutured into place. (E, F) The scaffold was secured into place with four sutures placed at the wound margins at 12-, 3-, 6-, and 9-o’clock positions. (Color version of figure is available online.)
Table 1 –
Postsurgical weight and CBC monitoring.
| Dog | Initial weight (lbs) | Weight week 2 (lbs) | Weight week 4 (lbs) | Weight week 6 (lbs) | CBC |
|---|---|---|---|---|---|
| Dog 1 (mucosectomy only) | 60.4 | 59.1 | 58.6 | 58.6 | Normal |
| Dog 2 (mucosectomy only) | 50.7 | 46.9 | 46.1 | 51.5 | Normal |
| Dog 3 (SIS-ECM) | 45.5 | 46.5 | 48.0 | 48.0 | Normal |
| Dog 4 (SIS-ECM) | 47.5 | 46.0 | 48.0 | 48.0 | Normal |
| Dog 5 (SIS-ECM) | 46.5 | 46.0 | 46.5 | 46.5 | Normal |
| Dog 6 (SIS-ECM) | 49.0 | 49.0 | 49.0 | 49.5 | Normal |
CBC = complete blood count.
Biopsy collection and histology
The tissue remodeling response was monitored via biweekly biopsy with endoscopic guidance under light sedation and via water enema. A 2–3 mm diameter specimen was collected from the scaffold implant site at distal, middle, and proximal sites and was fixed in neutral buffered formalin. Hematoxylin and eosin staining, Alcian blue staining, and proliferating cell nuclear antigen (PCNA) immunolabeling were conducted to evaluate the remodeling response. Semiquantitative histomorphologic scoring was completed by two blinded scorers according to previously established criteria for hyperplasia, goblet cell loss, crypt changes, and villous blunting as shown in Table 2. Cumulative scores were averaged between two blinded scorers. Proliferating cells and mucin-expressing cells were quantified with a CellProfiler Image analysis software pipeline.
Table 2 –
Histomorphologic scoring criteria.
| Criterion | Description | Score |
|---|---|---|
| Hyperplasia | Increase in epithelial cell numbers in longitudinal crypts (i.e., crypt elongation) | Minimal <25%—1 |
| Mild: 25%−35%—2 | ||
| Moderate: 36%−50%−3 | ||
| Marked: >51%—4 | ||
| Goblet cell loss | Reduction of goblet cell numbers | Minimal: <20%—1 |
| Mild: 21%−35%—2 | ||
| Moderate: 36%−50%—3 | ||
| Marked: >50%−4 | ||
| Crypt changes | Inflammation or changes in crypt architecture | No change to crypt architecture relative to healthy—0 |
| Neutrophils between crypts—1 | ||
| Crypt erosion (loss of surface epithelium)—2 | ||
| Mucosa devoid of crypts—3 | ||
| Villous blunting | Change in villous to crypt ratio length | Mild: villous-to-crypt length ratio of 2:1 or 3:1—1 |
| Moderate: villous-to-crypt length ratio of 1:1 or 2:1—2 | ||
| Villous atrophy—3 |
Colonoscopy
Colonoscopy was conducted biweekly postoperative and at the time of sacrifice to examine changes in gross colonic appearance following surgery. Images and video were recorded to visualize location of biopsy collection.
Statistical analysis
A one-way analysis of variance with post hoc Tukey test was used to determine differences in the percentage of PCNA+ and mucin+ expressing cells across groups (n = 6 images per animal). All data are reported as mean ± standard error.
Results
Clinical outcomes
All dogs (including controls) recovered well from the surgical procedure. The mucosectomy only control group animals developed intractable stricture and vesicocolonic fistula. All other dogs had an uneventful recovery showing normal appetite, normal hydration, normal activity, and no change in weight as shown in Table 1.
ECM bioscaffolds promote colonic mucosal remodeling
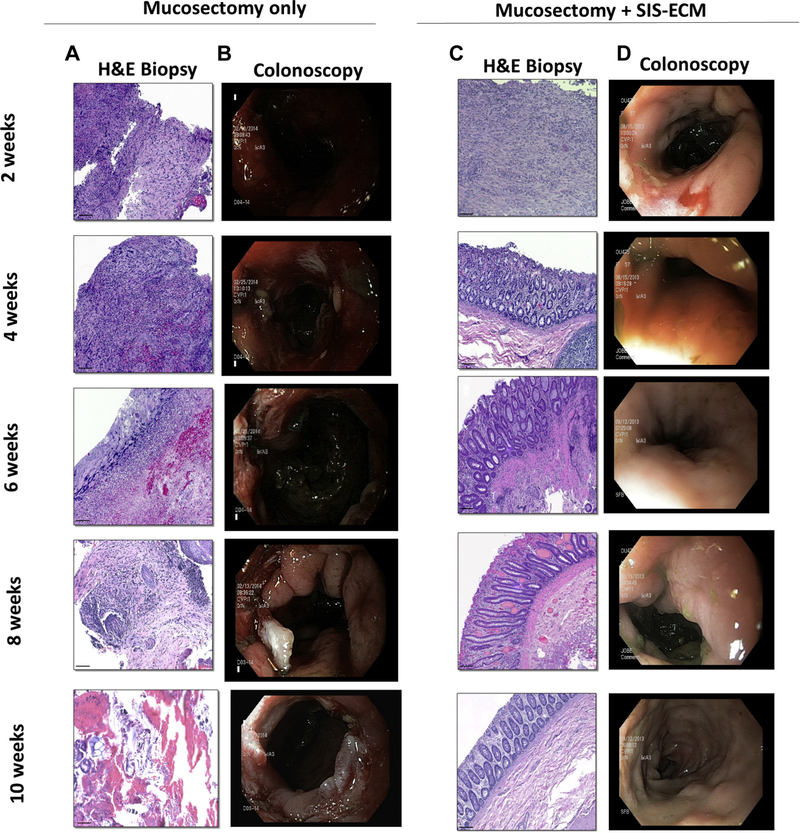
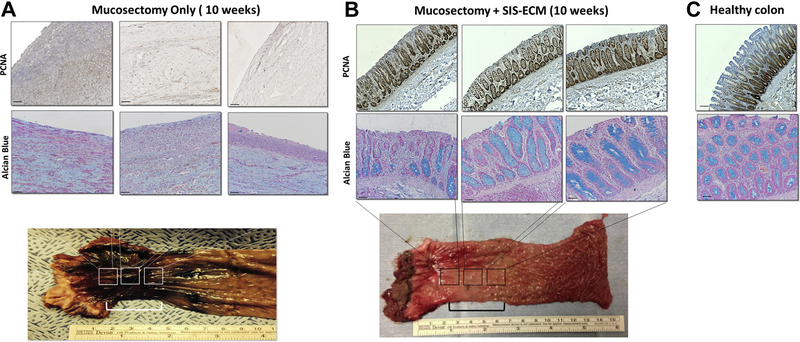
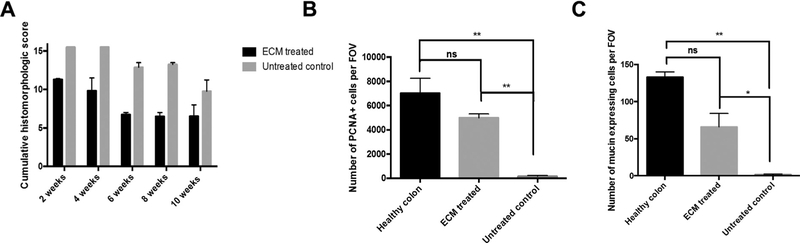
Hematoxylin and eosin (H&E) staining of mucosal biopsies and matched colonoscopy images showed that mucosectomy only control showed active bleeding, scar tissue formation, and inflammation. The control animals also showed no signs of neomucosal tissue growth at the defect site over 10 wk (Fig. 2A,B). The ECM-treated animals however showed near complete replacement of the ECM scaffold material with new colonic mucosa complete with crypt-like structures and an intact basement membrane as early as 4 wk postoperatively (Fig. 2C). The early remodeling response included a robust mononuclear cell infiltrate within this neomucosa. By 6 wk, ECM-treated animals showed diminished numbers of mononuclear cells and restoration of colonic mucosa with remarkable similarity to normal colonic architecture (Fig. 2D). In contrast, the mucosectomy only control group was characterized by necrotic debris, a disorganized layer of connective tissue, a dense mononuclear cell infiltrate, and a lack of intact epithelial tissue up to 10 wk (Fig. 2A). Site appropriate tissue deposition was evaluated using proliferating cell nuclear antigen (PCNA) and Alcian blue staining (Fig. 3). Cumulative scores averaged across two blinded scores show a reduction in score associated with colonic remodeling with ECM treatment when compared to the untreated controls (Fig. 4A). Quantification of PCNA and mucin shows an increased number of PCNA+ cells and an increase in mucin-expressing cells with ECM treatment, similar to that of a healthy colon and significantly higher than the untreated control (Fig. 4B,C).
Fig. 2 –
Temporal mucosal remodeling by ECM bioscaffolds. (A) H&E staining of mucosal biopsies from the mucosectomy only control animals shows a robust mononculear cell infiltrate with inflammation and scarring consistently until 10 wk.(B) Colonoscopies show gross appearance of scar tissue formation and stricture in the untreated controls. (C) ECM treatment was associated with a robust mononuclear cell infiltrate at early time points followed by site-appropriate tissue deposition following ECM treatment as early as 4 wk following mucosectomy. Intestinal crypts and well-organized neomucosa are apparent by 6 wk after scaffold placement. (D) Colonoscopies show restoration of normal gross appearance after ECM treatment. (Scale bars = 50 μm). (Color version of figure is available online.)
Fig. 3 –
ECM scaffolds promote mucosal remodeling. (A) Biopsies from mucosectomy only control animals show necrotic tissue, dense mononculear cell infiltrate, and a lack of an epithelial layer. (B) Biopsies from ECM-treated animals show the formation of neomucosal tissue with site-appropriate intestinal crypt formation and formation of goblet cells that appear histologically similar to (C) healthy colon. (Scale bars = 50 μm). (Color version of figure is available online.)
Fig. 4 –
ECM bioscaffolds promote site appropriate tissue deposition. (A) Combined histologic score at each time point was quantified and compared between groups. (B) Cells expressing PCNA were quantified and compared using a one-way analysis of variance. (C) The number of mucin-expressing cells was quantified and compared using a one-way analysis of variance. (*P < 0.05, **P < 0.01, error bars represent standard error).
ECM bioscaffold placement mitigates stricture formation following colonic mucosectomy
After 6 wk, the mucosectomy only control animals showed severe stricture and one control animal developed vesicocolonic fistula as noted by gross observation (Fig. 3A). At 6 wk, ECM-treated animals showed near complete remodeling of the colonic mucosa with a glistening and uniform surface, and no evidence of hemorrhage or ulceration (Fig. 3B).
ECM bioscaffolds promote site appropriate tissue formation
Biopsies stained for PCNA and Alcian blue show restoration of crypt-like structures with the presence of proliferating cells at the base of the crypts and goblet cells along the entire length of the crypts in the dogs treated with an ECM bioscaffold (Fig. 3B). Colonoscopy of the ECM-treated animals showed a remarkably normal gross appearance as early as 6 wk postoperative (Fig. 2D).
Discussion
The present study shows that placement of a bioscaffold composed of ECM following mucosal resection alters the default healing response and promotes site-appropriate colonic mucosal remodeling. ECM placement prevented stricture formation and scarring. EMR has been shown to be particularly useful for managing polyp formation and lesions of the colorectum5; however, stricture formation and bleeding are frequently associated complications that can limit the use of this organ preserving approach. In the present study, colonoscopy and gross explant analysis of the ECM-treated animals showed complete little or no stricture formation and minimal to no bleeding or scar tissue formation when compared to the untreated control animals.
The mechanisms by which ECM bioscaffolds promote constructive tissue remodeling have been at least partially identified by numerous preclinical and clinical studies. These mechanisms include the degradation of the scaffold material with subsequent release of proregenerative components (e.g., matricryptic peptides) that promote repair through modulation of the innate immune response (specifically the macrophage response) and recruitment of endogenous stem/progenitor cells.16–23 Consistent with previous findings, the bioscaffold in the present study appeared completely degraded by 2 wk and was replaced with site-appropriate colonic epithelium. A recent report shows that a hydrogel form of ECM has the ability to mitigate the proinflammatory environment in a rodent model of inflammatory bowel disease.22 Although not evaluated in the present study, an analysis of the macrophage response, especially macrophage phenotype, following mucosal resection and ECM bioscaffold placement represents an important area of future work.
The therapeutic potential of an ECM bioscaffold for esophageal repair has been previously reported.11–14 An endoscopic technique was used for circumferential, long segment en bloc resection of neoplastic esophageal mucosa followed by placement of an ECM bioscaffold. Results showed restoration of normal esophageal squamous epithelium, minimal stricture formation, and the entire length of the reconstituted esophageal mucosa remained disease-free,12 and a clinical trial is underway to further evaluate the safety and efficacy of this approach (ClinicalTrials.gov identifier: NCT02396745). The findings in the esophageal study mimic those of the present study that shows constructive colonic mucosal remodeling with endoscopic placement of an ECM bioscaffold. These experiences provide promising preliminary evidence for a bioscaffold-based regenerative medicine approach that can enable and support aggressive endomucosal resection of colorectal neoplasia without stricture formation and without the need for colectomy. The resection of cancerous lesions in the colon remains one of the few curative treatment options for neoplasia (T1–T4). Radical resection and local lesion excision are effective procedures but are associated with up to 20%−30% complication rates.24 Local excision, in particular, has the possibility of oncologically incomplete surgery.25 In addition, patients with T2 cancers who present with comorbidities that prevent full colonic resection could benefit from resection with the placement of such scaffolds to promote healing of large tissue defects.
The present study used SIS-ECM to repair the mucosal resection for two reasons. First, SIS-ECM is a Food and Drug Administration approved biologic scaffold, allowing for expedited clinical translation in this application. Second, SISECM has been previously used in the esophagus to achieve mucosal repair. The present study builds on the results in the esophagus by treating the colonic mucosa with the same material. The ability of ECM derived from the source tissue which is to be treated (i.e., homologous ECM) to promote superior remodeling outcomes when compared to heterologous ECM is not yet known. There is not yet an established, clear advantage to utilizing colonic-ECM for colon remodeling applications. Whether colonic ECM can promote better remodeling outcomes when compared to SIS-ECM represents an important area of future work.
The present study investigated the potential of ECM bioscaffolds to restore mucosal architecture in a short-segment resection of 4 cm in an otherwise healthy animal model. Future work should investigate the ability of ECM bioscaffolds to facilitate the same constructive remodeling outcomes with longer segment mucosal resection. Limitations of the present study include the small number of animals evaluated and the lack of an analysis of functional recovery of the colon; however, the histologic evidence of mucosal remodeling and clinical parameters such as weight maintenance is noteworthy.
Conclusion
Methods for reduction of complications including stricture, bleeding, and fistula formation associated with surgical procedures to preserve colorectal function after resection are of great interest and have obvious clinical utility. The present pilot study suggests that the use of bioscaffolds composed of mammalian ECM can replace resected colorectal tissue and provide an inductive template for constructive colon remodeling. This approach could provide a valuable clinical option for colorectal cancer patients and patients with other bowel disease to prevent common surgical complications and is worthy of further study.
Acknowledgment
Authors’ contributions: J.L.D., T.J.K., N.T., and S.F.B. conceived the study and experimental design. T.J.K., J.L.D., and N.T. prepared materials. S.S., D.N., and D.H. completed animal surgeries. T.J.K. assisted with animal surgeries. T.J.K., D.C., and J.L.D. collected tissue samples. S.S. and T.J.K. performed colonoscopies. T.J.K., D.C., and J.L.D. collected histology images and analyzed results. J.L.D. composed the manuscript. J.L.D., T.J.K., N.T., S.S., D.N., D.H., and S.F.B. edited the manuscript.
Footnotes
Disclosure
The authors declare no conflicts of interest to disclose.
REFERENCES
- 1.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. [DOI] [PubMed] [Google Scholar]
- 2.Sax HC. New treatment for patients with short-bowel syndrome: growth hormone, glutamine and a modified diet. JPEN J Parenter Enteral Nutr. 1996;20:375–376. [DOI] [PubMed] [Google Scholar]
- 3.El-Shami K, Oeffinger KC, Erb NL, et al. American cancer Society colorectal cancer Survivorship Care Guidelines. CA Cancer J Clin. 2015;65:428–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morino M, Risio M, Bach S, et al. , S. European Association for Endoscopic, C. European Society of. Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg Endosc. 2015;29:755–773. [DOI] [PubMed] [Google Scholar]
- 5.Tutticci N, Sonson R, Bourke MJ. Endoscopic resection of subtotal and complete circumferential colonic advanced mucosal neoplasia. Gastrointest Endosc. 2014;80:340. [DOI] [PubMed] [Google Scholar]
- 6.Gomez V, Racho RG, Woodward TA, et al. Colonic endoscopic mucosal resection of large polyps: is it safe in the very elderly? Dig Liver Dis. 2014;46:701–705. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Zhang XH, Ge J, Yang CM, Liu JY, Zhao SL. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal tumors: a meta-analysis. World J Gastroenterol. 2014;20:8282–8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo HM, Zhang XQ, Chen M, Huang SL, Zou XP. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol. 2014;20:5540–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146:652–660.e1. [DOI] [PubMed] [Google Scholar]
- 10.Bahin FF, Naidoo M, Williams SJ, et al. Prophylactic endoscopic coagulation to prevent bleeding after wide-field endoscopic mucosal resection of large sessile colon polyps. Clin Gastroenterol Hepatol. 2015;13:724–730.e1–2. [DOI] [PubMed] [Google Scholar]
- 11.Londono R, Badylak SF. Regenerative medicine Strategies for esophageal repair. Tissue Eng Part B Rev. 2015;21:393–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badylak SF, Hoppo T, Nieponice A, Gilbert TW, Davison JM, Jobe BA. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A. 2011;17:1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieponice A, Ciotola FF, Nachman F, et al. Patch esophagoplasty: esophageal reconstruction using biologic scaffolds. Ann Thorac Surg. 2014;97:283–288. [DOI] [PubMed] [Google Scholar]
- 14.Hoppo T, Badylak SF, Jobe BA. A novel esophageal-preserving approach to treat high-grade dysplasia and superficial adenocarcinoma in the presence of chronic gastroesophageal reflux disease. World J Surg. 2012;36:2390–2393. [DOI] [PubMed] [Google Scholar]
- 15.Badylak SF, Kropp B, McPherson T, Liang H, Snyder PW. Small intestinal submucosa: a rapidly resorbed bioscaffold for augmentation cystoplasty in a dog model. Tissue Eng. 1998;4:379–387. [DOI] [PubMed] [Google Scholar]
- 16.Meng FW, Slivka PF, Dearth CL, Badylak SF. Solubilized extracellular matrix from brain and urinary bladder elicits distinct functional and phenotypic responses in macrophages. Biomaterials. 2015;46:131–140. [DOI] [PubMed] [Google Scholar]
- 17.Brown BN, Sicari BM, Badylak SF. Rethinking regenerative medicine: a macrophage-centered approach. Front Immunol. 2014;5:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sicari BM, Dziki JL, Siu BF, Medberry CJ, Dearth CL, Badylak SF. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials. 2014;35:8605–8612. [DOI] [PubMed] [Google Scholar]
- 19.Brown BN, Londono R, Tottey S, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8:978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huleihel L, Hussey GS, Naranjo JD, et al. Matrix-bound nanovesicles within ECM bioscaffolds. Sci Adv. 2016;2:e1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dziki JL, Sicari BM, Wolf MT, Cramer MC, Badylak SF. Immunomodulation and Mobilization of progenitor cells by extracellular matrix bioscaffolds for Volumetric Muscle loss treatment. Tissue Eng Part A. 2016;22:1129–1139. [DOI] [PubMed] [Google Scholar]
- 22.Keane TJ, Dziki J, Sobieski E, et al. Restoring mucosal Barrier function and Modifying macrophage phenotype with an extracellular matrix hydrogel: potential Therapy for ulcerative colitis. J Crohns Colitis. 2017;11:360–368. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal V, Tottey S, Johnson SA, Freund JM, Siu BF, Badylak SF. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng Part A. 2011;17:2435–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heafner TA, Glasgow SC. A critical review of the role of local excision in the treatment of early (T1 and T2) rectal tumors. J Gastrointest Oncol. 2014;5:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endreseth BH, Myrvold HE, Romundstad P, et al. Norwegian Rectal Cancer, Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon Rectum. 2005;48:1380–1388. [DOI] [PubMed] [Google Scholar]