Abstract
Purpose:
The definitive diagnosis of necrotizing enterocolitis (NEC) is typically at an advanced stage, indicating the need for sensitive and noninvasive diagnostic modalities. Near infrared spectroscopy (NIRS) has been utilized to noninvasively measure intraabdominal tissue oxygenation and to diagnose NEC, but specificity is lacking, in part because sensors are limited to a narrow band of the electromagnetic spectrum. Here, we introduce the concept of broadband optical spectroscopy (BOS) as a noninvasive method to characterize NEC.
Methods:
NEC was induced in 7-day old mice by gavage feeding with formula supplemented with enteric bacteria plus hypoxia. Transabdominal spectroscopy was performed daily using a broad-spectrum halogen light source coupled with a spectroradiometer capable of detection from 400 to 1800 nm.
Results:
A feature extraction algorithm was developed based on the spectral waveforms from mice with NEC. When subsequently tested on cohorts of diseased and control mice by a blinded examiner, noninvasive BOS was able to detect disease with 100% specificity and sensitivity.
Conclusions:
We reveal that the use of BOS is able to accurately and noninvasively discriminate the presence of NEC in a mouse model, thus introducing a noninvasive early diagnostic modality for this devastating disease.
Keywords: Necrotizing enterocolitis, Hyperspectral imaging, Prematurity
Necrotizing Enterocolitis (NEC) is among the most devastating of neonatal diseases, [1] with mortality rates as high as 30% with modern treatment. [2] The pathophysiology of NEC is not fully understood, while its clinical presentation is varied and nonspecific, making early diagnosis challenging. Previous approaches to NEC diagnosis have included risk factor identification, biomarkers, and noninvasive imaging such as ultrasound or near-infrared spectroscopy (NIRS). [3] The use of NIRS in this context typically refers to commercially available oximeters that detect light absorbance or reflectance in 2–5 wavelengths, generally between 700 and 850 nm, where there is minimal overlap of the absorption spectra of oxygenated and deoxygenated hemoglobin. [4] Though this approach is useful to measure tissue oxygen saturation, it has not yet been demonstrated sufficiently sensitive or specific for NEC to gain traction in clinical practice.
In this study we introduce the concept of broadband optical spectroscopy (BOS) as a diagnostic tool for NEC, demonstrated in a murine model. Utilizing a spectroradiometer capable of detection of wavelengths ranging from 400 to 1800 nm with 1 nm resolution, we have constructed a system that, in addition to measuring tissue oxygenation, captures other potential biologic chromophores present during disease progression. The breadth and increased granularity of this methodology require advanced machine-learning statistics to decipher, but it has obvious advantages over commercial NIRS probes.
1. Methods
All animal experiments were approved by an institutional animal care and use committee (Johns Hopkins ACUC MO14M362). As initial validation for the use of BOS for intraabdominal disease, a cohort of mice with dextran sulfate sodium (DSS) induced enteritis, which is a well-established model of intestinal inflammation, [5] was studied against normal controls. 4-week-old C57BL/6 mice were administered 4% ad libitum in drinking water for 9 days, and disease progression was assessed by recording body weight, stool consistency and the presence of fecal occult blood. Severity of the disease was confirmed by gross examination of colon at necropsy, with measurement of colon length. After establishment of BOS measurement protocols and techniques, NEC was induced in 7-day old C57BL/6 mice by gavage feeding with formula supplemented with enteric bacteria plus daily induction of transient hypoxia (5% oxygen, 10 min). Finally, a model of infectious sepsis was created to evaluate the specificity of BOS for NEC. A cecal slurry was created by harvesting the cecal contents of a sacrificed adult mouse and preparing an injectable solution according to a previously validated protocol. [6] This slurry was intraperitoneally injected into breastfed age-matched mice of the same strain, and BOS measurements were subsequently obtained.
Transabdominal spectroscopy was performed using a broad-spectrum halogen light source coupled with a spectroradiometer (Labspec 5000; ASD Inc.; Boulder, CO) capable of detection with 1 nm resolution from 400 to 1800 nm with a 100 ms acquisition time. A fiberoptic cable comprising channels for light source and detection was fashioned into a small-aperture probe and connected to the light source and detector (Fig. 1). This probe was gently placed onto the mouse abdomen in multiple locations for data acquisition (Fig. 2). Measurements were performed by an investigator blinded to the presence or absence of disease. At the completion of the multiday experiment or as clinical condition required, the mice were euthanized and disease severity was assessed with histologic confirmation.
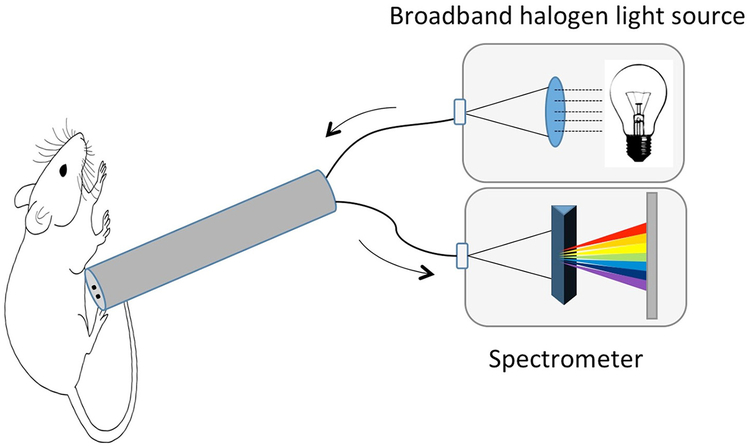
Fig. 1.
Depiction of hardware setup: a fiberoptic probe was constructed with channels to transmit a broadband halogen light source as well as detect reflected spectra via a spectroradiometer.
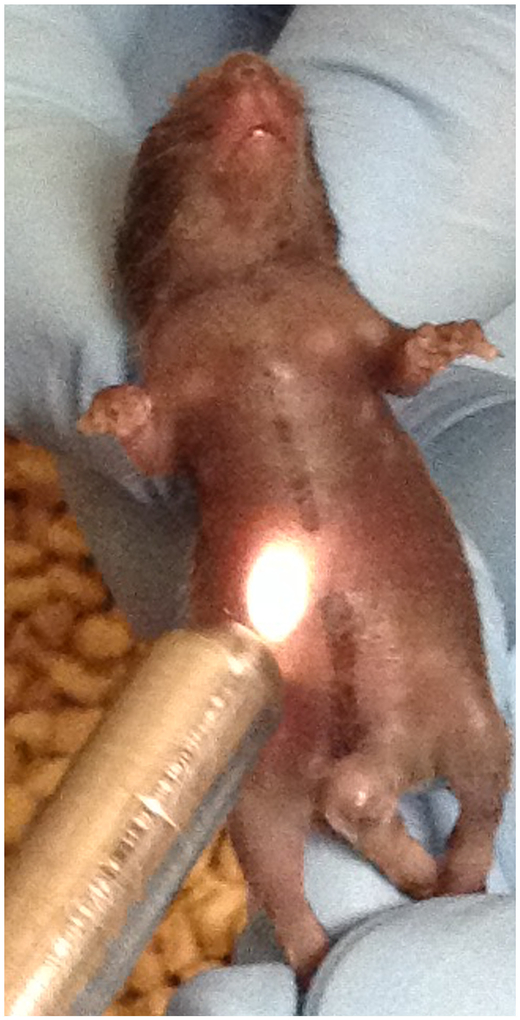
Fig. 2.
Example of data acquisition with the lit probe being gently placed noninvasively onto the lower abdomen of the juvenile mouse.
Data were analyzed using linear discriminant analysis (LDA) to classify features of the reflectance waveforms. LDA plots all features of a spectrum in multidimensional space and subsequently tests and assigns a threshold that permits the most accurate distinction between groups of data. All data processing and analysis were conducting offline with algorithms developed using the Matlab 2016a platform (Mathworks Inc.; Natick, MA). Given the raw spectral signals with assigned class labels of breastfed, NEC, or sepsis; our analysis transformed spectra from a training dataset of advanced NEC versus normal into a single feature that allowed for the greatest degree of separation between the two classes. A feature value threshold was then assigned to generate a classifier for prospective day-by-day testing. For comparison of multiple groups such as NEC versus breastfed versus infectious sepsis, characteristics of the spectra in the near infrared (700–1100 nm) and shortwave infrared (> 1100 nm) were analyzed separately. Nonparametric distributions were compared for statistical significance with a Mann–Whitney U test.
2. Results
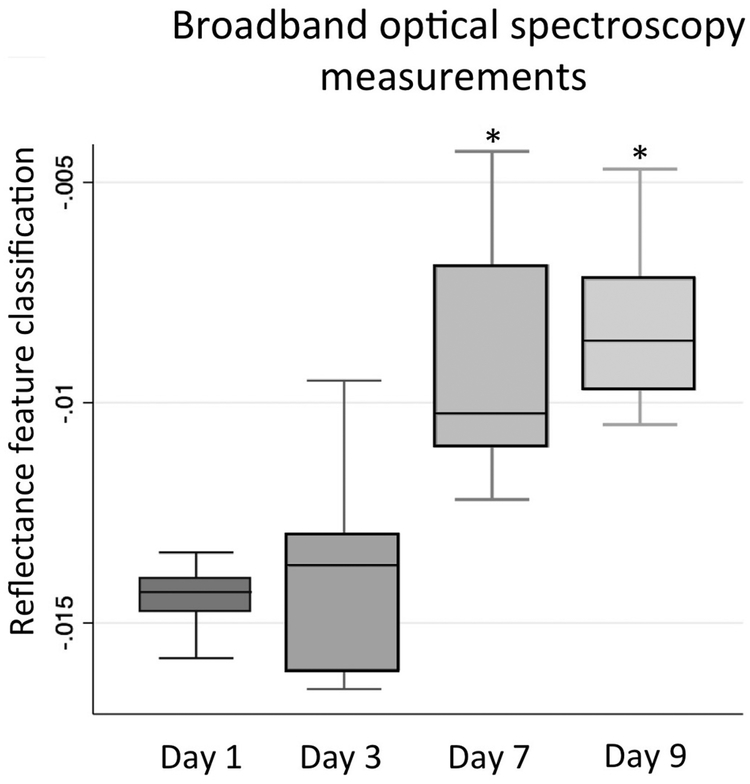
Measurements of 7 mice with DSS-colitis and 7 breastfed controls were taken at multiple daily intervals. Signal changes were detected using LDA feature extraction as early as 3 days after initiation of the experiment, concordant with the presence of fecal occult blood and days earlier than the onset of other clinical symptoms. Clinical symptoms of severe colitis were uniformly evident by 9 days with grossly bloody stools, diarrhea and significant weight loss compared to age-matched healthy controls (DSS final weight 12.8 ± 1.2 g vs controls 25.7 ± 1.4 g). Classification of spectral waveforms documented progressive alteration of tissue reflectance and could reliably differentiate disease on day 9 from lack of disease on day 1 with 100% sensitivity and specificity (Fig. 3). At necropsy, the colon of the diseased mice was significantly diseased as well as decreased in length compared to controls (DSS length 3.6 ± 0.5 cm vs 8.8 ±0.2 cm).
Fig. 3.
BOS measurements in a cohort of mice with DSS enteritis depicting a changing value as disease progresses. Asterisk represents statistically significant change from Day 1 baseline.
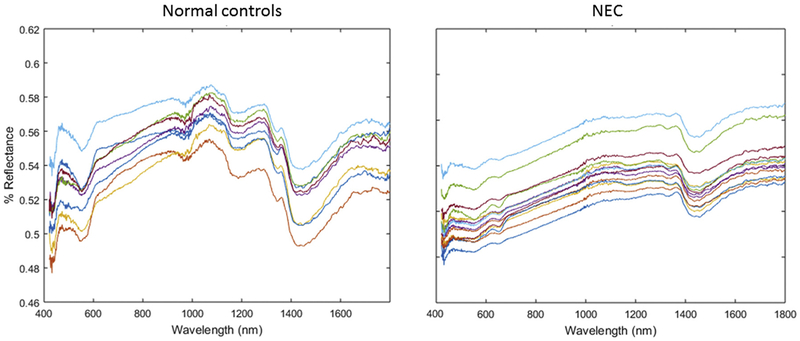
Spectra were then obtained from 20 mice with advanced NEC (Day 5 of disease induction, immediately prior to sacrifice) and 20 age-matched breastfed controls. A representative sample is graphed in Fig. 4. BOS demonstrated predictable patterns among mice in each respective group, and the spectra were grossly different between control and diseased mice.
Fig. 4.
A representative sample of broadband optical spectroscopy in breastfed and diseased mice with NEC. Each line represents a different mouse. BOS demonstrated predictable patterns among mice in each respective group, and the spectra were grossly different between breastfed and diseased.
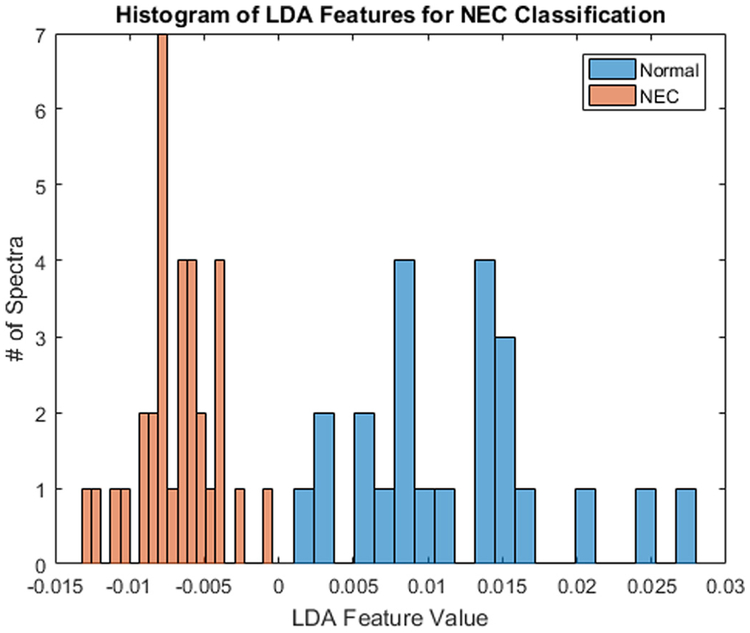
An LDA feature extraction algorithm was developed based on the spectral waveforms from mice with known NEC compared to breastfed. Following development of the LDA feature, when subsequently tested on cohorts of 35 diseased and 25 control mice by a blinded examiner, the mean (SD) unitless LDA value was −0.0068 (0.0026) for NEC mice, compared to 0.0117 (0.0066) in controls (Fig. 5). There was no overlap in values, allowing detection of disease with 100% specificity and sensitivity.
Fig. 5.
Histogram comparison of unitless LDA feature values based on the spectral waveforms from mice with NEC compared to normal breastfed control.
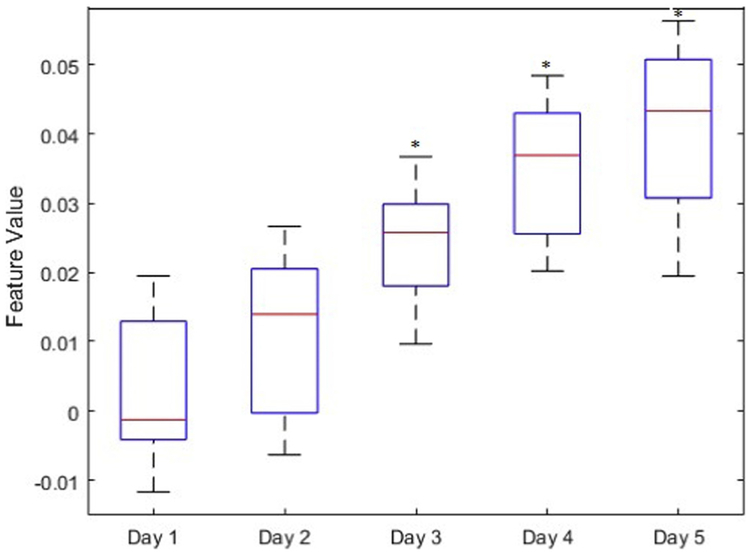
On a separate cohort of 14 mice undergoing induction of NEC, BOS measurements were obtained daily and the unitless LDA feature was calculated for each subject (Fig. 6). Statistically significant changes from baseline were first noted on Day 3, at which time the mice were noted to be hypoactive but not yet lethargic or otherwise clinically ill.
Fig. 6.
Box and whisker plot demonstrating median, interquartile range, and range for unitless LDA of BOS measurements in a NEC cohort. Statistically significant changes from baseline (asterisk) were first noted on Day 3, prior to the development of clinical symptoms.
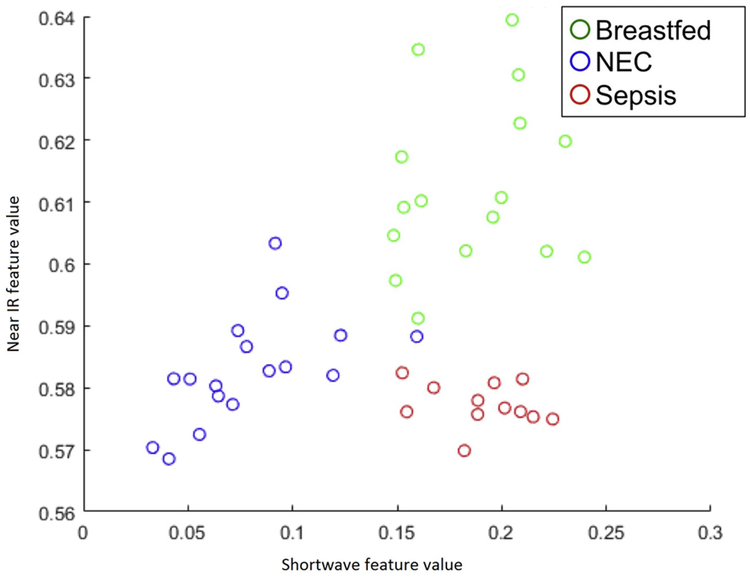
A cohort of mice with infectious abdominal sepsis was created via intraperitoneal injection of cecal slurry as described above. BOS readings were taken 48 h after injection and compared to previously unstudied groups of NEC and breastfed control. By plotting a unitless feature representing spectral traits in the known regions of blood proteins and water absorption, spectra from the mice with NEC were able to be grossly discriminated from both breastfed and sepsis groups (Fig. 7).
Fig. 7.
Scatterplot of the near infrared (700–1100 nm) and shortwave infrared (>N1100 nm) LDA feature value of breastfed, NEC, and infectious sepsis mice. Each circle represents an individual mouse.
3. Discussion
The fulminant nature of NEC is a hurdle to major breakthroughs in treatment, and preventive strategies have remained elusive despite decades of risk factor research. However, the onset of NEC may be accompanied by potentially detectable buildup of cellular factors and metabolic byproducts. Early diagnosis could conceivably offer a reduction in morbidity and mortality by helping to avoid advanced disease requiring laparotomy or peritoneal drainage. This study is the first to our knowledge to apply a hyperspectral approach to NEC identification using a wider electromagnetic band than commercially available NIRS. Here, we introduce the concept of BOS as a diagnostic modality for NEC.
The use of NIRS as a potential tool for the detection of NEC has been previously reported. Zamora et al. described the transabdominal use of neonatal cerebral oximeters in a piglet model of NEC and found on post hoc analysis that low tissue oxygen saturations predicted the development of NEC. [7] In a separate paper from the same group, this finding was confirmed in neonates, though the significance of low saturations occurring days to weeks before the clinical onset of NEC was unclear. [8] Schat et al. used similar technology from a different manufacturer and observed neonates in the 48 h after the onset of symptoms suspicious for NEC. [9] Their results were notable for no difference between neonates with NEC and normal controls, although there was a suggestion that complicated versus uncomplicated NEC could be discriminated. These studies are provocative but highlight the limitations of the commercial probes; most specifically that only tissue oxygenation is being measured and not any disease-specific chromophores. As a result, this approach is unlikely to distinguish between NEC and other causes of hypoperfusion such as hypovolemia, sepsis, or cardiac disease. A further drawback is that each manufacturer uses proprietary algorithms, precluding comparison across studies and investigators. By broadening the band of wavelengths measured and recording 1400 points with each reading, we believe that BOS mitigates these disadvantages and could have promise as a clinical tool in the neonatal intensive care unit. The system we have constructed was able to discern gross differences between normal and NEC in a mouse model and when LDA statistics were employed, BOS was capable of discrimination with 100% sensitivity and specificity.
Regarding questions of pathophysiology using this technology, interestingly it is not necessary to fully elucidate disease-specific chromophores in order to detect a difference in spectra. Our group has noted in adult colon cancer that discrimination can be performed even without full mechanistic understanding. [10] However, we speculate that the presence of specific ischemic byproducts or an increase in cellular expression of receptors with specific absorbances may play a role. Despite knowing some of the basics of tissue absorption for decades, [11] the makeup of contributions to in vivo autofluorescence is not fully described. Examples of commonly identified cellular chomophores include NAPDH [12] and lactate, [13] and there are likely countless others. As the pathophysiology of NEC is better understood, [14] there will undoubtedly be testable candidates for contributing chromophores.
The most valuable role for translational BOS research is regarding early prediction capability. Early identification of NEC may avoid the need for laparotomy or peritoneal drainage, which are major drivers of morbidity, cost, and hospital stay among this group of neonates.[2] Our DSS enteritis model was successful in creating predictable time-course disease that we could serially measure with BOS. Furthermore, daily measurements of NEC demonstrated progressive signal changes over the course of a 5-day period. Not only was disease advancement noted in spectral analysis, but statistically significant changes preceded the development of overt clinical signs of illness by days in some cases. Additionally in our work, BOS has been able to discriminate NEC not only from breastfed control, but also from infectious abdominal sepsis. This bodes well for translational applications that we have planned for future studies. If successful, we imagine that BOS could be an additional vital sign obtained on premature infants. Other pediatric surgical diseases such as esophageal atresia and diaphragmatic hernia that have been studied with NIRS [15,16] may also be characterized by differences in spectral reflectance.
The strength of this study is the use of robust technology applied to NEC in a novel way. Limitations of the work include the use of a mouse model, which may not fully recapitulate human disease and does not offer insight into performance of the technology under circumstances such as pneumoperitoneum. We are currently unable to predict whether skin tone, formula regimen, or presence of pneumatosis affects the signal characteristics of BOS. Prospective large-cohort animal or human studies must be undertaken to have sufficient rate of subjects develop disease while being serially measured. Lastly, as expected with new equipment, current costs of construction preclude wide-scale construction and must be evaluated for benefit that will justify resource allocation.
In conclusion, we reveal that BOS is able to accurately and noninvasively discriminate the presence of NEC in a mouse model, thus introducing a noninvasive early diagnostic modality for this devastating disease.
References
- [1].Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hull MA, Fisher JG, Gutierrez IM, et al. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective cohort study. J Am Coll Surg 2014;218:1148–55. [DOI] [PubMed] [Google Scholar]
- [3].Eaton S, Rees CM, Hall NJ. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology 2017;111:423–30. [DOI] [PubMed] [Google Scholar]
- [4].Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 2009;103:i3–i13. [DOI] [PubMed] [Google Scholar]
- [5].Chassaing B, Aitken JD, Malleshappa M, et al. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 2014;104 10.1002/0471142735.im1525s104 [Unit 15.25]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Starr ME, Steele AM, Saito M, et al. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS ONE 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zamora IJ, Stoll B, Ethun CG, et al. Low abdominal NIRS values and elevated plasma intestinal fatty acid-binding protein in a premature piglet model of necrotizing enterocolitis. PLoS ONE 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Patel AK, Lazar DA, Burrin DG, et al. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr Crit Care Med 2014;15:735–41. [DOI] [PubMed] [Google Scholar]
- [9].Schat TE, Schurink M, Van Der Laan ME, et al. Near-infrared spectroscopy to predict the course of necrotizing enterocolitis. PLoS ONE 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beaulieu RJ, Goldstein SD, Singh J, et al. Automated diagnosis of colon cancer using hyperspectral sensing. Int J Med Robot 2018. 10.1002/rcs.1897 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [11].Anderson RR, Parrish JA. The optics of human skin. J Invest Dermatol 1981;77:13–9. [DOI] [PubMed] [Google Scholar]
- [12].Yim SK, Yun SJ, Yun CH. A continuous spectrophotometric assay for NADPH-cytochrome P450 reductase activity using 1,1-diphenyl-2-picrylhydrazyl. J Biochem Mol Biol 2004;37:629–33. [DOI] [PubMed] [Google Scholar]
- [13].Lafrance D, Lands LC, Burns DH. Measurement of lactate in whole human blood with near-infrared transmission spectroscopy. Talanta 2003;60:635–41. [DOI] [PubMed] [Google Scholar]
- [14].Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 2016;13:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Conforti A, Giliberti P, Mondi V, et al. Near infrared spectroscopy: experience on esophageal atresia infants. J Pediatr Surg 2014;49:1064–8. [DOI] [PubMed] [Google Scholar]
- [16].Conforti A, Giliberti P, Landolfo F, et al. Effects of ventilation modalities on near-infrared spectroscopy in surgically corrected CDH infants. J Pediatr Surg 2016;51:349–53. [DOI] [PubMed] [Google Scholar]