Abstract
Background:
MNS1 (meiosis-specific nuclear structural protein 1) is necessary for motile cilia function, such as sperm flagella or those found in the embryonic primitive node. While little is known regarding the function or expression pattern of MNS1 in the embryo, co-immunoprecipitation experiments in sperm have determined that MNS1 interacts with ciliary proteins also important during development. Establishment of morphogenic gradients is dependent on normal ciliary motion in the primitive node beginning during gastrulation (gestational day (GD) 7 in the mouse, 2nd – 3rd week of pregnancy in humans), a critical window for face, eye, and brain development and particularly susceptible to perturbations of developmental signals. The current study investigates the role of Mns1 in craniofacial defects associated with gastrulation-stage alcohol exposure.
Methods:
On GD7, pregnant Mns1+/− dams were administered two doses of ethanol (5.8 g/kg total) or vehicle four hours apart to target gastrulation. On GD17, fetuses were examined for ocular defects by scoring each eye on a scale from 1 – 7 (1 = normal, 2 – 7 = defects escalating in severity). Craniofacial and brain abnormalities were also assessed.
Results:
Prenatal alcohol exposure (PAE) significantly increased the rate of defects in wild-type fetuses, as PAE fetuses had an incidence rate of 41.18% compared to a 10% incidence rate in controls. Furthermore, PAE interacted with genotype to significantly increase the defect rate and severity in Mns1+/− (64.29%) and Mns1−/− mice (92.31%). PAE Mns1−/− fetuses with severe eye defects also presented with craniofacial dysmorphologies characteristic of Fetal Alcohol Syndrome and midline tissue loss in the brain, palate, and nasal septum.
Conclusions:
These data demonstrate that a partial or complete knockdown of Mns1 interacts with PAE to increases susceptibility to ocular defects and correlating craniofacial and brain anomalies, likely though interaction of alcohol with motile cilia function. These results further our understanding of genetic risk factors that may underlie susceptibility to teratogenic exposures.
INTRODUCTION
Prenatal alcohol exposure (PAE) results in an array of developmental abnormalities, including growth retardation and craniofacial malformations, collectively known as Fetal Alcohol Spectrum Disorders (FASD). Recent conservative estimates put the rate of FASD in the US at ~5% of live births each year (May et al., 2018), making FASD the leading preventable cause of birth defects in this country. Susceptibility to alcohol-related birth defects is modified by genetic (Eberhart and Parnell, 2016) and environmental factors, including variations in alcohol metabolism (Edenberg, 2007), maternal nutrition status (Shankar et al., 2007, May et al., 2016), and timing and degree of alcohol exposure during the prenatal period (Maier et al., 1997). The molecular and genetic mechanisms involved in alcohol teratogenesis include pathways governing apoptosis (Dunty et al., 2001), oxidative stress (Brocardo et al., 2011), metabolic competition with retinoic acid (Johnson et al., 2007), and epigenetic modifications to chromatin (Veazey et al., 2015, Laufer et al., 2013). The use of rodent and zebrafish models of PAE has elucidated the importance of genetic variation on risk of alcohol teratogenesis. Identification of specific gene-alcohol interactions is an important step in understanding the range of outcomes resulting from PAE and developing targeted therapies to treat affected individuals.
The current study examines the role of meiosis specific nuclear structural protein 1 (MNS1) in susceptibility to craniofacial, ocular, and central nervous system (CNS) defects resulting from first trimester-equivalent alcohol exposure. Originally identified in mouse (Mus musculus) spermatocytes (Furukawa et al., 1994), the function and localization of MNS1 remains largely unexplored. Studies of MNS1 function and expression have largely focused on sperm, where it plays an important role in flagellar motility (Vadnais et al., 2014, Zhou et al., 2012). Mns1 knockout (KO) mice display numerous defects in sperm tails, including a short flagellar length and disorganized axonemes (Zhou et al., 2012). In addition, Mns1−/− mice display motile cilia defects, resulting in a high rate of hydrocephalus and situs inversus, this latter finding implicating MNS1 in nodal cilia function during gastrulation, the peak period of alcohol-induced craniofacial malformations.
Interestingly, preliminary RNA-seq analyses from our laboratory identified Mns1 as one of the top 25% most common RNAs in post-implantation mouse embryos (gestational days [GD] 7–10). However, information on MNS1 function during embryonic development is practically non-existent. Due to the importance of motile cilia within the primitive node in circulation of morphogens and axis development, we hypothesized that alterations to MNS1 could contribute to development of craniofacial dysmorphologies, such as those characteristic of Fetal Alcohol Syndrome (FAS). The current study investigates the interaction of Mns1 and alcohol exposure during gastrulation (2nd-3rd week of gestation in humans, GD7 in mice). This developmental time point was chosen because gastrulation-stage exposure results in a high incidence of craniofacial defects involving the eyes, face, and brain (Cook et al., 1987). In addition, left-right symmetry is established during this window, making normal motile cilia function particularly critical (Sulik et al., 1994). This study assesses susceptibility to craniofacial and ocular defects in Mns1 mutant mice (Mns1+/+, Mns1+/−, Mns1−/−) exposed to alcohol on GD7. Mice with severe eye defects further examined for concomitant brain and CNS abnormalities. Understanding Mns1 function in the developing brain will provide new information regarding 1) the unexplored role of MNS1 during embryonic development and 2) the interaction of PAE and motile cilia function in relation to alcohol teratogenesis.
MATERIALS AND METHODS
Animals.
B6:129S4-Mns1 mice (MMRRC Stock No: 37095-JAX) were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in a temperature and humidity-controlled environment on a 12:12 light/dark cycle with ad libitum access to food (Prolab Isopro RMH 3000, LabDiet, St. Louis, MO) and water. Males were housed singly and females housed up to 5 per cage in standard Techniplast cages with nesting material and shelter. A Mns1+/− male mouse was mated with 1–2 Mns1+/− nulliparous female mice for up to 2 hours. GD0 was defined as the beginning of the breeding period in which a copulation plug was found. Dams found to have a copulation plug in the same mating session were housed together in groups of up to 5. Nine PAE and eight vehicle litters were used; all fetuses from each litter were included in analyses. In total, 58 PAE fetuses and 60 vehicle-treated fetuses were used. Specific n’s per genotype are listed in the results and in Figure 3. All procedures involving animals were approved by the IACUC at UNC – Chapel Hill, in accordance with NIH and AAALAC guidelines.
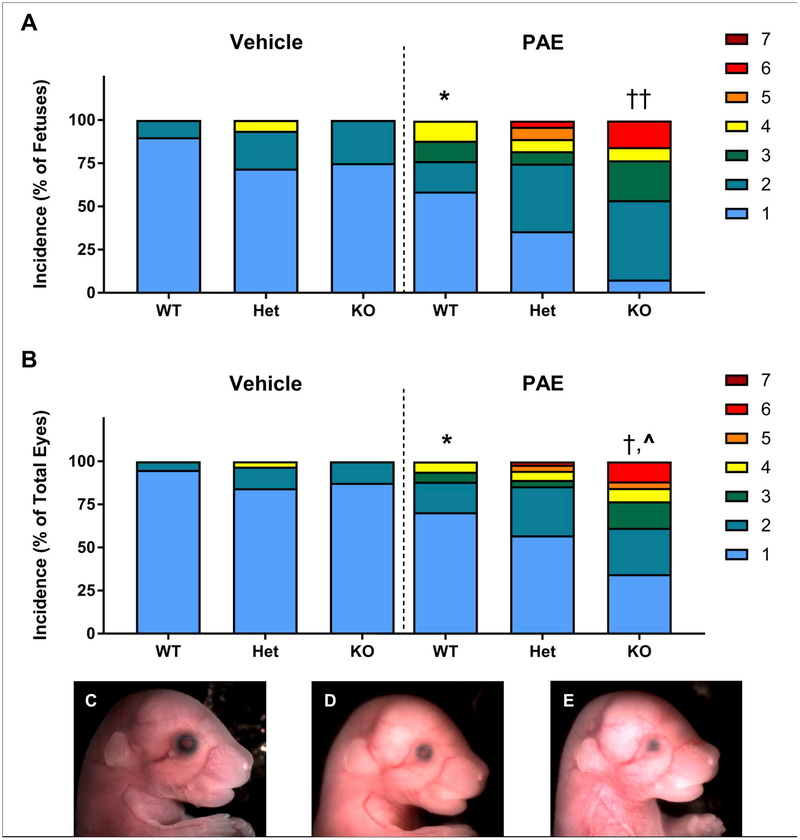
Figure 3.
Incidence and severity of ocular defects following GD7 alcohol exposure. A) Incidence of ocular defects expressed as percent of total fetuses. The most severely affected eye for each fetus was counted for this graph. For the analysis, each fetus was scored as “affected” or “unaffected” based on the presence or absence of a defect in either eye. PAE significantly increased the incidence of ocular defects in WT fetuses (* = p < 0.05 PAE-WT vs. Veh-WT). Within the PAE group, alcohol interacted with genotype to significantly increase the rate of ocular defects per fetus (†† = p < 0.01, PAE-KO vs. PAE-WT). Heterozygous mice did not significantly differ from either KO or WT. Genotype did not affect the rate of spontaneous eye defects in the vehicle-treated group. B) Incidence of ocular defects expressed as the percent of total eyes (two per fetus). These data demonstrate that for some affected fetuses, only one eye was malformed. Severity of eye defects determined that the total number of “non-subtle” eye defects was significantly greater in PAE WT fetuses compared to vehicle-treated WT (* = p < 0.05 PAE-WT vs. Veh-WT). Within the PAE group, alcohol interacted with genotype to significantly increase the rate of non-subtle eye defects († = p < 0.05, PAE-KO vs. PAE-WT; ^ = p < 0.05, PAE-KO vs. PAE-Het). Genotype did not affect the eye defect severity in the vehicle-treated group. For panels A and B, n’s for each group: Veh-WT: 20, Veh-Het: 32, Veh-KO: 8, PAE-WT: 17, PAE-Het: 28; PAE-KO: 13. C) The eye of a vehicle-treated fetus (score: 1). Following GD7 alcohol exposure, severe eye defects were observed in both Mns1+/− and Mns1−/− fetuses, including microphthalmia, the presence of colobomas (D; score: 4), and partial anophthalmia (E; score: 6).
Alcohol exposure paradigm.
On GD7, pregnant dams were administered two intraperitoneal injections, 4 h apart, of 25% (vol/vol) ethanol in a Lactated Ringer’s solution at a dose of 2.9 g/kg (5.8 g/kg total; PAE). This dose results in maternal peak blood alcohol concentrations averaging ~440 mg/dL (measured 30 min after the second dose) (O’Leary‐Moore et al., 2010). The control group was injected with an equivalent volume of vehicle (Ringer’s) under the same treatment scheme. Following injection, dams remained undisturbed until GD17.
Assessment of ocular defects.
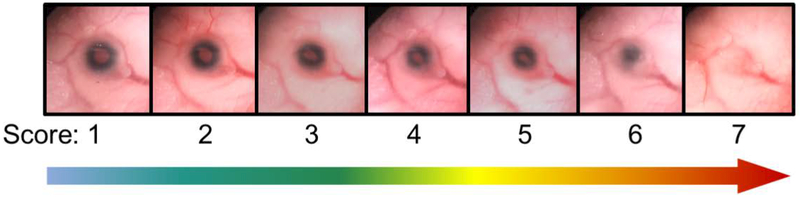
On GD17, dams were euthanized by CO2 asphyxiation followed by cervical dislocation. Fetuses were dissected from the placental sacs and euthanized in chilled 1× phosphate-buffered saline (PBS). Weight and crown-rump length were measured. Craniofacial and ocular dysmorphologies were assessed using a dissecting microscope and images of the philtrum and eyes of each fetus were captured using a Nikon SMZ-U Stereoscopic Zoom microscope (Nikon Corporation, Melville, NY) and QCapture Suite software version 2.98.2 (QImaging, British Columbia, Canada). Defects were categorized with a modified version of a previously used eye scoring paradigm (Gilbert et al., 2016). Briefly, eyes were scored based on overall size of the eye and the shape of the iris. Eyes were given a score from 1 – 7 with normal eyes scoring as “1” (representative images can be seen in Figure 1). For eyes with defects, the following criteria were used: “2” demonstrated normal size with an abnormally shaped pupil, “3” indicated a misshapen pupil and mild microphthalmia, “4” indicated moderate microphthalmia and the presence of a coloboma, “5” demonstrated severe microphthalmia and a coloboma, “6” was given to eyes with severe microphthalmia and lack of a pupil, and the most severe defect, anophthalmia, was scored as “7”. All eyes were initially scored from images by a trained experimenter blinded to treatment and genotype and, as needed, validated by blinded independent researchers. Following imaging, embryos were fixed in Bouin’s solution.
Figure 1.
Eye defect scoring system used in the current study. A score of 1 indicated a normal eye. Scores of 2 – 7 indicate abnormal eyes with the severity of the defect increasing with higher numbers. Defects include microphthalmia (small eye size), coloboma (abnormality of the iris or lens, seen in scores 4 and 5), and partial to complete anophthalmia (scores 6–7).
Genotyping.
Tails were collected for genotyping and stored in 70% EtOH. Genotyping was performed by Mouse Genotype (Escondido, CA) using the primers and cycling parameters recommended by Jackson Laboratories (Common Forward: TCGCTGTCAGAGCAGTCAGT; WT Reverse: GACTCTCGTGCTTCAGTCTGG; Mutant Reverse: GCCAGAGGCCACTTGTGTAG).
Histology.
Heads of GD17 embryos fixed in Bouin’s solution were rinsed in 1× PBS, changed daily, for one week and a final rinse in 70% ethanol. The heads were processed with a Leica TP1020 tissue processor (Leica Camera AG, Wetzlar, Germany), dehydrated with a graded ethanol series, cleared with toluene, and infiltrated with paraffin (Paraplast Plus®). Once infiltrated, the heads were embedded in paraffin and serially sectioned in the coronal plane at 10μm on a rotary microtome. Every fifth slide of each fetus was stained using a hematoxylin and eosin protocol to display tissue features. The slides were digitally photographed using a Nikon Eclipse E600 (Nikon Corporation, Melville, NY) and examined for dysmorphologies. If a significant abnormality was found, slides surrounding the affected area were stained and examined as necessary.
Little data has been published regarding the Mns1 mutant phenotype, aside from the original paper characterizing this strain (Zhou et al., 2012). To add additional information to the public record regarding strain characteristics of these mice and the function of Mns1 in brain development, two untreated male adolescent Mns1−/− mice showing physical signs of hydrocephaly and one untreated wild-type mouse were deeply anesthetized with 250 mg/kg tribromoethanol and transcardially perfused with 1× PBS followed by 10% formalin. Brains were removed and stored in 10% formalin at room temperature. The tissue was then processed for histology via paraffin embedding as described, apart from increased incubation in 70% EtOH for up to two days to ensure complete removal of formalin and maintain tissue integrity. Sections (every fifth) were stained with hematoxylin and eosin to assess the extent of the hydrocephalus.
Statistics.
Weights and crown-rump length were analyzed using a two-way analysis of variance (ANOVA). To analyze ocular defect prevalence, a defect score (1 = defect present, 0 = both eyes normal) was generated for each fetus. Groups were compared using χ2 tests for a priori analyses comparing the effects of PAE in wild-type (WT) mice and then between genotypes (WT vs. heterozygous vs. KO) within each treatment. If a significant effect of genotype was found between the three groups, further χ2 tests were run to determine differences between specific genotypes. Statistical significance was set as p < 0.05 and conducted using GraphPad Prism version 7.04 for Windows (GraphPad Software, La Jolla, CA, www.graphpad.com).
RESULTS
Characterization of litters and knockout animals.
On GD17, fetuses were examined for ocular, craniofacial, and CNS defects. To better characterize the Mns1 heterozygous and knockout phenotype, litter statistics were analyzed. Litter sizes and resorption rates did not differ between the vehicle and PAE groups (p = 0.21 and 0.69, respectively). Litters averaged 6.4 fetuses for PAE dams and 7.5 fetuses for vehicle-treated dams. There was an average of 0.89 resorptions per litter for PAE litters and 1.13 for control litters; the genotypes of these resorptions could not be determined. In total, 20 WT, 32 heterozygotes, and 8 KO were generated for the vehicle-treated group. For the PAE group, 17 WT, 28 heterozygous, and 13 KO were generated.
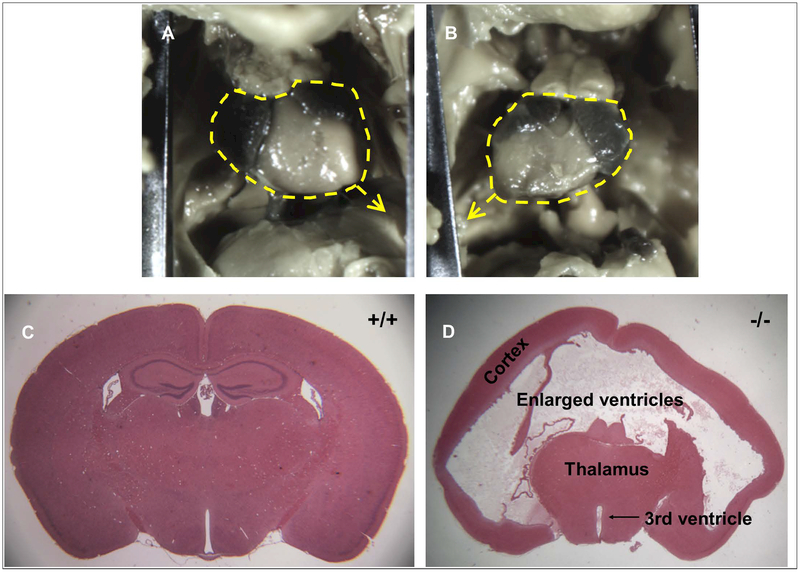
Overall, litters consisted 31.4% of WT, 50.8% of heterozygous, and 17.8% of KO mice. These rates slightly differ from the expected 25%/50%/25% genotype distribution expected for breeding heterozygous mice and suggest a possible increased risk of embryonic lethality for Mns1 KO. Lower than expected rates of Mns1−/− pups were also reported in the original description of this transgenic strain (Zhou et al., 2012). Mns1−/− fetuses had a 67% rate of situs inversus (Figure 2A–B), while no incidents of situs inversus were observed for WT or heterozygous animals. The variable penetrance rate of situs inversus in the knockouts was also observed in the original description of this strain, where 36% of embryos exhibited inverted left-right symmetry (Zhou et al., 2012). It is possible that deviation from normal incidence rates is due to the increased embryonic lethality in Mns1−/− mice (Wilson et al., 2016), as these values do not count the suspected Mns1−/−embryos that died in utero.
Figure 2.
Characterization of Mns1−/− mice. In 67% of Mns1−/− mice, situs inversus was observed. A) An example of a fetal heart from a vehicle-treated, WT fetus with the apex of the heart pointing towards the mouse’s left. B) In KO mice displaying situs inversus, the apex of the heart was pointing towards the animal’s right. C) Brain of an untreated adolescent WT mouse showing normal brain structure. This section is from similar bregma coordinates as the brain of an untreated adolescent KO mouse (D). This brain shows severe hydrocephalus that has created enlarged ventricles and compressed the cortical tissue. The hippocampus, visible at this bregma (~−1.34 mm) in the normal brain, is not visible in the brain with hydrocephalus as it emerges more posteriorly due to tissue compression.
The average mass for GD7 fetuses across vehicle and PAE groups was 0.79 ± 0.01 g (mean ± SEM) and the average crown-rump length was 1.73 ± 0.01 cm (Table 1). Two-way ANOVAs (genotype × treatment) revealed no significant differences between groups in length or weight. However, for length, there was a trend towards a main effects of treatment (F(2, 112) = 2.69, p = 0.072) and, for weight, there was a trend towards a main effect of genotype (F(2, 112) = 3.697, p = 0.057). While not statistically significant, it suggests that PAE fetuses tended to be shorter in length (1.716 cm) and Mns1−/− trended towards having a lower weight (0.766 g).
Table 1.
Gross morphological analyses following 5.8 g/kg alcohol on GD7. On GD17, fetuses did not differ in weight or crown-rump length between treatments or genotypes. Ocular defect rates were determined as the presence or absence of a defect in either eye and expressed as the percent of fetuses with a defect. Wild-type (WT) PAE mice had a significantly higher defect rate compared to the vehicle-treated WT (indicated by §). Genotype significantly interacted with PAE to increase defect rates in knockout mice compared to PAE WT (indicated by ^). The percent of defects scored as 3 or above is based on the total number of eyes scored. PAE alone increased the number of defects scored ≥3 compared to vehicle-treated mice, and genotype interacted with PAE to increase the rate of severe defects even further.
| Vehicle | PAE | |||||
|---|---|---|---|---|---|---|
| Mns1 | WT | Heterozygous | Knockout | WT | Heterozygous | Knockout |
| Weight (g) | 0.81±0.06 | 0.81±0.1 | 0.78±0.06 | 0.75±0.1 | 0.78±0.09 | 0.76±0.08 |
| Crown-rump length (cm) | 1.75±0.07 | 1.73±0.1 | 1.69±0.09 | 1.72±0.08 | 1.75±0.09 | 1.69±0.08 |
| Incidence (% of fetuses with defect) | 10 | 26.47 | 25 | 41.18§ | 64.29 | 92.31^ |
| % of defects scored ≥ 3 | 0 | 2.9 | 0 | 11.8 | 14.3 | 38.5 |
Heads of two untreated male adolescent Mns1−/− mice were also processed for histology to further characterize genotypic differences caused by complete deletion of Mns1. These mice displayed symptoms of hydrocephaly prior to sacrifice, including an enlarged skull, a hunched body, lack of grooming behavior, and lethargy. Compared to the normal adolescent brain (Figure 2C), severe hydrocephalus was evident in both animals (Figure 2D). The brains displayed enlarged ventricles and compressed cortical and subcortical tissue. Notably, hydrocephalus was not overtly detectable in any of the examined fetuses suggesting that this abnormality arises later in development.
Ocular defects.
Eye shape and size were assessed in GD17 embryos using a well-developed scale of defect severity that rates eyes from 1–7 with higher numbers indicating more severe defects (Figure 1) (Gilbert et al., 2016). Defect rates were based on the presence or absence of a defect in either eye. PAE significantly increase incidence of WT fetuses with eye defects (χ2 = 4.852, p = 0.0276) (Table 1, Figure 3A). Vehicle-treated WT fetuses had a defect rate of 10% while PAE fetuses had a defect rate of 41.18%. In vehicle-treated animals, genotype alone did not significantly affect the number of eye defects (p = 0.34), suggesting that MNS1 function is not necessary to normal eye development under normal conditions. However, PAE interacted significantly with genotype to increase incidence and severity of eye defects. Within the PAE group, there was an overall significant effect of genotype (χ2 = 8.344, p = 0.0154). KO mice had significantly higher defect rates than the WT mice (χ2 = 8.294, p = 0.004), with 92.31% of mice displaying defects (compared to 41.18% of WT fetuses). There was a trend for KO to have higher defect rates compared to heterozygous mice (χ2 = 3.551, p = 0.059), with 64.29% of heterozygotes exhibiting defects. Incidence rate did not differ between PAE heterozygote and WT fetuses (p = 0.13). These data are also displayed as the percent of total eyes (two per fetus) for each score (1–7) (Figure 3B). Rates of PAE-induced defects did not differ between the left and right eyes (χ2 = 0.8899, p = 0.64).
The eye defects in the PAE group were rated as more severe than those in the control group and also increased in severity based on genotype (Figures 3A and 3B). The most severe defect in the vehicle group was a rating of 4 (representative image Figure 3D), representing 1.6% of total eyes of vehicle-treated fetuses. Both of these eye were found in vehicle-treated heterozygotes and represented the most severe score in 6.25% of fetuses in this group. Conversely, the most severe defect recorded in the PAE group was a rating of 6 (2.6% of total eyes scored). A score of 6 (Figure 3E) was rated as the most severe eye in 3.6% of PAE heterozygous mice and 15.38% of PAE KO mice. Overall, 18.9% of total eyes of PAE fetuses were rated as “non-subtle” (3 or greater in severity) compared to 1.6% of total eyes of vehicle-treated mice. PAE WT fetuses had significantly more non-subtle defects (11.76%) compared to the vehicle-treated WT fetuses (0%) (χ2 = 4.975, p = 0.0257). Within the PAE group, defect severity also increased with loss of Mns1 (χ2 = 8.375, p = 0.015). PAE KO mice displayed more non-subtle defects compared to both the WT (χ2 = 5.87, p = 0.0154) and heterozygous genotypes (χ2 = 6.057, p = 0.0138). While the majority of eyes in the WT and heterozygote groups were rated as a 1 or 2 (88.2% and 85.7% of total eyes, respectively), 38.5% of total eyes in the KO group were rated as ≥ 3 (Figure 3B). Genotype did not impact the number of non-subtle eye defects in the vehicle-treated group (χ2 = 1.78, p = 0.41).
Craniofacial and CNS dysmorphologies.
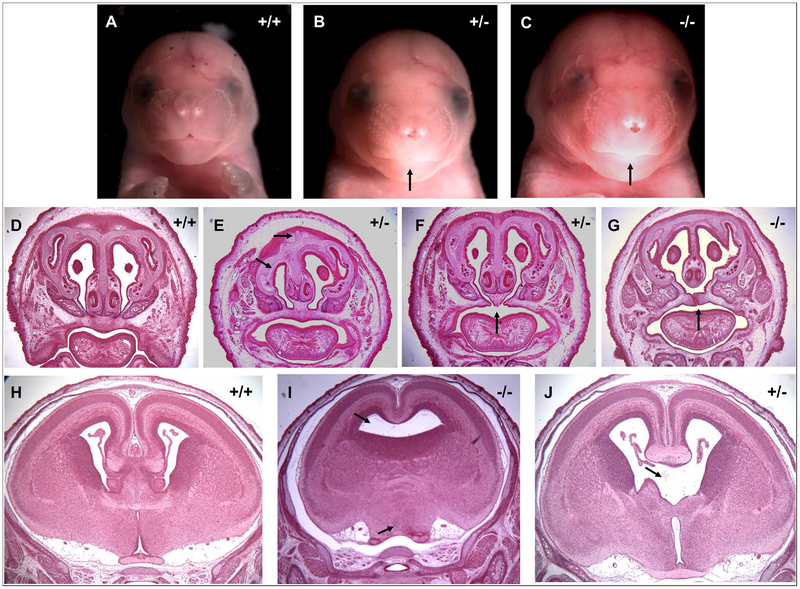
A subset of heterozygous or KO embryos with severe eye defects (>3) were coronally sectioned and stained with hematoxylin and eosin for observation of gross anatomical defects. In two cases, facial dysmorphologies characteristic of the features associated with FAS were observed, including a thin upper lip, smooth philtrum, and decreased facial width (Figure 4B, C). Other observed craniofacial abnormalities include nasal cavity and septum deformities (Figure 4E) and the presence of a cleft palate (Figure 4F, G), a feature associated with both FAS (DeRoo et al., 2008) and genetic ciliopathies (Brugmann et al., 2010, Cortés et al., 2015). CNS abnormalities including dysgenesis of the corpus callosum, holoprosencephaly (Figure 4I), and loss of midline septal brain tissue (Figure 4J) were also observed.
Figure 4.
Craniofacial and CNS defects (black arrows) following GD7 alcohol exposure in Mns1+/− and Mns1−/− mice. A, D, H) Examples of the face (A), nasal cavity and palate (D), and brain (H) of vehicle-treated WT fetuses. B – C) Two PAE fetuses displaying a thin upper lip and smooth philtrum similar to the classic facial dysmorphologies associated with FAS. E) Nasal cavity and septum defects in a PAE fetus. F-G) Cleft palates and improper fusion of the nasal septum to the palatal shelf in PAE fetuses. I) Holoprosencephaly and J) dysgenesis of midline septal tissue in the PAE fetal brain.
DISCUSSION
The current study used a mouse model of early gestational alcohol exposure to test whether knockdown of the Mns1 gene alters susceptibility to alcohol teratogenicity. PAE significantly interacted with genotype to increase the number and severity of birth defects compared to rates observed in vehicle-treated fetuses. Defects were most prevalent and severe (≥ 3 score) in alcohol-exposed Mns1+/− and Mns1−/− fetuses compared to both the PAE WT and vehicle-treated fetuses of the same genotype. Fetuses with severe eye defects also presented with numerous craniofacial and CNS abnormalities, including cleft palate, nasal cavity and septal deformities, holoprosencephaly, and agenesis of several midline brain structures. This study suggests that loss of the Mns1 gene increases susceptibility to ocular, craniofacial, and CNS defects resulting from PAE.
Loss of Mns1 may alter susceptibility to alcohol teratogenesis through disruption of normal motile cilia structure and function. Beginning during gastrulation, motile cilia in the embryonic primitive node distribute morphogens such as sonic hedgehog (Shh) and retinoic acid in a counter-clockwise rotation to determine regular left-right asymmetry (Sulik et al., 1994, Tsukui et al., 1999, Babu and Roy, 2013, Ryan and Belmonte, 2000). As described in the current study and previous work characterizing Mns1−/− mice, these mice have a high incidence of situs inversus (Zhou et al., 2012), likely due to disruption of important morphogenic gradients caused by abnormal motile cilia function following loss of Mns1. Loss of another cilia gene, Kif3b, is also correlated with a 50% incidence of situs inversus (Nonaka et al., 1998). In addition, Mns1−/− mice often develop postnatal hydrocephalus, as demonstrated in Figure 3. Ciliated ependymal cells lining the brain ventricles aid in the flow of cerebrospinal fluid (CSF), and abnormal motile cilia can lead to improper flow of CSF through the ventricles and, eventually, hydrocephalus (Narita and Takeda, 2015, Fliegauf et al., 2007).
While the exact role of MNS1 in cilia has yet to be determined, much can be learned from examining other proteins with which MNS1 is known to interact. In the sperm, MNS1 co-immunoprecipitates with the anterograde intraflagellar transport protein KIF3A (Lehti et al., 2013). Kif3a knockdown disrupts transport of MNS1 down the sperm flagella, causing MNS1 to localize abnormally in the sperm acrosome and manchette (Lehti et al., 2013). If MNS1 is related to protein trafficking up the axoneme, disruption of MNS1 could prevent transport of large complexes necessary for normal motile cilia function. Full knockdown of Kif3a is embryonic lethal by GD10, with embryos displaying defects similar to Mns1−/− mice including situs inversus and abnormal sperm head and flagella morphology (Takeda et al., 1999). Conditional Kif3a knockdown prevents the formation of non-motile primary cilia in neural crest cells that go on to form part of the face and brain. Expectedly, the conditional Kif3a KO display a spectrum of craniofacial defects that can present as facial thinning or widening, including cleft palates (Liu et al., 2014). The present study indicates that MNS1 is not strictly critical for craniofacial development in the healthy embryo, as loss of Mns1 did not affect the incidence of eye defects or other craniofacial abnormalities in vehicle-treated animals. However 5/8 of these fetuses exhibited situs inversus, demonstrating disruption of motile cilia function. The extent to which motile cilia impact craniofacial development beyond left-right axis formation remains to be elucidated. While there were no craniofacial abnormalities in the vehicle-treated KO mice, it is clear that there was a significant Mns1-alcohol interaction in craniofacial development, suggesting an intersection with other developmental pathways. One pathway through which the loss of Mns1 may increase craniofacial defect incidence and severity following GD7 alcohol exposure is through disruption of the Shh pathway. Non-motile cilia, or primary cilia, are critical for normal transduction of the Shh pathway and conditional Kif3a KO exhibit increased Shh responsiveness in the facial mesenchyme and altered expression of Shh pathway genes, including Shh, Gli1, Ptc, and Gli3 in the neural crest (Liu et al., 2014). Further work is needed to determine whether MNS1 localizes to or has any function in primary cilia specifically, although the data presented here would suggest a significant role of MNS1 in primary cilia function.
Besides KIF3A, MNS1 also co-immunoprecipitates with MFN2 (Vadnais et al., 2014), a protein necessary for mitochondrial fusion known to interact with the pro-apoptotic protein BAX and the anti-apoptotic protein BCL-2 (Zhao et al., 2015). High levels of MFN2 downregulate apoptosis. Unpublished work from our lab has shown that Bax knockdown reduces rates of ocular defects in PAE mice, supporting the important role of apoptotic pathways in the genesis of alcohol-related malformations. While currently speculative, loss of Mns1 may disrupt MFN2 interactions with apoptosis-related proteins resulting in increased cell death in the embryo that manifests as craniofacial defects when challenged with an environmental stressor such as alcohol. Further studies are needed to determine colocalization of MNS1 with other proteins in both the normally developing embryo and following PAE.
The current study demonstrated that partial or full loss of Mns1 increases susceptibility to ocular, craniofacial, and CNS defects following gastrulation-stage alcohol exposure in mice. This study is one of the first to investigate the role of MNS1 in the embryo and show that MNS1 has a role in craniofacial development. Whether disruption of either motile or primary cilia act as a pathogenic mechanism of alcohol teratogenesis remains under investigation, although our data suggest that MNS1 plays a role in primary cilia function. Future research should focus on uncovering MNS1 function in the developing embryo through determination of cell localization and protein-protein interactions. Through these studies, the role of MNS1 in craniofacial development, both in the normal and PAE fetus, can be established. Together, these data further demonstrate another aspect of the genetic factors that can modify susceptibility to PAE.
Highlights.
The MNS1 protein is associated with normal motile cilia function
Motile cilia distribute establish the morphogenic gradient during gastrulation
Mns1 knockdown increases incidence of eye defects after gastrulation-stage alcohol
Affected fetuses also exhibit craniofacial and brain abnormalities
Mns1 function is involved in embryonic development and alcohol teratogenesis
Acknowledgements.
The authors have no conflicts of interest to declare. Thank you to Dr. Eric Fish, Haley Mendoza-Romero, Debbie Dehart, and Divya Venkatasubmaranian for their technical assistance on this project.
Funding: Funding to support this research was provided by the National Institutes of Health/National Institute of Alcohol and Alcoholism (NIH/NIAAA) grants: U01AA021651, P60AA011605 and R00AA018697 to SEP, F32AA026479 to KEB, and conducted as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD).
REFERENCES
- Babu D, Roy S (2013) Left–right asymmetry: cilia stir up new surprises in the node. Open biology 3:130052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocardo PS, Gil-Mohapel J, Christie BR (2011) The role of oxidative stress in fetal alcohol spectrum disorders. Brain research reviews 67:209–225. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Allen NC, James AW, Mekonnen Z, Madan E, Helms JA (2010) A primary cilia-dependent etiology for midline facial disorders. Human molecular genetics 19:1577–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CS, Nowotny AZ, Sulik KK (1987) Fetal alcohol syndrome: eye malformations in a mouse model. Archives of Ophthalmology 105:1576–1581. [DOI] [PubMed] [Google Scholar]
- Cortés CR, Metzis V, Wicking C (2015) Unmasking the ciliopathies: craniofacial defects and the primary cilium. Wiley Interdisciplinary Reviews: Developmental Biology 4:637–653. [DOI] [PubMed] [Google Scholar]
- DeRoo LA, Wilcox AJ, Drevon CA, Lie RT (2008) First-trimester maternal alcohol consumption and the risk of infant oral clefts in Norway: a population-based case-control study. American journal of epidemiology 168:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunty WC, Chen Sy, Zucker RM, Dehart DB, Sulik KK(2001) Selective Vulnerability of Embryonic Cell Populations to Ethanol‐Induced Apoptosis: Implications for Alcohol‐Related Birth Defects and Neurodevelopmental Disorder. Alcoholism: Clinical and Experimental Research 25:1523–1535. [PubMed] [Google Scholar]
- Eberhart JK, Parnell SE (2016) The genetics of fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research 40:1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ (2007) The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Research & Health 30:5. [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H (2007) When cilia go bad: cilia defects and ciliopathies. Nature reviews Molecular cell biology 8:880. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Inagaki H, Naruge T, Tabata S, Tomida T, Yamaguchi A, Yoshikuni M, Nagahama Y, Hotta Y (1994) cDNA cloning and functional characterization of a meiosis-specific protein (MNS1) with apparent nuclear association. Chromosome Research 2:99–113. [DOI] [PubMed] [Google Scholar]
- Gilbert MT, Sulik KK, Fish EW, Baker LK, Dehart DB, Parnell SE (2016) Dose-dependent teratogenicity of the synthetic cannabinoid CP-55,940 in mice. Neurotoxicology and teratology 58:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CS, Zucker RM, Hunter ES, Sulik KK (2007) Perturbation of retinoic acid (RA)‐mediated limb development suggests a role for diminished RA signaling in the teratogenesis of ethanol. Birth Defects Research Part A: Clinical and Molecular Teratology 79:631–641. [DOI] [PubMed] [Google Scholar]
- Laufer BI, Mantha K, Kleiber ML, Diehl EJ, Addison SM, Singh SM (2013) Long-lasting alterations to DNA methylation and ncRNAs could underlie the effects of fetal alcohol exposure in mice. Disease models & mechanisms 6:977–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti MS, Kotaja N, Sironen A (2013) KIF3A is essential for sperm tail formation and manchette function. Molecular and cellular endocrinology 377:44–55. [DOI] [PubMed] [Google Scholar]
- Liu B, Chen S, Johnson C, Helms J (2014) A ciliopathy with hydrocephalus, isolated craniosynostosis, hypertelorism, and clefting caused by deletion of Kif3a. Reproductive Toxicology 48:88–97. [DOI] [PubMed] [Google Scholar]
- Maier SE, Chen WJA, Miller JA, West JR (1997) Fetal Alcohol Exposure and Temporal Vulnerability: Regional Differences in Alcohol‐Induced Microencephaly as a Function of the Timing of Binge‐Like Alcohol Exposure During Rat Brain Development. Alcoholism: Clinical and Experimental Research 21:1418–1425. [DOI] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G (2018) Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA 319:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hamrick KJ, Corbin KD, Hasken JM, Marais A-S, Blankenship J, Hoyme HE, Gossage JP (2016) Maternal nutritional status as a contributing factor for the risk of fetal alcohol spectrum disorders. Reproductive Toxicology 59:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K, Takeda S (2015) Cilia in the choroid plexus: their roles in hydrocephalus and beyond. Frontiers in cellular neuroscience 9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N (1998) Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95:829–837. [DOI] [PubMed] [Google Scholar]
- O’Leary‐Moore SK, Parnell SE, Godin EA, Dehart DB, Ament JJ, Khan AA, Johnson GA, Styner MA, Sulik KK (2010) Magnetic resonance microscopy‐based analyses of the brains of normal and ethanol‐exposed fetal mice. Birth Defects Research Part A: Clinical and Molecular Teratology 88:953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AK, Belmonte JCI (2000) Establishing a Left‐Right Axis in the Embryo. IUBMB life 50:1–11. [DOI] [PubMed] [Google Scholar]
- Shankar K, Ronis MJ, Badger TM (2007) Effects of pregnancy and nutritional status on alcohol metabolism. Alcohol Research & Health 30:55. [PMC free article] [PubMed] [Google Scholar]
- Sulik K, Dehart D, Inagaki T, Carson J, Vrablic T, Gesteland K, Schoenwolf G (1994) Morphogenesis of the murine node and notochordal plate. Developmental Dynamics 201:260–278. [DOI] [PubMed] [Google Scholar]
- Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N (1999) Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A−/− mice analysis. The Journal of cell biology 145:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukui T, Capdevila J, Tamura K, Ruiz-Lozano P, Rodriguez-Esteban C, Yonei-Tamura S, Magallón J, Chandraratna RA, Chien K, Blumberg B (1999) Multiple left-right asymmetry defects in Shh−/− mutant mice unveil a convergence of the Shh and retinoic acid pathways in the control of Lefty-1. Proceedings of the National Academy of Sciences 96:11376–11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadnais ML, Lin AM, Gerton GL (2014) Mitochondrial fusion protein MFN2 interacts with the mitostatin-related protein MNS1 required for mouse sperm flagellar structure and function. Cilia 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey KJ, Parnell SE, Miranda RC, Golding MC (2015) Dose-dependent alcohol-induced alterations in chromatin structure persist beyond the window of exposure and correlate with fetal alcohol syndrome birth defects. Epigenetics & chromatin 8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Geyer SH, Reissig L, Rose J, Szumska D, Hardman E, Prin F, McGuire C, Ramirez-Solis R, White J, Galli A, Tudor C, Tuck E, Mazzeo CI, Smith JC, Robertson E, Adams DJ, Mohun T, Weninger WJ (2016) Highly variable penetrance of abnormal phenotypes in embryonic lethal knockout mice. Wellcome open research 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Zhang Y, Liu Q, Xiang W (2015) Mfn2 affects embryo development via mitochondrial dysfunction and apoptosis. PloS one 10:e0125680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Yang F, Leu NA, Wang PJ (2012) MNS1 is essential for spermiogenesis and motile ciliary functions in mice. PLoS genetics 8:e1002516. [DOI] [PMC free article] [PubMed] [Google Scholar]