Abstract
In the treatment of both Type 1 and Type 2 diabetes mellitus, maintaining a euglycemic state represents one of the key challenges. Improper dosing and administration of glucose-lowering drugs is associated with an increased risk of recurrent hypoglycemia episodes. In addition, the risk of adverse cardiovascular events in diabetic patients, particularly myocardial infarctions and strokes, is well-established. Current research indicates a potential link between the baseline risk of cardio/cerebrovascular events in diabetic patients and exposure to hypoglycemia. In this review of the literature we aim to determine if a relationship exists between recurrent hypoglycemia and adverse neurovascular events.
Keywords: diabetes, hypoglycemia, cerebrovascular event, stroke
Introduction
Chronic hyperglycemia in diabetic patients can lead to a variety of long-term complications1–5. Of particular importance is the increased risk of cardiovascular diseases, which is reported as the leading cause of death in diabetic patients6, 7. Tight and aggressive glucose management treatment with pharmacological agents has been shown to reduce the risk for some macrovascular and microvascular pathologies, but such treatments also put patients at increased risk for subclinical and severe hypoglycemia8. During hypoglycemia, the autonomic nervous system acts to increase release of catecholamines in an attempt to restore normal concentrations by increasing hepatic production of glucose9. These increased systemic catecholamine levels can lead to unintended consequences, such as increased platelet aggregation, fatal arrhythmias and chronic cardiac inflammation10. Repeated episodes of hypoglycemia can weaken the body’s neuroglycopenic response to low blood glucose11. Preclinical studies indicate that hypoglycemia may activate procoagulant pathways. However, not all clinical studies support the notion that exposure to hypoglycemia increases the risk of cardiovascular complications. In this review, we aim to review the existing literature to determine if hypoglycemia has any effect on cerebrovascular events in diabetic patients.
Incidence of hypoglycemia in diabetes management
The American Diabetes Associated Workgroup on Hypoglycemia defines hypoglycemia as “all episodes of abnormally low plasma glucose concentration that expose the individual to potential harm”12. Several categories of hypoglycemia exist, including severe hypoglycemia (requiring another individual to assist with treatment), symptomatic and asymptomatic hypoglycemia (both of which have blood glucose ≤70 mg/dL but with variable symptoms), probable hypoglycemia (symptoms reported, but not confirmed by glucose measurement) and relative hypoglycemia (symptoms present, but glucose is still >70 mg/dL)12.
Although hypoglycemia presents with similar symptoms in type 1 diabetes (T1D) and type 2 diabetes (T2D), the differing pathologies of the diseases cause subtle differences in the course of these hypoglycemic episodes. Repeated episodes of hypoglycemia in a short amount of time increase the risk of development of hypoglycemia-associated autonomic failure (HAAF), in which the body’s neurogenic responses to low blood sugar are blunted, leading to defective glucose counter-regulation and hypoglycemia unawareness13. These repeated hypoglycemic episodes shift the systemic thresholds needed to induce these autonomic responses to lower blood glucose levels11. Owing to the onset of hypoglycemia unawareness in HAAF as well as the inability to properly measure and control blood glucose during sleep, it has been hypothesized that both T1D and T2D patients experience mild and moderate hypoglycemic episodes much more frequently than previously thought. Macleod et al.14 performed a retrospective clinical study on a randomly selected sample of diabetic patients (both T1D and T2D) and showed evidence of severe hypoglycemia at an event rate of 170 per 100 patient-years and 73 per 100 patient-years in insulin-treated T1D and T2D patients, respectively14. Several other studies also made similar observations15, 16. Studies involving continuous blood glucose monitoring have shown that as many as 68% of T1D (3 days of monitoring)17 and 49% of T2D (5 days of monitoring)18 recorded systemic glucose levels below 60 mg/dl.
Impaired autonomic responses in old age may increase older diabetic patients’ susceptibility to experiencing recurrent hypoglycemia19. An earlier study to evaluate incidences of hypoglycemia in sulfonylurea (SU)- or insulin-treated older diabetic patients observed that elderly persons using multiple medications and those who are frequently hospitalized, are at greater risk for hypoglycemia19,20. Although difficult to estimate, repeated exposure to hypoglycemia in older individuals may lead to deterioration of general heath, disability and poor outcomes19,21.
Despite recent advances in new regimens for blood glucose control, the fear of hypoglycemia still looms large22. There have been a small number of clinical trials in recent years showing that the incidence of hypoglycemia with the use of insulin analogs and oral hypoglycemic agents is an undeniable reality23, 24. The landmark clinical trials, such as Action to Control Cardiovascular Risk in Diabetes (ACCORD)25 and Diabetes Control and Complications Trial (DCCT)26, mostly concentrated on the hypoglycemia incidence in developed nations. However, it is as much of a reality in developing nations with huge population of diabetic patients, including India and China. The treatment of diabetes and the availability of appropriate clinical expertise to manage the condition varies slightly from country to country. The under-reporting of self-treated hypoglycemia events only adds to the seriousness of the issue as the risks and incidence of hypoglycemia can be underestimated. The Hypoglycemia Assessment Tool (HAT) study conducted recently in developing countries on 27,585 insulin-treated diabetic patients showed an incidence rate of hypoglycemia higher than previously reported27. There have been a few European studies that have also demonstrated the presence of self-treated hypoglycemia28, 29. As pointed out in a recent review on hypoglycemia in diabetic patients, the protocols of close glucose monitoring followed rigorously in clinical trials may not be an accurate representation of the real incidence of hypoglycemia in actual clinical practice30.
The effect of hypoglycemia on procoagulant mechanisms
In this section we discuss potential mechanisms by which exposure to hypoglycemia (single or repeated) accelerate procoagulant mechanisms. Mechanisms described below are summarized in Figure 1.
Figure 1.
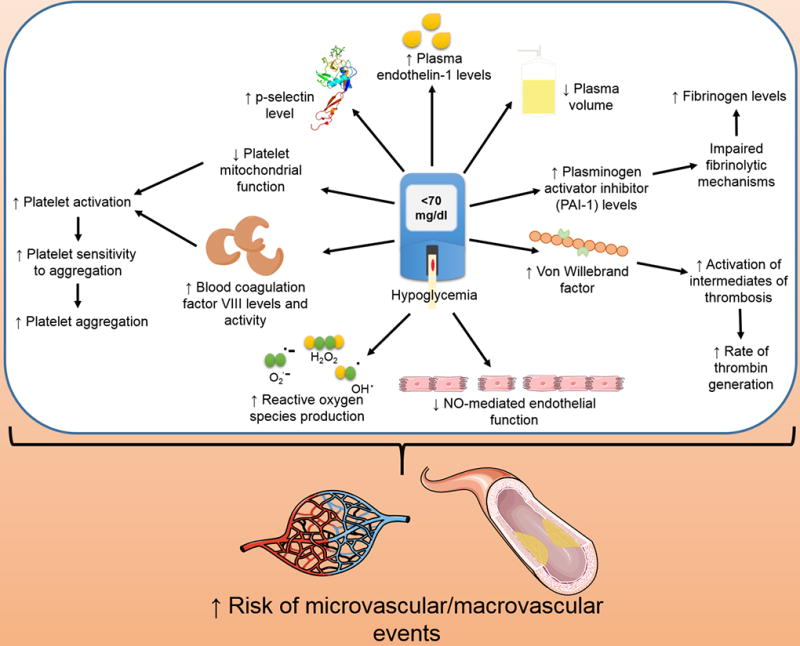
Schematic diagram summarizing procoagulant mechanisms activated by hypoglycemia.
The relation of hypoglycemia with thrombotic and hemostatic mechanisms
Disturbances in platelet activity can lead to bleeding disorders and thrombosis. Hypoglycemia appears to be associated with platelet activation31, 32. Hypoglycemia accelerates vascular complications in diabetes by increasing platelet aggregation as well as fibrinogen formation. Studies have shown that acute hypoglycemia impairs fibrinolytic balance, increases pro-inflammatory responses, platelet activation and coagulation biomarkers as well as reduces NO-mediated endothelial function33. Further studies have also shown that hypoglycemia induces an increase in circulating levels of vascular adhesion molecules (markers of endothelial cell damage), interleukin (IL)-6, and P-selectin (markers of platelet activation)34,35. Injury to endothelial cells and activation of platelets can result in a procoagulant state. Previous studies have confirmed that hypoglycemia has an inhibitory effect on fibrinolytic mechanisms35. Hypoglycemia also has a detrimental effect on calcium homeostasis and platelet mitochondrial integrity34. Altered mitochondrial calcium homeostasis and mitochondrial dysfunction can result in platelet activation36. Hypoglycemia decreases the activated partial thromboplastin time (aPTT) and increases levels of fibrinogen and factor VIII37. With recurrent transient hypoglycemic episodes, repeated occurrences may have additive effects that accentuate inflammation-based processes that include atherogenesis and other thrombotic complications. Other pro-inflammatory changes include an increase in the plasma concentration of IL-6, IL-8,IL-1β and other pro-inflammatory mediators that includes leukocytosis, reactive oxygen species (ROS) generation, lipid peroxidation, and increased levels of tumor necrosis factor-α (TNF-α)38. It is well established that proinflammatory mediators can increase systemic thrombogenicity by affecting multiple pathways39, 40.
Atherosclerosis is one of the determining factors associated with ischemic stroke. Platelets have been reported as one of the key factors in developing atherothrombosis41, 42. Platelets play a significant role in the pathology of cerebral ischemia through their participation in the generation of thromboemboli that may initiate stroke symptoms. More often, activated platelets particularly are a major contributor to the large-vessel subtype of ischemic stroke that occurs following atherosclerotic plaque rupture43. These detrimental effects would add to the previously demonstrated relationship between hypoglycemia on ischemic stroke. Collectively, therefore, hypoglycemia can trigger a sequence of events that may induce stroke.
Hypoglycemia stimulates the autonomic nervous system and brings about sympathoadrenal activation to facilitate the release of catecholamines44. This promotes secondary effects on the circulatory system, including a decrease in plasma volume45; an increase in leukocyte and erythrocyte counts46, 47, and platelet aggregation48. In addition to these changes in the formed elements of blood, hypoglycemia has substantial hemorheological effects. Hypoglycemia is shown to increase blood coagulation factor VIII activity via a β2 adrenoceptor-mediated process49. This was further confirmed and supported by other relevant studies50,51. Hypoglycemia leads to a rise in Von Willebrand factor52 and accelerates the rate of thrombin generation50.
Endothelin-1 (ET-1) is one of the major endothelins that acts as a potent vasoconstrictor. In an earlier clinical trial, Wright et al. investigated the effect of acute symptomatic hypoglycemia in adult T1D patients on plasma ET-1 response53. The goal of the study was to determine the mechanism by which acute insulin-induced hypoglycemia leads to vasoconstriction and triggers microvascular and macrovascular events. Plasma ET-1 levels were observed to increase substantially during hypoglycemia. Though these results adequately help to implicate the role of ET-1 in altered vascular hemodynamics during hypoglycemia, whether the observed effect is directly due to hypoglycemia, or secondary due to hypoglycemia-induced hormonal response, or insulin administration remains to be determined. In another study, Trovati et al. observed that insulin-induced hypoglycemia results in an increased platelet sensitivity to aggregating agents like ADP and thrombin in vitro potentially due to hypoglycemia-related alpha-adrenoceptor activation54.
Another clinical study determined the difference that euglycemia and hypoglycemia had on fibrinolytic balance and pro-inflammatory mechanisms35. This study included thirty five healthy and twenty four T1D volunteers who previously never experienced any episode of hypoglycemia and had intact autonomic function. Pro-inflammatory markers, such as vascular cel adhesion molecular (VCAM), intercellular adhesion molecular (ICAM), E-Selectin and vascular endothelial growth factor (VEGF), were increased during the hyperinsulinemic hypoglycemia episodes. These results suggest the possibility of hypoglycemia leading to atheroma formation, as these proteins are normally present on cell surfaces and are expressed during inflammation to promote leukocyte adhesion which can lead to plaque and atheroma formation. Another study observed increased CD40 expression and platelet–monocyte aggregation during acute hypoglycemia in both non-diabetic and T1D subjects55. A recent study on 45 healthy subjects compared inflammatory and pro-atherosclerotic biomarkers released during euinsulinemic hyperglycemia observed an increase in plasminogen activator inhibitor-1 (PAI-1) and TNF-α levels during hypoglycemia56. PAI-1 is a protein that inhibits activators of plasminogen and fibrinolysis like tissue plasminogen activator (tPA) while TNF-α has been shown to facilitate activation of the coagulation cascade57. These studies demonstrate activation of hemostatic mechanisms by exposure to hypoglycemia.
Hemostatic activation is a major contributor to the progression of ischemic stroke. Elevated levels of several inflammatory and rheological variables are reported to be associated with ischemic stroke. Past studies have provided strong evidence of increased thrombin generation and fibrin turnover with associated altered fibrinolytic activity and derailed endothelial function in acute stroke58–63. Further studies have revealed an elevated thrombin–antithrombin complex (TAT) and fibrin D-dimer levels in patients with progressing stroke63. This literature supports the notion that exposure to hypoglycemia may increase the risk of ischemic stroke via activation of hemostatic mechanisms.
Hypoglycemia and free radicals
Hypoglycemia plays a major role in enhancing diabetes-related vascular complications10. There is evidence that indicates a deleterious impact of hypoglycemia on the blood-brain barrier (BBB)64–66. Compromised BBB function during hypoglycemia has been reported to trigger the pathogenesis of secondary brain injuries. Importantly, lower glucose levels inhibit the protective physiological effects of flow-induced shear stress on vasculature and endothelial responses to flow by abundant accumulation of ROS67. Reduced glutathione functions as a major anti-oxidant in the brain. In hypoglycemia, impaired synthesis of glutathione subjects the brain to considerable oxidative stress53. Glucose reintroduction after hypoglycemic coma has been reported to generate superoxide and nitrotyrosine immunoreactivity and is suggested to be the main cause of stimulation of oxidative stress through the activity of NADPH oxidase68,69. Selective increase in lipo-peroxidation due to hypoglycemia leads to oxidative stress70 that has been confirmed in animal studies71. Animal studies have also confirmed increased mitochondrial ROS production during both hypoglycemia72 and in cultured neurons during glucose deprivation73. ROS are also known to activate several intermediates of thrombosis, including platelets74. These studies suggest that the hypoglycemic condition promotes the production of ROS and reactive nitrogen species (ROS/RNS) that might create a pro-thrombotic state.
Incidence and morbidity of stroke in diabetes
The importance of diabetes as a risk factor for the occurrence of stroke is well-known. There have been several studies proving an association between hyperglycemia and an increased risk of stroke75,76. The Framingham study was the first to report an increased occurrence of ischemic stroke in both men and women with diabetes77. The Copenhagen Stroke Study and the GCNKSS study provided epidemiological link between diabetes and stroke78,79. There are other studies that also support this link80,81. The U.S. Nationwide Inpatient Sample has shown an increase of about 27% in the absolute number of hospitalizations for acute ischemic stroke in patients with co-morbid diabetes (T2D) when compared to an overall 17% decrease in acute ischemic stroke hospitalizations without diabetes from 1997 to 200682. The rate of stroke in diabetic patients has declined by about 53% between 1990 and 201083.
Incidence of hypoglycemia and the risk of cerebrovascular events
Preclinical studies provide strong evidence that hypoglycemia may accelerate procoagulant mechanisms. In view of this, below we provide a summary of studies that support and oppose this premise.
Effect of prior hypoglycemia and cerebrovascular events
A study conducted by Rathmann et al. comparing a group of T2D patients receiving a dipeptidyl peptidase-4 inhibitor (DPP4-I) and another group receiving a SU only for two years observed a 5-fold decrease in frequency of recorded hypoglycemia in DPP4-I users compared to those on SU84. The hazard ratio for stroke/transient ischemic attack (TIA) was 0.57 (p < 0.001) in DPP4-I users compared to SU users. Similarly, hazard ratios for macrovascular disease, coronary heart disease, myocardial infarction, and peripheral vascular disease in DPP4-I treated patients were 0.74 (p < 0.001), 0.74 (p < 0.001), 0.81 (p < 0.05), and 0.73 (p < 0.001) compared to SU-treated patients, respectively. This study suggests that increased incidences of hypoglycemia may be linked to increased risk of stroke/TIA and other macrovascular diseases in SU-treated diabetic patients. Another prospective study was conducted by Gitt et al. to confirm earlier studies that treatment with DPP4-I resulted in decreased incidences of hypoglycemia. Patients received dual treatment with either metformin and sulfonylureas (SUs) or metformin and DPP4 inhibitors85. These two groups were followed for 12 months to study the frequency of hypoglycemia in these patients. It was observed that the patients on metformin + SU had a greater frequency of episodes of hypoglycemia than the patients on metformin + DPP4-I. The group on metformin + SU showed a substantial increase in stroke/TIA incidences when compared to patients on DPP4-I + metformin (2% vs 0.2%; p < 0.05). It is plausible that increased incidences of stroke/TIA in the SU + metformin group may be due to higher incidences of hypoglycemia observed in this group. It should be noted that these two studies were observational ones that did not adequately explore if hypoglycemia was an independent predictor of stroke/TIA. Although basic experimental data suggest the association of hypoglycemia and stroke, future clinical studies aimed to determine this association are warranted. The Veterans Affairs Diabetes Trial (VADT) reported significantly more episodes of hypoglycemia in the intensive-therapy group than in the standard-therapy group6. Patients with BMI of ≥27 and <27 were treated with metformin plus rosiglitazone or glimepiride plus rosiglitazone, respectively. However, this study did not find any significant effect of intensive therapy on the rates of major cardiovascular events when compared to standard therapy.
A prospective study of participants with diagnosed diabetes from the Atherosclerosis Risk in Communities (ARIC) aimed to determine the link between severe hypoglycemia and cardiovascular disease observed that prior sever hypoglycemia exposure increases the risk of coronary heart disease, all-cause mortality and cardiovascular mortality86. However, no association between prior exposure to severe hypoglycemia and stroke was observed86. Recently published secondary analysis of the double-blind Trial Comparing Cardiovascular Safety of Insulin Degludec vs Insulin Glargine in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events (DEVOTE) trial also evaluated the association between exposure to severe hypoglycemia and a major adverse cardiovascular event (MACE), defined as either cardiovascular death, non-fatal myocardial infarction or non-fatal stroke87. This study observed an association between exposure to severe hypoglycemia and all-cause mortality. However a non-significant difference was observed in the risk of MACE in individuals who experienced severe hypoglycemia (hazard ratio 1.38, p = .080) vs. those who did not. Similarly a non-significant increase in the risk for non-fatal stroke was observed in individuals those who had (hazard ratio 1.81, p = .085) vs. those who had not experienced severe hypoglycemia. However, a recently published study using data from the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) study observed that the adjusted hazard ratio for MACE risk in patients who experienced serious hypoglycemia events (hazard ratio 2.42, p = .007) was significantly higher than those without serious hypoglycemia exposure88. However, this study did not report effects on non-fatal stroke separately. Another recent study evaluated the association between hypoglycemia and cardiovascular disease in SUs-treated T2D observed that an increasing frequency of hypoglycemia exposure was associated with increased stroke risk89. The strongest association between exposure to hypoglycemia and stroke risk was observed when patients were exposed to 3 or more hypoglycemic events (relative risk 1.57)89. It should be noted that the effect of mild or moderate hypoglycemia was not evaluated in the ARIC, DEVOTE, or EXAMINE populations. It is plausible that the risk of cardio- and cerebrovascular events may be already higher in studied patients owing to their high risk for exposure to mild or moderate hypoglycemia, and if so, this may also mask (i.e. lower the hazard ratio) the effect of severe hypoglycemia on studied cardio- and cerebrovascular events.
The effects of intensive glucose control, involving the use of gliclazide (modified release) as compared to standard glucose control in T2D patients was undertaken with 11,140 patients from over 200 medical centers spanning over 20 countries by the ADVANCE Collaborative group. After a follow-up period of 5 years, there was a significant decrease in the overall HbA1C values in the intensive group (6.5%) than in the standard control group (7.3%). Hypoglycemia was more common in the intensive control group (2.7%) than the standard control group (1.5%) [Hazard ratio 1.86; 95% CI 1.4 to 2.4 and p < .001]. However, the study showed no statistically significant difference in the outcome of nonfatal stroke in the two groups90. In the ACCORD Trial, participants belonging to the intensive glucose control group experienced more episodes of hypoglycemia (self-monitored blood glucose < 70 mg/dl) than the standard control group (1.06 and 0.29 episodes per week, respectively)91, 92. In both original and nine year follow-up studies, the odds ratio for nonfatal stroke was lower in the intensive glucose control group91–93. However, this difference was not statistically significant. These effects may be explained as gliclazide has anti-oxidant properties and thus may also lower the impact of procoagulant pathways activated by hypoglycemia exposure94. Similarly, studies also reported that other SUs also improve platelet function95,96. For example, glibenclamide and glimepiride was able to suppress thrombin-stimulated increase in intracellular calcium and thrombin-induced arachidonic acid metabolism in platelets95. However, gliclazide showed no effect on above-mentioned parameters95. Another study reported that both gliclazide and glyburide treatment in noninsulin-dependent diabetic subjects normalized ADP-induced platelet aggregation when compared to a diet alone group of noninsulin-dependent diabetic subjects96. It should be noted that this study did not observe any correlation between plasma glucose levels and platelet function suggesting that these effects may be from the drug and not plasma glucose level96. It is possible that beneficial effects of glucose-lowering medications used to treat T2D patients may mask some of the deleterious effects of hypoglycemia on cerebrovascular outcomes.
Effect of acute post-stroke hypoglycemia and cerebrovascular events
The UK Glucose Insulin in Stroke Trial (GIST-UK) that determined if intensive insulin therapy (variable-dose-insulin by glucose-potassium insulin: GKI infusion) immediately after acute ischemic stroke reduces death at 90 days observed that episodes of hypoglycemia (<72 mg/dl over a period >30 min) occurred in 73 patients belonging to the GKI infusion group97, 98. Plasma glucose of lower than 72 mg/dl was also observed in an additional 187 patients belonging to the GKI group; however, these patients did not meet the temporal requirement. This study also noted that 7 patients receiving GKI had a recurrent stroke within 72 h after the initiation of treatment compared to only 3 patients in the control arm. However, this difference was not statistically significant.
The Treatment of Hyperglycemia in Ischemic Stroke (THIS) trial: a randomized, multicenter, blinded feasibility, and tolerability trial evaluating the effect of aggressive hyperglycemia correction using intravenous insulin vs standard care during acute cerebral infarction, noted that 35% of patients receiving aggressive treatment had at least one episode of hypoglycemia (<60 mg/dL). Only 3% of these patients had neurologic symptoms, and majority of them (64%) were asymptomatic99. This trial did not observe any correlation between the hypoglycemic episodes and worsened clinical outcomes. The Glucose Regulation in Acute Stroke Patients (GRASP) trial: a prospective, randomized, multicenter, trial evaluated the feasibility and safety of two insulin infusion protocols (tight glucose control (70–110 mg/dL) and loose glucose control (200 mg/dL)), and compared with usual care (glucose levels 70–300 mg/dL)100. This trial reported hypoglycemic (<55 mg/dL) episodes in 30% of patients in the tight glucose control group. This trial also did not report any significant differences in serious adverse events among study groups.
In summary, some clinical evidence suggests that exposure to hypoglycemia increases the risk of cerebrovascular events in diabetic patients. It is also possible that hypoglycemia may indirectly affect stroke risk, as increased incidences of hypoglycemia may also reflect underlying poor health per se that predisposes to adverse vascular outcomes. A systematic review aimed to summarize literature on the effect of hypoglycemia on cardiovascular risk in diabetic patients found that several studies identified hypoglycemia, via increasing thrombotic tendency and other mechanisms, as contributing to cardiovascular risk in diabetic patients10. Another similar review by Moheet and Seaquist concluded that although acute hypoglycemia may lead to a prothrombotic state, current knowledge based on large clinical trials does not establish a link between hypoglycemia and adverse cardiovascular outcomes101. A recent review indicated a potential link between hemorrhagic transformation observed in stroke patients and exposure to hypoglycemia post-stroke102. Similarly, future pre-clinical and clinical studies aimed to determine association between hypoglycemia and the risk of stroke are warranted.
Conclusions
Diabetic patients compared to non-diabetic subjects have both a higher risk of stroke and poor stroke outcomes. Frequent exposure to hypoglycemia is observed in both T1D and T2D patients. Pre-clinical studies suggest that hypoglycemia may increase the risk of stroke in diabetic patients. This premise is not well-supported by clinical studies. The effects of hypoglycemia in diabetic patients on normal and pathological hemostatic pathways warrant further investigation. It would be also important to identify any sub-group of diabetic patients that are more susceptible to a hypoglycemia-induced pro-hypercoagulable state.
Acknowledgments
The present study is supported by NIH Grant NS073779. We would like to thank Dr. Brant Watson for critical reading of this manuscript.
Footnotes
Author contributions
LS, DC, PB, DS, SK, and KD: contributed to the conception, design of the article, contributed in writing parts of the manuscript and critically revising the manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Atreja A, Kalra S. Infections in diabetes. J Pak Med Assoc. 2015;65:1028–1030. [PubMed] [Google Scholar]
- 2.Singla R, Gupta Y, Kalra S. Musculoskeletal effects of diabetes mellitus. J Pak Med Assoc. 2015;65:1024–1027. [PubMed] [Google Scholar]
- 3.Starup-Linde J, Vestergaard P. Management of endocrine disease: Diabetes and osteoporosis: cause for concern? Eur J Endocrinol. 2015;173:R93–99. doi: 10.1530/EJE-15-0155. [DOI] [PubMed] [Google Scholar]
- 4.Jin HY, Baek HS, Park TS. Morphologic Changes in Autonomic Nerves in Diabetic Autonomic Neuropathy. Diabetes Metab J. 2015;39:461–467. doi: 10.4093/dmj.2015.39.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalil H. Diabetes microvascular complications-A clinical update. Diabetes Metab Syndr. 2016;11(Suppl 1):S133–S139. doi: 10.1016/j.dsx.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 7.Writing Group, M. Mozaffarian D, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 8.Buehler AM, Cavalcanti AB, Berwanger O, et al. Effect of tight blood glucose control versus conventional control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Cardiovasc Ther. 2013;31:147–160. doi: 10.1111/j.1755-5922.2011.00308.x. [DOI] [PubMed] [Google Scholar]
- 9.Exton JH. Mechanisms of hormonal regulation of hepatic glucose metabolism. Diabetes Metab Rev. 1987;3:163–183. doi: 10.1002/dmr.5610030108. [DOI] [PubMed] [Google Scholar]
- 10.Hanefeld M, Duetting E, Bramlage P. Cardiac implications of hypoglycaemia in patients with diabetes - a systematic review. Cardiovasc Diabetol. 2013;12:135. doi: 10.1186/1475-2840-12-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51:724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 12.Workgroup on Hypoglycemia & American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 13.Cryer PE. Hypoglycemia-Associated Autonomic Failure in Diabetes: Maladaptive, Adaptive, or Both? Diabetes. 2015;64:2322–2323. doi: 10.2337/db15-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLeod KM, Hepburn DA, Frier BM. Frequency and morbidity of severe hypoglycaemia in insulin-treated diabetic patients. Diabet Med. 1993;10:238–245. doi: 10.1111/j.1464-5491.1993.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly LA, Morris AD, Frier BM, et al. Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabet Med. 2005;22:749–755. doi: 10.1111/j.1464-5491.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- 16.U. K. Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 17.Boland E, Monsod T, Delucia M, et al. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24:1858–1862. doi: 10.2337/diacare.24.11.1858. [DOI] [PubMed] [Google Scholar]
- 18.Gehlaut RR, Dogbey GY, Schwartz FL, et al. Hypoglycemia in Type 2 Diabetes--More Common Than You Think: A Continuous Glucose Monitoring Study. J Diabetes Sci Technol. 2015;9:999–1005. doi: 10.1177/1932296815581052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdelhafiz AH, Rodriguez-Manas L, Morley JE, et al. Hypoglycemia in older people - a less well recognized risk factor for frailty. Aging Dis. 2015;6:156–167. doi: 10.14336/AD.2014.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shorr RI, Ray WA, Daugherty JR, et al. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997;157:1681–1686. [PubMed] [Google Scholar]
- 21.Abdelhafiz AH, Koay L, Sinclair AJ. The effect of frailty should be considered in the management plan of older people with Type 2 diabetes. Future Sci OA. 2016;2:FSO102. doi: 10.4155/fsoa-2015-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.IDF. IDF Diabetes Atlas 8th Edition. International Diabetes Federation; Brussels: 2017. www.diabetesatlas.org. [Google Scholar]
- 23.Agesen RM, Kristensen PL, Beck-Nielsen H, et al. Effect of insulin analogues on frequency of non-severe hypoglycaemia in patients with type 1 diabetes prone to severe hypoglycaemia: The HypoAna trial. Diabetes Metab. 2016;42:249–255. doi: 10.1016/j.diabet.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Kristensen PL, Hansen LS, Jespersen MJ, et al. Insulin analogues and severe hypoglycaemia in type 1 diabetes. Diabetes Res Clin Pract. 2012;96:17–23. doi: 10.1016/j.diabres.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 25.Bonds DE, Kurashige EM, Bergenstal R, et al. Severe hypoglycemia monitoring and risk management procedures in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:80i–89i. doi: 10.1016/j.amjcard.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes. 1997;46:271–286. [PubMed] [Google Scholar]
- 27.Khunti K, Alsifri S, Aronson R, et al. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18:907–915. doi: 10.1111/dom.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostenson CG, Geelhoed-Duijvestijn P, Lahtela J, et al. Self-reported non-severe hypoglycaemic events in Europe. Diabet Med. 2014;31:92–101. doi: 10.1111/dme.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weitgasser R, Lopes S. Self-reported frequency and impact of hypoglycaemic events in insulin-treated diabetic patients in Austria. Wien Klin Wochenschr. 2015;127:36–44. doi: 10.1007/s00508-014-0626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heller S, Amiel SA, Khunti K, et al. Hypoglycaemia, a global cause for concern. Diabetes Res Clin Pract. 2015;110:229–232. doi: 10.1016/j.diabres.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Mikhailidis DP, Barradas MA, Maris A, et al. Fibrinogen mediated activation of platelet aggregation and thromboxane A2 release: pathological implications in vascular disease. J Clin Pathol. 1985;38:1166–1171. doi: 10.1136/jcp.38.10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda H, Kishikawa H, Shinohara M, et al. Effect of alpha 2-adrenoceptor antagonist on platelet activation during insulin-induced hypoglycaemia in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1988;31:657–663. doi: 10.1007/BF00278748. [DOI] [PubMed] [Google Scholar]
- 33.Joy NG, Tate DB, Younk LM, et al. Effects of Acute and Antecedent Hypoglycemia on Endothelial Function and Markers of Atherothrombotic Balance in Healthy Humans. Diabetes. 2015;64:2571–2580. doi: 10.2337/db14-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartley PS, Savill JS, Brown SB. Hypoglycaemia predisposes platelets to death by affecting calcium homeostasis and mitochondrial integrity. Platelets. 2007;18:103–112. doi: 10.1080/09537100600760822. [DOI] [PubMed] [Google Scholar]
- 35.Gogitidze Joy N, Hedrington MS, Briscoe VJ, et al. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care. 2010;33:1529–1535. doi: 10.2337/dc09-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zharikov S, Shiva S. Platelet mitochondrial function: from regulation of thrombosis to biomarker of disease. Biochem Soc Trans. 2013;41:118–123. doi: 10.1042/BST20120327. [DOI] [PubMed] [Google Scholar]
- 37.Dalsgaard-Nielsen J, Madsbad S, Hilsted J. Changes in platelet function, blood coagulation and fibrinolysis during insulin-induced hypoglycaemia in juvenile diabetics and normal subjects. Thromb Haemost. 1982;47:254–258. [PubMed] [Google Scholar]
- 38.Razavi Nematollahi L, Kitabchi AE, Stentz FB, et al. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism. 2009;58:443–448. doi: 10.1016/j.metabol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood. 2016;128:753–762. doi: 10.1182/blood-2016-05-718114. [DOI] [PubMed] [Google Scholar]
- 40.Ghasemzadeh M, Hosseini E. Platelet-leukocyte crosstalk: Linking proinflammatory responses to procoagulant state. Thromb Res. 2013;131:191–197. doi: 10.1016/j.thromres.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 41.Freynhofer MK, Bruno V, Wojta J, et al. The role of platelets in athero-thrombotic events. Curr Pharm Des. 2012;18:5197–5214. doi: 10.2174/138161212803251899. [DOI] [PubMed] [Google Scholar]
- 42.Roquer J, Segura T, Serena J, et al. Endothelial dysfunction, vascular disease and stroke: the ARTICO study. Cerebrovasc Dis. 2009;27(Suppl 1):25–37. doi: 10.1159/000200439. [DOI] [PubMed] [Google Scholar]
- 43.Cherian P, Hankey GJ, Eikelboom JW, et al. Endothelial and platelet activation in acute ischemic stroke and its etiological subtypes. Stroke. 2003;34:2132–2137. doi: 10.1161/01.STR.0000086466.32421.F4. [DOI] [PubMed] [Google Scholar]
- 44.Garber AJ, Cryer PE, Santiago JV, et al. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest. 1976;58:7–15. doi: 10.1172/JCI108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hilsted J, Bonde-Petersen F, Madsbad S, et al. Changes in plasma volume, in transcapillary escape rate of albumin and in subcutaneous blood flow during hypoglycaemia in man. Clin Sci (Lond) 1985;69:273–277. doi: 10.1042/cs0690273. [DOI] [PubMed] [Google Scholar]
- 46.Fisher BM, Hepburn DA, Smith JG, et al. The effect of alpha-adrenergic blockade on responses of peripheral blood cells to acute insulin-induced hypoglycaemia in humans. Eur J Clin Invest. 1990;20:51–55. doi: 10.1111/j.1365-2362.1990.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 47.Frier BM, Corrall RJ, Davidson NM, et al. Peripheral blood cell changes in response to acute hypoglycaemia in man. Eur J Clin Invest. 1983;13:33–39. doi: 10.1111/j.1365-2362.1983.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 48.Hutton RA, Mikhailidis D, Dormandy KM, et al. Platelet aggregation studies during transient hypoglycaemia: a potential method for evaluating platelet function. J Clin Pathol. 1979;32:434–438. doi: 10.1136/jcp.32.5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corrall RJ, Webber RJ, Frier BM. Increase in coagulation factor VIII activity in man following acute hypoglycaemia: mediation via an adrenergic mechanism. Br J Haematol. 1980;44:301–305. doi: 10.1111/j.1365-2141.1980.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 50.Ibbotson SH, Catto A, Davies JA, et al. The effect of insulin-induced hypoglycaemia on factor VIII:C concentrations and thrombin activity in subjects with type 1 (insulin-dependent) diabetes. Thromb Haemost. 1995;73:243–246. [PubMed] [Google Scholar]
- 51.Mikhailidis DP, Barradas MA, Hutton RA, et al. The effect of non-specific beta-blockade on metabolic and haemostatic variables during hypoglycaemia. Diabetes Res. 1985;2:127–134. [PubMed] [Google Scholar]
- 52.Fisher BM, Quin JD, Rumley A, et al. Effects of acute insulin-induced hypoglycaemia on haemostasis, fibrinolysis and haemorheology in insulin-dependent diabetic patients and control subjects. Clin Sci (Lond) 1991;80:525–531. doi: 10.1042/cs0800525. [DOI] [PubMed] [Google Scholar]
- 53.Wright RJ, Macleod KM, Perros P, et al. Plasma endothelin response to acute hypoglycaemia in adults with Type 1 diabetes. Diabet Med. 2007;24:1039–1042. doi: 10.1111/j.1464-5491.2007.02199.x. [DOI] [PubMed] [Google Scholar]
- 54.Trovati M, Anfossi G, Cavalot F, et al. Studies on mechanisms involved in hypoglycemia-induced platelet activation. Diabetes. 1986;35:818–825. doi: 10.2337/diab.35.7.818. [DOI] [PubMed] [Google Scholar]
- 55.Wright RJ, Newby DE, Stirling D, et al. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care. 2010;33:1591–1597. doi: 10.2337/dc10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joy NG, Perkins JM, Mikeladze M, et al. Comparative effects of acute hypoglycemia and hyperglycemia on pro-atherothrombotic biomarkers and endothelial function in non-diabetic humans. J Diabetes Complications. 2016;30:1275–1281. doi: 10.1016/j.jdiacomp.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Poll T, Jansen PM, Van Zee KJ, et al. Tumor necrosis factor-alpha induces activation of coagulation and fibrinolysis in baboons through an exclusive effect on the p55 receptor. Blood. 1996;88:922–927. [PubMed] [Google Scholar]
- 58.Ageno W, Finazzi S, Steidl L, et al. Plasma measurement of D-dimer levels for the early diagnosis of ischemic stroke subtypes. Arch Intern Med. 2002;162:2589–2593. doi: 10.1001/archinte.162.22.2589. [DOI] [PubMed] [Google Scholar]
- 59.Altes A, Abellan MT, Mateo J, et al. Hemostatic disturbances in acute ischemic stroke: a study of 86 patients. Acta Haematol. 1995;94:10–15. doi: 10.1159/000203964. [DOI] [PubMed] [Google Scholar]
- 60.Lip GY, Blann AD, Farooqi IS, et al. Abnormal haemorheology, endothelial function and thrombogenesis in relation to hypertension in acute (ictus < 12 h) stroke patients: the West Birmingham Stroke Project. Blood Coagul Fibrinolysis. 2001;12:307–315. doi: 10.1097/00001721-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 61.McConnell JP, Cheryk LA, Durocher A, et al. Urinary 11-dehydro-thromboxane B(2) and coagulation activation markers measured within 24 h of human acute ischemic stroke. Neurosci Lett. 2001;313:88–92. doi: 10.1016/s0304-3940(01)02260-1. [DOI] [PubMed] [Google Scholar]
- 62.Tohgi H, Konno S, Takahashi S, et al. Activated coagulation/fibrinolysis system and platelet function in acute thrombotic stroke patients with increased C-reactive protein levels. Thromb Res. 2000;100:373–379. doi: 10.1016/s0049-3848(00)00356-x. [DOI] [PubMed] [Google Scholar]
- 63.Uchiyama S, Yamazaki M, Hara Y, et al. Alterations of platelet, coagulation, and fibrinolysis markers in patients with acute ischemic stroke. Semin Thromb Hemost. 1997;23:535–541. doi: 10.1055/s-2007-996132. [DOI] [PubMed] [Google Scholar]
- 64.Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 65.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 66.Palmiotti CA, Prasad S, Naik P, et al. In vitro cerebrovascular modeling in the 21st century: current and prospective technologies. Pharm Res. 2014;31:3229–3250. doi: 10.1007/s11095-014-1464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kemeny SF, Figueroa DS, Clyne AM. Hypo- and hyperglycemia impair endothelial cell actin alignment and nitric oxide synthase activation in response to shear stress. PLoS One. 2013;8:e66176. doi: 10.1371/journal.pone.0066176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suh SW, Gum ET, Hamby AM, et al. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suh SW, Hamby AM, Gum ET, et al. Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J Cereb Blood Flow Metab. 2008;28:1697–1706. doi: 10.1038/jcbfm.2008.61. [DOI] [PubMed] [Google Scholar]
- 70.Haces ML, Montiel T, Massieu L. Selective vulnerability of brain regions to oxidative stress in a non-coma model of insulin-induced hypoglycemia. Neuroscience. 2010;165:28–38. doi: 10.1016/j.neuroscience.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Patockova J, Marhol P, Tumova E, et al. Oxidative stress in the brain tissue of laboratory mice with acute post insulin hypoglycemia. Physiol Res. 2003;52:131–135. [PubMed] [Google Scholar]
- 72.McGowan JE, Chen L, Gao D, et al. Increased mitochondrial reactive oxygen species production in newborn brain during hypoglycemia. Neurosci Lett. 2006;399:111–114. doi: 10.1016/j.neulet.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 73.Isaev NK, Stelmashook EV, Dirnagl U, et al. Mitochondrial free radical production induced by glucose deprivation in cerebellar granule neurons. Biochemistry (Mosc) 2008;73:149–155. doi: 10.1134/s0006297908020053. [DOI] [PubMed] [Google Scholar]
- 74.Fuentes E, Palomo I. Role of oxidative stress on platelet hyperreactivity during aging. Life Sci. 2016;148:17–23. doi: 10.1016/j.lfs.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 75.Abbott RD, Donahue RP, MacMahon SW, et al. Diabetes and the risk of stroke. The Honolulu Heart Program. JAMA. 1987;257:949–952. [PubMed] [Google Scholar]
- 76.Barrett-Connor E, Khaw KT. Diabetes mellitus: an independent risk factor for stroke? Am J Epidemiol. 1988;128:116–123. doi: 10.1093/oxfordjournals.aje.a114934. [DOI] [PubMed] [Google Scholar]
- 77.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 78.Kissela BM, Khoury J, Kleindorfer D, et al. Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care. 2005;28:355–359. doi: 10.2337/diacare.28.2.355. [DOI] [PubMed] [Google Scholar]
- 79.Jorgensen H, Nakayama H, Raaschou HO, et al. Stroke in patients with diabetes. The Copenhagen Stroke Study. Stroke. 1994;25:1977–1984. doi: 10.1161/01.str.25.10.1977. [DOI] [PubMed] [Google Scholar]
- 80.Karapanayiotides T, Piechowski-Jozwiak B, van Melle G, et al. Stroke patterns, etiology, and prognosis in patients with diabetes mellitus. Neurology. 2004;62:1558–1562. doi: 10.1212/01.wnl.0000123252.55688.05. [DOI] [PubMed] [Google Scholar]
- 81.Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 82.Towfighi A, Markovic D, Ovbiagele B. Current national patterns of comorbid diabetes among acute ischemic stroke patients. Cerebrovasc Dis. 2012;33:411–418. doi: 10.1159/000334192. [DOI] [PubMed] [Google Scholar]
- 83.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 84.Rathmann W, Kostev K, Gruenberger JB, et al. Treatment persistence, hypoglycaemia and clinical outcomes in type 2 diabetes patients with dipeptidyl peptidase-4 inhibitors and sulphonylureas: a primary care database analysis. Diabetes Obes Metab. 2013;15:55–61. doi: 10.1111/j.1463-1326.2012.01674.x. [DOI] [PubMed] [Google Scholar]
- 85.Gitt AK, Bramlage P, Binz C, et al. Prognostic implications of DPP-4 inhibitor vs. sulfonylurea use on top of metformin in a real world setting - results of the 1 year follow-up of the prospective DiaRegis registry. Int J Clin Pract. 2013;67:1005–1014. doi: 10.1111/ijcp.12179. [DOI] [PubMed] [Google Scholar]
- 86.Lee AK, Warren B, Lee CJ, et al. The Association of Severe Hypoglycemia With Incident Cardiovascular Events and Mortality in Adults With Type 2 Diabetes. Diabetes Care. 2018;41:104–111. doi: 10.2337/dc17-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pieber TR, Marso SP, McGuire DK, et al. DEVOTE 3: temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortality. Diabetologia. 2018;61:58–65. doi: 10.1007/s00125-017-4422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heller SR, Bergenstal RM, White WB, et al. Relationship of glycated haemoglobin and reported hypoglycaemia to cardiovascular outcomes in patients with type 2 diabetes and recent acute coronary syndrome events: The EXAMINE trial. Diabetes Obes Metab. 2017;19:664–671. doi: 10.1111/dom.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nunes AP, Iglay K, Radican L, et al. Hypoglycaemia seriousness and weight gain as determinants of cardiovascular disease outcomes among sulfonylurea users. Diabetes Obes Metab. 2017;19:1425–1435. doi: 10.1111/dom.13000. [DOI] [PubMed] [Google Scholar]
- 90.Advance Collaborative Group. Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 91.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122:844–846. doi: 10.1161/CIRCULATIONAHA.110.960138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Accord Study Group. Nine-Year Effects of 3.7 Years of Intensive Glycemic Control on Cardiovascular Outcomes. Diabetes Care. 2016;39:701–708. doi: 10.2337/dc15-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Papanas N, Maltezos E. Oral antidiabetic agents: anti-atherosclerotic properties beyond glucose lowering? Curr Pharm Des. 2009;15:3179–3192. doi: 10.2174/138161209789057995. [DOI] [PubMed] [Google Scholar]
- 95.Ozaki Y, Yatomi Y, Kume S. Effects of oral hypoglycaemic agents on platelet functions. Biochem Pharmacol. 1992;44:687–691. doi: 10.1016/0006-2952(92)90404-7. [DOI] [PubMed] [Google Scholar]
- 96.Klaff LJ, Kernoff L, Vinik AI, et al. Sulfonylureas and platelet function. Am J Med. 1981;70:627–630. doi: 10.1016/0002-9343(81)90585-4. [DOI] [PubMed] [Google Scholar]
- 97.Henderson JN, Allen KV, Deary IJ, et al. Hypoglycaemia in insulin-treated Type 2 diabetes: frequency, symptoms and impaired awareness. Diabetic medicine : a journal of the British Diabetic Association. 2003;20:1016–1021. doi: 10.1046/j.1464-5491.2003.01072.x. [DOI] [PubMed] [Google Scholar]
- 98.Gray CS, Hildreth AJ, Sandercock PA, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) The Lancet. Neurology. 2007;6:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- 99.Bruno A, Kent TA, Coull BM, et al. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008;39:384–389. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]
- 100.Johnston KC, Hall CE, Kissela BM, et al. Glucose Regulation in Acute Stroke Patients (GRASP) trial: a randomized pilot trial. Stroke. 2009;40:3804–3809. doi: 10.1161/STROKEAHA.109.561498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moheet A, Seaquist ER. Hypoglycemia as a driver of cardiovascular risk in diabetes. Curr Atheroscler Rep. 2013;15:351. doi: 10.1007/s11883-013-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Klingbeil KD, Koch S, Dave KR. Potential link between post-acute ischemic stroke exposure to hypoglycemia and hemorrhagic transformation. Int J Stroke. 2017 doi: 10.1177/1747493017743797. 1747493017743797. [DOI] [PMC free article] [PubMed] [Google Scholar]


