Abstract
Background
Alcohol involvement has familial associations with bulimic symptoms (i.e., binge eating, inappropriate compensatory behaviors), with several studies indicating a genetic overlap between the two. It is unclear whether overlapping familial risk with alcohol involvement extends to other eating disorder symptoms. Understanding the genetic overlap between alcohol involvement and other eating disorder symptoms may aid in more targeted interventions for comorbid alcohol use-eating disorder symptoms. Thus, we investigated associations between alcohol involvement and two core eating disorder symptoms: drive for thinness and body dissatisfaction in adolescent female and male twins.
Methods:
We assessed three levels of alcohol involvement: alcohol use in the last month, having ever been intoxicated, and alcohol intoxication frequency via self-report. The Eating Disorder Inventory-II assessed drive for thinness and body dissatisfaction. Sex-specific biometrical twin modeling examined the genetic overlap between alcohol involvement and eating disorder symptoms.
Results:
Phenotypic associations between alcohol involvement, drive for thinness, and body dissatisfaction were significantly greater in girls compared with boys. A majority of the associations between alcohol involvement and drive for thinness and body dissatisfaction in girls, but not boys, met our threshold for twin modeling (phenotypic r > .20). Moderate genetic correlations were observed between the three aspects of alcohol involvement and drive for thinness. Moderate genetic correlations were observed between alcohol use and intoxication frequency and body dissatisfaction.
Conclusions:
Together with the literature on alcohol involvement and bulimic symptoms, these findings suggest a generalized association between alcohol involvement and eating disorder symptoms in girls, whereas this association may be symptom specific in boys. Genetic correlations indicate that the amount and direction of this genetic overlap differs across specific symptoms. When intervening on comorbid alcohol involvement and eating disorder symptoms, it may be important to target specific eating disorder symptoms.
Keywords: alcohol use, eating disorder, disordered eating, comorbidity, twin study
Alcohol use disorders and eating disorders share overlapping familial risk (e.g., Munn-Chernoff & Baker 2016). Specifically, there is support for shared genetic risk between alcohol involvement and bulimia nervosa (BN) and its core symptoms (i.e., binge eating and inappropriate compensatory behaviors) (Baker et al. 2010; Munn-Chernoff & Baker 2016). Although the prevalence of an alcohol use disorder is also significantly elevated in anorexia nervosa (AN) compared with controls (Hudson et al. 2007; Root et al. 2010), no studies have examined the underpinnings of this elevated comorbidity. Further, the majority of studies examining the genetic association between alcohol involvement and BN and its core symptoms have focused on binge eating and inappropriate compensatory behaviors in adult female samples (Baker et al. 2010; Munn-Chernoff et al. 2013; Munn-Chernoff et al. 2015a; Munn-Chernoff et al. 2015b; Slane, Burt & Klump 2012). A recent study supports that this association may function similarly in adolescent girls and boys (Baker et al. 2017). However, it remains unclear whether the shared familial risk with alcohol involvement in adults and adolescents extends beyond BN, binge eating, and inappropriate compensatory behaviors.
Specifically, alcohol involvement may be related to additional core eating disorder symptoms such as drive for thinness and body dissatisfaction. Drive for thinness is the excessive desire to pursue thinness along with concern over weight and dieting whereas body dissatisfaction is the discontent with one’s body shape stemming from the belief that different areas of the body are too large (Garner 1991). Both can be considered transdiagnostic eating disorder symptoms in that they are typically observed in both AN and BN. In comparison to some bulimic symptoms (e.g., binge eating), drive for thinness and body dissatisfaction characterize the cognitive symptoms of the eating disorders whereas binge eating and inappropriate compensatory behaviors represent behavioral symptoms. However, similar to the associations observed with binge eating and inappropriate compensatory behaviors, alcohol use is positively associated with both drive for thinness and body dissatisfaction (Granner et al. 2002; Keski-Rahkonen et al. 2005; von Ranson et al. 2002).
Importantly, investigating the associations between alcohol involvement and specific eating disorder symptoms may be more informative compared with diagnosis in understanding shared familial risk. Early family studies reported mixed results as to whether there was a familial overlap between alcohol use disorders and eating disorders (e.g., Kaye et al. 1996; Lilenfeld et al. 1998; von Ranson, McGue, & Iacono 2002). However, twin studies indicate that alcohol involvement and eating disorder symptoms do share familial associations (Baker et al. 2010; Kendler et al. 1995; Munn-Chernoff et al. 2013; Munn-Chernoff et al. 2015a), specifically shared genetic overlap. In their review, Munn-Chernoff and Baker (2016) hypothesized that the differences observed in findings between family and twin studies may be attributable to focusing on diagnoses versus symptoms. Phenotypic studies have supported that alcohol involvement may be associated with some behavioral eating disorder symptoms such as dieting, binge eating, or compensatory behaviors, but yet not others such as food restriction (Dansky et al. 2000; Gadalla & Piran 2007; Root et al. 2010).
Indeed, an acquired preparedness model has been proposed to explain the significant association between alcohol involvement and eating disorder symptoms, which may vary by eating disorder symptom. According to this model, learned expectancies about alcohol and thinness/eating transact with personality traits, which in turn leads to maladaptive alcohol use and/or eating behaviors (Fischer et al. 2012). Initial evidence suggests that differing personality traits may interact with learned experiences to uniquely predict specific eating disorder symptoms. For example, negative urgency, a facet of impulsivity, has been shown to lead to expectancies related to reinforcement from eating, which predicts later binge eating (Combs et al. 2012), whereas negative affect leads to later thinness expectancies (Davis & Smith in press), and thinness expectancies are related to drive for thinness and body dissatisfaction (Atlas 2004; Stice & Whitenton 2002). Given that alcohol use is also associated with negative urgency and negative affect (Colder & Chassin 1997; Smith & Cyders 2016), negative urgency may be an underlying factor contributing to the comorbidity of alcohol involvement and bulimic symptoms, whereas trait negative affect/emotionality may link alcohol involvement and symptoms such as drive for thinness and body dissatisfaction.
Not only may the genetic associations between alcohol involvement and eating disorders vary based on specific eating disorder symptoms, but they may also vary based on differing levels of alcohol involvement (Munn-Chernoff et al. 2015b). For example, Baker and colleagues (2017) reported a stronger genetic association between lifetime alcohol intoxication and bulimic symptoms (genetic correlation of .76) compared with alcohol use in the last month (genetic correlation of .42) and frequency of alcohol intoxication (genetic correlation of .38). This study also showed differences between the sexes in genetic associations between different levels of alcohol involvement and bulimic symptoms: although females overall showed stronger genetic associations than males, the difference of this strength varied between levels of alcohol involvement. Thus, continuing to investigate familial associations between different levels of alcohol involvement and specific eating disorder symptoms beyond the core behavioral symptoms of BN of binge eating and inappropriate compensatory behaviors, as well as sex differences in these associations, is critical.
Therefore, we examined the familial associations between various levels of alcohol involvement and the additional core eating disorder symptoms of drive for thinness and body dissatisfaction in adolescent girls and boys. Given previous empirical evidence that the familial overlap between alcohol involvement and eating disorder symptoms may be more pertinent at the symptom-level versus the diagnostic-level (Munn-Chernoff & Baker 2016) and since the prevalence of alcohol use peaks at young adulthood (e.g., Grant et al. 2015; Teesson et al. 2010), we investigated three levels of non-diagnostic alcohol involvement: alcohol use in the last month, lifetime alcohol intoxication, and frequency of alcohol intoxication in an adolescent twin sample. First, we explored the observed phenotypic associations between these various levels of alcohol involvement and drive for thinness and body dissatisfaction, as well as how similar these phenotypic associations were in adolescent girls and boys. Second, we examined the familial association between alcohol involvement and drive for thinness and body dissatisfaction, using a bivariate twin design, hypothesizing that genetic overlap will be observed.
Materials and Methods
Sample
Participants were drawn from the longitudinal Swedish Twin study of Child and Adolescent Development (TCHAD). TCHAD has been described in detail elsewhere (Lichtenstein et al. 2007). Twins were 16–17-years-old when information about eating disorder symptoms was assessed. The sample for the current study consisted of 219 and 229 male and female monozygotic (MZ) twin pairs and 157 and 175 male and female dizygotic (DZ) twin pairs, respectively. As has been described previously, computer algorithms of questionnaire responses were used to determine zygosity of twins (Lichtenstein et al. 2007). This project was approved by the Ethics Committee of Karolinska Institutet, Stockholm, Sweden, and the University of North Carolina Institutional Review Board.
Measures
Three levels of alcohol involvement were assessed via self-report including alcohol use in the last month, having ever been intoxicated, and frequency of alcohol intoxication (Baker et al. 2017). To assess alcohol use in the last month, participants were asked if they drank beer, wine, or liquor in the last month. Response options included: (0) No; (1) Yes, once; and (2); Yes, several times. Having ever been intoxicated indicated whether or not the participant has ever experienced alcohol intoxication in their lifetime (yes/no). Frequency of alcohol intoxication was assessed with the question, “how often do you get drunk when you drink alcohol?”. Response options included: (0) never been intoxicated; (1) get intoxicated when drink alcohol sometimes (i.e., sometimes, only at parties); or (2) get intoxicated often (i.e., every time drinking, always). Participants who denied any alcohol use were coded as missing for having ever been intoxicated and frequency of alcohol intoxication because each variable is conditional on having initiated use. This practice is recommended, as including non-users can bias genetic estimates (Agrawal et al. 2016).
Eating disorder symptoms were examined with the Eating Disorder Inventory-II (EDI) (Garner 1991), which was designed to measure behaviors and attitudes relevant to eating disorders. Participants in TCHAD completed only the drive for thinness, body dissatisfaction, and bulimia subscales of the EDI. For the current study, only the drive for thinness (i.e., cognitions and behaviors that reflect an excessive desire to be thin) and body dissatisfaction (i.e., discontent with one’s body) subscales are included as bulimia symptoms have been explored elsewhere (Baker et al., 2017). The EDI has been translated and validated with a Swedish female population (Nevonen et al. 2006; Norring & Sohlberg 1988) and functions similarly in each sex (Baker et al. 2009; Olivardia et al. 1995; Spillane et al. 2004). Cronbach’s alpha coefficients were acceptable for this sample and were estimated at .87 and .90 for drive for thinness and body dissatisfaction for girls and at .81 for drive for thinness and .83 for body dissatisfaction for boys.
For the EDI, missing data were treated in the following manner: if the participant responded to at least 75% of the subscale items, missing values were mean imputed. Subscale scores were considered missing if less than 75% of the subscale items were available. After scoring, three categories were created for these subscales due to the categorical nature of the alcohol involvement data: (0) a score of zero for the subscale; (1) a score above zero and below one standard deviation above the mean; and (2) a score one standard deviation above the mean and beyond.
Statistical Methods
First, sex-specific correlations were used to examine phenotypic associations between alcohol involvement and drive for thinness and body dissatisfaction. Fischer r-to-z was applied to assess how similar all phenotypic bivariate associations were in girls and boys. Second, a Cholesky decomposition (Figure 1) was used to examine the genetic and environmental associations between alcohol involvement and eating disorder symptoms. To limit the number of statistical analyses being completed to those with an empirical rationale and to remain consistent with our previous work (Baker et al., 2017), twin modeling was based on results from the sex-specific phenotypic correlations. Specifically, only those bivariate associations that showed a sex-specific correlation of at least .20 were followed-up in twin modeling.
Figure 1.
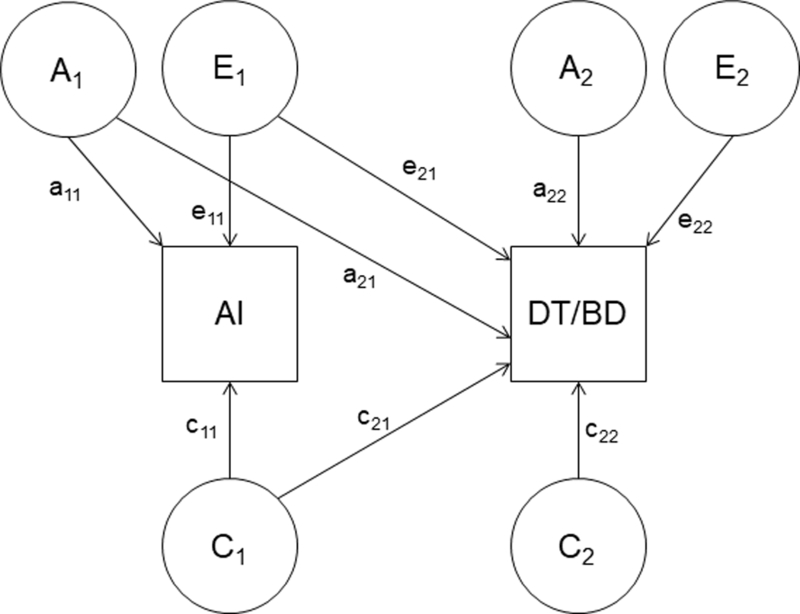
Cholesky decomposition of genetic and environmental covariance between alcohol involvement (AI) and drive for thinness (DT) or body dissatisfaction (BD)
The Cholesky decomposition assumes three sources of liability for alcohol involvement, eating disorder symptoms, and their overlap: additive genetic (A: the cumulative effects of multiple genes), common environmental (C: the similar environmental factors shared by twins and families), and individual-specific environmental (E: the environmental factors that are distinct between twins and measurement error) factors. The model estimates the liability for alcohol involvement and eating disorder symptoms due to genetic and environmental factors independently along with estimates of the genetic and environmental associations between the two phenotypes. The genetic and environmental associations are estimated via genetic (ra: calculated as a21*a11 / square root (a112*(a212+a222)) from Figure 1), common environment (rc: calculated as c21*c11 / square root (c112*(c212+c222)) from Figure 1)), and individual-specific environment (re: calculated as e21*e11 / square root (e112*(e212+e222)) from Figure 1) correlations. These correlations provide estimates of the proportion of variance two phenotypes share due to genetic and environmental factors.
The fit of the full ACE model was compared with two nested submodels: the AE model and the CE model. The fit of the models was compared using the negative log-likelihood of the models. Given certain regularity conditions, the difference in twice the negative log-likelihood of the models is distributed as a chi-square and a significant change in chi-square between the full model and the submodel indicates the model can be rejected. Model fit was also assessed with Akaike Information Criterion (AIC) (Akaike 1987). A lower AIC value indicates a better balance between parsimony and goodness-of-fit and as such models with lower AIC values are preferred.
An initial best-fit model was selected based on chi-square change and AIC. After determining the initial best-fit model, we compared additional nested submodels to this initial best-fitting model in order to assess the importance of familial covariance (i.e., genetic or common environmental covariance). Specifically, these additional submodels respectively dropped the genetic or common environmental covariance path (a21 or c21 from Figure 1) or the genetic or common environmental path specific to the eating disorder symptom (a22 or c22 from Figure 1) from the model.
Finally, within the model, alcohol involvement was entered first and drive for thinness/body dissatisfaction second. Notably, the genetic and environmental correlations and model-fits are invariant to variable ordering; however, from this, we can secondarily determine the proportion of variance in eating disorder symptoms that are accounted for by alcohol involvement from the covariance paths (a21, c21, e21 from Figure 1). These estimates are calculated as follows: genetic (a22 / a21 + a22), common environment (c22 / c21 + c22), and individual-specific environment (e22 / e21 + e22). Although these estimates are not distinct from the genetic and environmental correlations as both are determined, in part, from the covariance path estimates, each provides slightly different information about etiological relatedness. The genetic and environmental correlations provide information about the proportion of overlapping factors between two phenotypes, whereas the covariance estimates tell us the variance in one variable that is accounted for by a second variable. Although model-fits and genetic and environmental correlations are invariant to variable order, we reversed the order of the variables in the final, best-fit model in order to calculate the variance in alcohol involvement accounted for by eating disorder symptoms.1 The Cholesky decomposition was fitted in Mx (Neale 1991) using a categorical, raw data approach, which allows information from both complete twin pairs (n = 171 MZ male; 199 MZ female; 129 DZ male; 149 DZ female) and incomplete twin pairs (n = 48 MZ male; 30 MZ female; 28 DZ male; 26 DZ female) to be included. Separate twin models, based on the sex-specific phenotypic correlations, were completed for girls and boys.
Results
Descriptive Statistics
As can be seen in Table 1 and reported elsewhere (Baker et al. 2017), the frequency of alcohol involvement was similar in girls and boys. Means scores for both drive for thinness (M = 2.10, SD = 4.10 for girls; M = 0.33, SD = 1.40 for boys) and body dissatisfaction (M = 5.70, SD = 6.00 for girls; M = 2.10, SD = 3.60 for boys) were higher in girls than boys. A majority of the sample scored a zero on the drive for thinness subscale (0 = 57.9% for girls and 84.4% for boys; 1 = 31.0% for girls, 9.5% for boys; 2 = 12.1% for girls, 18.0% for boys). A majority of girls scored a one on the body dissatisfaction subscale (0 = 24.2%, 1 = 58.2%, 2 = 18.0%), whereas the majority of boys scored a zero (0 = 56.0%; 1 = 30.0%; 2 =14.4%).
Table 1.
Frequency of Alcohol Involvement by Sex (Boys Shown in Parentheses)
| Alcohol Involvement Frequency | |||
|---|---|---|---|
| No | Once/Sometimes |
Often/Yes |
|
| Alcohol Use in Last Montha |
44.2%; n = 371 (50.0%; n = 348) |
27.1%; n = 228 (25.5%; n = 181) |
29.0%; n= 241 (25.5%; n = 181) |
| Ever Intoxicated | 23.0%; n = 139 (30.0%; n = 147) |
-- | 77.5%; n = 475 (71.0%; n = 357) |
| Intoxication Frequency |
55.1%; n = 474 (61.0%; n = 453) |
18.2%; n = 157 (16.2%; n = 121) |
27.0%; n = 230 (23.1%; n = 172) |
Note. = Variable coded as no, once, and several times. % = percentage of sample. n = number of individuals in sample.
Phenotypic Associations
All of the observed phenotypic correlations between alcohol involvement and drive for thinness and body dissatisfaction were significantly greater in girls compared with boys (Table 2). Further, all phenotypic associations between alcohol involvement and drive for thinness for girls were above .20; these were included in follow-up twin modeling. Associations with alcohol use in the last month and frequency of alcohol intoxication also met the .20 threshold for body dissatisfaction. In contrast, no associations for boys met this threshold.
Table 2.
Phenotypic Correlations and 95% Confidence Intervals between Alcohol Involvement and Eating Disorder Symptoms
| Level of Alcohol Involvement |
Girls | Boys | ||
|---|---|---|---|---|
|
DT |
BD | DT | BD | |
| Use in the Last Month | 0.22* (.13; .31) |
0.22* (.14; .31) |
.07 (−.05; .20) |
.12 (.02; .22) |
| Ever Intoxicated | 0.32* (.20; .44) |
0.18* (.07; .31) |
.16 (−.01; .33) |
−.01 (−.14; .13) |
| Intoxication Frequency | 0.26* (.17; .35) |
0.20* (.12; .30) |
.13 (−.01; .25) |
.10 (−.03; .17) |
Note. DT = drive for thinness. BD = body dissatisfaction.
Fisher r-to-z transformation indicating a significant difference between girls and boys (all p’s < .01).
Within-trait cross-twin correlations are presented in Table 3. In girls, the presence of genetic effects was indicated for all phenotypes: MZ correlations were nearly twice that of the DZ correlations for most associations. In boys, genetic effects were most strongly indicated for alcohol use in the last month and body dissatisfaction. In both sexes, MZ correlations indicated that genetic factors may be more salient than individual-specific effects, as these correlations were substantial for all three levels of alcohol involvement. Cross-twin cross-trait correlations for those phenotypic correlations meeting the threshold for twin modeling are also provided in Table 3. Because the cross-twin cross-trait correlations were similar in MZ and DZ twins for the bivariate associations between alcohol involvement and drive for thinness and body dissatisfaction in girls, this suggest that common environment may play a role in overlap.
Table 3.
Twin Correlations (95% Confidence Interval) for Alcohol Involvement and Eating Disorder Symptoms
| MZF | DZF | MZM | DZM | |
|---|---|---|---|---|
| Within-Trait, Cross-twin Correlations | ||||
| Drank Alcohol in Last Month | .75 (.65; .84) |
.56 (.40; .71) |
.80 (.72; .89) |
.57 (.40; .73) |
| Ever Intoxicated | .94 (.87; 1.00) |
.46 (.10; .85) |
.84 (.70; .98) |
.70 (.60; 1.00) |
| Intoxication Frequency | .82 (.74; .90) |
.65 (.51; .80) |
.90 (.85; .96) |
.73 (.60; .85) |
| DT | .68 (.57; .80) |
.46 (.29; .63) |
.36 (.10; .62) |
.35 (.06; .64) |
| BD | .70 (.60; .80) |
.37 (.19; .54) |
.50 (.34; .64) |
.26 (.06; .47) |
|
Cross-twin, Cross-trait Correlations for DT |
||||
| Drank Alcohol in Last Month | .21 (.09; .33) |
.21 (.07; .35) |
-- | -- |
| Ever Intoxicated | .34 (.17; .51) |
.14 (−.10; .37) |
-- | -- |
| Intoxication Frequency | .23 (.11; .36) |
.20 (.06; .36) |
-- | -- |
|
Cross-twin, Cross-trait Correlations for BD |
||||
| Drank Alcohol in Last Month | .19 (.01; .31) |
.20 (.06; .33) |
-- | -- |
| Intoxication Frequency | .23 (.11; .35) |
.14 (−.01; .28) |
-- | -- |
Note. MZF=monozygotic female twins; DZF=dizygotic female twins; MZM=monozygotic male twins; DZM=dizygotic male twins. DT=drive for thinness; BD=body dissatisfaction.
Twin Analyses
Model fitting results and parameter estimates are provided in Table 4. Genetic and environmental correlations from the full and best-fit models are provided in Table 5.
Table 4.
Twin Model Parameter Estimates and Model Fitting Results for Alcohol Involvement and Eating Disorder Symptoms in Girls
| Alcohol Involvement Parameter Estimates |
Eating Disorder Symptom Parameter Estimates |
Fit-Statistics |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | a2 | c2 | e2 | a2 | c2 | e2 | −2lnL | Df | χ2 diff (p) |
AIC |
| Alcohol Use in Last Month | ||||||||||
| Drive for Thinness | ||||||||||
| ACE | 47 (14;80) |
29 (0;57) |
25 (17;35) |
56 (17;76) |
12 (0;45) |
32 (22;44) |
2906.50 | 1563 | -- | −220.00 |
| AE |
77 (67;83) |
-- |
23 (16;33) |
69 (58;78) |
-- |
31 (22;42) |
2910.20 | 1566 |
3.70 (.30) |
−222.00 |
| CE | -- | 65 (55;73) |
35 (27;45) |
-- | 56 (45;65) |
44 (35;55) |
2922.20 | 1566 | 16.00 (<.01) |
−210.00 |
| AE-no a21 | 75 (65;82) |
-- | 25 (18;35) |
67 (55;77) |
-- | 33 (23;45) |
2930.00 | 1567 | 16.00 (<.001) |
−208.00 |
| AE-no a22 | 65 (50;77) |
-- | 35 (23;51) |
32 (16;50) |
-- | 68 (51;84) |
2971.00 | 1567 | 60.01 (<.001) |
−163.23 |
| Body Dissatisfaction | ||||||||||
| ACE | 46 (14;81) |
30 (9;58) |
24 (16;34) |
81 (46;90) |
3 (0;37) |
15 (11;22) |
3064.00 | 1564 | -- | −64.10 |
| AE |
77 (68;84) |
-- |
23 (16;32) |
85 (78;90) |
-- |
15 (11;22) |
3067.10 | 11567 |
3.20 (.37) |
−67.00 |
| CE | -- | 66 (57;74) |
31 (26;43) |
-- | 70 (61;77) |
30 (23;39) |
3096.50 | 1567 | 33.00 (<.001) |
−38.00 |
| AE-no a21 | 75 (66;83) |
-- | 25 (17;34) |
84 (77;90) |
-- | 16 (11;23) |
3084.00 | 1568 | 17.00 (<.001) |
−52.10 |
| AE-no a22 | 50 (20;70) |
-- | 50 (30;72) |
64 (30;80) |
-- | 36 (20;62) |
3158.03 | 1568 | 91.00 (<.001) |
22.03 |
| Ever Intoxicated from Alcohol | ||||||||||
| Drive for Thinness | ||||||||||
| ACE | 50 (23;83) |
46 (13;71) |
4 (1;11) |
56 (17;77) |
12 (0; 45) |
32 (22;44) |
2116.00 | 1448 | -- | −781.00 |
| AE | 70 (58;78) |
-- | 31 (22;43) |
97 (91;99) |
-- | 3 (1;9) |
2123.00 | 1451 | 7.30 (.06) |
−780.00 |
| CE | -- | 83 (74;90) |
17 (10;26) |
-- | 56 (45;65) |
44 (35;55) |
2136.00 | 1451 | 20.23 (<.001) |
−766.02 |
| ACE- no a21 |
47 (20;77) |
50 (20;73) |
4 (2;12) |
53 (14;68) |
15 (3;47) |
32 (23;45) |
2217.00 | 1449 | 1.23 (.30) |
−781.20 |
| ACE-no no a22 |
44 (14;80) |
51 (17;76) |
5 (1;14) |
34 (0;72) |
30 (0;60) |
37 (26;50) |
2120.30 | 1449 | 5.00 (<.05) |
−778.00 |
| ACE-no c21 |
55 (27;86) |
42 (10;66) |
4 (1;11) |
61 (22;78) |
7 (0;40) |
32 (22;44) |
2116.43 | 1449 | .83 (.40) |
−781.60 |
|
ACE- no c22 |
50 (23;84) |
46 (13;71) |
4 (1;11) |
65 (36;77) |
4 (0;30) |
31 (22;42) |
2116.00 | 1449 |
.23 (.60) |
−782.20 |
| Frequency of Alcohol Intoxication | ||||||||||
| Drive for Thinness | ||||||||||
| ACE | 47 (17;81) |
35 (3;61) |
18 (11;27) |
57 (17;78) |
12 (0;45) |
32 (22;42) |
2770.00 | 1577 | -- | −385.40 |
| AE |
84 (77;88) |
-- |
16 (11;24) |
70 (58;78) |
-- |
31 (22;42) |
2774.10 | 1580 |
5.50 (.14) |
−386.00 |
| CE | -- | 72 (63;80) |
28 (26;37) |
-- | 56 (45;65) |
44 (35;55) |
2787.00 | 1580 | 18.10 (<.001) |
−373.32 |
| AE-no a21 | 81 (72;88) |
-- | 19 (12;28) |
66 (54;76) |
-- | 34 (24;46) |
2792.23 | 1581 | 18.15 (<.001) |
−370.00 |
| AE-no a22 | 78 (64;87) |
-- | 22 (13;36) |
24 (9;41) |
-- | 76 (59;91) |
2844.32 | 1581 | 70.23 (<.001) |
−317.70 |
| Body Dissatisfaction | ||||||||||
| ACE | 47 (18;81) |
35 (3;61) |
18 (12;26) |
84 (66;90) |
1 (0;37) |
15 (10;22) |
2950.00 | 1578 | -- | −206.20 |
| AE |
84 (16;90) |
-- |
16 (11;24) |
85 (78;90) |
-- |
15 (10;22) |
2954.60 | 1581 |
5.00 (.20) |
−207.42 |
| CE | -- | 73 (64;80) |
27 (20;36) |
-- | 70 (62;77) |
30 (23;38) |
1581 | 40.00 (<.001) |
−175.21 | |
| AE-no a21 | 82 (74;88) |
-- | 18 (12;26) |
68 (57;78) |
-- | 32 (22;43) |
2871.80 | 1582 | 15.30 (<.001) |
−192.00 |
| AE-no a22 | 74 (60;84) |
-- | 26 (16;40) |
26 (12;42) |
-- | 74 (58;90) |
2930.00 | 1582 | 70.34 (<.001) |
−119.30 |
Note. Final best fit model shown in bold. 95% confidence interval for parameter estimates in parentheses. a2 = heritability. c2 = common environmental estimate. e2 = individual-specific environmental estimate. −2lnL = difference in twice the negative log-likelihood of the models. Df = degrees of freedom. χ2 diff (p) = chi-square difference between full model and submodel and the associated p-value. AIC = Akaike Information Criterion. a21 = genetic covariance path from Figure 1. a22 = residual genetic effect path for unique genetic effects on eating disorder symptom from Figure 1. c21 = common environment covariance path from Figure 1. c22 = residual common environment effect path for unique common environmental effects on eating disorder symptom from Figure 1.
Table 5.
Observed Correlations (95% Confidence Interval) from Full, Initial, and Final Best Fit Twin Models for Alcohol Involvement and Eating Disorder Symptoms in Adolescent Girls
| Drive for Thinness | Body Dissatisfaction | |||||
|---|---|---|---|---|---|---|
| ra | rc | re | ra | rc | re | |
| Drink in Last Month | ||||||
| ACE | .17 (−.41; .75) |
.72 (−1; 1) |
.05 (−.21; .32) |
.30 (−.10; .71) |
1.00 (−1; 1) |
.10 (−.15; .34) |
| AE |
.31 (.16; .46) |
-- |
.03 (−.22; .28) |
.33 (.18; .47) |
-- |
.10 (−.15; .33) |
| Ever been Intoxicated | ||||||
| ACE | .32 (−.23; .91) |
.51 (−1; 1) |
.32 (−.51; .67) |
-- | -- | -- |
|
ACE-no unique common environment |
.27 (−.10; .82) |
1.00a (1; 1) |
.35 (−.50; .99) |
-- | -- | -- |
| Frequency of Intoxication | ||||||
| ACE | .50 (−.01; 1) |
−.05 (−1; 1) |
.10 (−.18; .38) |
.41 (.03; .82) |
.99 (−1; 1) |
−.06 (−.32; .21) |
| AE |
.32 (.18; .46) |
-- |
.12 (−.15; .38) |
.34 (.18; .48) |
-- |
−.04 (−.05; .30) |
Note. Final best-fit model shown in bold. a2 = heritability. c2 = common environmental estimate. e2 = individual-specific environmental estimate. ra = genetic correlation. rc = common environmental correlation. re = individual-specific environmental correlation.
= the confidence interval for the correlation in this bivariate association in the best-fit model is observed at +1.00; +1.00 because there are no common environmental effects specific to drive for thinness that are not shared with having ever been intoxicated.
Alcohol Use in the Last Month
For the alcohol use in the last month and drive for thinness association, comparing the fit of the ACE model to the AE and CE model indicated that the AE model was the initial best-fitting model. Therefore, two additional follow-up submodels examining the importance of familial covariance were examined: an AE model without genetic covariance and an AE model without unique genetic effects for drive for thinness. Both follow-up models had a significant change in chi-square and were rejected. The best-fit AE model showed an estimated ra of .31 (95% CI: .16; .46) and re of .03 (95% CI: −.22; .28). Moreover, alcohol use in the last month accounted for 10% of the total heritability of drive for thinness, whereas individual-specific environmental factors accounted for 1%. Notably however, the confidence interval for the individual-specific environmental correlation included zero so the significance of this overlap is unclear. Reversing the order of the variables in the model to determine how much the genetic and individual-specific environmental effects drive for thinness accounted for in alcohol use revealed 9% and <1%, respectively.
For alcohol use in the last month and body dissatisfaction, the AE model was the initial best-fit model and final best-fitting model. This model observed an estimated ra of .33 (95% CI: .18; .47) and re was estimated at .10 (95% CI: −.15; .33), suggesting alcohol use in the last month accounted for 12% of the total heritability of body dissatisfaction and 1% of the total individual-specific environmental effects for body dissatisfaction. Results were similar with the variables reversed such that body dissatisfaction accounted for 12% of the total heritability of alcohol use in the last month and <1% of the total individual-specific environmental effects.
Ever been Intoxicated
According to the change in chi-square and AIC, the ACE model was the initial best-fitting model for ever been intoxicated-drive for thinness. Therefore, follow-up submodels made the following comparisons to the ACE model: an ACE model without genetic covariance, an ACE model without common environmental covariance, an ACE model without unique genetic effects for drive for thinness, and an ACE model without unique common environmental effects for drive for thinness. The ACE model without unique common environmental effects for drive for thinness was the final, best-fitting model for the association between ever been intoxicated and drive for thinness. Examining this best-fit model showed an estimated ra of .27 (95% CI: −.07; .82); rc of 1.00 (95% CI: 1.00; 1.00); and re of .35 (95% CI: −.50; .99). Having ever been intoxicated accounted for 8% of the total heritability and 13% of the total individual-specific environmental effects for drive for thinness, but the confidence intervals for the genetic and individual-specific environmental correlations did include zero. However, we were unable to drop the genetic covariance path from the model, suggesting that genetic covariance is important. The common environmental effects for drive for thinness were entirely accounted for by common environmental effects shared with alcohol intoxication. Reversing the order of the variables showed a similar pattern: 8% of the total heritability, 100% of the common environmental effects, and <1% of the individual-specific environmental effects for having ever been intoxicated were accounted for by drive for thinness.
Alcohol Intoxication Frequency
Model comparisons showed that the AE model was the best-fitting model for alcohol intoxication frequency-drive for thinness. Both follow-up submodels testing the importance of this genetic covariance could be rejected as a significantly worse fit; thus, the AE model was the final, best-fitting model. This model estimated ra at .32 (95% CI: .18; .46) and re at .12 (95% CI: −.15; .38), indicating that alcohol intoxication frequency accounted for 10% of the total heritability of drive for thinness and 2% of the total individual-specific environmental effects. Similarly, drive for thinness accounted for 11% of the total heritability for alcohol intoxication frequency and <1% of the individual-specific environmental effects.
For body dissatisfaction, the AE model was the final best-fitting model after all model comparisons. Examining this best-fit AE model showed an estimated ra of .34 (95% CI: .18; .48) and re of −.04 (95% CI: −.05; .30). Thus, alcohol intoxication frequency accounted for approximately 9% of the total heritability of body dissatisfaction and 1% of the total individual-specific environmental effects for body dissatisfaction. In the reverse model, body dissatisfaction accounted for 12% of the heritability and <1% of the individual-specific environmental effects for alcohol intoxication frequency.
Discussion
The purpose of this study was to expand on the literature examining the familial association between alcohol involvement and eating disorder symptoms to eating disorder symptoms beyond the core behavioral symptoms of BN (i.e., binge eating and inappropriate compensatory behaviors). Similar to previous observations with bulimic symptomatology, we found a moderate phenotypic and genetic association between alcohol involvement and drive for thinness and body dissatisfaction in adolescent girls, indicating that alcohol involvement associations extend beyond the behavioral symptoms of BN (Baker et al. 2017). Indeed, these findings suggest generalized phenotypic and genetic associations across eating disorder symptoms in girls (Baker et al. 2017; Holderness et al. 1994; Munn-Chernoff & Baker 2016).
In general, alcohol involvement showed small-to-moderate phenotypic correlations with drive for thinness and body dissatisfaction in girls. In contrast, there were minimal associations between all aspects of alcohol involvement and drive for thinness and body dissatisfaction in boys, and no associations met our .20 threshold for twin modeling. This diverges from previous studies showing a significant association between alcohol involvement and bulimic symptoms in boys (Baker et al. 2017; Stickley et al. 2015). Thus, it may be that whereas adolescent girls display a global association between various levels of alcohol involvement across eating disorder symptoms,2 the association in adolescent boys is specific and unique to bulimic symptoms. This global association in girls may be related to the fact eating disorder symptoms, in general, are more common in girls (American Psychiatric Association 2013; Ellis et al. 2012) and engaging in risky behaviors and behavioral disorders are more common in boys (Merikangas et al. 2010). Indeed, drive for thinness and body dissatisfaction do not require engagement in specific behaviors for the symptom to be present whereas the presence of binge eating and/or inappropriate compensatory behaviors (i.e., behaviors) is required for the presence of bulimic behaviors. Thus, associations with alcohol involvement in boys may be restricted to behavioral eating disorder symptoms versus both behavioral and cognitive symptoms in girls.
Our findings also expand previous work on alcohol involvement and eating disorder symptoms in females showing that the familial association is not exclusive to binge eating and/or inappropriate compensatory behaviors and extends to drive for thinness and body dissatisfaction—two additional core eating disorder symptoms. We also extend this literature by showing that alcohol involvement and eating disorder symptoms account for a similar amount of genetic and environmental variance within one another in specific alcohol-eating disorder symptom bivariate associations. However, across bivariate relationships, genetic associations with alcohol involvement may be stronger with certain eating disorder symptoms than others, given that the genetic associations observed in this study were lower than what has been observed between alcohol involvement and bulimic symptoms. Whereas a previous study found that alcohol intoxication accounted for 57% of the heritability in bulimic symptoms for girls (Baker et al. 2017), having ever been intoxicated only accounted for approximately 10% of the total heritability of drive for thinness. Similarly, genetic factors for alcohol use in the last month accounted for 17% of the total heritability of bulimic symptoms but here only accounted for 10% of the heritability for drive for thinness and 12% for body dissatisfaction. This suggests that although there is a familial association between aspects of alcohol involvement and both the cognitive and behavioral symptoms of eating disorders in girls, the genetic association appears to be strongest with the behavioral symptoms. Additionally, while we cannot directly examine an acquired preparedness model, these findings partially support this model given that shared genetic factors were observed, which may be attributable to an underlying disposition such as negative affectivity.
In regard to common environmental effects, although having ever been intoxicated accounted for all of the common environmental effects for both drive for thinness and bulimic symptoms, they were in the opposite direction, such that an inverse association was previously observed between alcohol intoxication and bulimic symptoms (Baker et al. 2017). Notably, the common environmental effects for both drive for thinness and bulimic symptoms were quite small (i.e., 4% for drive for thinness). Given the significant associations observed with alcohol involvement and/or eating disorder symptoms, common environmental factors such as parental psychopathology (Bould et al. 2015; Chandy et al. 1994; Tully et al. 2008) and/or peer groups shared by co-twins (Neiderhiser et al. 2013) may account for increasing risk for drive for thinness and alcohol intoxication, yet it is uncertain what factors may influence the risk for alcohol intoxication and bulimic symptoms in opposite directions. Taken together, it appears that though there are generally similar phenotypic associations observed between various aspects of alcohol involvement and eating disorder symptoms for girls, the amount and direction of this genetic/familial overlap differs across specific symptoms.
These findings support our hypothesis that shared familial overlap with alcohol involvement extends beyond bulimic symptoms—in particular genetic overlap—suggesting several potential implications. Specifically, our findings suggest that focusing on comorbid alcohol involvement and specific eating disorder symptoms rather than disorder-level associations may be important for treatment. Interventions for boys in comorbid alcohol-eating disorder treatment should focus on behavioral eating disorder symptoms (i.e., bulimic symptoms), whereas interventions for girls should target cognitive and behavioral symptomatology. Further, these results have important implications for future genomics studies, particularly cross-disorder studies, providing initial evidence of which symptom-level associations are important to address.
Some limitations of the current study should be considered. For one, our statistical power was limited as indicated by some confidence intervals including zero. This may have influenced our ability to detect significant shared familial effects in the final models. For example, the confidence intervals for ever been intoxicated and drive for thinness included zero, but we could not drop genetic covariance from the model; this indicates its importance but that we did not have the power to detect significance. Further, some cross-twin cross-trait correlations suggested common environment may be important for associations with drive for thinness and body dissatisfaction, which were not replicated in the twin model results. However, twin correlations only provide a potential indication of twin model results and do not always predict them definitively. The bivariate model fitting analyses involve simultaneous modeling of all correlations, not just a discrete examination of the cross-twin cross-trait correlation. Still, it is possible significant common environmental overlap exists and we did not have the power to detect it. Despite possible limited statistical power, ours is the first study to explore whether alcohol involvement and eating disorder symptoms other than binge eating and inappropriate compensatory behaviors share etiological factors in a non-adult sample of both girls and boys; thus, answering a novel question and providing directions for future research in larger sample sizes.
Second, significant common environmental effects have been observed for alcohol use and eating disorder symptoms at younger ages (Baker et al. 2011; Klump et al. 2007). Common environmental covariance may be important for alcohol involvement and drive for thinness/body dissatisfaction in childhood, which we are not able to examine here. Third, the drive for thinness and body dissatisfaction subscales on the EDI had to be categorized because the alcohol involvement data were categorical. However, it is expected that the distribution of the categories for drive thinness and body dissatisfaction would be comparable to the distribution of previous studies, as the mean scores for these subscales were similar to other community samples (Nevonen et al. 2006; Slane et al. 2014; Stice et al. 2004). Finally, our Swedish sample may impact the generalizability of the results given that the frequency of alcohol involvement in our sample was less than the lifetime prevalence of alcohol involvement previously observed at similar ages in the United States (Johnston et al. 2013).
Our results indicate a shared genetic association between alcohol involvement and eating disorder symptoms in girls that cuts across various levels of alcohol use and eating disorder symptoms. However, some of these genetic associations are stronger than others, which differs from the phenotypic associations observed, which were generally similar in girls across alcohol use and eating disorder symptoms. Minimal phenotypic associations were observed between alcohol involvement and drive for thinness and body dissatisfaction in adolescent boys, indicating that boys may have a specific and unique association between alcohol involvement and bulimic/behavioral eating disorder symptoms (Baker et al. 2017). These findings suggest that targeting alcohol involvement and specific eating disorder symptoms in intervention programs may be beneficial. Future research should continue to explore the relationship between alcohol involvement and specific eating disorder symptoms, especially with respect to sex differences. Research should also address the etiological association between specific alcohol use disorder symptoms and eating disorder symptomatology. Ultimately, better understanding of genetic associations between specific aspects of alcohol involvement and specific eating disorder symptoms will improve prevention and treatment efforts and aid in building genomic models of comorbidity.
Acknowledgments
Dr. Baker was supported by grant NIH K01MH106675. Dr. Munn-Chernoff was supported by grant NIH K01AA025113. Dr. Larsson has served as a speaker for Eli-Lilly and Shire and has received a research grant from Shire; all outside the submitted work. Dr. Lichtenstein has served as a speaker for Medice outside the submitted work.
Footnotes
Model-fits and correlations were compared in the reversed order models and results remained invariant to variable order as expected: final, best-fit models remained identical and correlation estimates remained near identical (data not shown).
Phenotypic correlations observed here for girls (r’s = .18 to .32) are similar to those observed between alcohol involvement and bulimic symptoms for this same sample of girls (r’s = .21 to .33; Baker et al., 2017)
Contributor Information
Jessica H. Baker, Department of Psychiatry, University of North Carolina at Chapel Hill.
Leigh C. Brosof, Department of Psychological and Brain Sciences, University of Louisville.
Melissa A. Munn-Chernoff, Department of Psychiatry, University of North Carolina at Chapel Hill.
Paul Lichtenstein, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet.
Henrik Larsson, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet and Department of Medical Science Örebro University.
Hermine H. Maes, Department of Genetics, Virginia Commonwealth University.
Kenneth S. Kendler, Department of Psychiatry, Virginia Commonwealth University.
References
- Agrawal A, Edenberg HJ, & Gelernter J (2016) Meta-analyses of genome-wide association data hold new promise for addiction genetics. J Stud Alcohol Drugs 77:676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H (1987) Factor analysis and AIC. Psychometrika 52:317–332. [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Atlas JG (2004) Interpersonal sensitivity, eating disorder symptoms, and eating/thinness expectancies. Current Psychol 22:368. [Google Scholar]
- Baker JH, Maes HH, Larsson H, Lichtenstein P, Kendler KS (2011) Sex differences and developmental stability in genetic and environmental influences on psychoactive substance consumption from early adolescence to young adulthood. Psychol Med 41:1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Maes HH, Lissner L, Aggen SH, Lichtenstein P, Kendler KS (2009) Genetic risk factors for disordered eating in adolescent males and females. J Abnorm Psychol 118:576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Mitchell KS, Neale MC, Kendler KS (2010) Eating disorder symptomatology and substance use disorders: prevalence and shared risk in a population based twin sample. Int J Eat Disord 43:648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Munn-Chernoff MA, Lichtenstein P, Larsson H, Maes H, Kendler KS (2017) Shared familial risk between bulimic symptoms and alcohol involvement during adolescence. J Abnorm Psychol 126:506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bould H, Koupil I, Dalman C, DeStavola B, Lewis G, Magnusson C (2015) Parental mental illness and eating disorders in offspring. Int J Eat Disord 48:383–391. [DOI] [PubMed] [Google Scholar]
- Chandy JM, Harris L, Blum RW, Resnick MD (1994) Disordered eating among adolescents whose parents misuse alcohol: protective and risk factors. Int J Addict 29:505–516. [DOI] [PubMed] [Google Scholar]
- Cohen J (1992) A power primer. Psychol Bull 112:155–159. [DOI] [PubMed] [Google Scholar]
- Colder CR, Chassin L (1997) Affectivity and impulsivity: Temperament risk for adolescent alcohol involvement. Psychol Addict Behav 11:83–97. [Google Scholar]
- Combs JL, Pearson CM, Zapolski TC, Smith GT (2012) Preadolescent disordered eating predicts subsequent eating dysfunction. J Pediatr Psychol 38:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansky BS, Brewerton TD, Kilpatrick DG (2000) Comorbidity of bulimia nervosa and alcohol use disorders: results from the National Women’s Study. Int J Eat Disord 27:180–190. [DOI] [PubMed] [Google Scholar]
- Davis HA, Smith GT (in press) An integrative model of risk for high school disordered eating. J Abnorm Psychol [DOI] [PMC free article] [PubMed]
- Ellis BJ, Del Giudice M, Dishion TJ, Figueredo AJ, Gray P, Griskevicius V, Hawley PH, Jacobs WJ, James J, Volk AA, Wilson DS (2012) The evolutionary basis of risky adolescent behavior: implications for science, policy, and practice. Dev Psychol 48:598–623. [DOI] [PubMed] [Google Scholar]
- Fischer S, Settles R, Collins B, Gunn R, Smith GT (2012) The role of negative urgency and expectancies in problem drinking and disordered eating: testing a model of comorbidity in pathological and at-risk samples. Psychol Addict Behav 26:112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadalla T, Piran N (2007) Co-occurrence of eating disorders and alcohol use disorders in women: a meta analysis. Arch Womens Ment Health 10:133–140. [DOI] [PubMed] [Google Scholar]
- Garner D (1991) Eating Disorders Inventory-2: Professional Manual Psychological Assessment Resources, Inc., Florida. [Google Scholar]
- Granner ML, Black DR, Abood DA (2002) Levels of cigarette and alcohol use related to eating-disorder attitudes. Am J Health Behav 26:43–55. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan JW, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA 72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderness C, Brooks-Gunn J, Warren M (1994) Co-morbidity of eating disorders and substance abuse. Review of the literature. Int J Eat Disord 16:1–35. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC (2007) The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 61:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE (2013) Monitoring the future national results on drug use: 2012 overview of key findings adolescent drug use Institute for Social Research, The University of Michigan, Ann Arbor. [Google Scholar]
- Kaye WH, Lilenfeld LR, Plotnicov K, Merikangas KR, Nagy L, Strober M, Bulik CM, Moss H, Greeno CG (1996) Bulimia nervosa and substance dependence: association and family transmission. Alcohol Clin Exp Res 20:878–881. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Martin NG, Heath AC, Eaves LJ (1995) Self‐report psychiatric symptoms in twins and their nontwin relatives: are twins different?. Am J Med Genet A 60:588–591. [DOI] [PubMed] [Google Scholar]
- Keski‐Rahkonen A, Bulik CM, Neale BM, Rose RJ, Rissanen A, Kaprio J (2005) Body dissatisfaction and drive for thinness in young adult twins. Int J Eat Disord 37:188–199. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA, McGue M, Iacono WG (2007) Changes in genetic and environmental influences on disordered eating across adolescence: a longitudinal twin study. Arch Gen Psychiatry 64:1409–1415. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Svartengren M (1997) Genes, environments, and sex: factors of importance in atopic diseases in 7–9-year-old Swedish twins. Allergy 52:1079–1086. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Tuvblad C, Larsson J, Carlström E (2007) The Swedish Twin Study of CHild and Adolescent Development: the TCHAD-Study. Twin Res Hum Genet 10:67–73. [DOI] [PubMed] [Google Scholar]
- Lilenfeld LR, Kaye WH, Greeno CG, Merikangas KR, Plotnicov K, Pollice C, Rao R, Strober M, Bulik CM, Nagy L (1998) A controlled family study of anorexia nervosa and bulimia nervosa: psychiatric disorders in first-degree relatives and effects of proband comorbidity. Arch Gen Psychiatry 55:603–610. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J (2010) Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 49:980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn-Chernoff MA, Baker JH (2016) A primer on the genetics of comorbid eating disorders and substance use disorders. Eur Eat Disord Rev 24:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn-Chernoff MA, Duncan AE, Grant JD, Wade TD, Agrawal A, Bucholz KK, Madden PAF, Martin NG, Heath AC (2013) A twin study of alcohol dependence, binge eating, and compensatory behaviors. J Stud Alcohol Drugs 74:664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn-Chernoff MA, Grant JD, Agrawal A, Sartor CE, Werner KB, Bucholz KK, Madden PAF, Heath AC, Duncan AE (2015a). Genetic overlap between alcohol use disorder and bulimic behaviors in European American and African American women. Drug Alcohol Depend 153:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn‐Chernoff MA, Grant JD, Bucholz KK, Agrawal A, Lynskey MT, Madden PA, Heath AC, Duncan AE (2015b) Bulimic behaviors and early substance use: findings from a cotwin‐control study. Alcohol Clin Exp Res 39:1740–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M (1991). Statistical Modelling with Mx Richmond, VA. [Google Scholar]
- Neiderhiser JM, Marceau K, Reiss D (2013) Four factors for the initiation of substance use by young adulthood: A 10-year follow-up twin and sibling study of marital conflict, monitoring, siblings, and peers. Dev Psychopathol 25:133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevonen L, Clinton D, Norring C (2006) Validating the EDI-2 in three Swedish female samples: eating disorder patients psychiatric outpatients and normal controls. Nord J Psychiatry 60:44–50. [DOI] [PubMed] [Google Scholar]
- Norring C, Sohlberg S (1988) Eating Disorder Inventory in Sweden: description, cross-cultural comparison, and clinical utility. Acta Psychiatr Scand 78:567–575. [DOI] [PubMed] [Google Scholar]
- Olivardia R, Pope H, Mangweth B, Hudson J (1995) Eating disorders in college men. Am J Psychiatry 152:1279–1285. [DOI] [PubMed] [Google Scholar]
- Root TL, Pisetsky EM, Thornton L, Lichtenstein P, Pedersen NL, Bulik CM (2010). Patterns of comorbidity of eating disorders and substance use in a large population-based sample of Swedish females. Psychol Med 40:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slane JD, Burt SA, Klump KL (2012) Bulimic behaviors and alcohol use: shared genetic influences. Behav Genet 42:603–613. [DOI] [PubMed] [Google Scholar]
- Slane JD, Klump KL, McGue M, Iacono WG (2014) Developmental trajectories of disordered eating from early adolescence to young adulthood: a longitudinal study. Int J Eat Disord 47:793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Cyders (2016) Integrating affect and impulsivity: The role of positive and negative urgency in substance use risk. Drug Alcohol Depend 163:S3–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane NS, Boerner LM, Anderson KG, & Smith GT (2004) Comparability of the Eating Disorder Inventory-2 between women and men. Assessment 11:85–93. [DOI] [PubMed] [Google Scholar]
- Stice E (2002) Risk and maintenance factors for eating pathology: a meta-analytic review. Psychol Bull 128:825–848. [DOI] [PubMed] [Google Scholar]
- Stice E, Burton EM, Shaw H (2004) Prospective relations between bulimic pathology, depression, and substance abuse: unpacking comorbidity in adolescent girls. J Consult Clin Psychol 72:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Whitenton K (2002) Risk factors for body dissatisfaction in adolescent girls: A longitudinal investigation. Developmental Psychol 38:669–678. [DOI] [PubMed] [Google Scholar]
- Stickley A, Koyanagi A, Koposov R, McKee M, Murphy A, Ruchkin V (2015) Binge drinking and eating problems in Russian adolescents. Alcohol Clin Exp Res 39:540–547. [DOI] [PubMed] [Google Scholar]
- Teesson M, Hall W, Slade T, Mills K, Grove R, Mewton L, Baillie A, Haber P (2010) Prevalence and correlates of DSM‐IV alcohol abuse and dependence in Australia: findings of the 2007 National Survey of Mental Health and Wellbeing. Addiction 105:2085–2094. [DOI] [PubMed] [Google Scholar]
- Tully EC, Iacono WG, McGue M. (2008) An adoption study of parental depression as an environmental liability for adolescent depression and childhood disruptive disorders. Am J Psychiatry 165:1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ranson K, Iacono W, McGue M (2002) Disordered eating and substance use in an epidemiological sample: I. Associations within individuals. Int J Eat Disord 31:389–403. [DOI] [PubMed] [Google Scholar]


