Abstract
Objective:
Multiple sclerosis (MS) is less prevalent in African Americans (AAs) than Caucasians (CAs) but in the former the disease course tends to be more severe. In order to clarify the MRI correlates of disease severity in AAs, we performed a multimodal brain MRI study to comprehensively assess the extent of grey matter (GM) damage and the degree of functional adaptation to structural damage in AAs with MS.
Methods:
In this cross-sectional study, we characterized GM damage in terms of focal lesions and volume loss and functional adaptation during the execution of a simple motor task on a sample of 20 AAs and 20 CAs with MS and 20 healthy controls (CTRLs).
Results:
In AAs, we observed a wider range of EDSS scores than CAs, with multisystem involvement being more likely in AAs (p<0.01). While no significant differences were detected in lesion loads and global brain volumes, AAs showed regional atrophy in the posterior lobules of cerebellum, temporo-occipital and frontal regions in comparison with CAs (p<0.01), with cerebellar atrophy being the best metric in differentiating AAs from CAs (p=0.007, AUC=0.96 and p=0.005, AUC=0.96, respectively for right and left cerebellar clusters). In AAs, the functional analysis of cortical activations showed an increase in task-related activation of areas involved in high level processing and a decreased activation in the medial prefrontal cortex compared to CAs.
Interpretation:
In our study, the direct comparison of AAs and CAs points to cerebellar atrophy as the main differences between subgroups.
Keywords: Grey matter, African Americans, Multiple Sclerosis
Introduction
Multiple sclerosis (MS) is less prevalent in African Americans (AAs) than in Caucasians (CAs) (case/control ratio 0.45 vs 1.04, respectively)1 but the disease course tends to be more severe in the former, likely due to the concurrence of both genetic susceptibility and exposure to environmental risk factors2–4. Although socioeconomics may play a role in clinical outcomes, the disability difference between AAs and CAs also exists when the comparison is controlled for education, income, and insurance status5. Clinically, AAs not only present a lower age at onset6, a preponderance of optico-spinal MS and an increased occurrence of multifocal signs and symptoms4, but also a greater risk for secondary progression7 and poorer responses to disease-modifying therapies8. Retrospective MRI studies have suggested that the more severe course is associated with the higher white matter (WM) lesion load and diffuse microstructural damage in normal appearing WM, rather than greater whole brain and/or grey matter (GM) atrophy9–11. However, while all previous studies have been consistent in reporting a higher number of WM lesions in AAs compared to CAs, GM lesions have never been investigated and the findings related to GM atrophy are controversial ranging from no difference to significant decrease in GM global volume and global cortical thickness in AAs and compared to their CAs counterparts 10–12. To date, a comprehensive analysis of GM involvement in AAs is still lacking, despite the key role of GM focal and diffuse damage in contributing to clinical disability and in driving the extent and pace of MS progression13–19. Therefore, here we present the results of a multimodal brain MRI study to comprehensively assess the presence and extent of global and regional GM damage in AAs with MS. We hypothesized that greater focal and diffuse damage of clinically relevant cortical and subcortical GM regions may account for the difference in disease severity between AAs and CAs. Additionally, since greater disability in AAs may be the consequence of poor tissue repair capability and/or exhaustion of adaptive compensatory mechanisms, we investigated the functional correlates of a simple motor task in AAs versus CAs in order to explore possible differences in functional adaptation between the two racial subgroups.
Methods
Study population
Forty patients, with a diagnosis of clinically definite MS according to the International Panel diagnostic criteria20 and a relapsing-remitting (RR) course21 were prospectively enrolled. Patients underwent clinical evaluation including the Expanded Disability Status Scale (EDSS)22 and the 9-Hole Peg Test (9-HPT)23. The presence of multisystem involvement, defined as moderate disability in multiple functional systems (EDSS ≥ 4), was evaluated for each patient. To be included in the study, patients had to be of AA or CA ancestry, right handed, and relapse- and corticosteroid treatment-free for at least 3 months preceding the MRI examination. Ethnicity was self-identified by the patient at the time of clinic registration. Exclusion criteria consisted of pre-existing medical conditions of depression, drug or alcohol abuse and brain pathology other than MS. Twenty age- and gender-matched right-handed healthy volunteers of CA ancestry served as healthy controls (CTRLs). Approval for the study was obtained from the Institutional Review Board of the Mount Sinai School of Medicine, and all participants provided informed written consent.
Image acquisition
All subjects underwent a brain MRI on a 3T scanner (Philips Achieva, The Netherlands) with an 8-channel SENSE phased-array head coil. MRI acquisition included: (a) dual echo (DE) turbo spin echo (TSE) sequence (TR/TE1/TE2: 2500/10/80 ms; 50 contiguous axial slices; voxel size: 1×1×3 mm3); (b) 3D T1 fast field echo (FFE) sequence (TR/TE/TI: 7.5/3.4/900 ms; 162 sagittal slices; voxel size: 1×1×1 mm3); (c) DIR sequence (TR/TE/TI: 1100/25/3400 ms; 50 contiguous axial slices; voxel size 1×1×3 mm3).
Finally, in a subsample of 34 subjects (10 CTRLs, 12 AAs, 12 CAs), the following EPI sequence was utilized for the fMRI analysis: TR/TE: 3000/30 ms; number of volumes: 80; 36 contiguous axial slices; voxel size: 2.6×2.6×3.7 mm3.
fMRI Task design
The fMRI experiment used a block design (ABAB), where four epochs of activation were alternated with four epochs of rest (30 sec/epoch). All subjects were instructed to perform a simple motor task consisting of repetitive, calibrated and conjugated flexion-extension of the last four fingers of the right hand. In order to guide movement amplitude, a hard ball was placed in the palm of the hand and held in place with the subject’s thumb and adhesive tape. Patients and controls were trained before performing the experiments and monitored visually during scanning to ensure accurate task performance.
Image Analysis
Lesion count and volumes
T2-hyperintense, T1-hypointense lesion volume (LV), and cortical lesion (CL) volume were measured on the DE T2-weighted, T1-weighted and DIR images, respectively, using a semi-automated segmentation technique based on user-supervised local thresholding (Jim version 5; Xinapse Systems, Northants, England) (http://www.xinapse.com). All lesions were identified in consensus by two experienced observers (MI with more than 10 years and SC with 5 years of experience in brain imaging). In order to further improve accuracy of CLs identification, these lesions were identified according to published guidelines24. T1-hypointense lesions were identified as areas of focal hypointensity compared to the surrounding normal-appearing white matter, visible on the T1-weighted sequence and corresponding to a region of high signal intensity on the T2-weighted images.
WM and GM lesion probability maps
For each patient subgroup (AAs and CAs), WM and GM lesion probability maps (LPMs) were obtained using the FMRIB Software Library - FSL v5 (www.fmrib.ox.ac.uk/fsl/), as previously described25. In the resulting LPM, the intensity of each voxel represents the probability of that voxel to be a lesion in that patients’ subgroup.
Global and regional brain volume measures
All volumetric analyses were conducted on high-resolution 3D FFE T1-weighed images after correcting for the impact of WM lesions (lesions in-painting, LI)26. Calculation of global brain volumes was computed in FSL’s SIENAX, obtaining, for each subject, normalized brain volume (NBV), GM volume (GMV) and WM volume (WMV)27. Voxel-based morphometry (VBM) analysis was performed via SPM 12. The segmented GM images were normalized using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) algorithm28. The normalized segmented GM maps (2×2×2 mm3 voxel size) provided by DARTEL were then modulated by the Jacobian determinants (derived from the spatial normalization procedure) in order to preserve the local GM volumes. Images were smoothed using a 8mm FWHM isotropic Gaussian kernel.
fMRI Data Analysis
Preprocessing of functional data was conducted as described in Saiote, et al.29. Briefly, to model the on-off periods of the right hand motor task activity, one explanatory variable (EV) was defined and convolved with the hemodynamic response function (HRF) while the 24 motion parameters calculated during motion correction were added as confound EVs. Mean CSF and WM signals were added to the general linear model (GLM) as covariates of no interest. Boundary-based registration (BBR)30 was used to register each individuals’ functional data to their corresponding T1-weighted brain images followed by a linear affine 12 degrees of freedom registration of each individuals’ T1-weighted brain image to standard MNI space (MNI152 brain template, voxel size: 2mm)31.
Statistical Analysis
Between groups comparisons in terms of clinical and MRI variables, as well as correlation and classification accuracy analyses, were carried out using Statistical Package for Social Science (SPSS Inc, v. 19.0, Chicago, Ill).
Demographic, clinical and volumetric statistical analyses
A Chi-square test was used to compare groups in terms of gender and treatment type. Nonparametric Mann-Whitney and Kruskal-Wallis tests were used for between-group comparisons of demographic (age) and clinical (EDSS, disease duration, treatment exposure) parameters, as appropriate. A binary logistic regression was used to compare AAs and CAs with respect to the presence of multisystem involvement. For this analysis, the presence of multisystem involvement was the dependent variable and the logistic model included patient subgroups (AAs or CAs) with age, gender and disease duration as covariates. Analysis of variance (ANOVA) was used for between-group comparison of MRI measures (T2LV, T1LV, CLs count and volume, NBV, GMV, WMV) correcting for age and gender.
Voxelwise statistical analyses
The probability of lesion occurrence in AAs and CAs was compared with a voxel-wise nonparametric statistic as implemented in FSL’s randomise, entering age, gender and T2LV as covariates of no interest (5000 permutations; p<0.05, Threshold-Free Cluster Enhancement-TCFE corrected for multiple comparisons).
For the VBM analysis, the normalized modulated GM maps were compared via GLM based on Gaussian random field theory32. The design matrix included age, gender and ICV as nuisance variables. Results were considered significant for p<0.01, FWE-corrected at cluster level (0.05/3, as three different contrasts were probed). Where significant differences emerged, signal intensity values were extracted for correlation purposes.
Finally, fMRI group level analyses were completed using the mixed effects model FSL FLAME. One sample t-tests were used to model group mean activation and two-sample t-tests were used to model comparison between subgroups. The results were converted to Z-values and then thresholded at Z=2.3, with significance level set at p<0.05 and FWE corrected for multiple comparisons at cluster level.
Anatomical labeling of GM regions was derived from the Talairach atlas33.
Correlation analyses and classification accuracy
Correlations between MRI metrics that differed between AAs and CAs and clinical disability were probed using a partial correlation analysis, with age, gender and disease duration as covariates of no interest. Logistic regression models were computed to assess the utility of each MR measure for the differentiation of CAs from AAs after adjusting for age, gender and disease duration. Mean classification accuracy was calculated to show the predictive power of the MR measures during the classification process by measuring area under the curve (AUC) of the free-response receiver operating characteristic (ROC) analysis.
All reported p values are two-sided with statistical significance defined as p<0.05. Given the exploratory nature of the study, no correction for multiple comparisons was performed, with the exception of the voxelwise statistical analyses. Therefore, results have to be considered as descriptive.
Results
Demographic and clinical characteristics of the study population are shown in Table 1. The comparison of the three groups (CTRLs, AAs, CAs) in terms of demographics showed a trend of difference for gender (p=0.048) but not for age (p=0.841). AAs and CAs were not significantly different in terms of age, gender, age at disease onset, disease duration, EDSS, treatment type or treatment exposure at the time of MRI (p>0.05 for all). In both subgroups, 90% of the subjects were on disease-modifying therapies, with the following distribution: 61% of AAs and CAs on interferon β-1a treatment (33% on Avonex®, 28% on Rebif® in both subgroups), 11% of AAs and 17% of CAs on glatiramer acetate, 11% of AAs and 22% of CAs on natalizumab and 17% of AAs on rituximab. Although not significantly different, AAs presented a wider range of EDSS scores, a higher percentage of subjects with moderate involvement of multiple functional systems (30% of AAs with EDSS ≥ 4 vs 0% of CAs with EDSS ≥ 4) and a higher 9-HPT score mean value compared to CAs (Table 1). Multisystem involvement was significantly predicted by race, considering age, gender and disease duration as covariates (p<0.01).
Table 1.
Demographic and clinical characteristics of the study population.
| AAs | CAs | CTRLs | p-value* | p-value§ | |
|---|---|---|---|---|---|
| M/F (n) | 5/15 | 6/14 | 8/12 | 0.723a | 0.048 a |
| Age at onset [yrs] | 28.6 ± 7.3 | 28.4 ± 6.4 | - | 0.841b | - |
| Age at MRI [yrs] | 34.6 ± 10.4 | 33.8 ± 6.3 | 33.4±10.4 | 0.820b | 0.841 c |
| Disease duration [yrs] | 6.0 ± 4.3 | 5.3 ± 4.4 | - | 0.620b | - |
| EDSS score (median and range) | 1.5 (1.0-6.0) | 1.7 (1.0-3.5) | - | 0.640b | - |
| 9-HPT score [sec] | 25.0 ± 7.2 | 21.6 ± 4.5 | - | 0.157b | - |
| Patient under treatment (n) | 18/20 | 18/20 | - | 1a | - |
| Treatment exposure [yrs] | 3.0 ± 3.3 | 4.6 ± 4.5 | - | 0.277b | - |
Abbreviations: AAs=African Americans; CAs=Caucasians; CTRLs=controls; EDSS= Expanded Disability Status Score; F=female; M=male; n=number; yrs=years; 9-HPT=9-hole peg test. Unless otherwise specified, all values are expressed as mean ± standard deviation.
Chi-square test.
Mann-Whitney test.
Kruskal-Wallis test.
AAs vs CAs.
AA vs CAs vs CTRLs.
Lesion count and volumes
There were no WM lesions detected on T2- and T1-weighted images in CTRLs. Although AAs had a greater mean T2LV, T1LV, CLs volume and count compared to CAs, the difference did not reach the statistical significance (Table 2).
Table 2.
Lesion and brain volume measures in patients and CTRLs.
| AAs | CAs | CTRLs | pa | pb | pc | |
|---|---|---|---|---|---|---|
| T2 LV (mL) | 3.60 ± 3.91 | 3.03 ± 2.44 | - | - | - | 0.508 |
| T1 LV (mL) | 0.57 ± 0.43 | 0.52 ± 0.62 | - | - | - | 0.743 |
| CLs (n) | 8.79 ± 6.53 | 6.60 ± 4.68 | - | - | - | 0.149 |
| CL-V (mL) | 0.33 ± 0.28 | 0.22 ± 0.18 | - | - | 0.072 | |
| NBV (mL) | 1511.37 ± 110.49 | 1485.35 ± 75.52 | 1499.14 ± 84.00 | 0.977 | 0.591 | 0.357 |
| NWMV (mL) | 706.06 ± 56.94 | 693.14 ± 33.70 | 703.09 ± 52.67 | 0.961 | 0.497 | 0.399 |
| NGMV (mL) | 805.31 ± 56.05 | 792.21 ± 41.37 | 796.04 ± 44.06 | 0.910 | 0.796 | 0.354 |
Abbreviations: AAs=African Americans; CA=Caucasians; CL=cortical lesions; CL-V=cortical lesions volume; LV=lesion volume; NBV=normalized brain volume; NWMV=normalized white matter volume; NGMV=normalized grey matter volume.
ANOVA age and gender corrected (AAs vs CTRLs; p<0.05).
ANOVA age and gender corrected (CAs vs CTRLs; p<0.05).
ANOVA age and gender corrected (AAs vs CAs; p<0.05).
All the values are expressed as mean ± SD.
WM and GM LPMs
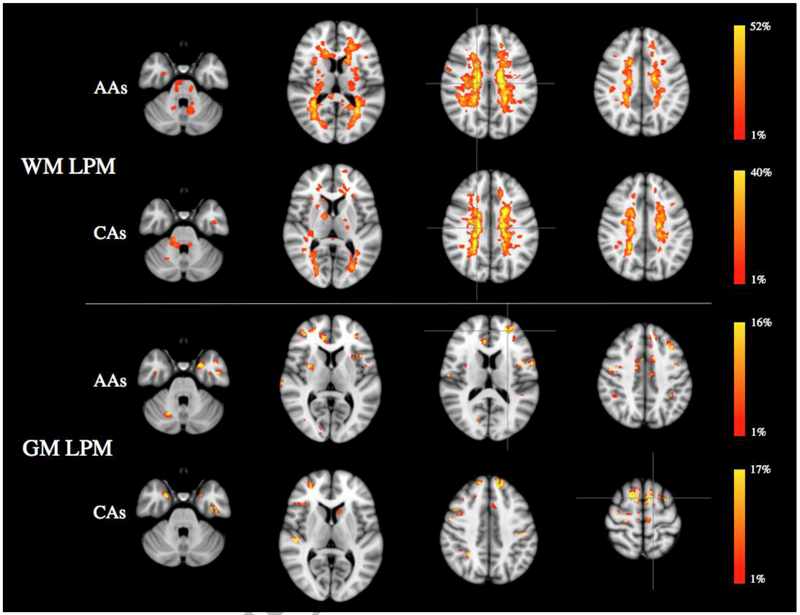
The distributions of T2 lesions and CLs were similar in AAs and CAs (Figure 1), with no significant difference in regional WM and GM lesion frequency between the two subgroups of patients. The peak of WM lesion frequency was in the right periventricular WM for both racial subgroups, with AAs showing higher probabilities than CAs (52.3% vs 40%). The peak of GM lesion frequency was in the left superior frontal gyrus (15.8%) in AAs and in the left middle frontal gyrus (16.7%) in CAs.
Figure 1.
White matter and grey matter lesion probability maps in African American and Caucasian MS patients, overlaid on the MNI standard brain template, showing the probability of each voxel to contain a lesion in each racial subgroup (p<0.05, TCFE corrected for multiple comparisons). Peaks of WM and GM lesion frequency are identified by crosshairs. The color scale denotes the probability range. Abbreviations: AAs=African-American; CAs=Caucasians; WM=white matter; GM=grey matter; LPM=lesion probability map.
Global normalized brain volume measures
Compared to CTRLs, AAs and CAs showed no differences in terms of global brain volumes (p>0.05). Similarly, no differences in NBV, GMV or WMV were detected when comparing the two racial subgroups (p>0.05).
Mean values of normalized brain volumes and results of the between group comparisons are reported in Table 2.
Voxel-based GM volume measure
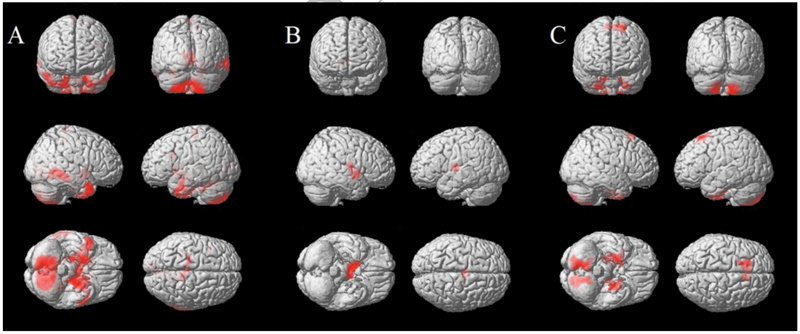
Compared to CTRLs, AAs showed lower GM volumes in the uncus, cerebellum, inferior temporal gyrus and paracentral lobule (all with p≤0.001), whereas CAs showed lower GM volume in the thalamus (p=0.003) (Figure 2A and B). When the two racial subgroups were compared, AAs showed lower GM volumes in the cerebellum, uncus, supplementary motor area, and visual associative cortex compared to CAs (lingual gyrus p=0.004, all others p<0.001) (Figure 2C). There were no areas of relative GM atrophy detected in CAs compared to AAs.
Figure 2.
Results of the Voxel Based Morphometry analysis showing significant differences between groups. Areas of relative GM reduction in African American MS patients compared to controls (A), Caucasian MS patients compared to controls (B) and African American compared to Caucasian patients (C) are showed in red, overlaid on the 3D brain surface rendering of a single normal subject provided in SPM12 (p<0.01, FWE-corrected at cluster level).
A complete list of the results of the VBM analysis is reported in Table 3.
Table 3.
Regions showing significant grey matter loss in MS patients (p<0.01, FWE-corrected at cluster level).
| Comparison | Regions | Side | BA | MNI coordinates (x,y,z) | T value | Cluster size | p-value |
|---|---|---|---|---|---|---|---|
| AAs<CTRLs | Uncus | L | 6 | 26, −8, −50 | 6.32 | 16403 | < 0.001 |
| CBL | R | - | 16, −58, −58 | 5.99 | 5591 | < 0.001 | |
| CBL | L | - | −32, −54, −22 | 5.37 | 1019 | 0.001 | |
| ITG | R | 37 | 48, −54, −4 | 4.91 | 1193 | < 0.001 | |
| PCL | R | 3 | 14, −34, 60 | 4.67 | 1130 | < 0.001 | |
| CAs<CTRLs | Thalamus | R | - | 8, −8, 12 | 4.95 | 730 | 0.003 |
| AAs<CAs | CBL | R | - | 12, −74, −50 | 6.37 | 1649 | < 0.001 |
| Uncus/FG | R | 38 | 24, −8, −46 | 6.30 | 1400 | < 0.001 | |
| Uncus/FG | L | 38 | −24, −10, −48 | 5.82 | 1263 | < 0.001 | |
| SFG | L | 6 | −20, 22, 60 | 5.74 | 1065 | < 0.001 | |
| CBL | L | - | −12, −66, −60 | 5.68 | 1397 | < 0.001 | |
| LG | L | 18 | 0, −68, 6 | 4.95 | 720 | 0.004 |
Abbreviations: AAs=African Americans; BA=Brodmann area; CAs=Caucasians; CBL=cerebellum; CTRLs=controls; FG=fusiform gyrus; ITG=inferior temporal gyrus; L=left; LG=lingual gyrus; PCL=paracentral lobule; R=right; SFG=superior frontal gyrus.
Task-Associated fMRI Activations: Between-Group Comparisons
There were no differences in age or gender between the three groups (CTRLs, AAs, CAs) included in the fMRI analysis (p=0.870 and p=0.648, respectively), with the two patient subgroups (AAs and CAs) also showing no differences in terms of age at onset (p=0.713), disease duration (p=0.887), EDSS (p=0.478), and treatment exposure (p=0.514).
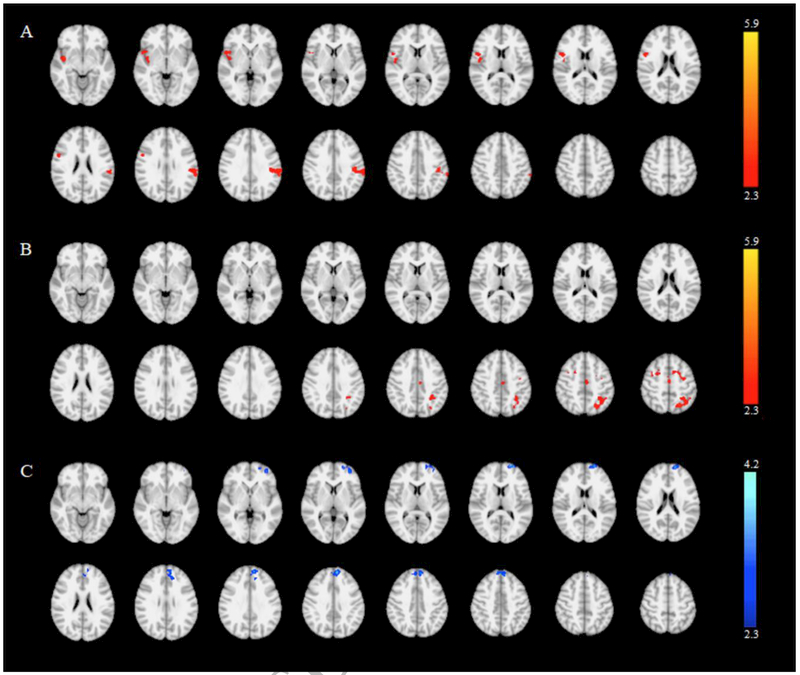
Compared to CTRLs, AAs showed an increased activation of the contralateral parietal cortex as well as ipsilateral insular cortex (Figure 3A), while CAs showed an increased activation of the contralateral premotor cortex and precuneus (Figure 3B). Finally, when comparing CAs and AAs, the latter group showed reduced activation of the contralateral medial frontal gyrus (Figure 3C). Results of the between group comparisons are listed in Table 4.
Figure 3.
Relative activations during the performance of the motor task in African-American patients vs. controls (A), Caucasian patients vs. controls (B) and African-American vs. Caucasian patients (C). Clusters showing a significant difference in activation are shown overlaid on the MNI standard brain template (p<0.05, cluster corrected for multiple comparisons). The color scale represents the Z value.
Table 4.
Between-group comparison of cortical activation maps (p<0.05, cluster corrected for multiple comparisons).
| Comparison | Regions | Side | BA | MNI coordinates (x,y,z) | Cluster Size | Max z |
|---|---|---|---|---|---|---|
| AAs > CTRLs | IPL | L | 40 | −64, −34, 34 | 525 | 3.56 |
| Insula | R | 13 | 42, −2, −8 | 516 | 3.13 | |
| CAs > CTRLs | SFG | L | 6 | −24, −2, 68 | 1515 | 3.27 |
| PC | L | 7 | −22, −64, 54 | 722 | 3.41 | |
| AAs < CAs | MedFG | L | 9 | −8, 52, 34 | 799 | −4.22 |
Anatomical coordinates in the standard MNI space, maximum z-values and cluster size for clusters showing significantly different activation in patient subgroups vs CTRLs and in AAs vs CAs (two-sample t-test, FEW-corrected for multiple comparisons at cluster level, p<0.05). Abbreviations: AAs=African-Americans, BA=Brodmann area, CAs=Caucasians, CTRLs=controls, IPL=inferior parietal lobule, L=left, MedFG=medial frontal gyrus, PC=precuneus, , R=right, SFG=superior frontal gyrus.
Correlations between MRI parameters and clinical measures
In patients, significant correlations were identified between EDSS and clusters of atrophy in the right and left cerebellum (r=−0.356, p=0.030 and r=−0.331, p=0.046, respectively), left uncus (r=−0.340, p=0.039) and left secondary visual cortex (r=−0.361, p=0.028). Significant correlations were also identified between 9-HPT and clusters of atrophy in right and left cerebellum (r=−0.415, p=0.011 and r=−0.378, p=0.021, respectively) and left secondary visual cortex (r=−0.405, p=0.013).
Classification accuracy analysis
Among the MRI metrics that differed between racial subgroups, different indices of regional atrophy were able to differentiate AAs from CAs patients (supplementary motor area atrophy p=0.004, AUC=0.93; left uncus atrophy p=0.003, AUC=0.92; right uncus atrophy p=0.003, AUC=0.93; visual association cortex atrophy p=0.008, AUC=0.92), with the best predictors being the clusters of cerebellar atrophy (p=0.007, AUC=0.96 and p=0.005, AUC=0.96, respectively for right and left cerebellar clusters).
Discussion
Our study confirms and expands the current knowledge about GM involvement in MS patients of AA ancestry, providing a comprehensive characterization of GM in terms of structural damage and functional reorganization.
Structural damage-GM and WM lesion loads
Regarding focal damage, the highest tendency of lesion accrual in MS patients of AA ancestry appears not limited, as previously reported, to the WM tissue9,10 but seems also to affect the cortex in terms of CLs. Nonetheless, the higher CL prevalence we report in our AA population has to be interpreted with caution, considering that the between-group difference in terms of CL only shows a significance trend and that the DIR sequence utilized for CL identification does not represent the gold standard in terms of sensitivity for CL classification34.
Additionally, we identified a higher WM lesion load and higher lesion frequency in WM tracts in AAs compared to CAs, but this difference, in contrast with previous reports9–12, did not reach the statistical significance. A possible explanation of this discrepancy could be researched in the lower disease duration and mean age at MRI of our population compared to those previously analysed9–12. Furthermore, it should be noted that other characteristics of our population, such as immunomodulatory therapy, could also have influenced our findings. Indeed, although the two racial subgroups did not differ in terms of treatment exposure, a higher percentage of AAs were on treatment with anti-inflammatory monoclonal antibodies compared to CAs (38% vs 22%).
Structural damage-GM atrophy
After exploring the presence and distribution of focal damage, we evaluated GM in terms of atrophy through the estimation of global brain volumes and voxel-wise analysis. No differences were identified between MS patients and healthy controls in terms of global brain volumes and, although this finding might be surprising, the lack of significance could be related to the small sample size and the relative lack of sensitivity of a global measure, as mean values for each group show agreement with the literature, with AAs presenting larger brain volumes than CAs.9,10
The two racial subgroups (AAs and CAs) showed different clusters of regional GM atrophy when compared to healthy controls. Although the lack of an AA control group might limit the interpretation of these findings, as the observed difference in volumes could be driven by race rather than being MS-specific, the few data available in the literature suggest that race-specific differences in brain volume would justify the presence of larger, rather than smaller global and regional volumes in African-Americans in comparisons with Caucasians 35,36
While no differences in global brain volumes were detected in AAs in comparison with CAs, the voxel-wise analysis revealed the presence of significant cerebellar atrophy in AAs compared to CAs, mainly involving the posterior lobules. Additional areas of GM loss were disclosed in the temporo-occipital and frontal regions. In line with previous findings9,10, global brain volumes did not differ between racial subgroups but, increasing the sensitivity of the analysis through the exploration of regional volumetry, we found clusters of GM loss in motor areas (i.e., supplementary motor area and cerebellum), parahippocampal regions and occipital lobe in AAs. The presence of GM volume loss in these specific areas in AAs in comparison with CA MS patients could provide the background for the more severe impairment in learning and memory function as well as the gait difficulties reported in AAs5,9,37. Our results about regional atrophy only partially overlap with the findings of a recent study reporting cortical thickness differences between AAs and CAs11. This lack of complete concordance could be related to methodological differences in GM atrophy estimation, (i.e., the lower sensitivity of the cortical thickness estimation method in detecting subtle atrophy, especially in medial cortical areas, limbic and temporal regions, compared to voxel-based analysis38). Furthermore, the lack of complete concordance could also be related to the population characteristics, as the patients included in our study were younger, with shorter disease duration and with lower EDSS scores. Finally, it is possible to speculate that the pattern of GM atrophy described here could represent the initial stage of the widespread involvement reported in more advanced phases of the disease11.
Indeed, the concurrent damage of different brain areas could represent the anatomical substrate of the multisystemic involvement that is one of the major characteristics of the disease course in AAs4. In our population, although AAs and CAs did not differ in terms of individual measures of clinical disability (EDSS, 9-HPT), AAs showed a higher frequency of multisystem involvement. The direct comparison of the two racial subgroups highlights the presence of more severe GM damage in AAs with regional GM atrophy emerging as the main differences between AAs and CAs patients. When exploring the ability of different MR metrics to differentiate the AA from CA patients, cerebellar atrophy emerged as the best predictor. Cerebellar atrophy has recently been reported as a sensitive and clinically meaningful outcome measure in progressive MS39,40. Similarly, cerebellar damage could represent a crucial factor for disability expression in AAs.
Functional adaptation
Previous studies have demonstrated how structural damage in MS impacts functional activity and how hyper-activation of the sensorimotor network might occur as an attempt to maintain motor ability41,42. In our MS population, the functional analysis of cortical activations, although limited in power by the small size of our sample, showed, as expected, an increase in task-related activation in key regions of the sensorimotor network when compared to CTRLs. However, while CAs presented an increase in activation in the premotor area and associative cortex responsible for visuo-spatial imagery43, AAs showed an increased activity in areas involved in higher level processing, such as tactile object recognition and integration of somatosensory information44,45. Furthermore, when a direct comparison between the two racial subgroups was performed, AAs showed a decreased activation in the medial prefrontal cortex compared to CAs, an area known to play a role in the selection and inhibitory control of hand movements46. Similar to what has been reported in progressive MS patients41,47, the pattern of increased activity observed in AAs suggests that the recruitment of multimodal integration regions during the execution of a simple motor task represents a consequence of the prominent structural damage observed in this population, while the reduced activation in the prefrontal cortex might indicate a decrease in the compensatory ability of AAs compared to CAs.
Study limitations
When testing the impact of structural damage on clinical outcomes, we found moderate associations between infra-tentorial and temporo-occipital GM damage and clinical disability. The lack of stronger correlations, although disappointing, could be partially explained by the observation that regions of GM damage accrual were mainly located in non-motor areas, while the available clinical scores were reflecting motor capabilities. For this reason, the lack of neuropsychological examination, together with the relatively small sample size, has to be considered as one of the main limitations of this study. Other limitations have to be taken into account, such as the lack of spinal cord damage quantification, the lack of data regarding participants’ socioeconomic status and the absence of an AA control group. The latter, in particular, might explain the higher, although not significant, mean values for global brain volumes observed in AAs in comparison with controls. Notwithstanding all the acknowledged limitations, the present study is the first attempt to fully characterize the extent and regional distribution of GM damage in AAs. Further studies on a broader population are needed to fully elucidate the possible causes of the severe clinical presentation and progression of MS in AAs, but our exploratory findings provide the basis for future investigation on the pathological substrates of inter-racial difference in MS.
Acknowledgements
This study was supported in part by the National Multiple Sclerosis Society (NMSS RG 5120A3/1) to MI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest
Nothing to report.
References
- 1.Kurtzke JF, GW Beebe, Norman JE Jr.. Epidemiology of multiple sclerosis m U . S . veterans : I. Race, sex, and geographic distribution. Neurology 1979;29(53): 1228–1235. [DOI] [PubMed] [Google Scholar]
- 2.Johnson BA, Wang J, Taylor EM, et al. Multiple sclerosis susceptibility alleles in African Americans. [Internet]. Genes Immun 2010; 11(4):343–50.Available from: 10.1038/gene.2009.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isobe N, Gourraud P-A, Harbo HF, et al. Genetic risk variants in African Americans with multiple sclerosis. [Internet]. Neurology 2013;81(3):219–27.Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3770164&tool=pmcentrez&rend ertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cree B a C, Khan O, Bourdette D, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology 2004;63(11):2039–2045. [DOI] [PubMed] [Google Scholar]
- 5.Marrie RA, Cutter G, Tyry T, et al. Does multiple sclerosis-associated disability differ between races? Neurology 2006;66(8): 1235–1240. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan RJ, Martin R a, Zuniga M, et al. Nursing home residents with multiple sclerosis: comparisons of African American residents to white residents at admission. Mult. Scler 2004;10(6):660–667. [DOI] [PubMed] [Google Scholar]
- 7.Kister I, Chamot E, Bacon JH, et al. Rapid disease course in African Americans with multiple sclerosis. Neurology 2010;75(3):217–223. [DOI] [PubMed] [Google Scholar]
- 8.Cree BAC, Reich DE, Khan O, et al. Modification of Multiple Sclerosis Phenotypes by African Ancestry at HLA. Arch. Neurol 2009;66(2):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstock-Guttman B, Ramanathan M, Hashmi K, et al. Increased tissue damage and lesion volumes in African Americans with multiple sclerosis. Neurology 2010;74(7):538–544. [DOI] [PubMed] [Google Scholar]
- 10.Howard J, Battaglini M, Babb JS, et al. MRI correlates of disability in African-Americans with multiple sclerosis. PLoS One 2012;7(8):8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Kawaz M, Monohan E, Morris E, et al. Differential Impact of Multiple Sclerosis on Cortical and Deep Gray Matter Structures in African Americans and Caucasian Americans [Internet]. J. Neuroimaging 2016;1–6.Available from: http://doi.wiley.com/10.1111/jon.12393 [DOI] [PubMed] [Google Scholar]
- 12.Seraji-Bozorgzad N, Khan O, Cree BAC, et al. Cerebral Gray Matter Atrophy Is Associated with the CSF IgG index in African American with Multiple Sclerosis [Internet]. J. Neuroimaging 2017; 1–5.Available from: http://doi.wiley.com/10.1111/jon.12435 [DOI] [PubMed] [Google Scholar]
- 13.Dalton CM, Chard DT, Davies GR, et al. Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain 2004; 127(5): 1101–1107. [DOI] [PubMed] [Google Scholar]
- 14.Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: A longitudinal study. Ann. Neurol 2008;64(3):255–265. [DOI] [PubMed] [Google Scholar]
- 15.Calabrese M, De Stefano N, Atzori M, et al. Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis [Internet]. Arch. Neurol 2007;64(10): 1416–1422.Available from: 10.1001/archneur.64.10.1416%5Cnhttp://archneur.jamanetwork.com/data/Journals/NEUR/7442/noc70051_1416_1422.pdf [DOI] [PubMed] [Google Scholar]
- 16.Calabrese M Cortical Lesions and Atrophy Associated With Cognitive Impairment in Relapsing-Remitting Multiple Sclerosis. 2009;66(9): 1144–1150. [DOI] [PubMed] [Google Scholar]
- 17.Calabrese M, Rinald F, Mattisi I, et al. The predictive value of gray matter atrophy in clinically isolated syndromes. Neurology 2011;77:257–263. [DOI] [PubMed] [Google Scholar]
- 18.Benedict RHB, Bruce JM, Dwyer MG, et al. Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch. Neurol 2006;63(9): 1301–1306. [DOI] [PubMed] [Google Scholar]
- 19.Moraal B, Pouwels PJ, Vrenken H, et al. Accumulation of cortical lesions in MS: relation with cognitive impairment. [Internet]. Mult. Scler 2009;15(6):708–714.Available from: http://msj.sagepub.com/cgi/doi/10.1177/1352458509102907%5Cnpapers3://publication/doi/10.1177/1352458509102907 [DOI] [PubMed] [Google Scholar]
- 20.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol 2011;69(2):292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lublin FD, Reingold SC, Cohen J a, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. [Internet]. Neurology 2014;83:1–9.[cited 2014 Jul 14 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24871874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 23.Mathiowetz V, Weber K, Kashman N, Volland G. Adult Norms For The Nine Hole Peg Test Of Finger Dexterity. Occup. Ther. J. Res 1985;5(1):24–38. [DOI] [PubMed] [Google Scholar]
- 24.Geurts JJG, Roosendaal SD, Calabrese M, et al. Consensus recommendations for MS cortical lesion scoring using double inversion recovery MRI. Neurology 2011;76(5):418–24. [DOI] [PubMed] [Google Scholar]
- 25.Rossi F, Giorgio A, Battaglini M, et al. Relevance of brain lesion location to cognition in relapsing multiple sclerosis. [Internet]. PLoS One 2012;7(11):e44826[cited 2014 Sep 29 ] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3489883&tool=pmcentrez&rend ertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battaglini M, Jenkinson M, De Stefano N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum. Brain Mapp 2012;33(9):2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17:479–489. [DOI] [PubMed] [Google Scholar]
- 28.Ashburner J A fast diffeomorphic image registration algorithm. Neuroimage 2007;38(1):95–113. [DOI] [PubMed] [Google Scholar]
- 29.Saiote C, Tacchino A, Brichetto G, et al. Resting-state functional connectivity and motor imagery brain activation. Hum. Brain Mapp 2016;37(11):3847–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration [Internet]. Neuroimage 2009;48(1):63–72.Available from: 10.1016/j.neuroimage.2009.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17(2):825–841. [DOI] [PubMed] [Google Scholar]
- 32.Friston KJ, Holmes AP, Poline JB, et al. Analysis of fMRI time-series revisited. Neuroimage 1995;2(1):45–53. [DOI] [PubMed] [Google Scholar]
- 33.Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, et al. Bias between MNI and talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp 2007;28(11):1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sethi V, Yousry T a, Muhlert N, et al. Improved detection of cortical MS lesions with phase-sensitive inversion recovery MRI. [Internet]. J. Neurol. Neurosurg. Psychiatry 2012;83(9):877–82.[cited 2014 Oct 20 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22807559 [DOI] [PubMed] [Google Scholar]
- 35.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. [Internet]. Arch. Neurol 2008;65(8): 1053–61.Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2692286&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isamah N, Faison W, Payne ME, et al. Variability in frontotemporal brain structure: The importance of recruitment of African Americans in neuroscience research. PLoS One 2010;5(10):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naismith RT, Trinkaus K, Cross AH. Phenotype and prognosis in African-Americans with multiple sclerosis: a retrospective chart review. Mult. Scler 2006;12(6):775–781. [DOI] [PubMed] [Google Scholar]
- 38.Clerx L, Jacobs HIL, Burgmans S, et al. Sensitivity of different MRI-techniques to assess gray matter atrophy patterns in Alzheimer’s disease is region-specific. [Internet]. Curr. Alzheimer Res 2013; 10(9):940–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23947582 [DOI] [PubMed] [Google Scholar]
- 39.Inglese M, Petracca M, Mormina E, et al. Cerebellar volume as imaging outcome in progressive multiple sclerosis. PLoS One 2017; 12(4): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cocozza S, Petracca M, Mormina E, et al. CEREBELLAR LOBULE ATROPHY AND DISABILITY IN PROGRESSIVE MS. JNNP 2017; [DOI] [PubMed] [Google Scholar]
- 41.Rocca M a Matthews PM, Caputo D, et al. Evidence for widespread movement-associated functional MRI changes in patients with PPMS. [Internet]. Neurology 2002;58(6):866–872.Available from: http://www.ncbi.nlm.nih.gov/pubmed/11914400 [DOI] [PubMed] [Google Scholar]
- 42.Valsasina P, Rocca MA, Absinta M, et al. A multicentre study of motor functional connectivity changes in patients with multiple sclerosis. Eur. J. Neurosci 2011;33(7): 1256–1263. [DOI] [PubMed] [Google Scholar]
- 43.Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006;129(3):564–583. [DOI] [PubMed] [Google Scholar]
- 44.Milner TE, Franklin DW, Imamizu H, Kawato M. Central control of grasp: Manipulation of objects with complex and simple dynamics [Internet]. Neuroimage 2007;36(2):388–395.Available from: http://dx.doi.Org/10.1016/j.neuroimage.2007.01.057 [DOI] [PubMed] [Google Scholar]
- 45.Kurth F, Zilles K, Fox PT, et al. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct 2010; 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Jong BM, Paans AMJ. Medial versus lateral prefrontal dissociation in movement selection and inhibitory control. Brain Res 2007; 1132(1): 139–147. [DOI] [PubMed] [Google Scholar]
- 47.Ceccarelli A, Rocca M a, Valsasina P, et al. Structural and functional magnetic resonance imaging correlates of motor network dysfunction in primary progressive multiple sclerosis. [Internet]. Eur. J. Neurosci 2010;31(7): 1273–80.[cited 2014 Sep 2 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/20345920 [DOI] [PubMed] [Google Scholar]