Abstract
Background:
We studied the utility of the tumor suppressor Tristetraprolin (TTP, ZFP36) as a clinically relevant biomarker of aggressive disease in prostate cancer patients after radical prostatectomy (RP).
Methods:
TTP RNA expression was measured in an RP cohort of patients treated at Moffitt Cancer Center (MCC) and obtained from six publically available RP datasets with biochemical recurrence (BCR) (total n=1,394) and/or metastatic outcome data (total n=1,222). TTP protein expression was measured by immunohistochemistry in a tissue microarray of 153 MCC RP samples. The time to BCR or metastasis based on TTP RNA or protein levels was calculated using Kaplan-Meier analysis. Univariable and multivariable Cox proportional hazard models were performed on multiple cohorts to evaluate if TTP is a clinically relevant biomarker, and to assess if TTP improves upon the Cancer of the Prostate Risk Assessment postsurgical (CAPRA-S) score for predicting clinical outcomes.
Results:
In all of the RP patient cohorts, prostate cancer with low TTP RNA or protein levels had decreased time to BCR or metastasis versus TTP-High tumors. Further, the decreased time to BCR in TTP-Low prostate cancer was more pronounced in low-grade tumors. Finally, pooled survival analysis suggests that TTP RNA expression provides independent information beyond CAPRA-S to predict BCR.
Conclusions:
TTP is a promising prostate cancer biomarker for predicting which RP patients will have poor outcomes, especially for low-grade prostate cancer patients.
Impact:
This study suggests that TTP RNA expression can be used to enhance the accuracy of CAPRA-S to predict outcomes in patients treated with RP.
Keywords: prostate cancer, Tristetraprolin, TTP, biomarker
Introduction
While proving beneficial to many men with prostate cancer, testing for serum prostate-specific antigen (PSA) levels has resulted in an epidemic of prostate cancer overtreatment, causing hundreds of thousands of men with a low-risk of disease-specific death to have unnecessary harmful side effects (1–4). Indeed, potentially harmful side effects from overtreatment were an important factor in the United States Preventive Services Task Force’s (USPSTF) 2012 recommendation that healthy men should not be screened with PSA testing (5). However, a 2017 update issued by the USPSTF states that clinicians should discuss the potential benefits and harms of PSA testing with men between 55 and 69 years old (6). As an alternative to aggressively treating patients with low-risk prostate cancer, there is a growing emphasis towards utilizing active surveillance. While this is an appropriate alternative for many low-risk patients, ~30% of patients in long-term active surveillance studies were ultimately reclassified as harboring aggressive disease and required therapeutic intervention (7–9). Thus, there is a dire unmet need for improved prognostic prostate cancer biomarkers.
Tristetraprolin (TTP, ZFP36), an RNA-binding protein involved in controlling mRNA stability, impairs tumor growth and development in a variety of malignancies, including prostate cancer (10–19). In a previous study that established TTP’s functions as a tumor suppressor in prostate cancer, analysis of TTP RNA expression in a single prostate cancer patient dataset suggested that low TTP-expressing primary tumors may have increased rates of biochemical recurrence (BCR) compared to high TTP-expressing prostate cancer (19). To more fully assess whether TTP is a prognostic biomarker that identifies prostate cancer patients with an increased risk of poor clinical outcomes, we evaluated two prostate cancer patient cohorts from Moffitt Cancer Center (MCC) for TTP RNA or TTP protein expression, as well as six independent prostate cancer genomic datasets having post-surgical clinical follow-up from multiple medical institutions. Our findings establish that TTP alone is a clinically relevant prostate cancer biomarker that provides an improvement over clinical and pathologic variables to predict which patients may harbor aggressive disease.
Materials and Methods
Study Design and Patient Selection
This study analyzed TTP RNA or protein expression and associated clinical endpoint data (BCR and/or metastatic development) from eight studies (four cohort, two case-cohort, and two case-control) of RP patients with or without postoperative treatment at multiple US medical centers: Memorial Sloan Kettering Cancer Center (Taylor et al., n = 131) (20), MCC (Das et al., n = 306 and Mahajan et al. tissue microarray (TMA), n = 153) (21–23), Johns Hopkins Medical Institute (Ross et al., n = 260) (24), Mayo Clinic (Karnes et al., n = 235 and Erho et al., n = 545) (25, 26), Cleveland Clinic (Klein et al., n = 182) (27), and The Cancer Genome Atlas prostate adenocarcinoma (TCGA PRAD n = 333; multiple US medical centers) (28). (An overview of the prostate cancer patient study cohorts is available as Supplementary Table S1.) For the MCC patient cohorts, Das et al. and Mahajan et al., BCR was determined by clinical documentation of a single post-operative PSA ≥ 0.2 ng/ml or two consecutive post-operative PSA values of 0.2 ng/ml.
From the Das et al. cohort, 306 patients were selected for analyses if they had post-surgical clinical follow-up data (Table 1) and quantitative Real Time-PCR (qRT-PCR) successfully measured TTP and β-actin RNA expression (21, 22). The Das et al. cohort also includes 115 patient-matched normal adjacent tissue samples. From the Mahajan et al. TMA cohort, 153 primary prostate cancer patients whose primary treatment was RP and had post-surgical clinical follow-up data were selected for analyses (Table 1) (23). From the TCGA PRAD cohort, 280 patients had post-surgical clinical follow-up data and were selected for analyses (28). All primary prostate cancer patients in the Taylor et al., Ross et al., Karnes et al., Erho et al., and Klein et al. cohorts were included in these studies. CAPRA-S scores were calculated for the cohort and case-cohort studies, Das et al., Mahajan et al., Taylor et al., Ross et al., and Karnes et al., but not for TCGA PRAD data, which lacks necessary histopathological data for CAPRA-S. There were no other inclusion or exclusion criteria.
Table 1.
Clinico-Pathological & Demographic Characteristics of Moffitt Cancer Center Prostate Cancer Patient Cohorts
| Clinico-Pathological/ Demographic Characteristics | Das et al. Cohort (21, 22) n = 306 (%) | Mahajan et al. Cohort (23) n = 153 (%) | |
|---|---|---|---|
| pGleason Score | ≤ 3+3 | 149 (48.7) | 31 (20.3) |
| 3+4 | 90 (29.4) | 38 (24.8) | |
| 4+3 | 11 (3.6) | 38 (24.8) | |
| ≥ 8 | 16 (5.2) | 46 (30.1) | |
| Unknown | 40 (13.1) | 0 (0) | |
| Pathological Stage | pT2 | 210 (68.6) | 105 (68.6) |
| pT3 | 54 (17.6) | 38 (24.8) | |
| pT4 | 0 (0) | 2 (1.3) | |
| Unknown | 42 (13.7) | 8 (5.2) | |
| PSA (ng/ml) | ≤ 6 | 130 (42.5) | 72 (47.0) |
| > 6 – 10 | 76 (24.8) | 48 (31.3) | |
| > 10 – 20 | 34 (11.1) | 18 (11.8) | |
| > 20 | 11 (3.6) | 9 (5.9) | |
| Missing | 55 (18.0) | 6 (3.9) | |
| CAPRA-S | 0 – 2 | 165 (53.9) | 57 (37.2) |
| 3 – 5 | 76 (24.8) | 60 (39.2) | |
| 6 – 12 | 15 (4.9) | 30 (19.6) | |
| Incomplete | 50 (16.3) | 6 (3.9) | |
| Age at Diagnosis (Years) | Median | 60 | 60 |
| Inter Quartile Range (IQR) | 55 – 65 | 54 – 65 | |
| Median Follow Up (Months) | 104 | 102 | |
| Era of Prostatectomy | ≤ 2000 | 128 (41.8) | 38 (24.8) |
| > 2000 | 178 (58.2) | 115 (75.2) | |
| Race | Black | 13 (4.2) | 6 (3.9) |
| White | 293 (95.7) | 146 (95.4) | |
Abbreviations: pGleason, pathological Gleason; PSA, prostate specific antigen; CAPRA-S, Cancer of the Prostate Risk Assessment postsurgical.
RNA Preparation and Analyses
For the Das et al. cohort, total RNA isolation from formalin-fixed, paraffin-embedded (FFPE) prostate tissues was previously described (21, 22). cDNA was synthesized and qRT-PCR was performed as previously described (29). Data analyses used the ΔΔCt method, where levels of B-actin mRNA served as the internal control, and then the calculated results were converted to log2 expression. Oligonucleotides for qRT-PCR were as follows: β-actin (β-actin FOR3 5’-AAGGTGACAGCAGTCGGTTG-3’ and β-actin REV3 5’-CGGCCACATTGTGAACTTTG-3’) (30) and TTP (TTP FOR100 5’-CCACTCCTATCAGCGTCT-3’ and TTP REV100 5’-CGCTGCTGGCATATTCAT-3’).
For samples retrieved from the Decipher GRID database (Ross et al., Karnes et al., Erho et al., and Klein et al.), specimen selection, RNA extraction, and microarray hybridization was performed in a Clinical Laboratory Improvement Amendments-certified laboratory (GenomeDx Biosciences) and was previously described (24–26). Quality control was performed using Affymetrix Power Tools. Normalization was done using the single channel array normalization (SCAN) algorithm and expression was summarized using the median expression of probesets that map to exonic regions of the gene.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on a Ventana Medical Systems (VMS) Discovery XT using VMS’s UltraMap-AP Kit (VMS #760–153). Antigen retrieval consisted of a timed application of Standard CC1 (VMS # 950–500) at pH of 8.0 and Ventana Antibody Block (VMS #760–4204). LifeSpan Biosciences TTP antibody (LS-B5606) was diluted 1:200 or Ventana Rabbit IgG antibody was diluted 1:1 for a final concentration of 5μg/mL in Dako antibody diluent (S0809). Primary antibodies were incubated for 32 min at room temperature. This was followed by hematoxylin (VMS #760–2021) and Bluing Reagent (VMS #760–2037), four minutes for each cellular counterstain. TTP protein levels in the Mahajan et al. TMA samples were semi-quantitatively measured by a Modified H Scoring method in a blinded fashion by a research pathologist in the MCC Tissue Core. For this method, staining intensity (0 = negative, 1 = weak, 2 = moderate, 3 = strong) and percentage of positive cells (0 = 0%, 1 = 1% to 33%, 2 = 34% to 66%, 3 ≥ 67%) were determined. These two values were multiplied to generate a composite TTP IHC score (range = 0 to 9), and the scores were stratified as TTP-Low = 0 to 3 and TTP-High = 4 to 9. Images were taken using an Aperio AT2 Scanner (Leica Biosystems).
Western blot analyses
Protein from 293T cells was disrupted in lysis buffer (50mM HEPES, pH7.5, 150mM NaCl, 1mM EDTA, 2.5mM EGTA, and 0.1% Tween-20 with 1mM PMSF, 10mM β-glycerophosphate, 1mM NaF, 1mM NaVO4, and complete mini tablet protease inhibitor [Roche]) by sonication at 4°C two times until all cells were lysed. PANC-1 whole cell lysate was acquired (Santa Cruz Biotechnology, Inc. #sc-364380). Protein (30μg per lane) was separated a 10% SDS-polyacrylamide gel, transferred to a PVDF membrane, and blotted for antibodies specific for TTP (LifeSpan Biosciences #LS-B5606) and β-actin (Sigma #AC-15).
Statistical Analyses
Normalized gene expression obtained from the patient datasets was converted to log2 expression. Differences in TTP RNA expression in normal adjacent versus tumor tissue were tested by paired t-test. Differences in TTP RNA expression in patients that progressed to BCR versus those that remained indolent and patients that developed metastatic disease versus those that remained indolent were tested by Mann-Whitney U. Both tests were performed using MATLAB version 9.3 (The MathWorks, Inc.). Statistical analyses of time to BCR and time to metastasis was performed using a log-rank test, calculated using R software version 3.4.0. (http://www.R-project.org). In case-cohort studies, survival analysis was weighted using the Lin-Ying method for case-cohort design to estimate cohort parameters (31–33).
Hazard ratios and 95% confidence intervals for univariable analysis (UVA) and multivariable analysis (MVA) were estimated using Cox proportional hazard models in R software version 3.4.0. To account for cohort heterogeneity in pooled hazard ratio estimates, stratified Cox models were fit.
All statistical tests were two-sided with P values <0.05 considered statistically significant.
RESULTS
TTP is a Biomarker of BCR Risk Assessment Following Radical Prostatectomy
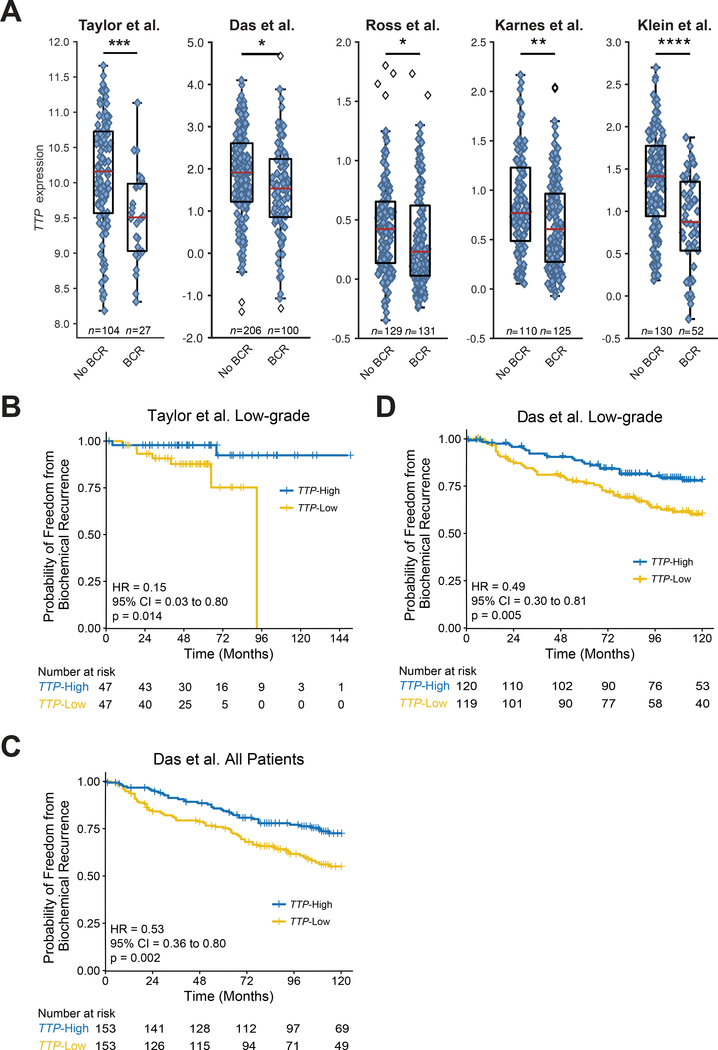
To assess the relationship between TTP mRNA expression and BCR, the Taylor et al., Das et al., Ross et al., Karnes et al., and Klein et al. datasets were evaluated. In all five datasets, patients that progressed to BCR had tumors with lower TTP RNA expression levels at the time of RP than patients whose tumors remained indolent (Fig. 1A). In addition, analysis of the Das et al. cohort found that TTP mRNA levels were lower in prostate tumor tissues versus patient-matched normal adjacent tissues (Supplementary Fig. S1A).
Figure 1.
TTP RNA is a biomarker for BCR risk assessment. A, Box-and-whisker plots of multiple prostate cancer patient cohorts (Taylor et al., Das et al., Ross et al., Karnes et al., and Klein et al.) showing TTP RNA expression at prostatectomy in prostate tumors that remained indolent (No BCR) versus tumors that developed BCR (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, two-sided Mann-Whitney U test). B, C, and D, Kaplan-Meier curves showing the time to BCR in primary prostate cancer patients separated into TTP-High and TTP-Low subtypes based on median TTP RNA levels for pathologically low-grade patients in the Taylor et al. cohort (B), all patients in the Das et al. cohort (C), and pathologically low-grade patients in the Das et al. cohort (D) (p-values, Mantel-Cox log-rank test).
To test if TTP mRNA expression levels at RP correlate with time to BCR, patients in the Taylor et al. and Das et al. cohorts were separated into two subtypes, TTP-High and TTP-Low, based on the median TTP expression level in each dataset. Our previous analysis revealed that TTP-Low patients in the Taylor et al. study had an decreased time to BCR compared to men with TTP-High prostate cancer (19). Here, using the Taylor cohort, we found that in pathologically low-grade prostate cancer (defined herein as pathological Gleason score (pGS) ≤ 7(3+4) tumors), patients with low expression of TTP also had a decreased time to BCR compared to TTP-High patients (Fig. 1B). To validate our results, we repeated these analyses and found that Das et al. patients with low TTP expressing prostate cancer also had a decreased time to BCR versus TTP-High patients (Fig. 1C). This difference was also present in the subset of low-grade patients within the Das et al. cohort, as men in this subset with TTP-Low tumors had a decreased time to BCR (Fig. 1D and Supplementary Fig. S1B). The Taylor and Das datasets have too few pathologically high-grade prostate cancer patients (defined here as pGS ≥ 7(4+3) tumors), 36 and 27 respectively, to properly analyze the relationship between TTP expression and BCR in this population; however, from the high-grade tumors that are present in these cohorts, the time to BCR was similar for both TTP-Low and TTP-High patients (Supplementary Fig. S1C and D). The TCGA PRAD dataset also showed decreased expression of TTP in patients that developed BCR, and a decreased time to BCR in men with TTP-Low tumors compared to patients with TTP-High tumors (Supplementary Fig. S2A and B). The clinical follow-up time for the TCGA PRAD study is limited (median 24 months), and there are very few BCR events in the low-grade subset; therefore, it was not possible to analyze this dataset for BCR in pGS subcategories. The Ross et al. and Karnes et al. studies were designed to have metastasis as the study endpoint, and Klein et al. is a case-control study, so the time to BCR also cannot be accurately assessed from these cohorts. Overall, these results provide strong evidence that TTP mRNA levels may be a promising prognostic biomarker of prostate cancer that distinguishes which patients with low-grade disease progress to BCR.
TTP Protein Levels Predict Risk for Progression to Aggressive Prostate Cancer
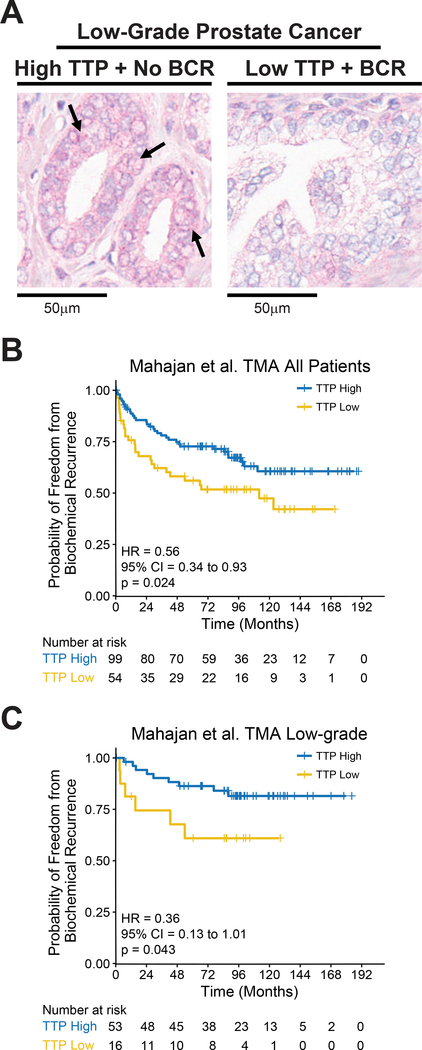
An IHC staining assay was developed to measure TTP protein expression in prostate tissues to test if TTP protein levels could be utilized as a clinical biomarker for identifying patients with an increased chance of BCR (Fig. 2A). To validate that the TTP antibody is specific for TTP, an immunoblot assay was performed on cells known to express TTP, PANC-1, and cells lacking TTP protein, 293T (Supplementary Fig. S3A). Specific antigen binding by the TTP antibody was confirmed by IHC staining of sections from the same FFPE prostate cancer sample using the TTP antibody and a non-specific rabbit IgG antibody (Supplementary Fig. S3B). Using this protocol TTP protein levels were measured in 153 primary prostate cancer samples from the Mahajan et al. study (Table 1) (23). Similar to TTP RNA analyses, patients with low TTP protein expression had a decreased time to BCR versus patients with TTP-High tumors (Fig. 2B). Once again, in the low-grade population, tumors with low TTP protein levels had a decreased time to BCR compared to those with high TTP expression (Fig. 2C). In addition, TTP protein levels did not predict for BCR in high-grade patients (Supplementary Fig. S3C).
Figure 2.
TTP protein levels predict risk for progression to BCR. A, Representative images (20x magnification) of TTP immunohistochemistry (IHC) showing TTP protein expression in low-grade primary prostate tumors collected at prostatectomy that remained indolent (No BCR) or developed BCR. Arrows indicate cells with high TTP protein levels (pink). B, Kaplan-Meier curves showing the time to BCR in primary prostate cancer patients from the Mahajan et al. TMA cohort separated into TTP-High and TTP-Low subtypes based on composite TTP IHC scoring for all patients (B) and low-grade patients (C) (p-values, Mantel-Cox log-rank test).
TTP Is a Clinically Relevant Biomarker of BCR Risk Assessment
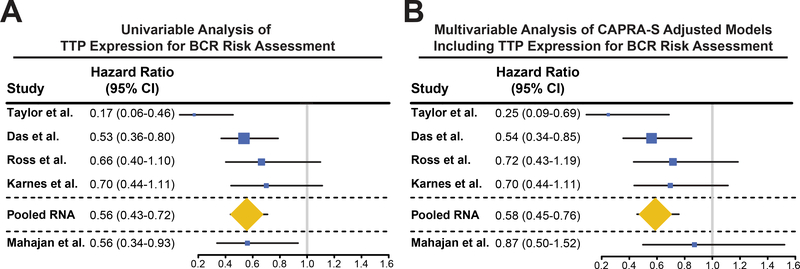
To determine if TTP RNA can be utilized as a prognostic biomarker for identifying RP patients that will have poor outcomes, Cox proportional hazard models were estimated for the Das et al., Taylor et al., Ross et al., and Karnes et al. datasets. Univariable analyses (UVA) of TTP expression in these cohorts indicate that TTP levels measured at prostatectomy can identify which prostate cancer patients develop BCR (Fig. 3A). Further, stratified Cox regression analysis of the pooled UVA from all of these datasets further supports the conclusion that TTP RNA is a clinically relevant biomarker of BCR (Fig. 3A). In addition, UVA of TTP protein expression in the Mahajan et al. TMA suggests that TTP protein is also a clinically relevant biomarker for BCR (Fig. 3A).
Figure 3.
TTP is a clinically relevant biomarker of BCR risk assessment. A and B, Forest plots of TTP’s univariable (A) and multivariable (B) Cox hazard ratios for BCR of multiple prostate cancer patient cohorts (Taylor et al., Das et al., Ross et al., Karnes et al., Pooled RNA, and Mahajan et al.). The size of the boxes correlates with the number of patients in each cohort.
The Cancer of the Prostate Risk Assessment postsurgical (CAPRA-S) score is a clinical risk calculation commonly used by physicians to predict the development of aggressive prostate cancer following RP based on preoperative PSA, pGS, surgical margins, extracapsular extension, seminal vesicle invasion, and lymph node invasion (34). Assessment of CAPRA-S and TTP RNA expression revealed that TTP levels are reduced in patients with high-risk CAPRA-S scores compared to patients with low-risk CAPRA-S in the Taylor cohort. Importantly, no other differences were observed between the Taylor et al. and Das et al. datasets, showing that there is no interaction between these two predictive indicators (Supplementary Fig. S4). Further, to evaluate if TTP RNA can improve clinical risk assessment for RP patients, hazard ratios were calculated for CAPRA-S adjusted models including TTP expression using multivariable analyses (MVA) for the same patient cohorts as the UVA. Similar to UVA, stratified Cox regression analysis of the pooled MVA hazard ratios shows that the addition of TTP RNA expression provides independent prognostic information beyond CAPRA-S, suggesting that TTP RNA may improve the ability to predict the patients who are likely to develop BCR after RP (Fig. 3B). In addition, the MVA of CAPRA-S adjusted to include TTP protein in the Mahajan et al. TMA suggests that TTP protein might improve risk prediction (Fig. 3B), but this analysis did not reach the level of significance so further evaluation is needed in a larger patient cohort to confirm this result.
TTP Expression Predicts Risk for Progression to Metastatic Disease
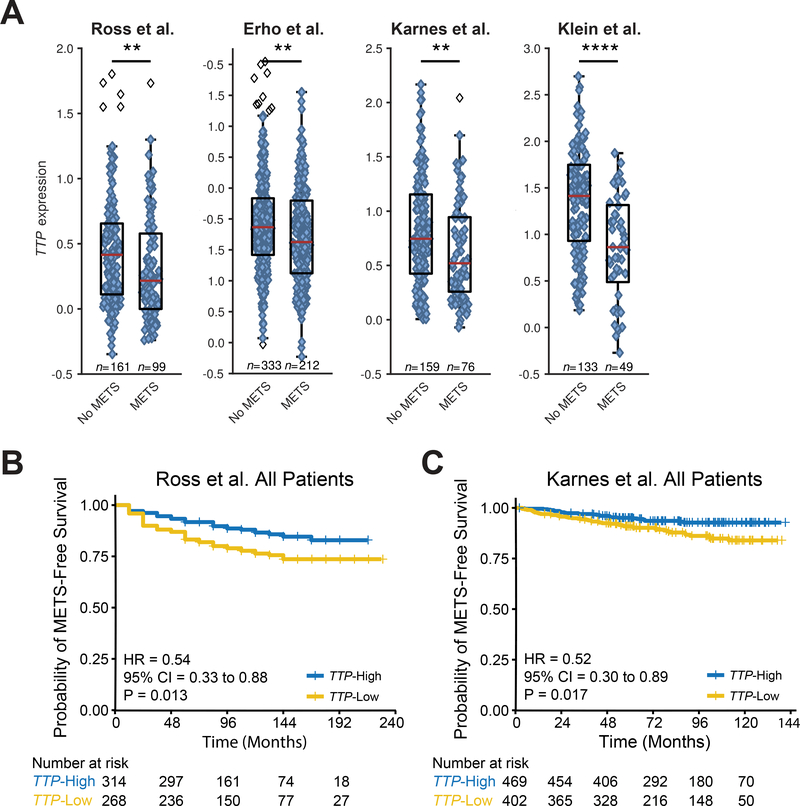
In addition to BCR, we assessed whether TTP RNA is a potential biomarker that discriminates which patients might progress to metastatic disease by testing four prostate cancer expression datasets: Ross et al., Erho et al., Karnes et al., and Klein et al. Patients in each of these studies that developed metastatic disease had lower TTP RNA levels at RP than men whose tumors remained indolent (Fig. 4A). Erho et al. and Klein et al. are case-control studies, so the time to metastatic disease cannot be accurately assessed. However, Ross et al. and Karnes et al. are case-cohort studies with metastasis as the study design endpoint, so patients in these cohorts were analyzed for time to metastasis. These analyses revealed that men with low TTP expression after RP progressed to metastatic disease more rapidly than patients with TTP-High prostate cancer (Fig. 4B and C). Both UVA and MVA of TTP expression and metastatic progression in prostate tumors in these two cohorts demonstrated that TTP RNA can function as a prognostic biomarker for identifying RP patients who are at an increased risk of metastasis (Table 2), both when taken as an independent biomarker and when added alongside CAPRA-S.
Figure 4.
TTP RNA expression predicts risk for progression to metastatic disease. A, Box-and-whisker plots of multiple patient cohorts (Ross et al., Erho et al., Karnes et al., and Klein et al.) showing TTP RNA levels at prostatectomy in prostate tumors that remained indolent (No METS) versus tumors that progressed to metastasis (METS) (**p < .01, ****p < .0001, two-sided Mann-Whitney U test). B and C, Kaplan-Meier curves showing the time to metastasis in primary prostate cancer patients separated into TTP-High and TTP-Low subtypes based on median TTP RNA expression levels in the Ross et al. (B) and Karnes et al. (C) cohorts (p-values, Mantel-Cox log-rank test).
Table 2.
Univariable and Multivariable Analysis of TTP RNA Expression and Metastasis
| UVA |
MVA |
||||
|---|---|---|---|---|---|
| Cohort | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | |
| Ross et al. (24) | 0.54 (0.33 to 0.88) | .013 | 0.62 (0.35 to 1.11) | .109 | |
| Karnes et al. (25) | 0.52 (0.30 to 0.89) | .017 | 0.54 (0.30 to 0.97) | .041 | |
Abbreviations: UVA, univariable analysis; MVA, multivariable analysis.
DISCUSSION
The epidemic in overtreatment of low-risk prostate cancer underscores the need to develop superior biomarkers that identify patients with an increased risk of developing aggressive disease. Here we validate the tumor suppressor TTP as a biomarker for predicting which prostate cancer patients may have poor outcomes following RP. Specifically, by evaluating TTP expression levels and its association with clinical outcomes in seven independent cohorts of prostate cancer patients (n = 1,939) from multiple medical centers, prostate tumors that developed BCR and/or metastatic disease were found to express lower levels of TTP RNA versus tumors that remained indolent. Further, TTP-Low prostate cancer progressed to BCR and metastasis at increased rates compared to TTP-High prostate cancer, especially in men with low-grade tumors. In addition, UVA establish TTP as a clinically relevant prognostic biomarker for identifying which RP patients may have poor outcomes. Finally, stratified proportional hazard modeling found that TTP RNA expression provides prognostic information distinct from what CAPRA-S already provides. Thus, the addition of TTP RNA expression as a variable to CAPRA-S might improve predictions of which RP patients harbor aggressive disease.
The National Comprehensive Cancer Network guidelines classify Stage T1-T2a prostate tumors with a GS ≤ 6 and PSA ≤ 10 ng/ml as very low-risk or low-risk disease, and men in these risk categories that have a life expectancy ≥ 10 years are candidates for active surveillance (35). However, many men in low-risk categories immediately undergo aggressive treatment despite the common adverse long-term side effects. Therefore, new clinical tests are needed to identify men that have low chances of failure on active surveillance. Our data repeatedly demonstrate that patients with low-grade tumors and low TTP expression have a significantly increased rate of BCR following RP versus men with low-grade tumors and high TTP levels. Thus, TTP may be a particularly beneficial biomarker for prognosticating which clinically low-risk prostate cancer patients will have a more aggressive clinical course.
A major strength of our study design is the validation of TTP’s ability to function as a biomarker in multiple genomic platforms, as well as in a multi-institutional cohort of prostate cancer patients across the United States. This is one of the larger studies to date of expression and outcomes in PCa (BCR total n=1,394 and metastatic outcome total n=1,222). Thus, this study provides strong evidence for the generalizability of TTP as a biomarker to identify inherently aggressive prostate tumors among patients thought to have clinically low-grade disease. However, there is the caveat that valid inference regarding generalizability in minority populations may be limited at this time, and that further study is needed for African American men in particular due to few known prognostic biomarkers for this population which is unduly affected by poor prostate cancer outcomes. Further, given that Gleason scores are changed ~30% of time from the initial biopsy score to the pathologic score following RP (36), an additional strength of our study is that it utilizes pGS, thus providing stringent analysis of prostate cancer outcomes in patients with true low-grade disease. Finally, we developed an IHC method to stain for TTP in prostate tissues and will perform future studies to determine if TTP protein expression from biopsy samples has the ability to predict prostate cancer outcomes. This could be of high clinical relevance as a tool that enables clinicians to more accurately risk-stratify their patients for appropriate treatment recommendations.
We recognize that this study has limitations. Specifically, our studies only assessed tissue from RP, which is more easily accessible than biopsy material. Accordingly, there is as yet no formal proof that TTP is a useful biomarker that will predict success or failure for active surveillance. However, given our findings, we submit that a long-term prospective study examining TTP expression in biopsies in newly diagnosed prostate cancer patients is warranted.
In conclusion, the tumor suppressor TTP is a clinically relevant prognostic prostate cancer biomarker that could be used in determining which patients will have poor clinical outcomes at the time of RP, and may improve the effectiveness of CAPRA-S. Notably, TTP performs particularly well as a biomarker in low-grade prostate cancer patients where much controversy exists on appropriate management options. The ability to use TTP to more accurately identify clinically low-risk patients who may harbor aggressive disease will enable physicians to more accurately risk-stratify their patients for appropriate therapy recommendations and will prevent overtreatment of potentially indolent disease.
Supplementary Material
Acknowledgements
The authors thank Dr. Nupam Mahajan for generously providing the prostate cancer tissue microarray utilized in these studies. We also thank Dr. Alex Lopez and Jeanette Rheinhardt of the Moffitt Cancer Center Tissue Core for their expert technical assistance. The authors thank Hyun Y. Park for the technical assistance that she provided for this study. Dr. John L. Cleveland also acknowledges the generous support of Lesa France Kennedy.
Grant Support
This work was supported in part by a Moffitt Cancer Center Team Science grant (to J.Y.P., A.E.B., and R.J.R.), NIH/NCI R01 grants CA128813 (to J.Y.P.) and CA167093 (to J.L.C.), Prostate Cancer Foundation Young Investigator Award (to K.Y.), American Cancer Society MRSG-17–108-01-TBG (to K.Y.), the Cortner Couch Endowed Chair, College of Medicine at the University of South Florida (to J.L.C.), and NCI Comprehensive Cancer Center Grant P30-CA076292 awarded to the H. Lee Moffitt Cancer Center & Research Institute.
Footnotes
Disclosure of Potential Conflicts of Interest: E. Davicioni holds ownership interest (including patents) in GenomeDx Biosciences. A.E. Ross holds ownership interest (including patents) in and is a consultant/advisory board member for GenomeDx Biosciences. E.A. Klein holds consulting or advisory roles for GenomeDx Biosciences, Berg, and Genomic Health. R.J. Karnes reports receiving other commercial research support from GenomeDx Biosciences. No potential conflicts were disclosed by the other authors.
References
- 1.Chabner BA, Smith M. Call it cancer. The oncologist. 2012;17:149–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, et al. Overdiagnosis and overtreatment of prostate cancer. European urology. 2014;65:1046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickel JC, Speakman M. Should we really consider Gleason 6 prostate cancer? BJU international. 2012;109:645–6. [DOI] [PubMed] [Google Scholar]
- 4.Lin K, Lipsitz R, Miller T, Janakiraman S, Force USPST. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Annals of internal medicine. 2008;149:192–9. [DOI] [PubMed] [Google Scholar]
- 5.Moyer VA, Force USPST. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2012;157:120–34. [DOI] [PubMed] [Google Scholar]
- 6.Bibbins-Domingo K, Grossman DC, Curry SJ. The US Preventive Services Task Force 2017 Draft Recommendation Statement on Screening for Prostate Cancer: An Invitation to Review and Comment. Jama. 2017;317:1949–50. [DOI] [PubMed] [Google Scholar]
- 7.Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7. [DOI] [PubMed] [Google Scholar]
- 8.Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–90. [DOI] [PubMed] [Google Scholar]
- 9.Dall’Era MA, Konety BR, Cowan JE, Shinohara K, Stauf F, Cooperberg MR, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112:2664–70. [DOI] [PubMed] [Google Scholar]
- 10.Brennan SE, Kuwano Y, Alkharouf N, Blackshear PJ, Gorospe M, Wilson GM. The mRNA-destabilizing protein tristetraprolin is suppressed in many cancers, altering tumorigenic phenotypes and patient prognosis. Cancer research. 2009;69:5168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young LE, Sanduja S, Bemis-Standoli K, Pena EA, Price RL, Dixon DA. The mRNA binding proteins HuR and tristetraprolin regulate cyclooxygenase 2 expression during colon carcinogenesis. Gastroenterology. 2009;136:1669–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HH, Son YJ, Lee WH, Park YW, Chae SW, Cho WJ, et al. Tristetraprolin regulates expression of VEGF and tumorigenesis in human colon cancer. Int J Cancer. 2010;126:1817–27. [DOI] [PubMed] [Google Scholar]
- 13.Sanduja S, Kaza V, Dixon DA. The mRNA decay factor tristetraprolin (TTP) induces senescence in human papillomavirus-transformed cervical cancer cells by targeting E6-AP ubiquitin ligase. Aging. 2009;1:803–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milke L, Schulz K, Weigert A, Sha W, Schmid T, Brune B. Depletion of tristetraprolin in breast cancer cells increases interleukin-16 expression and promotes tumor infiltration with monocytes/macrophages. Carcinogenesis. 2013;34:850–7. [DOI] [PubMed] [Google Scholar]
- 15.Bourcier C, Griseri P, Grepin R, Bertolotto C, Mazure N, Pages G. Constitutive ERK activity induces downregulation of tristetraprolin, a major protein controlling interleukin8/CXCL8 mRNA stability in melanoma cells. American journal of physiology Cell physiology. 2011;301:C609–18. [DOI] [PubMed] [Google Scholar]
- 16.Fallahi M, Amelio AL, Cleveland JL, Rounbehler RJ. CREB targets define the gene expression signature of malignancies having reduced levels of the tumor suppressor tristetraprolin. PLoS One. 2014;9:e115517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei ZR, Liang C, Feng D, Cheng YJ, Wang WM, Yang DJ, et al. Low tristetraprolin expression promotes cell proliferation and predicts poor patients outcome in pancreatic cancer. Oncotarget. 2016;7:17737–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HH, Lee SR, Leem SH. Tristetraprolin regulates prostate cancer cell growth through suppression of E2F1. Journal of microbiology and biotechnology. 2014;24:287–94. [DOI] [PubMed] [Google Scholar]
- 19.Berglund AE, Scott KE, Li W, Yang C, Fernandez MR, Schaub FX, et al. Tristetraprolin disables prostate cancer maintenance by impairing proliferation and metabolic function. Oncotarget. 2016;7:83462–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010;18:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das DK, Naidoo M, Ilboudo A, Park JY, Ali T, Krampis K, et al. miR-1207–3p regulates the androgen receptor in prostate cancer via FNDC1/fibronectin. Exp Cell Res. 2016;348:190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das DK, Osborne JR, Lin HY, Park JY, Ogunwobi OO. miR-1207–3p Is a Novel Prognostic Biomarker of Prostate Cancer. Translational oncology. 2016;9:236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahajan K, Challa S, Coppola D, Lawrence H, Luo Y, Gevariya H, et al. Effect of Ack1 tyrosine kinase inhibitor on ligand-independent androgen receptor activity. The Prostate. 2010;70:1274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross AE, Johnson MH, Yousefi K, Davicioni E, Netto GJ, Marchionni L, et al. Tissue-based Genomics Augments Post-prostatectomy Risk Stratification in a Natural History Cohort of Intermediate- and High-Risk Men. European urology. 2016;69:157–65. [DOI] [PubMed] [Google Scholar]
- 25.Karnes RJ, Bergstralh EJ, Davicioni E, Ghadessi M, Buerki C, Mitra AP, et al. Validation of a genomic classifier that predicts metastasis following radical prostatectomy in an at risk patient population. The Journal of urology. 2013;190:2047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erho N, Crisan A, Vergara IA, Mitra AP, Ghadessi M, Buerki C, et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS One. 2013;8:e66855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein EA, Yousefi K, Haddad Z, Choeurng V, Buerki C, Stephenson AJ, et al. A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy. European urology. 2015;67:778–86. [DOI] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas Research N. The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163:1011–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rounbehler RJ, Fallahi M, Yang C, Steeves MA, Li W, Doherty JR, et al. Tristetraprolin impairs myc-induced lymphoma and abolishes the malignant state. Cell. 2012;150:563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong H, Zhu M, Cui F, Wang S, Gao X, Lu S, et al. Quantitative assessment of short amplicons in FFPE-derived long-chain RNA. Scientific reports. 2014;4:7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin DY, Ying Z. Cox Regression with Incomplete Covariate Measurements. Journal of the American Statistical Association. 1993;88:1341–9. [Google Scholar]
- 32.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. Journal of clinical epidemiology. 1999;52:1165–72. [DOI] [PubMed] [Google Scholar]
- 33.Therneau TM, Li H. Computing the Cox model for case cohort designs. Lifetime data analysis. 1999;5:99–112. [DOI] [PubMed] [Google Scholar]
- 34.Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: A straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011;117:5039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohler JL, Antonarakis ES, Armstrong AJ, al. e. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. NCCN Guidelines. 2017;Version 2. [Google Scholar]
- 36.Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. European urology. 2012;61:1019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.